Abstract
Insect pest management relies mainly on neurotoxic insecticides, including neonicotinoids, leaving residues in the environment. There is now evidence that low doses of insecticides can have positive effects on pest insects by enhancing various life traits. Because pest insects often rely on sex pheromones for reproduction, and olfactory synaptic transmission is cholinergic, neonicotinoid residues could modify chemical communication. We recently showed that treatments with different sublethal doses of clothianidin could either enhance or decrease behavioural sex pheromone responses in the male moth, Agrotis ipsilon. We investigated now effects of the behaviourally active clothianidin doses on the sensitivity of the peripheral and central olfactory system. We show with extracellular recordings that both tested clothianidin doses do not influence pheromone responses in olfactory receptor neurons. Similarly, in vivo optical imaging does not reveal any changes in glomerular response intensities to the sex pheromone after clothianidin treatments. The sensitivity of intracellularly recorded antennal lobe output neurons, however, is upregulated by a lethal dose 20 times and downregulated by a dose 10 times lower than the lethal dose 0. This correlates with the changes of behavioural responses after clothianidin treatment and suggests the antennal lobe as neural substrate involved in clothianidin-induced behavioural changes.
Keywords: sublethal insecticide dose, olfactory receptor neurons, antennal lobe, calcium imaging, electrophysiology, moth
1. Introduction
Neurotoxic insecticides, including neonicotinoids, are of paramount importance in pest management, and despite recent efforts, their effects on the insect nervous system are still not well understood. Neonicotinoids act selectively on the insect central nervous system as agonists of the nicotinic acetylcholine receptors (nAChRs), and thus disturb synaptic transmission [1,2]. They are highly efficient because of their systemic action for crop protection (i.e. their distribution in all organs of a treated plant). Neonicotinoids, such as the widely used clothianidin, have insecticidal effects on a broad range of insect pests ([3] and references therein). Owing to their widespread use and long half-life in anaerobic soil, pesticide residues accumulate in the environment [4]. In addition to the known lethality at high doses, these pesticide residues may have negative effects on target insects, thus improving their pest control effects. They can also have negative effects on non-target insects such as honeybees, for which several studies showed that neonicotinoids decrease survival, and impair foraging behaviour and learning and memory functions [5–7]. On the other hand, stimulatory effects on various life traits associated with low doses of insecticides have been reported, and this is currently becoming recognized as a general toxicological phenomenon called hormesis, characterized by inhibition at high doses and stimulation at low doses by the same toxic compound [8–10].
Most animals, including agricultural pest insects, rely on olfaction to find their mating partners. Because synaptic transmission in sensory systems, including olfaction, is mainly cholinergic, neonicotinoid residues could modify the chemical communication system and consequently decrease or even increase reproductive capacities in pest insects. In moths, males are attracted by female-produced sex pheromones [11]. They detect the sex pheromone through olfactory receptor neurons (ORNs) on their antennae. Attraction behaviour is elicited owing to central processing in the macroglomerular complex (MGC) of the primary olfactory centre, the antennal lobe (AL), and higher brain centres such as the mushroom bodies and the lateral protocerebrum [12,13].
In insects, behavioural sex pheromone responses are submitted to modulation as a function of the physiological state or experience [14,15]. In the black cutworm moth, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae), a worldwide pest insect [16], for example, the neuronal basis of age- and mating-state-dependent modulation of pheromone-guided behaviour has been investigated. The response threshold of AL neurons in this species is modified depending on age and mating state, whereas peripheral responses to the sex pheromone do not vary [15]. We also recently showed that low doses of clothianidin induced differential effects on male orientation towards the pheromone in a wind tunnel in A. ipsilon [17]. Orientation behaviour was improved after intoxication with 10 ng clothianidin per moth, corresponding to the LD20, whereas orientation behaviour was disturbed by a treatment with 0.25 ng per moth, corresponding to a dose 10 times lower than the LD0 [17].
To investigate the neural mechanisms underlying such behavioural modifications, we tested the effects of the two above-mentioned clothianidin doses on individual and global response thresholds of AL input (ORNs) and output (projection neurons) neurons in vivo. In correlation with behavioural changes after clothianidin treatment, the sensitivity of AL output neurons but not ORNs was modified in a dose-dependent manner.
2. Material and methods
(a). Insects
Experiments were performed with laboratory-reared adult males of A. ipsilon fed on an artificial diet [18] in individual cups until pupation. Pupae were sexed, and males and females were kept separately at 22°C in an inversed light–dark cycle (16 h : 8 h light : dark photoperiod). Newly emerged adults were removed every day from hatching containers and given access to 20% sucrose solution ad libitum. The day of emergence was considered as day 0. Four-day-old virgin males of A. ipsilon were treated with the solvent or insecticide, and experiments were performed at the age of 5 days.
(b). Chemicals
Clothianidin (99% purity) was first dissolved in dimethyl sulfoxide (DMSO) and dilutions (0.25 and 10 ng) were prepared with 20% sucrose solution. The concentrated solution was stored in the freezer, and diluted solutions were stored for no more than 4 days in the refrigerator protected from light. All chemicals were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France), unless stated otherwise.
(c). Clothianidin intoxication
Oral application of clothianidin was accomplished as described previously [17]. Briefly, 4-day-old virgin males were restrained in plastic pipette tips with their head protruding before the onset of the scotophase. They were then fed with either 10 µl of clothianidin-contained solution or the corresponding DMSO control solution and kept in plastic containers until the next day. Intoxications were performed with the two doses that were previously shown to induce effects on behavioural responses: 0.25 and 10 ng per moth. As control group, insects were fed with a solution of DMSO corresponding to the concentration of DMSO in the clothianidin solutions (5 × 10−4% or 2 × 10−2% for the 0.25 ng or 10 ng clothianidin doses, respectively). We used DMSO treatments and untreated sugar-fed males as controls in this study. Note that individual clothianidin doses were always tested in parallel with the corresponding DMSO concentrations, whereas experiments with different clothianidin doses were done at different times during the year for imaging and intracellular recording experiments, and absolute values are therefore not directly comparable among each other owing to varying effects over time.
(d). Odour stimulation
For stimulation in calcium imaging and intracellular recordings, an artificial behaviourally active pheromone blend containing (Z)-7-dodecen-1-yl acetate (Z7–12:OAc), (Z)-9-tetradecen-1-yl acetate (Z9–14:OAc) and (Z)-11-hexadecen-1-yl acetate (Z11–16:OAc) at a ratio of 4 : 1 : 4 was used [19–21] in order to compare physiological results with the previously acquired behavioural data [17]. For single-sensillum recordings, only the major pheromone component, Z7–12:OAc, was used, because ORNs are tuned to individual pheromone compounds. Responses at the peripheral and AL level are nevertheless comparable, because the vast majority of AL neurons respond to the major pheromone component [22]. Whereas ORNs are selectively only responding to a single compound, certain AL neurons also respond to other compounds in addition [23]. Responses to the major compound are generally very similar to responses to the pheromone blend, and only very few AL neurons are blend-specific in A. ipsilon [24]. For all experiments, pheromone stimuli were diluted in decadic steps in hexane and applied on a filter paper introduced in a Pasteur pipette. For single sensillum recordings, doses from 0.1 ng to 1 µg of Z7–12:Ac were used (lower doses did not elicit any responses). For calcium imaging experiments, the pheromone blend was tested at doses from 0.01 to 10 ng to limit the number of stimuli, critical for these experiments. Preliminary experiments have shown that higher stimulus doses did not further increase responses. For intracellular recordings, the pheromone blend was used at doses from 0.01 pg to 100 ng. A minimum evaporation time of 30 min was respected. To avoid mechanical stimulation at the odour onset, the antennae were constantly superfused by a humidified and charcoal-filtered air stream (70 l h−1). An air pulse of 200 ms for peripheral and intracellular recordings and 1 s for imaging experiments (10 l h−1) passing through a Pasteur pipette containing the stimulus on a filter paper was introduced into the constant air stream by means of a stimulation device (CS55 Syntech, Kirchzarten, Germany).
(e). Single-sensillum recordings
One sensillum was recorded per insect. Insect preparation and recordings were performed as described earlier [25], but using electrolytically sharpened tungsten wires (TW5-6, Science Products, Hofheim, Germany) instead of tip recordings. The recording electrode was inserted at the base of a long sensillum trichodeum located on an antennal branch. The reference electrode was inserted in the antennal stem. Recordings were done using an EX1 amplifier with a 4002 headstage (Dagan, MN, USA). The biological signal was amplified (×1000), high-pass (1 Hz) and low-pass (3 kHz) filtered and sampled at 10 kHz via a 16-bit acquisition board (CRIO-9215, National Inst., Nanterre, France) under Labview (National Inst.). Odour stimuli were applied with interstimulus intervals of at least 1 min. Four different parameters were used to compare the mean ORN activities between treatments. Spontaneous activities were measured for 30 s on each sensillum. Action potential frequencies were calculated during the first second after stimulus onset. Post-stimulus time histograms (PSTHs) have a 50 ms binning and the first 24 bins (1.2 s) after stimulus onset were compared between treatments. Finally, cumulative threshold curves were established as a function of stimulus dose. Because in our experimental conditions responses never began before 200 ms after stimulus onset, the neuron response threshold was determined as the lowest concentration that elicited at least four action potentials between 200 and 350 ms after stimulus onset.
(f). Calcium imaging
For calcium imaging, moths were restrained individually in Plexiglas chambers, as described earlier [26]. Ten microlitres of dye solution (50 mg calcium green 2-AM; Molecular Probes, Eugene, OR, USA) dissolved in 50 ml Pluronic F-127 (20% in DMSO) was bath-applied at 4°C for at least 1 h on the opened head capsule. After washing with Ringer, recordings were done using a TILL Photonics imaging system (Martinsried, Germany) with an epifluoresence microscope (Olympus BX-51WI, Olympus, Hamburg, Germany) equipped with a 10× water immersion objective. 1004 × 1002 pixel images were taken with a 14-bit monochrome CCD camera (Andor iXON) for 20 s at a rate of 5 Hz. If possible, three runs of stimulations were recorded for each experimental insect. The interstimulus interval was approximately 60 s.
Identification of activated regions within the MGC of one AL for each animal was done by superposing activity maps of all stimulations. Raw data were analysed using custom-made software written in IDL (Research Systems Inc., CO, USA) and Visual Basic (Microsoft Excel) as described earlier [26]. Response intensities (difference between recorded signal and the estimated background normalized by the background, ΔF/F) and the time course of responses were averaged over all recordings within the same treatment.
(g). Intracellular recordings
For intracellular recordings, moths were immobilized in a cut disposable pipette tip, the head protruding. The brain was exposed by removing the cuticle and overlaying tissue from the AL as described previously [24]. Standard intracellular recording techniques were used [27]. AL neurons in the MGC were penetrated randomly by inserting a recording electrode, filled with 3 M KCl, close to the entrance of the antennal nerve. Data were recorded and analysed off-line using Autospike 32 software (Syntech, Kirchzarten, Germany). The neuron response threshold was determined, as previously described, as the lowest concentration, which elicited a net pheromone response exceeding the net hexane response by at least 20% (where the net response corresponds to the odour/hexane response minus the spontaneous activity before stimulus onset [28]). Data are presented as cumulative threshold curves as a function of stimulus dose threshold distributions.
(h). Statistical analyses
ORN spontaneous activities were compared between treatments using a one-way ANOVA. The effects of dose and treatment on the ORN response frequency and on the Ca2+ response were evaluated statistically using two-way ANOVAs for repeated measures. The effects of dose and treatment on the PSTHs were compared with an ANOVA for repeated measures. As data did not meet the sphericity criteria, the degree of freedom (d.f.) of ANOVAs was adjusted according to Greenhouse & Geisser [29]. The proportion of ORNs and AL neurons responding to sex pheromone at different thresholds were compared statistically between treatments with an R × C test of independence, by using a G-test and applying the Williams correction [30].
3. Results
(a). Effects of clothianidin on olfactory receptor neuron spontaneous activity and pheromone responses
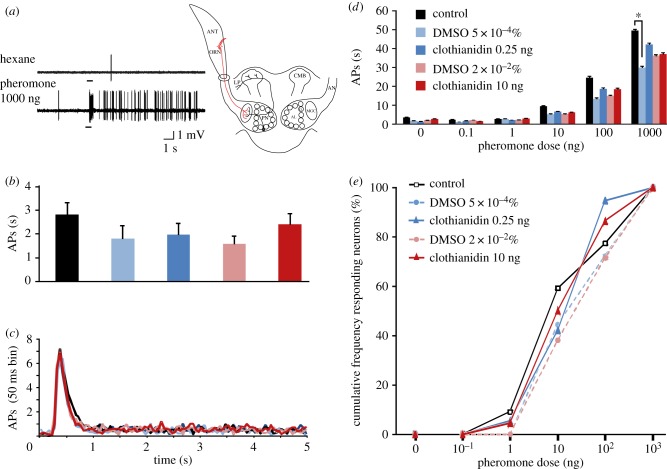
The mean spontaneous activity of ORNs and their responses to five doses of the main pheromone compound and a control (hexane) were compared between males subjected to five different treatments (two doses of clothianidin and their respective DMSO controls, as well as sugar-fed control males). The mean spontaneous firing activities did not differ significantly between males from the five treatments (F4,106 = 1.11, p = 0.35; figure 1b). ORNs exhibited excitatory responses to the major pheromone component (figure 1a). The average time courses of these responses (number of spikes fired per 50 ms bin) were not significantly different between treatments (F14.9,394.9 = 1.44, p = 0.13; figure 1c). Responses of the recorded ORNs, measured as the number of action potentials fired in 1 s, were highly similar but not equal across all treatments (F4,97 = 3.48, p = 0.01; figure 1d). However, there was only a significant difference in responses to 1000 ng of Z7–12:Ac between sugar-fed control males and 5.10−4% of DMSO-treated males (post hoc Tukey's honest significant difference test, p = 0.01). When considering only males treated with either of the two different doses of clothianidin and the corresponding DMSO controls, no difference in response was observed (F3,76 = 1.19, p = 0.32). Finally, no significant difference was detected in the cumulative threshold curves between treatments, in particular when comparing the clothianidin-treated groups with their corresponding DMSO controls (clothianidin 0.25 ng versus DMSO: G = 1.59, d.f. = 4, p = 0.81; clothianidin 10 ng versus DMSO: G = 1.48, d.f. = 4, p = 0.83; figure 1e).
Figure 1.
Clothianidin does not affect ORN responses. (a) Example of recording traces from an ORN in a trichoid sensillum stimulated with hexane (control stimulus) and 1000 ng of the major sex pheromone component (Z7–12:OAc) in a male treated with 0.25 ng clothianidin. Bar beneath recording indicates stimulus duration (200 ms). The sketch indicates the recording level. (b) Average spontaneous activity of ORNs recorded from males of five treatment groups (sugar-fed controls, 0.25 ng and 10 ng clothianidin, 5×10−4% and 2×10−2% DMSO) (mean ± s.e.m., n = 21–23). (c) Average time courses of responses to 1000 ng of Z7–12:OAc expressed as the number of action potentials (APs) fired per 50 ms bin. For clarity, data are presented in curves and not as classical post-stimulus time histograms (n = 18–22). (d) Average responses to different doses of Z7–12:OAc and hexane from males of the five treatment groups (mean ± s.e.m., n = 18–22). (e) Cumulative percentage of tested ORNs responding to Z7–12:OAc at different thresholds in males of the five treatment groups (n = 18–22).
(b). Effects of clothianidin on the glomerular activation pattern within the antennal lobe
Stimulation with the different pheromone doses induced typical biphasic calcium signals within the MGC, which reached their maximum approximately 2 s after odour onset (figure 2a). Treatment with 0.25 ng clothianidin did not change MGC response intensities to different pheromone doses compared with animals treated with the corresponding DMSO dose (main effect treatment: F1,17 = 0.28, p = 0.61; figure 2b). The treatment × pheromone dose interaction was not significant (F4,68 = 0.77, p = 0.55). Similarly, MGC response intensities observed in males treated with 10 ng clothianidin did not differ from those observed in males treated with the corresponding DMSO control (main effect treatment: F1,25 = 1.78, p = 0.19; figure 2b). The treatment × pheromone dose interaction was again not significant (F4,100 = 0.59, p = 0.67). When comparing clothianidin- and DMSO-treated males with sugar-fed males, no differences were found between the corresponding groups (main effect treatment: F4,52 = 2.48, p = 0.06; figure 2b). Thus, treatments with two different clothianidin doses had no effect on response intensities to different pheromone doses in the male A. ipsilon MGC.
Figure 2.
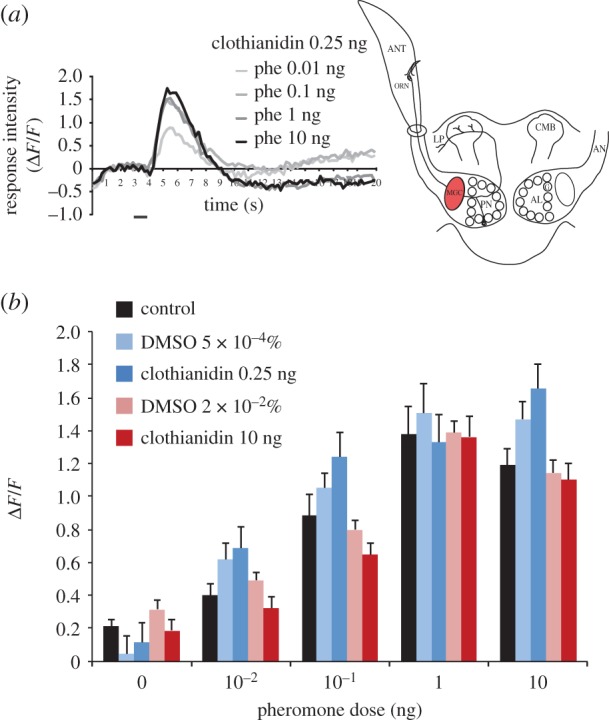
Clothianidin does not affect global AL input responses. (a) Average time course of in vivo calcium responses to four pheromone (phe) doses in males treated with 0.25 ng clothianidin (n = 9). Bar beneath recording indicates stimulus duration (1 s). The sketch indicates the recording level. (b) Average response intensity to different pheromone doses in males of five different treatment groups (sugar-fed controls, 0.25 and 10 ng clothianidin, 5×10−4% and 2×10−2% DMSO; mean±s.e.m. n = 8–14).
(c). Effects of clothianidin on antennal lobe neuron thresholds
Intracellularly recorded AL neurons showed predominantly excitatory responses to the pheromone, followed in most cases (over 90%) by an inhibitory period [22], which is characteristic for MGC projection neuron responses [22] (figure 3a). The cumulative threshold curve obtained from neurons recorded in males treated with 10 ng clothianidin was significantly shifted to lower sex pheromone doses when compared with AL neurons in DMSO-treated controls (G = 30.55, d.f. = 7, p ≤ 0.0001; figure 3b). On the contrary, AL neurons in 0.25 ng clothianidin-treated males show a cumulative threshold curve, which is shifted significantly to higher sex pheromone doses when compared with DMSO-treated males (G = 29.28, d.f. = 7, p ≤ 0.0001; figure 3b). Both threshold curves for DMSO-treated males were not significantly different from the curve for sugar-fed control males (G = 6.98, d.f. = 7, p = 0.43).
Figure 3.
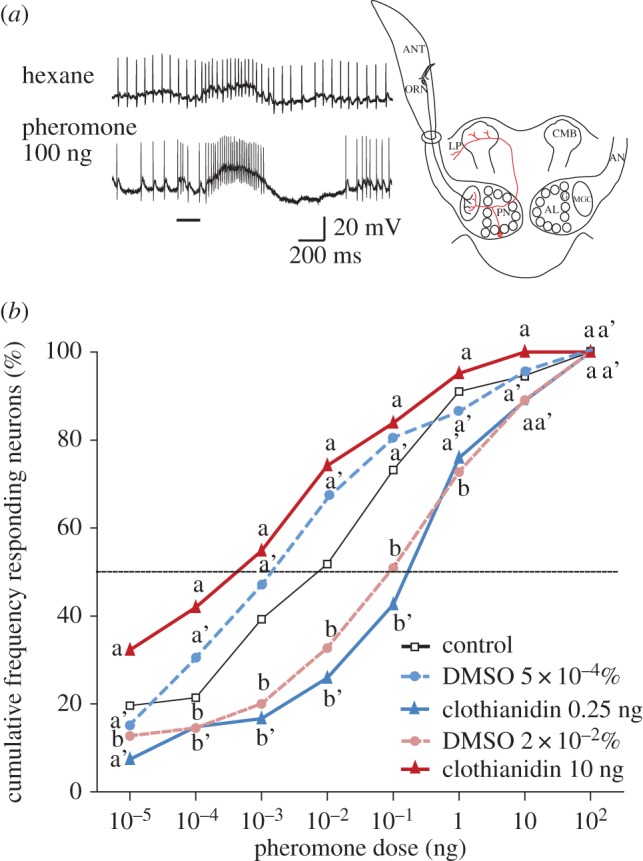
Clothianidin intoxication affects response thresholds of AL neurons. (a) Example of intracellular recording traces of an AL neuron from a clothianidin-treated (10 ng) male stimulated with hexane and the sex pheromone. Bar beneath recording indicates stimulus duration (200 ms). The sketch indicates the recording level. (b) Cumulative percentage of tested AL neurons responding to the pheromone blend at different thresholds in males of five different treatment groups (sugar-fed controls, 0.25 ng and 10 ng clothianidin, 5×10−4% and 2×10−2% DMSO; n = 43–66 in 20–27 males). Values with the same letters are not significantly different (clothianidin 10 ng: a, b; clothianidin 0.25 ng: a′, b′).
4. Discussion
In this study, we show that the dose-dependent modifications of pheromone-guided behaviour observed after clothianidin intoxication [17] might originate from the effect of insecticide treatments on the sensitivity of AL neurons to the sex pheromone. Indeed, single sensillum recordings and calcium imaging approaches show no significant effect of clothianidin at the used doses when compared with the corresponding solvent controls on the responses of individual ORNs and on the global input response in the AL. By contrast, intracellular recordings demonstrate that this insecticide induces an increase or decrease in sensitivity of AL neurons depending on the clothianidin concentration used.
Low doses of other insecticides (two pyrethroids and DDT) have been shown to affect the peripheral detection of sex pheromone in two species of Mamestra [31]. However, these insecticides differ in their target from neonicotinoids: pyrethroids and DDT interact with sodium channels, modifying the excitability of the spike initiation site of ORNs [31]. We recorded only from the major type of pheromone sensilla and can thus not exclude that the sensitivity of ORNs situated in other sensilla might be influenced by low doses of clothianidin. However, the absence of clothianidin effects on the sensitivity of pheromone-responding ORNs is not surprising when taking into consideration that, to our current knowledge, sensory neurons do not receive cholinergic input and probably do not express nAChRs. Nevertheless, modulation of the sensory input delivered by ORNs to the AL could occur via feedback synapses from AL local neurons (i.e. via presynaptic inhibition). Such effects would not be sufficient to record differences at the level of the antennal sensilla but could potentially be revealed by optical imaging of AL input activity. However, optical imaging responses to the pheromone were not modified by either of the two tested clothianidin doses when compared with responses in males treated with the corresponding DMSO concentration. Therefore, we assume that clothianidin indeed only affects pheromone-sensitive AL neurons or that the inhibitory feedback to ORNs might be too locally restricted to have an effect on the net calcium signal. Nevertheless, the absence of effects in the MGC might not mirror a general rule. Feedback from LNs on ORNs could potentially be different for the plant-odour-processing parts of the AL, and therefore the effect of clothianidin on plant odour responses should be tested in the future. The modulatory mechanisms elicited by neonicotinoids in intact neural networks are not well understood so far. In the honeybee, bath application of low doses of clothianidin and imidiacloprid (1–100 nM) on isolated brain preparations lead to sustained nAChR activation and reduced Kenyon cell responses to acetylcholine stimulation [32]. Thus, it might be possible that the 0.25 ng clothianidin dose reduces olfactory responses in the A. ipsilon AL owing to sustained nAChR activation. However, these data are not easily comparable with our results, because we do not know which concentrations of the insecticide reach the central nervous system after treatment through feeding, and effects on Kenyon cells might be different from effects on AL neurons. The behavioural effects of the different low doses of clothianidin certainly originate from combined effects on different neuronal levels.
The modulation of AL neuron sensitivity underlying behavioural plasticity in response to sex pheromone has been shown in different contexts in male noctuid moths, including A. ipsilon. Up- and downregulation of AL sensitivity has been revealed as a function of adult maturation, mating and experience, whereas no or only minor sensitivity changes occur in the peripheral olfactory system [33–38]. Although the cellular and molecular mechanisms of the regulation of AL sensitivity are largely unknown, hormones (juvenile hormone, ecdysone) and biogenic amines (octopamine and dopamine) have been identified as neuromodulators involved in the different forms of behavioural plasticity in A. ipsilon males [28,35,37,39]. Here, we identified changes of pheromone sensitivity caused by the insecticide clothianidin at the same level as for other types of plasticity, even if the cellular and molecular mechanisms might be different in this case. The sensitivity of MGC neurons decreased after treatment with a very low dose (0.25 ng) and increased after treatment with a still relatively low dose (10 ng). Even though we did not stain intracellularly recorded neurons, the observed response patterns of the majority of the neurons corresponded with those observed in projection neurons in A. ipsilon [22,25]. Electrical properties of AL neurons or more complex effects at the AL network level might participate in the observed changes. It is also possible that the modulation of AL neuron sensitivity is caused by increased dopamine release, which has been shown to be caused by clothianidin treatments in the rat brain [40]. Even though nothing is known about clothianidin effects on dopamine release in insects so far, the recently observed role of the dopamine/steroid receptor DopEcR in regulating the sensitivity of pheromone-responding AL neurons [28] would allow this type of modulation.
Numerous studies have shown a negative effect of neonicotinoid insecticides on cognitive processes (i.e. learning and memory), especially in beneficial insects, such as the honeybee (review in [41]). However, reports on effects of these insecticides on sensory systems are rare. We show here that a dose of clothianidin below the LD0 (0.25 ng) decreases the sensitivity of AL neurons, whereas intoxication with a dose corresponding to the LD20 (10 ng) increases AL neuron sensitivity, i.e. a 100-fold lower pheromone dose is necessary to elicit a response in neurons after intoxication compared with solvent (DMSO)-treated males. This result can be compared with findings in the honeybee, showing that nicotine, a potent ligand of nAChRs, at doses of 10−5 to 10−6 M can increase the sensitivity of the gustatory system to sugar, and can improve olfactory memory, even though the underlying neural mechanisms have not been investigated [42].
The differential effects of clothianidin on the central nervous pheromone responses may be explained by the involvement of distinct nAChR subtypes with different affinities to this ligand. Different subunit combinations generate nAChRs with different pharmacological properties [43]. Certain nicotinic receptor subunits have in addition been shown to be differentially expressed in different parts of the honeybee brain, including areas treating olfactory information such as the ALs [42,44–46]. We hypothesize that different nAChR types with different affinities to clothianidin are expressed in different neuron types within the AL network, which could cause the opposing effects of different doses. In order to investigate which subunits are expressed in which neuron types, it would be necessary to develop antibodies against different receptor subunits and use them for immunocytochemical stainings of the brain. The knowledge on differential expression of nAChR subunits in different neuron types and different insect species linked with specific affinity of different neonicotinoids to the resulting receptors would provide a very powerful tool to study neural networks of sensory systems. In addition, this knowledge would provide important information for the development of new, species-specific neonicotinoids to achieve more efficient pest control and to avoid harming beneficial insects such as pollinators.
Acknowledgements
We thank Corinne Chauvet, Lydie Garnier and Cyril Le Corre for insect rearing and two anonymous referees for helpful comments on an earlier version of the manuscript.
Data accessibility
Data are available upon request from the corresponding author: sylvia.anton@angers.inra.fr.
Authors' contributions
K.K.R. carried out part of the electrophysiological experiments, participated in the design of experiments and data analysis, and drafted the manuscript. N.D. and J.L.C. carried out the imaging experiments, and N.D. participated in data analysis and helped draft the manuscript. E.D. and G.R. carried out part of the electrophysiological experiments. H.T.-L. participated in the design of experiments, and helped draft the manuscript. P.L. and C.G. participated in the design of experiments and data analysis, and helped draft the manuscript. S.A. designed and coordinated the study, participated in part of the electrophysiological experiments and data analysis, and drafted the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a French National Funding Agency grant no. ANR-12 ADAP- 0012-01, and by a grant from the ‘Région Pays de la Loire’ to S.A. and C.G.
References
- 1.Matsuda K, Kanaoka S, Akamatsu M, Sattelle DB. 2009. Diverse actions and target-site selectivity of neonicotinoids: structural insights. Mol. Pharmacol. 76, 1–10. ( 10.1124/mol.109.055186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown LA, Ihara M, Buckingham SD, Matsuda K, Sattelle DB. 2006. Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J. Neurochem. 99, 608–615. ( 10.1111/j.1471-4159.2006.04084.x) [DOI] [PubMed] [Google Scholar]
- 3.Nauen R, Ebbinghaus-Kintscher U, Salgado VL, Kaussmann M. 2003. Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic. Biochem. Physiol. 76, 55–69. ( 10.1016/S0048-3575(03)00065-8) [DOI] [Google Scholar]
- 4.Goulson D. 2013. Review: an overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. ( 10.1111/1365-2664.12111) [DOI] [Google Scholar]
- 5.Henry M, Béguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. ( 10.1126/science.1215039) [DOI] [PubMed] [Google Scholar]
- 6.Blacquiere T, Smagghe G, van Gestel CA, Mommaerts V. 2012. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21, 973–992. ( 10.1007/s10646-012-0863-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfray HCJ, Blacquiere T, Field LM, Hails RS, Petrokofsky G, Potts SG, Raine NE, Vanbergen AJ, McLean AR. 2014. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B 281, 20140558 ( 10.1098/rspb.2014.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrese EJ, Baldwin LA. 2003. Hormesis: the dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 43, 175–197. ( 10.1146/annurev.pharmtox.43.100901.140223) [DOI] [PubMed] [Google Scholar]
- 9.Guedes RN, Cutler GC. 2014. Insecticide-induced hormesis and arthropod pest management. Pest Manage. Sci. 70, 690–697. ( 10.1002/ps.3669) [DOI] [PubMed] [Google Scholar]
- 10.Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Stout JC, Wright GA. 2015. Bees prefer foods containing neonicotinoid pesticides. Nature 521, 74–76. ( 10.1038/nature14414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roelofs WL. 1995. Chemistry of sex attraction. Proc. Natl Acad. Sci. USA 92, 44–49. ( 10.1073/pnas.92.1.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anton S, Homberg U. 1999. Antennal lobe structure. In Insect olfaction (ed. Hansson BS.), pp. 98–125. Berlin, Germany: Springer. [Google Scholar]
- 13.Todd JL, Baker TC. 1999. Function of peripheral olfactory organs. In Insect olfaction (ed. BS Hansson), pp. 67–96. Berlin, Germany: Springer. [Google Scholar]
- 14.Anderson P, Anton S. 2014. Experience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant Cell Environ. 37, 1826–1835. ( 10.1111/pce.12342) [DOI] [PubMed] [Google Scholar]
- 15.Anton S, Dufour M-C, Gadenne C. 2007. Plasticity of olfactory-guided behaviour and its neurobiological basis: lessons from moths and locusts. Entomol. Exp. Appl. 123, 1–11. ( 10.1111/j.1570-7458.2007.00516.x) [DOI] [Google Scholar]
- 16.Rings RW, Arnold FJ, Keaster AJ, Musick GJ. 1974. Worldwide annotated bibliography of the black cutworm Agrotis ipsilon, Hufnagel. Ohio Agric. Res. Dev. Cent. Res. Circ. 198, 1–106. [Google Scholar]
- 17.Rabhi KK, Esancy K, Voisin A, Crespin L, Le Corre J, Tricoire-Leignel H, Anton S, Gadenne C. 2014. Unexpected effects of low doses of a neonicotinoid insecticide on behavioral responses to sex pheromone in a pest insect. PLoS ONE 9, e114411 ( 10.1371/journal.pone.0114411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poitout S, Buès R. 1974. Elevage de plusieurs espèces de lépidoptères sur milieu artificiel simplifié. Ann. Zool. Ecol. Anim. 2, 79–91. [Google Scholar]
- 19.Gemeno C, Haynes K. 1998. Chemical and behavioral evidence for a third pheromone component in a North American population of the black cutworm moth, Agrotis ipsilon. J. Chem. Ecol. 24, 999–1011. ( 10.1023/a:1022398318465) [DOI] [Google Scholar]
- 20.Picimbon JF, Gadenne C, Bécard JM, Clément JL, Sreng L. 1997. Sex pheromone of the French black cutworm moth, Agrotis ipsilon (Lepidoptera: Noctuidae): identification and regulation of a multicomponent blend. J. Chem. Ecol. 23, 211–230. ( 10.1023/B:JOEC.0000006355.13207.91) [DOI] [Google Scholar]
- 21.Causse R, Buès R, Barthes J, Toubon J. 1988. Mise en évidence expérimentale de nouveaux constituants des phéromones sexuelles de Scotia ipsilon et Mamestra suasa. In Médiateurs chimiques: comportement et systématique des lépidoptères. Coll. INRA no. 46 (ed. INRA), pp. 75–82. Paris, France: Institut National de la Recherche Agronomique. [Google Scholar]
- 22.Jarriault D, Gadenne C, Rospars J-P, Anton S. 2009. Quantitative analysis of sex-pheromone coding in the antennal lobe of the moth Agrotis ipsilon: a tool to study network plasticity. J. Exp. Biol. 212, 1191–1201. ( 10.1242/jeb.024166) [DOI] [PubMed] [Google Scholar]
- 23.Jarriault D, Gadenne C, Lucas P, Rospars J-P, Anton S. 2010. Transformation of the sex pheromone signal in the noctuid moth Agrotis ipsilon: from peripheral input to antennal lobe output. Chem. Senses 35, 705–715. ( 10.1093/chemse/bjq069) [DOI] [PubMed] [Google Scholar]
- 24.Gadenne C, Anton S. 2000. Central processing of sex pheromone stimuli is differentially regulated by juvenile hormone in a male moth. J. Insect Physiol. 46, 1195–1206. ( 10.1016/S0022-1910(00)00040-8) [DOI] [PubMed] [Google Scholar]
- 25.Barrozo RB, Jarriault D, Deisig N, Gemeno C, Monsempes C, Lucas P, Gadenne C, Anton S. 2011. Mating-induced differential coding of plant odour and sex pheromone in a male moth. Eur. J. Neurosci. 33, 1841–1850. ( 10.1111/j.1460-9568.2011.07678.x) [DOI] [PubMed] [Google Scholar]
- 26.Deisig N, et al. 2012. Differential interactions of sex pheromone and plant odour in the olfactory pathway of a male moth. PLoS ONE 7, e33159 ( 10.1371/journal.pone.0033159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen TA, Hildebrand JG. 1987. Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J. Comp. Physiol. A 160, 553–569. ( 10.1007/BF00611929) [DOI] [PubMed] [Google Scholar]
- 28.Abrieux A, Duportets L, Debernard S, Gadenne C, Anton S. 2014. The GPCR membrane receptor, DopEcR, mediates the actions of both dopamine and ecdysone to control sex pheromone perception in an insect. Front. Behav. Neurosci. 8, 312 ( 10.3389/fnbeh.2014.00312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhouse SW, Geisser S. 1959. On methods in the analysis of profile data. Psychometrika 24, 95–112. ( 10.1007/BF02289823) [DOI] [Google Scholar]
- 30.Sokal RR, Rohlf FJ. 1995. Biometry: the principles and practice of statistics in biological research. New York, NY: WH Freeman. [Google Scholar]
- 31.Lucas P, Renou M. 1992. Electrophysiological study of the effects of deltamethrin, bioresmethrin, and DDT on the activity of pheromone receptor neurones in two moth species. Pestic. Biochem. Physiol. 43, 103–115. ( 10.1016/0048-3575(92)90024-T) [DOI] [Google Scholar]
- 32.Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA, Connolly CN. 2013. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634–1638. ( 10.1038/ncomms2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson P, Hansson BS, Nilsson U, Han Q, Sjöholm M, Skals N, Anton S. 2007. Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem. Senses 32, 483–491. ( 10.1093/chemse/bjm017) [DOI] [PubMed] [Google Scholar]
- 34.Anderson P, Sadek MM, Hansson BS. 2003. Pre-exposure modulates attraction to sex pheromone in a moth. Chem. Senses 28, 285–291. ( 10.1093/chemse/28.4.285) [DOI] [PubMed] [Google Scholar]
- 35.Anton S, Gadenne C. 1999. Effect of juvenile hormone on the central nervous processing of sex pheromone in an insect. Proc. Natl Acad. Sci. USA 96, 5764–5767. ( 10.1073/pnas.96.10.5764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadenne C, Dufour MC, Anton S. 2001. Transient post-mating inhibition of behavioural and central nervous responses to sex pheromone in an insect. Proc. R. Soc. Lond. B 268, 1631–1635. ( 10.1098/rspb.2001.1710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadenne C, Renou M, Sreng L. 1993. Hormonal control of pheromone responsiveness in the male black cutworm, Agrotis ipsilon. Experientia 49, 721–724. ( 10.1007/bf01923960) [DOI] [Google Scholar]
- 38.Guerrieri F, Gemeno C, Monsempes C, Anton S, Jacquin-Joly E, Lucas P, Devaud J-M. 2012. Experience-dependent modulation of antennal sensitivity and input to antennal lobes in male moths (Spodoptera littoralis) pre-exposed to sex pheromone. J. Exp. Biol. 215, 2334–2341. ( 10.1242/jeb.060988) [DOI] [PubMed] [Google Scholar]
- 39.Jarriault D, et al. 2009. Age-dependent plasticity of sex pheromone response in the moth, Agrotis ipsilon: combined effects of octopamine and juvenile hormone. Horm. Behav. 56, 185–191. ( 10.1016/j.yhbeh.2009.04.005) [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira IM, Nunes BVF, Barbosa DR, Pallares AM, Faro LRF. 2010. Effects of the neonicotinoids thiametoxam and clothianidin on in vivo dopamine release in rat striatum. Toxicol. Lett. 192, 294–297. ( 10.1016/j.toxlet.2009.11.005) [DOI] [PubMed] [Google Scholar]
- 41.Desneux N, Decourtye A, Delpuech JM. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106. ( 10.1146/annurev.ento.52.110405.091440) [DOI] [PubMed] [Google Scholar]
- 42.Thany SH, Gauthier M. 2005. Nicotine injected into the antennal lobes induces a rapid modulation of sucrose threshold and improves short-term memory in the honeybee Apis mellifera. Brain Res. 1039, 216–219. ( 10.1016/j.brainres.2005.01.056) [DOI] [PubMed] [Google Scholar]
- 43.Lansdell SJ, Millar NS. 2000. The influence of nicotinic receptor subunit composition upon agonist, α–bungarotoxin and insecticide (imidacloprid) binding affinity. Neuropharmacology 39, 671–679. ( 10.1016/S0028-3908(99)00170-7) [DOI] [PubMed] [Google Scholar]
- 44.Thany S, Lenaers G, Crozatier M, Armengaud C, Gauthier M. 2003. Identification and localization of the nicotinic acetylcholine receptor alpha3 mRNA in the brain of the honeybee, Apis mellifera. Insect Mol. Biol. 12, 255–262. ( 10.1046/j.1365-2583.2003.00409.x) [DOI] [PubMed] [Google Scholar]
- 45.Thany SH, Crozatier M, Raymond-Delpech V, Gauthier M, Lenaers G. 2005. Apisα2, Apisα7-1 and Apisα7-2: three new neuronal nicotinic acetylcholine receptor α-subunits in the honeybee brain. Gene 344, 125–132. ( 10.1016/j.gene.2004.09.010) [DOI] [PubMed] [Google Scholar]
- 46.Dupuis JP, Gauthier M, Raymond-Delpech V. 2011. Expression patterns of nicotinic subunits 2, 7, 8, and 1 affect the kinetics and pharmacology of ACh-induced currents in adult bee olfactory neuropiles. J. Neurophysiol. 106, 1604–1613. ( 10.1152/jn.00126.2011) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the corresponding author: sylvia.anton@angers.inra.fr.