Abstract
The evolution of separate males and females is an important evolutionary transition that has occurred multiple times in flowering plants. While empirical studies have stressed the potential importance of natural enemies and organismal interactions in the evolution of separate sexes, there has been no treatment of natural enemies in the theoretical literature. We investigated the effects of disease on the evolution of females in gynodioecious populations composed of females and hermaphrodites, where sex is determined by the interaction of cytoplasmic male sterility (CMS) and nuclear restorer genes. When females are significantly more resistant than hermaphrodites, disease drives an increase in the frequency of females and sex determination becomes nuclear, creating the pre-conditions for the evolution of separate males and females. However, when females are only moderately more resistant, disease drives changes in the frequency of CMS and restorer alleles, but has little effect on the frequency of females. We discuss our results in the context of the evolution of mating systems and cyto-nuclear epistasis.
Keywords: disease, sex-specific resistance, dioecy, gynodioecy, cytoplasmic male sterility, cyto-nuclear epistasis
1. Introduction
The partitioning of reproduction into two separate sexes is a remarkably important evolutionary transition that ensures the production of outcrossed progeny and also results in separate ecologies for females and males. Sex as a mechanism of producing genetically diverse progeny has been studied extensively [1–3], and infectious disease is established as a major selective force driving this evolution [4–6]. The evolution of sexual dimorphism following the emergence of separate sexes has also been studied extensively and influences both levels of disease exposure [7–9] as well as immunity [10,11]. However, the question of whether disease plays a role in the incipient evolution of separate sexes has been largely omitted from the evolutionary discussion. Here we use a modelling approach to ask whether infectious disease can drive the evolution of females in hermaphroditic populations and set the conditions for the evolution of separate sexes.
In flowering plants, the evolution of females from hermaphrodites is widely considered to be the first step towards the evolution of separate males and females, or dioecy [12–14]. Females will evolve if genes conferring male sterility arise and provide a fitness advantage for females over hermaphrodites. As females become more frequent, the fraction of hermaphrodite fitness that comes from male function increases, eventually allowing the spread of female sterility alleles and the evolution of males [15]. Thus, under this conceptual model, the evolution of a female-biased sex ratio can be viewed as a critical prerequisite for the evolution of dioecy [16].
The evolution of dioecy also requires an important transition in the genetic basis of sex. Sex determination in dioecious species is nuclear, indeed, an important caveat for the spread of a female sterility gene is that it must be tightly linked on the same chromosome as the male sterility gene [15,17]. However in gynodioecious populations (females and hermaphrodites), sex is often determined by cytoplasmic male sterility (CMS) genes, which arise in the mitochondria and are thought to act as ‘selfish’ genes that increase the fitness of the mitochondria at the expense of the whole organism [18–21]. In response, many nuclear genomes have evolved ‘restorer’ genes capable of counteracting CMS genes and restoring male function [22–24]. In populations that are polymorphic at both the CMS and nuclear restorer loci, sex is determined by the epistatic interaction between these loci [25,26]. Sex determination can become nuclear if selection sweeps the male sterile cytoplasm to fixation, leaving the nuclear restorer gene polymorphic [27,28]. Thus, fixation of the CMS cytotype and evolution of nuclear sex-determination can also be viewed as a critical prerequisite for the evolution of dioecy.
Disease has the potential to be a powerful agent of selection for females. In animals, females have been shown to experience lower rates of disease exposure than males, due to different social interactions [8,29]. Females have also been shown to invest more in immunity and other forms of resistance [10,11] because female fitness is often maximized by longevity rather than reproduction. These female-specific advantages with respect to disease, and natural enemies in general, have also been found in both dioecious [7,30–32] and gynodioecious plants [33–36] leading several authors to argue that natural enemies may play an important role in the evolution of separate sexes [37–40]. However, we still lack a clear understanding of how selection from disease interacts with the complex genetic basis of sex in gynodioecious populations to drive the evolution of mating systems.
Here we combine a cyto-nuclear genetic model of gynodioecy [41] with a classic susceptible-infected (SI) model of disease dynamics [42] to investigate how sex-specific resistance to disease affects the evolution of females and the genetic basis of sex. We first ask whether disease can drive the evolution of gynodioecy from hermaphroditism by providing a selective advantage for females. We then ask whether disease can drive the evolution of gynodioecy in ways that favour the evolution of dioecy by increasing female frequency and shifting the genetic basis of sex from cyto-nuclear to nuclear.
Our results show that selection from disease can have strong, and often unexpected effects on the evolution of plant mating systems. In hermaphroditic populations, we show that small increases in female resistance can drive the evolution of gynodioecy in the presence of disease. In gynodioecious populations, we show that when females are much more resistant than hermaphrodites, disease can drive populations towards dioecy by increasing the frequency of females and shifting the genetic basis of sex from cyto-nuclear to nuclear. However, when females are only moderately more resistant, disease may drive a ‘cryptic’ evolutionary response, detectable only through changes in CMS and restorer frequencies rather than changes in female frequency.
2. The model
To investigate the effect of disease on the evolution of mating systems, we first constructed a genetic model of cyto-nuclear gynodioecy that consisted of two maternally inherited cytotypes (one male fertile, one male sterile) and a single nuclear restorer locus with two alleles, similar to previous genetic models of gynodioecy [27,41]. We then introduced numerical dynamics and population regulation by incorporating a density-dependent birth rate and fixed mortality rate. Then, we overlaid an SI disease model on this numerical framework to investigate the sex-specific effects of disease. We examined the dynamics under two different disease transmission modes: frequency-dependent transmission (observed in vector borne and sexually transmitted diseases) and classic mass-action transmission (observed in wind and splash borne diseases).
(a). Genetic model
Our genetic model followed that of Dufay et al. [41] for an outcrossing plant. Sex was determined by the interaction between cytotype and a single nuclear restorer locus (table 1). The male fertile cytotype, cms, always produced hermaphrodites. The male sterile cytotype, CMS, caused individuals to become female due to a loss of male function, unless a restorer allele, R, was present. Previous theoretical work has shown that stable cyto-nuclear gynodioecy can only be maintained if a female fertility advantage and a cost of restoration are included [27,41,43]. Females were assumed to have a female fertility advantage, a, and a dominant, constitutive cost associated with the restorer allele, cr, was applied to the male component of fertility of individuals carrying at least one restorer allele ([41]; table 1). We also included a cost, cc, associated with carrying a male sterile cytotype because previous work has shown that stable cyto-nuclear gynodioecy is not possible without cc < 1 [41]. Under these parameters, all genotypes with a restorer allele bear a cost of decreased pollen production, but the cost is higher in genotypes where the restorer is expressed. The recursion equations for determining the number and frequency of genotypes in the next generation follow those of Dufay et al. [41] and are given in the electronic supplementary material, appendix A.
Table 1.
Genotypes and their associated phenotypes and fertilities.
| genotype | cytotype | nuclear genotype | phenotype | female fertility | male fertility |
|---|---|---|---|---|---|
 |
cms | RR | hermaphrodite | 1 | 1-cr1 |
 |
cms | Rr | hermaphrodite | 1 | 1-cr |
 |
cms | rr | hermaphrodite | 1 | 1 |
 |
CMS | RR | hermaphrodite | cc2 | 1-cr |
 |
CMS | Rr | hermaphrodite | cc | 1-cr |
| GF | CMS | rr | female | cc*a3 | 0 |
1cost of restoration.
2cost of CMS cytoplasm.
3female fertility advantage.
(b). Numerical population regulation
To incorporate numerical population dynamics, we added a constant death rate, μ, to all genotypes and a density-dependent population regulation term, k, to the birth rate, where k represents the strength of density dependence [44]. The number of individuals of genotype Gi in the next generation can then be represented as:
| 2.1 |
where Zi is the number of new zygotes of genotype Gi prior to population regulation (electronic supplementary material, appendix A) and N is the total population size.
(c). Disease dynamics
We added disease dynamics by overlaying an SI epidemic model [42]. For simplicity, we assumed that the disease was completely sterilizing, but did not affect host mortality. Individuals that became infected were transferred from one of the six healthy classes to a diseased class, D, in which there was no differentiation between sexes. The dynamics are represented by the following equations:
| 2.2 |
and
| 2.3 |
where β is the transmission coefficient, and T is the transmission mode. For diseases with frequency-dependent transmission, T = D/N. For diseases with classic mass-action transmission, T = D.
To determine whether sex-specific differences in disease resistance can drive the evolution of sex ratio we assumed separate transmission coefficients for hermaphrodites (βH) and females (βF). Females were more resistant when βF < βH. The number of individuals of each genotype in each time step was calculated from the following equations:
| 2.4 |
| 2.5 |
and
| 2.6 |
where  is the number of hermaphrodites with genotype Hi and GF is the number of females.
is the number of hermaphrodites with genotype Hi and GF is the number of females.
(d). Simulations
We used the above model to investigate two questions. First we asked whether disease could drive the evolution of females in conditions where they would otherwise not be maintained. We assumed there was no inherent female fertility advantage (a = 1) and ran invasion simulations in which a single diseased individual was introduced into a population of hermaphrodites with a low frequency of restored hermaphrodites (starting conditions: 990 cms_rr, 10 CMS_Rr).
Second, we asked whether the introduction of disease into populations at stable cyto-nuclear gynodioecy could help set the conditions for dioecy by increasing the frequency of females and driving the evolution of nuclear sex-determination. We ran simulations using parameter combinations that have previously been shown to result in stable cyto-nuclear equilibrium [41] and then introduced a single diseased individual after the population reached a stable equilibrium. We ran each simulation for 5 000 time steps following the introduction of disease and found that all simulations reached stable equilibrium (less than 1% change in female frequency) within this time frame. All simulations were run with both frequency- and density-dependent diseases. We ran additional simulations using the frequency-dependent disease model to evaluate the effects of the different values of female fertility advantage, costs of restoration, and starting conditions. All work was carried out using R v. 2.12.0 (The R foundation for statistical computing).
3. Results
(a). Effect of disease on the invasion of females
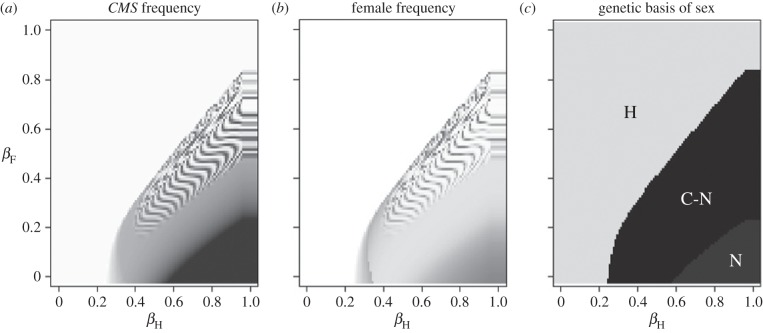
When females were more resistant than hermaphrodites (βF < βH), the introduction of disease allowed females to invade and persist in populations without any inherent fertility advantage (figure 1). Disease spread and persisted at a stable equilibrium within populations as long as βH > 0.2, the background mortality rate. When disease was not present, females were not able to invade and the CMS cytotype was quickly lost. Large differences in female resistance ( ) resulted in fixation of the CMS cytotype and a transition to nuclear sex-determination (figure 1c). Smaller differences in female resistance resulted in the maintenance of cyto-nuclear sex-determination, either through stable point-equilibria or through stable-limit cycles.
) resulted in fixation of the CMS cytotype and a transition to nuclear sex-determination (figure 1c). Smaller differences in female resistance resulted in the maintenance of cyto-nuclear sex-determination, either through stable point-equilibria or through stable-limit cycles.
Figure 1.
Level of female-specific disease resistance required for disease to drive the evolution of gynodioecy from hermaphroditism. A single diseased individual was introduced into a hermaphroditic population with a low starting CMS frequency (0.01), and no inherent female fertility advantage (a = 1). (a) Increase in the frequency of the CMS cytotype, (b) increase in the frequency of females. For both, darker colours indicate a larger increase. Wavy lines indicate parameter values that resulted in stable-limit cycles of CMS and females rather than stable point equilibria. (c) Genetic basis of sex H = hermaphroditism (loss of the CMS cytotype), C-N = cyto-nuclear sex-determination (maintenance of polymorphism in both the CMS and R loci), N = nuclear sex-determination (fixation of the CMS, and maintenance of polymorphism at the R locus). Disease transmission was frequency dependent. Disease was not maintained when βH < 0.2. Other model parameters: cc = 0.9, a*cc = 0.9, cr = 0.25, μ = 0.2, k = 0.001.
(b). Effect of disease in populations at stable gynodioecy
(i). Effect of disease on female frequency
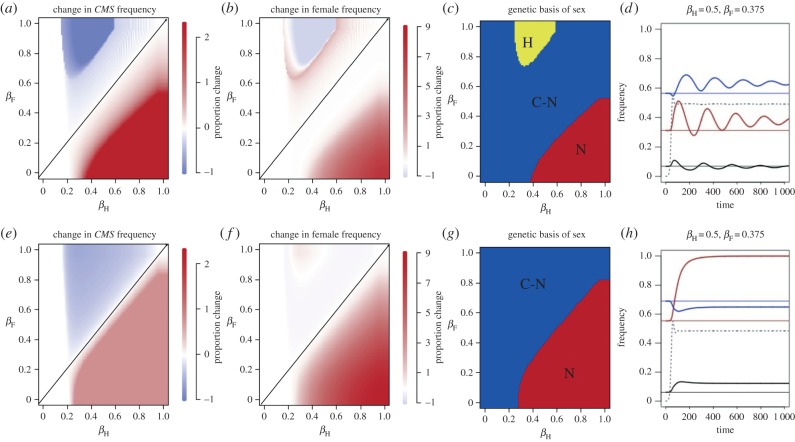
Introducing disease with frequency-dependent transmission into populations in which females were already maintained at a stable equilibrium through an inherent fertility advantage (a > 1) affected the evolution of females in unexpected ways (figure 2). In all cases, the CMS cytotype increased when females were more resistant than hermaphrodites and decreased when females were less resistant than hermaphrodites (figure 2a,e). However, changes in female frequency did not necessarily correspond to changes in CMS frequency (figure 2b,f). Instead we found that females only increased in frequency when they were significantly more resistant than hermaphrodites ( ) or, surprisingly, under some parameter combinations when they were less resistant than hermaphrodites (βF > βH; figure 2b,f). Moderate levels of female resistance (βF < βH) resulted in no change or drove very small decreases (less than 0.0035) in female frequency compared with pre-disease equilibrium (figure 2b,d and electronic supplementary material, figure S1). Results were qualitatively similar when disease transmission was density dependent (electronic supplementary material, figure S2).
) or, surprisingly, under some parameter combinations when they were less resistant than hermaphrodites (βF > βH; figure 2b,f). Moderate levels of female resistance (βF < βH) resulted in no change or drove very small decreases (less than 0.0035) in female frequency compared with pre-disease equilibrium (figure 2b,d and electronic supplementary material, figure S1). Results were qualitatively similar when disease transmission was density dependent (electronic supplementary material, figure S2).
Figure 2.
Effect of sex-specific disease resistance on the evolution of females and the genetic basis of sex in populations at stable cyto-nuclear gynodioecy. Top and bottom rows show the effect of introducing disease with different levels of sex-specific resistance into two populations with different pre-disease equilibrium levels. Females are more resistant to disease than hermaphrodites below the diagonal. (a–d) a = 1.5, a*cc = 1.35, initial CMS frequency = 0.31, female frequency = 0.068. (e–h) a = 2, a*cc = 1.8, CMS = 0.55, female = 0.061. (a,e) Change in the frequency of the equilibrium frequency of the CMS cytotype following the introduction of disease. (b,f) Change in the frequency of females. Red indicates an increase in frequency relative to disease-free equilibrium, and blue indicates a decrease in frequency relative to disease-free equilibrium. Darker shades correspond to a greater magnitude of change. (c,g) Change in the genetic basis of sex (figure 1). (d,h) Change in the frequency of the CMS cytotype (red lines), nuclear restorer (blue lines), and females (black lines) when females are 25% more resistant than hermaphrodites. Thin lines indicate pre-disease equilibrium; dotted grey line indicates disease prevalence. For all simulations, disease transmission was frequency dependent. Other model parameters: cc = 0.9, cr = 0.25, μ = 0.2, k = 0.001.
As in the simulations without a female advantage, models that included a female advantage also required a minimum threshold value of βH for disease persistence. For both frequency- and density-dependent diseases, the hermaphrodite transmission rate had a much larger effect on disease prevalence than female transmission (electronic supplementary material, figure S3). Indeed, disease was able to persist even when females were completely resistant βF = 0 (electronic supplementary material, figure S3). This asymmetry in the epidemiological effects of βH and βF helps explain why the CMS allele can be maintained even when females are less resistant than hermaphrodites. For a frequency-dependent disease, we can calculate the relative per capita fertility of females (WF) to hermaphrodites (WH) as:
| 3.1 |
where P* is disease prevalence at equilibrium. In the case where hermaphrodites are more resistant (βH < βF), P* is also low, which reduces the overall contribution of disease resistance to female fertility (electronic supplementary material, figure S3). Thus, unless a*cc is very close to 1, females will still have net fertility advantage over hermaphrodites.
(ii). Effect of disease on the genetic basis of sex
Strong selection for females by disease led to fixation of the CMS cytotype, and sex determination shifted from cyto-nuclear to purely nuclear (figure 2c). When selection for females by disease was strong enough to sweep the CMS cytotype to fixation, the response to selection was in the predicted direction, and females increased in the presence of disease (figure 2b, lower right corner). However, when selection for females by disease was not strong enough to sweep the CMS cytotype to fixation (figure 2c,g blue regions), the response to selection was complex, and female frequency either did not change or decreased slightly in the presence of disease (figure 2b,f, white regions below the diagonal; electronic supplementary material, figure S1). This counter-intuitive response arose because the nuclear restorer allele also increased in frequency as a result of selection (figure 2d). Populations responded in the opposite direction of selection when females were selected against (βF > βH), as long as selection was not strong enough to drive the loss of the CMS cytotype (figure 2b,f, red region above diagonal). In this case, selection by disease drove a decrease in the frequency of the CMS cytotype and an even greater decrease in the frequency of the nuclear restorer allele, leading to a net increase in female frequency (electronic supplementary material, figure S4).
(iii). Effect of an inherent female fertility advantage
Increasing the inherent female fertility advantage from a = 1.5 to 2 significantly changed the response to selection from disease (figure 2e–h). Females and nuclear sex-determination evolved under a broader range of βF and βH (figure 2f,g). Increasing a increased the frequency of the CMS cytotype prior to the introduction of disease from 0.33 to 0.55, moving it closer to fixation. We ran additional simulations where we held a constant and varied the starting CMS frequencies and found that the evolutionary response to disease did not depend on the starting frequency of CMS, and was entirely predicted by a (electronic supplementary material, figure S5).
(iv). Effect of the cost of restoration
Lowering the cost of restoration, cr, increased the disease-free equilibrium frequency of the CMS and females, but the cost had relatively little effect on the response to disease (electronic supplementary material, figure S6). Moderate costs that ranged between cr = 0.1 and 0.3 did not cause any detectable difference in the response to disease. Very low costs, cr = 0.05 resulted in a stronger increase in females in response to disease, whereas high costs, cr = 0.50, resulted in either a decrease in females (electronic supplementary material, figure S6) or negligible change (electronic supplementary material, figure S6), depending on the level of inherent female advantage.
(v). Effect of disease on the heritability of sex
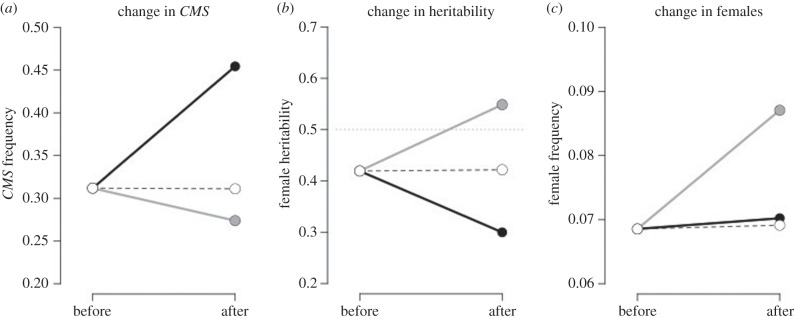
Phenotypic response to selection is determined by the strength of selection and heritability of the trait, R = h2s. Thus, in the cases where evolution occurs in the opposite direction of selection (e.g. when sex determination is cyto-nuclear), the heritability of sex must be negative. To test this, we calculated the proportion of female progeny produced by females under different selection regimes. We found that increasing selection for females from disease led to an increase in the overall frequency of the CMS cytotype (figure 3a, black line), but led to a decrease in the heritability of the CMS_rr gene combination that gives rise to females in this scenario (figure 3b). The net result was that the frequency of females did not change in response to selection from disease (figure 3c). In contrast, we found that selection against females when they were less resistant to disease led to a decrease in the frequency of the CMS cytotype, but an increase in the heritability of the CMS_rr gene combination (figure 3, grey lines). The net result in this case was that females evolved in the opposite direction of selection from disease.
Figure 3.
Effect of selection on the heritability and evolution of females when sex determination is cyto-nuclear. (a) Frequency of the CMS cytotype before and after the introduction of disease. (b) Female heritability, measured as the proportion of female offspring that are female. (c) Change in female frequency. Black lines denote females are more resistant than hermaphrodites (βH = 0.5, βF = 0.3). Grey lines, females are more susceptible than hermaphrodites (βH = 0.3, βF = 0.5). Dashed grey lines, no sex-specific difference in resistance (βH = βF = 0.4). Other parameters: a = 1.5, cc = 0.9, a*cc = 1.35, cr = 0.25, μ = 0.2, k = 0.001.
4. Discussion
Empirical studies have shown that female plants are often less affected by disease and herbivory than hermaphrodites [33–35]. For example, López-Villavicencio et al. [36] found that exposing Gypsophila repens flowers to the spores of the sterilizing fungal pathogen Microbotryum violaceum led to signficiant reductions in fruit and seed production in hermaphrodite plants but did not affect the fertility of female plants. Such observations have led to the hypothesis that natural enemies may drive an increase in the evolution of females [37–39]. Our results show that disease can indeed be a powerful selective force driving the evolution of mating systems. We show that disease can facilitate the invasion and maintenance of females into populations even when sex-specific differences in disease resistance are small, and could therefore play a role in the incipient evolution of gynodioecy. We also show that in some cases the introduction of disease into gynodioecious populations can help set the conditions for dioecy by driving the evolution of female-biased sex ratios and shifting the genetic basis of sex from cyto-nuclear to nuclear. However, we also found that disease can drive the evolution of mating systems in unexpected ways: when females are only moderately more resistant than hermaphrodites, the introduction of disease has little effect on female frequency. Additionally, when females are less resistant than hermaphrodites, disease can drive an increase in female frequency. The complexity of these results highlights the necessity of integrating sex-specific measures of disease resistance into explicit genetic models of sex determination.
(a). Effect of disease on the evolution of nuclear sex determination
Nuclear sex-determination appears to be a key factor in determining the phenotypic response to selection from disease. When selection for females was strong enough to drive the CMS cytotype to fixation, sex determination became nuclear, and the evolution of females occurred in the same direction as selection. When selection was not strong enough to fix the CMS cytotype, sex determination remained cyto-nuclear and the evolution of females was highly unpredictable. Predicting the relative level of female resistance needed to drive the CMS cytotype to fixation is therefore critical in predicting how the sex ratio will evolve in response to disease.
Our results show that differences in female resistance combine with underlying differences in female fertility to drive the evolution of nuclear gynodioecy in the presence of disease. Dufay et al. [41] showed that female fertility has to be twice that of hermaphrodites for the CMS to reach fixation (a*cc > 2). We find that if there is no inherent female fertility advantage (a = 1), then females must be approximately twice as resistant as hermaphrodites for disease to drive the evolution of nuclear sex-determination. When females have a preexisting fertility advantage (a > 1), smaller levels of female-specific resistance can push relative female fitness above 2, and drive the CMS cytotype to fixation. Exact solutions are difficult because disease prevalence also changes under different transmission rates. However, as long as the hermaphrodite transmission is large enough to sustain disease (βH > μ), this general rule of thumb seems to predict fixation:
| 4.1 |
Thus in order to predict whether disease is likely to drive the evolution of nuclear sex-determination and an increase in female frequency, one must measure both the relative infection rate of females and hermaphrodites and the inherent differences in seed production.
An important cautionary note is that observations of sex ratios and measures of sex-specific levels of resistance do not provide any predictive power on their own. While intuition might suggest that populations with a higher proportion of females prior to disease are likely to be closer to the critical ‘tipping’ point for disease to drive populations into nuclear sex-determination, differences in the cost of restoration can mask large differences in fertility advantages and result in populations with similar sex ratios but very different evolutionary potentials. For example, consider two gynodioecious populations with similar CMS and female frequencies but different advantages and costs: population A has a higher inherent fertility advantage, and a higher cost of restoration (a = 1.6, cr = 0.5). Population B has a lower inherent fertility advantage, but also a lower cost of restoration (a = 1.3, cr = 0.3). At equilibrium, population A has a lower frequency of females (4%) than population B (8%). Armed with only sex ratio data, we might assume that females in population B have a higher fertility advantage. However, if we introduce a disease where βH = 0.4, and βF = 0.2, into both populations, the outcomes are extremely different: in population A, the disease drives the CMS cytotype to fixation and female frequency increases fourfold to 16%. However in population B, and the introduction of the same disease does not drive the cytotype to fixation and female frequency declines slightly to 7.8%. Thus in this example, the population with a lower starting frequency of females appears to respond much more strongly to the same disease than the population with a higher initial female frequency.
(b). Effect of disease on sex ratio when sex determination is cyto-nuclear
When the combined effect of female disease resistance and underlying female fertility advantages were not strong enough to drive the evolution of nuclear sex-determination (equation (4.1)), the evolutionary response to disease was complex. In most cases, female frequency did not change at all, even when females were considerably more resistant than hermaphrodites. In other cases, female frequency evolved in the opposite direction of selection; females decreased slightly in frequency when they were more resistant and increased when they were more susceptible. These counter-intuitive responses stem from the fact that when the trait is determined by a specific cyto-nuclear combination, selection and heritability are no longer independent. Indeed, a key finding from our results is that increased selection for a specific cyto-nuclear combination can simultaneously decrease the heritability of that combination. In our case, this negative relationship occurred because the heritability of the cyto-nuclear combination that gave rise to females (CMS_rr) was entirely determined by the frequency of the restorer allele in the pollen pool. Females can produce either female (CMS_rr) or restored hermaphrodite (CMS_Rr) progeny, depending on the frequency of R in the pollen. In the case where females were more resistant, the introduction of disease resulted in an increase in both of these progeny genotypes in the next generation. Because females cannot produce pollen, the initial increase in these two genotypes disproportionately increased the frequency of R in the pollen. The over-representation of the R allele in the pollen in turn increased the proportion of female ovules that were fertilized by R pollen, which decreased the heritability of the CMS_rr combination and prevented any phenotypic change in female frequency. In the case where females were less resistant than hermaphrodites (but still maintained an overall seed fertility advantage), the unrestored hermaphrodite (cms_rr) became the most fit genotype in the population because it did not suffer any costs associated with restoration. However an initial increase in the cms_rr genotype led to an increased frequency of r in the pollen, which increased the heritability of the CMS_rr gene combination and led to a net increase in the frequency of females.
There is growing evidence that cytoplasmic genomes provide a rich source of genetic variation for the expression of many other life-history traits besides male sterility, including metabolism [45] and longevity [46]. Moreover, it appears that a large proportion of this variation is driven by epistatic interactions between cytoplasmic and nuclear genes [24,47]. Experimental work in a variety of plant, animal, and fungal species [48–50] have repeatedly shown that novel combinations of cytoplasmic and nuclear genomes can result in fitness differences, suggesting reciprocal selection on organelle and nuclear genomes [49,51]. These observations have led to the recent conclusion that cyto-nuclear interactions are ‘a key unit on which natural selection acts’ [47]. However, we still have a limited understanding of how traits controlled by cyto-nuclear epistasis respond to selection. Our results illustrate how remarkably non-intuitive the evolutionary outcome of these epistatic interactions can be.
(c). Role of natural enemies in the evolution of mating systems
While our focus was on the effects of disease, our results appear to be quite general and likely apply to other natural enemies or even other external sources of selection for females. As long as the transmission rate to hermaphrodites was above the mortality rate, disease persisted stably within the population and exerted a relatively constant selection pressure. In addition, the insights generated from our results do not appear to be strongly determined by specific transmission dynamics, as the disease transmission mode did not have a strong effect on the evolutionary outcomes. Indeed, for the most part, sex-specific differences in disease resistance appeared to act simply as an additional type of female fertility advantage. However, our model only considered a single uniform population and therefore may not have captured one of the most important differences between biotic selection from ecological interactions and selection from inherent differences in seed production: spatial heterogeneity.
In natural plant populations, disease incidence is often both spatially and temporally heterogeneous, with pathogens only persisting at the metapopulation level [52–55]. Spatial variation in sex ratio has also been observed in many different gynodioecious species [56–60] and there is good reason to believe that, in many species, metapopulation dynamics play a large role in the maintenance of gynodioecy [56,61,62]. McCauley & Taylor [63] showed that once among population differences in sex ratio are established, females can be maintained at the metapopulation-level through frequency-dependent selection, and can persist even in the absence of fertility advantages or costs. Spatial heterogeneity in disease could play an important role in establishing these initial differences in among-deme sex ratios. Our results show that disease can facilitate the invasion of females into hermaphrodite populations even in the absence of inherent fertility advantages. If selection is strong enough to establish significantly different sex ratios among populations, females could potentially be maintained through metapopulation dynamics long after epidemics have passed.
5. Conclusion
The evolution of females in gynodioecious populations has been studied extensively by theoretical biologists [27,64–66], but despite growing empirical evidence of sex-specific differences in interactions with natural enemies [33,35,37,39,67] no aspect of community ecology has ever been incorporated into these models. Our results illustrate that the inclusion of natural enemies generates novel insights into the evolution of mating systems. We show that even in environments where females have a fitness advantage with respect to disease resistance, the response to selection can be complex and depends on the genetic basis of sex and existing differences in female fertility. In some cases, disease can help set the conditions for the evolution of dioecy by driving the evolution of nuclear sex-determination and increasing female frequency. In other cases, disease may have strong effects on the genetic composition of a population, but little discernable effect on the sex ratio.
Acknowledgements
We thank Janis Antonovics for his encouragement and helpful discussions over coffee. We also thank the two anonymous reviewers who provided excellent comments and suggestions on the manuscript.
Data accessibility
No primary data were used. The R-code for the model has been uploaded as part of the electronic supplementary material.
Authors' contributions
I.M. developed the model. E.B. conceived the project, and helped develop the model. Both authors prepared the manuscript.
Competing interests
The authors have no conflicting interests to declare.
Funding
We gratefully acknowledge the support of the National Science Foundation: DEB1115895 to J.A. and REU grant to I.M.
References
- 1.Hamilton WD. 1980. Sex versus non-sex versus parasite. Oikos 35, 282–290. ( 10.2307/3544435) [DOI] [Google Scholar]
- 2.Lively CM. 1996. Host-parasite coevolution and sex. Bioscience 46, 107–114. ( 10.2307/1312813) [DOI] [Google Scholar]
- 3.Morran LT, Schmidt OG, Gelarden IA, Parish RCI, Lively CM. 2011. Running with the Red Queen: host-parasite coevolution selects for biparental sex. Science 333, 216–219. ( 10.1126/science.1206360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lively CM, Craddock C, Vrijenhoek RC. 1990. Red queen hypothesis supported by parasitism in sexual and clonal fish. Nature 344, 864–866. ( 10.1038/344864a0) [DOI] [Google Scholar]
- 5.Busch J, Neiman M, Koslow JM. 2004. Evidence for maintenance of sex by pathogens in plants. Evolution 58, 2584–2590. ( 10.1111/j.0014-3820.2004.tb00886.x) [DOI] [PubMed] [Google Scholar]
- 6.Koskella B, Lively CM. 2009. Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution 63, 2213–2221. ( 10.1111/j.1558-5646.2009.00711.x) [DOI] [PubMed] [Google Scholar]
- 7.Kaltz O, Shykoff JA. 2001. Male and female Silene latifolia plants differ in per-contact risk of infection by a sexually transmitted disease. J. Ecol. 89, 99–109. ( 10.1046/j.1365-2745.2001.00527.x) [DOI] [Google Scholar]
- 8.Perkins SE, Ferrari MF, Hudson PJ. 2008. The effects of social structure and sex-biased transmission on macroparasite infection. Parasitology 135, 1561–1569. ( 10.1017/S0031182008000449) [DOI] [PubMed] [Google Scholar]
- 9.Grear DA, Luong LT, Hudson PJ. 2012. Sex-biased transmission of a complex life-cycle parasite: why males matter. Oikos 121, 1446–1453. ( 10.1111/j.1600-0706.2012.20358.x) [DOI] [Google Scholar]
- 10.Zuk M, McKean KA. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26, 1009–1024. ( 10.1016/S0020-7519(96)80001-4) [DOI] [PubMed] [Google Scholar]
- 11.Zuk M, Stoehr AM. 2002. Immune defense and host life history. Am. Nat. 160(Suppl), S9–S22. ( 10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 12.Barrett SCH. 2002. The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284. ( 10.1038/nrg776) [DOI] [PubMed] [Google Scholar]
- 13.Charlesworth D. 2006. Evolution of plant breeding systems. Curr. Biol. 16, R726–R735. ( 10.1016/j.cub.2006.07.068) [DOI] [PubMed] [Google Scholar]
- 14.Dufay M, Champelovier P, Käfer J, Henry JP, Mousset S, Marais GAB. 2014. An angiosperm-wide analysis of the gynodioecy-dioecy pathway. Ann. Bot. 114, 539–548. ( 10.1093/aob/mcu134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlesworth B, Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997. ( 10.1086/283342) [DOI] [Google Scholar]
- 16.Spigler RB, Ashman T-L. 2012. Gynodioecy to dioecy: are we there yet? Ann. Bot. 109, 531–543. ( 10.1093/aob/mcr170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlesworth B. 1991. The evolution of sex chromosomes. Science 251, 1030–1033. ( 10.1126/science.1998119) [DOI] [PubMed] [Google Scholar]
- 18.Budar F, Touzet P, De Paepe R. 2003. The nucleo-mitochondrial conflict in cytoplasmic male sterilities revisited. Genetica 117, 3–16. ( 10.1023/A:1022381016145) [DOI] [PubMed] [Google Scholar]
- 19.Touzet P, Budar F. 2004. Unveiling the molecular arms race between two conflicting genomes in cytoplasmic male sterility? Trends Plant Sci. 9, 565–568. ( 10.1016/j.tplants.2004.10.001) [DOI] [PubMed] [Google Scholar]
- 20.Delph LF, Touzet P, Bailey MF. 2007. Merging theory and mechanism in studies of gynodioecy. Trends Ecol. Evol. 22, 17–24. ( 10.1016/j.tree.2006.09.013) [DOI] [PubMed] [Google Scholar]
- 21.McCauley DE, Bailey MF. 2009. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Ann. Bot. 104, 611–620. ( 10.1093/aob/mcp141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnable PS, Wise RP. 1998. The molecular basis of cytoplasmic male sterility and fertility restoration. Annu. Rev. Plant Biol. 65, 175–180. ( 10.1016/s1360-1385(98)01235-7) [DOI] [Google Scholar]
- 23.Fujii S, Bond CS, Small ID. 2011. Selection patterns on restorer-like genes reveal a conflict between nuclear and mitochondrial genomes throughout angiosperm evolution. Proc. Natl Acad. Sci. USA 108, 1723–1728. ( 10.1073/pnas.1007667108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caruso CM, Case AL, Bailey MF. 2012. The evolutionary ecology of cytonuclear interactions in angiosperms. Trends Plant Sci. 17, 638–643. ( 10.1016/j.tplants.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 25.Van Damme JMM, Hundscheid MPJ, Ivanovic S, Koelewijn HP. 2004. Multiple CMS-restorer gene polymorphism in gynodioecious Plantago coronopus. Heredity 93, 175–181. ( 10.1038/sj.hdy.6800490) [DOI] [PubMed] [Google Scholar]
- 26.Garraud C, Brachi B, Dufay M, Touzet P, Shykoff JA. 2011. Genetic determination of male sterility in gynodioecious Silene nutans. Heredity 106, 757–764. ( 10.1038/hdy.2010.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delannay X, Gouyon PH, Valdeyron G. 1981. Mathematical study of the evolution of gynodioecy with cytoplasmic inheritance under the effect of a nuclear restorer gene. Genetics 99, 169–181. [PMC free article] [PubMed] [Google Scholar]
- 28.Maurice S, Belhassenj E, Couvet D. 1994. Evolution of dioecy: can nuclear-cytoplasmic interactions select for maleness? Heredity 73, 346–354. ( 10.1038/hdy.1994.181) [DOI] [PubMed] [Google Scholar]
- 29.Ferrari N, Cattadori IM, Nespereira J, Rizzoli A, Hudson PJ. 2003. The role of host sex in parasite dynamics: field experiments on the yellow-necked mouse Apodemus flavicollis. Ecol. Lett. 7, 88–94. ( 10.1046/j.1461-0248.2003.00552.x) [DOI] [Google Scholar]
- 30.Boecklen WJ, Price PW, Mopper S. 1990. Sex and drugs and herbivores: sex-biased herbivory in Arroyo willow (Salix lasiolepis). Ecology 71, 581–588. ( 10.2307/1940311) [DOI] [Google Scholar]
- 31.Jing S, Coley P. 1990. Dioecy and herbivory—the effect of growth-rate on plant defense in Acer negundo. Oikos 58, 369–377. ( 10.2307/3545228) [DOI] [Google Scholar]
- 32.Alexander HM, Antonovics J. 1995. Spread of anther-smut disease (Ustilago violacea) and in a genetically character correlations variable experimental population of Silene alba. J. Ecol. 83, 783–794. ( 10.2307/2261415) [DOI] [Google Scholar]
- 33.Marshall M, Ganders FR. 2001. Sex-biased seed predation and the maintenance of females in a gynodioecious plant. Am. J. Bot. 88, 1437–1443. ( 10.2307/3558451) [DOI] [PubMed] [Google Scholar]
- 34.Collin C, Pennings P, Rueffler C, Widmer A, Shykoff J. 2002. Natural enemies and sex: how seed predators and pathogens contribute to sex-differential reproductive success in a gynodioecious plant. Oecologia 131, 94–102. ( 10.1007/s00442-001-0854-8) [DOI] [PubMed] [Google Scholar]
- 35.Cole DH, Ashman T-L. 2005. Sexes show differential tolerance to spittlebug damage and consequences of damage for multi-species interactions. Am. J. Bot. 92, 1708–1713. ( 10.3732/ajb.92.10.1708) [DOI] [PubMed] [Google Scholar]
- 36.López-villavicencio AM, Branca A, Giraud T, Shykoff JA. 2005. Sex-specific effect of Microbotryum (Uredinales) spores on healthy plants of the gynodioecious Gypsophila repens (Caryophyllaceae). Am. J. Bot. 92, 896–900. ( 10.3732/ajb.92.5.896) [DOI] [PubMed] [Google Scholar]
- 37.Ashman T. 2002. The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83, 1175–1184. ( 10.1890/0012-9658(2002)083%5B1175:TROHIT%5D2.0.CO;2) [DOI] [Google Scholar]
- 38.Ashman T. 2007. The evolution of separate sexes : a focus on the ecological context. In Ecology and evolution of flowers (eds Harder LD, Barrett SCH), pp. 204–222. Oxford, UK: Oxford University Press. [Google Scholar]
- 39.Steets JA, Wolf DE, Auld JR, Ashman T, Steets A. 2007. The role of natural enemies in the expression and evolution of mixed mating in hermaphroditic plants and animals. Evolution 61, 2043–2055. ( 10.1111/j.1558-5646.2007.00184.x) [DOI] [PubMed] [Google Scholar]
- 40.Vega-Frutis R, Munguía-Rosas MA, Varga S, Kytöviita M-M. 2013. Sex-specific patterns of antagonistic and mutualistic biotic interactions in dioecious and gynodioecious plants. Perspect. Plant Ecol. Evol. Syst. 15, 45–55. ( 10.1016/j.ppees.2012.10.004) [DOI] [Google Scholar]
- 41.Dufay M, Touzet P, Maurice S, Cuguen J. 2007. Modeling the maintenance of male-fertile cytoplasm in a gynodioecious population. Heredity 99, 349–356. ( 10.1038/sj.hdy.6801009) [DOI] [PubMed] [Google Scholar]
- 42.Anderson RM, May RM. 1981. The population dynamics of microparasites and their invertebrate hosts. Phil. Trans. R. Soc. Lond. B 291, 451–524. ( 10.1098/rstb.1981.0005) [DOI] [Google Scholar]
- 43.Bailey MF, Delph LF, Lively CM. 2003. Modeling gynodioecy: novel scenarios for maintaining polymorphism. Am. Nat. 161, 762–776. ( 10.1086/374803) [DOI] [PubMed] [Google Scholar]
- 44.Antonovics J, Thrall PH. 1994. The cost of resistance and the maintenance of genetic polymorphism in host-pathogen systems. Proc. R. Soc. Lond. B 257, 105–110. ( 10.1098/rspb.1994.0101) [DOI] [Google Scholar]
- 45.Arnqvist G, Dowling DK, Eady P, Gay L, Tregenza T, Tuda M, Hosken DJ. 2010. Genetic architecture of metabolic rate: environment specific epistasis between mitochondrial and nuclear genes in an insect. Evolution 64, 3354–3363. ( 10.1111/j.1558-5646.2010.01135.x) [DOI] [PubMed] [Google Scholar]
- 46.Rand DM, Fry A, Sheldahl L. 2006. Nuclear-mitochondrial epistasis and drosophila aging: introgression of Drosophila simulans mtDNA modifies longevity in D. melanogaster nuclear backgrounds. Genetics 172, 329–341. ( 10.1534/genetics.105.046698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobler R, Rogell B, Budar F, Dowling DK. 2014. A meta-analysis of the strength and nature of cytoplasmic genetic effects. J. Evol. Biol. 27, 2021–2034. ( 10.1111/jeb.12468) [DOI] [PubMed] [Google Scholar]
- 48.Galloway LF, Fenster CB. 2007. The effect of nuclear and cytoplasmic genes on fitness and local adaptation in an annual legume, Chamaecrista fasciculata. Evolution 53, 1734–1743. ( 10.2307/2640436) [DOI] [PubMed] [Google Scholar]
- 49.Burton RS, Pereira RJ, Barreto FS. 2013. Cytonuclear genomic interactions and hybrid breakdown. Annu. Rev. Ecol. Evol. Syst. 44, 281–302. ( 10.1146/annurev-ecolsys-110512-135758) [DOI] [Google Scholar]
- 50.Paliwal S, Fiumera AC, Fiumera HL. 2014. Mitochondrial-nuclear epistasis contributes to phenotypic variation and coadaptation in natural isolates of Saccharomyces cerevisiae. Genetics 198, 1251–1265. ( 10.1534/genetics.114.168575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sloan DB. 2015. Using plants to elucidate the mechanisms of cytonuclear co-evolution. New Phytol. 205, 1040–1046. ( 10.1111/nph.12835) [DOI] [PubMed] [Google Scholar]
- 52.Thrall PH, Burdon JJ. 2002. Evolution of gene-for-gene systems in metapopulations: the effect of spatial scale of host and pathogen dispersal. Plant Pathol. 51, 169–184. ( 10.1046/j.1365-3059.2002.00683.x) [DOI] [Google Scholar]
- 53.Antonovics J. 2004. Long-term study of a plant-pathogen metapopulation. In Ecology, genetics, and evolution of metapopulations (eds Hanski I, Gaggiotti O), pp. 471–488. New York, NY: Academic Press, Inc. [Google Scholar]
- 54.Laine A. 2005. Spatial scale of local adaptation in a plant-pathogen metapopulation. J. Evol. Biol. 18, 930–938. ( 10.1111/j.1420-9101.2005.00933.x) [DOI] [PubMed] [Google Scholar]
- 55.Carlsson-Granér U, Giles BE, Thrall PH. 2014. Patterns of disease and host resistance in spatially structured systems. Eur. J. Plant Pathol. 138, 499–511. ( 10.1007/s10658-013-0316-2) [DOI] [Google Scholar]
- 56.Taylor DR, Trimble S, McCauley DE. 1999. Ecological genetics of gynodioecy in Silene vulgaris: relative fitness of females and hermaphrodites during the colonization process. Evolution 53, 745–751. ( 10.2307/2640714) [DOI] [PubMed] [Google Scholar]
- 57.Medrano M, Alonso C, Herrera CM. 2005. Mating system, sex ratio, and persistence of females in the gynodioecious shrub Daphne laureola L. (Thymelaeaceae). Heredity 94, 37–43. ( 10.1038/sj.hdy.6800550) [DOI] [PubMed] [Google Scholar]
- 58.Cuevas E, Parker IM, Molina-Freaner F. 2008. Variation in sex ratio, morph-specific reproductive ecology and an experimental test of frequency-dependence in the gynodioecious Kallstroemia grandiflora (Zygophyllaceae). J. Evol. Biol. 21, 1117–1124. ( 10.1111/j.1420-9101.2008.01530.x) [DOI] [PubMed] [Google Scholar]
- 59.Dufay M, Cuguen J, Arnaud JF, Touzet P. 2009. Sex ratio variation among gynodioecious populations of sea beet: can it be explained by negative frequency-dependent selection? Evolution. 63, 1483–1497. ( 10.1111/j.1558-5646.2009.00653.x) [DOI] [PubMed] [Google Scholar]
- 60.Landergott U, Schneller JJ, Holderegger R, Thompson JD. 2009. Sex-ratio variation and spatial distribution of nuclear and cytoplasmic sex-determining genes in gynodioecious Thymus praecox across altitudinal gradients. Evol. Ecol. Res. 11, 23–42. [Google Scholar]
- 61.Couvet D, Ronce O, Gliddon C. 1998. The maintenance of nucleocytoplasmic polymorphism in a metapopulation: the case of gynodioecy. Am. Nat. 152, 59–70. ( 10.1086/286149) [DOI] [PubMed] [Google Scholar]
- 62.Olson MS, McCauley DE. 2002. Mitochondrial DNA diversity, population structure, and gender association in the gynodioecious plant Silene vulgaris. Evolution 56, 253–262. ( 10.1111/j.0014-3820.2002.tb01335.x) [DOI] [PubMed] [Google Scholar]
- 63.McCauley DE, Taylor DR. 1997. Local population structure and sex ratio: evolution in gynodioecious plants. Am. Nat. 150, 406–419. ( 10.1086/286072) [DOI] [PubMed] [Google Scholar]
- 64.Charlesworth D, Ganders FR. 1979. The population genetics of gynodioecy with cytoplasmic-genic male sterility. Heredity 43, 213–218. ( 10.1038/hdy.1979.76) [DOI] [Google Scholar]
- 65.Gouyon AP, Vichot F, van Damme JMM. 1991. Nuclear-cytoplasmic male sterility: single-point equilibria versus limit cycles. Am. Nat. 137, 498–514. ( 10.1086/285179) [DOI] [Google Scholar]
- 66.Dornier A, Dufay M. 2013. How selfing, inbreeding depression, and pollen limitation impact nuclear-cytoplasmic gynodioecy: a model. Evolution 67, 2674–2687. ( 10.1111/evo.12142) [DOI] [PubMed] [Google Scholar]
- 67.Clarke GL, Brody AK. 2015. Gender inequality in pre-dispersal seed predation contributes to female seed set advantage in a gynodioecious species. Ecology 96, 1309–1317. ( 10.1890/14-1513.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No primary data were used. The R-code for the model has been uploaded as part of the electronic supplementary material.