Abstract
In cooperatively breeding vertebrates, the existence of individuals that help to raise the offspring of non-relatives is well established, but unrelated helpers are less well known in the social insects. Eusocial insect groups overwhelmingly consist of close relatives, so populations where unrelated helpers are common are intriguing. Here, we focus on Polistes dominula—the best-studied primitively eusocial wasp, and a species in which nesting with non-relatives is not only present but frequent. We address two major questions: why individuals should choose to nest with non-relatives, and why such individuals participate in the costly rearing of unrelated offspring. Polistes dominula foundresses produce more offspring of their own as subordinates than when they nest independently, providing a potential explanation for co-founding by non-relatives. There is some evidence that unrelated subordinates tailor their behaviour towards direct fitness, while the role of recognition errors in generating unrelated co-foundresses is less clear. Remarkably, the remote but potentially highly rewarding chance of inheriting the dominant position appears to strongly influence behaviour, suggesting that primitively eusocial insects may have much more in common with their social vertebrate counterparts than has commonly been thought.
Keywords: cooperative breeding, eusocial, direct fitness, inheritance, social wasps, Polistes
1. Introduction
In the Hymenoptera (ants, bees and wasps), indirect fitness obtained through helping genetic relatives has been the main paradigm used to understand when natural selection should favour the evolution of eusociality [1]. There are several good reasons for this. A practical reason is that associations of non-relatives are rare in the social Hymenoptera. Another reason is the existence of completely sterile workers in some ants, for which only indirect fitness benefits are possible through rearing the offspring of a related queen, usually the workers' mother. In most other ants, and in taxa such as honeybees and yellowjacket wasps, although workers are capable of laying eggs, they are still less fecund than queens and cannot mate, so that they can lay only haploid, male eggs. However, there are a few situations in which eusociality involving non-relatives is comparatively frequent. One example is the cooperation between unrelated foundresses that routinely occurs in some ant species [2]. Cooperation in this case appears to be mutualistic, for example, through increasing the chance that nests survive brood-raiding by other colonies. But cooperation usually breaks down as soon as the first workers mature to adulthood. At this point, the queens fight until only one remains alive. A second context in which unrelated colony members occur is so-called worker drifting, where workers from one colony enter an unrelated colony, either strategically or because they are lost. This has been less well investigated, but drifters in at least some taxa appear to be intraspecific social parasites, laying male eggs in recipient colonies [3].
In this paper, we discuss a third context in which unrelated individuals may often nest together: co-foundress associations of primitively eusocial wasps. In primitively eusocial insect societies, individuals known as subordinates or helpers sacrifice their own reproduction at least temporarily, and help to rear the offspring of another individual known as the queen or dominant. The defining feature of these societies, however, is that all individuals, including the helpers, are potentially capable of mating and independent reproduction. Primitively eusocial societies have therefore been the key testing grounds for theories concerning the origin of eusociality. Given that all individuals can potentially reproduce directly in these societies, cooperation between non-relatives seems intrinsically more likely to be evolutionarily stable. In this review, after a brief introduction to primitively eusocial wasps, we first document the occurrence of unrelated co-foundresses in paper wasps (Polistes). We then address two major questions: (i) why do some females become non-reproductive subordinates with unrelated nest-mates; (ii) why do these unrelated subordinates participate in costly helping behaviour? We consider both ultimate and proximate (mechanistic) answers to these questions.
2. Natural history of primitively eusocial wasps
Primitively eusocial wasps comprise the vespid subfamilies Polistinae (paper wasps) and Stenogastrinae (hover wasps), plus the little-studied lineage of apoid wasps that includes the genus Microstigmus [4]. These wasps have life-histories analogous to cooperatively breeding vertebrates. Indeed, Gadagkar [5] has previously advocated referring to both groups as ‘eusocial’.
Polistes paper wasps are the best-studied genus of primitively eusocial wasps, including more than 200 species that occur throughout most of the world [6]. In seasonal habitats, the nesting cycle begins in spring when overwintered females (foundresses) start building their characteristic paper nests attached to plants, rocks, man-made structures, etc. (figure 1). Foundresses have already been inseminated, usually by a single male, soon after emerging from their natal nests the previous autumn. In some populations, almost all nests have only a single foundress, whereas in other populations some or almost all nests have more than one foundress, with 10 or more not infrequent in some populations of P. dominula [7]. On multiple foundress nests, typically one ‘dominant’ foundress lays most or all of the eggs, while the others (‘subordinates') forage for insect prey which is pulped up and fed to larvae. Larvae mature to adulthood in late spring/early summer, denoting the end of the foundress phase of the nesting cycle. The first female offspring become workers on their natal nests. Workers help the foundress to rear further offspring, some of which are reproductives of both sexes. After mating with reproductives from other nests, the males die, and the female reproductives enter diapause to become the next year's new foundresses.
Figure 1.

Newly founded nest of Polistes dominula on a cactus at our Spanish field site, with three individually marked co-foundresses. Photo: M. Tiradon. (Online version in colour.)
3. Unrelated co-foundresses in Polistes
Co-foundresses in some Polistes species are close relatives: mean co-foundress relatedness is high in these species (≈0.6), with the majority of co-foundresses being full sisters [8–10]. However, mean relatedness is much lower (0.13–0.53) in other species such as P. aurifer [11] and P. dominula [12–16]. In these species, completely unrelated pairs of co-foundresses are common, with 15% (Spain) or 35% (Italy) of foundresses being unrelated in populations of P. dominula [13,15] (figure 2). In the Spanish populations, more than 80% of co-foundress groups contain at least one foundress that is unrelated or only distantly related to other group members [16]. Because P. dominula is by far the best studied of the species in which unrelated co-foundresses are common, we will focus on it in the remainder of this article.
Figure 2.
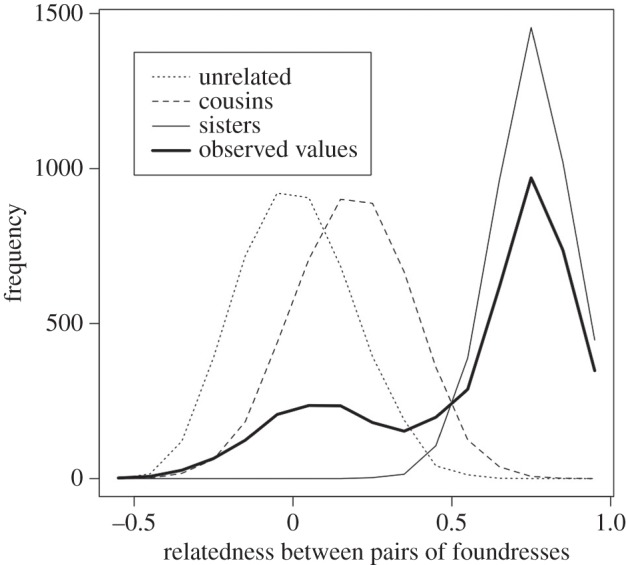
Distribution of relatedness between nest-mate pairs in our Spanish study population (thick solid line). Other lines represent expected distributions for populations comprising entirely sisters, cousins or unrelated nest-mate pairs. This figure is reproduced from Leadbeater et al. [13, fig. 2].
The significant proportion of unrelated subordinates in P. dominula at first sight appeared paradoxical, because the dominant foundress produces most of the offspring at any one time [15]. In fact, assuming that subordinates can obtain only indirect fitness, the increase in group productivity per subordinate seemed so small that even full sisters of the dominant would do better to nest independently [17]! A more recent, large-scale field study of the Spanish population of P. dominula, however, provides an unexpected explanation for this paradox based on direct fitness for subordinates, a possibility that has tended to be neglected in social insects. By genotyping offspring produced on more than 200 nests throughout the colony cycle, the study showed that although most subordinates produce few offspring, a minority are extremely productive, so that on average, subordinates produce more offspring of their own than lone breeders [12]. This is especially true towards the end of the colony cycle, the time when nearly all of the reproductive offspring are produced [12]. One-third of these subordinate-produced offspring represent eggs laid by subordinates in the presence of the original dominant, but the most important route for subordinate reproduction is via inheritance of the dominant position. Although the original dominant retains her position until the end of the colony cycle on more than 85% of nests, so that the chance of inheritance for individual subordinates is small, the reproductive payoff for the few that do inherit is large, especially in larger, more productive groups.
Nest inheritance, and laying occasional eggs while still a subordinate, provide an explanation for why some foundresses might choose to join groups of non-relatives in P. dominula. However, this does not explain why unrelated subordinates should carry out costly helping to rear offspring of the (unrelated) dominant. One adaptive explanation that could help to account for this involves group augmentation. Group augmentation benefits are direct benefits that a helper obtains through the increase in group size to which her helping effort leads [18]. There is little evidence that individual survival is greater in larger groups of primitively eusocial wasps [19], or that larger groups are better able to defend the nest against predators [6]. However, it is likely that group augmentation benefits do operate. If an unrelated subordinate helps to rear more offspring of the current dominant, she is likely to have more helpers and be more productive if she later inherits dominance herself: group productivity is positively correlated with group size, at least at the foundress stage [7,12], and is known to also correlate with group size at the worker stage where this has been investigated in other social insects [20]. This mechanism, of course, relies on worker offspring being unable to detect that the dominant is no longer their mother, or being prepared to rear the offspring of a less closely related individual: the evidence available suggests that this is the case [12,21–23].
4. Behavioural differences between unrelated subordinates and relatives of the dominant
An obvious question, given the above findings, is whether unrelated subordinates use behavioural strategies different from those used by relatives of the dominant. Because unrelated subordinates cannot obtain indirect fitness benefits through rearing the dominant's offspring, might they tailor their behaviour towards inheritance? In the field, offspring genotyping shows that subordinates that are sisters and cousins of the dominant have the same direct fitness (number of offspring of their own) as unrelated subordinates [12]. Furthermore, group productivity in the Spanish population is unaffected by mean relatedness between the cofoundresses on a nest. However, breaking total direct fitness down into its components, it was found that unrelated subordinates produce significantly fewer offspring as subordinates (in the presence of the dominant) than do relatives of the dominant. This does not lead to lower direct fitness overall for unrelated subordinates, however, because unrelated subordinates also tend to produce more offspring through inheritance than do relatives of the dominant [12, supplementary material].
Because subordinates are not sterile, they are likely to face a trade-off between helping effort and future direct fitness benefits [24]. For example, by investing more in rearing offspring of the dominant, subordinates suffer reduced survival [24], and therefore have a smaller chance of eventually inheriting the dominant position themselves. We might therefore expect unrelated subordinates to forage less than their related counterparts: unrelated subordinates can obtain group augmentation benefits by foraging (see §3), but not the indirect benefits that relatives of the dominant simultaneously obtain. Indeed, Queller et al. [15] found a tendency for more distant relatives of the dominant to forage less, although this was only a marginally significant result from observations conducted in small laboratory cages.
To further elucidate the strategies of unrelated cofoundresses, Leadbeater et al. [13,25] compared the behaviour of related and unrelated subordinates in more detail in the field, in key contexts where the two kinds of wasps might be expected to behave differently. These contexts included two costly aspects of helping behaviour itself: foraging for larval food; and nest defence when presented with a foreign ‘usurper’. And two contexts where unrelated subordinates might be expected to show greater aggression than relatives of the dominant: aggression immediately after experimental removal of the dominant, when the opportunity for inheritance arises; and aggression when the original dominant was returned to the nest, several days after her removal. The latter context is useful because it can lead to serious, escalated fights between the original dominant and the rank 2, fights of a kind rarely seen otherwise [26]. Leadbeater et al. [13] also tested whether unrelated subordinates tend to occupy higher inheritance ranks, as expected if they behave strategically to maximize their direct fitness.
A first analysis of these behavioural data suggested that unrelated subordinates behave no differently to relatives of the dominant [13]: they forage as much, are no less aggressive in nest defence, and no more aggressive when the opportunity to inherit arises or when an experimentally removed dominant returns to the nest. Furthermore, unrelated subordinates occupy no higher inheritance ranks than relatives of the dominant. However, a wasp's position in the queue to inherit was correlated with her size, and intriguingly, incorporating an interaction between size and relatedness into the analysis produced somewhat different results, suggesting that the effect of relatedness might depend upon an individual's chances of inheritance [25]. First, relatives of the dominant foraged more if they were smaller in size (and so lower down the inheritance queue), but this pattern was absent in unrelated subordinates. This suggests that related subordinates with little chance of inheritance might opt to invest more in the current brood, while those that have better direct fitness prospects forage less, in order to reduce their own chance of mortality. Unrelated subordinates are not sensitive to the probability of inheritance, presumably because it represents their only source of fitness, whether probable or not. However, this contrasts with the second finding, that unrelated subordinates were more aggressive following dominant removal if they were larger, but no such pattern occurred for relatives. It is unclear why small, unrelated subordinates appear not to fight for the dominant position, and more data are clearly needed for a full interpretation. These patterns do, however, suggest that related and unrelated subordinates might help for different reasons. For example, relatives of the dominant might adjust their effort voluntarily, according to the trade-off between current indirect- and future direct-fitness benefits, while unrelated subordinates might be forced by the threat of eviction or punishment to work harder than they would otherwise choose in some contexts (pay-to-stay [27,28]).
5. Recognition errors: mechanistic constraints that could lead to low co-foundress relatedness
Although some of the evidence just discussed suggests that unrelated subordinates might behave differently to relatives of the dominant in some contexts, it is worth considering the hypothesis that at least some unrelated subordinates have simply made recognition errors. One of the cues widely believed to be involved in kin- or nest-mate recognition in social insects is the cuticular hydrocarbon profile, the complex mixture of lipids found on the insect cuticle. However, differences between individuals within the same social group can be blurred through transfer of odours during allogrooming and other physical contact. The resulting gestalt odour, common to all colony members, facilitates recognition of non-nest-mates but reduces the potential for within-nest kin recognition [29]. This constraint could mean that foundresses are unable to detect when they are unrelated to the dominant, and so cannot adjust their behaviour adaptively. However, less closely related pairs of P. dominula cofoundresses do in fact have less similar hydrocarbon profiles, suggesting that hydrocarbon cues contain useful information even within colonies [25,30]. But relatedness itself is a better predictor of behaviour than hydrocarbon differences [25]. This suggests either that wasps do not use hydrocarbon cues, or that specific components, rather than the overall hydrocarbon profile that has been measured to date, are the cues involved in recognition [31].
Even if odour-mixing makes it difficult for nest-mates to assess individual relatedness, one might expect wasps that are not yet part of a group to retain informative cues that could be used to avoid joining non-relatives. The indirect fitness benefits available to subordinate relatives of the dominant in P. dominula are usually much larger than the direct benefits, suggesting that given a choice, foundresses should prefer to nest with relatives [12]. However, at least some unrelated foundresses have relatives in other groups, suggesting that they might have made recognition errors [12]. A mechanistic constraint that may limit the usefulness of hydrocarbon cues, even when first joining an existing group, is odour transfer during hibernation. After reaching adulthood in late summer, females that will become foundresses the following spring overwinter in tight clusters of sometimes hundreds of individuals, often in crevices or behind old nests. Thus, even though females have informative cues on first reaching adulthood on their natal nests [30], these cues may become blurred during hibernation [32]. Indeed, relatedness between wasps in the same hibernation cluster is relatively low (≈0.3) [16], with cluster-mates at least sometimes originating from different nests and occasionally even being different species [16,33]. Furthermore, when wasps from different localities, with distinct hydrocarbon profiles, are placed in mixed clusters in the laboratory, their profiles become more similar [32]. Subsequently, wasps that had been forced to hibernate in mixed clusters show less of a preference for co-founding nests with other wasps originating from the same locality [32,34].
Mechanistic constraints that reduce the utility of hydrocarbon cues could help to explain the high frequency of unrelated subordinates in P. dominula, and could reduce the ability of foundresses to respond appropriately to relatedness with their nest-mates. Variation between foundresses in the constraints experienced could also introduce noise into analyses aimed at testing for correlations between behaviour and relatedness. For example, some pairs of unrelated co-foundresses could have reached adulthood on the same natal nest then hibernated in a large cluster with foundresses from many other nests. At the opposite extreme, other unrelated foundresses could have matured on separate nests then hibernated with only close relatives. These latter foundresses should be less likely to co-found nests in spring, and if they do co-found, should be more likely to express behaviours appropriate to their low relatedness.
Although mechanistic constraints might result in foundresses having limited cues to detect relatedness, it remains unclear why the frequency of unrelated cofoundresses is so much higher in P. dominula than in some other Polistes species where the vast majority of nest-mates are close relatives [8,10]. Most Polistes species probably overwinter in hibernation clusters where recognition cues could become obscured [32]. One characteristic that could help to explain the high frequency of unrelated cofoundresses is that P. dominula appears to be an invasive species [35], so that perhaps there is a high frequency of immigrants with no relatives in the population. Coupled with the low reproductive success obtained when nesting alone, so that foundresses should prefer to co-found even with unrelated nest-mates [12], this has the potential to explain the existence of unrelated nest-mates. Arguing against this explanation, however, low relatedness is also found in populations where single foundress nests are more successful and more common [15]. A second characteristic of at least some of the P. dominula populations that have been studied [12,13,25] is that nests occur at high density. This has the potential to interfere with another aspect of recognition: philopatry. After leaving winter hibernation, a foundress typically founds a nest close to the position of her own natal nest the previous year [36,37]. Foundresses might be more likely to make mistakes when their natal nests are close to other nests.
6. Concluding remarks
In this review, we have focused on the species that has become the best-studied of all the primitively eusocial wasps. Thanks to its remarkable social biology, combined with the accessibility of its nesting sites, Polistes dominula now offers an extraordinary depth of insight into the evolutionary drivers of behaviour in eusocial groups where helpers truly have an option to breed alone. The picture that emerges is one of a eusocial insect society that has more in common with cooperatively breeding vertebrate groups, in which the presence of non-relatives is widespread [38,39], than had previously been conceived. Like their vertebrate counterparts [39], cofoundress associations are shaped and maintained by both direct and indirect fitness components. Unrelated helpers are therefore more likely to be symptom of the fact that direct fitness benefits can maintain cooperation [38] than just a maladaptive by-product of kin recognition errors. Moreover, such direct fitness benefits may well have played a role in precluding the evolution of a committed worker caste in primitively eusocial groups; it makes no evolutionary sense to forgo reproductive potential if the prospect of nest inheritance is real [40].
Many unanswered questions nevertheless remain. In order to further understand the origins of unrelated cofoundresses in Polistes, it would be useful to trace the history of foundresses in nature, from the natal nests where they reached adulthood to the other females with which they share hibernation clusters and the spring nesting associations that they eventually form. This would allow us to test whether foundresses with different histories behave differently, and thus the extent to which recognition errors might lead to variation in behaviour. A second approach that could be fruitful for understanding variation in behaviour is the application of biological market theory [41] to cofoundress associations. The work discussed above has considered individual nests in isolation. In temperate Polistes, however, large numbers of foundresses initiate new nests synchronously in spring, and switching between different nests is common [10,34,42]. Foundresses may have a choice of nests to potentially join, and residents on existing nests may have a choice of potential joiners. Whether this truly represents a biological market, and if so how partner choice and market forces influence behaviour, remain to be investigated.
Authors' contributions
J.F. drafted the manuscript, which was then discussed and edited by both authors. Both authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Much of this research was funded by NERC grant NE/E017894/1 to J.F. E.L. was funded by a Leverhulme Trust Early Career Fellowship during the writing of the manuscript.
References
- 1.Hamilton WD. 1964. The genetical evolution of social behaviour. I. J. Theoret. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi G, Strassmann JE. 1999. Cooperation among unrelated individuals: the ant foundress case. Trends Ecol. Evol. 14, 477–482. ( 10.1016/S0169-5347(99)01722-X) [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Vaamonde C, Koning JW, Brown RM, Jordan WC, Bourke AFG. 2004. Social parasitism by male-producing reproductive workers in a eusocial insect. Nature 430, 557–560. ( 10.1038/nature02769) [DOI] [PubMed] [Google Scholar]
- 4.Matthews RW. 1991. Evolution of social behaviour in Sphecid wasps. In The social biology of wasps (eds Ross KG, Matthews RW), pp. 570–602. Ithaca, NY: Cornell University Press. [Google Scholar]
- 5.Gadagkar R. 1994. Why the definition of eusociality is not helpful to understand its evolution and what should we do about it. Oikos 70, 485–488. ( 10.2307/3545789) [DOI] [Google Scholar]
- 6.Reeve HK. 1991. Polistes. In The social biology of wasps (eds Ross KG, Matthews RW), pp. 99–148. Ithaca, NY: Cornell University Press. [Google Scholar]
- 7.Shreeves G, Cant MA, Bolton A, Field J. 2003. Insurance-based advantages for subordinate co-foundresses in a temperate paper wasp. Proc. R. Soc. Lond. B 270, 1617–1622. ( 10.1098/rspb.2003.2409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field J, Solís CR, Queller DC, Strassmann JE. 1998. Social and genetic structure of paper wasp cofoundress associations: tests of reproductive skew models. Am. Nat. 151, 545–563. ( 10.1086/286140) [DOI] [PubMed] [Google Scholar]
- 9.Reeve HK, Starks PT, Peters JM, Nonacs P. 2000. Genetic support for the evolutionary theory of reproductive transactions in social wasps. Proc. R. Soc. Lond. B 267, 75–79. ( 10.1098/rspb.2000.0969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seppa P, Queller DC, Strassmann JE. 2002. Reproduction in foundress associations of the social wasp, Polistes carolina: conventions, competition, and skew. Behav. Ecol. 13, 531–542. ( 10.1093/beheco/13.4.531) [DOI] [Google Scholar]
- 11.Liebert AE, Nonacs P, Wayne RK. 2005. Solitary nesting and reproductive success in the paper wasp Polistes aurifer. Behav. Ecol. Sociobiol. 57, 445–456. ( 10.1007/s00265-004-0875-5) [DOI] [Google Scholar]
- 12.Leadbeater E, Carruthers JM, Green JP, Rosser NS, Field J. 2011. Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333, 874–876. ( 10.1126/science.1205140) [DOI] [PubMed] [Google Scholar]
- 13.Leadbeater E, Carruthers JM, Green JP, van Heusden J, Field J. 2010. Unrelated helpers in a primitively eusocial wasp: is helping tailored towards direct fitness? PLoS ONE 5, e0011997 ( 10.1371/journal.pone.0011997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liebert AE, Starks PT. 2006. Taming of the skew: transactional models fail to predict reproductive partitioning in the paper wasp Polistes dominulus. Anim. Behav. 71, 913–923. ( 10.1016/j.anbehav.2005.09.005) [DOI] [Google Scholar]
- 15.Queller DC, Zacchi F, Cervo R, Turillazzi S, Henshaw MT, Santorelli LA, Strassmann JE. 2000. Estimating relatedness. Nature 405, 784–787. ( 10.1038/35015552) [DOI] [PubMed] [Google Scholar]
- 16.Zanette LRS, Field J. 2008. Genetic relatedness in early associations of Polistes dominulus: from related to unrelated helpers. Mol. Ecol. 17, 2590–2597. ( 10.1111/j.1365-294X.2008.03785.x) [DOI] [PubMed] [Google Scholar]
- 17.Nonacs P, Liebert AE, Starks PT. 2006. Transactional skew and assured fitness return models fail to predict patterns of cooperation in wasps. Am. Nat. 167, 467–480. ( 10.1086/501168) [DOI] [PubMed] [Google Scholar]
- 18.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196. ( 10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shreeves G, Field J. 2002. Group size and direct fitness in social queues. Am. Nat. 159, 81–95. ( 10.1086/324125) [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Vaamonde C, Raine NE, Koning JW, Brown RM, Pereboom JJM, Ings TC, Ramos-Rodriguez O, Jordan WC, Bourke AFG. 2009. Lifetime reproductive success and longevity of queens in an annual social insect. J. Evol. Biol. 22, 983–996. ( 10.1111/j.1420-9101.2009.01706.x) [DOI] [PubMed] [Google Scholar]
- 21.Field J, Foster W, Shreeves G, Sumner S. 1998. Ecological constraints on independent nesting in facultatively eusocial hover wasps. Proc. R. Soc. Lond. B 265, 973–977. ( 10.1098/rspb.1998.0386) [DOI] [Google Scholar]
- 22.Hughes CR, Beck MO, Strassmann JE. 1987. Queen succession in the social wasp, Polistes annularis. Ethology 76, 124–132. ( 10.1111/j.1439-0310.1987.tb00678.x) [DOI] [Google Scholar]
- 23.Gamboa GJ. 1996. Kin recognition in social wasps. In Natural history and evolution of paper-wasps (eds Turillazzi S, West-Eberhard MJ), pp. 161–177. Oxford, UK: Oxford University Press. [Google Scholar]
- 24.Cant MA, Field J. 2001. Helping effort and future fitness in cooperative animal societies. Proc. R. Soc. Lond. B 268, 1959–1964. ( 10.1098/rspb.2001.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leadbeater E, Dapporto L, Turillazzi S, Field J. 2013. Available kin recognition cues may explain why wasp behavior reflects relatedness to nest mates. Behav. Ecol. 25, 344–351. ( 10.1093/beheco/art113) [DOI] [Google Scholar]
- 26.Cant MA, English S, Reeve HK, Field J. 2006. Escalated conflict in a social hierarchy. Proc. R. Soc. B 273, 2977–2984. ( 10.1098/rspb.2006.3669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokko H, Johnstone RA, Wright J. 2002. The evolution of parental and alloparental effort in cooperatively breeding groups: when should helpers pay to stay? Behav. Ecol. 13, 291–300. ( 10.1093/beheco/13.3.291) [DOI] [Google Scholar]
- 28.Field J, Cant M. 2007. Direct fitness, reciprocity and helping: a perspective from primitively eusocial wasps. Behav. Process 76, 160–162. ( 10.1016/j.beproc.2007.01.019) [DOI] [PubMed] [Google Scholar]
- 29.van Zweden JS, Brask JB, Christensen JH, Boomsma JJ, Linksvayer TA, D'ettorre P. 2010. Blending of heritable recognition cues among ant nestmates creates distinct colony gestalt odours but prevents within-colony nepotism. J. Evol. Biol. 23, 1498–1508. ( 10.1111/j.1420-9101.2010.02020.x) [DOI] [PubMed] [Google Scholar]
- 30.Dani FR, et al. 2004. Can cuticular lipids provide sufficient information for within-colony nepotism in wasps? Proc. R. Soc. Lond. B 271, 745–753. ( 10.1098/rspb.2003.2646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S. 2001. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 62, 165–171. ( 10.1006/anbe.2001.1714) [DOI] [Google Scholar]
- 32.Dapporto L, Pansolli C, Turillazzi S. 2004. Hibernation clustering and its consequences for associative nest foundation in Polistes dominulus (Hymenoptera Vespidae). Behav. Ecol. Sociobiol. 56, 315–321. ( 10.1007/s00265-004-0800-y) [DOI] [Google Scholar]
- 33.Starks PT. 2003. Natal nest discrimination in the paper wasp, Polistes dominulus. Ann. Zool. Fenn. 40, 53–60. [Google Scholar]
- 34.Zanette LRS, Field J. 2011. Founders versus joiners: group formation in the paper wasp Polistes dominulus. Anim. Behav. 82, 699–705. ( 10.1016/j.anbehav.2011.06.025) [DOI] [Google Scholar]
- 35.Liebert AE, et al. 2006. Genetics, behavior and ecology of a paper wasp invasion: Polistes dominulus in North America. Ann. Zool. Fenn. 43, 595–624. [Google Scholar]
- 36.Klahn JE. 1979. Philopatric and nonphilopatric foundress associations in the social wasp Polistes fuscatus. Behav. Ecol. Sociobiol. 5, 417–424. ( 10.1007/BF00292528) [DOI] [Google Scholar]
- 37.Strassmann JE. 1983. Nest fidelity and group size among foundresses of Polistes annularis (Hymenoptera: Vespidae). J. Kansas Entomol. Soc. 56, 621–634. [Google Scholar]
- 38.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245 ( 10.1098/rspb.2013.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clutton-Brock T. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72. ( 10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 40.Bourke AFG. 1999. Colony size, social complexity and reproductive conflict in social insects. J. Evol. Biol. 12, 245–257. ( 10.1046/j.1420-9101.1999.00028.x) [DOI] [Google Scholar]
- 41.Noe R, Hammerstein P. 1995. Biological markets. Trends Ecol. Evol. 10, 336–339. ( 10.1016/S0169-5347(00)89123-5) [DOI] [PubMed] [Google Scholar]
- 42.Seppa P, Queller DC, Strassmann JE. 2012. Why wasp foundresses change nests: relatedness, dominance, and nest quality. PLoS ONE 7, e45386 ( 10.1371/journal.pone.0045386) [DOI] [PMC free article] [PubMed] [Google Scholar]


