Abstract
Many bats are extremely social. In some cases, individuals remain together for years or even decades and engage in mutually beneficial behaviours among non-related individuals. Here, we summarize ways in which unrelated bats cooperate while roosting, foraging, feeding or caring for offspring. For each situation, we ask if cooperation involves an investment, and if so, what mechanisms might ensure a return. While some cooperative outcomes are likely a by-product of selfish behaviour as they are in many other vertebrates, we explain how cooperative investments can occur in several situations and are particularly evident in food sharing among common vampire bats (Desmodus rotundus) and alloparental care by greater spear-nosed bats (Phyllostomus hastatus). Fieldwork and experiments on vampire bats indicate that sharing blood with non-kin expands the number of possible donors beyond kin and promotes reciprocal help by strengthening long-term social bonds. Similarly, more than 25 years of recapture data and field observations of greater spear-nosed bats reveal multiple cooperative investments occurring within stable groups of non-kin. These studies illustrate how bats can serve as models for understanding how cooperation is regulated in social vertebrates.
Keywords: by-product mutualism, group augmentation, partner choice, reciprocity, vampire bats, spear-nosed bats
1. Introduction
With over 1300 described species, bats are the second most speciose order of mammals and exhibit extraordinary social diversity, from species that roost solitarily to those that form stable groups, dynamic social networks or seasonal aggregations in excess of a million individuals [1,2]. Bats are also extremely long-lived for their size, with individuals from several species known to survive over 30 years [3]. Relatedness within social groups is often low, and interactions frequently occur between distant kin and non-kin [4,5].
Many mutually beneficial behaviours in bats—such as foraging together or huddling for warmth—appear to emerge from selfish individual actions. Evolutionary explanations of cooperation typically distinguish between such by-product mutualisms and outcomes resulting from cooperative investments that require time or energy and can be exploited [6–8]. Some authors restrict their definition of ‘cooperation’ to only the latter case, requiring that cooperative traits evolved because they benefit others (e.g. [7], but see [9]). In other words, not all cooperative outcomes require evolved cooperative investments. Because by-product mutualisms provide the simplest explanations for cooperative outcomes, they are useful null hypotheses. However, simple mutualisms can lead to escalating selective pressures for individuals to invest differently among partners according to their returns [10,11]. Hence, many presumptive by-product mutualisms may often require complex and contingent decision-making by one or both participants [12,13].
Key social behaviours in bats involve roosting, foraging, feeding and caring for offspring. For potential cooperative behaviours in each of these situations, we considered two questions. Do the actors each make a costly cooperative investment? If so, what ensures a cooperative return? We indicate which cases are supported by data or remain speculative at present and merit additional study, and then discuss details of what we consider to be the clearest cases involving cooperative investments. Blood sharing in common vampire bats (Desmodus rotundus) shows how kin-biased helping can mask an important role for direct fitness benefits, while pup guarding in greater spear-nosed bats (Phyllostomus hastatus) exemplifies how complex cooperative investments can occur within stable groups of non-kin.
2. Cooperation at the roost
As in many birds [14], communal roost sites provide a potential public good for bats. Benefits of roost sharing include dilution of predation risk [15], information sharing or exploitation [16,17], social thermoregulation [18] and access to mates. Additional benefits may arise from long-term relationships formed by roosting together for extended periods. Cooperative investments can occur when bats advertise, defend or construct roost sites.
Echolocating bats constantly broadcast their location while flying, and this public information can be used to locate or even advertise communal roost sites. Many tree-cavity roosting bats form subgroups that move among several roosts but belong to a larger more stable social network (e.g. [19–22]). The resulting associations may be partially kin-biased, but active recruitment of unrelated individuals indicates that direct fitness benefits are important [23]. For example, Bechstein's bats, Myotis bechsteini, form long-term relationships [24] and recruit non-kin to roosts. In experiments introducing novel artificial roosts, naive bats were recruited to new sites by experienced bats independent of kinship [25]. Given that colony members do not forage in close proximity [26], roost discovery must either involve active leading or overt advertisement. Some individuals appear to explore and share roost discoveries more often than others [25]. The degree to which such roles are alternated has yet to be determined.
While at or near roost sites, several bat species actively recruit roostmates with low-frequency social calls [27,28]. These calls travel farther than echolocation calls and convey more information, such as individual identity [28]. For instance, disc-winged bats (Thyroptera tricolor) roost in large furled leaves, and recruit additional group members [29] to these ephemeral roosts using an antiphonal calling system [27,30]. Flying bats respond preferentially to group members, but those bats already in roosts vocally respond to both group and non-group mates, either because they cannot discriminate using calls or because adding more individuals is not costly to the bats already in the roost [30].
Some bats build their own roosts by chewing cavities in termite nests [31] or by chewing, folding or pulling leaves into ‘tents' that can last weeks to years [32–34]. Roost construction appears to be under sexual selection because shelters are typically constructed by single males [31,35] and occupied by them and one or more females [36]. However, females also make tents in some species [37]. Whether or not their construction costs are reduced through cooperative tent-building is unknown.
Many bats in temperate regions aggregate during the winter to hibernate. Hibernating bats often cluster, possibly to reduce loss of water [38] or energy required for arousal [39], similar to how huddling reduces energy loss in social rodents [40] and some cooperatively breeding birds [41]. In the spring, females move to warmer sites for pup rearing, while males roost alone or join bachelor colonies. To prepare again for hibernation, females and males converge at hibernacula in ‘swarming’ aggregations, within which promiscuous mating occurs [42]. Because these aggregations contain multiple species from large areas, they may result simply from a limited number of suitable sites. However, mark–recapture data at swarming sites indicate that some males stay together over multiple nights and young-of-the-year often arrive together [42]. The causes and consequences of these associations are not known, but there is evidence that interactions between pups can influence the development of subsequent social relationships [43].
Outside of primates, cooperative defence of females by a coalition of males is uncommon [44], with notable exceptions including lions, Panthera leo [45], horses, Equus caballus [46], dolphins, Tursiops sp. [47] and two neotropical bat species, Artibeus jamaicensis and Saccopteryx bilineata. In both bat species, dominant and related subordinate males cooperatively defend female groups from intruding males [48,49]. In S. bilineata, harem male tenure is longer and lifetime breeding success is greater when more males are present to help exclude intruders from a colony [48]. Similarly, the presence of subordinate male A. jamaicensis reduced the number of visits by satellite males to groups of females defended by dominant males [50]. In both species, the presence of subordinates improved the direct fitness of the dominant and subordinates had increased breeding opportunities. However, the relative importance of direct and indirect fitness benefits has yet to be determined for either species.
3. Cooperative foraging
Bats that use echolocation to hunt aerial prey increase their call rate and decrease frequency just prior to capture. The resulting ‘feeding buzz’ indicates prey presence to others in the area. Many studies [51,52] have shown that playbacks of feeding buzzes attract conspecifics. Such eavesdropping is typically viewed as social parasitism in which the feeding buzz of one bat enables a competitor to discover food, similar to how terns use plunge dives of conspecifics to locate fish schools [53]. However, when bats elect to forage together, they can more easily attend to each other's feeding buzzes. In a suitably heterogeneous environment, foraging together can be viewed as a cooperative investment that increases collective search area and improves detection of ephemeral food patches [54]. Several studies suggest that, as in some swallows [55], some insectivorous bats exhibit social foraging when hunting for unpredictable but patchy prey. For example, two neotropical bats (Noctilio albiventris and Molossus molossus) forage for insects over water in small groups [56,57] and female evening bats (Nycticeius humeralis) have greater foraging success when they follow a previously successful forager [58]. Detailed description of social foraging comes from a recent study of greater mouse-tailed bats (Rhinopoma microphyllum) carrying a microphone and GPS recorder. Bats that foraged close enough to hear conspecific feeding buzzes expanded their prey patch detection radius [59]. At this point, we do not know how widespread such social foraging might be. Given that most experimental work has focused on echolocation strategies under competitive situations where prey cannot be shared [60,61], considerable opportunity exists to pursue this topic in captive and field situations.
Bats may cooperate, not only to discover food patches, but also to defend or exploit them. This hypothesis is consistent with observations of greater spear-nosed bats giving group-specific calls that attract groupmates when foraging on rich food sources, such as large flowering trees [62], and with observations of vampire bats that preferentially defend or tolerate others at wound sites on livestock [19]. A potential but unconfirmed case of strategic patch exploitation involves flower-visiting bats that might consistently fly together to avoid feeding from previously visited plants or in formation to avoid previously visited flowers [63–65] in a manner similar to that hypothesized for finch flocks in the desert [66].
4. Food sharing
In some species, bats actively share food with others. Some adult bats provision their young with prey (e.g. Vampyrum spectrum [1] and Micronycteris microtis [67]), but regular food sharing among adults has only been reported for the three species of blood-feeding vampire bats [68–70]. Most food sharing among common vampire bats, D. rotundus, involves mothers regurgitating to dependent young [71], as occurs in many birds and mammals. However, adult bats that are well fed will also regurgitate to bats in need. This situation occurs regularly because 18% of bats fail to obtain a blood meal [68] and unfed bats are susceptible to starvation owing to their limited ability to store energy [72,73]. By receiving regurgitations, a hungry bat can regain 20% of its mass lost from 24 h of fasting [68,74]. Such donations can, therefore reduce a recipient's risk of mortality by starvation. Successful foragers can obtain a large blood meal, so the costs of sharing are low and the benefits of receiving are high [68].
Regurgitated blood sharing likely evolved from extended maternal care. Once symmetrical helping is established through kin selection, the direct fitness benefits of reciprocal help can be greater at all levels of kinship, leading bats to base decisions on repeated social interactions rather than just kinship [75]. A similar scenario may account for some cases of cooperative breeding in birds [76,77]. Below, we summarize how this scenario is consistent with the relative importance of social factors that affect vampire bat food-sharing decisions.
(a). Social predictors of food sharing in vampire bats
Wild female vampire bats form stable associations with both kin and non-kin [19]. Regurgitations between adults correlated independently with both kinship and co-roosting association but occurred only between bats that roosted together at least 60% of the time [68]. Attempts to induce food sharing in captivity among unfamiliar bats failed unless the animals had been together for months [78]. Hence, food sharing is biased towards familiar partners, which are often, but not always, related.
Previous help is more important than kinship for explaining variation in donation rates among familiar individuals. In a captive group of bats of mixed relatedness, both food and grooming received were more important than kinship for predicting donation rates across dyads, with prior food received nearly nine times more predictive than kinship [74]. Measures of captive food sharing are relevant to what occurs in the wild because regurgitation durations between adult bats are similar in the wild and in captivity (figure 1) despite different methods of observation.
Figure 1.
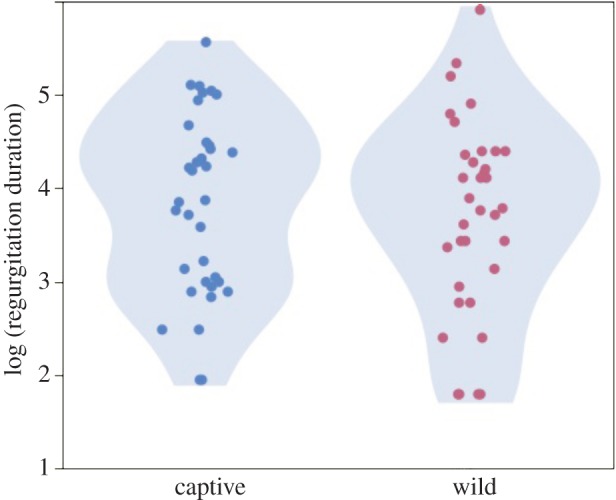
Distributions of regurgitation donations (more than 5 s) between adult (more than 2 years) wild [68] and a random sample of captive vampire bats [74]. N = 36 regurgitations in both cases (points jittered to prevent overlap). Shaded areas show probability density. Mean duration (and boot-strapped 95% confidence interval) of regurgitations were 68.0 s (52–85 s) and 65.9 s (49–87 s) in captive and wild bats, respectively. Kinship did not predict regurgitation duration in either captive (r2 = 0.0005, p = 0.9) or wild bats (r2 = 0.005, p = 0.7, permuted linear regression). (Online version in colour.)
(b). Why do vampire bats feed non-kin?
Non-kin food sharing is common among familiar bats even if the individuals are first housed together as adults. Given the opportunity to accrue both direct and indirect fitness benefits by exclusively helping kin, why should vampire bats ever feed non-kin? Recent work shows that helping non-kin expands the network of possible donors beyond that possible if sharing were limited to close kin [79]. When primary donors, many of whom were relatives, were prevented from sharing, females that previously shared food with more non-kin were fed by more individuals and received more food [79]. When kin donors are unavailable, non-kin can therefore act as a ‘safety net’.
Ideally, testing reciprocity would involve not only inducing acts of reciprocal cooperation, but also measuring the extent to which individuals shift their investments either away from or towards partners whose ability to reciprocate has been diminished or enhanced [6,13]. For example, Norway rats, Rattus norvegicus, trained in a food delivery task give food based on past help received [80,81] but testing such contingency among animals with long-term social bonds can be more difficult for two key reasons. First, as shown in several primate studies [82–84], food sharing between pairs of vampire bats is balanced only over extended periods [74] rather than via short-term matching. Detecting change from symmetry requires, therefore, prolonged experiments. In an attempt to determine how vampire bats respond to non-reciprocation, we prevented food sharing between targeted pairs of bats with a history of sharing so that each could only be fed by other bats for several weeks. When sharing was then allowed between the pair, five targeted donors refused to share any blood while six others increased their relative contributions [79]. These divergent responses suggest that vampire bats do not follow a simple tit-for-tat rule [85] and may use alternative strategies for dealing with non-reciprocation.
Second, an imbalance in one service, such as food sharing, can potentially be compensated by another service, such as allogrooming. Such multiple benefits maintain social bonds in many primates [12,86,87] and likely also do so in vampire bats. Social grooming and food sharing are correlated in vampire bats [74,88] and appear to be regulated by some shared hormonal mechanisms, such as oxytocin [89]. However, unlike food sharing, social grooming does not require a hungry recipient, is not constrained by contributions from other partners, and can occur at any time. Measuring precisely how food sharing is regulated within long-term bonds will require controlled studies that manipulate the ability of bats to give and receive, while controlling for social factors such as the kinship and experience of potential partners, as well as other forms of cooperation.
5. Cooperative care of young
Maternal care may have served as the origin for more complex cooperative investments in other species besides vampire bats. For example, pups have been observed to nurse from non-maternal females, which can sometimes be explained as an inability of females to fully prevent milk parasitism [90] or as a by-product benefit, if ‘milk dumping’ occurs during times of excess production [91]. Group augmentation in which helpers gain direct fitness benefits by increasing their own group's size [92,93] might also be important in cases where female bats preferentially allonurse female offspring that remain in the colony as adults [91].
Many female bats leave newborn pups in clusters called crèches [94]. As in penguins [95], crèching likely provides multiple by-product benefits; however, some benefits, such as heat, could be an exploitable public good. By investing less in self-warming, a pup in a crèche could conserve energy by exploiting the heat generated by its neighbours [96]. In response, pups might selectively huddle with warmer individuals to prevent such ‘cheating’.
A particularly intriguing case of cooperative offspring care occurs in greater spear-nosed bats (P. hastatus). Females of this neotropical species roost in the ceilings of caves in discrete groups of 10–25 individuals (figure 2a). Each group is defended year round by a single male that sires 64–100% of the offspring in the group [5]. Both male and female offspring disperse from their natal group during their first year. Dispersing males join all-male bachelor groups while dispersing females either join an existing group or form a new group with other first-year females. Females first give birth to a single pup in their second year and usually every subsequent year until they die. Between 1990 and 2015, we conducted 20 field trips to Trinidad, West Indies in which we captured and banded 4179 bats and recaptured 1664 individuals—some up to 22 times. By capturing pups attached to lactating females we inferred maternity for 1134 offspring. Below, we use these data [97] to provide insight into how and why cooperation occurs in this species.
Figure 2.
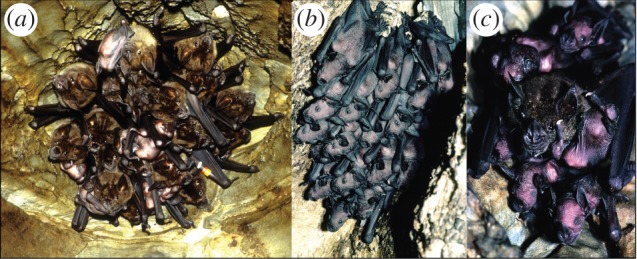
Photos of greater spear-nosed bats illustrating (a) a female group in a cave ceiling depression, (b) a crèche of pups and (c) an adult female babysitting pups. (Online version in colour.)
(a). Female greater spear-nosed bats form unrelated groups
Prior studies show that female groups are very stable [5]. Our new analyses confirm this conclusion (e.g. four members of one group were recaptured together for 15 years) but also reveal that females occasionally switch groups during their lives. Adult females (N = 1137) roost in 1.53 ± 0.03 (mean ± s.e.) groups. The average age estimate of an adult female is 4.3 ± 0.1 years, but many individuals live much longer including one female that was recaptured after 20 years. Dominant males (N = 130) are 4.4 ± 0.1 years old and retain tenure for 1.34 ± 0.06 years, although two adult males remained with a female group for four years.
Given these demographics, females could potentially have either paternal or maternal relatives in their social group. To evaluate these possibilities, we assumed that all pups born in a social group in a year are sired by the same male to obtain an upper estimate of the number of paternal half-sibs in a female group. A total of 206 females were banded as pups and subsequently recaptured as reproductive adults in a group. Of these, 74 females had at least one potential paternal half-sib in the same group. But those 74 potential half-sibs came from 33 different dominant males. Given the number of groups, this results in an upper limit of 1.9 half-sibs per group. In other words, even 100% paternity by dominant males produces only two paternal half-sibs per group. Maternal half-sibs in the same group are even less likely. Out of 80 female maternal half-sib pairs, only three pairs were recaptured as adults. The females in two pairs were never caught in the same group while the females in the third pair were recaptured in the same group once, but recaptured in different groups in four other years.
These inferences from mark–recapture data are consistent with estimates of average group relatedness using genetic markers (r = 0.04 ± 0.04 for nine groups using three allozyme loci [4] and r = 0.01 ± 0.01 for seven groups using five microsatellite loci [98]). Thus, as in feral horses [99] and some cooperatively breeding birds [76], nearly all females within a social group are unrelated.
(b). Female greater spear-nosed bats babysit unrelated pups
One striking feature of reproduction in greater spear-nosed bats is within-group reproductive synchrony; while births in the same cave can differ by a month or more, most female groupmates give birth within a span of a few days [5,100]. Group-specific reproductive synchrony leads to group crèches (figure 2b), where each female leaves its pup while foraging [62]. However, at night a single adult often can be seen in a crèche (figure 2c). To gain insight into why some individuals delay foraging to stay with pups, we conducted daily censuses for 14 days in 2001 on 25 groups containing individually marked bats [62,101,102]. During this period, the number of pups increased from 22 to 203.
These observations indicate that females delay foraging to ‘babysit’—similar to what has been observed in meerkats, Suricata suricatta [103]. Evening group censuses revealed a positive association between the presence of pups and the presence of an adult female (χ2 = 41.14, p < 0.0001, d.f. = 137) and the number of females positively correlated with the number of pups (Spearman's r = 0.44, p < 0.0001). Females were never observed in roosting sites without pups. However, when pups were in a group's site, at least one female was present 73% of the time. By contrast, male presence was rare and independent of pup presence (χ2 = 3.23, p = 0.07, d.f. = 137).
We identified 144 individually marked females in eight groups where babysitting was monitored. Thirty-eight per cent acted as a babysitter a total of 89 times, with some individuals babysitting on five different days. Thus, babysitting was not distributed equally among group members, but it was predicted by female age (χ2 = 8.0, p = 0.005, estimated by toothwear or capture data), pup age (χ2 = 17.4, p < 0.0001), and whether females were lactating (χ2 = 12.3, p = 0.0005). Compared with non-babysitters, babysitters were older (6.5 ± 0.4 versus 5.0 ± 0.3 years) and their pups were younger (4.7 ± 0.8 versus 7.8 ± 0.2 days of age). Females were often babysitters on the day they gave birth (11 of 15 possible opportunities). By contrast, non-lactating females (eight non-reproductive, 47 pregnant) never babysat.
During this same period, we observed individually marked females in one group using infrared illumination and found that three of the five female babysitters flew from the roosting site in the cave ceiling to the floor or wall to visit a pup that was not their own but was from their group. Additional observations of this behaviour, which we summarize below, indicate that this prevents attacks by foreign females from other groups in the same cave [98].
(c). Female greater spear-nosed bats guard unrelated fallen pups
Non-volant pups frequently fall from the cave ceiling to the cave floor, where they are vulnerable to predation [98]. After falling they typically crawl to and partially up a wall, but the only way for them to return to their roost site is if an adult carries them. Fallen pups produce loud isolation calls [104] that attract multiple adult females. By staging pup falls from groups with individually marked bats we found that when mothers visited their pups, they retrieved them, but 95% of visits to pups were by females who were not their mothers [98]. These non-maternal visits took drastically different forms depending on the visiting female's social group affiliation. Females from the same social group as the pup often remained near the pup for up to 30 min until the mother arrived. By contrast, females from different social groups attacked pups, sometimes fatally. If a pup was bitten, it typically vocalized loudly, which attracted other females, often including some from its own social group, who then would fight each other. These observations indicate that females actively guard pups of unrelated groupmates against lethal attacks by females from other groups. Given the low reproductive rate and high infant mortality [102], pup guarding should strongly influence female reproductive success.
(d). Why do female greater spear-nosed bats help unrelated pups?
Females that babysit are in an ideal position to hear and respond to fallen pups, including their own, from their social group. Guarding reduces the chance that fallen pups will be killed before retrieval can occur, but how does the guarding female benefit by responding to a pup that is not hers? One possible explanation is that pup survival improves social thermoregulation. By helping other offspring survive, a mother helps keep her own pup warm, potentially reducing the energy needed for thermoregulation. Newborn pups lack fur and in the absence of adults can only generate heat through metabolism of brown adipose fat. The striking birth synchrony within groups and dense clustering of naked pups in crèches suggest that young pups save more energy by roosting in a crèche with similar-aged individuals than in a group of mixed ages. Because the degree of birth synchrony is not solely owing to environmental cues or male mating behaviour, it must result from cues shared by females in a group [100], which implies that selection has favoured within-group synchrony. If so, the thermoregulatory benefits of crèching should be greatest before fur appears, and alloparental care should be most beneficial during this vulnerable period. Consistent with this hypothesis, we failed to observe either babysitting or pup guarding when pups were older than two weeks of age and had fur.
Social thermoregulation could lead to a cooperative dilemma if pups reduce thermogenesis to exploit social warming as described above [96]. How might this ‘huddler's dilemma’ be resolved? We suggest several possible answers. First, as noted above, pups might exhibit partner choice by moving next to warmer neighbours. Second, babysitters might detect and exclude pups that are acting as a significant heat sink. Third, indirect fitness benefits could mitigate the conflict because most pups in a crèche are related as paternal half-sibs. Metabolism of brown adipocytes is influenced by several imprinted genes in mice [105,106]. In contrast to mice, where pups may be maternal half-sibs and thermogenesis is under a maternally expressed promoter [105], we predict that heat generation is under paternal genetic control in greater spear-nosed bats. These alternatives are hypothetical but merit testing.
Given how long females roost together, those that invest in babysitting or guarding groupmate pups can receive other cooperative returns at a later time or in other contexts. For example, adult female groupmates also cooperate while foraging. After leaving roosts, bats give loud calls that advertise and defend feeding sites from bats in other groups [62]. In some ways, these group-specific calls are similar to those given by amazon parrots, Amazona palliata [107], killer whales, Orcinus orca [108] or chimpanzees, Pan troglodytes [109] in that they are learned gradually after an individual joins a group [110], which prevents non-groupmates from falsely signalling group membership. Unlike most bats, groups of non-kin female spear-nosed bats are remarkably stable in membership and size. This group stability can be explained if new potential group members are less able to provide cooperative services compared to more experienced and familiar members. As in many primates, stable groups provide the greatest opportunities for cooperative benefits to accrue in multiple contexts.
6. Conclusion
By-product benefits can occur whenever individuals have the potential to share a common resource, but evidence for costly cooperative investments among non-kin remains relatively rare among bats. However, the social interactions of only a few species have been studied in detail and intrinsic biases almost certainly favour some explanations for cooperation over others. While correlations between cooperation and kinship are relatively straightforward to assess, it is typically more difficult to demonstrate direct fitness benefits. This bias is even larger when direct fitness returns accrue across many types of exchanges and over long timespans. Long-term studies on meerkats [111] and baboons (Papio cynocephalus ursinus) [112] show that the direct fitness benefits of cooperative behaviours may be missed by short-term studies. This is undoubtedly also true for bats. For example, consider that a three year field study of 184 marked vampire bats led to only 33 observations of food sharing between adult bats [68]—an infrequent helping behaviour with a large fitness effect. As a consequence, reciprocal sharing could not have been detected from field observations alone. Similarly, while a role for kinship was immediately obvious after assessing relatedness, the role of direct fitness benefits required years of observation and controlled experiments. Comparing both helping symmetry and kinship as predictors can help tease these factors apart [113], but determining the mechanisms responsible for ensuring mutual benefits, such as reciprocity or group augmentation, requires additional study. Long-term field studies remain crucial to uncovering the key drivers of non-kin cooperative behaviour in the wild [114].
We anticipate that new and improved tracking technologies, such as proximity data-loggers and on-board GPS with audio [115], supplemented by temperature sensors and observations made with infrared video will help reveal if and when free-ranging bats compete or cooperate within roosts or while foraging. To assess the adaptive significance of any cooperative behaviour in bats, we believe it is critical to first understand the socioecology of the species and natural patterns of cooperative behaviour. Informed hypotheses can then be tested using controlled experiments that address possible adaptive features of cooperative decision-making.
Acknowledgements
We thank Jack Bradbury, Christina Riehl, Bill Wcislo and an anonymous reviewer for comments on the manuscript. Our recent work on cooperation in bats has been supported by the National Science Foundation (IOS-0308642 and IOS-1311336), the Ford Foundation of the National Academy of Sciences, the Animal Behavior Society, the Society for the Study of Evolution and the American Society of Mammalogists.
Data accessibility
Original data analysed in this paper can be accessed at http://hdl.handle.net/1903/17156.
Authors' contributions
All authors helped to conceive, draft and revise the manuscript. Data on babysitting by P. hastatus were collected by G.S.W. and K.M.B. Data on group stability and longevity of female P. hastatus were collected by G.S.W., K.M.B. and D.M.A. Analysis of data was performed by G.S.W.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.McCracken GF, Wilkinson GS. 2000. Bat mating systems. In Reproductive biology of bats (eds Crichton EG, Krutszch PH), pp. 321–362. New York, NY: Academic Press. [Google Scholar]
- 2.Kerth G. 2008. Causes and consequences of sociality in bats. Bioscience 58, 737–746. ( 10.1641/B580810) [DOI] [Google Scholar]
- 3.Wilkinson GS, South JM. 2002. Life history, ecology and longevity in bats. Aging Cell 1, 124–131. ( 10.1046/j.1474-9728.2002.00020.x) [DOI] [PubMed] [Google Scholar]
- 4.McCracken GF. 1987. Genetic structure of bat social groups. In Recent advances in the study of bats (eds Racey PA, Fenton MB, Rayner JMV), pp. 281–298. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.McCracken GF, Bradbury JW. 1981. Social organization and kinship in the polygynous bat Phyllostomus hastatus. Behav. Ecol. Sociobiol. 8, 11–34. ( 10.1007/BF00302840) [DOI] [Google Scholar]
- 6.Noë R. 2006. Cooperation experiments: coordination through communication versus acting apart together. Anim. Behav. 71, 1–18. ( 10.1016/j.anbehav.2005.03.037) [DOI] [Google Scholar]
- 7.West S, Griffin A, Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672. ( 10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 8.Bshary R, Bergmüller R. 2008. Distinguishing four fundamental approaches to the evolution of helping. J. Evol. Biol. 21, 405–420. ( 10.1111/j.1420-9101.2007.01482.x) [DOI] [PubMed] [Google Scholar]
- 9.Connor RC. 2010. Cooperation beyond the dyad: on simple models and a complex society. Phil. Trans. R. Soc. B 365, 2687–2697. ( 10.1098/rstb.2010.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor RC. 1996. Partner preferences in by-product mutualisms and the case of predator inspection in fish. Anim. Behav. 51, 451–454. ( 10.1006/anbe.1996.0042) [DOI] [Google Scholar]
- 11.Foster KR, Wenseleers T. 2006. A general model for the evolution of mutualisms. J. Evol. Biol. 19, 1283–1293. ( 10.1111/j.1420-9101.2005.01073.x) [DOI] [PubMed] [Google Scholar]
- 12.Cheney DL. 2011. Extent and limits of cooperation in animals. Proc. Natl Acad. Sci. USA 108, 10 902–10 909. ( 10.1073/pnas.1100291108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter GG. 2014. The reciprocity controversy. Anim. Behav. Cogn. 1, 368–386. (doi:0.12966/abc.08.11.2014) [Google Scholar]
- 14.Ward P, Zahavi Z. 1973. The importance of certain assemblages of birds as ‘information centres’ for food finding. Ibis 115, 517–534. ( 10.1111/j.1474-919X.1973.tb01990.x) [DOI] [Google Scholar]
- 15.Hamilton WD. 1971. Geometry for the selfish herd. J. Theor. Biol. 31, 295–311. ( 10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson GS. 1995. Information transfer in bats. Symp. Zool. Soc. Lond. 67, 345–360. [Google Scholar]
- 17.Safi K, Kerth G. 2007. Comparative analyses suggest that information transfer promoted sociality in male bats in the temperate zone. Am. Nat. 170, 465–472. ( 10.1086/520116) [DOI] [PubMed] [Google Scholar]
- 18.Gilbert C, McCafferty D, Le Maho Y, Martrette JM, Giroud S, Blanc S, Ancel A. 2010. One for all and all for one: the energetic benefits of huddling in endotherms. Biol. Rev. Camb. Philos. Soc. 85, 545–569. ( 10.1111/j.1469-185X.2009.00115.x) [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson GS. 1985. The social organization of the common vampire bat. I. Pattern and cause of association. Behav. Ecol. Sociobiol. 17, 111–121. [Google Scholar]
- 20.Patriquin KJ, Palstra F, Leonard ML, Broders HG. 2013. Female northern myotis (Myotis septentrionalis) that roost together are related. Behav. Ecol. 24, 949–954. ( 10.1093/beheco/art012) [DOI] [Google Scholar]
- 21.Metheny JD, Kalcounis-Rueppell MC, Willis CK, Kolar KA, Brigham RM. 2008. Genetic relationships between roost-mates in a fission–fusion society of tree-roosting big brown bats (Eptesicus fuscus). Behav. Ecol. Sociobiol. 62, 1043–1051. ( 10.1007/s00265-007-0531-y) [DOI] [Google Scholar]
- 22.Fleischmann D, Kerth G. 2014. Roosting behavior and group decision making in two syntopic bat species with fission–fusion societies. Behav. Ecol. 25, 1240–1247. ( 10.1093/beheco/aru117) [DOI] [Google Scholar]
- 23.Carter GG, Wilkinson GS. 2013. Cooperation and conflict in the social lives of bats. In Bat evolution, ecology, and conservation (eds Adams R, Pedersen S), pp. 225–242. New York, NY: Springer Science Press. [Google Scholar]
- 24.Kerth G, Perony N, Schweitzer F. 2011. Bats are able to maintain long-term social relationships despite the high fission–fusion dynamics of their groups. Proc. R. Soc. B 278, 2761–2767. ( 10.1098/rspb.2010.2718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerth G, Reckardt K. 2003. Information transfer about roosts in female Bechstein's bats: an experimental field study. Proc. R. Soc. Lond. B 270, 511–515. ( 10.1098/rspb.2002.2267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melber M, Fleischmann D, Kerth G. 2013. Female Bechstein's bats share foraging sites with maternal kin but do not forage together with them—results from a long-term study. Ethology 119, 793–801. ( 10.1111/eth.12123) [DOI] [Google Scholar]
- 27.Chaverri G, Gillam EH, Vonhof MJ. 2010. Social calls used by a leaf-roosting bat to signal location. Biol. Lett. 6, 441–444. ( 10.1098/rsbl.2009.0964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold BD, Wilkinson GS. 2011. Individual specific contact calls of pallid bats (Antrozous pallidus) attract conspecifics at roosting sites. Behav. Ecol. Sociobiol. 65, 1581–1593. ( 10.1007/s00265-011-1168-4) [DOI] [Google Scholar]
- 29.Buchalski MR, Chaverri G, Vonhof MJ. 2014. When genes move farther than offspring: gene flow by male gamete dispersal in the highly philopatric bat species Thyroptera tricolor. Mol. Ecol. 23, 464–480. ( 10.1111/mec.12593) [DOI] [PubMed] [Google Scholar]
- 30.Chaverri G, Gillam EH, Kunz TH. 2013. A call-and-response system facilitates group cohesion among disc-winged bats. Behav. Ecol. 24, 481–487. ( 10.1093/beheco/ars188) [DOI] [Google Scholar]
- 31.Dechmann DKN, Kerth G. 2008. My home is your castle: roost making is sexually selected in the bat Lophostoma silvicolum. J. Mammal. 89, 1379–1390. ( 10.1644/08-MAMM-S-061.1) [DOI] [Google Scholar]
- 32.Sagot M, Stevens RD. 2012. The evolution of group stability and roost lifespan: perspectives from tent-roosting bats. Biotropica 44, 90–97. ( 10.1111/j.1744-7429.2011.00774.x) [DOI] [Google Scholar]
- 33.Kunz TH, Lumsden LF. 2003. Ecology of cavity and foliage roosting bats. In Bat ecology (eds Kunz TH, Fenton MB), pp. 3–89. Chicago, IL: Chicago University Press. [Google Scholar]
- 34.Chaverri G, Kunz TH. 2010. Ecological determinants of social systems: perspectives on the functional role of roosting ecology in the social behavior of tent-roosting bats. Adv. Study Behav. Behav. Ecol. Trop. Anim. 42, 275–318. ( 10.1016/S0065-3454(10)42009-4) [DOI] [Google Scholar]
- 35.Balasingh J, Koilraj J, Kunz TH. 1995. Tent construction by the short-nosed fruit bat Cynopterus sphinx (Chiroptera, Pteropodidae) in Southern India. Ethology 100, 210–229. ( 10.1111/j.1439-0310.1995.tb00326.x) [DOI] [Google Scholar]
- 36.Kunz TH, McCracken GF. 1996. Tents and harems: apparent defense of foliage roosts by tent-making bats. J. Trop. Ecol. 12, 121–137. ( 10.1017/S0266467400009342) [DOI] [Google Scholar]
- 37.Rodriguez-Herrera B, Medellin RA, Gamba-Rios M. 2006. Tent building by female Ectophylla alba (Chiroptera: Phyllostomidae) in Costa Rica. Acta Chiropterol. 8, 557–560. ( 10.3161/1733-5329(2006)8%5B557:TBBFEA%5D2.0.CO;2) [DOI] [Google Scholar]
- 38.Thomas DW, Cloutier D. 1992. Evaporative water loss by hibernating little brown bats, Myotis lucifugus. Physiol. Zool. 65, 443–456. [Google Scholar]
- 39.Boyles JG, Storm JJ, Brack V. 2008. Thermal benefits of clustering during hibernation: a field test of competing hypotheses on Myotis sodalis. Funct. Ecol. 22, 632–636. ( 10.1111/j.1365-2435.2008.01423.x) [DOI] [Google Scholar]
- 40.Kotze J, Bennett NC, Scantlebury M. 2008. The energetics of huddling in two species of mole-rat (Rodentia: Bathyergidae). Physiol. Behav. 93, 215–221. ( 10.1016/j.physbeh.2007.08.016) [DOI] [PubMed] [Google Scholar]
- 41.Napper CJ, Sharp SP, McGowan A, Simeoni M, Hatchwell BJ. 2013. Dominance, not kinship, determines individual position within the communal roosts of a cooperatively breeding bird. Behav. Ecol. Sociobiol. 67, 2029–2039. ( 10.1007/s00265-013-1613-7) [DOI] [Google Scholar]
- 42.Burns LE, Broders HG. 2015. Who swarms with whom? Group dynamics of Myotis bats during autumn swarming. Behav. Ecol. 26, 866–876. ( 10.1093/beheco/arv017) [DOI] [Google Scholar]
- 43.Ancillotto L, Serangeli MT, Russo D. 2012. Spatial proximity between newborns influences the development of social relationships in bats. Ethology 118, 331–340. ( 10.1111/j.1439-0310.2011.02016.x) [DOI] [Google Scholar]
- 44.Smith JE, Van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE. 2010. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 284–303. ( 10.1093/beheco/arp181) [DOI] [Google Scholar]
- 45.Packer C, Gilbert DA, Pusey AE, O'Brien SJ. 1991. A molecular genetic analysis of kinship and cooperation in African lions. Nature 351, 562–565. ( 10.1038/351562a0) [DOI] [Google Scholar]
- 46.Feh C. 1999. Alliances and reproductive success in Camargue stallions. Anim. Behav. 57, 705–713. ( 10.1006/anbe.1998.1009) [DOI] [PubMed] [Google Scholar]
- 47.Randic S, Connor RC, Sherwin WB, Krutzen M. 2012. A novel mammalian social structure in Indo-Pacific bottlenose dolphins (Tursiops sp.): complex male alliances in an open social network. Proc. R. Soc. B 279, 3083–3090. ( 10.1098/rspb.2012.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagy M, Knornschild M, Voigt CC, Mayer F. 2012. Male greater sac-winged bats gain direct fitness benefits when roosting in multimale colonies. Behav. Ecol. 23, 597–606. ( 10.1093/beheco/ars003) [DOI] [Google Scholar]
- 49.Ortega J, Guerrero JA, Maldonado JE. 2008. Aggression and tolerance by dominant males of Artibeus jamaicensis: strategies to maximize fitness in harem groups. J. Mammal. 89, 1372–1378. ( 10.1644/08-MAMM-S-056.1) [DOI] [Google Scholar]
- 50.Ortega J, Arita HT. 2002. Subordinate males in harem groups of Jamaican fruit-eating bats (Artibeus jamaicensis): satellites or sneaks? Ethology 108, 1077–1091. ( 10.1046/j.1439-0310.2002.00836.x) [DOI] [Google Scholar]
- 51.Fenton MB. 2003. Eavesdropping on the echolocation and social calls of bats. Mamm. Rev. 33, 193–204. ( 10.1046/j.1365-2907.2003.00019.x) [DOI] [Google Scholar]
- 52.Gillam EH. 2007. Eavesdropping by bats on the feeding buzzes of conspecifics. Can. J. Zool. 85, 795–801. ( 10.1139/Z07-060) [DOI] [Google Scholar]
- 53.Gochfeld M, Burger J. 1982. Feeding enhancement by social attraction in the sandwich tern. Behav. Ecol. Sociobiol. 10, 15–17. ( 10.1007/BF00296391) [DOI] [Google Scholar]
- 54.Bhattacharya K, Vicsek T. 2014. Collective foraging in heterogeneous landscapes. J. R. Soc. Interface 11, 20140674 ( 10.1098/rsif.2014.0674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown CR. 1999. Enhanced foraging efficiency through information centers: a benefit of coloniality in cliff swallows. Ecology 69, 602–613. ( 10.2307/1941009) [DOI] [Google Scholar]
- 56.Dechmann DK, Kranstauber B, Gibbs D, Wikelski M. 2010. Group hunting—a reason for sociality in molossid bats? PLoS ONE 5, e9012 ( 10.1371/journal.pone.0009012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dechmann DKN, Heucke SL, Giuggioli L, Safi K, Voigt CC, Wikelski M. 2009. Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. R. Soc. B 276, 2721–2728. ( 10.1098/rspb.2009.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson GS. 1992. Information transfer at evening bat colonies. Anim. Behav. 44, 501–518. ( 10.1016/0003-3472(92)90059-I) [DOI] [Google Scholar]
- 59.Cvikel N, Egert Berg K, Levin E, Hurme E, Borissov I, Boonman A, Amichai E, Yovel Y. 2015. Bats aggregate to improve prey search but might be impaired when their density becomes too high. Curr. Biol. 25, 206–211. ( 10.1016/j.cub.2014.11.010) [DOI] [PubMed] [Google Scholar]
- 60.Chiu C, Xian W, Moss CF. 2008. Flying in silence: echolocating bats cease vocalizing to avoid sonar jamming. Proc. Natl Acad. Sci. USA 105, 13 116–13 121. ( 10.1073/pnas.0804408105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiu C, Xian W, Moss CF. 2009. Adaptive echolocation behavior in bats for the analysis of auditory scenes. J. Exp. Biol. 212, 1392–1404. ( 10.1242/jeb.027045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkinson GS, Boughman JW. 1998. Social calls coordinate foraging in greater spear-nosed bats. Anim. Behav. 55, 337–350. ( 10.1006/anbe.1997.0557) [DOI] [PubMed] [Google Scholar]
- 63.Howell DJ. 1979. Flock foraging in nectar-feeding bats: advantages to the bats and to the host plants. Am. Nat. 114, 23–49. ( 10.1086/283452) [DOI] [Google Scholar]
- 64.Sazima I, Sazima M. 1977. Solitary and group foraging: two flower-visiting patterns of the lesser spear-nosed bat (Phyllostomus discolor). Biotropica 9, 213–215. ( 10.2307/2387882) [DOI] [Google Scholar]
- 65.Wilkinson GS. 1987. Altruism and cooperation in bats. In Recent advances in the study of bats (eds Racey PA, Fenton MB, Rayner JMV), pp. 299–323. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 66.Cody ML. 1971. Finch flocks in the Mohave desert. Theor. Popul. Biol. 2, 142–158. ( 10.1016/0040-5809(71)90012-8) [DOI] [PubMed] [Google Scholar]
- 67.Geipel I, Kalko EK, Wallmeyer K, Knörnschild M. 2013. Postweaning maternal food provisioning in a bat with a complex hunting strategy. Anim. Behav. 85, 1435–1441. ( 10.1016/j.anbehav.2013.03.040) [DOI] [Google Scholar]
- 68.Wilkinson GS. 1984. Reciprocal food sharing in the vampire bat. Nature 308, 181–184. ( 10.1038/308181a0) [DOI] [Google Scholar]
- 69.Carter GG, Skowronski M, Faure P, Fenton MB. 2008. Antiphonal calling allows individual discrimination in white-winged vampire bats. Anim. Behav. 76, 1343–1355. ( 10.1016/j.anbehav.2008.04.023) [DOI] [Google Scholar]
- 70.Elizalde-Arellano C, Lopez-Vidal JC, Arroyo-Cabrales J, Medellin RA, Laundre JW. 2007. Food sharing behavior in the hairy-legged vampire bat Diphylla ecaudata. Acta Chiropterol. 9, 314–319. ( 10.3161/1733-5329(2007)9%5B314:FSBITH%5D2.0.CO;2) [DOI] [Google Scholar]
- 71.Schmidt U, Manske U. 1973. Die Jugendentwicklung der Vampirfledermaus (Desmodus rotundus). Z. Saugetierkunde 38, 14–33. [Google Scholar]
- 72.Freitas MB, Passos CBC, Vasconcelos RB, Pinheiro EC. 2005. Effects of short-term fasting on energy reserves of vampire bats (Desmodus rotundus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 140, 59–62. ( 10.1016/j.cbpc.2004.09.023) [DOI] [PubMed] [Google Scholar]
- 73.Freitas MB, Queiroz JF, Gomes CID, Collares-Buzato CB, Barbosa HC, Boschero AC, Gonçalves CA, Pinheiro EC. 2013. Reduced insulin secretion and glucose intolerance are involved in the fasting susceptibility of common vampire bats. Gen. Comp. Endocrinol. 183, 1–6. ( 10.1016/j.ygcen.2012.11.023) [DOI] [PubMed] [Google Scholar]
- 74.Carter GG, Wilkinson GS. 2013. Food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Proc. R. Soc. B 280, 20122573 ( 10.1098/rspb.2012.2573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilkinson GS. 1988. Reciprocal altruism in bats and other mammals. Ethol. Sociobiol. 9, 85–100. ( 10.1016/0162-3095(88)90015-5) [DOI] [Google Scholar]
- 76.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245 ( 10.1098/rspb.2013.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cockburn A. 2013. Cooperative breeding in birds: toward a richer conceptual framework. In Cooperation and its evolution (eds Sterelney K, Joyce R, Calcott B, Fraser B), pp. 223–245. Cambridge, MA: MIT Press. [Google Scholar]
- 78.Carter G, Wilkinson G. 2013. Does food sharing in vampire bats demonstrate reciprocity? Commun. Integr. Biol. 6, e25783 ( 10.4161/cib.25783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carter GG, Wilkinson GS. 2015. Social benefits of non-kin food sharing by female vampire bats. Proc. R. Soc. B 282, 20152524 ( 10.1098/rspb.2015.2524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rutte C, Taborsky M. 2008. The influence of social experience on cooperative behaviour of rats (Rattus norvegicus): direct vs generalised reciprocity. Behav. Ecol. Sociobiol. 62, 499–505. ( 10.1007/s00265-007-0474-3) [DOI] [Google Scholar]
- 81.Dolivo V, Taborsky M. 2015. Norway rats reciprocate help according to the quality of help they received. Biol. Lett. 11, 20140959 ( 10.1098/rsbl.2014.0959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaeggi AV, De Groot E, Stevens JM, Van Schaik CP. 2013. Mechanisms of reciprocity in primates: testing for short-term contingency of grooming and food sharing in bonobos and chimpanzees. Evol. Hum. Behav. 34, 69–77. ( 10.1016/j.evolhumbehav.2012.09.005) [DOI] [Google Scholar]
- 83.Seyfarth RM, Cheney DL. 2012. The evolutionary origins of friendship. Annu. Rev. Psychol. 63, 153–177. ( 10.1146/annurev-psych-120710-100337) [DOI] [PubMed] [Google Scholar]
- 84.Sabbatini G, Vizioli ADB, Visalberghi E, Schino G. 2012. Food transfers in capuchin monkeys: an experiment on partner choice. Biol. Lett. 8, 757–759. ( 10.1098/rsbl.2012.0534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Axelrod R, Hamilton WD. 1981. The evolution of cooperation. Science 211, 1390–1396. ( 10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 86.Schino G. 2007. Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behav. Ecol. 18, 115–120. ( 10.1093/beheco/arl045) [DOI] [Google Scholar]
- 87.Silk JB, House BR. 2011. Evolutionary foundations of human prosocial sentiments. Proc. Natl Acad. Sci. USA 108, 10 910–10 917. ( 10.1073/pnas.1100305108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilkinson GS. 1986. Social grooming in the common vampire bat, Desmodus rotundus. Anim. Behav. 34, 1880–1889. ( 10.1016/S0003-3472(86)80274-3) [DOI] [PubMed] [Google Scholar]
- 89.Carter GG, Wilkinson GS. 2015. Intranasal oxytocin increases social grooming and food sharing in the common vampire bat Desmodus rotundus. Horm. Behav. 75, 150–153. ( 10.1016/j.yhbeh.2015.10.006) [DOI] [PubMed] [Google Scholar]
- 90.McCracken GF, Gustin MK. 1991. Nursing behavior in Mexican free tailed bat maternity colonies. Ethology 89, 305–321. ( 10.1111/j.1439-0310.1991.tb00376.x) [DOI] [Google Scholar]
- 91.Wilkinson GS. 1992. Communal nursing in evening bats. Behav. Ecol. Sociobiol. 31, 225–235. ( 10.1007/BF00171677) [DOI] [Google Scholar]
- 92.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196. ( 10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clutton-Brock TH. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72. ( 10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 94.Wilkinson GS. 2003. Social and vocal complexity in bats. In Animal social complexity: intelligence, culture and individualized societies (eds de Waal FB, Tyack PL), pp. 322–341. Cambridge, MA: Harvard University Press. [Google Scholar]
- 95.Le Bohec C, Gauthier-Clerc M, Le Maho Y. 2005. The adaptive significance of creches in the king penguin. Anim. Behav. 70, 527–538. ( 10.1016/j.anbehav.2004.11.012) [DOI] [Google Scholar]
- 96.Haig D. 2008. Huddling: brown fat, genomic imprinting and the warm inner glow. Curr. Biol. 18, R172–R174. ( 10.1016/j.cub.2007.12.040) [DOI] [PubMed] [Google Scholar]
- 97.Wilkinson GS, Bohn KM, Adams DM. 2015. Mark-recapture data on greater spear-nosed bats in Trinidad, West Indies, 1990–2015. In DRUM See http://hdl.handle.net/1903/17156 ( 10.13016/M2840H) [DOI]
- 98.Bohn KM, Moss CF, Wilkinson GS. 2009. Pup guarding by greater spear-nosed bats. Behav. Ecol. Sociobiol. 63, 1693–1703. ( 10.1007/s00265-009-0776-8) [DOI] [Google Scholar]
- 99.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853. ( 10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Porter TA, Wilkinson GS. 2001. Birth synchrony in greater spear-nosed bats (Phyllostomus hastatus). J. Zool. 253, 383–390. ( 10.1017/S0952836901000358) [DOI] [Google Scholar]
- 101.Boughman JW. 2006. Selection on social traits in greater spear-nosed bats, Phyllostomus hastatus. Behav. Ecol. Sociobiol. 60, 766–777. ( 10.1007/s00265-006-0220-2) [DOI] [Google Scholar]
- 102.Stern AA, Kunz TH. 1998. Intraspecific variation in postnatal growth in the greater spear-nosed bat. J. Mammal. 79, 755–763. ( 10.2307/1383086) [DOI] [Google Scholar]
- 103.Clutton-Brock TH, Brotherton PNM, O'Riain MJ, Griffin AS, Gaynor D, Sharpe L, Kansky R, Manser MB, McIlrath GM. 2000. Individual contributions to babysitting in a cooperative mongoose, Suricata suricatta. Proc. R. Soc. Lond. B 267, 301–305. ( 10.1098/rspb.2000.1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bohn KM, Wilkinson GS, Moss CF. 2007. Discrimination of infant isolation calls by female greater spear-nosed bats, Phyllostomus hastatus. Anim. Behav. 73, 423–432. ( 10.1016/j.anbehav.2006.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu SH, Yu DW, Lee E, Eckhaus M, Lee R, Corria Z, Accili D, Westphal H, Weinstein LS. 1998. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein α-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc. Natl Acad. Sci. USA 95, 8715–8720. ( 10.1073/pnas.95.15.8715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G. 2004. The imprinted signaling protein XLαs is required for postnatal adaptation to feeding. Nat. Genet. 36, 818–826. ( 10.1038/ng1397) [DOI] [PubMed] [Google Scholar]
- 107.Wright TF, Wilkinson GS. 2001. Population genetic structure and vocal dialects in an amazon parrot. Proc. R. Soc. Lond. B 268, 609–616. ( 10.1098/rspb.2000.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yurk H, Barrett-Lennard L, Ford JKB, Matkin CO. 2002. Cultural transmission within maternal lineages: vocal clans in resident killer whales in southern Alaska. Anim. Behav. 63, 1103–1119. ( 10.1006/anbe.2002.3012) [DOI] [Google Scholar]
- 109.Crockford C, Herbinger I, Vigilant L, Boesch C. 2004. Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology 110, 221–243. ( 10.1111/j.1439-0310.2004.00968.x) [DOI] [Google Scholar]
- 110.Boughman JW. 1998. Vocal learning by greater spear-nosed bats. Proc. R. Soc. Lond. B 265, 227–233. ( 10.1098/rspb.1998.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Russell A, Young A, Spong G, Jordan N, Clutton-Brock T. 2007. Helpers increase the reproductive potential of offspring in cooperative meerkats. Proc. R. Soc. B 274, 513–520. ( 10.1098/rspb.2006.3698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104. ( 10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schino G, Aureli F. 2010. The relative roles of kinship and reciprocity in explaining primate altruism. Ecol. Lett. 13, 45–50. ( 10.1111/j.1461-0248.2009.01396.x) [DOI] [PubMed] [Google Scholar]
- 114.Clutton-Brock T, Sheldon BC. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. ( 10.1016/j.tree.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 115.Cvikel N, Levin E, Hurme E, Borissov I, Boonman A, Amichai E, Yovel Y. 2015. On-board recordings reveal no jamming avoidance in wild bats. Proc. R. Soc. B 282, 20142274 ( 10.1098/rspb.2014.2274) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data analysed in this paper can be accessed at http://hdl.handle.net/1903/17156.


