Abstract
The molecular events that drive Wnt-induced regulation of glycogen synthase kinase 3β (GSK-3β) activity are poorly defined. In this study, we found that protein kinase Cζ (PKCζ) and GSK-3β interact mainly in colon cancer cells. Wnt stimulation induced a rapid GSK-3β redistribution from the cytoplasm to the nuclei in malignant cells and a transient PKC-mediated phosphorylation of GSK-3β at a different site from serine 9. In addition, while Wnt treatment induced a decrease in PKC-mediated phosphorylation of GSK-3β in nonmalignant cells, in malignant cells, this phosphorylation was increased. Pharmacological inhibition and small interfering RNA (siRNA)-mediated silencing of PKCζ abolished all of these effects, but unexpectedly, it also abolished the constitutive basal activity of GSK-3β. In vitro activity assays demonstrated that GSK-3β phosphorylation mediated by PKCζ enhanced GSK-3β activity. We mapped Ser147 of GSK-3β as the site phosphorylated by PKCζ, i.e., its mutation into alanine abolished GSK-3β activity, resulting in β-catenin stabilization and increased transcriptional activity, whereas phosphomimetic replacement of Ser147 by glutamic acid maintained GSK-3β basal activity. Thus, we found that PKCζ phosphorylates GSK-3β at Ser147 to maintain its constitutive activity in resting cells and that Wnt stimulation modifies the phosphorylation of Ser147 to regulate GSK-3β activity in opposite manners in normal and malignant colon cells.
INTRODUCTION
Glycogen synthase kinase 3 (GSK-3) was first discovered in 1980 as a protein kinase that inactivates glycogen synthase (1). Since then, GSK-3 has been revealed as one of the master regulators that play central roles in a diverse range of signaling pathways, including those activated by Wnts, Hedgehog, growth factors, cytokines, and G protein-coupled ligands. GSK-3 is involved in the regulation of many cellular functions, and more than 40 proteins have been reported to be phosphorylated by the kinase, suggesting that its activity is tightly regulated (2). Numerous studies have pointed to an association of GSK-3 dysregulation, particularly hyperactivation, with the onset and progression of human diseases, including diabetes mellitus, obesity, inflammation, neurological disorders, and cancer (3).
A distinct feature of GSK-3 is its constitutive kinase activity in resting cells, which is inhibited in response to cellular signaling mediated by growth factors, cytokines, and hormones via phosphorylation of Ser21 in GSK-3α and Ser9 in GSK-3β (4–6). Several kinases can phosphorylate these serines, including Akt, protein kinase A (PKA), and p90Rsk. 12-O-Tetradecanoyl phorbol 13-acetate (TPA)-sensitive isoforms of protein kinase C (PKC) have also been shown to be involved in agonist-induced inactivation of GSK-3β by phosphorylating the enzyme at Ser9 (7, 8). It has also been reported that in vitro, GSK-3β is inactivated in the same manner by particular forms of PKC: conventional α, β, and γ isoforms and the novel δ and η isoforms (9, 10). Interestingly, these experiments showed that neither PKCε nor atypical PKCζ phosphorylates GSK-3β at Ser9 (9, 10) and that, in contrast, the related GSK-3α is not a substrate for any of these PKC isozymes (9). However, the mechanisms of GSK-3 regulation are varied and not yet fully understood; precise control appears to be achieved by a combination of phosphorylation, localization, and sequestration by a number of GSK-3-binding proteins (6).
Wnt signaling is a key pathway in embryonic development and adult homeostasis (11, 12) and has been defined as one of the most important contributors to tumorigenesis. Indeed, aberrant Wnt signaling is a hallmark of the majority of colorectal cancers. GSK-3β is a central player in the canonical pathway that operates through regulating the phosphorylation and degradation of the transcription coactivator β-catenin. In the absence of Wnt stimulation, β-catenin is assembled into the so-called destruction complex, consisting of GSK-3β, casein kinase 1 (CK1), adenomatous polyposis coli (APC), and axin. This complex directs a series of phosphorylation events in β-catenin that targets it for degradation via the proteosome (11, 12). Stimulation by Wnt leads to inhibition of β-catenin breakdown and to phosphorylation of the coreceptor LRP5/6 by GSK-3β, followed by inhibition of GSK-3β activity by a mechanism not yet fully understood (11–13). In this respect, it has been demonstrated that the canonical Wnt signaling pathway employs a distinct mechanism for regulating GSK-3β that is independent of N-terminal-domain serine phosphorylation or tyrosine phosphorylation and instead relies on protein-protein interactions and intracellular sequestration (6, 11, 14).
It is well known that the Wnt and PKC signaling pathways are both involved in colon carcinogenesis and tumor progression. Recent studies have found that specific interactions between GSK-3β and atypical PKC are a key component of the Par complex (15, 16) participating in polarity determination. We have recently reported that atypical PKCζ plays an important role in the positive regulation of the canonical Wnt pathway by controlling nuclear β-catenin localization in colon cancer cells (17). In this study, we found that PKCζ and GSK-3β interact specifically mainly in colon cancer cells. Furthermore, we found that upon cell treatment with Wnts, GSK-3β activity is rapidly and transiently activated in cancer cells as a result of PKCζ-mediated phosphorylation at Ser147. Unexpectedly, we also found that PKCζ-mediated phosphorylation of Ser147 is required to maintain the constitutive basal activity of GSK-3β under resting conditions in both nonmalignant and malignant cells.
MATERIALS AND METHODS
Reagents and antibodies.
Antibodies against GSK-3β were from the following sources: rabbit polyclonal antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and mouse monoclonal antibody was from Millipore (Billerica, MA). The rabbit monoclonal antibody against phospho-Ser (P-Ser)-PKC substrate was purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal antibodies against Akt and against P-Thr308-Akt were obtained from Santa Cruz Biotechnology. Protein A-Sepharose, the pseudosubstrate-specific PKCζ inhibitor, and the GSK-3β inhibitor IX [(2′Z,3′E)-6-bromoindirubin-3′-oxime (BIO)] were from Calbiochem/Merck (Darmstadt, Germany). GSM (GSK-3 substrate peptide) was obtained from Millipore.
Plasmids.
The plasmid encoding human GSK-3β (HA-GSK3β wt pcDNA3; Addgene plasmid 14753) was obtained from Addgene, a nonprofit organization devoted to facilitating the sharing of plasmids among scientists. For knockdown PKCζ experiments, we utilized the control (the empty plasmid pSUPER) or the pSUPER.PKCzeta.RNAi plasmid donated by Alex Toker to Addgene (Addgene plasmid 10803), whose construction and effectiveness have been described previously (18). The pTOPFlash and pFOPFlash reporter plasmids were obtained from Upstate Biotechnology (Lake Placid, NY).
Cell culture.
Malignant RKO (human colon carcinoma) and SW480 (human colorectal adenocarcinoma) cells and nonmalignant 112CoN (human colon) and IEC-18 (mouse intestine) cells were all obtained from the American Type Culture Collection (ATCC) (Manassas, VA). RKO and 112CoN cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), antibiotics (120 mg/ml penicillin and 200 mg/ml streptomycin), and 2 mM l-glutamine. SW480, HEK293, and IEC-18 cells were maintained in DMEM F-12 supplemented with 5% FBS, antibiotics, and 2 mM glutamine. For IEC-18 cells, the medium was also supplemented with 0.1 IU/ml insulin, which was removed from the medium 12 h before each experiment. All cells were cultured in a humidified 5% CO2 incubator at 37°C. The human cell lines were authenticated by DNA profiling using short tandem repeat (STR) analysis on an AmpFlSTR Identifiler PCR Amplification System at the Instituto Nacional de Medicina Genómica (INMEGEN) in México City. RKO human malignant cells display normal canonical Wnt signaling (expressing wild-type APC protein) and are responsive to Wnt ligand in comparison with SW480 human malignant cells, which express a truncated version of APC and have constitutively active Wnt signaling.
Incubation with Wnt ligands and pharmacological inhibition of PKCζ.
For pharmacological PKCζ inhibition in RKO and SW480 cells, serum-starved cells (2% serum instead of 10%) were incubated in the absence or presence of the myristoylated PKCζ-selective inhibitor (20 μM) for 1 h. Then, the cells were incubated in the absence or presence of Wnt3a or -5a ligand (100 ng/ml) for 5 min and then washed and lysed.
Immunofluorescence analysis.
112CoN, RKO, and SW480 cells were grown on coverslips. The cells were washed with phosphate-buffered saline (PBS), fixed in ice-cold methanol for 10 min, washed in PBS, and blocked with 1% IgG-free bovine serum albumin (BSA) for 1 h. The cells were incubated overnight at 4°C with the corresponding primary antibodies in blocking solution, washed three times with PBS, and incubated for 1 h in darkness at room temperature with secondary antibodies (fluorescein isothiocyanate [FITC]-conjugated goat anti-rabbit antibody or rhodamine goat anti-mouse antibody). After washing, the coverslips were mounted with the antifade reagent Vectashield. Cell fluorescence was examined using a confocal microscope (Leica TCS SP5) with a krypton-argon laser.
Western blotting.
Protein samples (100 μg) were separated by SDS-10 or 12% PAGE, followed by electrophoretic transfer onto nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked with 5% nonfat dry milk and incubated overnight at 4°C with the corresponding primary antibody. Detection was achieved using a SuperSignal kit (Pierce, Rockford, IL) with a horseradish peroxidase-conjugated secondary antibody. An actin antibody was utilized as a control for equal loading.
Immunoprecipitation.
Confluent cells were washed and homogenized in ice-cold lysis buffer containing 20 mM Tris-HCl, pH 7.5, 10 mM EGTA, 2 mM EDTA, 0.5% Triton X-100, and a mixture of protease inhibitors and protein phosphatase inhibitors. Aliquots of cell extracts (1 mg/ml) were incubated overnight with 1 μg/ml of primary antibody at 4°C with gentle shaking. Then, 20 μl protein A-Sepharose (30%) was added, and incubation continued for 2 h. The immune complexes were then washed three times with buffer A (50 mM Tris-HCl, 0.6 M NaCl, 1% Triton X-100, 0.5% Nonidet P-40, pH 8.3) supplemented with 0.1 mg/ml trypsin inhibitor and 1 mM phenylmethylsulfonyl fluoride (PMSF) and once with buffer B (20 mM Tris HCl, pH 7.5, 0.15 M NaCl) containing protease and phosphatase inhibitors.
PKCζ knockdown.
PKCζ silencing was performed by transient transfection of cells with 2 μg of pSuperPKCζ-RNAi plasmid, constructed and reported by Alex Toker (18) and obtained from Addgene (catalog no. 10803), or with control plasmid (the empty plasmid pSUPER) using Lipofectamine 2000. Silencing efficiency was analyzed by Western blotting and flow cytometry.
Fluorescence-activated cell sorter (FACS) analysis.
The cells were detached and dissociated in 10 mM EDTA solution. The cell suspension was washed, resuspended in PBS supplemented with 4% fetal calf serum (staining buffer), stained with the corresponding primary antibody (rabbit polyclonal anti-pThr308-Akt or goat polyclonal anti-pSer9-GSK-3β), and then incubated with the secondary antibody. Cells stained with the secondary antibody alone were employed as a negative control.
In vitro GSK-3β activity assay.
GSK-3β was immunoprecipitated from cells incubated for 5 min in the absence (vehicle) or presence of Wnt ligands (100 ng/ml). The immune complexes were washed twice with buffer A (50 mM Tris-HCl, 0.6 M NaCl, 0.5% [vol/vol] Triton X-100, 0.5% [vol/vol] IGEPAL [pH 8.3]) containing protease and phosphatase inhibitors and once with buffer B (50 mM Tris-HCl, 0.15 M NaCl, 50 mM 2-mercaptoethanol, pH 7.5). Kinase activity was initiated by resuspending the immunoprecipitates in 50 μl of the assay mixture, consisting of kinase buffer (40 mM Tris, 20 mM MgCl2, pH 7.5) plus [γ-32P]ATP and 4 mg/ml of GSK-3 substrate (GSM), in the absence or presence of the GSK-3β inhibitor BIO (5 μM). The reactions proceeded for 20 min at 30°C and were then terminated by the addition of 50 μl of SDS-PAGE sample buffer to the reaction mixture, which was boiled for 5 min and analyzed by SDS-PAGE (18% [wt/vol] gel) and autoradiography. The data were quantified by densitometric analysis performed in both Coomassie-stained gels and the corresponding autoradiographs. The specific phosphorylation was determined as the ratio of phosphorylated protein to the total protein content normalized with respect to control cells.
Site-directed mutagenesis.
The human GSK-3β S147A and S147E mutants were generated by employing a QuikChange site-directed mutagenesis kit obtained from Stratagene (La Jolla, CA) according to the manufacturer's instructions and utilizing the following primers: S147A forward, 5′-GTTGCCAGACACTATGCTCGAGCCAAACAGACGCTC-3′, and reverse, 5′-GAGCGTCTGTTTGGCTCGAGCATAGTGTCTGGCAAC-3′, and S147E forward, 5′-GTTGCCAGACACTATGAACGAGCCAAACAGACGCTC-3′, and reverse, 5′-GAGCGTCTGTTTGGCTCGTTCATAGTGTCTGGCAAC-3′. Both mutant constructions were verified by sequencing.
β-Catenin/TCF transcriptional activity reporter assay.
Cells were seeded on 24-well plates at a density of 1.2 × 105 to 1.8 × 105 cells per well. Twenty-four hours after seeding, the cells were placed in a serum-free medium and transfected with 1 μg of a reporter plasmid (pTOPFlash or the control plasmid pFOPFlash), with 0.05 μg of the pRL luciferase plasmid (transfection control), and with 1 μg of plasmid HA-GSK3β-wt or HA-GSK3β-S147A. The luciferase reporter activity in the cell lysates was measured 24 h after transfection using a Dual Luciferase assay kit (Promega, Madison, WI). The activity was normalized with respect to the activity of Renilla luciferase or with respect to the protein content of each sample.
Statistical analysis.
The data are expressed as means and standard errors of the mean (SEM). Statistical analysis of the data was performed using the Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
PKCζ and GSK-3β coimmunoprecipitate in a reciprocal manner and colocalize in both nonmalignant and malignant colon cells.
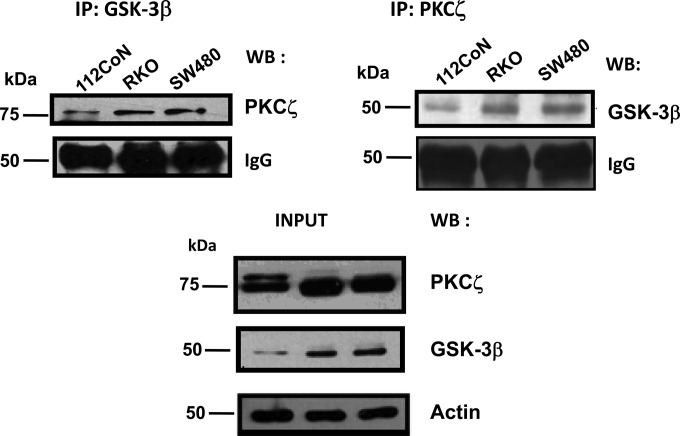
PKC has been shown to be involved in agonist (growth factors, cytokines, and hormones but not Wnts)-induced inactivation of GSK-3β by phosphorylating the enzyme at Ser9 (7, 8). It has also been reported that in vitro GSK-3β, but not GSK-3α, is inactivated in this way by particular isozymes of PKC, but not by others, such as PKCε and PKCζ (9, 10, 14). Because we have previously reported that the atypical PKCζ participates in the regulation of nuclear β-catenin localization, playing an important role in the positive regulation of the canonical Wnt pathway (17), we hypothesized that PKCζ probably does not interact in vivo with GSK-3β. To our surprise, coimmunoprecipitation studies clearly showed that GSK-3β interacts in cells with PKCζ, because they coimmunoprecipitated in a reciprocal manner from 112CoN human colon nonmalignant cells and from RKO and SW480 human colon cancer cells exhibiting normal Wnt signaling and constitutively active Wnt signaling, respectively (Fig. 1).
FIG 1.
PKCζ interacts with GSK-3β. PKCζ or GSK-3β was immunoprecipitated from cell lysates obtained from nonmalignant 112CoN cells or from malignant human RKO and SW480 cells. Immunoprecipitates (IP) were analyzed by Western blotting (WB) for the presence of the indicated proteins. The results are representative of three independent experiments using different cell preparations. IgG is shown as a control for immunoprecipitation equal loading, and actin antibody was used as a control for equal loading of cell extracts.
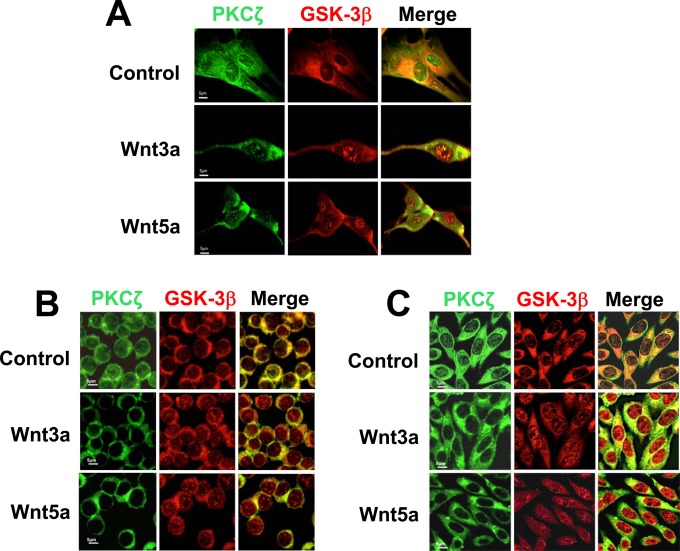
Consistent with these results, immunofluorescence assays followed by confocal microscopy analysis, depicted in Fig. 2, revealed colocalization of GSK-3β with PKCζ under basal conditions in the cytoplasm and in some regions of the nucleus in both normal (Fig. 2A) and malignant (Fig. 2B and C) cells. To investigate whether their colocalization changes as a result of Wnt stimulation, the cells were treated for 5, 15, and 30 min with canonical (Wnt3a) or noncanonical (Wnt5a) ligands. As can be observed in Fig. 2, although colocalization of GSK-3β with PKCζ remained upon Wnt treatment, stimulation of cells with Wnt3a or Wnt5a provoked rapid changes (detected at 5 min) in the intracellular distribution of GSK-3β and PKCζ: they induced a GSK-3β redistribution from cytoplasm to nucleus in both nonmalignant (Fig. 2A) and malignant (Fig. 2B and C) cells, but whereas the localization of PKCζ did not change in nonmalignant cells, Wnt treatment produced an exit of PKCζ from the nucleus to the cytoplasm in malignant cells. Analysis of the time course of stimulation with Wnt ligands indicated that these results were maintained for 30 min in nonmalignant 112CoN cells (see Fig. S1A in the supplemental material) but not in malignant cells because the Wnt-induced exit of PKCζ was transient (see Fig. S1B and C in the supplemental material), appearing to be located again in both the nucleus and the cytoplasm after 30 min of treatment.
FIG 2.
GSK-3β colocalizes with PKCζ in both normal and malignant cells. (A) Wnt ligands (100 ng/ml; 5 min) induce GSK-3β redistribution from cytoplasm to nuclei in nonmalignant 112CoN cells. (B and C) In malignant RKO and SW480 cells, Wnt ligands cause an exit of PKCζ from the nucleus to the cytoplasm and a redistribution of GSK-3β from the cytoplasm to the nucleus. In all cases, the cells were fixed, permeabilized, and coimmunostained with antibodies against GSK-3β and PKCζ. Fluorescence was analyzed by laser confocal microscopy. PKCζ was visualized with FITC-conjugated goat anti-rabbit antibody and GSK-3β with rhodamine-conjugated goat anti-mouse antibody. The data are representative of the results of three independent experiments.
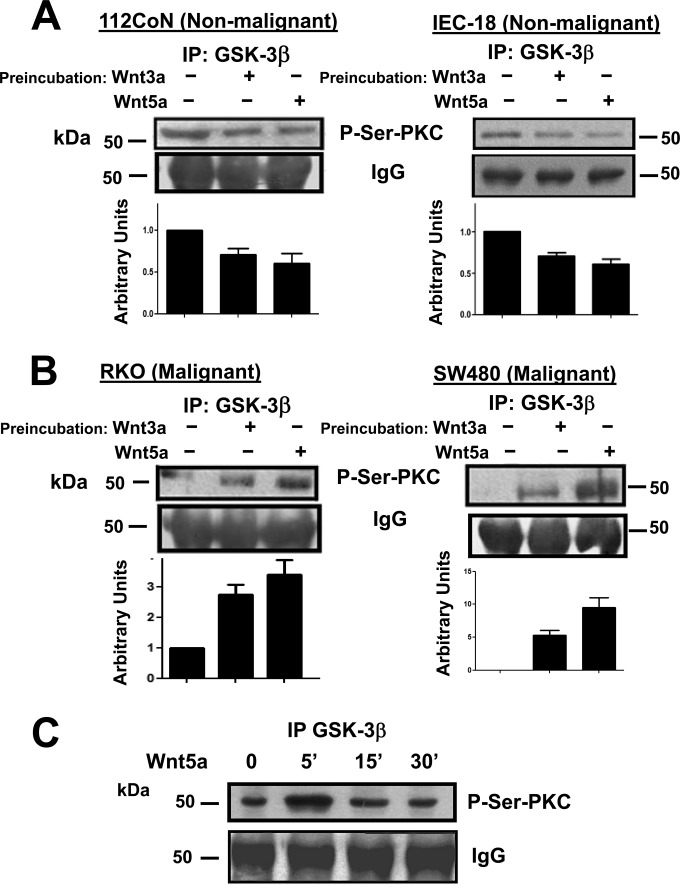
Wnt3a and Wnt5a ligands increase the PKC-mediated phosphorylation of GSK-3β in cancer cells and induce the opposite effect in nonmalignant cells.
To investigate the biochemical meaning of the interaction between PKCζ and GSK-3β, we first examined whether GSK-3β could be a PKC substrate in cells upon Wnt stimulation, employing an antibody that specifically recognizes phospho-serine-PKC substrates (Cell Signaling). Serum-starved nonmalignant or malignant cells were incubated in the absence or presence of canonical Wnt3a or noncanonical Wnt5a for 5 min. GSK-3β was immunoprecipitated from the cell lysates and analyzed by Western blotting. The results presented in Fig. 3A indicated that in 112CoN or IEC-18 nonmalignant cells, GSK-3β exhibited basal PKC-mediated phosphorylation that was diminished as a result of 5 min of treatment with either Wnt3a or Wnt5a ligand. Interestingly, and in an opposite manner, in RKO or SW480 cancer cells, basal PKC-mediated phosphorylation of GSK-3β was nearly negligible, and the 5-min stimulation of cells with Wnt3a or -5a induced an increase in PKC-mediated GSK-3β phosphorylation (Fig. 3B). The phosphorylation of GSK-3β by PKC occurs rapidly and transiently, as can be observed in the time course illustrated in Fig. 3C, because after 5 min of RKO cell treatment with Wnt5a, PKC-mediated phosphorylation of GSK-3β began to return to the basal state.
FIG 3.
Wnt3a and Wnt5a ligands increase the PKC-mediated phosphorylation of GSK-3β in cancer cells and induce the opposite effect in nonmalignant cells. (A and B) Nonmalignant (A) or malignant (B) cells were incubated in the absence or presence of Wnt3a or -5a ligand for 5 min. GSK-3β was immunoprecipitated from the cell lysates, and the immunoprecipitates were analyzed by Western blotting using the anti-P-Ser-PKC substrate antibody. The results shown are representative of at least three independent experiments using different cell preparations. A densitometric analysis was performed to estimate the changes in Wnt-induced GSK-3β phosphorylation levels with respect to the levels found in basal nonstimulated cells. The bar graphs represent the means and SEM from the results of at least three independent assays. (C) Time course of stimulation of RKO cells with Wnt5a (100 ng/ml). GSK-3β was immunoprecipitated from cell lysates obtained at each time point indicated and analyzed by Western blotting using the anti-P-Ser-PKC substrate antibody. IgG is shown as a control for immunoprecipitation equal loading. The results shown are representative of three independent experiments using different cell preparations.
Wnt agonists increase the PKC-mediated phosphorylation of GSK-3β in cancer cells at a different site from Ser9.
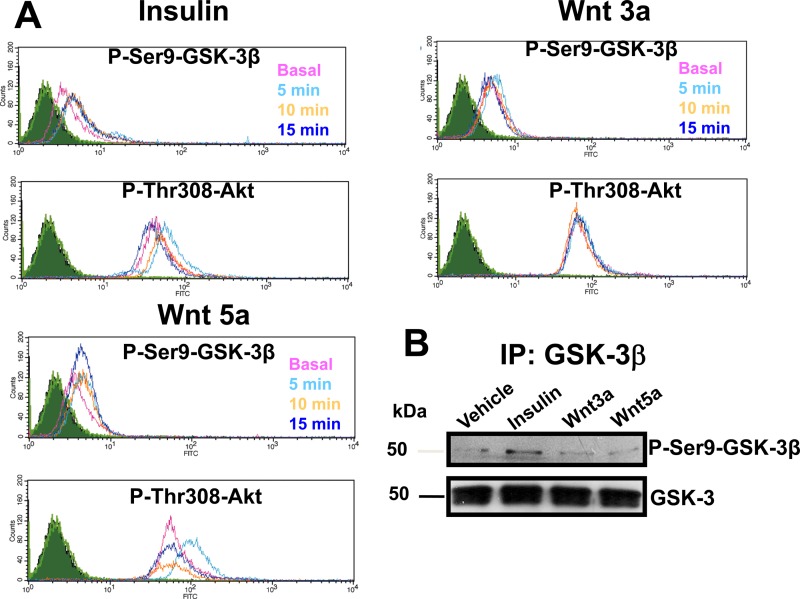
In order to confirm that canonical Wnt ligands do not produce GSK-3β inactivation by inducing its phosphorylation at serine 9, we tested whether the phosphorylation of GSK-3β mediated by PKC in RKO cancer cells (responsive to Wnt ligands) occurs at this site as a result of Wnt stimulation. As shown in Fig. 4A, the time course of GSK-3β phosphorylation at Ser9 obtained upon Wnt3a or -5a treatment of cells in comparison with insulin, which is well known to induce phosphorylation of Ser9 of GSK-3β vía Akt activation and phosphorylation at Thr308 (5, 6), indicated that canonical Wnt3a does not induce the phosphorylation of GSK-3β at serine 9, nor did it induce phosphorylation of Akt at Thr308. It can also be observed that, although Wnt5a produced only transient Akt phosphorylation at Thr308, it did not increase phosphorylation of GSK-3β at Ser9 in the same manner (Fig. 4A). To confirm these results, we performed an immunoblot analysis of GSK-3β immunoprecipitated from RKO cells stimulated in the absence or presence of insulin or Wnts for 5 min. As can be seen in Fig. 4B, only insulin treatment of cells induced the phosphorylation of GSK-3β at Ser9, reproducing the flow cytometry results.
FIG 4.
Wnt stimulation does not induce GSK-3β phosphorylation at Ser9. (A) FACS analysis of the time course of stimulation of cells with or without Wnts or insulin. Serum-starved RKO cells were incubated in the absence (time zero [Basal]) or presence of Wnt3a or -5a (100 ng/ml) or in the absence or presence of insulin (0.3 IU/ml) for 5, 10, and 15 min. The cells were stained with the corresponding primary antibody (anti-pSer9-GSK-3β or anti-pThr308-Akt), as described in Materials and Methods, and analyzed by flow cytometry. Shown are overlapping histograms of the time courses obtained from at least three independent experiments. (B) Western blot analysis of the time course of stimulation of cells with or without Wnts or insulin. Serum-starved RKO cells were incubated in the absence (time zero [Vehicle]) or presence of Wnt3a or -5a (100 ng/ml) or in the absence or presence of insulin (0.3 IU/ml) for 5 min. GSK-3β was immunoprecipitated from RKO cells and analyzed by Western blotting using anti-pSer9-GSK-3β or anti-GSK-3 antibody and developed with a horseradish peroxidase-conjugated secondary antibody. The results shown are representative of the results of at least three independent experiments using different cell preparations.
PKCζ phosphorylates GSK-3β in colon cells.
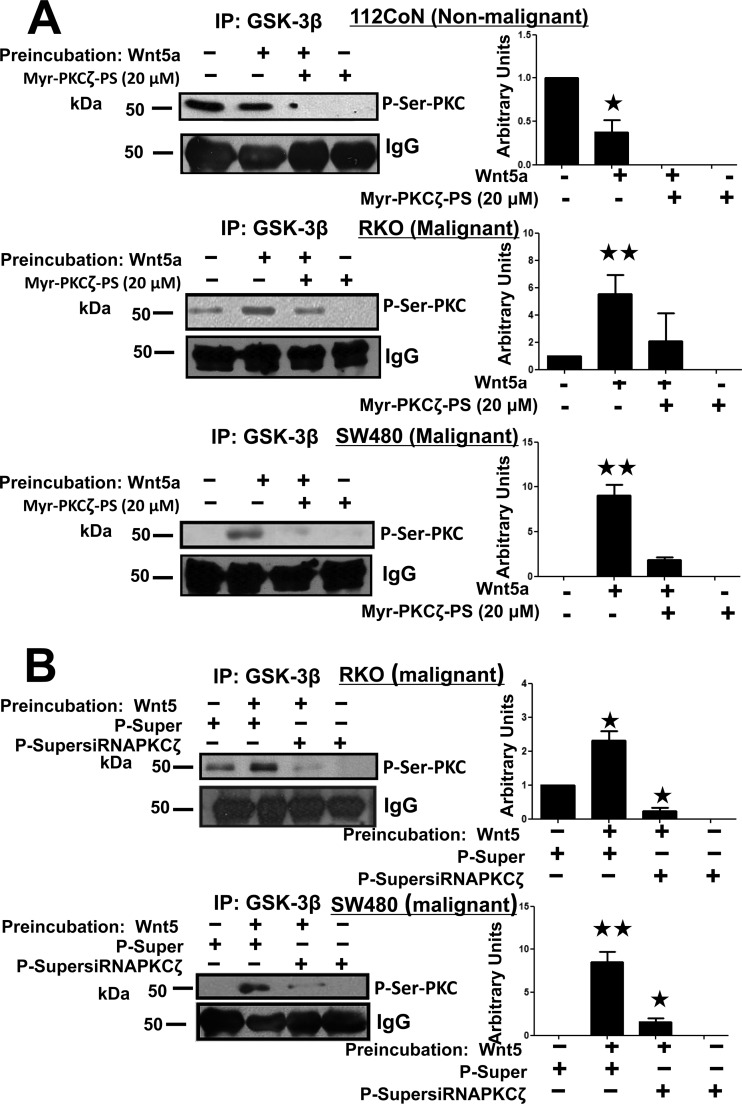
To investigate which PKC isoform phosphorylates GSK-3β in response to Wnt stimulation, we first examined the effect of PKCζ inhibition on GSK-3β phosphorylation mediated by PKC. We used the Wnt5a ligand, which has been reported to transduce mainly in a noncanonical way, such as Wnt/Ca2+ signaling, activating calcium-dependent conventional PKC isoforms. Nonmalignant 112CoN or RKO and SW480 cancer cells were incubated for 1 h in the absence or presence of the selective PKCζ-myristoylated pseudosubstrate inhibitor and then in the absence or presence of Wnt5a for 5 min. GSK-3β was immunoprecipitated from cell extracts and analyzed by Western blotting using an anti-phosphoserine-PKC substrate antibody. Unexpectedly, the results obtained clearly indicated that in normal 112CoN cells, inhibition of PKCζ abolished basal PKC-mediated phosphorylation of GSK-3β and reduced the phosphorylation induced by Wnt5a (Fig. 5A). Consistent with the results shown in Fig. 3A, in both RKO and SW480 cancer cells, there was less basal phosphorylation (RKO cells) or no basal phosphorylation (SW480 cells) compared with normal 112CoN or IEC-18 cells. However, Wnt5a stimulation produced an increase in the PKC-mediated phosphorylation of GSK-3β that was also abolished by PKCζ inhibition in the absence of Wnt5a stimulation or greatly reduced in the presence of Wnt5a stimulation (Fig. 5A). In order to confirm that the observed effects were mediated by PKCζ, we utilized an RNA interference (RNAi) approach to transiently block PKCζ expression. It is noteworthy that the plasmids utilized were previously constructed and successfully probed by Alex Toker (18), and we also employed these plasmids in a previous study (17). As can be observed in Fig. S2 in the supplemental material, a great reduction in the PKCζ protein level was observed 36 h after transfection of RKO or SW480 cells with 2 μg small interfering RNA (siRNA) plasmid in comparison with a control plasmid. The siRNA-transfected RKO cells were serum starved and then incubated in the absence or presence of Wnt5a. GSK-3β was immunoprecipitated from cell extracts and analyzed by Western blotting with the anti-phospho-Ser-PKC substrate antibody. The results presented in Fig. 5B confirmed that the effects observed in the GSK-3β phosphorylation status in colon cancer cells were mediated by the atypical PKCζ. Taken together, these results clearly indicated that GSK-3β is a substrate of PKCζ in colon cells at a different site from Ser9 and that Wnt stimulation induces rapid and transient changes in the phosphorylation status of GSK-3β mediated by PKCζ in opposite manners in normal or cancer cells.
FIG 5.
PKCζ inhibition blocks GSK-3β phosphorylation. (A) Nonmalignant 112CoN cells and malignant RKO or SW480 serum-starved cells were incubated in the absence or presence of the myristoylated (Myr) PKCζ-selective inhibitor (20 μM) for 1 h. Then, the cells were incubated in the absence or presence of Wnt5a (100 ng/ml) for 5 min. GSK-3β was immunoprecipitated from the cell lysates, and the immunoprecipitates were analyzed by Western blotting using the anti-P-Ser-PKC substrate antibody. The results shown are representative of at least three independent experiments using different cell preparations. A densitometric analysis of the changes in Wnt-induced GSK-3β-specific phosphorylation levels with respect to the levels found in basal nonstimulated cells is shown on the right, and the data represent the means and SEM from at least three independent assays. *, P < 0.05; **, P < 0.01. (B) PKCζ knockdown decreases the phosphorylation of GSK-3β. PKCζ-silenced RKO and SW480 cells were serum starved for 6 h and then incubated in the absence or presence of 100 ng/ml Wnt5a for 5 min. GSK-3β was immunoprecipitated from cell extracts, and the immunoprecipitates were analyzed by Western blotting with the anti-P-Ser-PKC substrate antibody. The results shown are representative of at least three independent experiments using different cell preparations. The densitometric analysis shown in the bar graphs represents the means and SEM from at least three independent assays. *, P < 0.05; **, P < 0.01.
The PKCζ-induced phosphorylation of GSK-3β stimulates GSK-3β activity.
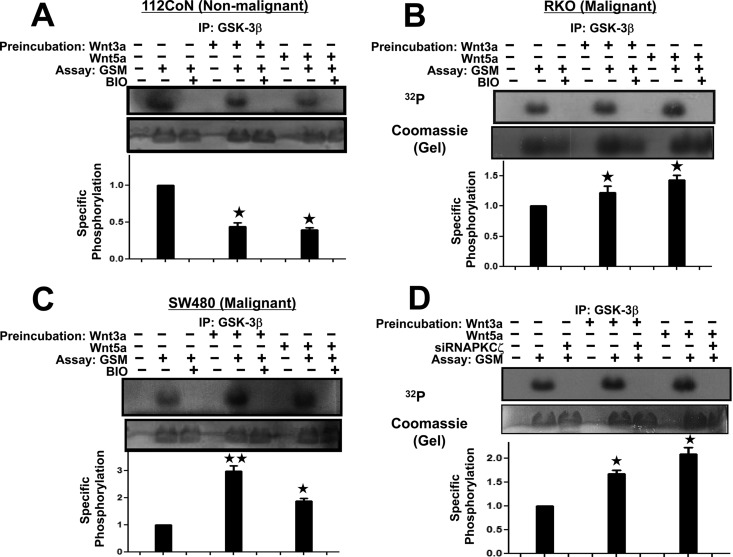
To investigate the effect of the phosphorylation produced by PKCζ on GSK-3β activity, we first incubated the serum-starved nonmalignant or malignant cells in the absence (vehicle) or presence of Wnt ligands for 5 min and then proceeded to immunoprecipitate GSK-3β in order to analyze the effect of each treatment on its activity in vitro, as described in Materials and Methods. Kinase activity was measured with the GSK-3β immune complexes obtained from the cell extracts in the absence or presence of the added substrate (GSM peptide) and in the absence or presence of the selective GSK-3β inhibitor BIO to validate assay specificity. Figure 6A illustrates how the GSK-3β obtained from normal 112CoN cells was inhibited upon Wnt3a or -5a treatment, because the amount of 32P-labeled GSM produced was less than that produced by the GSK-3β obtained from untreated 112CoN control cells. In marked contrast, the activity of GSK-3β obtained from RKO or SW480 cancer cells (Fig. 6B and C, respectively) was increased in comparison with untreated control cancer cells, producing more 32P-labeled GSM substrate. As expected, phosphorylation of the GSM substrate was abolished in vitro in all cases when the inhibitor of GSK-3β, BIO, was added to the assay mixture. Thus, these results indicated that phosphorylation induced in GSK-3β by PKCζ stimulates enzymatic activity. This is consistent with the decrease in the PKC-mediated phosphorylation of GSK-3β previously observed in nonmalignant cells (Fig. 3A), as well as with the increase in PKC-mediated phosphorylation of GSK-3β previously observed upon Wnt treatment of cancer cells (Fig. 3B).
FIG 6.
PKCζ-induced phosphorylation of GSK-3β stimulates GSK-3β activity. (A to C) Serum-starved nonmalignant 112CoN cells (A) or malignant RKO (B) or SW480 (C) cells were incubated in the absence (vehicle) or presence of Wnt ligands for 5 min and then washed and lysed. GSK-3β was immunoprecipitated to analyze the effect of each treatment on its activity in vitro. Kinase activity was measured with the GSK-3β immune complexes in the absence or presence of added substrate (GSM peptide) and in the absence or presence of the selective GSK-3β inhibitor BIO to validate the specificity of the assay. Representative autoradiograms with their corresponding Coomassie blue-stained gels are shown (in panel B, the tiny white line that appears between the third and fourth lanes from the left corresponds to an anomaly in the original stained gel). The autoradiograms and their corresponding stained gels were quantified with an image densitometer, and the specific phosphorylation was determined as the ratio of phosphorylated protein to the total protein content. The values plotted are means and SEM for at least three experiments with different cell preparations. *, P < 0.05; **, P < 0.01. (D) Silencing of PKCζ in RKO cancer cells inhibited the enhancement of GSK-3β activity induced by Wnt treatment. RKO cells were transfected with the control scrambled-RNAi plasmid or with the pSuperPKCζ-RNAi plasmid and 30 h posttransfection were serum starved for 6 h and then incubated in the absence (vehicle) or presence of Wnt3a or -5a agonist for 5 min. GSK-3β was immunoprecipitated to analyze its kinase activity in vitro in the absence or presence of added substrate (GSM peptide). A representative autoradiogram with its corresponding Coomassie blue-stained gel is shown. The autoradiogram and its corresponding stained gel was quantified with an image densitometer to determine the specific phosphorylation, normalized with respect to control cells. The values plotted are means and SEM for at least three experiments with different cell preparations. *, P < 0.05.
The PKCζ-induced phosphorylation of GSK-3β is required to maintain constitutive basal GSK-3β activity.
To confirm that the transient effects on GSK-3β activity observed upon Wnt treatment of cells are the result of phosphorylation of GSK-3β mediated by PKCζ, we again utilized an RNAi approach to transiently block PKCζ expression in colon cancer cells. RKO cells were transfected with the control scrambled-RNAi plasmid or with pSuperPKCζ-RNAi plasmid and, 30 h posttransfection, incubated in the absence (vehicle) or presence of Wnt3a or -5a agonist for 5 min. GSK-3β was immunoprecipitated from the cell extracts to examine its activity in vitro. Figure 6D shows how the silencing of PKCζ in RKO cancer cells inhibited enhancement of GSK-3β activity induced by Wnt3a or -5a stimulation of cells, but in addition, and unexpectedly, the PKCζ blockade of expression also abolished constitutive basal GSK-3β activity.
Taken together, our results indicate the following: (i) that PKCζ activity is required to maintain constitutive basal activity of GSK-3β in colon cells, (ii) that Wnt stimulation induces rapid and transient changes (5 min) in the phosphorylation status of GSK-3β mediated by PKCζ, and (iii) that the changes induced by Wnts are opposite in normal and in cancer cells (in nonmalignant cells, PKCζ-mediated phosphorylation and GSK-3β activity decrease upon Wnt treatment, whereas in cancer cells, PKCζ-mediated phosphorylation of GSK-3β and its activity are transiently increased).
PKCζ phosphorylates GSK-3β at serine 147.
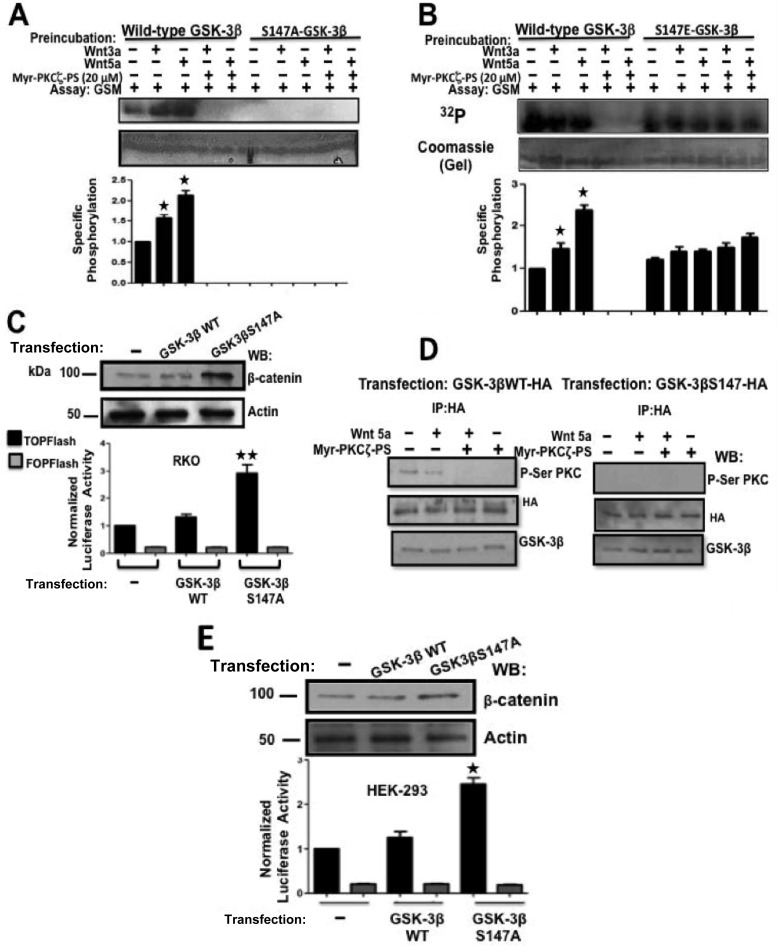
Since we demonstrated here that Wnt agonists induce the phosphorylation of GSK-3β at a site that is not serine 9, we next investigated which GSK-3β site or sites are phosphorylated by PKCζ. An in silico analysis of the GSK-3β amino acid sequence shown in Fig. S3 in the supplemental material, employing the PhosphoMotif Finder program (http://www.dabi.temple.edu/disphos/pred/predict) or the NetphosK 2.0 program (http://www.expasy.org) showed that GSK-3β possesses several putative PKC phosphorylation sites, three of which display the classical PKC consensus sequence with high probability scores but only one of which exactly matched the consensus sequence recognized by the P-Ser-PKC substrate antibody utilized in our experiments: R/K0–2Ser XhydrophobicR/K. This consensus sequence is located only around serine 147 of GSK-3β. Therefore, we decided to construct an epitope-tagged GSK-3β-hemagglutinin (HA) mutant with Ser147 replaced by alanine (GSK-3β/S147A-HA), and a phosphomimetic GSK-3β mutant with Ser147 replaced by glutamic acid (GSK-3β/S147E-HA) and to examine the effects of these mutations on GSK-3β activity upon Wnt stimulation. To this end, RKO cells were transfected with a plasmid encoding wild-type GSK-3β–HA or with plasmids encoding GSK-3β–HA mutants. The levels of wild-type or mutant GSK-3β expression obtained in transfected RKO cells with respect to the endogenous GSK-3β level are presented in Fig. S4 in the supplemental material. RKO cells expressing epitope-tagged mutant or control wild-type GSK-3β were incubated in the absence or presence of PKCζ-myristoylated pseudosubstrate inhibitor (1 h) and then in the absence or presence of Wnt5a for 5 min. GSK-3β was immunoprecipitated from cell extracts (anti-HA immunoprecipitation) to be analyzed for its catalytic ability in vitro. To our surprise, the results depicted in Fig. 7A revealed that the replacement of S147 with alanine was sufficient not only to abolish the enhancement of GSK-3β activity induced by Wnt agonists but also to abolish the basal activity of GSK-3β in the same manner as it inhibits PKCζ. On the other hand, Fig. 7B revealed that the phosphomimetic GSK-3β/S147E-HA mutant displayed constitutive basal GSK-3β activity, which was not significantly further increased as a result of Wnt stimulation and which was insensitive to PKCζ inhibition. In view of these results, we reasoned that in cells expressing the GSK-3β/S147A-HA mutant, the loss of basal GSK-3β activity would increase β-catenin levels. To examine this, RKO cells were transiently cotransfected with pTOPFlash or pFOPFlash (control) β-catenin/TCF transcriptional activity reporter plasmid and with a plasmid encoding wild-type GSK-3β–HA or a plasmid encoding the GSK-3β/S147A-HA mutant. At 24 h posttransfection, β-catenin levels in cell extracts were analyzed by Western blotting, and the reporter activity was examined. Indeed, as shown in Fig. 7C, β-catenin protein levels, as well as the β-catenin transcriptional activity, were increased in RKO cells expressing the GSK-3β/S147A-HA mutant. To explore whether this type of GSK-3β regulation occurs in other cell types, HEK-293T cells expressing wild-type GSK-3β–HA or the GSK-3β/S147A-HA mutant were incubated in the absence or presence of the PKCζ-myristoylated pseudosubstrate inhibitor and then in the absence or presence of Wnt5a for 5 min. GSK-3β was immunoprecipitated from the HEK293T cell extracts and analyzed by Western blotting, using the anti-phospho-serine-PKC substrate antibody. The results presented in Fig. 7D confirmed that GSK-3β was regulated by Wnt5a in the same way as in nonmalignant colon cells, since inhibition of PKCζ abolished basal PKC-mediated phosphorylation of GSK-3β and reduced the phosphorylation induced by Wnt5a. In addition, it can also be confirmed that the anti-phospho-Ser-PKC substrate antibody specifically recognizes the phosphorylated Ser147 in GSK-3β. Consistent with these results, Fig. 7E shows how HEK293T cells cotransfected with the pTOPFlash or pFOPFlash reporter plasmid and with a plasmid encoding wild-type GSK-3β–HA or a plasmid encoding GSK-3β/S147A-HA displayed the same effect as in colon cells: β-catenin protein levels, as well as β-catenin transcriptional activity, were increased in cells expressing the GSK-3β/S147A-HA mutant. Taken together, our results indicate that GSK-3β is phosphorylated in cells at Ser147 by PKCζ to maintain its constitutive basal activity, that short Wnt3a or -5a treatment of cells can transiently modulate this basal activity in opposite fashions in nonmalignant and cancer cells, and that this is a widely used mechanism for GSK-3β regulation mediated by PKCζ and Wnts in cell systems other than colon epithelial cells.
FIG 7.
Ser147-to-alanine mutation inactivates GSK-3β, whereas the phosphomimetic mutation of Ser147 to glutamic acid allows constitutive activation of GSK-3. (A and B) Twenty-four hours posttransfection, RKO cells expressing epitope-tagged (HA) mutant or control wild-type GSK-3β were incubated for 1 h in the absence or presence of the selective PKCζ-myristoylated pseudosubstrate inhibitor (20 μM) and then in the absence or presence of 100 ng/ml of Wnt5a for 5 min. GSK-3β was immunoprecipitated (with anti-HA antibody) from cell extracts to be analyzed for its catalytic ability in vitro. Representative autoradiograms with their corresponding Coomassie blue-stained gels are shown. The data were quantified by densitometric analysis performed in both Coomassie-stained gels and the corresponding autoradiographs to determine the specific phosphorylation, normalized with respect to control cells. The values plotted are means and SEM for at least three experiments with different cell preparations. *, P < 0.05. (C and E) RKO or HEK293T cells were transiently cotransfected with pTOPFlash or pFOPFlash (control) reporter plasmid, with 0.05 μg of the pRL luciferase plasmid (transfection control), and with a plasmid encoding wild-type (WT) GSK-3β–HA or a plasmid encoding a GSK-3β/S147A-HA mutant. At 24 h posttransfection, β-catenin levels were analyzed by Western blotting in cell extracts, and the luciferase activity was assayed. The activity was normalized with respect to the activity of Renilla luciferase or with respect to the protein content in each sample. All of the assays were performed in triplicate, and the data represent the means and SEM from at least three independent assays. *, P < 0.05; **, P < 0.01. (D) Nonmalignant HEK293T cells expressing wild-type GSK-3β–HA or GSK-3β/S147A-HA were serum starved for 7 h and then incubated in the absence or presence of the myristoylated PKCζ-selective inhibitor (20 μM) for 1 h. Then, the cells were incubated in the absence or presence of Wnt5a (100 ng/ml) for 5 min. GSK-3β was immunoprecipitated with anti-HA antibody from cell lysates, and the immunoprecipitates were analyzed by Western blotting using the indicated antibodies. The results shown are representative of at least three independent experiments using different cell preparations.
DISCUSSION
GSK-3β is a serine threonine kinase involved in the regulation of a diverse range of signaling pathways, including those activated by Wnts, and has been linked to the onset and progression of human diseases, such as Alzheimer's disease, diabetes, and cancer. The mechanisms regulating GSK-3 have proven to be a topic of great debate, because they are varied and not fully understood. In this regard, despite the fact that the molecular mechanisms of Wnt-induced regulation of GSK-3β activity have been intensively studied in recent years, they remain unclear. These studies have shown that the canonical Wnt signaling pathway employs a distinct mechanism for regulating GSK-3β that is independent of N-terminal-domain serine phosphorylation or tyrosine phosphorylation and instead relies on protein-protein interactions and intracellular sequestration (13, 19, 20).
PKC isozymes are commonly dysregulated in many types of cancers, such as colon cancer, and it has been demonstrated that they may act both as oncogenes (21) and as tumor suppressors (22), depending on the cell context and on which protein adaptors interact with PKC isoforms. In this respect, the atypical isoforms, PKCι and PKCζ, have been demonstrated to be critical components of cell survival signal transduction pathways, frequently suppressing apoptosis by activation of prosurvival NF-κB and MAPK signaling pathways (21, 23, 24), but it has also been reported that the loss of PKCζ in mice results in enhanced intestinal tumorigenesis and in increased stem cell activity (25, 26). These atypical isoforms differ from other PKCs in that their catalytic activity is not dependent upon diacylglycerol, calcium, or phosphatidylserine. Instead, their activity can be regulated by 3-phosphoinositides produced as the result of phosphatidylinositol (PI) 3-kinase (PI3K) activation induced by growth factors, by phosphorylation by the phosphoinositide-dependent kinase PDK1, and through specific protein-protein interactions (27).
With respect to the role played by PKC isozymes in Wnt signaling, the first evidence of possible cross talk between PKC and canonical Wnt signaling emerged with the observation that some PKC isozymes phosphorylate (at Ser9) and inactivate GSK-3β in vitro (9, 10). It is now well known that PKC is one of the key targets of noncanonical Wnt signaling, particularly in the Wnt/Ca2+ pathway, and we previously demonstrated that PKCζ plays an important role in the positive regulation of the canonical Wnt pathway by controlling nuclear β-catenin localization in colon cancer cells (17).
The interaction of PKCζ with GSK-3β had been previously described. Etienne-Manneville and Hall (15) reported that GSK-3β physically associates with PKCζ, forming a complex with Par6. Both fluorescence resonance energy transfer (FRET) and immunofluorescence studies (28) confirmed the existence and close association of these proteins with PKCζ at the meiotic spindle. Interestingly, it was observed that when the two proteins are precipitated together after scratch-induced migration, phosphorylated GSK-3β (at Ser9) cannot be detected in the PKCζ precipitate, indicating that GSK-3β phosphorylation at Ser9 leads to its dissociation from PKCζ (15). Here, we report, to our knowledge, an unprecedented function for atypical PKCζ in regulation of the constitutive basal activity of GSK-3β and in regulation of its activity by Wnt ligands. Our data demonstrate that PKCζ positively regulates GSK-3β activity by phosphorylating serine 147 in order to maintain the constitutive activity of GSK-3β in resting cells. Furthermore, we found that Wnt stimulation induces a rapid and transient modification in the PKCζ-mediated Ser147 phosphorylation status and GSK-3β activity, which results, in nonmalignant cells, in a reduction of the GSK-3β basal activity but in enhancement of GSK-3β activity in colon cancer cells. Although all these findings at the cellular level need to be further validated using animal models, they have substantial implications for an understanding of the mechanisms of Wnt signaling and of GSK-3β regulation. In canonical Wnt signaling, GSK-3β plays a dual role: besides its well-known negative role targeting β-catenin for ubiquitylation and proteosomal degradation in the absence of Wnt stimulation, GSK-3β activity is required to transduce the signal at the cell membrane upon Wnt stimulation in order to phosphorylate the cytoplasmic tail of the LRP5 coreceptor and to induce signalosome formation at the membrane, a step that is widely recognized to be crucial for Wnt signaling activation (29). However, Wnt-induced signalosome formation at the plasma membrane is the key step that precedes inhibition of GSK-3β. How signalosomes lead to a block of β-catenin phosphorylation by GSK-3β remains an open question, and there are two main models that describe how this might occur (13, 30). Our data provide insight into the events that precede LRP phosphorylation and are consistent with the time courses reported for LRP phosphorylation and signalosome formation. In this respect, Bilic et al. (29) showed that LRP6 signalosome-containing aggregates of phospho-LRP6, Frizzled (Fzd), Dvl, axin, and GSK-3β are formed at and under the plasma membrane 15 min after Wnt addition. Thus, the time course of Wnt-induced upregulation that we observed here (peaking at 5 min) correlates in cancer cells with the time required for GSK-3β to be activated to phosphorylate LRP5/6 at PPPSP repeats.
Here, we also found that Wnt stimulation modifies the phosphorylation of Ser147 to regulate GSK-3β activity in opposite manners in normal and malignant colon cells. A possible explanation of this is that in cancer cells, Wnts increase the activity of GSK-3β only during the first 5 min after stimulation by increasing the phosphorylation of Ser147 in GSK-3β to phosphorylate LRP5 and to hyperactivate Wnt signaling, but after 15 min, the activity returns to the basal level. In nonmalignant cells, Wnts diminish the activity of GSK-3β during the first 5 min upon stimulation, probably delaying signalosome formation, whereas β-catenin accumulates, but after 30 min of Wnt stimulation, the final effect is the same in both cell types: GSK-3β is inhibited once the signalosome is formed.
Our data are consistent with previously reported evidence for ligand-stimulated elevation of GSK-3 activity. Plyte et al. (31) demonstrated that in Dictyostelium, extracellular cyclic AMP (cAMP) acting via the serpentine receptor cAR3 causes a rise in GSKA kinase activity, which regulates cell type patterning during initial stages of multicellularity. The timing of this rise correlates with the requirement for the Dictyostelium homolog of GSK-3, GSKA, to specify the cell fate (31). Later, Kim et al. (32) identified a novel cAMP-activated protein tyrosine kinase, ZAK1, that is involved in cAR3-mediated activation of GSK-3.
Our results also indicate that Wnt3a, a prototype canonical ligand, and Wnt5a, a prototype noncanonical ligand, both induce an increase in GSK-3β activity. However, the consequences produced by each one in the cell are different, depending on which receptor-coreceptor complex interacts with the ligand at the membrane: canonical Wnt3a promotes transient upregulation of GSK-3β to participate in signalosome formation and phosphorylation of LRP5/6, and noncanonical Wnt5a promotes Ror2 phosphorylation at Ser864 to induce cell migration (27, 28). In this respect, Yamamoto et al. first demonstrated that Ror2 is phosphorylated on serine/threonine residues upon stimulation of cultured cells expressing Ror2 endogenously with Wnt5a but not with Wnt3a and that inhibition or depletion of GSK-3β blocked this phosphorylation and cell migration (33). Consistent with this, Grumolato et al. (34) demonstrated that prototype canonical Wnt3a and noncanonical Wnt5a ligands specifically trigger completely unrelated endogenous coreceptors—LRP5/6 and Ror1/2, respectively—through a common mechanism involving their Wnt-dependent coupling to the Fzd/coreceptor and recruitment of shared components, including Dvl, axin, and GSK-3. The authors identified Ror2 Ser864 as a critical residue phosphorylated by GSK-3β and required for noncanonical receptor activation by Wnt5a, analogous to the priming phosphorylation of LRP6 in response to Wnt3a (34).
Finally, here, we found that Wnt stimulation induced rapid GSK-3β redistribution from cytoplasm to nuclei in both nonmalignant and malignant cells and a transient exit of PKCζ from nucleus to cytoplasm in malignant cells, and, to our knowledge, this correlation between nuclear GSK-3β and Wnt signaling has not been previously demonstrated. In this regard, although the mechanisms governing the intracellular localization of GSK-3β are not fully elucidated, GSK-3β is known to be located at the cytoplasm, nucleus, and mitochondria (4). Moreover, it has been reported that the nuclear localization of GSK-3β is dynamic and cell cycle dependent (35) and that nuclear GSK-3β plays a role in controlling the nuclear/cytoplasmic distribution of several proteins, such as cyclin D1, STAT, GATA-4, c-Myc, NRF2, Snail, and p53 (36). In addition, there is evidence that nuclear GSK-3β can form a complex with β-catenin, thereby lowering the levels of β-catenin/TCF-dependent transcription and negatively affecting canonical Wnt signaling at the nuclei (36). However, other reports have shown that depletion of nuclear GSK-3β or pharmacological inhibition of its kinase activity impaired survival and proliferation of cultured colon cancer cells (37). Thus, the biochemical and physiological meaning of the GSK-3β redistribution from the cytoplasm to the nucleus as a result of Wnt stimulation observed in this study in nonmalignant and malignant cells remains to be elucidated.
Taking all of this experimental evidence, and our findings reported here, together reveals multifaceted roles of GSK-3β in Wnt signaling, thus establishing a central role for the kinase in strict regulation of its activity, which is different in normal and malignant cells.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from Universidad Nacional Autónoma de México (DGAPA-UNAM IN226111 and IN215514) and from CONACYT (CB2010-151731). Nydia Tejeda-Muñoz is a Ph.D. student in the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM), and received a fellowship from CONACYT (260991).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00828-15.
REFERENCES
- 1.Embi N, Rylatt DB, Cohen P. 1980. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 107:519–527. [PubMed] [Google Scholar]
- 2.Jope RS, Johnson GV. 2004. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, Pan W. 2010. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi-Yanaga F. 2013. Activator or inhibitor? GSK-3 as a new drug target. Biochem Pharmacol 86:191–199. doi: 10.1016/j.bcp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Frame S, Cohen P. 2001. The renaissance of GSK3. Nat Rev Mol Cell Biol 2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 6.Kaidanovich-Beilin O, Woodgett JR. 2011. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci 4:40. doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen RH, Ding WV, McCormick F. 2000. Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem 275:17894–17899. [DOI] [PubMed] [Google Scholar]
- 8.Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC. 1996. Wingless inactivates glycogen synthase kinase-3 via an intracelular signalling pathway which involves a protein kinase C. EMBO J 15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 9.Goode N, Hughes K, Woodgett JR, Parker PJ. 1992. Differential regulation of glycogen synthase kinase-3β by protein kinase C isotypes. J Biol Chem 267:16878–16882. [PubMed] [Google Scholar]
- 10.Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. 2002. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol 22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens J. 2013. Everything you would like to know about Wnt signaling. Sci Signal 6:pe 17. [Google Scholar]
- 12.Clevers H, Nusse R. 2012. Wnt/β-catenin signaling and disease. Cell 149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. 2010. Wnt signaling requires the sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. 2005. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J 24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etienne-Manneville S, Hall A. 2003. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 16.Schlessinger K, McManus EJ, Hall A. 2007. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol 178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luna-Ulloa B, Hernández-Maqueda J, Santoyo-Ramos P, Castañeda-Patlán MC, Robles-Flores M. 2011. Protein kinase C ζ is a positive modulator of canonical Wnt signaling pathway in tumoral colon cell lines. Carcinogenesis 32:1615–1624. doi: 10.1093/carcin/bgr190. [DOI] [PubMed] [Google Scholar]
- 18.Storz P, Döppler H, Toker A. 2004. Protein kinase C delta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol 24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimelman D, Xu W. 2006. Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 20.Mishra R. 2010. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer 9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields AP, Regala RP. 2007. Protein kinase Cι: human oncogene, prognostic marker and therapeutic target. Pharmacol Res 55:487–497. doi: 10.1016/j.phrs.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari FB, Hunter T, Brognard J, Newton AC. 2015. Cancer-associated protein kinase C mutations reveal kinase's role as tumor suppressor. Cell 160:489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berra E, Diaz-Meco MT, Dominguez I, Municio MM, Sanz L, Lozano J, Chapkin RS, Moscat J. 1993. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell 74:555–563. doi: 10.1016/0092-8674(93)80056-K. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Park M, Shin N, Kim G, Kim YG, Shin JS, Kim H. 2012. High mobility group box-1 is phosphorylated by protein kinase C zeta and secreted in colon cancer cells. Biochem Biophys Res Commun 424:321–326. doi: 10.1016/j.bbrc.2012.06.116. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, Castilla EA, Chen J, Yajima T, Porollo A, Medvedovic M, Brill LM, Plas DR, Riedl SJ, Leitges M, Diaz-Meco MT, Richardson AD, Moscat J. 2013. Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell 152:599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF, Yajima T, Campos A, Aza-Blanc P, Leitges M, Diaz-Meco MT, Moscat J. 2015. Repression of intestinal stem cell function and tumorigenesis through direct posphorylation of β-catenin and Yap by PKCζ. Cell Rep 10:740–754. doi: 10.1016/j.celrep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. 2000. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signaling and cell polarity. Nat Cell Biol 2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 28.Baluch DP, Capco DG. 2008. GSK3β mediates acentromeric spindle stabilization by activated PKCζ. Dev Biol 317:46–58. doi: 10.1016/j.ydbio.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. 2007. Wnt induces LRP6 Signalosomes and promotes Dishevelled-dependent LRP6 phosphorylation. Science 316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 30.Metcalfe C, Bienz M. 2011. Inhibition of GSK3 by Wnt signalling: two contrasting models. J Cell Sci 124:3537–3544. doi: 10.1242/jcs.091991. [DOI] [PubMed] [Google Scholar]
- 31.Plyte SE, O'Donovan E, Woodgett JR, Hardwood AJ. 1999. Glycogen synthase kinase-3 (GSK-3) is regulated during Dictyostelium development via the serpentine receptor cAR3. Development 126:325–333. [DOI] [PubMed] [Google Scholar]
- 32.Kim L, Liu J, Kimmel AR. 1999. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell 99:399–408. doi: 10.1016/S0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. 2007. Wnt5a modulates glucogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells 12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 34.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. 2010. Canonical and noncanonical Wnts use a common mechanism to actívate completely unrelated coreceptors. Genes Dev 24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diehl JA, Cheng M, Roussel MR, Sherr CJ. 1998. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caspi M, Zilberberg A, Eldar-Finkelman H, Rosin-Arbesfeld R. 2008. Nuclear GSK-3β inhibits the canonical Wnt signalling pathway in a β-catenin phosphorylation-independent manner. Oncogene 27:3546–3555. doi: 10.1038/sj.onc.1211026. [DOI] [PubMed] [Google Scholar]
- 37.Shakoori A, Ougolkov A, Yu ZW, Zhang B, Modarressi MH, Billadeau DD, Mai M, Takahashi Y, Minamoto T. 2005. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun 334:1365–1373. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.