Abstract
In situ gels are systems which are applied as solutions or suspensions and are capable of undergoing rapid sol-to-gel transformation triggered by external stimulus such as temperature, pH etc. on instillation. The aim of the present study was to formulate and evaluate pH responsive in-situ gel for ophthalmic delivery. Ciprofloxacin hydrochloride is popularly used as a broad spectrum antibiotic in the treatment of corneal ulcers of ocular infections. However, rapid dilution on instillation, wash out, poor retention of drug concentration delimit the therapeutic benefits of the drug when used in form of conventional eye drops. Sodium alginate, an ophthalmic gel forming mucoadhesive polymer was chosen as polymer which undergoes instantaneous gel formation due to formation of calcium alginate by virtue of its interaction with divalent cation (Ca+2) present in lachrymal fluid. Hydroxy Propyl Methyl Cellulose (HPMC K4M and E5 0LV) was further incorporated as a viscosity enhancer in order to achieve the desired consistency so as to facilitate sustained drug release. The developed formulations were evaluated for clarity, pH measurement, gelling capacity, drug content, rheological study, and in vitro drug release. Thus, in situ gel based systems containing gums can be a valuable approach for ophthalmic drug delivery when compared to conventional systems.
Keywords: Ophthalmic delivery, In situ gel, Sol–gel transition, pH
Graphical abstract
1. Introduction
One of the major limitations faced in ophthalmic delivery is the attainment and retention of optimum drug concentration at the site of action within the eye. Various ophthalmic dosage forms, like solutions, ointments, gels and polymeric inserts have been investigated in an attempt to extend the ocular residence time of medications for topical application to the eye [1]. The corneal contact time has been increased to varying degrees by these dosage forms. But, they have not been unanimously accepted, because of blurred vision (e.g. ointments) or lack of patient compliance (e.g. inserts).
The eye drops have very poor bioavailability due to their rapid washout during lachrymation in eyes. Most of the systems are applied as solutions or suspensions. The rapid pre-corneal elimination observed with conventional ocular formulations ends in poor drug bio-availability. Ease of administration in case of highly viscous solution and gel forms retard its use and patient compliance. The blurred vision and the lachrymation are associated with the dosage form involving hydrogel.
So, these may be overcome by fabricating the drug as a formulation that undergoes instantaneous in situ gel formation upon ophthalmic administration. They undergo gelation after instillation due to physico-chemical changes occurring in the eye. It increases the pre-corneal residence time and better bioavailability of drug can be achieved by formulating in situ gel. The present work describes the “formulation and evaluation of in situ gel forming ophthalmic formulation containing ciprofloxacin hydrochloride”. In which in situ phase transition occurs on the surface of the cornea. At time of instillation dosage form is in solution phase and soon later upon coming in contact with calcium ion with surrounding pH of 7.4 it turns into transparent gel depo. Thus, this type of formulation has benefit of both solutions as well gels they may improve the retention time of the formulation as well the drug, accuracy and ease of administration [1], [2], [3], [4].
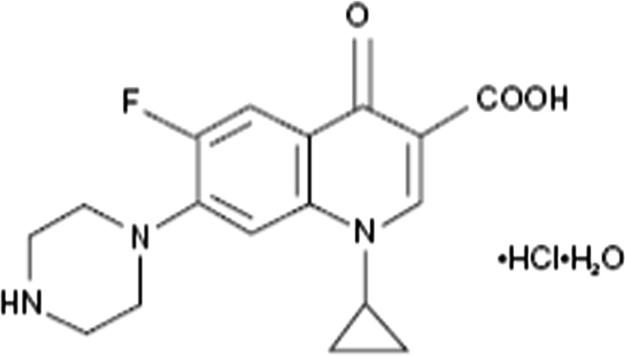
Ciprofloxacin hydrochloride is a pale yellow, crystalline powder which contains Fluoroquinolone group. Ciprofloxacin hydrochloride is used as an antibacterial agent in the treatment of corneal ulcers caused by susceptible strains of bacteria, including Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumonia, Streptococcus and conjunctivitis, bacterial (treatment) of conjunctivitis caused by Haemophilus influenzae, S. aureus, S. epidermidis, S. pneumoniae, and Streptococcus [5], [6] (Fig. 1).
Fig. 1.
Structure of ciprofloxacin hydrochloride.
Ciprofloxacin's bactericidal action is due to interference with the enzyme DNA gyrase, which is needed for the synthesis of bacterial DNA. It inhibits this enzyme hence will not allow multiplication of bacterial cell.
In situ gelling systems consist of polymer that exhibit sol-to-gel phase transitions in the cul-de-sac which improves patient compliance due to change in specific physico-chemical parameters like pH, temperature and ionic strength in the environment [8]. The sol-to-gel phase transition on the eye surface depending on the different methods employed which consist of thermo-sensitive, ion-activated and electric-sensitive, magnetic field-sensitive, ultrasonic-sensitive and chemical material-sensitive varieties. But above them the most commonly methods are as follows:
-
1.
pH-triggered system (e.g. cellulose acetate hydrogen phthalate latex),
-
2.
temperature dependent system (eg. pluronics and tetronics), and
- 3.
The present work is based on the
-
1.
pH triggered system and
-
2.
ion activated system.
In situ gel based drug delivery systems consist of active pharmaceutical ingredients, polymer, co-polymer and excipients. Sodium alginate, an ophthalmic gel forming mucoadhesive polymer was chosen, as the polymer and Hydroxy Propyl Methyl Cellulose (HPMC) as copolymer.
Sodium alginate, family of linear un-branched polysaccharides, the sodium salt of alginic acid, is a natural hydrophilic polysaccharide containing two types of monomers, β-d-mannuronic acid (M units) and α-l glucuronic acid (G units) residues.
The polymer, Sodium alginate, which undergoes instantaneous gel formation due to formation of calcium alginate by virtue of its interaction with divalent cation (Ca+2) present in lachrymal fluid (pH 7.4). Alginate can be ionically crosslinked in the presence of divalent cations.
Hydroxy Propyl Methyl Cellulose (HPMC) is incorporated as a viscosity enhancer to further aid in accomplishment of sustained drug delivery. HPMC is semisynthetic, inert, viscoelastic polymer which is non-ionic nontoxic, a good carrier for pharmaceutical application which exhibits high swelling capacity [11], [12], [13], [14].
2. Materials and method
Ciprofloxacin hydrochloride was obtained from chitin chem (Vadodara), Sodium alginate and Hydroxy propyl methyl cellulose (HPMC E5 0LV and K4M) were obtained from Dutt enterprise, Renchem Company. All other chemicals and reagents were of analytical grade procured from CDH chemicals.
2.1. Analytical method development
2.1.1. Determination of λmax of ciprofloxacin hydrochloride
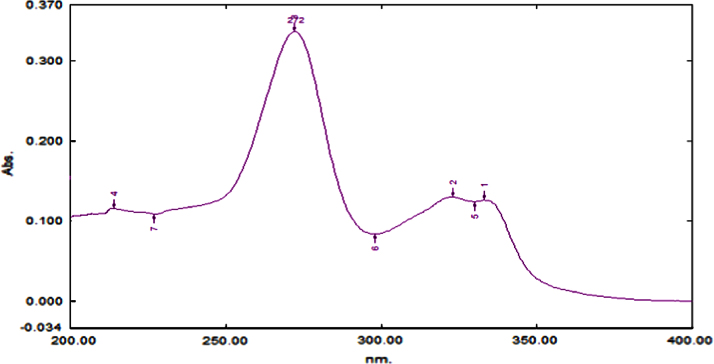
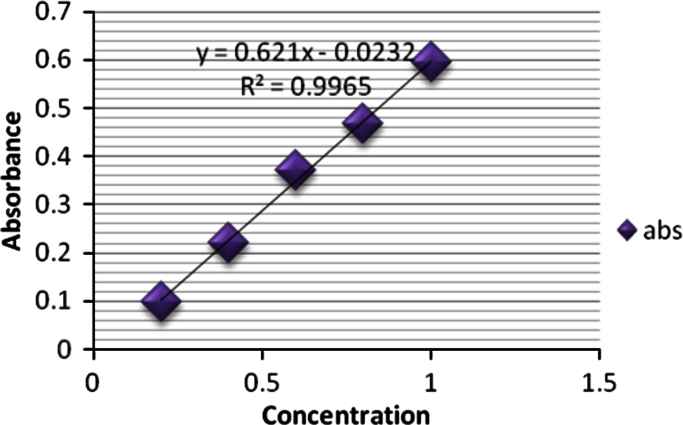
For the determination of absorption maxima, stock solution (1000 µg/ml) was prepared by weighing 100 mg (0.1 g) drug and dissolving it in 100 ml volumetric flask and making the volume to the mark with Methanol. 10 ml of standard stock solution was taken in 100 ml volumetric flask and making the volume to the mark with methanol to make 100 µg/ml of ciprofloxacin. Serial dilutions with concentrations 2, 4, 6, 8 and 10 µg/ml were prepared by transferring 0.2, 0.4, 0.6, 0.8 and 1.0 ml of the stock solution in 10 ml volumetric flask and makeup the volume with phosphate buffer 7.4 up to the mark. The resulting solution was scanned between 200 and 400 nm using UV–visible spectrophotometer UV 1400, Shimadzu [15] (Fig. 2, Fig. 3).
Fig. 2.
UV visible spectra of ciprofloxacin hydrochloride at 272 nm.
Fig. 3.
calibration curve of Ciprofloxacin Hydrochloride.
2.2. Formulation development and evaluation
2.2.1. Formulation of ciprofloxacin hydrochloride in situ gel
Table 1.
Formulation of in situ gel.
| Ingredients | IG-1 | IG-2 | IG-3 | IG-4 |
|---|---|---|---|---|
| Ciprofloxacin HCl (g) | 0.3 | 0.3 | 0.3 | 0.3 |
| Sodium alginate (g) | 0.5 | 1.0 | 1.5 | 0.5 |
| HPMC (g) | 0.5 | 0.5 | 0.5 | – |
| Distilled water (ml) | 100 | 100 | 100 | 100 |
Fig. 4.
Formulation of in situ gel [2].
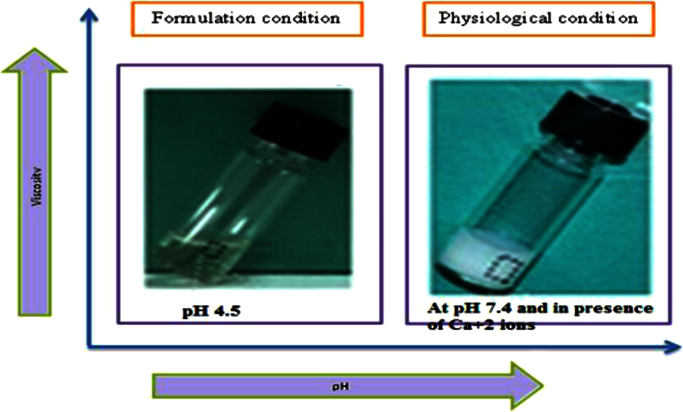
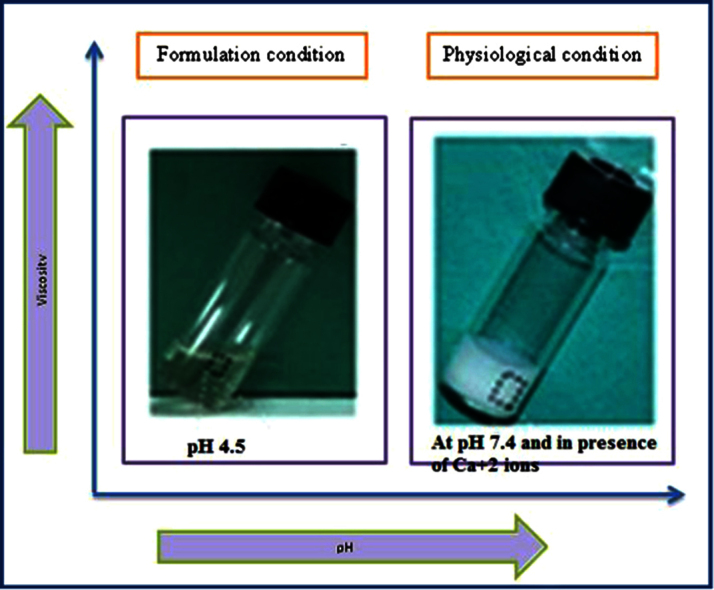
Fig. 5.
Formulations and physiological condition of in situ gel [16].
2.3. Characterization of formulation
2.3.1. FT-IR studies
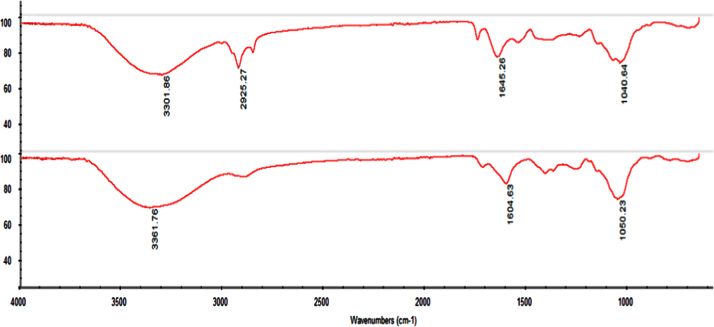
The IR spectra were recorded on Thermo Nicolet, Avatar 370. FTIR spectra of sodium alginate, HPMC and the drug (Ciprofloxacin HCl) were obtained. Spectral scanning was done in the range between 4000 and 500 cm−1 [6], [17]. FT-IR study of drug and polymer mixture along with water (in situ mixture) is shown in Fig. 6.
Fig. 6.
FT-IR study of drug and polymer mixture along with water (in situ mixture).
2.3.2. Clarity
Clarity test was observed by visual inspection under a good light, viewed against a black and white background, with the contents set in motion with a swirling action. Also it was observed for formation of turbidity or any unwanted particles dispersed in the solution [3], [18], [19].
2.3.3. Gelling capacity
The gelling capacity of the prepared formulation was determined by placing a drop of the formulation in a beaker containing 50 ml of freshly prepared concentrated calcium chloride solution and was visually observed for gelling time. Coding for the gelling capacity described in Table 2 [2], [3].
Table 2.
Coding for the gelling capacity.
| Observation | Coding |
|---|---|
| No gelation | – |
| Gelation occurred in few minutes and remained for few hour | + |
| Gelation immediate, remained for few hour | ++ |
| Gelation immediate, and for extended period | +++ |
| Very stiff gel | ++++ |
2.3.4. Rheological studies
The primitive ophthalmic solution, suspension, and ointment dosage forms are clearly no longer sufficient to combat these diseases, and current research and development efforts to design better therapeutic systems are the primary focus of this research work. The aim of the present investigation is to formulate an in situ gel and from our prior knowledge we know that gels show thixotropic behaviour, so rheological studies are to be performed.
The viscosity measurements were carried out using Brookfield viscometer model DVII. The developed formulations were placed in the sampler tube using spindle no. 4. Viscosity of the prepared formulations was measured by using Research Rotator and Oscillatory Rheometer. The gel under study was placed in the small sample holder and the spindle was lowered perpendicularly into it. The spindle was rotated at varying speeds and the suitable speed was selected [20]. Rheological studies of formulation are shown in Table 4.
Table 4.
Rheological studies of formulation.
| Formulation code | Viscosity of solution (Pa s) | Viscosity of in situ gel (Pa s) |
|---|---|---|
| IG-1 | 0.0163 | 89.5 |
| IG-2 | 0.0447 | 266 |
| IG-3 | 0.189 | 856 |
| IG-4 | 0.00466 | 0.147 |
2.3.5. Measurement of pH
Each formulated batch, pH was measured using pH metre which was previously calibrated using standard buffers of pH 4 and pH 7 as per the established procedure [17], [21].
2.3.6. Drug content
1 ml of the developed formulation was dissolved in 100 ml phosphate buffer (pH=7.4) followed by spectrophotometrically estimation of the aliquot to determine drug concentration [2].
Appearance, pH, gelling capacity and drug content (results of in situ gel) is shown in Table 3.
Table 3.
Results of in situ gel.
| Formulation code | Appearance | pH | Gelling capacity | % Drug content |
|---|---|---|---|---|
| IG-1 | Transparent solution | 6.55 | ++ | 84.07 |
| IG-2 | Transparent solution | 6.58 | ++ | 87.00 |
| IG-3 | Transparent solution | 6.53 | +++ | 92.43 |
| IG-4 | Transparent solution | 6.49 | − | 82.13 |
2.3.7. In vitro dissolution studies
Dissolution studies of samples were performed using Franz diffusion apparatus and phosphate buffer (pH=7.4) as a dissolution medium. Phosphate buffer with pH 7.4 will simulate the lachrymal fluid [23]. The temperature was maintained at 37±0.5 °C with the speed of rotation maintained at 100 rpm. The samples were withdrawn at various time intervals and analysed spectrophotometrically for the drug content [5].
3. Results and discussion
The two main prerequisites of an in situ gelling system are viscosity and gelling capacity. Aqueous solutions of varying concentrations of HPLC and alginate were prepared and evaluated for viscosity and gelling capacity. Different concentrations of HPMC and sodium alginate were tailored and their effects were observed. Of them the one with suitable consistency required for in situ gelling was considered for further study.
Alginate forms stable hydrogel in the presence of certain divalent cations (e.g. Ca2+ and Sr2+) through the ionic interaction between the cation and the carboxyl functional group of G moieties located on the polymer chain [16], [20].
3.1. FT-IR studies
Drug-excipients compatibility study was performed by FTIR technique. The IR spectra of the solution were taken, which indicate no interaction between ciprofloxacin HCl and polymers [6], [17].
FT-IR spectrum of drug and polymer mixture shows characteristic peaks at 3364 cm−1 indicates the presence of carboxylic group ,1643 cm−1 exhibits alkenes, 1420 cm−1 indicates the presence of aromatic ring, 1396 cm−1 exhibits carboxylic acids, 1342 cm−1 indicates alkyl halides, 1311 cm−1 indicates ester, 1273 cm−1 indicates alkyl halide, and 1227 cm−1 indicates amine oxide.
From the spectral study it was observed that there was no significant change in the peaks of drug polymer mixture. Hence, no specific interaction was observed between the drug and the polymers used in the formulations (Fig. 7).
Fig. 7.
FT-IR spectra of drug and optimized formulation.
3.2. Clarity and pH
The formulations (IG1–IG4) were prepared by using various concentrations of sodium alginate along with HPMC in different ratios. All the formulations prepared were clear without any turbidity and suspended particles or impurities.
The pH of in situ gel solution was found to be around 6.49–6.58 (Table 3) for all the formulations. The formulation IG 3 has 6.53 pH which is an acceptable range for ophthalmic preparations.
3.3. Gelling capacity
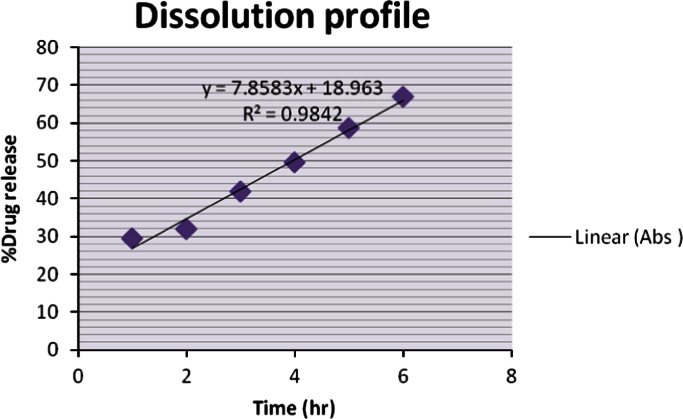
Gelling capacity is coded as describe in Table 2. According that IG 3 shows immediate gelation and for extended period (Fig. 8).
Fig. 8.
Dissolution study of in situ gel.
3.4. Rheological studies
The viscosity was directly dependent on the polymeric content of the formulations. Addition of HPMC led to increase in the viscosity of formulations and exhibited more pseudo-plasticity (IG1–IG3) as compared to IG 4 batch prepared without HPMC. The higher concentration of sodium alginate and HPMC among the developed formulations IG 3 gives good results which are selected as optimized batch.
The formulation which is in the solution form should have an optimum viscosity that will allow for easy instillation into the eye, which would undergo a rapid sol to gel transition.
3.5. In vitro dissolution studies
3.5.1. For in situ gel: 3 mg
Percentage drug release in case of in situ gel of Ciprofloxacin hydrochloride was found to be 67.02% release in 7 h. Thus the in vitro dissolution test indicated the sustained release nature of in situ gel of Ciprofloxacin hydrochloride.
4. Conclusion
Ciprofloxacin hydrochloride was successfully formulated as in situ gel-forming eye drops using sodium alginate and HPMC. Thus the above results demonstrate that the alginate and HPMC mixture can be used as an in situ gelling vehicle to enhance ocular bioavailability and patient compliance [22].
Physicochemical characterization and in vitro drug release studies indicated that the developed formulation (IG 3) may prove to be a viable alternative to conventional eye drops and ointment in terms of ease of administration with added benefits of sustained drug release which may ultimately result into improved patient compliance.
Conflicts of interest
There is no conflict of interest.
Acknowledgements
I am thankful to my guide Ms. Shraddha Parmar for giving her advices and her critical reviews, which always encouraged me to improve myself for the better. I take this opportunity to express my endless gratitude and indebtedness and a special thanks to Mrs. Bhavna Patel and Mr. Tapan R. Shah for their supervision, advice, and valuable guidance. I heartily thank my friend Viral for her valuable time and support at every stage.
I thank DST, New Delhi for the assistance in general and for the PURSE central facility Research Rotator Oscillatory Rheometer sponsored under PURSE program grant.
references
- 1.Gratieri T., Gelfuso G.M., de Freitas O.D., Rocha E.M., Lopez R.F. Enhancing and sustaining the topical ocular delivery of fluconazole using chitosan solution and poloxamer/chitosan in situ forming Gel. Eur. J. Pharm. Biopharm. 2011;79(2):320–327. doi: 10.1016/j.ejpb.2011.05.006. 21641994 [DOI] [PubMed] [Google Scholar]
- 2.Thimmasetty M.K., Mandal S., Prabhushankar G.L., Geetha M.S. Formulation and evaluation of an in situ gel of amoxicillin trihydrate. Int. J. Pharm. Investig. 2012;2(2):78–82. doi: 10.4103/2230-973X.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijaya C., Goud K.S. Ion-activated in situ gelling ophthalmic delivery systems of azithromycin. Indian J. Pharm. Sci. 2011;73(6):615–620. doi: 10.4103/0250-474X.100234. 23112394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majithiya R.J., Ghosh P.K., Umrethia M.L., Murthy R.S. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):67. doi: 10.1208/pt070367. 17025248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Indian Pharmacopoeia, The Indian Pharmacopoeia Commission: Ghaziabad, India, Published by Ministry of Health and Public Welfare, Government of India, New Delhi vol. 2, 2010, p. 1032.
- 6.Nagesh C., Patil M., Chandrashekhara S., Sutar R. A novel in situ gel for sustained ophthalmic delivery of ciprofloxacin hydrochloride and dexamethasone design and characterization. Pharm. Lett. 2012;4(3):821–827. [Google Scholar]
- 8.Liu Z., Li J., Nie S., Liu H., Ding P., Pan W. Study of an alginate/Hpmc-Based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006;315(1–2):12–17. doi: 10.1016/j.ijpharm.2006.01.029. 16616442 [DOI] [PubMed] [Google Scholar]
- 9.Mohanambal E., Arun K., Sathali A. Formulation and evaluation of pH-triggered in situ gelling system of levofloxacin. Ind. J. Pharm. Educ. Res. 2011;45(1):58–64. [Google Scholar]
- 10.Chu K., Chen L., Xu W. Preparation of A paeonol-containing temperature-sensitive in situ gel and its preliminary efficacy on allergic rhinitis. Int. J. Mol. Sci. 2013;14(3):6499–6515. doi: 10.3390/ijms14036499. 23525047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowda D.V., Tanuja D., Khan M.S., Desai J., Shivakumar H.G. Formulation and evaluation of in-situ gel of diltiazem hydrochloride for nasal delivery. Pharm. Lett. 2011;3(1):371–381. [Google Scholar]
- 12.Geethalakshmi A., Karki R., Jha S.K., Venkatesh D.P., Nikunj B. Sustained ocular delivery of brimonidine tartrate using ion activated in situ gelling system. Curr. Drug Deliv. 2012;9(2):197–204. doi: 10.2174/156720112800234530. 22283647 [DOI] [PubMed] [Google Scholar]
- 13.Wee S., Gombotz W.R. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998;31(3):267–285. doi: 10.1016/s0169-409x(97)00124-5. 10837629 [DOI] [PubMed] [Google Scholar]
- 14.Khairnar P.S., Walke P.S., Narkhede M.R., Nehete J.Y. Formulation and in-vitro evaluation of thermoreversible rizatriptan benzoate nasal gel. Int. J. Pharm. Pharm. Sci. 2011;3(4):250–256. [Google Scholar]
- 15.Chelladurai S., Mishra M., Mishra B. Design and evaluation of bioadhesive in-situ nasal Gel of ketorolac tromethamine. Chem. Pharm. Bull. 2008;56(11):1596–1599. doi: 10.1248/cpb.56.1596. 18981612 [DOI] [PubMed] [Google Scholar]
- 16.〈www.Pharmatutor.org〉 (accessed 10.2.14).
- 17.Nisha G.S., Maithil P., Charyulu R.N. Formulation and development of nasal in situ gels of triptans for antimigraine activity. Int. J. Res. Pharm. Biomed. Sci. 2012;3(2):861–868. [Google Scholar]
- 18.Dholakia M., Thakkar V., Patel N., Gandhi T. Development and characterisation of thermo reversible mucoadhesive moxifloxacin hydrochloride in situ ophthalmic gel. J. Pharm. Bioallied Sci. 2012;4:S42–S45. doi: 10.4103/0975-7406.94138. 23066202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S., Vyas S.P. Carbopol/chitosan based Ph triggered in situ gelling system for ocular delivery of timolol maleate. Sci. Pharm. 2010;78(4):959–976. doi: 10.3797/scipharm.1001-06. 21179328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel M., Thakkar H., Kasture P.V. Preparation and evaluation of Thermoreversible formulations of flunarizine hydrochloride for nasal delivery. Int. J. Pharm. Pharm. Sci. 2010;2(4):116–120. [Google Scholar]
- 21.Liu Y., Liu J., Zhang X., Zhang R., Huang Y., Wu C. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. Aaps PharmSciTech. 2010;11(2):610–620. doi: 10.1208/s12249-010-9413-0. 20354916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z., Li J., Nie S., Liu H., Ding P., Pan W. Study of an alginate/HPMC-Based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006;315(1–2):12–17. doi: 10.1016/j.ijpharm.2006.01.029. 16616442 [DOI] [PubMed] [Google Scholar]
- 23.Mundada A.S., Shrikhande B.K. Formulation and evaluation of ciprofloxacin hydrochloride soluble ocular drug insert. Curr. Eye Res. 2008;33(5):469–475. doi: 10.1080/02713680802023104. 18568884 [DOI] [PubMed] [Google Scholar]