Abstract
Endothelial cells (EC) play a central role in inflammation. E-selectin and ICAM-1 expression are essential for leukocyte recruitment and are good markers of EC activation. Most studies of EC activation are done in vitro using isolated mediators. The aim of the present study was to examine the relative importance of pattern recognition systems and downstream mediators in bacteria-induced EC activation in a physiological relevant human model, using EC incubated with whole blood. HUVEC were incubated with human whole blood. Escherichia coli– and Staphylococcus aureus–induced EC activation was measured by E-selectin and ICAM-1 expression using flow cytometry. The mAb 18D11 was used to neutralize CD14, and the lipid A analog eritoran was used to block TLR4/MD2. C5 cleavage was inhibited using eculizumab, and C5aR1 was blocked by an antagonist. Infliximab and canakinumab were used to neutralize TNF and IL-1β. The EC were minimally activated when bacteria were incubated in serum, whereas a substantial EC activation was seen when the bacteria were incubated in whole blood. E. coli–induced activation was largely CD14-dependent, whereas S. aureus mainly caused a C5aR1-mediated response. Combined CD14 and C5 inhibition reduced E-selectin and ICAM-1 expression by 96 and 98% for E. coli and by 70 and 75% for S. aureus. Finally, the EC activation by both bacteria was completely abolished by combined inhibition of TNF and IL-1β. E. coli and S. aureus activated EC in a CD14- and C5-dependent manner with subsequent leukocyte secretion of TNF and IL-1β mediating the effect.
Introduction
Direct bacterial tissue damage and bacteria-induced inflammation are important causes of both severe morbidity and mortality worldwide. Both Gram-negative and Gram-positive bacteria can cause severe disease. Although historically Gram-negative bacteria have been the main cause of severe infections, with Escherichia coli being the most common, there is an increasing prevalence of Gram-positive–induced infections, in particular with Staphylococcus aureus (1).
In recent years, the role of endothelial cells (EC) in inflammation has been recognized both as central and undervalued (2–4). From the basic point that EC activation is necessary for leukocyte recruitment to the recent discovery that inhibiting increased vascular permeability in sepsis seems to alleviate most of the symptoms of the condition in murine models (5–7), it has been demonstrated that a better evaluation of EC activation has a central part in the quest for understanding and modulating inflammation.
A large number of models have been used to examine EC activation, with HUVEC being a common surrogate. Using HUVEC, multiple potential activating factors have been found in in vitro studies. However, as the field of sepsis has duly demonstrated, there is a long path from simple isolated cell models to the clinic, a path riddled with wrong turns and empty promises.
To narrow this gap, we have developed a novel model to look at human EC activation in a more complex system using an already established whole blood model, that is, coincubating lepirudin anticoagulated whole blood with HUVEC monolayers (8).
Recognition of bacteria by the innate immune system is mediated by pattern recognition receptors. There are several different classes of these, where TLRs play a central role, particularly in detection of LPS from Gram-negative bacteria through TLR4, but also motifs on Gram-positive bacteria, particularly lipoproteins, recognized by TLR2 (9). CD14 is a promiscuous coreceptor to several of the TLRs, and it plays a central role in recognition both by TLR4 and TLR2 (10).
The complement system is an important recognition system comprising both fluid-phase and cell-bound components. There are three pathways of pathogen recognition (classical, lectin, and alternative pathways) that all lead to activation of the C3 convertases, which proteolyze C3 into C3a, an anaphylatoxin with a range of effector functions, and C3b, which acts as an opsonin on bacteria and other exogenous or endogenous structures. With further activation of C3, the C5 convertase is constructed, which activates C5, leading to the release of C5a, the most potent anaphylatoxin in this system, and assembly of the terminal C5b-9 complex. This complex can either be formed in the fluid phase as sC5b-9 or assembled on a membrane as the membrane attack complex, which may lyse Gram-negative bacteria. Complement system activation is strictly regulated by endogenous inhibitors at different steps of the cascade (11–13).
We have earlier shown that combined inhibition of CD14 and one of the key complement components, at the level of either C3 or C5, can more or less completely attenuate cytokine release and leukocyte activation in whole blood after bacteria-induced activation both with Gram-negative and Gram-positive bacteria (14–16). Furthermore, in a murine sepsis model, we have recently shown that combined inhibition reduces both inflammation and mortality (17).
The aim of the present study was to evaluate the mechanisms behind bacteria-induced EC activation in our novel whole blood and HUVEC model. Using E. coli and S. aureus, we examined the effect of inhibition both at the recognition stage, inhibiting complement activation and CD14, and at the downstream mediator stage, inhibiting TNF and IL-1β.
Materials and Methods
Reagents
Sterile PBS with and without Ca2+ and Mg2+ and EDTA were purchased from Sigma-Aldrich (St. Louis, MO). CryoTubes (polypropylene) were purchased from Nunc (Roskilde, Denmark). Lepirudin (Refludan) was purchased from Pharmion (Copenhagen, Denmark) and used at a final concentration of 50 μg/ml in whole blood. A 4% stock solution made of paraformaldehyde was purchased from Sigma-Aldrich. BSA 30% was purchased from Biotest (Dreieich, Germany) and trypsin/EDTA was from Invitrogen (Carlsbad, CA). A complete EC growth medium was purchased from Cell Applications (San Diego, CA). Heat-inactivated E. coli strain LE392 (ATCC 33572) and S. aureus Cowan strain 1 (ATCC 12598) were obtained from American Type Culture Collection (Manassas, VA).
Abs used for flow cytometric detection of EC surface proteins were FITC-conjugated mouse anti-human ICAM-1 (CD54, clone BBIG-I1) and isotype control (FITC-conjugated mouse IgG1, clone 11711), PerCP-conjugated mouse anti-human MCAM (CD146, clone 128018) (from R&D Systems, Minneapolis, MN), PE-conjugated mouse anti-human E-selectin (CD62E, clone 1.2B6) and isotype control (PE-conjugated mouse IgG1, clone 15H6) (both from SouthernBiotech, Birmingham, AL).
The following inhibitors were used: the C5-blocking Ab eculizumab (Soliris; Alexion), the TNF-blocking Ab infliximab (Remicade; Janssen Biologics), the IL-1β–blocking Ab canakinumab (Ilaris; Novartis), a blocking Ab to CD14 (r18D11) that was produced and used as described previously (18), the specific C5a receptor (C5aR1) antagonist (AcF[OPdChaWR]), which was a gift from Prof. John D. Lambris and synthesized as previously described (19), and the TLR4/MD2-blocking lipid A analog eritoran (E5564), which was provided by Eisai (Andover, MA). The CD20-blocking Ab rituximab (Mabthera; Roche) was used as a control Ab in all experiments.
Whole blood and HUVEC
The model for whole blood and HUVEC activation was described previously (8). Briefly, a modified version of an ex vivo whole blood model (20) was used combined with HUVEC (European Collection of Authenticated Cell Cultures, Salisbury, U.K.) seeded in 48-well plates (Costar, Corning, NY). HUVEC were used in passages two through five. On the day of the experiment, fresh human whole blood was obtained from healthy donors of both genders (age of 27–83 y), who had used no medications the previous week, and was anticoagulated with the thrombin-specific inhibitor lepirudin. In experiments with human serum, three separate pooled human sera were used. Confluent HUVEC monolayers were washed twice with sterile, 37°C PBS before the addition of 100 μl whole blood, pooled normal human serum, or growth medium with 2% FBS. The inhibitors anti-CD14 (15 μg/ml), eculizumab (100 μg/ml), C5aR1 antagonist (10 μM), infliximab (100 μg/ml), canakinumab (100 μg/ml), eritoran (1μM), rituximab as a control Ab (100 μg/ml), or PBS were added in a total volume of 20 μl and incubated for 4 min at 37°C prior to addition of PBS, heat-inactivated E.coli (105 bacteria/ml unless specified otherwise) or heat-inactivated S. aureus (107 bacteria/ml, unless specified otherwise). Samples were then incubated at 37°C with 5% CO2 for 4 h with gentle shaking. Thereafter, whole blood, serum, or medium was removed and EDTA (20 mM) was added before centrifugation for 15 min at 3000 × g at 4°C. Plasma or supernatant was stored at −70°C until analysis.
EC activation markers
After removal of medium, serum, or whole blood, HUVEC monolayers were gently washed twice with ice-cold PBS, fixed with 0.5% paraformaldehyde, and incubated at 4°C for 2.5 min, as described previously (21). After gentle washing with PBS, anti–ICAM-1-FITC and anti–E-selectin-PE, or their isotype controls, and anti–MCAM-PerCP were added and plates were incubated for 30 min at 4°C. Cells were washed twice with PBS, briefly trypsinated, and transferred to 5-ml polypropylene tubes (Sarstedt, Nuernbrecht, Germany), washed with PBS with 0.1% BSA, and run on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). HUVEC were gated as MCAM-positive cells. Median values were used for fluorescence intensity. Data were analyzed in FlowJo X (Tree Star, Ashland, OR).
Cytokine measurements
Analysis of plasma, serum, or medium concentrations of IL-1β, IL-6, IL-8, and TNF were done using multiplex technology. Single-plex beads were purchased from Bio-Rad Laboratories (Hercules, CA) and used according to the manufacturer’s recommendations.
Statistical analysis, data presentation, and ethical approval
All data were compiled in Prism 6 (GraphPad Software, San Diego, CA). Both absolute and relative values of groups were compared using a nonparametric Friedman test with a Dunn posttest. Data were paired for each donor to eliminate donor variation, and all groups were compared with noninhibited controls unless specified otherwise. The data are presented as absolute values in the figures, but for comparison relative values have been calculated and are presented in Supplemental Figs. 2–4. Informed written consent was obtained from each donor, and the local ethics committee approved the study.
Results
Bacteria-induced EC activation
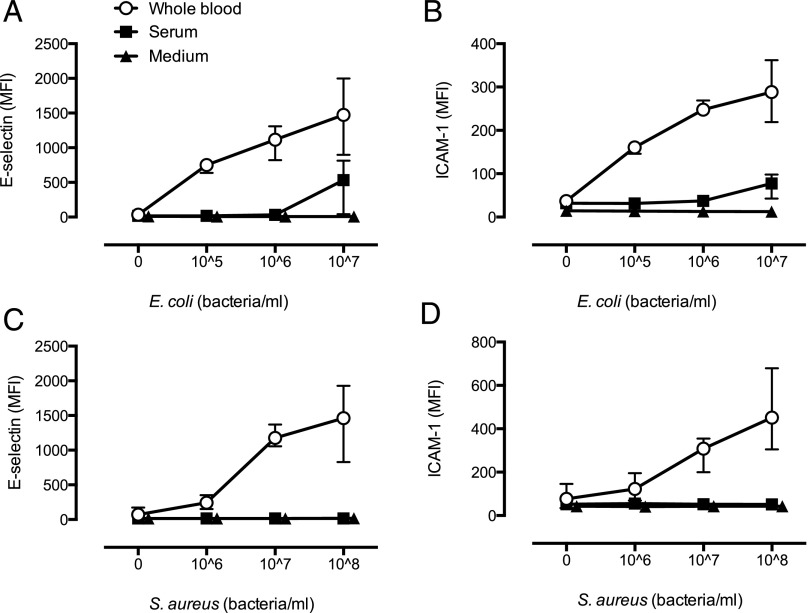
To evaluate activation of EC by Gram-negative bacteria, we incubated EC with incremental doses of E. coli in medium (no human serum), pooled normal human serum, or whole blood. E. coli in medium only could not induce EC activation. However, in the presence of human serum, 107 bacteria/ml caused a modest expression of E-selectin (Fig. 1A) and ICAM-1 (Fig. 1B). Notably, the sensitivity of the system was drastically increased with similar activation by 105 bacteria/ml in whole blood as 107 bacteria/ml in serum (Fig. 1A, 1B).
FIGURE 1.
E. coli and S. aureus cause dose-dependent EC activation in whole blood. Expression of E-selectin (A and C) and ICAM-1 (B and D) on EC after incubation of E. coli (A and B) or S. aureus (C and D) in growth medium, normal human serum, or in whole blood. Data are given as mean ± range of n = 3 and n = 4 for E. coli and S. aureus, respectively.
To evaluate activation of EC by Gram-positive bacteria, we incubated EC with incremental doses of S. aureus in medium (no human serum), pooled normal human serum, or whole blood. S. aureus did not activate EC in any bacterial concentration in medium only or in human serum. However, in the presence of whole blood, S. aureus caused a potent and dose-dependent upregulation of E-selectin (Fig. 1C) and ICAM-1 (Fig. 1D), although at a 100-fold increase in bacteria concentration compared with E. coli (Fig. 1C, 1D).
Inhibition of bacteria-induced EC activation in whole blood
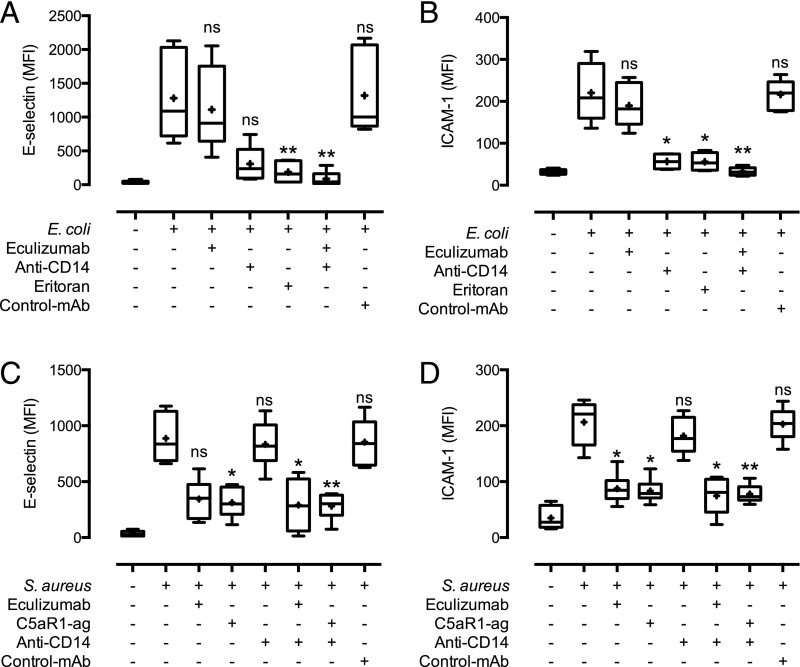
We have earlier shown that the inflammatory reaction induced by both E. coli and S. aureus in human whole blood can be attenuated by combined inhibition of CD14 and one of the key complement components (14, 16). To evaluate whether bacteria-induced EC activation also would be reduced by this regimen, we incubated whole blood on EC monolayers with either E. coli (105 bacteria/ml) or S. aureus (107 bacteria/ml) in addition to blocking mAbs to CD14 and C5, alone or in combination (Fig. 2, Supplemental Fig. 2).
FIGURE 2.
Complement (C5) and TLR (CD14 and TLR4/MD2) inhibition of whole blood attenuates EC activation by E. coli and S. aureus. Monolayers of EC were incubated with whole blood. The C5 inhibitors eculizumab or C5aR1 antagonist, anti-CD14, TLR4/MD2 antagonist eritoran, or combinations of these, as well as control Ab (rituximab) were added to the blood prior to the addition of 105 bacteria/ml E. coli (A and B) or 107 bacteria/ml S. aureus (C and D). E-selectin expression (A and C) and ICAM-1 expression (B and D) data are presented with median values (bars), mean values (+), and error bars from the 10th to 90th percentile with n = 6 donors. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with bacterial incubation without inhibitors.
E. coli–induced EC activation was largely attenuated by anti-CD14 alone, as evaluated by E-selectin (Fig. 2A, Supplemental Fig. 2A) and ICAM-1 (Fig. 2B, Supplemental Fig. 2B). We therefore included the TLR4/MD2 antagonist eritoran to evaluate to what extent the CD14 effect depended on TLR4 signaling. Eritoran had a comparable effect to anti-CD14 (Fig. 2A, 2B, Supplemental Fig. 2A, 2B).
S. aureus caused a time-dependent formation of C5b-9 in whole blood (Supplemental Fig. 1). Whole blood–induced EC activation was largely attenuated by complement inhibition alone, as demonstrated by the C5-blocking Ab eculizumab (Fig. 2C, 2D, Supplemental Fig. 2C, 2D). To evaluate whether this attenuating effect was due to the inhibition of C5a or C5b-9 formation, we also included a C5aR1 antagonist. The effect of the antagonist was virtually identical to that of eculizumab, indicating that the effect was mainly mediated through the C5a–C5aR1 interaction and not C5b-9 (Fig. 2C, 2D, Supplemental Fig. 2C, 2D). For both bacteria, combined inhibition of CD14 and C5 had a potent and similar degree of inhibition compared with single inhibition of either (Fig. 2, Supplemental Fig. 2).
Mediators of bacteria-induced EC activation in whole blood
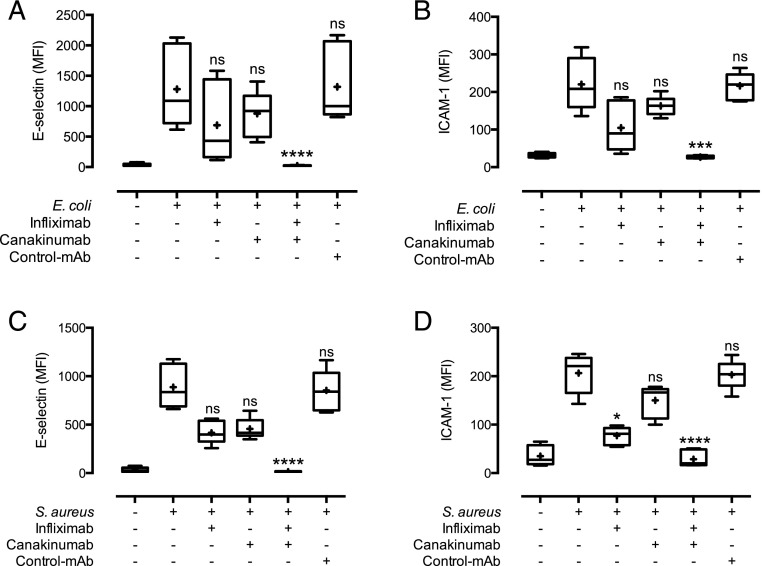
As activation of EC by E. coli and S. aureus was largely dependent on whole blood, we then evaluated potential downstream, secreted mediators causing this activation. TNF and IL-1β are commonly described EC activators (22). Thus, we evaluated to what extent they could explain the whole blood bacteria-induced EC activation using Abs already in clinical use, namely infliximab (anti-TNF) and canakinumab (anti–IL-1β). TNF and IL-1β release was detectable after 2 h and increased over time after activation with both E. coli and S. aureus (Supplemental Fig. 1). Notably, although single inhibition of TNF or IL-1β only modestly reduced activation of EC, combined TNF and IL-1β inhibition completely abolished the expression of E-selectin (Fig. 3C, Supplemental Fig. 3C) and ICAM-1 (Fig. 3D, Supplemental Fig. 3D) on the EC surface to background levels. This was the case for both E. coli– and S. aureus–induced activation.
FIGURE 3.
Complement (C5)- and TLR (CD14 and TLR4/MD2)-induced EC activation is mediated by TNF and IL-1β. Monolayers of EC were incubated with whole blood and the TNF-blocking Ab infliximab, the IL-1β–blocking Ab canakinumab, a combination of these, or control Ab (rituximab) prior to the addition of 105 bacteria/ml E. coli (A and B) or 107 bacteria/ml S. aureus (C and D). E-selectin expression (A and C) and ICAM-1 expression (B and D) data are presented as in Fig. 2 with n = 6 donors. ***p < 0.001, ****p < 0.0001 as compared with bacterial incubation without inhibitors.
Mechanisms of complement and CD14 inhibition
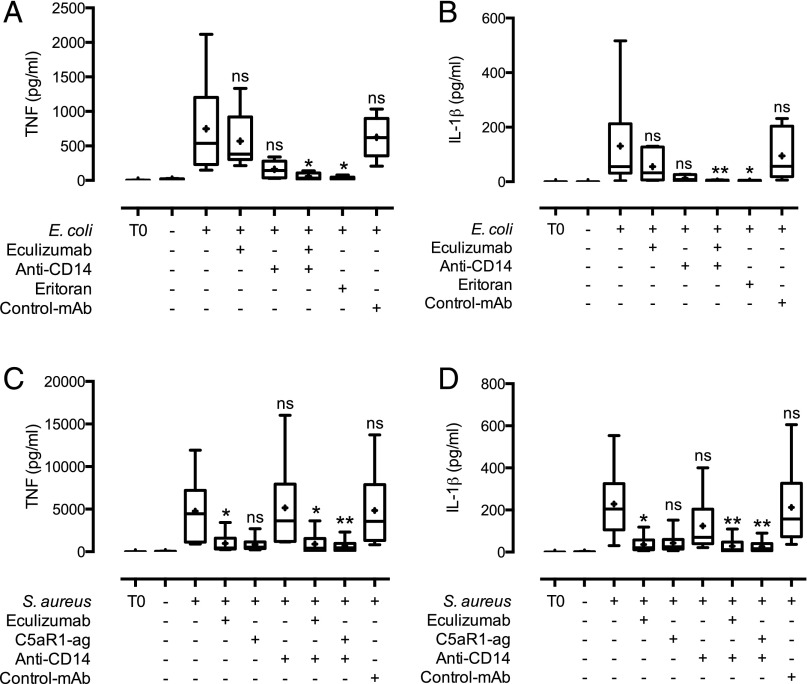
To evaluate whether the effect of CD14 and C5 inhibition was due to reduced secretion of TNF and IL-1β, we analyzed cytokine release in whole blood from E. coli and S. aureus activation in the presence of HUVEC. E. coli–induced TNF and IL-1β secretion was reduced to background levels by combined CD14 and C5 inhibition, and it was clearly reduced by single inhibition with anti-CD14 (Fig. 4A, 4B, Supplemental Fig. 4A, 4B). We included eritoran to examine whether the CD14 effect was mainly dependent on TLR4 activation, and eritoran also reduced TNF and IL-1β secretion to background levels (Fig. 4A, 4B, Supplemental Fig. 4A, 4B), indicating that TNF and IL-1β release induced by E. coli was mediated through TLR4 activation. S. aureus–induced TNF and IL-1β secretion was reduced to background levels by combined CD14 and C5 inhibition, as well as by single inhibition of C5 (Fig. 4C, 4D, Supplemental Fig. 4C, 4D). To evaluate the mechanism behind C5-induced TNF and IL-1β release, we included a specific C5aR1 antagonist, which also reduced their release to background levels, indicating that TNF and IL-1β release induced by S. aureus was mainly mediated through the C5a–C5aR1 interaction and not C5b-9.
FIGURE 4.
Bacteria-induced cytokine release in whole blood is attenuated by complement (C5) and TLR (CD14 and TLR4/MD2) inhibition. Plasma from whole blood incubated with monolayers of EC and 105 bacteria/ml E. coli (A and B) or 107 bacteria/ml S. aureus (C and D) and inhibitors was analyzed for TNF (A and C) and IL-1β (B and D). Data are presented as in Fig. 2 with n = 6 donors. *p < 0.05, **p < 0.01 as compared with bacterial activation without inhibitors.
Taken together, these findings suggest that targeting key upstream recognition molecules, that is, CD14 and complement, inhibits E. coli– and S. aureus–induced EC activation by preventing blood cells from releasing the downstream inflammatory mediators TNF and IL-1β.
Discussion
In the present study, we show a mechanistic link between upstream recognition of C5 and CD14 activation and downstream TNF and IL-1β release, demonstrating that mainly C5a–C5aR1 activation combined with CD14 interaction are responsible for the TNF- and IL-1β–dependent EC activation induced by both E. coli and S. aureus. Of particular interest is that the combined inhibition of either of the two upstream molecules C5 and CD14, or the two downstream cytokines TNF and IL-1β mediators, was required to obtain a virtually complete attenuation of the EC activation.
The central role of EC in inflammatory disease is becoming increasingly apparent, and thus the importance of understanding the mechanisms behind their activation can reveal potential targets of intervention in inflammatory conditions. However, with increasing appreciation of the cross-talk between systems formerly thought of as separate, we need to develop models that at least include some of this complexity if we are to successfully translate laboratory results into clinical efficacy. To a large extent, the evaluation of EC activation has been done in vitro in isolated cell systems, where the exposure of EC to different mediators is used to evaluate the role of these mediators in EC activation. Although the list of potential activators is increasing, the relative importance of these activators in clinical inflammation remains unclear, and thus which of those provide most promise for intervention is still elusive.
We have developed a novel model of EC activation by coincubating HUVEC with whole blood. As a necessary limitation of the model, coagulation has to be prevented. However, using the thrombin-specific agent lepirudin, only the end stage of the coagulation process is inhibited, providing a more physiological environment compared with the more commonly used heparin (23). HUVEC do not express ABO Ags, and consequently ABO matching between EC and whole blood donors is not necessary (24). In this system, we can examine the effect of specific mediators on EC, but also study cell–cell interactions between EC and leukocytes and cross-talk between different blood constituents, such as platelets, the complement system, RBCs, and leukocytes, as well as evaluate interventions that might alleviate EC activation.
Several studies have shown that HUVEC can react to LPS or E. coli directly mediated by TLR4, but the role of CD14 is less clear. Lloyd-Jones et al. (25) showed that both soluble and membrane-bound CD14 was necessary with low levels of LPS, whereas when the dose increased, the dependency of CD14 was eliminated. The potential role of TLR4 recognition by EC in vivo was further elaborated in a mouse model with TLR4 expressed exclusively in EC (26). It was found that this was sufficient to recruit leukocytes to tissue after local injection of LPS or E. coli, and limiting TLR4 expression to EC was beneficial, as it led to a larger number of immune cells recruited to the inflamed tissue instead of sequestered in the lungs (26). Thus, it has become clear that TLR4 on EC is capable of detecting bacterial stimuli, and this detection is sufficient to initiate a robust local inflammatory response. However, what role TLR4 activation on EC plays in inflammation in a complex, TLR4-sufficient, human system is less clear.
In our study, we found that although EC could be activated directly by E. coli in sufficient doses, this activation was dependent on plasma, which contains soluble CD14, and was 100-fold less potent than the response to whole blood–mediated E. coli–induced activation of EC. This is in line with previous studies demonstrating that plasma from E. coli–activated heparin anti-coagulated whole blood (27) or conditioned medium with LPS-activated THP-1 cells, a monocytic cell line (28), caused ∼100-fold more potent activation of EC than did activating EC directly with the same concentration of E. coli or LPS. Thus, it seems that although E. coli can directly activate EC, this does not play a significant role due to the shear scale of leukocyte-induced activation. However, as there are indications that EC-expressed TLR4 can more potently activate microvascular than macrovascular cells (29), whole blood–independent EC activation may play a larger role in the microvasculature than what we found in our model.
In a number of different models, we have previously shown that combined inhibition of CD14 and key complement components can attenuate inflammation induced by Gram-negative bacteria (15, 16, 20, 30–34), Gram-positive bacteria (14), and polymicrobial stimuli (17). We therefore intended to evaluate to what extent inhibiting whole blood inflammation would affect EC activation by using CD14- and C5-blocking Abs. We found that E. coli–induced whole blood–mediated activation of EC was largely CD14-dependent whereas S. aureus–induced activation was mediated by the complement system, mainly through C5a–C5aR1 interaction. We have earlier found that single inhibition is less efficient than combined inhibition, particularly with increased load of inflammatory stimuli (35). In the present study, we used fairly low concentrations of bacteria compared with previous studies in whole blood alone, which might explain the impressive effect of single inhibition. However, there is no doubt that a combined inhibitory approach would be superior when the causative agent is not known, as the effect of single CD14 blockage in S. aureus–induced inflammation or single complement inhibition in E. coli–induced activation is highly ineffective.
Several previous studies in less complex models have found an impressive effect of combined TNF and IL-1β inhibition in HUVEC activation. Already in 1995, Pugin et al. (36) demonstrated that combined use of a TNF-blocking Ab and an IL-1R antagonist could completely inhibit activation of HUVEC after stimulation with either plasma or highly diluted whole blood in medium incubated with the EC. Nooteboom et al. (37, 38) used media conditioned with plasma from LPS- and bacteria-activated heparin anticoagulated whole blood to look at EC expression of adhesion molecules and permeability, and Schildberger et al. (28, 39) used conditioned media from LPS-activated THP-1 cells, a monocytic cell line, to activate HUVEC. Both groups found a central role for TNF and IL-1β in EC activation.
Although these studies do take us a step closer to a clinically more relevant model of EC activation, there are some important shortcomings. Using activated plasma, mediators with short half-lives such as C5a, a central complement system anaphylatoxin postulated to activate EC (40–42), as well as cell–cell interaction between leukocytes and EC cannot be modeled. Furthermore, diluting the plasma or whole blood dramatically also entails a danger of including dilution-specific effects, as for instance the alternative pathway of the complement system is close to inactivated at plasma concentrations <6–10% (43).
We therefore evaluated to what extent TNF and IL-1β could account for the whole blood–induced activation in our model. We found a robust time-dependent release of TNF and IL-1β starting from 2 h of incubation, and that combined inhibition of TNF and IL-1β completely blocked HUVEC activation induced by bacteria-stimulated whole blood. Importantly, the central role of TNF and IL-1β seems to hold both with endogenous and exogenous stimuli, as we have previously shown in the same model that blocking TNF completely abrogated cholesterol crystal–induced activation of HUVEC (8). To examine whether the effects of C5 and CD14 blockage were mediated by their ability to eliminate the release of TNF and IL-1β, we analyzed cytokine release in the plasma from the experiments. Indeed, we found that TNF and IL-1β release from whole blood after stimulation with both Gram-negative and Gram-positive bacteria was completely eliminated by combined C5 and CD14 blockage, thus supporting the central role of these two cytokines in inducing EC activation
Thus, in our complex human model, EC are activated not directly by the bacteria, but indirectly, due to bacterial activation of leukocytes. This leads to the secretion of TNF and IL-1β, which then activate the EC. Interestingly, despite the large number of inflammatory mediators in the system, only TNF and IL-1β contributed significantly toward EC activation. One can speculate about why other inflammatory mediators did not seem to significantly contribute toward direct EC activation. EC receptors for activators such as C5a and LPS could be outcompeted by the shear number of receptors for these molecules on leukocytes. The lack of activation could also be due to a lower potency of these mediators, where the overwhelming scale of TNF- and IL-1β–induced activation drowns out their effect. No matter the mechanisms involved, our findings suggest the importance of not only describing mediators that are capable of activating the immune system, but also evaluating the relative importance of these mediators in complex systems. Although mediators are found to cause activation in in vitro single-cell systems, this might not translate into significant relevance as the complexity of the system increases.
In conclusion, this study demonstrates the ability of a novel whole blood and EC model to evaluate EC activation in a complex human ex vivo system. Doing so, we found that EC activation by both Gram-negative and Gram-positive bacteria could be eliminated in the upstream recognition phase by dual inhibition of C5 and CD14. We also found that CD14- and C5-mediated activation caused EC adhesion molecule expression due to subsequent leukocyte release of TNF and IL-1β, and blocking these mediators completely abrogated EC activation. Thus, both upstream combined targeting of C5 and CD14 and downstream combined targeting of TNF and IL-1β appear to be promising foci to modulate EC activation in inflammation.
Supplementary Material
This work was supported by the Research Council of Norway, the Norwegian Council on Cardiovascular Disease, the Northern Norway Regional Health Authority, the Southern and Eastern Norway Regional Health Authority, the Odd Fellow Foundation, and by European Community Seventh Framework Programme Grant 602699 (DIREKT).
The online version of this article contains supplemental material.
- EC
- endothelial cell.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Martin G. S., Mannino D. M., Eaton S., Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 2.Mai J., Virtue A., Shen J., Wang H., Yang X.-F. 2013. An evolving new paradigm: endothelial cells—conditional innate immune cells. J. Hematol. Oncol. 6: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding M., Kubes P. 2012. Innate immunity in the vasculature: interactions with pathogenic bacteria. Curr. Opin. Microbiol. 15: 85–91. [DOI] [PubMed] [Google Scholar]
- 4.van Hinsbergh V. W. M. 2012. Endothelium—role in regulation of coagulation and inflammation. Semin. Immunopathol. 34: 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H., Anand A. R., Ganju R. K. 2014. Slit2-Robo4 pathway modulates lipopolysaccharide-induced endothelial inflammation and its expression is dysregulated during endotoxemia. J. Immunol. 192: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London N. R., Zhu W., Bozza F. A., Smith M. C., Greif D. M., Sorensen L. K., Chen L., Kaminoh Y., Chan A. C., Passi S. F., Day C. W., et al. 2010. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci. Transl. Med. 2: 23ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones C. A., London N. R., Chen H., Park K. W., Sauvaget D., Stockton R. A., Wythe J. D., Suh W., Larrieu-Lahargue F., Mukouyama Y.-S., et al. 2008. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat. Med. 14: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nymo S., Niyonzima N., Espevik T., Mollnes T. E. 2014. Cholesterol crystal-induced endothelial cell activation is complement-dependent and mediated by TNF. Immunobiology 219: 786–792. [DOI] [PubMed] [Google Scholar]
- 9.Kumar H., Kawai T., Akira S. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388: 621–625. [DOI] [PubMed] [Google Scholar]
- 10.Di Gioia M., Zanoni I. 2015. Toll-like receptor co-receptors as master regulators of the immune response. Mol. Immunol. 63: 143–152. [DOI] [PubMed] [Google Scholar]
- 11.Kolev M., Le Friec G., Kemper C. 2014. Complement—tapping into new sites and effector systems. Nat. Rev. Immunol. 14: 811–820. [DOI] [PubMed] [Google Scholar]
- 12.Holers V. M. 2014. Complement and its receptors: new insights into human disease. Annu. Rev. Immunol. 32: 433–459. [DOI] [PubMed] [Google Scholar]
- 13.Mollnes T. E., Song W.-C., Lambris J. D. 2002. Complement in inflammatory tissue damage and disease. Trends Immunol. 23: 61–64. [DOI] [PubMed] [Google Scholar]
- 14.Skjeflo E. W., Christiansen D., Espevik T., Nielsen E. W., Mollnes T. E. 2014. Combined inhibition of complement and CD14 efficiently attenuated the inflammatory response induced by Staphylococcus aureus in a human whole blood model. J. Immunol. 192: 2857–2864. [DOI] [PubMed] [Google Scholar]
- 15.Lappegård K. T., Christiansen D., Pharo A., Thorgersen E. B., Hellerud B. C., Lindstad J., Nielsen E. W., Bergseth G., Fadnes D., Abrahamsen T. G., et al. 2009. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc. Natl. Acad. Sci. USA 106: 15861–15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brekke O.-L., Christiansen D., Fure H., Pharo A., Fung M., Riesenfeld J., Mollnes T. E. 2008. Combined inhibition of complement and CD14 abolish E. coli-induced cytokine-, chemokine- and growth factor-synthesis in human whole blood. Mol. Immunol. 45: 3804–3813. [DOI] [PubMed] [Google Scholar]
- 17.Huber-Lang M., Barratt-Due A., Pischke S. E., Sandanger Ø., Nilsson P. H., Nunn M. A., Denk S., Gaus W., Espevik T., Mollnes T. E. 2014. Double blockade of CD14 and complement C5 abolishes the cytokine storm and improves morbidity and survival in polymicrobial sepsis in mice. J. Immunol. 192: 5324–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau C., Gunnarsen K. S., Høydahl L. S., Andersen J. T., Berntzen G., Pharo A., Lindstad J. K., Ludviksen J. K., Brekke O.-L., Barratt-Due A., et al. 2013. Chimeric anti-CD14 IGG2/4 hybrid antibodies for therapeutic intervention in pig and human models of inflammation. J. Immunol. 191: 4769–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finch A. M., Wong A. K., Paczkowski N. J., Wadi S. K., Craik D. J., Fairlie D. P., Taylor S. M. 1999. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 42: 1965–1974. [DOI] [PubMed] [Google Scholar]
- 20.Mollnes T. E., Brekke O.-L., Fung M., Fure H., Christiansen D., Bergseth G., Videm V., Lappegård K. T., Köhl J., Lambris J. D. 2002. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 100: 1869–1877. [PubMed] [Google Scholar]
- 21.Gräbner R., Till U., Heller R. 2000. Flow cytometric determination of E-selectin, vascular cell adhesion molecule-1, and intercellular cell adhesion molecule-1 in formaldehyde-fixed endothelial cell monolayers. Cytometry 40: 238–244. [DOI] [PubMed] [Google Scholar]
- 22.Haskard D. O. 2008. Endothelial Activation in Inflammation: Lessons Learned from E-Selectin. In Vascular Complications in Human Disease: Mechanisms and Consequences. Abraham D., Dashwood M., Handler C., Coghlan G., eds. Springer, London, p. 77–93. [Google Scholar]
- 23.Bexborn F., Engberg A. E., Sandholm K., Mollnes T. E., Hong J., Nilsson Ekdahl K. 2009. Hirudin versus heparin for use in whole blood in vitro biocompatibility models. J. Biomed. Mater. Res. A 89: 951–959. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell J., Mille-Baker B., Laffan M. 2000. Human umbilical vein endothelial cells differ from other endothelial cells in failing to express ABO blood group antigens. J. Vasc. Res. 37: 540–547. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones K. L., Kelly M. M., Kubes P. 2008. Varying importance of soluble and membrane CD14 in endothelial detection of lipopolysaccharide. J. Immunol. 181: 1446–1453. [DOI] [PubMed] [Google Scholar]
- 26.Andonegui G., Zhou H., Bullard D., Kelly M. M., Mullaly S. C., McDonald B., Long E. M., Robbins S. M., Kubes P. 2009. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J. Clin. Invest. 119: 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nooteboom A., van der Linden C. J., Hendriks T. 2004. Modulation of adhesion molecule expression on endothelial cells after induction by lipopolysaccharide-stimulated whole blood. Scand. J. Immunol. 59: 440–448. [DOI] [PubMed] [Google Scholar]
- 28.Schildberger A., Rossmanith E., Weber V., Falkenhagen D. 2010. Monitoring of endothelial cell activation in experimental sepsis with a two-step cell culture model. Innate Immun. 16: 278–287. [DOI] [PubMed] [Google Scholar]
- 29.Lu Z., Li Y., Jin J., Zhang X., Lopes-Virella M. F., Huang Y. 2012. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arterioscler. Thromb. Vasc. Biol. 32: 1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorgersen E. B., Hellerud B. C., Nielsen E. W., Barratt-Due A., Fure H., Lindstad J. K., Pharo A., Fosse E., Tønnessen T. I., Johansen H. T., et al. 2010. CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs. FASEB J. 24: 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellerud B. C., Aase A., Herstad T. K., Naess L. M., Kristiansen L. H., Trøseid A.-M. S., Harboe M., Lappegård K. T., Brandtzaeg P., Høiby E. A., Mollnes T. E. 2010. Critical roles of complement and antibodies in host defense mechanisms against Neisseria meningitidis as revealed by human complement genetic deficiencies. Infect. Immun. 78: 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorgersen E. B., Pharo A., Haverson K., Axelsen A. K., Gaustad P., Kotwal G. J., Sfyroera G., Mollnes T. E. 2009. Inhibition of complement and CD14 attenuates the Escherichia coli-induced inflammatory response in porcine whole blood. Infect. Immun. 77: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brekke O.-L., Christiansen D., Fure H., Fung M., Mollnes T. E. 2007. The role of complement C3 opsonization, C5a receptor, and CD14 in E. coli-induced up-regulation of granulocyte and monocyte CD11b/CD18 (CR3), phagocytosis, and oxidative burst in human whole blood. J. Leukoc. Biol. 81: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 34.Sprong T., Møller A.-S. W., Bjerre A., Wedege E., Kierulf P., van der Meer J. W. M., Brandtzaeg P., van Deuren M., Mollnes T. E. 2004. Complement activation and complement-dependent inflammation by Neisseria meningitidis are independent of lipopolysaccharide. Infect. Immun. 72: 3344–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egge K. H., Barratt-Due A., Nymo S., Lindstad J. K., Pharo A., Lau C., Espevik T., Thorgersen E. B., Mollnes T. E. 2015. The anti-inflammatory effect of combined complement and CD14 inhibition is preserved during escalating bacterial load. Clin. Exp. Immunol. 181: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugin J., Ulevitch R. J., Tobias P. S. 1995. Tumor necrosis factor-α and interleukin-1β mediate human endothelial cell activation in blood at low endotoxin concentrations. J. Inflamm. 45: 49–55. [PubMed] [Google Scholar]
- 37.Nooteboom A., Bleichrodt R. P., Hendriks T. 2006. Modulation of endothelial monolayer permeability induced by plasma obtained from lipopolysaccharide-stimulated whole blood. Clin. Exp. Immunol. 144: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nooteboom A., van der Linden C. J., Hendriks T. 2005. Whole blood-mediated endothelial permeability and adhesion molecule expression: a model study into the effects of bacteria and antibiotics. J. Antimicrob. Chemother. 55: 150–156. [DOI] [PubMed] [Google Scholar]
- 39.Schildberger A., Buchacher T., Weber V., Falkenhagen D. 2011. Adsorptive modulation of inflammatory mediators dampens endothelial cell activation. Blood Purif. 32: 286–295. [DOI] [PubMed] [Google Scholar]
- 40.Foreman K. E., Glovsky M. M., Warner R. L., Horvath S. J., Ward P. A. 1996. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation 20: 1–9. [DOI] [PubMed] [Google Scholar]
- 41.Kilgore K. S., Shen J. P., Miller B. F., Ward P. A., Warren J. S. 1995. Enhancement by the complement membrane attack complex of tumor necrosis factor-α-induced endothelial cell expression of E-selectin and ICAM-1. J. Immunol. 155: 1434–1441. [PubMed] [Google Scholar]
- 42.Foreman K. E., Vaporciyan A. A., Bonish B. K., Jones M. L., Johnson K. J., Glovsky M. M., Eddy S. M., Ward P. A. 1994. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Invest. 94: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harboe M., Ulvund G., Vien L., Fung M., Mollnes T. E. 2004. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 138: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.