ABSTRACT
Tumor Necrosis Factor α (TNF) is a pleiotropic cytokine exhibiting a dual activity in oncoimmunology, either acting as a cytotoxic effector produced by leukocytes or behaving as an immunosuppressive molecule. We have just discovered that TNF signaling impairs the accumulation of tumor-infiltrating CD8+ T lymphocytes in experimental melanoma.
KEYWORDS: Apoptosis, high endothelial venules, melanoma, TNF, T lymphocytes, tumor-infiltrating CD8+ lymphocytes
TNF has been initially shown to be part of the cytotoxic effectors of the immune cells such as natural killers and CD8+ T lymphocytes. More recently, TNF has emerged as a putative immune suppressor cytokine, facilitating the accumulation and/or biological activity of regulatory T lymphocytes (Tregs)1 as well as myeloid-derived suppressor cells (MDSC).2 In addition, TNF triggered melanoma dedifferentiation, promoting melanoma relapse in an adoptive CD8+ T cell transfer protocol.3
We have recently demonstrated that TNF signaling facilitates the tumor growth of mouse melanoma exhibiting a high-expression level of major histocompatibility class I (MHC-Ihigh), while having no or minimal effects toward mouse melanoma expressing MHC-I at low levels (MHC-Ilow). As a matter of fact, we provided evidence that the tumor growth of MHC-Ihigh, but not that of MHC-Ilow melanoma, was impaired in TNF-deficient mice. Moreover, the administration of Etanercept, a soluble form of human TNF-R2, which efficiently neutralizes mouse TNF, significantly reduced the MHC-Ihigh melanoma growth.4
In terms of molecular mechanisms, we showed that TNF receptor 1 (TNF-R1), rather than TNF-R2, was required to transduce the pro-tumorigenic effect of TNF in melanoma. Indeed, the MHC-Ihigh melanoma growth was impaired in mice lacking TNF-R1, but not TNF-R2. The TNF-R1-dependent TNF signaling inhibited the accumulation of tumor-infiltrating CD8+ T lymphocytes (CD8+ TILs), including specific CD8+ T cells. The latter phenomenon likely accounted for the pro-tumorigenic role of TNF since immunodepletion of CD8+ T cells fully restored the MHC-Ihigh melanoma growth in TNF-deficient mice. Conversely, the antitumor effect of Etanercept was observed only in immunocompetent mice, TNF blockade having no effect in mice lacking IFNγ or CD8 as well as in nude mice.4
We demonstrated that TNF exerts a direct negative effect toward CD8+ T cells by inducing cell death of activated CD8+ T lymphocytes (Fig. 1). Although this finding is in agreement with a previous study,5 we provided genetic evidence that TNF-R1, rather than TNF-R2, is the key receptor that triggered activated CD8+ T apoptosis in response to TNF. To definitely demonstrate that TNF-R1 signaling directly impaired CD8+ TIL homeostasis, we performed an adoptive transfer protocol by injecting activated TNF-R1-deficient or proficient CD8+ T cells in melanoma tumors developed in CD8+-deficient mice. Three days later, the proportion of live CD8+ TILs was enhanced by TNF-R1 deficiency.4
Figure 1.
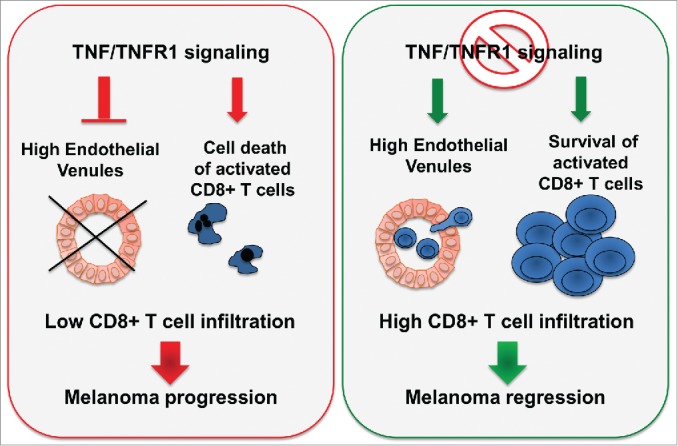
TNF impairs CD8+ TIL accumulation in melanoma. TNF-R1-dependent TNF signaling (i) limits the intra-tumor content of HEV, which are involved in lymphocyte extravasation, and (ii) induces activated CD8+ T cell death, thus facilitating melanoma escape from the immune system.
Because the pro-tumorigenic role of TNF was unlikely restricted to a direct effect on CD8+ T cells, we also analyzed the tumor microenvironment in TNF-deficient mice, focusing on tumor vasculature and immunosuppressor cells. Under our experimental conditions and in sharp contrast with recent studies highlighting the critical function of TNF-R2 in the accumulation of immunosuppressor cells,1,2 TNF deficiency or blockade was not associated with a reduction of the tumor content of both Tregs and MDSC. Moreover, MDSC immunodepletion by injecting an anti-Gr1 antibody did not result in an increased CD8+ TIL content in wild-type and TNF-deficient mice (Bertrand, Colacios and Ségui, unpublished data). Thus, the effect of TNF on the limitation of CD8+ T cell-dependent immune response was unlikely a consequence of the alteration of MDSC homeostasis and biological activity, at least under our experimental conditions.
The tumor vasculature was not drastically altered by TNF deficiency as evaluated by the quantification of CD31 staining (Bertrand and Ségui, unpublished data). However, the abundance of high endothelial venules (HEV), which are critical blood vessels for lymphocyte extravasation into lymph nodes and tumors,6,7 was increased in melanoma tumors from mice lacking TNF or TNF-R1. Of note, these vessels were surrounded by a large number of CD8+ TILs. Similar data were obtained in wild-type mice injected with Etanercept. Although it remains unclear how TNF signaling impairs HEV in melanoma, their increased frequency likely contributed to the accumulation of CD8+ TILs upon TNF blockade (Fig. 1).
Anti-TNF antibodies and Etanercept have been tested in patients affected with diverse advanced cancers, with no or limited clinical responses. To the best of our knowledge, only one melanoma patient was reported as a good responder to anti-TNF for several weeks.8 Our preclinical findings may be of great interest in the clinic since we have identified high MHC-I expression by melanoma cells as a critical point to condition the antitumor effects triggered by the TNF blockade strategy. In addition, we document that TNF blockade was efficient only in immunocompetent animals. MHC-I expression by melanoma may serve as a biomarker, and its assessment in tumor biopsies may help select patients for anti-TNF therapies in melanoma and possibly in other cancer types. In addition, a pre-existing infiltration of CD8+ TILs in tumor biopsies, that may reflect the immunogenicity of the tumor cells, is most likely a prerequisite to observe a clinical benefit of TNF blockade strategy. Clinical trials are obviously necessary to evaluate this tenet and to determine eligibility criteria for such a treatment. Because monotherapy based on anti-TNF may have limited effects, a combination of TNF blockade with emerging therapies (such as anti-CTLA4 or BRAFV600E inhibitors), which are associated with TNF production and tumor lymphocyte infiltration,9,10 may lead to a positive clinical outcome by enhancing the accumulation of CD8+ TILs in melanoma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by RITC (Recherche et Innovation Thérapeutique en Cancérologie), Cancéropôle Grand Sud-Ouest, INSERM and Paul Sabatier University (Toulouse III).
References
- 1.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol 2007; 179:154-61; PMID:17579033; http://dx.doi.org/ 10.4049/jimmunol.179.1.154 [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P et al.. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest 2012; 122:4094-104;PMID:23064360; http://dx.doi.org/ 10.1172/JCI64115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M et al.. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012; 490:412-6; PMID:23051752; http://dx.doi.org/ 10.1038/nature11538 [DOI] [PubMed] [Google Scholar]
- 4.Bertrand F, Rochotte J, Colacios C, Montfort A, Tilkin-Mariame AF, Touriol C, Rochaix P, Lajoie-Mazenc I, Andrieu-Abadie N, Levade T et al.. Blocking tumor necrosis factor α enhances CD8+ T cell-dependent immunity in experimental melanoma. Cancer Res 2015; 75(13):2619-28; PMID:25977337; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2524 [DOI] [PubMed] [Google Scholar]
- 5.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 1995; 377:348-51; PMID:7566090; http://dx.doi.org/ 10.1038/377348a0 [DOI] [PubMed] [Google Scholar]
- 6.Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard JP. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012; 1:829-39; PMID:23162750; http://dx.doi.org/ 10.4161/onci.20492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinet L, Garrido I, Girard JP. Tumor high endothelial venules (HEVs) predict lymphocyte infiltration and favorable prognosis in breast cancer. Oncoimmunology 2012; 1:789-90; PMID:22934284; http://dx.doi.org/ 10.4161/onci.19787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown ER, Charles KA, Hoare SA, Rye RL, Jodrell DI, Aird RE, Vora R, Prabhakar U, Nakada M, Corringham RE et al.. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-α inhibitor, in patients with advanced cancer. Ann Oncol 2008; 19:1340-6; PMID:18325912; http://dx.doi.org/ 10.1093/annonc/mdn054 [DOI] [PubMed] [Google Scholar]
- 9.Turcotte S, Gros A, Hogan K, Tran E, Hinrichs CS, Wunderlich JR, Dudley ME, Rosenberg SA. Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy. J Immunol 2013; 191:2217-25; PMID:23904171; http://dx.doi.org/ 10.4049/jimmunol.1300538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong DS, Vence L, Falchook G, Radvanyi LG, Liu C, Goodman V, Legos JJ, Blackman S, Scarmadio A, Kurzrock R et al.. BRAF(V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clin Cancer Res 2012; 18:2326-35; PMID:22355009; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-2515 [DOI] [PubMed] [Google Scholar]


