Abstract
Antibody-dependent cell-mediated cytotoxicity (ADCC) mediated through the IgG Fc receptor FcγRIIIa represents a major effector function of many therapeutic antibodies. In an attempt to further enhance natural killer (NK) cell-mediated ADCC, we combined therapeutic antibodies against CD20 and CD38 with recombinant immunoligands against the stimulatory NK cell receptors NKG2D or NKp30. These immunoligands, respectively designated as ULBP2:7D8 and B7-H6:7D8, contained the CD20 scFv 7D8 as a targeting moiety and a cognate ligand for either NKG2D or NKp30 (i.e. ULBP2 and B7-H6, respectively). Both the immunoligands synergistically augmented ADCC in combination with the CD20 antibody rituximab and the CD38 antibody daratumumab. Combinations with ULBP2:7D8 resulted in higher cytotoxicity compared to combinations with B7-H6:7D8, suggesting that coligation of FcγRIIIa with NKG2D triggered NK cells more efficiently than with NKp30. Addition of B7-H6:7D8 to ULBP2:7D8 and rituximab in a triple combination did not further increase the extent of tumor cell lysis. Importantly, immunoligand-mediated enhancement of ADCC was also observed for tumor cells and autologous NK cells from patients with hematologic malignancies, in which, again, ULBP2:7D8 was particularly active. In summary, co-targeting of NKG2D was more effective in promoting rituximab or daratumumab-mediated ADCC by NK cells than co-ligation of NKp30. The observed increase in the ADCC activity of these therapeutic antibodies suggests promise for a ‘dual-dual-targeting’ approach in which tumor cell surface antigens are targeted in concert with two distinct activating NK cell receptors (i.e. FcγRIIIa and NKG2D or B7-H6).
Keywords: ADCC, antibody, CD20, NK cells, NKp30, NKG2D
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- B7-H6
B7 homolog 6
- CI
combination index
- CLL
chronic lymphocytic leukemia
- DAP10
DNAX-activating protein of 10 kDa
- DLBCL
diffuse large B cell lymphoma
- FL
follicular lymphoma
- DRI
dose reduction index
- KIR
killer cell immunoglobulin-like receptor
- MCL
mantle cell lymphoma
- MRD
minimal residual disease
- NK
natural killer
- NKG2D
natural killer group 2 member D
- ULBP2
UL-16 binding protein 2.
Introduction
The introduction of antibodies into standard treatment regimens has improved the outcome of lymphoma and leukemia patients significantly.1,2 However, despite the success, certain subgroups of patients and particularly those with advanced disease stages are often refractory to immuno-chemotherapy. Thus, improving antibody therapy remains a major effort in translational research.
Various mechanisms of action are discussed to contribute to the efficacy of therapeutic antibodies in patients, including induction of apoptosis, complement-dependent cytotoxicity, ADCC, phagocytosis and T cell-based immune responses.2 The relative contribution of individual mechanisms is not fully understood and may vary for different antibodies and tumor entities. However, results from several animal models suggested that recruitment of effector cells by engagement of FcγR expressed by various effector cells and induction of cell-mediated cytotoxicity are important antibody functions in vivo.3-6 The importance of Fc receptor engagement by therapeutic antibodies is also evidenced by clinical observations. Patients homozygously expressing the FcRγIIIa allelic variant 158 V with high-affinity for the IgG Fc domain have higher response rates and show increased overall survival than individuals expressing the low affinity F allele.7-10 Although the precise role of different effector cell populations including NK cells, monocytes, macrophages or granulocytes in antibody therapy is still unclear, a contribution of NK cells, which constitute the major FcγRIIIa-positive effector cell population in the peripheral blood, has been suggested.11-13 Hence, improving the performance of antibodies by increasing their ability to recruit NK cells and their potency to induce ADCC is regarded as an attractive approach to advance antibody therapy for cancer patients.
Along this line various strategies were followed to enhance effector cell-mediated antibody functions. For example, a gain in ADCC capacity was achieved by engineering the antibody Fc domain to increase its affinity to activating FcγR including FcγRIIIa on NK cells.14,15 Alternatively, ADCC was enhanced by combination strategies, in which therapeutic antibodies were combined with a variety of drugs, cytokines or immunomodulatory antibodies, which either provided additional activating stimuli or blocked inhibitory molecules.16 Employing immunomodulatory antibodies in particular appeared attractive to improve ADCC by NK cells, whose cytotoxic functions are regulated by a complex interplay between sets of stimulatory and inhibitory cell surface receptors that may constitute potential targets for immune intervention.17-20 For example, NK cell-meditated ADCC was enhanced by agonistic antibodies triggering the co-stimulatory CD137 (4–1BB) molecule or by blocking antibodies masking inhibitory killer cell immunoglobulin-like receptors (KIR).21-24
Previous observations suggested that recombinant immunoligands can be employed to modulate ADCC.25-28 Recombinant immunoligands are equipped with bispecific binding abilities and combine a tumor-directed antibody fragment with a ligand of an activating or co-stimulatory surface receptor expressed by effector cells. Hence, in an attempt to attract NK cells to lymphomas, a single chain fragment variable (scFv) of the CD20 antibody 7D8 was fused to UL16-binding protein (ULBP) 2 or B7 homolog 6 (B7-H6),29-32 which are ligands of the activating NK cell receptors natural killer group 2 member D (NKG2D) and NKp30, respectively.33,34 By binding to lymphoma cells the immunoligands designated as ULBP2:7D8 and B7-H6:7D8 mimicked an induced self phenotype and thereby triggered NK cells to kill lymphoma and leukemia cells.29,30 Moreover, co-ligation of NKp30 and NKG2D enhanced NK cell cytotoxicity synergistically, and both molecules were able to increase ADCC by monoclonal antibodies. However, NKp30 and NKG2D have not been directly compared as potential targets for antibody combination approaches. Because NKG2D associates with DNAX-activating protein of 10 kDa (DAP10) and NKp30 pairs with FcεRIγ / CD3ζ adaptor chains like FcγRIIIa,18 the receptors employ different intracellular routes for signal transduction and thus may impact on ADCC differentially. Here, the capacity of ULBP2:7D8 and B7-H6:7D8 to boost NK cell-mediated ADCC was investigated in an attempt to design novel therapeutic co-stimulation approaches.
Results
Therapeutic antibodies, such as rituximab and daratumumab, effectively engage FcγRIIIa for NK cell-mediated tumor cell killing. We developed a co-stimulatory approach to further improve this important effector mechanism by the design of CD20-specific immunoligands ULBP2:7D8 and B7-H6:7D8. These immunoligands engage the activating NK cell receptors NKG2D and NKp30, respectively.
Because rituximab and the CD20 scFv 7D8 contained in the immunoligands recognize non-identical but cross-blocking epitopes, simultaneous binding to individual cells was analyzed. Despite competition, simultaneous binding of both rituximab and the immunoligands to individual lymphoma cells was detected (Fig. 1A), albeit at reduced levels as compared to single agents (Figs. 1B, C). Particularly this became obvious when rituximab was combined with B7-H6:7D8, which was employed at higher concentrations than ULBP2:7D8 to compensate for its previously observed lower cytotoxic capacity.29
Figure 1.
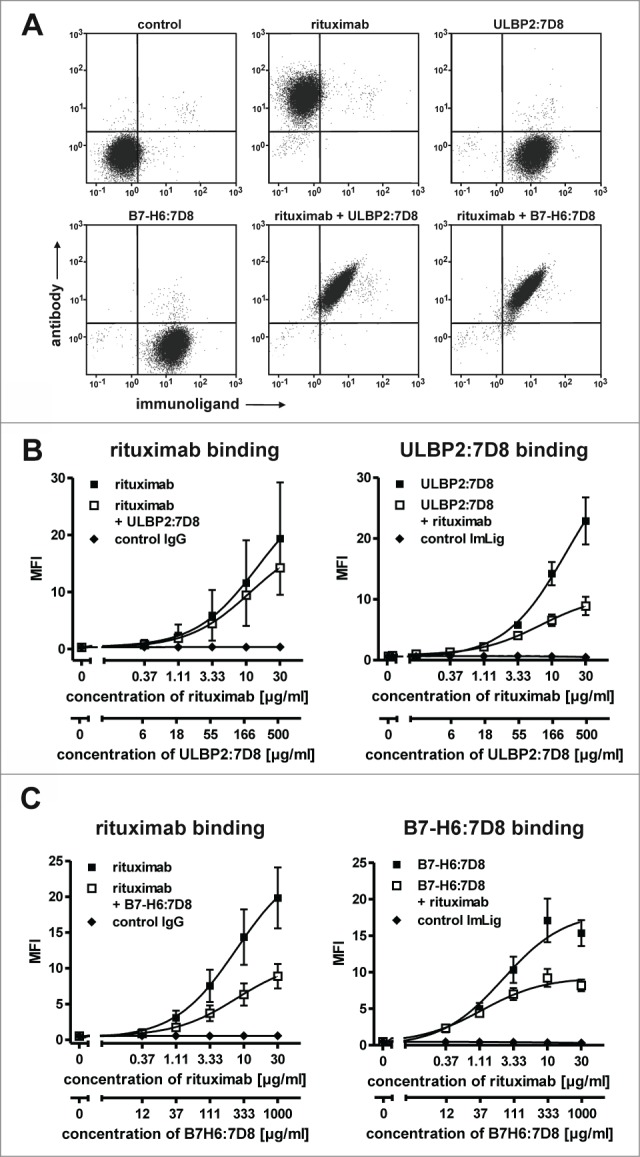
(A) Simultaneous binding of rituximab (200 nM) and the immunoligands ULBP2:7D8 (10 µM) and B7-H6:7D8 (25 µM) to Ramos cells was analyzed by 2-color flow cytometry. On the basis of pilot tests, the molecules were applied at distinct concentration ratios for compensation of different binding and cytotoxic activities (data not shown). Secondary antibodies conjugated to APC and FITC were used for detection of rituximab and the immunoligands, respectively. (B) Dose-dependent binding of rituximab (left panel) and ULBP2:7D8 (right panel) when either applied alone or in combination. As a control an isotype matched antibody was combined with the similar constructed immunoligand (ImLig) binding HER2 (MFI, mean fluorescence intensity). (C) Impact of B7-H6:7D8 on binding of rituximab (left) or vice versa (right). Data points indicate mean values ± SEM obtained in three independent experiments.
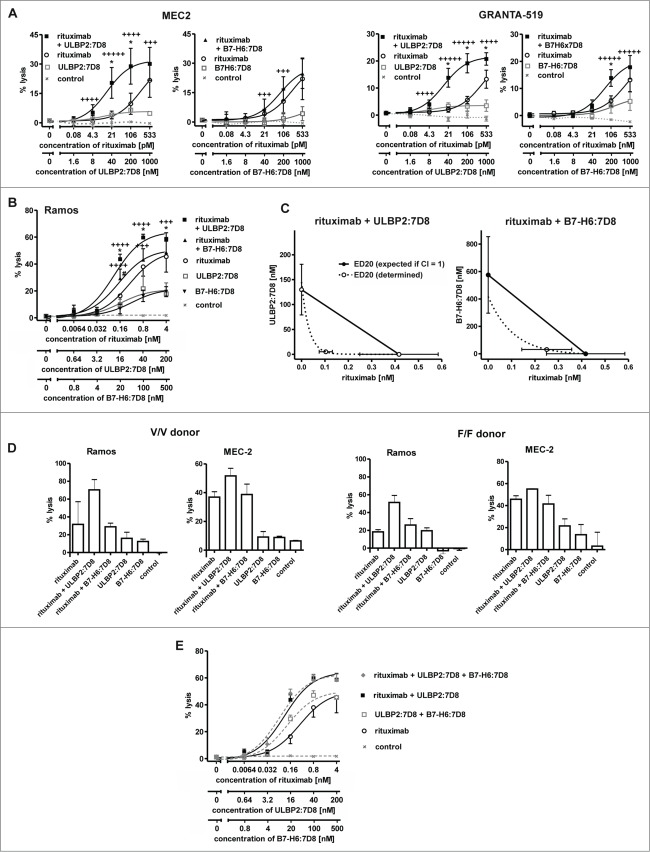
To compare the abilities of ULBP2:7D8 and B7-H6:7D8 to enhance ADCC, cytotoxic effects of combinations between rituximab and the immunoligands were determined by employing the chronic lymphocytic leukemia (CLL) line MEC2 and the mantle cell lymphoma (MCL) line GRANTA-519 as targets and allogeneic mononuclear cells (MNC) from healthy donors as effector cells (Fig. 2A). As a result, target cell lysis was significantly enhanced in the presence of either ULBP2:7D8 or B7-H6:7D8, indicating that the observed competition in binding was not detrimental for this effect (Fig. 1). Calculation of CI values revealed synergistic effects especially at low antibody concentrations (with more stars indicating greater synergy). Notably, stronger cytotoxic effects were observed when the antibody was combined with ULBP2:7D8 compared to B7-H6:7D8. Thus, ULBP2:7D8 enhanced ADCC efficiently even though it hardly mediated any detectable effects under these experimental conditions when applied as single agent. Synergy between rituximab and the immunoligands and an enhanced potency of ULBP2:7D8 to boost ADCC were also observed when purified NK cells were applied as effector cells (data not shown). In contrast to experiments with MNC, significant lysis of both MEC2 and GRANTA-519 cells was induced by ULBP2:7D8 and B7-H6:7D8 even when they were applied as single agents (data not shown). Similar results were obtained when Ramos Burkitt's lymphoma cells were analyzed as target cells (Fig. 2B). Again ULBP2:7D8 enhanced ADCC more efficiently than B7-H6:7D8. Synergy between the antibody and each immunoligand was indicated by calculated combination index (CI) and dose reduction index (DRI) values and was further demonstrated by isobologram analysis (Fig. 2C, Table 1). Whereas ULBP2:7D8 reliably boosted ADCC, B7-H6:7D8 was not effective with NK cells from some donors (data not shown). Overall ULBP2:7D8 was more efficacious than B7-H6:7D8 to boost ADCC. Of note, this was observed irrespective of the critical FcγRIIIa-V/F allotype at amino acid position 158 of the NK cells employed (Fig. 2D). Moreover, we determined the activation status of NK cells after incubation with lymphoma cells in the presence of rituximab and the immunoligands, either alone or in combination (Fig. S1). This was performed by analyzing the induced expression of the activation marker CD69 by flow cytometry. When rituximab was combined with ULBP2:7D8, CD69 expression was induced more efficiently and more NK cells were activated. Also B7-H6:7D8 enhanced NK cell activation in the presence of rituximab, but had a lower efficacy than ULBP2:7D8, in agreement with the results obtained in cytotoxicity experiments.
Figure 2.
For figure legend, see page 5.Figure 2. See previous page ULBP2:7D8 and B7-H6:7D8 boost rituximab-induced ADCC. (A) Cytotoxicity against MEC2 and GRANTA-519 cells induced by single agents and by two-drug combinations of rituximab with either ULBP2:7D8 or B7-H6:7D8. MNC were used as effector cells at an E:T cell ratio of 40:1. Data points represent mean values ± SEM from at least three different experiments. Trastuzumab was employed as a control. Synergistic effects were graded into very strong synergy (+++++, CI < 0.1) strong synergy (++++, CI = 0.1 − 0.3) and synergy (+++, CI = 0.3 – 0.7). Statistically significant differences between groups treated with the single agents or the combinations are indicated (*, p < 0.05). (B) NK cells were purified by MACS technology and analyzed as effector cells (E:T cell ratio: 10:1) for combinations between rituximab and ULBP2:7D8 or B7-H6:7D8. Ramos cells served as target cells. (++++, CI = 0.1 − 0.3; +++, CI = 0.3 – 0.7; *, p < 0.05). Please note that cytotoxic effects of rituximab and B7-H6:7D8 were previously published.29 (C) Isobologram analysis demonstrating synergy of immunoligands and rituximab in NK cell-mediated killing of Ramos cells. The doses of ULBP2:7D8 (left panel) and B7-H6:7D8 (right panel) resulting in 20% (ED20) lysis were plotted against equally effective doses of rituximab. The diagonal line connecting the ED20 values of the two agents (CI = 1) indicates theoretical combination doses which would be required to achieve equal effects, if additive effects were assumed (expected ED20 for CI = 1). Synergy between rituximab and the immunoligands is indicated by the experimentally determined combination doses locating below the corresponding additivity lines (antagonism would have been indicated by combination doses falling above the additivity lines). (D) Lysis of Ramos lymphoma and MEC2 CLL cells by NK cells homozygously expressing the FcγRIIIa V/V or F/F allotype at amino acid position 158 in the presence of rituximab and the immunoligands. Data points represent mean values ± SEM from triplicate determinations. (E) Cytotoxicity against Ramos cells induced by three-drug combinations of rituximab, ULBP2:7D8 and B7-H6:7D8 (Note: the data depicted in 2B and 2E were obtained in experiments performed in parallel, but were presented in different graphs for illustrative reasons).
Table 1.
Computer simulated CI and DRI values for combinations of rituximab, ULBP2:7D8 and B7-H6:7D8a
| CI values at lysis of |
DRI values at lysis of |
||||||
|---|---|---|---|---|---|---|---|
| Combination | ratio | 10% | 20% | 40% | 10% | 20% | 40% |
| rituximab + ULBP2:7D8 | 1:50 | 0.4 | 0.3 | 0.2 | 3.3 | 3.6 | 4.1 |
| 14.1 | 22.4 | 39.4 | |||||
| rituximab + B7-H6:7D8 | 1:125 | 0.5 | 0.5 | 0.6 | 2.1 | 2.0 | 1.9 |
| 13.3 | 22.6 | 43.0 | |||||
| ULBP2:7D8 + B7-H6:7D8 | 2.5:1 | 0.3 | 0.2 | 0.1 | 5.1 | 8.7 | 16.6 |
| 7.4 | 15.9 | 40.4 | |||||
| rituximab + ULBP2:7D8+B7-H6:7D8 | 1:50:125 | 0.6 | 0.4 | 0.3 | 2.2 | 3.1 | 4.6 |
| 9.4 | 19.0 | 44.4 | |||||
| 13.5 | 34.6 | 107.7 | |||||
Combination index (CI) and dose reduction index (DRI) were calculated from dose response curves using Ramos cells and purified NK cells for three different effect levels by computer simulation using CalcuSyn software. Upper, middle and lower DRI-values correspond to rituximab, ULBP2:7D8 and B7-H6:7D8, respectively.
Because expression of endogenous ligands for NKG2D and NKp30 may affect sensitivity of cells to both natural cytotoxicity and ADCC, the expression levels of known NKG2D and NKp30 cell surface ligands were determined by flow cytometry (Fig. S2). As a result, expression of some of the multiple NKG2D ligands was observed by MEC2 and GRANTA-519 cells, whereas Ramos cells hardly displayed any NKG2D ligands. B7-H6 was expressed by all three cell lines with GRANTA-519 cells exhibiting highest endogenous expression levels. However, exogenous coating of lymphoma cells with either ULBP2:7D8 or B7-H6:7D8 resulted in dramatic increases in the absolute amounts of cell-associated ULBP2 and B7-H6, respectively (Fig. S2).
On the basis of previous observations indicating that ULBP2:7D8 and B7-H6:7D8 induce NK cell cytotoxicity synergistically,29 the cytotoxic effects induced by triple combinations consisting of rituximab, ULBP2:7D8 and B7-H6:7D8 were analyzed. Thus, the molecules were applied either in three-drug or in two-drug combinations employing Ramos and purified NK cells (Fig. 2E). However, the three-drug combination consisting of B7-H6:7D8, ULBP2:7D8 and rituximab did not show any additional cytotoxic effects in comparison to the combination of rituximab and ULBP2:7D8.
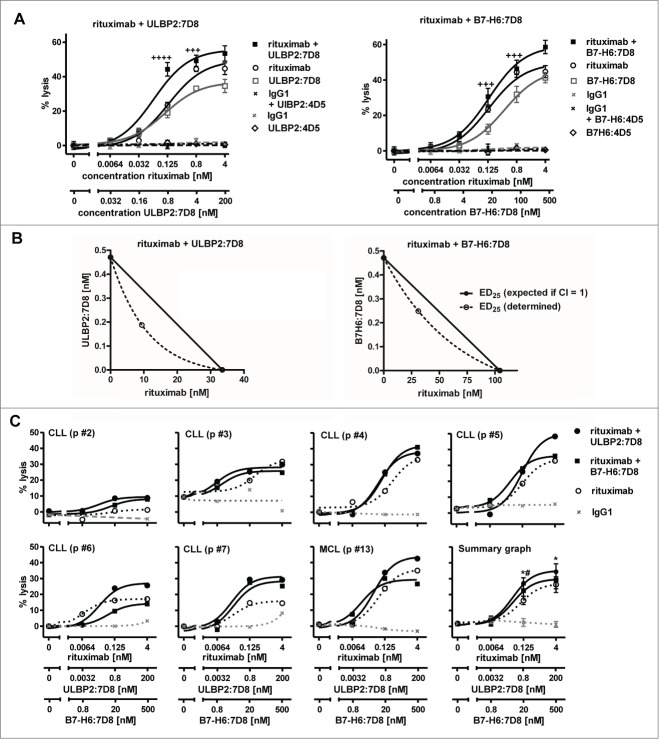
Furthermore, we determined whether the immunoligands were able to enhance ADCC against freshly isolated tumor cells (Fig. 3; Table S1), which similarly to cell lines had varying expression levels of diverse NKG2D ligands and B7-H6 (Fig. S3).Tumor cells were enriched from peripheral blood of CLL and MCL patients and analyzed as targets for allogeneic NK cells from healthy donors. Throughout all experiments rituximab-mediated ADCC was synergistically enhanced by ULBP2:7D8 (Fig. 3). Improved lysis was also achieved by B7-H6:7D8 in the majority of experiments, but in agreement with results obtained with cell lines, ULBP2:7D8 had a higher potency for increasing ADCC. In some cases, B7-H6:7D8 was unable to boost rituximab-dependent ADCC or even had inhibitory effects (e.g. CLL p #6, Fig. 3C).
Figure 3.
Enhanced killing of patients' tumor cells by combinations of rituximab and the immunoligands. (A) Both ULBP2:7D8 (left panel) and B7-H6:7D8 (right panel) increased rituximab-mediated ADCC against freshly isolated CLL cells (CLL p #1; ++++, CI = 0.1 − 0.3; +++, CI = 0.3 – 0.7). (B) Illustration of synergistic effects at the 25% effect level by isobolograms. (C) Cytotoxicity induced by varying concentrations of rituximab alone or in combination with either ULBP2:7D8 or B7-H6:7D8 against a panel of tumor cell samples. NK cells from healthy donors were applied as effector cells (E:T cell ratio = 20:1). Data are presented as mean percentage of lysis from triplicate determinations. Data acquired with different tumor samples were analyzed in a summary graph for statistical analysis. (* and # indicate statistical significant differences between rituximab as single agent or combined with either ULBP2:7D8 or B7-H6:7D8, respectively. p, patient).
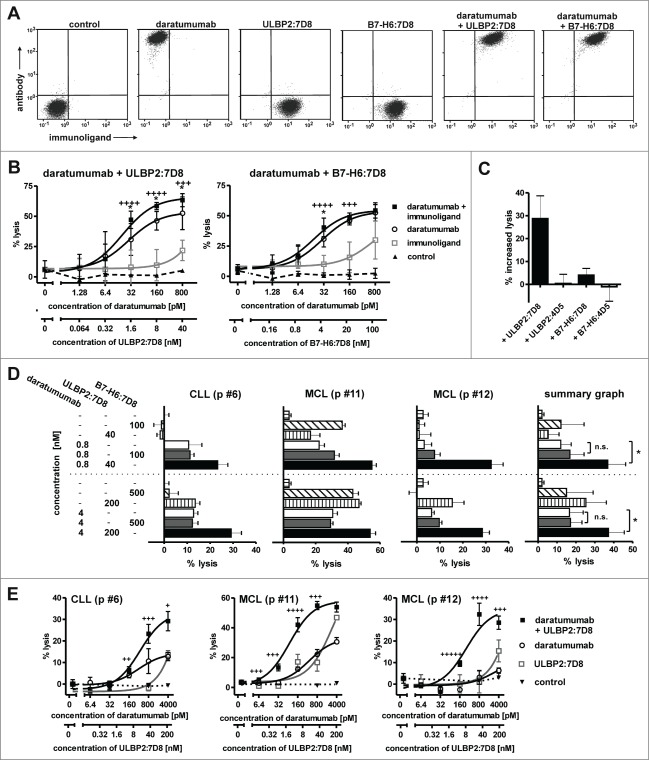
The combination of an antibody and an immunoligand that have a distinct tumor target specificity may allow more efficient engagement of NK cell trigger molecules, a concept we propose as ‘dual-dual-targeting’. To address this and to analyze a different target antigen, the immunoligands were combined with the CD38 antibody daratumumab. As expected, binding of ULBP2:7D8 and B7-H6:7D8 did not interfere with antibody binding due to recognition of different target antigens (Fig. 4A). Using Ramos cells, which co-express considerable amounts of both CD20 and CD38 (data not shown), ADCC by the IgG1 antibody was significantly enhanced by the immunoligands with ULBP2:7D8 again exhibiting a higher potential than B7-H6:7D8 (Fig. 4B). Importantly, ADCC was unaffected when daratumumab was combined with the control immunoligands ULBP2:4D5 and B7-H6:4D5 targeting HER2, suggesting that ULBP2:7D8 and B7-H6:7D8 required interaction with the target antigen to produce the additional stimulatory effects (Fig. 4C). Enhanced ADCC was also observed when freshly isolated CLL or MCL cells from patients were applied as target cells (Figs. 4D, E). Thus, these results underlined the previous observations obtained in combinations with rituximab. Moreover, it was formally excluded, that differential sensitization of target cells for ADCC by ULBP2:7D8 and B7-H6:7D8 was a consequence of differential cross-blocking effects between the antibody and the two immunoligands.
Figure 4.
(See previous page) ULBP2:7D8 and B7-H6:7D8 enhance ADCC by the CD38 antibody daratumumab. (A) Simultaneous binding of daratumumab (200 nM) and the immunoligands ULBP2:7D8 (10 µM) and B7-H6:7D8 (25 µM) to CD20 and CD38 double-positive Ramos cells was analyzed by flow cytometry. Secondary antibodies conjugated to APC and FITC were used for detection of daratumumab and the immunoligands, respectively. (B) Synergistic induction of cell lysis by combinations of daratumumab and ULBP2:7D8 (left graph) or daratumumab and B7-H6:7D8 (right graph). NK cells were effector cells and Ramos cells were analyzed as target cells (E:T ratio = 10: 1). A non-relevant IgG1 antibody served as control. *, Statistically significant differences of antibody combinations to the single agents (p < 0.05); ++++, strong synergism (CI ranges of 0.1 – 0.3), +++, synergism (CI ranges of 0.3 – 0.7). (C) To illustrate antigen-specific enhancement of ADCC, the percentages of increased lysis achieved by combining daratumumab (100 pM) with ULBP2:7D8 (8 nM) or B7-H6:7D8 (20 nM) were compared with results obtained by combinations of the antibody with equimolar concentrations of the control molecules B7-H6:4D5 and ULBP2:4D5. (D) Killing of MCL or CLL cells from patients (p) by daratumumab, ULBP2:7D8 and B7-H6:7D8 alone or in varying combinations. Data points represent mean values ± SEM of triplicate determinations. NK cells were effector cells at an E:T ratio of 20:1. For statistical analysis, patient samples were analyzed in a summary graph (*, p < 0.05). Please note that improved cytotoxic effects of the combination of daratumumab with ULBP2:7D8 were previously published 29. (E) Lysis of patient-derived tumor cells by combination of daratumumab and ULBP2:7D8 at varying concentrations. NK cells were used as effector cells. ++++, strong synergism (CI: 0.1 – 0.3), +++, synergism (CI: 0.3 – 0.7), ++, moderate synergism (CI: 0.7 – 0.85); + slight synergism (CI: 0.85 – 0.9).
NK cells from tumor patients were reported to have reduced effector functions and ADCC capacity.35 To investigate whether the immunoligands were capable to improve ADCC mediated by patients' NK cells, NK cells from lymphoma or leukemia patients were isolated from MNC. We included untreated patients (e.g. CLL p#1) as well as patients under rituximab maintenance therapy (e.g. DLBC p#14, FL p#17), who had no detectable malignant B cells in the peripheral blood at the time-point of NK cell isolation. NKG2D and NKp30 surface expression by these patient-derived NK cells was verified for some individual patient samples (Fig. 5A). The patient-derived NK cells were then analyzed as effector cells for combinations of rituximab and the immunoligands employing allogeneic Ramos and MEC2 target cells (Fig. 5B). Even though high antibody concentrations were applied, ULBP2:7D8 enhanced ADCC by NK cells derived from patients, whereas additional cytotoxic effects by B7H6:7D8 were not consistently observed. Thus, in agreement with results obtained with NK cells from healthy individuals, the combination of rituximab and ULBP2:7D8 triggered cytotoxicity more efficiently than the combination of rituximab and B7-H6:7D8.
Figure 5.
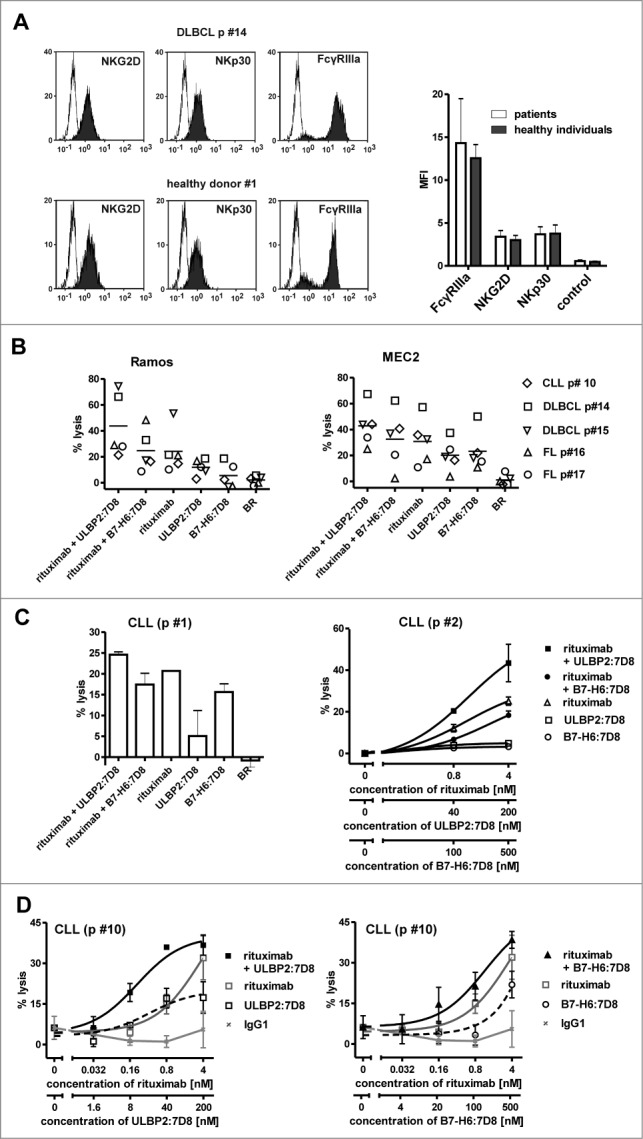
Tumor cell killing by patient-derived NK cells. (A) MNC from either healthy individuals or patients were prepared, stained with antibodies specific for CD56 (PC-7 conjugated), CD19 (Pacific Blue-conjugated), CD3 (FITC-conjugated) and either NKG2D or NKp30 (each PE-labeled), and analyzed by flow cytometry. CD56-positive, CD3- and CD19-negative NK cells were gated and the expression levels of the respective receptors were determined. Left panel: representative histogram analysis of the expression of the indicated receptors by MNC from a patient (DLBCL p# 14) or from a healthy individual (white peaks: control; gray peaks: receptor). Right panel: summary graph illustrating mean fluorescence intensity (MFI) for NKG2D, NKp30 and the control reaction obtained in different experiments with cells from different individuals. Data points represent mean values ± SEM (n = 4). (B) Patient-derived NK cells were employed as effector cells for combinations of rituximab (4 nM) and either ULBP2:7D8 (200 nM) or B7-H6:7D8 (500 nM) using allogeneic Ramos and MEC2 tumor cells as targets. The E:T ratio was 10:1. Data points represent mean values of triplicate determinations. The horizontal line indicates mean percentage of lysis (p, patient; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma). (C) NK cells from two CLL patients were isolated and applied as effector cells against autologous tumor cells (E:T ratio: 20:1). The concentration of rituximab was 4 nM, ULBP2:7D8 and B7-H6:7D8 were used at 200 and 500 nM, respectively, unless otherwise indicated. Data points represent mean values ± SEM of triplicate determinations. (D) After CLL p #10 was transplanted with allogeneic haematopoietic progenitor cells, NK cells from this patient were enriched and employed as effector cells against the same patient´s CLL target cells (E:T ratio: 20:1). Data represent mean values of triplicate wells ± SEM [++++, strong synergism (CI: 0.1–0.3), +++, synergism (CI: 0.3–0.7), ++, moderate synergism (CI: 0.7–0.85)].
Moreover, we were interested whether enhancement of ADCC was also achievable when autologous tumor cells were used as targets. These may inhibit NK cell cytotoxicity by the expression of HLA class I-molecules which are recognized by autologous NK cells through cognate KIR. From two patients, both NK cells and tumor cells were isolated and analyzed in cytotoxicity experiments (Fig. 5C). As a result, ULBP2:7D8 enhanced rituximab-mediated lysis of autologous tumor cells, whereas no benefits were observed with B7-H6:7D8 in these experiments. Since therapeutic antibodies may be promising for the eradication of minimal residual disease (MRD) cells in a post-transplantation setting when high E:T ratios are available, NK cells from a CLL patient who had received transplantation with HLA-matched allogeneic haematopoietic progenitor cells were isolated and tested as effector cells (Fig. 5D). In cytotoxicity reactions using these patient-derived donor NK cells, both ULBP2:7D8 and B7-H6:7D8 exerted biologic activity and enhanced ADCC by rituximab. This demonstrates that ULBP2:7D8 and, with certain restrictions, also B7-H6:7D8, are capable of enhancing ADCC by patient-derived but donor NK cells against tumor cells of the patient in vitro.
Discussion
Enhancing the potential of monoclonal antibodies to trigger ADCC is an attractive strategy to improve their efficacy.16 Here, we analyzed the ability of the two immunoligands ULBP2:7D8 and B7-H6:7D8 to boost ADCC by NK cells. Both ULBP2:7D8 and B7-H6:7D8 promoted ADCC by the therapeutic antibodies rituximab and daratumumab synergistically, indicating that NK cell cytotoxicity was enhanced by simultaneous engagement of FcγRIIIa and either NKG2D or NKp30. However, synergistic effects were more pronounced when the antibodies were combined with ULBP2:7D8, which exerted a reliable activity with NK cells from a broad range of different healthy individuals and tumor patients and which also was able to enhance maximum extent of ADCC.
ADCC by therapeutic antibodies was enhanced by various approaches including strategies which relied on modifying the antibody itself or on combining the antibody with other drugs or biologics tuning the NK cell activation threshold. Although antibody Fc engineering strategies are potent,15 it has been revealed recently that Fc engineering may impact different effector mechanisms differentially. For instance, the ADCC activity of Fc engineered antibodies with enhanced affinity to FcγRIII was improved with NK cells but diminished with granulocytes.36,37 Such potential limitations may be circumvented by combination strategies. Thus, employing native IgG1 antibodies and achieving improvements in ADCC by combining the antibodies with immunomodulatory drugs or biologics that do not interfere with other antibody effector functions may be advantageous in certain situations. In combination strategies NK cell-based ADCC was augmented by toll-like receptor agonists, immunomodulatory drugs, cytokines and antibodies.16,38,39 Antibody-based approaches that enhanced NK cell-mediated ADCC include anti-KIR antibodies blocking interaction with MHC class I molecules as well as agonistic CD137 antibodies.21,23 However, these antibodies are not directed to the tumor, whereas recombinant immunoligands such as ULBP2:7D8 and B7-H6:7D8 carry a tumor targeting moiety and are anticipated to trigger NK cell functions locally restricted to the tumor site.
NKG2D as well as NKp30 are constitutively displayed by virtually all resting human NK cells, in contrast to other activating NK cell receptors such as CD137 or NKp44, whose expression is induced upon activation. Moreover, both NKG2D and NKp30 function as primary stimulatory molecules, which induce NK cell cytotoxicity upon engagement. Thus, ULBP2:7D8 as well as B7-H6:7D8 triggered cytotoxicity of NK cells as single agents without co-stimulation. However, ULBP2:7D8 was more effective than B7-H6:7D8 in boosting ADCC. Although co-engagement of NKp30 enhanced ADCC in the vast majority of experiments, in some cases ADCC by rituximab was not enhanced or even diminished in the presence of B7-H6:7D8 (Fig. 3, e.g. p# 6). It was assumed that this may reflect different characteristics of the target cells or properties of NK cells from individual donors. For example, CD20 expression levels by tumor cells may determine the coating efficiency or influence the role of cross-blocking effects. Moreover, a potentially varying expression of endogenous ligands of NKp30, NKG2D or other stimulatory surface receptors such as NKp46 or DNAM-1 by the tumor cells may have contributed to the observed effects. Also the relative expression of different NKp30 splice variants by the applied NK cells may influence the cytotoxic potential of B7-H6:7D8.40 Interestingly, addition of B7-H6:7D8 to combinations of ULBP2:7D8 and rituximab did not further enhance NK cell cytotoxicity significantly. Thus, in this situation, NKG2D and FcγRIIIa signaling may have overruled the additional signal provoked by NKp30 engagement. The observed stronger co-stimulatory effect mediated by ULBP2:7D8 was also evident in combination with daratumumab, a CD38 antibody not competing with the recombinant immunoligands for antigen binding. Therefore, the more pronounced cross-blocking of rituximab binding was excluded as a major reason for the weaker co-stimulatory activity of B7-H6:7D8.
Since ULBP2:7D8 and B7-H6:7D8 were applied at concentrations at which they produced similar cytotoxic effects as single agents in the mean, the observed differences in target cell killing by the combinations are presumably not due to different cytotoxic potentials of the individual agents. Rather the differential effects may reflect peculiar characteristics of the receptors. One explanation for the observed results may be that NKG2D and NKp30 differ in their intracellular signaling pathways. NKp30 similarly to FcγRIIIa signals via FcεRIγ and CD3ζ polypeptides containing ITAM motifs for signal transduction.17 In contrast, NKG2D pairs with DAP10 and signals ITAM-independent.33 The concomitant activation of both FcεRIγ / CD3ζ chains and DAP10 triggered signaling may explain why ULBP2:7D8 had a higher potency to enhance ADCC than B7-H6:7D8. The strong synergy between ULBP2:7D8 and B7-H6:7D8 may be for the same reason. Previously, FcγRIIIa signaling was shown to be differentially promoted by other NK cell receptors.19 Whereas engagement of ITAM-independent receptors such as NKG2D enhanced FcγRIIIa–mediated intracellular Ca2+ mobilization, degranulation and cytotoxicity, engagement of the ITAM-dependent NKp46, which signals in a similar manner as NKp30, did not. These findings suggested that two ITAM-based receptors synergize weakly and may underline the importance of concomitant activation of different intracellular signaling routes, in agreement with our results. However, differences between ULBP2:7D8 and B7-H6:7D8 could also be due to the lytic immunological synapse formed more efficiently by engagement of NKG2D rather than NKp30. Co-ligation of activating receptors stabilizes the immunological synapse, as it has been demonstrated for NKG2D and the FcγRIIIa.41 Thus, it is conceivable that ligation of different activating NK cell receptors may differentially impact the stability of the immunological synapse.
Combining two IgG1 antibodies to co-target two different surface antigens on tumor cells may be advantageous. For example, dual targeting may promote antibody-effector functions, because more binding sites are available permitting more IgG1 molecules to attach to a single cell, or hinder tumor cells to escape by down-modulating just a single target antigen. However, as suggested by previous studies, improvements of ADCC were difficult to achieve by combinations of two tumor targeting IgG1 antibodies.30,42,43 Here we demonstrated that by employing the CD38 antibody daratumumab and the CD20-specific immunoligand ULBP2:7D8, two different antigens can be efficiently targeted and result in significantly and synergistically increased tumor cell killing. On the basis of these results we propose the ‘dual-dual-targeting’ concept.
In conclusion, the two recombinant immunoligands ULBP2:7D8 and B7-H6:7D8 enhanced NK cell-mediated ADCC induced by therapeutic antibodies in vitro using cell lines and patient-derived tumor cells. In particular ULBP2:7D8 boosted ADCC against a broad range of different target cells and had a higher efficacy as co-stimulatory molecule than B7-H6:7D8. Thus, combining therapeutic antibodies with antibody-derivatives engaging NKG2D may represent a promising approach to further increase the efficacy of antibody therapy. This approach may be especially promising for the eradication of MRD cells in a post-transplantation setting when high E:T ratios are achieved.
Materials and Methods
Cell culture
Ramos cells (DSMZ, The German Resource Centre for Biological Material) were cultured in RPMI 1,640 Glutamax-I medium (Invitrogen) supplemented with 10% fetal calf serum (FCS; Invitrogen), 100 units/mL penicillin and 100 µg/mL streptomycin (Invitrogen). MEC2 cells (DSMZ) were maintained in Iscove's MDM medium containing 20% FCS, 100 U/mL penicillin and 100 µg/mL streptomycin. GRANTA-519 (DSMZ) and Lenti-X 293T cells (Takara Bio Europe / Clontech) were kept in DMEM supplemented with 10% FCS, 100 U/mL penicillin and 100 µg/mL streptomycin.
Preparation of MNC and isolation of NK cells
Experiments were approved by the Ethics Committee of the Christian-Albrechts-University of Kiel (Kiel, Germany), in accordance with the Declaration of Helsinki. Blood was drawn after receiving the donors´ written informed consents. Preparation of MNC from peripheral blood of patients and healthy donors was performed as described previously.36 NK cells from healthy individuals were isolated by MACS technology following the manufacturer's protocols by negative selection using NK cell isolation kit (Miltenyi). NK cells from patients were enriched by positive selection employing CD56-beads (Miltenyi). Purified NK cells were directly employed in functional assays, or cultured over-night at a density of 2 × 106 cells/mL in RPMI 1,640 Glutamax-I medium supplemented with 10% FCS, 100 U/mL penicillin and 100 µg/mL streptomycin.
Antibodies and immunoligands
The construction of derivatives of the pSecTag2 HygroC vector for expression of B7-H6:7D8 and ULBP2:7D8 have been described previously.29,30 Expression vectors encoding the control immunoligands (i.e. B7-H6:4D5 and ULBP2:4D5, respectively) were generated by replacing the coding sequences for scFv 7D8 by those encoding scFv 4D5 derived from the humanized HER2-specific antibody 4D5–8.44 The immunoligands were transiently expressed in Lenti-X 293T cells by calcium-phosphate transfection (Invitrogen) and purified by affinity chromatography with nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen) as described earlier.29 Concentrations of purified proteins were estimated against a standard curve of BSA or determined by quantitative capillary electrophoresis using Experion™ Pro260 technology (BioRad) in accordance with the manufacturer's protocol. Rituximab and trastuzumab (Roche Pharma AG), which was used as control, were purchased. The CD38 antibody daratumumab was kindly provided by Genmab B.V.45
Flow cytometry
Flow cytometry was performed on Navios flow cytometer (Beckman Coulter) as described previously.29 The hexa-histidine-tagged recombinant immunoligands were detected with a secondary Alexa Fluor 488-coupled anti-penta His antibody (Qiagen). Human or chimeric antibodies were detected with allophycocyanin-labeled mouse anti-human Ig kappa light chain antibodies (BD Biosciences). Isolated tumor or NK cells were characterized by flow cytometry using FITC- or Pacific Blue-conjugated CD20 or CD19 antibodies, FITC or Krome Orange-conjugated CD3 antibodies, PC7-coupled CD56 antibodies, PE-conjugated CD16, anti-NKG2D and anti-NKp30 antibodies (Beckman Coulter) according to the manufacture´s recommendations. Expression of NKG2D and NKp30 ligands was analyzed with following antibodies: PE-conjugated anti-ULBP1 (clone 170818; R&D Systems), PE-conjugated anti-ULBP-2/5/6 (clone 165903; R&D Systems), PE-conjugated anti-ULBP3 (clone 166510; R&D Systems), PE-conjugated anti-ULBP4 (clone 709116; R&D Systems), PE-conjugated anti-MICA/B (clone 6D4; BD biosciences) and allophycocyanin-conjugated anti B7-H6 (clone 875001, R&D Systems). As controls, recommended corresponding isotype antibodies were used. Dead cells were excluded from analysis by staining with 7-AAD (BD biosciences).
Analysis of NK cell cytotoxicity
Cytotoxicity was analyzed in standard 3 h 51Cr release assays performed in 96-well microtiter plates in a total volume of 200 µL as described previously.46 Human NK cells or MNC were used as effector cells at the indicated E:T ratios.
Analysis of NK cell activation
Two hundred thousand NK cells were seeded together with equal numbers of Ramos cells in microtiter plates in a volume of 200 µL. Rituximab, B7-H6:7D8, ULBP2:7D8, trastuzumab or PBS were added. After 4 h cells were stained with antibodies against CD69 (PE-conjugated), CD56 (PC-7), CD19 (Pacific Blue) and CD3 (FITC) and analyzed by flow cytometry. CD56-positive, CD3- and CD19-negative NK cells were gated and the expression levels of CD69 were determined.
Data processing and statistical analyses
Graphical and statistical analyses were performed with GraphPad Prism 5.0 software. Potential outliers of triplicate determinations were detected by Grubbs' test. P-values were calculated using repeated measures ANOVA and the Bonferroni post-test. The null hypothesis was rejected for p < 0.05. DRI and CI values were calculated with CalcuSyn software (Biosoft) according to published procedures using the formula CIx = DA/DxA + DB/DxB, where DxA and DxB indicate doses of drug A and drug B alone producing x% effect, and DA and DB indicate doses of drugs A and B in combination producing equal effects.47 Per definition, synergistic effects at distinct effect levels were graded into very strong synergy (+++++, CI < 0.1) strong synergy (++++, CI = 0.1 − 0.3), synergy (+++, CI = 0.3 − 0.7), moderate synergy (CI = 0.7 – 0.85) and slight synergy (CI = 0.85 – 0.95).
Disclosure of Potential Conflicts of Interest
J.G.J.v.d.W. and P.W.H.I.P. are employees of Genmab, a biotechnology company that develops therapeutic mAbs, and own Genmab warrants and/or stock. They are named as inventors on Genmab-owned CD20 and CD38 Ab patents that have been licensed to GlaxoSmithKline and Janssen Biotech, respectively. The other authors have no financial conflicts of interest.
Acknowledgments
The authors thank Anja Muskulus, Britta von Below and Heidi Bosse for expert technical assistance.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This study was supported by research grants 110951 (to MP) from the Deutsche Krebshilfe e.V., Bonn, Germany, 2007.065.2 (to MP) from the Wilhelm Sander Stiftung, Neustadt, Germany, a research grant from the Werner und Lara Kreitz Stiftung (Bad Segeberg, Germany; to CK) and intramural funding from the Christian-Albrechts-University Kiel (to CK and MP).
References
- 1.Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol 2008; 26:1774-1777; PMID:18398141; http://dx.doi.org/ 10.1200/JCO.2007.15.7438 [DOI] [PubMed] [Google Scholar]
- 2.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-287; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 3.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 2000; 6:443-446; PMID:10742152; http://dx.doi.org/ 10.1038/74704 [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005; 310:1510-1512; PMID:16322460; http://dx.doi.org/ 10.1126/science.1118948 [DOI] [PubMed] [Google Scholar]
- 5.de Haij S, Jansen JH, Boross P, Beurskens FJ, Bakema JE, Bos DL, Martens A, Verbeek JS, Parren PWHI, van de Winkel JGJ et al.. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer Res 2010; 70:3209-3217; PMID:20354182; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-4109 [DOI] [PubMed] [Google Scholar]
- 6.Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res 2011; 71:5134-5143; PMID:21697279; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4222 [DOI] [PubMed] [Google Scholar]
- 7.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003; 21:3940-3947; PMID:12975461; http://dx.doi.org/ 10.1200/JCO.2003.05.013 [DOI] [PubMed] [Google Scholar]
- 8.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G et al.. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26:1789-1796; PMID:18347005; http://dx.doi.org/ 10.1200/JCO.2007.14.8957 [DOI] [PubMed] [Google Scholar]
- 9.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-758; PMID:11806974; http://dx.doi.org/ 10.1182/blood.V99.3.754 [DOI] [PubMed] [Google Scholar]
- 10.Persky DO, Dornan D, Goldman BH, Braziel RM, Fisher RI, Leblanc M, Maloney DG, Press OW, Miller TP, Rimsza LM. Fc gamma receptor 3a genotype predicts overall survival in follicular lymphoma patients treated on SWOG trials with combined monoclonal antibody plus chemotherapy but not chemotherapy alone. Haematologica 2012; 97:937-942; PMID:22271896; http://dx.doi.org/ 10.3324/haematol.2011.050419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ et al.. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood 2006; 108:2648-2654; PMID:16825493; http://dx.doi.org/ 10.1182/blood-2006-04-020057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol 2013; 4:76; PMID:23543707; http://dx.doi.org/ 10.3389/fimmu.2013.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, Modak S, Heller G, Dupont B, Cheung NK et al.. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest 2012; 122:3260-3270; PMID:22863621; http://dx.doi.org/ 10.1172/JCI62749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desjarlais JR, Lazar GA, Zhukovsky EA, Chu SY. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer's perspective. Drug Discov Today 2007; 12:898-910; PMID:17993407; http://dx.doi.org/ 10.1016/j.drudis.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 15.Kellner C, Derer S, Valerius T, Peipp M. Boosting ADCC and CDC activity by Fc engineering and evaluation of antibody effector functions. Methods 2014; 65:105-113; PMID:23851282; http://dx.doi.org/ 10.1016/j.ymeth.2013.06.036 [DOI] [PubMed] [Google Scholar]
- 16.Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, Levy R, Brody J. Combination strategies to enhance antitumor ADCC. Immunotherapy 2012; 4:511-527; PMID:22642334; http://dx.doi.org/ 10.2217/imt.12.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-49; PMID:21212348; http://dx.doi.org/ 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science 2004; 306:1517-1519; PMID:15567854; http://dx.doi.org/ 10.1126/science.1103478 [DOI] [PubMed] [Google Scholar]
- 19.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006; 107:159-166; PMID:16150947; http://dx.doi.org/ 10.1182/blood-2005-04-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 2009; 114:2657-2666; PMID:19628705; http://dx.doi.org/ 10.1182/blood-2009-01-201632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Muller A, Pachynski R, Czerwinski D, Coutre S et al.. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011; 117:2423-2432; PMID:21193697; http://dx.doi.org/ 10.1182/blood-2010-08-301945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW et al.. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 2012; 122:1066-1075; PMID:22326955; http://dx.doi.org/ 10.1172/JCI61226 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol 2008; 180:6392-6401; PMID:18424763; http://dx.doi.org/ 10.4049/jimmunol.180.9.6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A et al.. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014; 123:678-686; PMID:24326534; http://dx.doi.org/ 10.1182/blood-2013-08-519199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Strandmann EP, Hansen HP, Reiners KS, Schnell R, Borchmann P, Merkert S, Simhadri VR, Draube A, Reiser M, Purr I et al.. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood 2006; 107:1955-1962; PMID:16210338; http://dx.doi.org/ 10.1182/blood-2005-05-2177 [DOI] [PubMed] [Google Scholar]
- 26.Kellner C, Gramatzki M, Peipp M. Promoting natural killer cell functions by recombinant immunoligands mimicking an induced self phenotype. Oncoimmunology 2013; 2: e24481; PMID:23894708; http://dx.doi.org/ 10.4161/onci.24481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamova S, Cartellieri M, Feldmann A, Bippes CC, Bartsch H, Wehner R, Schmitz M, von Bonin M, Bornhauser M, Ehninger G et al.. Simultaneous engagement of the activatory receptors NKG2D and CD3 for retargeting of effector cells to CD33-positive malignant cells. Leukemia 2011; 25:1053-1056; PMID:21415850; http://dx.doi.org/ 10.1038/leu.2011.42 [DOI] [PubMed] [Google Scholar]
- 28.Vyas M, Koehl U, Hallek M, Strandmann EP. Natural ligands and antibody-based fusion proteins: harnessing the immune system against cancer. Trends Mol Med 2014; 20:72-82; PMID:24268686; http://dx.doi.org/ 10.1016/j.molmed.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 29.Kellner C, Maurer T, Hallack D, Repp R, van de Winkel JGJ, Parren PWHI, Valerius T, Humpe A, Gramatzki M, Peipp M. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J Immunol 2012; 189:5037-5046; PMID:23066150; http://dx.doi.org/ 10.4049/jimmunol.1201321 [DOI] [PubMed] [Google Scholar]
- 30.Kellner C, Hallack D, Glorius P, Staudinger M, Mohseni Nodehi S, de Weers M, van de Winkel JGJ, Parren PWHI, Stauch M, Valerius T et al.. Fusion proteins between ligands for NKG2D and CD20-directed single-chain variable fragments sensitize lymphoma cells for natural killer cell-mediated lysis and enhance antibody-dependent cellular cytotoxicity. Leukemia 2012; 26:830-834; PMID:22005785; http://dx.doi.org/ 10.1038/leu.2011.288 [DOI] [PubMed] [Google Scholar]
- 31.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C et al.. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 2009; 206:1495-1503; PMID:19528259; http://dx.doi.org/ 10.1084/jem.20090681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001; 14:123-133; PMID:11239445; http://dx.doi.org/ 10.1016/S1074-7613(01)00095-4 [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 1999; 285:730-732; PMID:10426994; http://dx.doi.org/ 10.1126/science.285.5428.730 [DOI] [PubMed] [Google Scholar]
- 34.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R et al.. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med 1999; 190:1505-1516; PMID:10562324; http://dx.doi.org/ 10.1084/jem.190.10.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farnault L, Sanchez C, Baier C, Le Treut T, Costello RT. Hematological malignancies escape from NK cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol 2012; 2012:421702; PMID:22899948; http://dx.doi.org/ 10.1155/2012/421702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG et al.. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 2008; 112:2390-2399; PMID:18566325; http://dx.doi.org/ 10.1182/blood-2008-03-144600 [DOI] [PubMed] [Google Scholar]
- 37.Derer S, Glorius P, Schlaeth M, Lohse S, Klausz K, Muchhal U, Desjarlais JR, Humpe A, Valerius T, Peipp M. Increasing FcgammaRIIa affinity of an FcgammaRIII-optimized anti-EGFR antibody restores neutrophil-mediated cytotoxicity. MAbs 2014; 6:409-421; PMID:24492248; http://dx.doi.org/ 10.4161/mabs.27457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu H, Yang Y, Gad E, Inatsuka C, Wenner CA, Disis ML, Standish LJ. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin Cancer Res 2011; 17:6742-6753; PMID:21918170; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Dietsch GN, Matthews MA, Yang Y, Ghanekar S, Inokuma M, Suni M, Maino VC, Henderson KE, Howbert JJ et al.. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res 2012; 18:499-509; PMID:22128302; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1625 [DOI] [PubMed] [Google Scholar]
- 40.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M et al.. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011; 17:700-707; PMID:21552268; http://dx.doi.org/ 10.1038/nm.2366 [DOI] [PubMed] [Google Scholar]
- 41.Deguine J, Breart B, Lemaitre F, Bousso P. Cutting edge: tumor-targeting antibodies enhance NKG2D-mediated NK cell cytotoxicity by stabilizing NK cell-tumor cell interactions. J Immunol 2012; 189:5493-5497; PMID:23183896; http://dx.doi.org/ 10.4049/jimmunol.1202065 [DOI] [PubMed] [Google Scholar]
- 42.Larbouret C, Gaborit N, Chardes T, Coelho M, Campigna E, Bascoul-Mollevi C, Mach JP, Azria D, Robert B, Pelegrin A. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors' down-regulation and dimers' disruption. Neoplasia 2012; 14:121-130; PMID:AMBIGUOUS; http://dx.doi.org/ 10.1593/neo.111602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klitgaard JL, Koefoed K, Geisler C, Gadeberg OV, Frank DA, Petersen J, Jurlander J, Pedersen MW. Combination of two anti-CD5 monoclonal antibodies synergistically induces complement-dependent cytotoxicity of chronic lymphocytic leukaemia cells. Br J Haematol 2013; 163:182-193; PMID:23927424; http://dx.doi.org/ 10.1111/bjh.12503 [DOI] [PubMed] [Google Scholar]
- 44.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992; 89:4285-4289; PMID:1350088; http://dx.doi.org/ 10.1073/pnas.89.10.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW et al.. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011; 186:1840-1848; PMID:21187443; http://dx.doi.org/ 10.4049/jimmunol.1003032 [DOI] [PubMed] [Google Scholar]
- 46.Repp R, Kellner C, Muskulus A, Staudinger M, Nodehi SM, Glorius P, Akramiene D, Dechant M, Fey GH, van Berkel PH et al.. Combined Fc-protein- and Fc-glyco-engineering of scFv-Fc fusion proteins synergistically enhances CD16a binding but does not further enhance NK-cell mediated ADCC. J Immunol Methods 2011; 373:67-78; PMID:21855548; http://dx.doi.org/ 10.1016/j.jim.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 47.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22:27-55; PMID:6382953; http://dx.doi.org/ 10.1016/0065-2571(84)90007-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.