Abstract
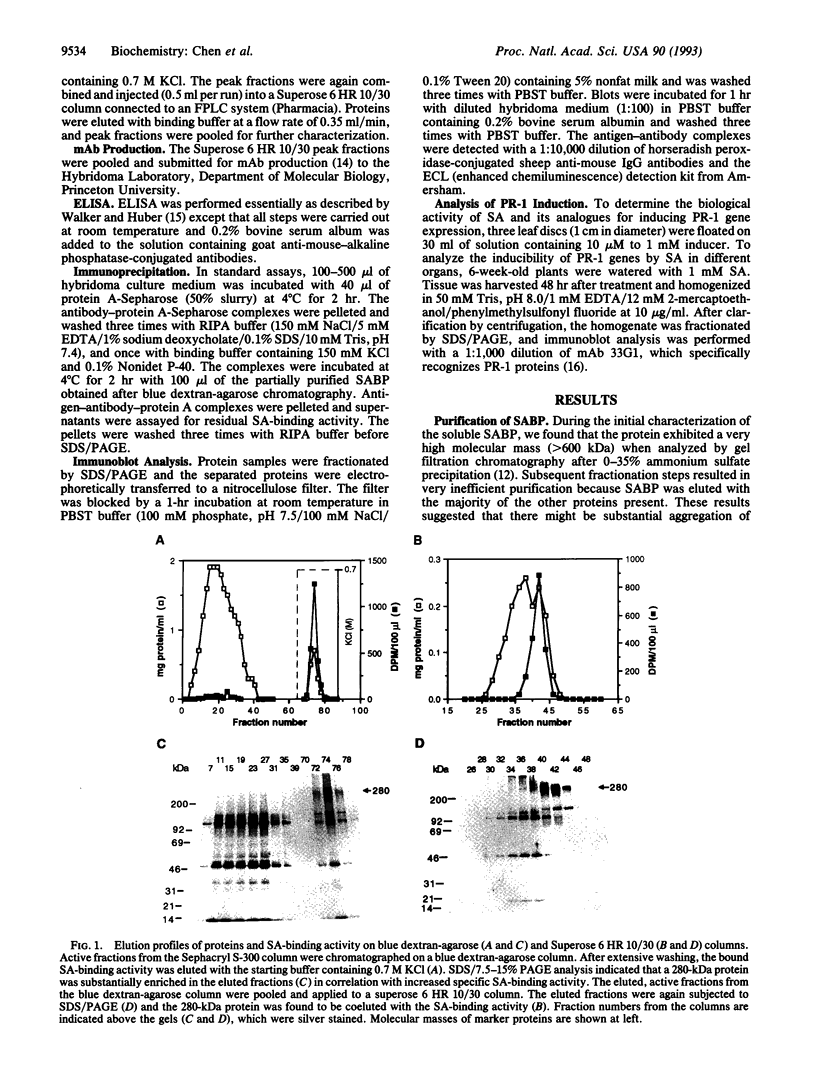
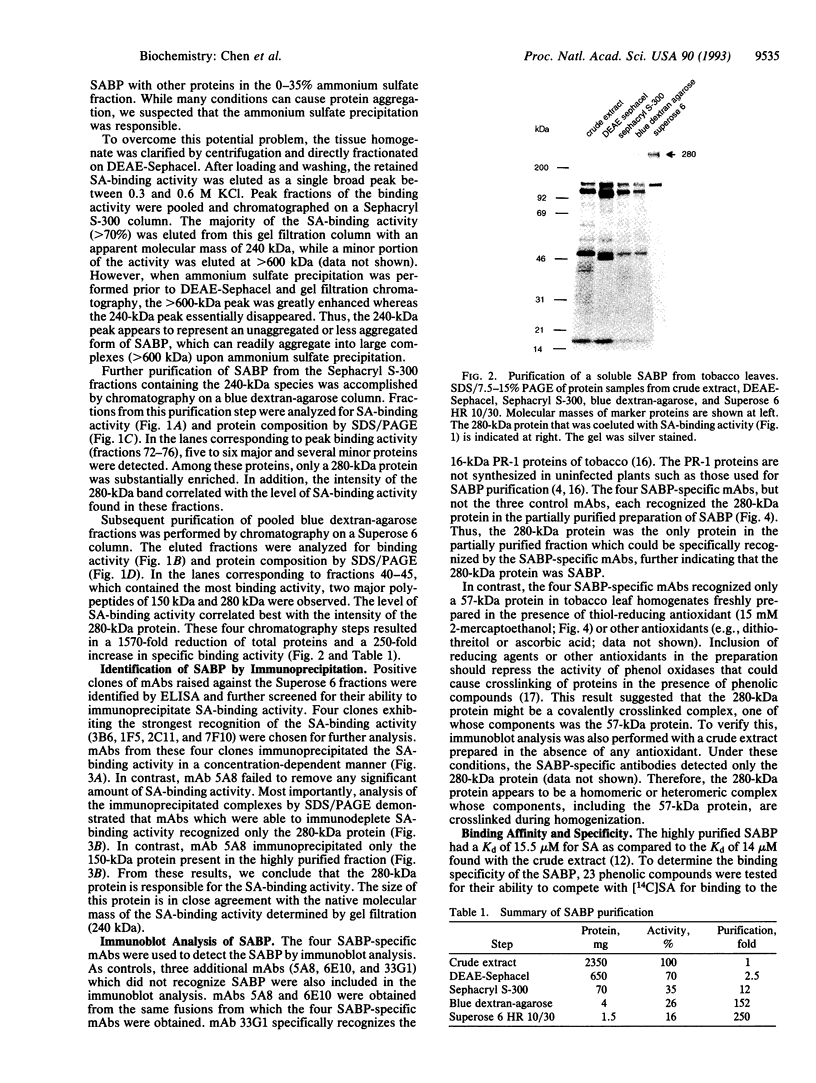
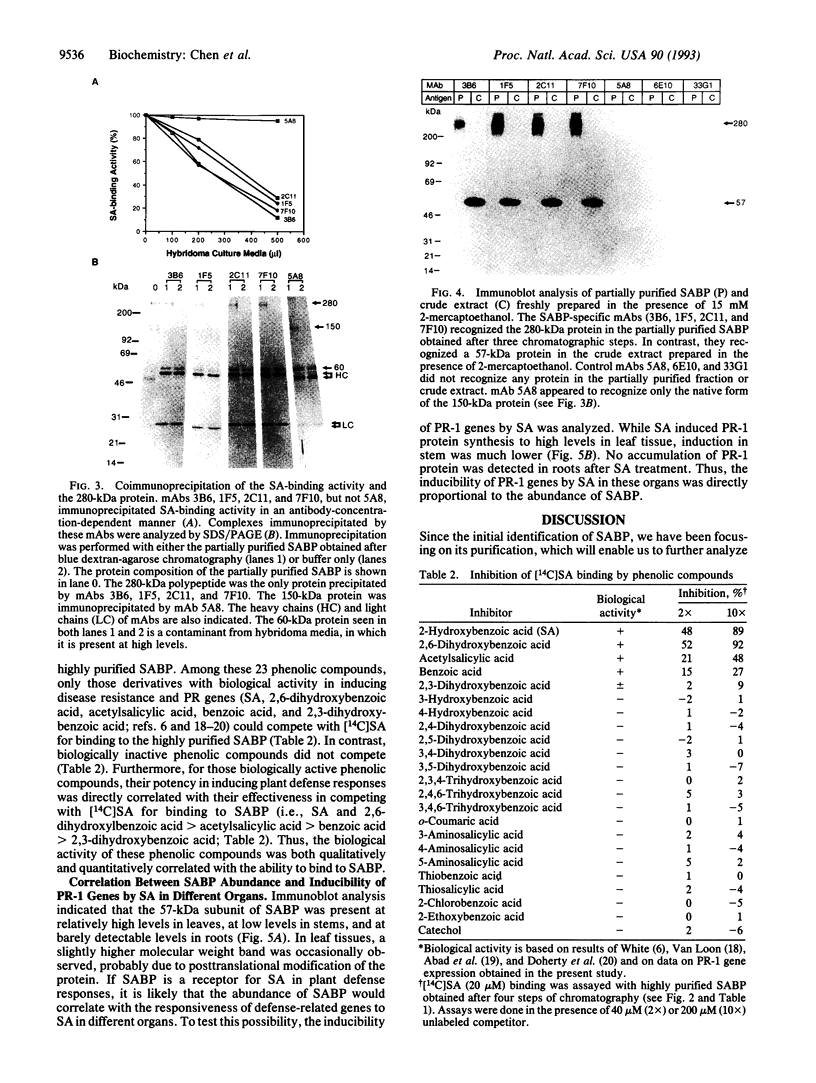
Previously, we identified a soluble salicylic acid (SA)-binding protein (SABP) in tobacco whose properties suggest that it may play a role in transmitting the SA signal during plant defense responses. This SA-binding activity has been purified 250-fold by conventional chromatography and was found to copurify with a 280-kDa protein. Monoclonal antibodies capable of immunoprecipitating the SA-binding activity also immunoprecipitated the 280-kDa protein, indicating that it was responsible for binding SA. These antibodies also recognized the 280-kDa protein in immunoblots of the partially purified SABP fraction or the crude extract. However, when the crude extract was prepared in the presence of antioxidants, only a 57-kDa protein was recognized. Since the SABP has a native molecular mass of 240 kDa, it appears that the SABP is a complex which contains a 57-kDa subunit and perhaps one or more additional proteins which are covalently crosslinked in the absence of antioxidants. The ability of a variety of phenolic compounds to compete with SA for binding to the SABP was both qualitatively and quantitatively correlated with their biological activity in inducing defense-related genes. Moreover, the inducibility of the pathogenesis-related (PR)-1 genes by SA was proportional to the abundance of the SABP in different organs. These correlations are consistent with a role for the SABP in perceiving and transducing the SA signal in plant defense.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad P., Marais A., Cardin L., Poupet A., Ponchet M. The effect of benzoic acid derivatives on Nicotiana tabacum growth in relation to PR-b1 production. Antiviral Res. 1988 Aug;9(5):315–327. doi: 10.1016/0166-3542(88)90026-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carr J. P., Dixon D. C., Nikolau B. J., Voelkerding K. V., Klessig D. F. Synthesis and localization of pathogenesis-related proteins in tobacco. Mol Cell Biol. 1987 Apr;7(4):1580–1583. doi: 10.1128/mcb.7.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Klessig D. F. Identification of a soluble salicylic acid-binding protein that may function in signal transduction in the plant disease-resistance response. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8179–8183. doi: 10.1073/pnas.88.18.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T., Friedrich L., Vernooij B., Negrotto D., Nye G., Uknes S., Ward E., Kessmann H., Ryals J. Requirement of salicylic Acid for the induction of systemic acquired resistance. Science. 1993 Aug 6;261(5122):754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. Salicylic Acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990 Nov 16;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Malamy J., Hennig J., Klessig D. F. Temperature-Dependent Induction of Salicylic Acid and Its Conjugates during the Resistance Response to Tobacco Mosaic Virus Infection. Plant Cell. 1992 Mar;4(3):359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Signer H., Ryals J., Ward E., Wyss-Benz M., Gaudin J., Raschdorf K., Schmid E., Blum W., Inverardi B. Increase in salicylic Acid at the onset of systemic acquired resistance in cucumber. Science. 1990 Nov 16;250(4983):1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Rasmussen J. B., Hammerschmidt R., Zook M. N. Systemic Induction of Salicylic Acid Accumulation in Cucumber after Inoculation with Pseudomonas syringae pv syringae. Plant Physiol. 1991 Dec;97(4):1342–1347. doi: 10.1104/pp.97.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Huber S. C. Purification and preliminary characterization of sucrose-phosphate synthase using monoclonal antibodies. Plant Physiol. 1989 Feb;89(2):518–524. doi: 10.1104/pp.89.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. R., Uknes S. J., Williams S. C., Dincher S. S., Wiederhold D. L., Alexander D. C., Ahl-Goy P., Metraux J. P., Ryals J. A. Coordinate Gene Activity in Response to Agents That Induce Systemic Acquired Resistance. Plant Cell. 1991 Oct;3(10):1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N., Silverman P., Wilson T. M., Kleier D. A., Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991 Aug;3(8):809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]