abstract
Human CD8+ effector T cells derived from CD45RO+CD62L+ precursors enriched for central memory (TCM) precursors retain the capacity to engraft and reconstitute functional memory upon adoptive transfer, whereas effectors derived from CD45RO+CD62L− precursors enriched for effector memory precursors do not. Here we sought to compare the engraftment fitness and function of CD8+ effector T cells derived from CD45RA+CD62L+ precursors enriched for naïve and stem cell memory precursors (TN/SCM) with that of TCM. We found that cytotoxic T cells (CTLs) derived from TCM transcribed higher levels of CD28, FOS, INFγ, Eomesodermin (Eomes), and lower levels of BCL2L11, maintained higher levels of phosphorylated AKT, and displayed enhanced sensitivity to the proliferative and anti-apoptotic effects of γ-chain cytokines compared to CTLs derived from TN/SCM. Higher frequencies of CTLs derived from TCM retained CD28 expression and upon activation secreted higher levels of IL-2. In NOD/Scid IL-2RγCnull mice, CD8+ TCM derived CTLs engrafted to higher frequencies in response to human IL-15 and mounted robust proliferative responses to an immunostimulatory vaccine. Similarly, CD8+ TCM derived CD19CAR+ CTLs exhibited superior antitumor potency following adoptive transfer compared to their CD8+ TN/SCM derived counterparts. These studies support the use of TCM enriched cell products for adoptive therapy of cancer.
Keywords: Antitumor activity, adoptive therapy, central memory T cell derived CTLs, engraftment fitness, naïve T cell derived CTLs
Abbreviations
- BM
bone marrow
- CD19CAR
CD19-specific chimeric antigen receptor
- CTLs
cytotoxic T cells
- EGFRt
truncated human EGFR
- ffLuc+
firefly luciferase
- IL-15R
IL-15 Receptor
- LCL
lymphoblastoid cell lines
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cells
- REM
rapid expansion method
- TCM
central memory T cells
- TN/SCM
naïve and stem cell memory T cells
Introduction
Adoptive transfer of in vitro expanded T cells is a therapeutic approach, that when coupled to genetic modification to express tumor targeting antigen receptors, can result in dramatic regressions of leukemia and lymphoma.1-4 While early data in the CD19-specific chimeric antigen receptor (CD19CAR) field is demonstrative of the efficacy of this approach in selected patients, the full potential of this emerging modality is hampered by the therapeutic failures arising from attenuated engraftment of CAR redirected T cells. Most active trials use patient derived peripheral blood mononuclear cells (PBMC) as a source of T cells for product manufacturing. Consequently, each product is composed of a heterogeneous population of T cells that is unique to the repertoire of the patient at the time of peripheral blood acquisition. It is reasonable to expect that the patient’s immune status based on underlying tumor type and tumor burden, prior cytotoxic therapies, and patient age will significantly affect the composition of T cells from which products are generated. Insufficient number of CAR redirected T cells capable of engrafting, amplifying, and persisting in the cell products is therefore a significant impediment to achieving reproducible and uniform therapeutic potency. We hypothesize that this untoward variable might be ameliorated by manufacturing T cell products of defined composition and specifically enriched for T cell subsets that harbor intrinsic capacity for sustained engraftment and antitumor functional outputs.
The attributes of T cells that confer engraftment fitness as manifested by the capacity to sustain a functional immune response following adoptive transfer of in vitro propagated effector T cells has been the subject of intensive investigation. We have demonstrated in a non-human primate model and human T cell NOD/Scid IL-2RγCnull (NSG) mouse model that CD8+ effector T cells derived from macaque CD62L+CD95+ or CD62L+CD45RO+central memory T cells (TCM), respectively, have the capacity to persist following adoptive transfer and re-populate functional memory niches.5,6 Consistently, Busch et al. demonstrated the self-renewal capacity and multipotency of single TCM in serial transfer design, indicating the stemness of TCM.7,8
Here, we compared the relative engraftment performance of human CD8+ effector cells derived from CD45RA+CD62L+ naïve/TSCM enriched precursors (TN/SCM) and CD45RO+CD62L+ TCM enriched precursors in vitro and in vivo using a NSG mouse engraftment model. Our data using a clinical applicable IL-2 based regimen demonstrate that CD8+ effector cells arising from polyclonal preparations of CD45RO+CD62L+ TCM enriched precursors exhibit superior performance in homeostatic cytokine driven engraftment, vaccine driven proliferation, and CD19CAR redirected antitumor potency in the NSG mouse model system, as compared to their CD45RA+CD62L+ TN/SCM enriched counterparts. Superior engraftment performance of CD45RO+CD62L+ TCM derived CD8+ effector cells in response to IL-15 in vivo was correlated with higher levels of IL-15 Receptor (IL-15R) expression and responsiveness, while augmented proliferation in vivo in response to vaccine challenge correlated with sustained CD28 expression on activated effector cells and enhanced autocrine IL-2 secretion. Lastly, TCM derived CD8+ effector cells lentivirally transduced to express a second generation CD19CAR exhibited enhanced antitumor efficacy as compared to their TN/SCM derived counterparts in a xenogeneic model of human lymphoma and leukemia. These data provide the rationale for embarking on clinical trials of CD19CAR T cell adoptive therapy using cell products derived from CD45RO+CD62L+ TCM enriched PBMC precursors.
Results
Phenotypic attributes and purification of CD8+ TN/SCM and CD8+ TCM from healthy donor peripheral blood
Human T cells can be segregated into TN/SCM and TCM based on differential expression of CD45 isoforms CD45RA and CD45RO.9,10 Using multiparameter flow cytometry, we analyzed peripheral blood samples from 8 healthy donors to determine the frequencies of CD8+ T cells expressing CD45RA+CD62L+ versus CD45RO+CD62L+. CD45RA and CD45RO double positive cells were excluded in these two populations. Based on forward and side scatter profiles to gate on CD8+ lymphocytes (Fig. 1A), we found that CD45RA+CD62L+ cells in the blood are more frequent (34.3±6.2%) than CD45RO+CD62L+ cells (16.3±2.7%) (p = 0.02) (Fig. 1B), which is consistent with other reports.11-13 Despite the differential expression of CD45RA and CD45RO, these resting/unstimulated CD62L+CD8+ T cells exhibited comparable frequencies of cells expressing CD28, CD27, and IL7Rα (Fig. 1C). As expected, significantly higher frequencies of CCR7+ cells were observed within the CD45RA+CD62L+ enriched TN/SCM cells (77.5±3.7% for TN/SCM and 47.8±6.5% for TCM p < 0.01).14 Likewise, CD45RO+CD62L+ CD8+ T cells have a significantly higher frequency of CD57 expression, a molecule associated with memory T cells with high cytolytic potential,15 and the expression of which is up-regulated in cells with a replicative history (10.4±2.2% for TN/SCM and 23.9±6.5% for TCM; Fig. 1C).16 We next sort-purified CD8+ cells (> 97% purity) from leukapheresis products obtained from the cohort of 4 healthy donors into CD45RA+CD45RO−CD62L+ and CD45RA−CD45RO+CD62L+ subsets for subsequent testing (Table 1).
Figure 1.
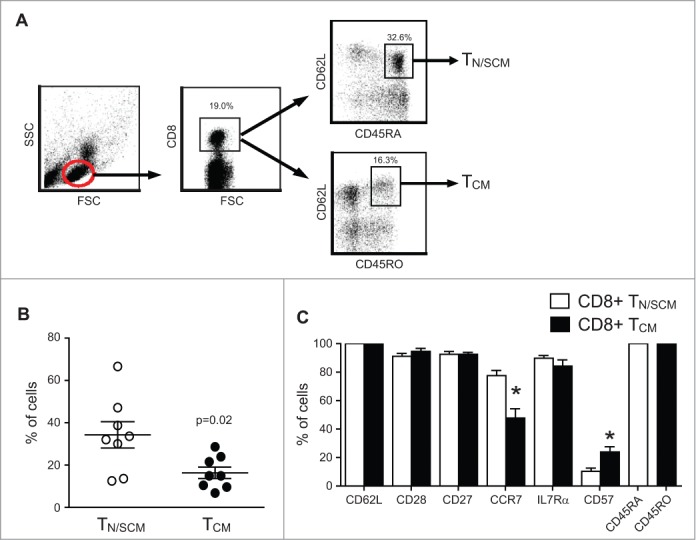
Frequency and phenotypic attributes of CD8+ TN/SCM and CD8+ TCM from healthy donor peripheral blood. (A) Representative gating/sorting strategy for CD8+CD45RA+CD62L+ TN/SCM vs. CD8+CD45RO+CD62L+ TCM. (B) Analysis of PBMC from eight different healthy donors gated on the CD8+ population and analyzed for CD45RA+CD62L+ TN/SCM and CD45RO+CD62L+ TCM by multicolor flow cytometry. (C) Percentages of immunoreactive cells in each population are indicated (mean + SEM, n = 8). * p< 0.05 when comparing TN/SCM and TCM using a paired Student’s t-test.
Table 1.
Purity of isolated CD8+CD45RA+CD62L+ CD8+ TN/SCM and CD8+CD45RO+CD62L+ CD8+TCM cells from healthy donors (HD).
| HD | %CD8+ TN/SCM | %CD8+TCM |
|---|---|---|
| 1 | 98 | 99 |
| 2 | 98 | 99 |
| 3 | 97 | 100 |
| 4 | 100 | 100 |
In vitro differentiation of TN/SCM and TCM precursors generates effector cytotoxic T cells (CTLs) with divergent gene expression profiles, and phenotypic/functional attributes
Purified CD8+ TN/SCM and CD8+ TCM were subject to a 14 day in vitro rapid expansion method (REM) stimulation17 including anti-CD3ε (OKT3), irradiated PBMC, and lymphoblastoid cell lines (LCLs) as feeders in the presence of recombinant human IL-2 (rhIL-2) 50 U/mL that has been successfully used in many applications18,19 (Fig. S1). We observed equivalent proliferative responses and cytolytic activity of effector CTLs derived from CD8+ TN/SCM and TCM precursors. These findings were reproduced even after additional REM stimulation cycles (Fig. S2A, B). In addition, each subset maintained equal telomere length through repetitive stimulations (Fig. S2C) and therefore subsequent differences in replicative potential, persistence and functional outputs in the in vivo NSG model could not be attributed to differences in the proclivity of either subset to enter replicative senescence.
To investigate the impact of different stimuli on the proliferative capacity of the T cell subsets, we subjected purified CD8+ TN/SCM, CD8+ TCM, and CD8+ memory stem cells (TSCM) characterized as CD45RA+CD62L+CD95+ 12,20 (Fig. S3A) to either REM stimulation (Fig. S3B, C) or OKT3 stimulation in the presence of irradiated allogeneic PBMC and rhIL-2 300U/mL (Fig. S3D, E) as described by Hinrichs et al. 11 We observed comparable levels of cell growth of all three subsets and CD28 expression was better retained in CD8+ TCM subset independent of the culture conditions.
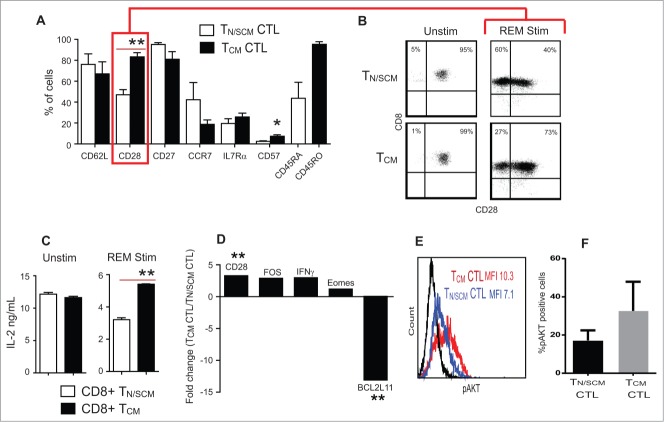
Consistent with effector cell differentiation during the first 14-day REM stimulation, we observed down regulation of CD45RA expression on TN/SCM derived CTL and incremental decreases in frequencies of T cells from both TN/SCM and TCM subsets of CD62L, CCR7 and IL-7Rα expression (Fig. 2A). In contrast, despite the initial comparable frequencies of CD28 expression on unstimulated CD8+ TN/SCM and CD8+ TCM precursors (91 ± 2% and 94 ± 2%, respectively), following REM stimulation, CD28 expression was retained preferentially on cultured cells derived from TCM precursors (78.3 ± 4.9%) than that of TN/SCM, (40 ± 2.8%) (P<0 .01) (Fig. 2A, B and Table 2). While both precursor subsets expressed CD28 and have equal capacity to secrete IL-2 upon TCR/CD28 triggering, decreased CD28 expression by TN/SCM derived CTLs upon differentiation to effector CTLs correlates with their reduced capacity to secrete IL-2 upon subsequent polyclonal activation using OKT3-LCL stimulator cells (Fig. 2C).
Figure 2.
Effector cells derived from CD8+ TCM precursors expressed higher levels of CD28 and IL-2 production upon stimulation. Purified CD8+ TN/SCM and CD8+ TCM were subject to 14 d in vitro REM stimulation in the presence of rhIL-2 (50U/mL). (A) Phenotypic analysis of differentiated effector cells derived from CD8+ TN/SCM (TN/SCM CTL) and CD8+ TCM (TCM CTL) following expansion. Mean percentages of immunoreactive cells + SEM from 4 different donors are presented (**p < 0.01, *p < 0.05). (B) Representive flow cytometric analysis of CD28 on the CTLs derived from CD8+ TN/SCM and CD8+ TCM. (C) Supernatants were collected after overnight co-incubation of either unstimulated CD8+ TN/SCM and CD8+TCM or stimulated CD8+ TN/SCM and CD8+TCM with OKT3 expressing LCL. Cytokine levels (means +SEM of triplicate wells) were determined using cytometric bead array. **p < 0.01. Representative data of 4 experiments are depicted. (D) After 14 d of stimulation, RNA was extracted and analyzed for genes that confer differentiation, survival, and apoptotic qualities of effector T cells and 4 control genes including housekeeping genes, RT control and positive PCR control. Medians of fold change of CD28, FOS, IFNγ, Eomes and BCL2L11 mRNA from cells derived from CD8+ TCM (TCM CTL) vs. CD8+ TN/SCM (TN/SCM CTL) from 4 individual donors are presented. **p < 0.01. (E) Effector T cells derived from CD8+ TN/SCM (TN/SCM CTL) (blue) and CD8+TCM (TCM CTL) (red) were stained for intracellular phosphorylated AKT (pAKT). Fluorochrome conjugated isotype matched antibody stained cells are shown in black. (F) Percentages of AKT+ cells from 4 donors are presented.
Table 2.
CD28 expression on CD8+ TN/SCM and CD8+TCM CTL.
| HD | %CD28 CD8+ TN/SCM | %CD28 CD8+TCM |
|---|---|---|
| 1 | 36.1 | 78.7 |
| 2 | 35.3 | 70.2 |
| 3 | 47.4 | 92.0 |
| 4 | 39.6 | 72.8 |
| Mean±SEM | 40.3±2.8 | 78.3±4.9 |
| p value | 0.008 |
| HD | MFI CD28 CD8+ TN/SCM | MFI CD28 CD8+TCM |
|---|---|---|
| 1 | 20 | 29 |
| 2 | 35 | 65 |
| 3 | 31 | 64 |
| 4 | 42 | 80 |
| Mean±SEM | 32±4.6 | 59.5±10.8 |
| p value | 0.0015 |
To further define the differences between the two effector CTL populations, we evaluated expression levels of transcriptionally regulated genes involved in CD28 signaling and T cell survival by RT-qPCR. Consistent with their higher CD28 expression by flow cytometric analysis, CD8+ TCM derived CTL cells contained a two-fold higher content of mRNA encoding CD28 and FOS, a downstream signaling molecule of CD28 which leads to IL-2 production (Fig. 2D).21-23 In line with these findings, intracellular phosphorylated AKT (pAKT), an effector molecule of co-stimulatory receptors such as CD28,24 was present in higher abundance in CD8+ TCM derived CTLs than in CD8+ TN/SCM derived CTLs (Fig. 2E, F) and the mRNA encoding the apoptosis facilitator BCL2L11 was 13-fold lower in TCM derived CTLs than that of TN/SCM derived CTLs. These data reveal cell intrinsic divergence of CD28 gene expression and CD28 costimulatory function in effector cells derived from TCM vs. TN/SCM precursors. As intrinsically programmed, CD8+ TCM derived CTLs transcribed higher levels of IFNγ and Eomesodermin (Eomes) than that from CD8+ TN/SCM after in vitro activation and expansion.25,26
CTLs derived from TCM precursors exhibited increased common γ-chain cytokine receptors and responsiveness to rhIL-2 and rhIL-15 than that from TN/SCM
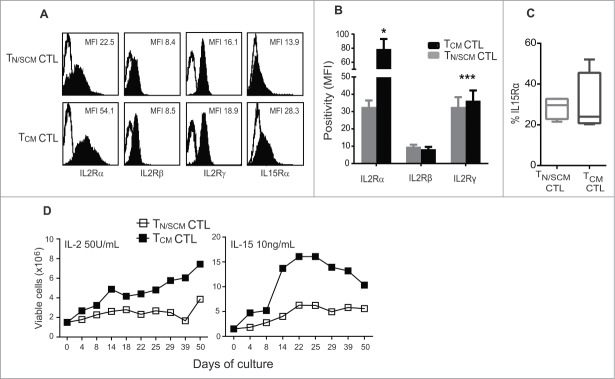
The capacity of TCM derived CD8+ CTLs to engraft and persist following adoptive transfer has been correlated with their sensitivity to the anti-apoptotic effects of homeostatic cytokines, in particular, IL-15.27-29 This augmented sensitivity has been attributed to cell intrinsic differences in expression levels of the γc-chain cytokine receptor subunits, including CD25 and CD132. To further compare TCM and T N/SCM derived CTLs, we evaluated the expression levels of the γ-chain cytokine receptor complex. We found that TCM derived CTLs expressed significantly higher levels of the α-chain (p < 0.05) and γ-chain (p < 0.001) of the IL-2 receptor, while the β-chain expression levels were equivalent (Fig. 3A and B). IL-15 receptor α-chain was higher on the TCM derived CTLs (Fig. 3A and C). We next subjected the REM expanded CTLs to prolonged culture (50 days) in either rhIL-2 (50U/mL) or recombinant human IL-15 (rhIL-15) (10ng/mL) without restimulation. Higher numbers of viable TCM derived CTLs persisted in both rhIL-2 and rhIL-15 supplemented cultures as compared to TN/SCM derived CTL (Fig. 3D). Similar findings were observed using CTLs from different donors (Fig. S4). These data demonstrate that TCM derived effectors retain higher expression levels of γ-chain cytokine receptor and have enhanced capacity to survive and expand in the presence of rhIL-2 or rhIL-15 than TN/SCM derived CTLs.
Figure 3.
Effector cells derived from CD8+TCM precursors displayed greater ability to persist and expand in vitro. Purified CD8+ TN/SCM and CD8+ TCM were expanded 14 d in vitro using a REM in the presence of rhIL-2 (50U/mL). (A) After 14 d of in vitro stimulation, the effector cells from each population (TN/SCM CTL and TCM CTL) were stained with antibodies to the indicated cytokine receptor and analyzed by flow cytometry. Histograms show the mean fluorescence intensities (MFIs) of γ-chain cytokine receptor positive cells (black) after subtraction from the isotype controls (open). Representative data of 4 experiments are depicted. (B) Positivity of IL-2 receptors from four different donors is presented. *p < 0.05, ***p < 0.001. (C) Percentages of IL-15Rα from a cohort of four donors are presented. (D) After the initial expansion, the TN/SCM CTL and TCM CTL cells were maintained in rhIL-2 (50U/mL) (left) or rhIL-15 (10ng/mL) (right). Cytokines were supplemented every other day. Viable cell numbers were determined by Guava ViaCount at the indicated time points.
In vitro expanded TCM derived CD8+ CTLs exhibit superior in vivo engraftment in response to homeostatic huIL-15 as compared to TN/SCM derived CD8+ CTLs
We examined the extent to which human CD8+ TCM and CD8+ TN/SCM derived CTLs engraft and persist in vivo. Three weeks after i.v. infusion, CD8+ TCM derived CTLs were detected in blood, bone marrow (BM) and spleen at levels significantly higher than in mice receiving TN/SCM derived CTLs (p < 0.01) (Fig. 4A). We did not observe a remarkably divergent phenotypic profile for the engrafting cells from each cohort of mice, besides a consistent and statistically higher frequency of CD28+ and IL-7Rα+ T cells in TCM derived CTL infused mice (Fig. 4B). To rule out the selective engraftment of an oligoclonal population that might be expected from a rare TSCM, and to establish that the superior engraftment fitness of TCM derived CD8+ CTLs is a general feature of CD45RO+CD62L+CD8+ T cells, we performed flow-based TCR Vβ spectratyping of T cells prior to adoptive transfer and on 21 day post engraftment. We demonstrate a highly polyclonal input cell population from each subset and an equally polyclonal, minimally skewed population of engrafting T cells (Fig. 4C). Unlike our previous observation that CD45RO+CD62L− effector memory derived CD8+ CTLs in the same model did not engraft, TN/SCM derived CD8+ CTLs exhibited engraftment fitness as reflected by these data, however, not at the level exhibited by TCM derived CTLs, presumably due in part to the greater sensitivity of these cells to limiting amounts of huIL-15 in vivo.
Figure 4.
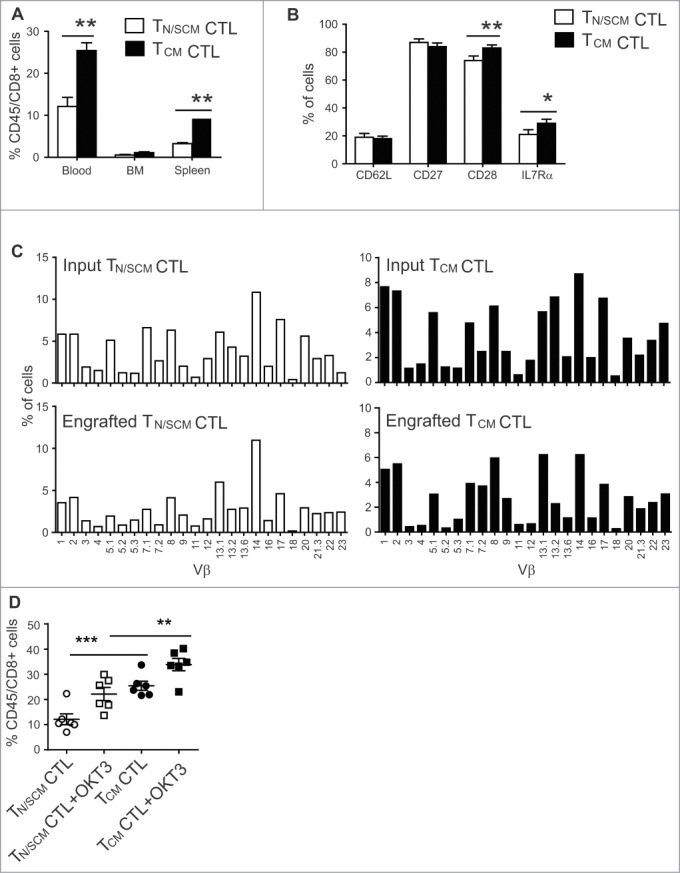
Effector cells derived from CD8+TCM precursors exhibited superior engraftment fitness in NSG mice. After 14 d of in vitro stimulation, effector cells derived from CD8+ TN/SCM (TN/SCM CTL) and CD8+TCM (TCM CTL) (107) were infused i.v. into huIL-15-replete NSG mice. (A) Three weeks after adoptive transfer, human T cells in the peripheral blood, BM and spleen of recipient mice were determined by flow cytometric analysis using antibodies specific for human CD45 and CD8. Means+SEM of a total of 6 mice per T cell subset in a representative experiment are depicted. **p < 0.01, using a Mann–Whitney test. (B) Percentages of CD62L, CD27, CD28, and IL7Rα positive cells on gated human CD45+ positive cells of pooled peripheral blood, BM and spleens are indicated. *p < 0.05 **p < 0.01 using a Mann–Whitney test. (C) TCR vβ repertoire of the effectors derived from CD8+ TN/SCM and CD8+TCM before and after infusion. Percentage (%) of human CD3+ cells that were positive for the indicated TCR vβ genes was determined by flow cytometry. (D) For in vivo stimulation, 107 irradiated OKT3-expressing LCL (+OKT3) were injected i.v. into mice that had been engrafted (3 days) with CD8+ TN/SCM or CD8+TCM derived CTLs. Human T cells in peripheral blood were determined 7 d post in vivo challenge. ***p < 0.001. **p < 0.01.
We next compared the extent to which CTL’s proliferate in vivo in response to an immunostimulatory vaccine. We quantitated the frequency of engrafted human T cells immediately before and seven days after OKT3-LCL challenge. Both subsets have equivalent fold expansion to vaccine (1.8 folds for CD8+ TN/SCM derived CTL and 1.3 folds for CD8+ TCM derived CTL in peripheral blood (Fig. 4D). These data demonstrate that once engrafted, both TCM and TN/SCM derived CTLs are able to mount a proliferative response to TCR stimulation.
CD19CAR CTLs derived from CD8+ TCM displayed superior persistence and antitumor potency as compared to that from CD8+ TN/SCM
To further compare the therapeutic potential, purified CD8+ TN/SCM and CD8+TCM were genetically modified by lentiviral transduction to express a second generation CD19CAR using CD28 as the costimulatory domain and a selectable marker, truncated EGFR (EGFRt) (CD19R:CD28:ζ/EGFRt).30 EGFRt+CD19CAR T cells were immunomagnetically enriched to 90% purity and underwent 3 cycles of REM stimulation and expansion. Studies were then performed using the CD19CAR CTLs derived from CD8+ TN/SCM (TN/SCM-CD19R) and CD8+ TCM (TCM-CD19R) (Fig. 5A) populations. The TCM-CD19R displayed higher fold expansion in vitro with comparable telomere length to that of TN/SCM-CD19R (Fig. 5B, C). They possessed similar phenotypic characteristics other than CCR7 expression (Fig. S5A) and exhibited equivalent CD19-specific effector function in vitro (Fig. S5B, C). Interestingly, after adoptive transfer into huIL-15 supplemented mice, human T cells were only detected in the BM of NSG mice that had been infused with TCM-CD19R (Fig. 5D). Therapy experiments were also performed using CD19+ ffluc+ LCLs that were inoculated i.v. into NSG mice. In vitro expanded TN/SCM-CD19R or TCM-CD19R were adoptively transferred into the tumor bearing, huIL-15-supplemented mice. After 20 days, tumor signals were significantly inhibited (p < 0.05) in the mice that received TCM-CD19R as compared to mice receiving TN/SCM-CD19R (Fig. 5E).
Figure 5.
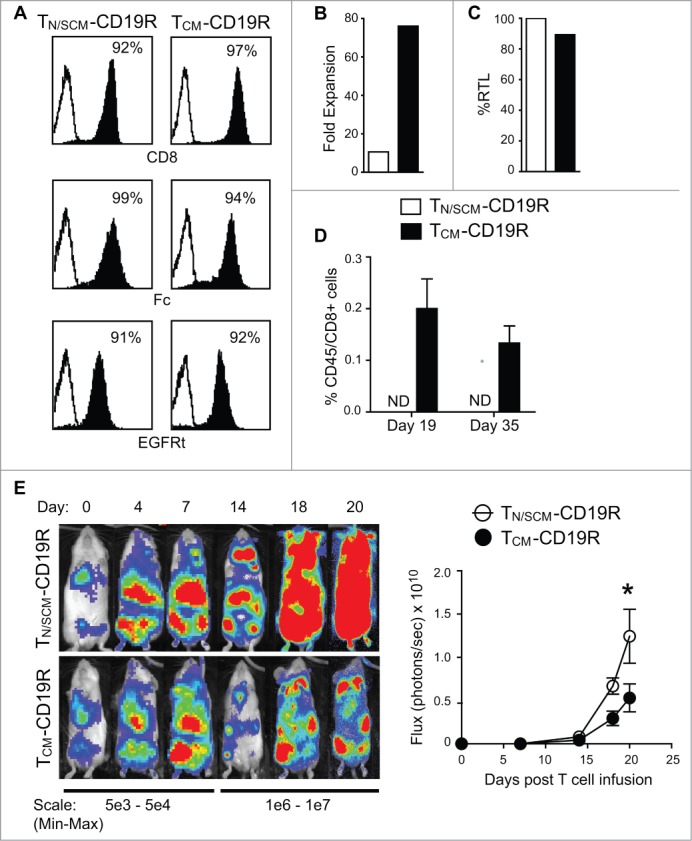
Engineered CD19 specific T cells derived from CD8+ TCM (TCM-CD19R) displayed superior engraftment fitness and antitumor activity as compared to that from CD8+ TN/SCM (TN/SCM-CD19R). CD8+ TN/SCM and CD8+ TCM that had been transduced to express a CD19-specitic CAR (CD19R:CD28:ζ) and huEGFRt selection marker, were enriched for EGFRt+ cells and further expanded by REM stimulation in the presence of rhIL-2 and rhIL-15. (A) Purified EGFRt+ populations after 3 cycles of REM were flow cytometrically analyzed for expression of CD8+ as well as the CAR and EGFRt transgenes (using anti-Fc and Erbitux reagents, respectively) (B) Fold expansion of the purified EGFRt+ TN/SCM-CD19R and TCM-CD19R cells within the third cycle of REM stimulation. (C) Relative telomere length of the expanded cells. (D) The expanded TN/SCM-CD19R and TCM-CD19R cells (107) were adoptively infused into huIL-15 reconstituted NSG mice. Human T cells in the BM were analyzed by flow cytometry at indicated time points (3 mice per time point). ND, not detectable. (E) CD19+ffluc+LCLs (106) were inoculated into NSG mice i.v. on day -4. Expanded TN/SCM-CD19R and TCM-CD19R cells (107) were adoptively transferred (i.v.) into the huIL-15-reconstituted, tumor-bearing mice on day 0. Tumors were monitored with biophotonic imaging. Representative mouse images and mean photon flux ± SEM of each group (N = 6) are depicted.*, p < 0.05 using Mann–Whitney t-test. Representative data from two separate experiments are presented.
Consistently, when the purified CD8+ TCM and CD8+ TN/SCM cells were transduced with lentivirus encoding CD19R(EQ) to ensure enhanced potency after adoptive transfer,31,32 TCM-CD19R displayed higher fold expansion in vitro (30 vs.8 folds for total cells and 47 vs.12 folds for CAR+ T cells) (Fig. S6A, B) and superior antitumor activity to TN/SCM-CD19R (Fig. S6C). Antitumor efficacy of 1 × 10^6 TCM-CD19R is comparable to that of 2 × 10^6 TN/SCM-CD19R as demonstrated in a titration in vivo experiment (Fig. S7).
Discussion
The nature of the infused T cells is known to be one major determinant for the persistence of transferred cells and correlates with therapeutic efficacy.5 Our previous studies in macaques and xenogeneic mouse models demonstrated that human CD8+ effector T cells derived from macaque CD62L+CD95+ TCM or human CD45RO+CD62L+ TCM precursors, respectively, retain the capacity to engraft and reconstitute functional memory upon adoptive transfer, whereas effectors derived from CD62L− TEM precursors do not.5,6,33 In support of this, clinical experience with adoptive transfer using bulk PBMC derived effector T cells for the treatment of CLL and ALL have suggested that some of the gene-engineered cells detected long-term in patients exhibit characteristics of TCM.1-4 Although it is impossible to track the precursors of the persisting cells in those trials, these results indicate that the efficacy of adoptively transferred T cells might be augmented by specifically engineering the T cell subset that exhibits the intrinsic capacity to persist in vivo. As an extension of our previous studies, in the present investigation, we sought to compare the in vivo performance of the TCM and TN/SCM derived effector cells using an IL-2 based REM system that is widely used in clinical trials of T cell therapy.18,19 Our data demonstrate that effector cells arising from polyclonal preparations of CD45RO+CD62L+ TCM enriched precursors exhibited superior engraftment fitness and remained the capacity to respond to in vivo stimulation via TCR, and display better CD19CAR redirected antitumor efficacy in the xenogeneic model of human lymphoma and leukemia, as compared to CTLs derived from the CD45RA+CD62L+ TN/SCM enriched precursors.
It is increasingly evident that in vitro attributes of ex vivo expanded T cells may correlate with their ability to persist after adoptive transfer.11,34 In our study, stimulation with REM resulted in the equivalent proliferative response and cytolytic activity of CTLs derived from TCM and TN/SCM precursors and each subset maintained equal telomere length through multiple rounds of stimulation. However, the qualitative difference of the two populations was revealed in more detailed analysis. Despite the comparable levels of CD28 expression on freshly isolated CD8+ TCM and CD8+ TN/SCM and the ability to secrete IL-2 upon OKT3 stimulation, after 14 days of in vitro expansion, the CD8+ TCM derived CTLs maintained higher levels of CD28, phosphorylated AKT, and autocrine IL-2 secretion than that of CD8+ TN/SCM derived CTLs. Additionally, CD8+ TN/SCM expressed higher levels of apoptotic facilitator BCL2L11 and low expression of CD28-associated FOS signaling at molecular levels. Apparently, these intrinsic attributes of the CTLs derived from TCM was not compromised by the acquisition of effector cell transcriptional program such as higher IFNγ and Eomes.35 These results led to the postulation that TCM derived CTLs would perform better in both persistence and function upon adoptive transfer compared to TN/SCM derived CTLs.
In contrast to our findings, others' studies have suggested that after in vitro stimulation, CD8+ naïve derived effector cells possess better features for immunotherapy as compared to CD8+ TCM derived effector cells such as longer telomere and higher expression of costimulatory molecules.11 The discrepancy could be ascribed to differences among the factors, such as selection of the T cell subsets from frozen/ overnight rested PBMC, which has been showed to induce CD62L shedding with potential biological changes,36 and subsequent gene modification in those studies. We speculate that different culture conditions such as addition of IL-7 and IL-1520 and various means of stimulation would also result in differential impacts on the activation and differentiation of TSCM, naive and memory T cells.12,37,38
To further study whether the phenotypic and functional attributes of CTLs derived from CD8+TCM correlate with their ability to persist and mount an effective antitumor response in vivo, ex vivo expanded T cells were adoptively transferred into NSG mice. CTLs arising from CD8+TCM cells exhibited an engraftment advantage with broad Vβ TCR repertoire in huIL15-supplemented NSG mice, consistent with higher expression of IL-15Rα and γ-chain receptors on the infused cells. Moreover, the persisting CTLs derived from CD8+TCM were also capable of expanding in vivo robustly in respond to OKT3 vaccine challenge, indicating CTLs derived from CD8+ TCM possess essential characteristics for improved engraftment, long term survival and retention of memory function after adoptive transfer. We also consistently found that lentiviral transduced and expanded CD19CAR+ CTL derived from CD8+ TCM exhibited superior engraftment fitness and enhanced antitumor activity compared to TN/SCM derived CD19 CAR CTL upon transfer into CD19+ tumor bearing mice.
Memory stem cells (TSCM) defined by Restifo' s group displayed the potential for the induction of long-term memory T cells in vivo, therefore, have direct relevance to the design of T cell immune therapy. However, like other somatic stem cells, the naturally occurring “memory stem cells” are rare in PBL, the frequency is around 2% of CD8+ cells in peripheral blood. Isolation of such a rare subset and obtaining a clinically relevant therapeutic dose without prolonged ex vivo culture are the obvious challenges.39 It is possible that TSCM, which are characterized as CD45RA+CD62L+CD95+ were captured in the TN/SCM that were examined in our study. However, we found that TCM derived cells still exhibited the best engraftment and antitumor activity in our mouse model. In addition, the culture conditions used in our study lack the preferential signals such as Wnt signaling required for the infrequent TSCM to expand. This might further contribute to the superior therapeutic activity of CD8+ TCM derived cells.
Overall, this work supports the idea that the CD8+ TCM described here is a promising candidate T cell population to consider for immediate immunotherapeutic use. We have developed a semi-closed manufacturing process for reproducibly generating genetically modified CD19-specific T cells from a defined population of TCM in a short period of time for clinical applications.40
Materials and methods
Cell lines
Unless stated otherwise, all cell lines were maintained in RPMI 1640 (Irvine Scientific) supplemented with 2mM L-glutamine, 25mM HEPES, and 10% heat-inactivated FCS (Hyclone), hereafter referred to as complete media (CM). PBMCs were transformed with Epstein–Barr virus to generate lymphoblastoid cell lines (LCL) as previously described.41 OKT3-LCL cells express membrane bound OKT3 and are grown in CM supplemented with 0.4mg/mL hygromycin.30 K562 and Sup B15 cells were obtained from ATCC and cultured as recommended.
Antibodies and flow cytometry
Human T cells were analyzed by flow cytometry after staining with fluorochrome-conjugated monoclonal antibodies (mAbs) to CD8+, CD62L, CD45RO, CD45RA, CD127, CD28, CD27, CD57,CD45, CD3, IFNγ, CD25 (IL-2Rα), CD122 (IL-2Rβ), and CD132 (IL-2Rγ) (BD Biosciences); CCR7 and IL-15Rα (R&D Systems); and phosphorylated AKT (pAKT) (Cell Signaling Technology). The IOTestBeta Mark T cell receptor (TCR) Vβ Repertoire Kit was purchased from Beckman Coulter. Isotype-matched mAbs served as controls. Data acquisition was performed on a FACSCalibur (BD Biosciences) and analyzed using FCS Express, Version 3 software (De Novo Software). Telomere length analysis was performed using flow-fish technique with Telomere PNA Kit/FITC obtained from Dako (Dako Denmark A/S, Denmark). Biotinylated Erbitux (Cetuximab) and streptavidin-PE were used to identify T cells that expressed truncated human EGFR (EGFRt), and surface expression of the CD19CAR was confirmed by staining with a biotinylated anti-Fc (Jackson ImmunoResearch Laboratories) and streptavidin-PE.
T cell isolation and stimulation
Leukapheresis products were obtained from the cohort of four healthy donors under protocols approved by the COHNMC Institutional Review Board. For purification of CD8+ TN/SCM and CD8+ TCM subsets, PBMCs were labeled with anti-CD45RO, -CD45RA, -CD62L, -CD95, and -CD8 mAbs, and CD8+CD62L+CD45RA+ TN/SCM, CD8+CD62L+CD45RO+ TCM and CD8+CD62L+CD45RA+ CD95+TSCM cells were sort-purified using a MoFlo MLS (Dako Cytomation). Freshly isolated CD8+TSCM, CD8+ TN/SCM and CD8+ TCM (5 × 105 each) were then propagated using the rapid expansion method (REM).17 Briefly, 106 T cells were stimulated with 30ng/mL anti-CD3ε (OKT3; Ortho Biotech), 5 × 107 γ-irradiated PBMCs (3500 cGy), and 107 γ-irradiated lymphoblastoid cell lines (LCLs, 8000 cGy) in 50mL culture media. Cultures were then supplemented with 50U/mL rhIL-2 (CellGenix) every 48 h for 14 d before in vitro analysis and adoptive transfer. In some cases, purified CD8+ TN/SCM, CD8+ TCM and CD8+ TSCM were stimulated with 30ng/mL OKT3, γ-irradiated PBMCs at 10:1 ratio (feeders: T cells). Cultures were then supplemented with 300U/mL rhIL-2 every 48 h for 14 d.
DNA constructs
The GFP-IMPDH2dm-2A-IL-15_pcDNA3.1(+) plasmid contains a fusion of the eGFP cDNA, which confers fluorescence and resistance to mycophenolic acid, followed by the 2A self-cleaving peptide sequence 42 and a human IL-15 cDNA. OKT3–2A-Hygro_pEK is an expression plasmid wherein an anti-human CD3ε scFV:Fc:CD28TM cDNA is formatted N-terminal to hygromycin phosphotransferase with an intervening 2A sequence 43 under the transcriptional control of the human EF-1α promoter. The CD19R:CD28:ζ/EGFRt -epHIV7 contains: (1) the CAR sequence consisting of the VH and VL gene segments of the CD19-specific FMC63 mAb, an IgG4 hinge-CH2CH3, the transmembrane, and cytoplasmic domains of the costimulatory molecule CD28, and the cytoplasmic domain of the CD3ζ chain44; (2) the ribosomal skip T2A sequence and (3) the truncated human EGFR (EGFRt) sequence as previous described.30 In some cases, the CD19R:CD28:ζ/EGFRt with mutations at two sites (L235E; N297Q) within the CH2 region on the IgG4-Fc spacers (CD19R(EQ)) was used (Fig. S8A and S8B).
Generation of CD8+ CD19-specific TN/SCM and TCM derived effector cells
Freshly isolated CD8+ TN/SCM and CD8+ TCM were activated with CD3/CD28 Dynabeads (Invitrogen), and transduced 3 d later with CD19R:CD28:ζ/EGFRt lentivirus at an MOI of 3. Ten days after the lentiviral transduction, the truncated human EGFR+ (EGFRt+) T cells were then enriched 30 and further expanded by REM in the presence of 50U/mL rhIL-2 and 0.5ng/mL rhIL-15 (CellGenix).
Cytokine production assays
Freshly isolated and expanded T cell products (105) were co-cultured in 96-well tissue culture plates with 105 of the indicated stimulator cells. Supernatants were harvested 18 h after stimulation and analyzed by cytometric bead array using the Bio-Plex Human Cytokine 17-Plex Panel (Bio-Rad Laboratories) (in triplicates) according to the manufacturer’s instructions. Intracellular IFNγ staining was performed and analyzed with flow cytometry.
Cytotoxicity assays
Four-hour 51Cr release assays were performed as previously described 45 using the indicated effector cells and 51Cr-labeled target cells.
Reverse transcriptase (RT) quantitative PCR (qPCR) analysis
After 14 d of stimulation, RNA was extracted with RNeasy Kits (Qiagen). RT-qPCR was performed using the RT2 Profiler PCR Array (SABiosciences) designed with customized primers for sequences of transcriptionally regulated genes shown to impart differentiation, survival, and apoptotic attributes to effector CD8+ T cells 11,46 and 4 control genes including house keeping genes PPIA (Peptidylprolyl isomerase A) (cyclophilin A) and RPL13A (Ribosomal protein L13a), control for reverse transcription, and positive PCR control. All the samples passed the RNA quality control, PCR array reproducibility, RT efficiency, and genomic DNA contamination according to the manufacturer’s instructions. Data were analyzed by ΔΔCt method using RT2 Profiler PCR Array Web portal software (http://www.sabiosciences.com). Medians of fold change of TCM versus TN/SCM derived CD8+ effectors from 4 individual donors are presented.
Xenograft model
All mouse experiments were approved by the COH Institutional Animal Care and Use Committee. Six- to 10-week old NSG mice were injected intravenously (i.v.) on day 0 with 1 × 107 freshly thawed T cell products. Irradiated (8000 cGy) NS0-huIL15 cells (2 × 107) were given intraperitoneally (i.p.) three times a week to provide a systemic supply of human IL-15 in vivo, as previously described.5 Human T cell engraftment was determined by flow cytometric analysis of harvested tissues based on staining with antibodies specific for human CD45 and CD8+. For in vivo stimulation, 107 irradiated OKT3-expressing LCLs were injected into the mice i.v. 3 d after i.v. administration of 107 T cells, and human T cells in peripheral blood were analyzed 7 d after challenge.
Biophotonic tumor imaging
1 × 106 CD19+ firefly luciferase+ (ffLuc+) LCLs or 0.5 × 106 CD19+ ffLuc+ acute lymphoid leukemic cells SupB15 were inoculated into NSG mice by i.v. injection. Anesthetized mice were imaged using a Xenogen IVIS 100 series system(Xenogen, Alameda, CA). Photons emitted from ffLuc+ tumor xenografts were quantified using the software program Living Image (Xenogen), and the bioluminescence signal was measured as total photon flux normalized for exposure time and surface area and expressed in units of photons (p) per second per cm2 per steradian (sr).
Statistical analysis
Analyses were performed using Prism (GraphPad Softward Inc.,). Mann–Whitney t- test was used for the statistical analysis of the in vivo data. Paired t-test (2-tailed) was used for the analysis of in vitro data. p < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Contribution: X.W. designed and performed research, collected, analyzed, and interpreted data, and co-wrote the manuscript; A.M, L.E.B, C.E.B., and C.B analyzed and interpreted data, and co-wrote the manuscript; C.W.W., R. U, E.T, B.A, and W.C performed research and collected data; S.J.F. analyzed and interpreted data; and M.C.J. designed research, analyzed and interpreted data, and co-wrote the manuscript. The authors thank Julie R. Ostberg for assistance in generating the Figures.
Funding
This work was supported by the National Institutes of Health (grants P50 CA107399, P01 CA030206, R01 CA136551, R01 CA114536, and AI053193) and the Lymphoma Research Foundation.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF et al.. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368:1509-18; PMID:23527958; http://dx.doi.org/ 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3:95ra73; PMID:21832238; http://dx.doi.org/ 10.1126/scitranslmed.3002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M et al.. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5:177ra38; PMID:23515080; http://dx.doi.org/ 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM et al.. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119:2709-20; PMID:22160384; http://dx.doi.org/ 10.1182/blood-2011-10-384388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 2011; 117:1888-98; PMID:21123821; http://dx.doi.org/ 10.1182/blood-2010-10-310599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest 2008; 118:294-305; PMID:18060041; http://dx.doi.org/ 10.1172/JCI32103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graef P, Buchholz VR, Stemberger C, Flossdorf M, Henkel L, Schiemann M, Drexler I, Hofer T, Riddell SR, Busch DH. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity 2014; 41:116-26; PMID:25035956; http://dx.doi.org/ 10.1016/j.immuni.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 8.Gattinoni L. Memory T cells officially join the stem cell club. Immunity 2014; 41:7-9; PMID:25035947; http://dx.doi.org/ 10.1016/j.immuni.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas ML, Lefrancois L. Differential expression of the leucocyte-common antigen family. Immunol Today 1988; 9:320-6; PMID:2978372; http://dx.doi.org/ 10.1016/0167-5699(88)91326-6 [DOI] [PubMed] [Google Scholar]
- 10.Terry LA, Brown MH, Beverley PC. The monoclonal antibody, UCHL1, recognizes a 180,000 MW component of the human leucocyte-common antigen, CD45. Immunology 1988; 64:331-6; PMID:2455685 [PMC free article] [PubMed] [Google Scholar]
- 11.Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S et al.. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 2011; 117:808-14; PMID:20971955; http://dx.doi.org/ 10.1182/blood-2010-05-286286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C et al.. A human memory T cell subset with stem cell-like properties. Nat Med 2011; 17:1290-7; PMID:21926977; http://dx.doi.org/ 10.1038/nm.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabin RL, Roederer M, Maldonado Y, Petru A, Herzenberg LA. Altered representation of naive and memory CD8+ T cell subsets in HIV-infected children. J Clin Invest 1995; 95:2054-60; PMID:7738172; http://dx.doi.org/ 10.1172/JCI117891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708-12; PMID:10537110; http://dx.doi.org/ 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol 2009; 85:88-97; PMID:18820174; http://dx.doi.org/ 10.1189/jlb.0208107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood KL, Twigg HL 3rd, Doseff AI. Dysregulation of CD8+ lymphocyte apoptosis, chronic disease, and immune regulation. Front Biosci (Landmark edition) 2009; 14:3771-81; PMID:19273309; http://dx.doi.org/ 10.2741/3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crossland KD, Lee VK, Chen W, Riddell SR, Greenberg PD, Cheever MA. T cells from tumor-immune mice nonspecifically expanded in vitro with anti-CD3 plus IL-2 retain specific function in vitro and can eradicate disseminated leukemia in vivo. J Immunol 1991; 146:4414-20; PMID:1674958 [PubMed] [Google Scholar]
- 18.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 1992; 257:238-41; PMID:1352912; http://dx.doi.org/ 10.1126/science.1352912 [DOI] [PubMed] [Google Scholar]
- 19.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ et al.. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008; 112:2261-71; PMID:18509084; http://dx.doi.org/ 10.1182/blood-2007-12-128843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F et al.. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013; 121:573-84; PMID:23160470; http://dx.doi.org/ 10.1182/blood-2012-05-431718 [DOI] [PubMed] [Google Scholar]
- 21.Jain J, Nalefski EA, McCaffrey PG, Johnson RS, Spiegelman BM, Papaioannou V, Rao A. Normal peripheral T-cell function in c-Fos-deficient mice. Mol Cell Biol 1994; 14:1566-74; PMID:8114694; http://dx.doi.org/ 10.1128/MCB.14.3.1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochi Y, Koizumi T, Kobayashi S, Phuchareon J, Hatano M, Takada M, Tomita Y, Tokuhisa T. Analysis of IL-2 gene regulation in c-fos transgenic mice. Evidence for an enhancement of IL-2 expression in splenic T cells stimulated via TCR/CD3 complex. J Immunol 1994; 153:3485-90; PMID:7930571 [PubMed] [Google Scholar]
- 23.Yaseen NR, Park J, Kerppola T, Curran T, Sharma S. A central role for Fos in human B- and T-cell NFAT (nuclear factor of activated T cells): an acidic region is required for in vitro assembly. Mol Cell Biol 1994; 14:6886-95; PMID:7935406; http://dx.doi.org/ 10.1128/MCB.14.10.6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, Spencer DM, Rooney CM. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther 2010; 18:2006-17; PMID:20842106; http://dx.doi.org/ 10.1038/mt.2010.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho BK, Wang C, Sugawa S, Eisen HN, Chen J. Functional differences between memory and naive CD8+ T cells. Proc Natl Acad Sci U S A 1999; 96:2976-81; PMID:10077622; http://dx.doi.org/ 10.1073/pnas.96.6.2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pihlgren M, Arpin C, Walzer T, Tomkowiak M, Thomas A, Marvel J, Dubois PM. Memory CD44(int) CD8+ T cells show increased proliferative responses and IFN-gamma production following antigenic challenge in vitro. Int Immunol 1999; 11:699-706; PMID:10330275; http://dx.doi.org/ 10.1093/intimm/11.5.699 [DOI] [PubMed] [Google Scholar]
- 27.Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8+ T cell pool. J Immunol 2010; 184:35-44; PMID:19949092; http://dx.doi.org/ 10.4049/jimmunol.0803355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokaji AI, Hockley DL, Kane KP. IL-15 transpresentation augments CD8+ T cell activation and is required for optimal recall responses by central memory CD8+ T cells. J Immunol 2008; 180:4391-401; PMID:18354159; http://dx.doi.org/ 10.4049/jimmunol.180.7.4391 [DOI] [PubMed] [Google Scholar]
- 29.Daudt L, Maccario R, Locatelli F, Turin I, Silla L, Montini E, Percivalle E, Giugliani R, Avanzini MA, Moretta A et al.. Interleukin-15 favors the expansion of central memory CD8+ T cells in ex vivo generated, antileukemia human cytotoxic T lymphocyte lines. J Immunother 2008; 31:385-93; PMID:18391757; http://dx.doi.org/ 10.1097/CJI.0b013e31816b1092 [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011; 118:1255-63; PMID:21653320; http://dx.doi.org/ 10.1182/blood-2011-02-337360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, Chang WC, Bretzlaff W, Starr R, Priceman S et al.. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid Fc receptor binding and improve T cell persistence and anti-tumor efficacy. Mol Ther 2014; 23:757-68; PMID:25366031; http://dx.doi.org/ 10.1038/mt.2014.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 2014; 3:125-35; PMID:25212991; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr Opin Immunol 2009; 21:224-32; PMID:19304470; http://dx.doi.org/ 10.1016/j.coi.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 2005; 115:1616-26; PMID:15931392; http://dx.doi.org/ 10.1172/JCI24480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang A, Chandran S, Shah SA, Chiu Y, Paria BC, Aghamolla T, Alvarez-Downing MM, Lee CC, Singh S, Li T et al.. The stoichiometric production of IL-2 and IFN-gamma mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci Transl Med 2012; 4:149ra20; PMID:22932225; http://dx.doi.org/ 10.1126/scitranslmed.3004306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparrow RL, Komodromou H, Tippett E, Georgakopoulos T, Xu W. Apoptotic lymphocytes and CD34+ cells in cryopreserved cord blood detected by the fluorescent vital dye SYTO 16 and correlation with loss of L-selectin (CD62L) expression. Bone Marrow Transplant 2006; 38:61-7; PMID:16788684; http://dx.doi.org/ 10.1038/sj.bmt.1705405 [DOI] [PubMed] [Google Scholar]
- 37.Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology 2002; 106:127-38; PMID:12047742; http://dx.doi.org/ 10.1046/j.1365-2567.2002.01447.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boesteanu AC, Katsikis PD. Memory T cells need CD28 costimulation to remember. Semin Immunol 2009; 21:69-77; PMID:19268606; http://dx.doi.org/ 10.1016/j.smim.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E et al.. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 2013; 123:594-9; PMID:23281401; http://dx.doi.org/ 10.1172/JCI66327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Naranjo A, Brown CE, Bautista C, Wong CW, Chang WC, Aguilar B, Ostberg JR, Riddell SR, Forman SJ et al.. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J Immunother 2012; 35:689-701; PMID:23090078; http://dx.doi.org/ 10.1097/CJI.0b013e318270dec7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelloquin F, Lamelin JP, Lenoir GM. Human B lymphocytes immortalization by Epstein-Barr virus in the presence of cyclosporin A. In Vitro Cell Dev Biol 1986; 22:689-94; PMID:3023278; http://dx.doi.org/ 10.1007/BF02621085 [DOI] [PubMed] [Google Scholar]
- 42.Donnelly ML, Hughes LE, Luke G, Mendoza H, ten Dam E, Gani D, Ryan MD. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J Gen Virol 2001; 82:1027-41; PMID:11297677; http://dx.doi.org/ 10.1099/0022-1317-82-5-1027 [DOI] [PubMed] [Google Scholar]
- 43.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 2004; 22:589-94; PMID:15064769; http://dx.doi.org/ 10.1038/nbt957 [DOI] [PubMed] [Google Scholar]
- 44.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, Smith DD, Forman SJ, Jensen MC, Cooper LJ. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res 2006; 66:10995-1004; PMID:17108138; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-0160 [DOI] [PubMed] [Google Scholar]
- 45.Stastny MJ, Brown CE, Ruel C, Jensen MC. Medulloblastomas expressing IL13Ralpha2 are targets for IL13-zetakine+ cytolytic T cells. J Pediatr Hematol Oncol 2007; 29:669-77; PMID:17921847; http://dx.doi.org/ 10.1097/MPH.0b013e3181468c68 [DOI] [PubMed] [Google Scholar]
- 46.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8+ T cell subsets. J Immunol 2005; 175:5895-903; PMID:16237082; http://dx.doi.org/ 10.4049/jimmunol.175.9.5895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.