Abstract
Recent breakthroughs in therapeutic modulation of immune cells have led to novel exciting treatments for cancers. Moreover, the cytokine milieu in the tumor microenvironment is important for appropriate immune surveillance. Here, we demonstrate that NF-κB activity in myeloid cells is essential for cytokine-mediated antitumor polarization of immune cells.
Keywords: cytokines, melanoma, myeloid cells, NF-κB, T cells, tumor-associated macrophages
Introduction
Myeloid cells are known to be a key component of the innate immune response during tumorigenesis. In some tumors, macrophages may constitute as much as 50% of the tumor cell population. Although, clearly, there are tumor-associated macrophages (TAMs) that are thought to enhance tumor growth and metastasis1 and immature myeloid cells, known as myeloid-derived suppressor cells, that block the activation of an antitumor T-cell response,2 there are also populations of myeloid cells that inhibit tumor progression and serve to enhance the immune response to the tumor.3 These myeloid cells can affect T-regulatory cells (Tregs), Th17 cells, and many other subgroups of immune cells that play key roles in the modulation of the immune response to the tumor.4 Many recent studies have evaluated the factors that modulate the plasticity of the pro- or antitumor phenotype of leukocytes in the tumor microenvironment.
The tumor itself likely affects the phenotype of the TAMs. Often, macrophages are classified based on the expression of transcription factors, scavenger receptors, membrane proteins, cytokines, chemokines, matrix, and enzymes involved in amino acid metabolism.5 NF–κB-mediated transcription is known to be a key regulator of the expression of numerous cytokines that influence the properties of both lymphoid and myeloid cells in the tumor microenvironment. Myeloid cells are also classified on the basis of their cytokine expression profile. Myeloid cells that produce interferon gamma (IFNγ), interleukin (IL)-12, IL-1β, and inducible nitric oxide synthase (iNOS) are usually regarded as antitumor myeloid cells, whereas those that produce IL-4, IL-13, transforming growth factor β (TGFβ), IL-10High, and arginase-1High are referred to as protumor myeloid cells, although this is a major oversimplification of this biology.5 Moreover, macrophages may produce PDL1 and CTLA4, which result in suppression of CD4-mediated CD8+ T-cell activation, resulting in a decrease in tumor-infiltrating lymphocytes with tumor cell-killing capacity.6 TAMs also produce cytokines that can favor or suppress dendritic cell maturation.
The tumor microenvironment influences the differentiation of monocytes.7 Protumor macrophages have been classified as either MHCIIhi/CD11bhi alternatively activated macrophages (AAMs) or CD11blow/VCAM1high TAMS, and these are different from myeloid-derived suppressor cells (MDSCs). Peripheral blood monocytes recruited into the tumor undergo a “tumor-elicited adaptive immune response,” which can be coupled to the cytokine milieu and, thus, replenish the population of AAMs in the tumor. The expansion of CD11blow/VCAM1hiTAMs within the tumor is associated with an increase in CD8+ TILs that are PD-1+/granzymeB− and promote immune tolerance. Both of these can ultimately promote tumor growth.
Prior work from several groups8,9 suggested that a reduction of myeloid NF-κB activity is associated with an antitumor myeloid phenotype, with enhanced tumor surveillance and a reduction in tumor growth. To further evaluate the role of NF-kB in the manifestation of the pro- or anti-tumor phenotype of myeloid cells, we crossed IKKβf/f mice8 with mice expressing lysosome-driven Cre-recombinase, allowing targeted deletion of IKKβ in myeloid cells to inhibit NF-κB activity in these cells. However, orthotopic implantation of syngeneic and allogenic melanoma tumor cells into C57Bl/6 mice with targeted deletion of IKKβ and reduced NF-κB activity in myeloid cells resulted in markedly enhanced tumor growth.10 This was accompanied by reduction in macrophage, neutrophil, dendritic cell, and T-cell infiltration into the tumor and enhanced expression of CCL11 by myeloid cells. We observed that the enhanced expression of CCL11 resulted in a reduced maturation of dendritic cells. CCL11 is known to be upregulated by the Th2 cytokines IL-4 and IL-13, and downregulated by the Th1 cytokine IFNγ. This CCL11-mediated block in dendritic cell maturation was accompanied by a reduction in the number of activated CD8+ T cells in the tumor. Loss of IKKβ in myeloid cells was also accompanied by reduced expression of the major histocompatibility class (MHC) II by macrophages, likely leading to poor antigen presentation to CD4+ T cells and failure to prime and activate CD8+ T cells. Moreover, the IKKβ−/− macrophages exhibited defective phagocytosis and reduced degradation of tumor cells in the phagosomes. We were using a tissue-specific inducible genetic model where the expression of the oncogene BRAFV600E and loss of PTEN−/− expression was evoked locally in melanocytes to allow development of melanoma; therefore, if these mice received a bone marrow transplant from mice with deletion of IKKβ in myeloid cells, the tumors also grew more rapidly as compared to mice with bone marrow transplants from mice where IKKβ is expressed in myeloid cells. Conversely, we observed that constitutive activation of the NF-κB pathway in myeloid cells resulted in marked inhibition of melanoma tumor growth.
These data demonstrate that, in allogenic and syngeneic mouse xenograft melanoma models as well as in genetically inducible melanoma models, NF-kB activity in myeloid cells is needed to successfully mount an antitumor immune response. The data also imply that, although systemic inhibition of NF-κB might be useful for organ transplant patients, it will likely enhance the growth and metastasis of tumors unless a method for tumor cell-specific delivery of the inhibitor could be developed. As an alternative, combining IKKβ inhibitors with broad-spectrum Janus kinase (JAK) inhibitors or cytokine-blocking antibodies that interrupt the IL-4, IL-13, and IL-10–mediated responses that drive the protumor myeloid cells might be therapeutically beneficial.
Figure 1.
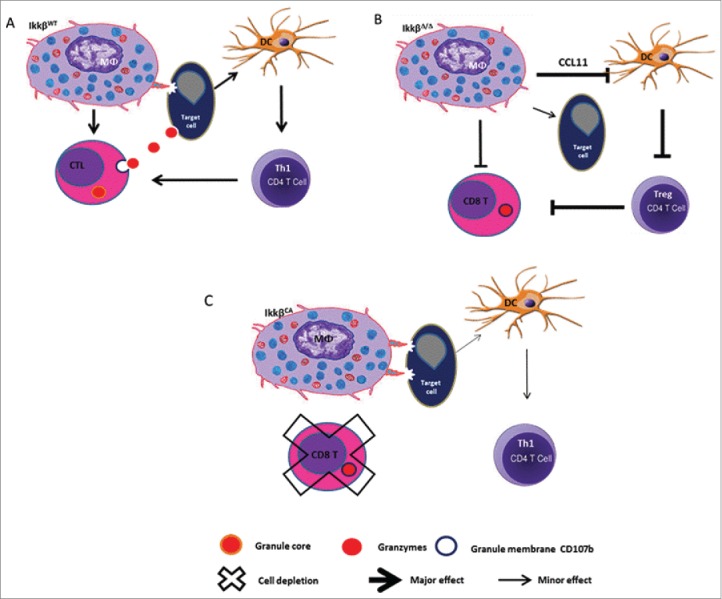
IKKβ activity in myeloid cells is required for rejection of allogenic melanoma and CD8+ T cells are important in this rejection. Knockout of IKKβ in myeloid cells confers a protumor phenotype to immune cells with enhanced tumor growth. Constitutive activation of IKKβ (CA) in myeloid cells blocks melanoma tumor outgrowth.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Jill Suttles, University of Louisville, for her review of this manuscript and insightful comments.
Funding
This work was supported by grants from the Department of Veterans Affairs (5101BX000196–04 and a Senior Research Career Scientist Award to Ann Richmond) and the NIH (grants CA116021 to Ann Richmond, CA116021-S1 to Ann Richmond, CA90625, 5T32CA119925–03, 1F32CA171895–01, GM084333, and CA68485).
References
- 1.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41:49–61; http://dx.doi.org/ 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature Rev Immunol 2012; 12:253–68; http://dx.doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011; 20:300–14; PMID:21907922; http://dx.doi.org/ 10.1016/j.ccr.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91–102; PMID:19647220; http://dx.doi.org/ 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al.. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014; 41:14–20; PMID:25035950; http://dx.doi.org/ 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loke P, Allison JP. Pd-l1 and pd-l2 are differentially regulated by th1 and th2 cells. Proc Nal Acad Sci USA 2003; 100:5336–41; http://dx.doi.org/ 10.1073/pnas.0931259100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science 2014; 344:921–5; PMID:24812208; http://dx.doi.org/ 10.1126/science.1252510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. Ikkbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118:285–96; PMID:15294155; http://dx.doi.org/ 10.1016/j.cell.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol 2006; 176:5023–32; PMID:16585599; http://dx.doi.org/ 10.4049/jimmunol.176.8.5023 [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Hawkins OE, Barham W, Gilchuk P, Boothby M, Ayers GD, Joyce S, Karin M, Yull FE, Richmond A. Myeloid IKKbeta promotes antitumor immunity by modulating ccl11 and the innate immune response. Cancer Res 2014; 74:7274–84; PMID:25336190; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]


