Abstract
Neutrophils are important innate immune cells involved in microbial clearance at the sites of infection. However, their role in cancer development is unclear. We hypothesized that neutrophils mediate antitumor effects in early tumorigenesis. To test this, we first studied the cytotoxic effects of neutrophils in vitro. Neutrophils were cytotoxic against tumor cells, with neutrophils isolated from tumor-bearing mice trending to have increased cytotoxic activities. We then injected an ELR+ CXC chemokine-producing tumor cell line into C57BL/6 and Cxcr2−/− mice, the latter lacking the receptors for neutrophil chemokines. We observed increased tumor growth in Cxcr2−/− mice. As expected, tumors from Cxcr2−/− mice contained fewer neutrophils. Surprisingly, these tumors also contained fewer CD8+ T cells, but more IL-17-producing cells. Replenishment of functional neutrophils was correlated with decreased IL-17-producing cells, increased CD8+ T cells, and decreased tumor size in Cxcr2−/− mice, while depletion of neutrophils in C57BL/6 mice showed the opposite effects. Results from a non-ELR+ CXC chemokine producing tumor further supported that functional neutrophils indirectly mediate tumor control by suppressing IL-17A production. We further studied the correlation of IL-17A and CD8+ T cells in vitro. IL-17A suppressed proliferation and IFNγ production of CD8+ T cells, while CD11b+Ly6G+ neutrophils did not suppress CD8+ T cell function. Taken together, these data demonstrate that, while neutrophils could control tumor growth by direct cytotoxic effects, the primary mechanism by which neutrophils exert antitumor effects is to regulate IL-17 production, through which they indirectly promote CD8+ T cell responses.
Keywords: CD11b+Ly6G+Cells, Neutrophils, Tumor, IL-17 and CD8+T cells
Introduction
The tumor environment is composed of tumor cells, stromal cells and tumor-infiltrating immune cells of both innate and adaptive lineages. These diverse cells communicate with each other by means of direct contact and/or indirect signaling that can be mediated by cytokine and chemokine secretion. This interplay may functionally alter and polarize immune cells in a way which either favors tumorigenesis or limits tumor growth.1,2
There is an emerging interest in the role of neutrophils in cancer. Recent evidence suggests that neutrophils can adopt either protumor or antitumor activity.2-9 Protumor neutrophils are thought to be functionally related to the recently described myeloid-derived suppressor cells (MDSCs).4,10-12 In mice, MDSCs are characterized by the co-expression of the myeloid lineage differentiation antigens Gr-1 and CD11b (Ly6C/G and α M-integrin, respectively).13,14 However, neutrophils, whether suppressive or not, express the same markers. While some properties of antitumor neutrophils have been described,7,8 the mechanisms by which neutrophils exert these effects have not yet been defined. As normal or tumor infiltrating neutrophils are indistinguishable by cell surface markers from g-MDSC, mechanistic studies on antitumor neutrophil function have been difficult. Thus, the effect of neutrophils on early tumor development remains obscure.
A considerable body of work suggests that neutrophil trafficking in the circulation is controlled, at least in part, by chemokines. Based on their structural properties, neutrophil-attracting chemokines are categorized in the ELR+ CXC chemokine family. In mice, there are five members, including CXCL1/KC, CXCL2/MIP-2 and CXCL5/LIX, that interact with a single receptor, CXCR2.15 These chemokines are critically involved not just in migration to inflammatory sites but in homeostatic regulation of neutrophil development and release from marrow. Despite considerable interest in these signals, no consensus has been reached as to their effect on tumor growth.
One important cytokine in inducing chemokine releases is IL-17. IL-17 plays a pivotal role in protecting the host against certain infectious microorganism through neutrophil recruitment, by inducing the production of granulocyte colony-stimulating factor (GCSF) and increasing the expression of CXC chemokines.16-21 Although IL-17 is detected in cancer patients and tumor-bearing mice,22-25 as with neutrophils the precise role of IL-17 during tumor development remains unclear. IL-17 has been shown to exert pro-tumor properties, as local blockade of IL-17A in a model of lung cancer enhanced antitumor immunity, characterized by increased IFNγ, diminished T-regulatory cell number and reduced tumor growth.26,27 We have previously demonstrated an intimate connection between neutrophil homeostasis and IL-17-producing cells.17 Not only does IL-17 induce granulopoiesis through G-CSF, but IL-17 responds rapidly to a perceived deficiency of neutrophils as one critical component of a feedback loop. Since neutrophils and IL-17-producing cells have been both shown in the tumor microenvironment, we sought to examine the neutrophil/IL-17 relationship within the context of early control of tumor growth.
In this paper, we demonstrate that deficiency of neutrophils, either by depletion or presence of chemotaxis-defective neutrophils, enhances early tumor growth. Furthermore, under such circumstances the tumor environment is altered, such that the tumor contains fewer CD8+ T cells and more IL-17-producing cells. Additionally, the major IL-17-producing cell population is altered from αβ T cells to γδ T cells. Adoptive transfer of active neutrophils inhibits IL-17 production, and increases CD8+ T cell number, with an associated decrease in tumor growth. In vitro T cell culture experiments indicate that IL-17A alone is sufficient to limit CD8+ T cell functionality, and that CD11b+Ly6G+ neutrophils, isolated from bone marrow of naive or tumor-bearing mice, do not show suppressive effects on CD8+ T cell function. While neutrophils may control tumor growth through direct cytotoxic effects, as other groups28 and our own in vitro experiments have shown, we believe that this is unlikely to be the primary mechanism by which neutrophils promote antitumor immunity. Rather we suggest that the primary role of neutrophils in promoting antitumor immunity is to control IL-17 secretion, and thus indirectly suppress tumor growth by promoting CD8+ T cell function. Since many cancer therapies may involve depletion of neutrophils, these findings provide new insights that can be employed for the development of novel cancer immunotherapies.
Materials and Methods
Animals
All mice were kept in SPF conditions in the animal facility of the Children's Hospital of Philadelphia. Cxcr2−/− and WT control mice were on a C57BL/6J background. Sex- and age-matched 6- to 10-week-old mice were used for experiments. Mouse experiments were conducted under oversight of the Institutional Appropriate Animal Care and Use Committee.
Cell line
The murine Lewis lung carcinoma (LLC) cell line (American Type Culture Collection) was propagated in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS, 2 mmol/L L-glutamine and 10 ug/mL penicillin/streptomycin. Mouse TC-1 lung cancer cells,29 were maintained in RPMI1640 medium (Gibco) supplemented with 10% fetal bovine serum (Georgia Biotechnology), 2 mmol/L L-glutamine and 10 ug/mL penicillin/streptomycin. AE-17 cells were also maintained in RPMI 1640 medium (with 2 mmol/L L-glutamine, 10 ug/mL penicillin/streptomycin, 25mM HEPES and 5% FCS).
Animal flank tumor models
Mice were injected on the right flank with 2 × 106 LLC cells or 1.2 × 106 TC-1 cells in C57B6 and Cxcr2−/− mice. 2 × 106 AE-17 cells were injected in BALB/c mice.
ELISA assay
Cytokines and chemokines were quantified by ELISA using kits specific for CXCL1/KC, CXCL2/MIP-2, CXCL5/LIX, G-CSF, and IL-17A according to the manufacturer's specifications (R&D Systems or eBioscience).
In vitro killing assay
In vitro killing assay was performed following the protocol of Reise and colleagues.30 Briefly, luciferase labeled cells (5000/well) were plated on a 96-well in MEM 0.5% FBS. Four hours later, purified neutrophils (100,000/well or 200,000/well) were added to the plated tumor cells and cocultured overnight. Following overnight incubation, luciferase activity was measured using the Clarity (Bio-Tek) microplate luminescence reader. In vitro killing experiments were repeated at least three times.
In vivo depletion of Ly-6G+ neutrophils
Neutrophil depletion was achieved using daily intraperitoneal injections of 250 ug 1A8 monoclonal Ab (anti-Ly-6G; BioXcell) starting one day before LLC flank injection. Control mice were injected with 250 ug 2A3 isotype control Ab (Rat IgG2a; BioXcell). Systemic neutrophil depletion was evaluated periodically with CBC count and manual blood differentials. Tumor and splenic neutrophil depletion was confirmed at the end of each experiment using flow cytometry.
Adoptive transfer of neutrophils
Mice were injected on the right flank with 2 × 106 LLC in Cxcr2−/− mice. 4 h later, mice were intravenously injected with 1 × 107 isolated BM neutrophils from C57BL/6 mice or with vehicle control (PBS). I.V. injections were repeated on day 2, 8 and 11.
Flow cytometry
To examine cytokine production, single cell suspensions of tumor, lung, bone marrow, and spleen were cultured at 37°C in RPMI 1640 containing 10% FBS, 1% penicillin/streptomycin and 1 ug/mL BFA (Sigma-Aldrich) for 4 h with 30 ng/mL PMA (Sigma-Aldrich) and 1 ug/mL ionomycin (Sigma-Aldrich). Unstimulated single cell suspensions were stained directly ex vivo. Cells were washed in PBS and stained with live/dead blue viability dye (Invitrogen) prior to surface staining. Fixation and intracellular staining were done using the FoxP3 fix/perm buffer kit (Ebioscience). The following antibodies were purchased from BioLegend: CD11b (M1/70), Ly6G (1A8), CD45.2 (104), TCRβ (H57–597), TCRγδ (GL3), IFNγ (XMG1.2), CD8α (53–6.7), CD4 (GK1.5). Antibody against IL-17A (eBio17B7) was purchased from Ebioscience. Flow cytometry was performed on an LSR Fortessa Flow Cytometer (BD Biosciences). Data were analyzed using Flowjo (Treestar).
T-cell proliferation and intracellular IFNγ production
For proliferation assay, purified splenic CD8+ T cells from C57BL/6 mice were labeled with 2.5 uM CellTrace ef670 (eBioscience) in PBS for 6 min at 37°C. The labeling reaction was quenched by addition of cold DMEM medium with 10% FCS. Sorted CD11b+Ly6G+ cells from spleens of LLC tumor-bearing mice or isolated neutrophils from bone marrows of naive C57BL/6 mice were cocultured with polyclonal-stimulated (5 ug/mL plate-bound anti-CD3ε (2C11) and 5 ug/mL anti-CD28 (37.51)) and ef670-labeled splenic CD8+ T cells at a series of ratios (4:1, 1:1, 1:4) in the presence or absence of 10ng/mL of rmIL-17A (Peprotech). The proliferation and IFNγ production of CD8+ T cells was evaluated 3 d later by flow cytometry as described above.
H&E and immunohistochemical staining
Portions of tumor were fixed in 10% neutral buffered formalin, routinely processed, embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin (H&E) for light microscopy. Additional tissue sections for immunohistochemistry were stained with primary antibodies for myeloperoxidase (MPO) (Dako A0398) with DAB (DAKO Cytomation) for antigen detection. Only cells with 2+ and 3+ intensity of staining were selected for analysis.
Statistical analyses
We performed all statistical analyses with the GraphPad Prism software (version 4). Data are presented as means ± SEM, and a p value less than 0.05 was considered significant. The number of mice used in each experiment is noted in the figure legends. We used a 1-way ANOVA, 2-way ANOVA or 2-tailed Student's t-test to compare data sets, as appropriate.
Results
LLC cells induce neutrophil chemotaxis by secreting ELR+CXC chemokine and neutrophils had a cytotoxic effect on these cells in vitro
A number of lung cancers have been described to produce neutrophil chemoattractants.31,32 In order to examine the role of neutrophils in early tumorigenesis, we first compared a variety of cell lines for the production of ELR+ CXC neutrophil chemokines. As seen in Fig. 1A, not all mesothelioma and NSCLC cell lines tested produced detectable amounts of both CXCL1 and CXCL5, suggesting that production of ELR+ CXC chemokines is highly cell line dependent. Of these cell lines, only one expressed both CXCL1 and CXCL5 and is syngeneic to our C57BL/6 recipient mice: the lung cancer cell line, LLC.33
Figure 1.
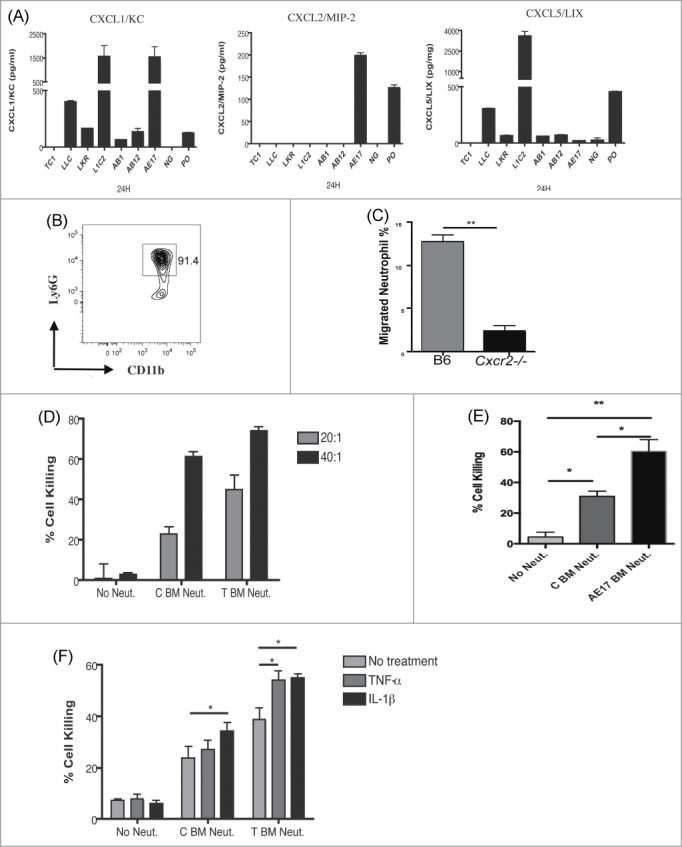
LLC cells induce neutrophil chemotaxis via secreting ELR+CXC chemokine and neutrophils had a cytotoxic effect on these cells in vitro. (A) The production of CXCL1, CXCL2 and CXCL5 by NSCLC and mesothelioma lines was measured by ELISA. (B) Neutrophils were purified from bone marrow of C57BL/6 mice and were analyzed by flow cytometry for purity by CD11b+Ly6G+ staining. (C) Purified neutrophils from C57BL/6 or Cxcr2−/− mice were assayed by transwell migration for chemotaxis toward LLC cells. Negative control was neutrophil migration toward a non-neutrophil secretion lung cancer cell line. Cells were counted from both the top and bottom of transwell plate after 16 h. (D) Neutrophils isolated from bone marrow of naive (C BM Neut) or tumor-bearing (T BM Neut) mice were cocultured with luciferase-labeled LLC cells at 20:1 and 40:1 neutrophil to tumor cell ratio. Following overnight incubation, luciferase activity was measured using the Clarity (Bio-Tek) microplate luminescence reader as a measure of cytotoxicity. (E) As above neutrophils isolated from bone marrow of naive (C BM Neut) or AE17 tumor bearing (AE17 BM Neut) mice were assayed for cytotoxicity against LLC cells, at a 20:1 neutrophil:tumor cell ratio. (F) Addition of 10ng/mL recombinant TNF-α or IL-1β induced enhanced neutrophil cytotoxic effect, measured as in D. *p <0.05 **p <0.01; ***p <0.001. Values are mean ± SEM, n = 3/experiment, representative of three experiments).
To test the chemotactic function of chemokines secreted by LLC cells, we utilized a transwell assay. Neutrophils were purified from the bone marrow of C57BL/6 or Cxcr2−/− mice, which lack the primary neutrophil receptor for ELR+ CXC chemokines, and tested for their ability to migrate toward tumor cells (Fig. 1B, C). After 16 h of incubation, neutrophils were collected from both top and bottom chambers and quantified. As seen in Fig. 1C, neutrophils isolated from C57BL/6 mice showed enhanced migration toward LLC cells, while neutrophils isolated from Cxcr2−/− mice were unresponsive. These data indicate that chemokine expression by LLC tumor cells induces CXCR2-dependent neutrophil chemotaxis.
To test whether neutrophils have a direct cytotoxic effect on LLC cells, we performed in vitro cell killing assays as previously described.30 Neutrophils isolated from bone marrow exerted cytotoxic effects and there was a trend for neutrophils isolated from LLC tumor-bearing mice to induce more cell killing than those from naive mice (Fig. 1D). The cytotoxic effect was cell number dependent (Fig. 1D). A similar killing pattern was observed with LLC cells cocultured with neutrophils from AE17-tumor bearing mice (Fig. 1E). We further tested whether inflammatory cytokines, specifically TNF-α and IL-1β, affected neutrophil cytotoxicity. Both TNF-α and IL-1β enhanced neutrophil-mediated cancer cell killing in our coculture model (Fig. 1F).
These results establish use of the LLC tumor cell line as an appropriate model for investigating the role of tumor infiltrating neutrophils. Further, they indicate that neutrophils may exert a direct cytotoxic effect on cancer cells, and this effect may be enhanced by cytokines such as TNF-α and IL-1β.
Heterotopic LLC tumors fail to recruit neutrophils and show enhanced tumorigenicity in Cxcr2−/− mice at early stages of tumorigenesis
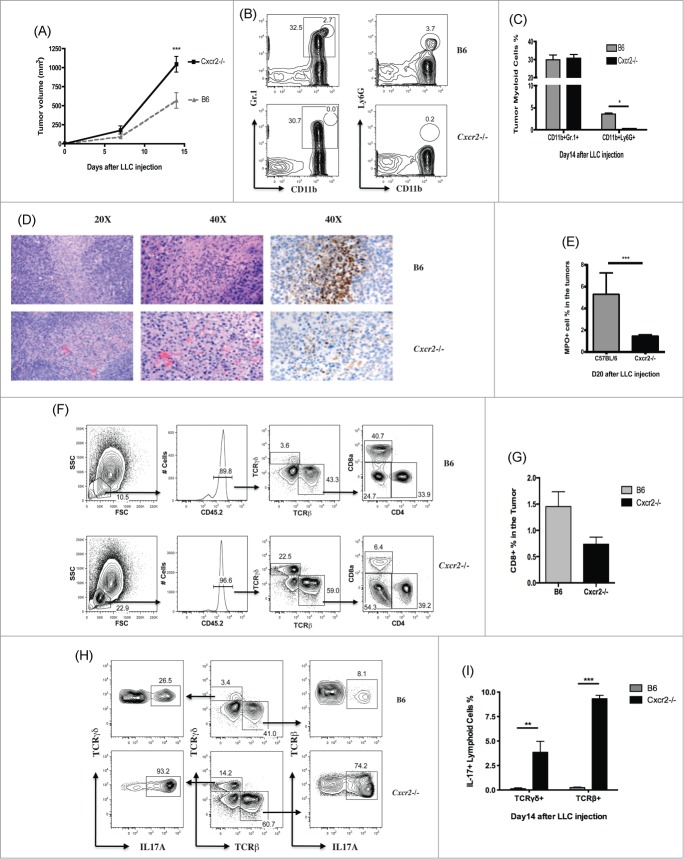
Since LLC cells express neutrophil chemokines and attract neutrophils in vitro in a CXCR2 dependent manner, we hypothesized that neutrophils might directly affect tumor growth in mice injected with LLC cells. To test the role of neutrophils in controlling LLC tumors, we injected 2 × 106 mouse LLC cells into the flanks of C57BL/6 and Cxcr2−/− mice. We observed increased growth of tumors in Cxcr2−/− recipients as compared to C57BL/6 controls within 2 weeks of injection (Fig. 2A).
Figure 2.
For figure legend, see page 6.
Recently, it has become clear that many cells labeled with Gr-1, often used as a neutrophil marker, actually represent heterogeneous cell populations. The percent of CD11b+Gr-1+ cells within tumors from LLC-injected C57BL/6 and Cxcr2−/− mice was not significantly different (29.8 ± 2.7% and 30.8 ± 2.1% CD11b + Gr-1+ cells in C57BL/6 and Cxcr2−/− tumors respectively, Fig. 2B, C), indicating that the accumulation of the large Gr-1+ population is not dependent on CXCR2. However, the population of bona fide neutrophils, which are CD11b + Ly6G+ and can also be identified by high expression of Gr-1, was significantly different between LLC-injected C57BL/6 and Cxcr2−/− mice (3.5 ± 0.3% and 0.2 ± 0.0% respectively, Fig. 2B, C).
In order to determine where neutrophils were localized within the LLC tumors, we used immunohistochemical staining of MPO to identify neutrophil activity within the tumor 14 d after injection. As seen in Fig. 2D, neutrophils were found largely within necrotic areas of the tumor tissue, and the percentages of MPO+ cells in the tumor was consistent with the percent of CD11b + Ly6G+ cells in the tumors as identified by flow cytometry (Fig. 2E). These results indicate that only the infiltration of CD11b + Ly6G+ neutrophils is CXCR2-dependent, and influx of other Gr-1+ cells is intact in CXCR2 deficient mice. We also observed that, consistent with decreased MPO+ cells in the tumor and increased tumor volume, the percent of tumor necrosis in LLC injected Cxcr2−/− mice was decreased relative to controls (Fig. S1A However, when tumors were examined 21 d after inoculation, we found that necrosis was increased in Cxcr2−/− mice (Fig. S1B). This phenomenon was previously observed by Kean and colleagues, and is likely related to loss of pro-angiogenic effects of CXCR2.15,34,35
In addition to increased tumor size, tumors isolated from Cxcr2−/− mice showed alterations in lymphoid populations other than neutrophils (Fig. 2F). The total CD8+ T cell percentage in the tumors of C57BL/6 mice was two-fold more than that of Cxcr2−/− mice (1.45 ± 0.28% and 0.74 ± 0.13% respectively), while the population of TCRγδ+ T cells was expanded in tumors from Cxcr2−/− mice (Fig. 2F, G). Strikingly, most of the lymphoid cells isolated from tumors of Cxcr2−/− mice (˜70%) were IL-17A positive; only about 3% of the same population was IL-17A positive in C57BL/6 mice (Fig. S1C). These IL-17 producers were enriched for TCRβ+ CD4+ cells (Fig. S1C). Of TCRγδ+ T cells found in the CD45.2+ lymphoid population of tumors from C57BL/6 (˜3% total lymphoid cells) and Cxcr2−/− (˜15% total lymphoid cells) mice, almost all of these TCRγδ+ T cells in Cxcr2−/− mice (90%), and many in C57BL/6 mice (25%), were IL-17-producing cells. There was a significant increase in both TCRβ and TCRγδ IL-17 producing cells between C57BL/6 and Cxcr2−/− mice (Fig. 2H, I).
Taken together, these data indicate that dysfunction of bona fide neutrophils (CD11b+ Ly6G+ cells), and no other CD11b+Gr-1+ cell populations, is associated with decreased CD8+T cell numbers, increased IL-17 production from several cell lineages, and enhanced tumor growth. However, from these data it is unclear whether the effect of neutrophil loss on tumor CD8+ T cells and IL-17-producing lymphoid cells is exerted within the tumor microenvironment, in the systemic compartment, or in other tissue sites.
Neutrophil depletion augments tumor growth rate, increases percentages of IL-17-producing cells, and reduces percentages of CD8+ T cells in the tumors
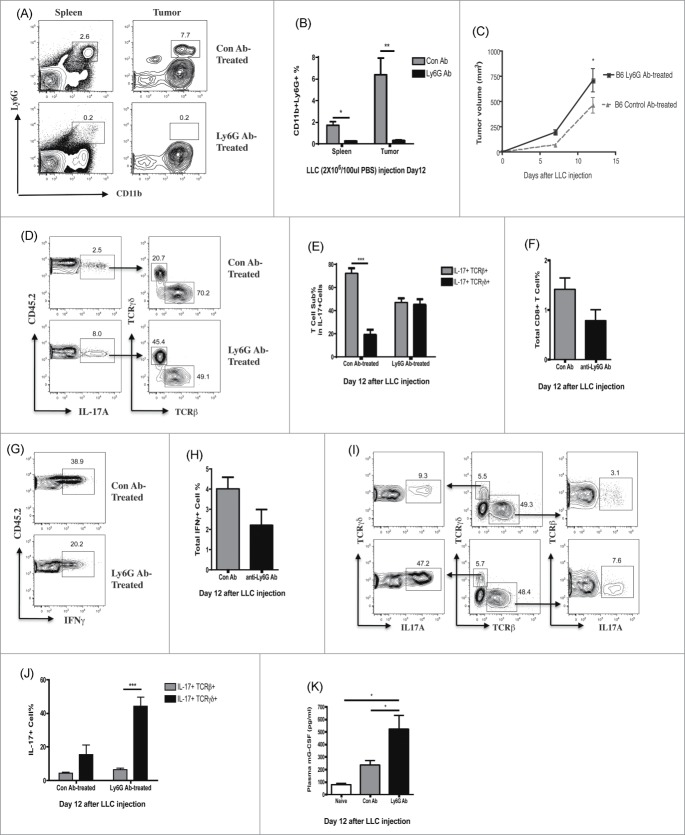
Since recruitment of CD11b + Ly6G+ cells is CXCR2-dependent, and these cells may affect tumor growth by altering the balance of tumor infiltrating IL-17-producing cells and CD8+T cells, we next tested directly the effects of CD11b + Ly6G+ cell depletion on tumor growth in C57BL/6 mice. Based on published7 and our own unpublished data, intermittent depletion of neutrophils by anti-Ly6G antibody post-tumor engraftment does not decrease, and may actually increase, neutrophil numbers in the circulation. On the other hand, daily neutrophil depletion appears highly effective until day 14 post-tumor engraftment.7 Based on these findings, we developed a new approach for neutrophil depletion. Neutrophil depletion was started the day before LLC flank injection via intraperitoneal injection of 250 ug anti-Ly6G antibody and depletion was performed daily until mice were sacrificed on day 12. Control mice were injected with 250 ug isotype control antibody.
Analysis of spleens and tumors showed that CD11b + Ly6G+ cells were dramatically reduced after antibody, but not isotype control, treatment (Fig. 3A, B). The CD11b + Gr-1high population was also specifically diminished after anti-Ly6G antibody treatment, again indicating that the Gr-1high cells represent the CD11b + Ly6G+ cell population (data not shown). Tumor progression and lymphocyte infiltration in neutrophil depleted tumor-bearing C57BL/6 mice phenocopied our observations of tumor-bearing Cxcr2−/− mice. Neutrophil depletion significantly augmented tumor growth (Fig. 3C) and increased the percentages of CD45.2 + IL-17-producing cells (from 3.11 ± 0.55% to 7.26 ± 1.66%) within the tumor (Fig. 3D). IL-17-producing cells were predominantly TCRβ+ T cells in isotype control treated mice, but TCRγδ+ and TCRβ+ T cells contributed equally to the IL-17 production in neutrophil-depleted mice (Fig. 3D, E). Neutrophil depletion also decreased the total percentages of tumor infiltrating CD8+ T cells (from 1.41 ± 0.23 to 0.78 ± 0.22) (Fig 3F). Consistent with a loss of CD8+ infiltrating T cells, neutrophil depletion led to decreased total IFNγ producing cells in the tumors (Fig. 3G, H). Although neutrophil depleted mice did not show skewing toward TCRγδ+ T cells within the tumor T cell population, TCRγδ+ T cells were much more likely to be IL-17-producing cells, similar to findings in tumor-bearing Cxcr2−/− mice (Fig. 3I, J). These higher levels of IL-17 in the tumors corresponded with increased plasma levels of G-CSF in the neutrophil-depleted group (Fig. 3K).
Figure 3.
For figure legend, see page 8.
These results are consistent with the concept that the absence of tissue-infiltrating neutrophils, whether through neutrophil deficiency or dysfunction, modifies the tumor microenvironment to increase IL-17 production and decrease CD8+ T cell number, leading to failure to control early tumorigenesis. This still leaves open the question, however, of whether this effect is exerted locally in the tumor, or at other sites.
Replenishment of functional neutrophils inhibits IL-17A production and reduces tumor growth
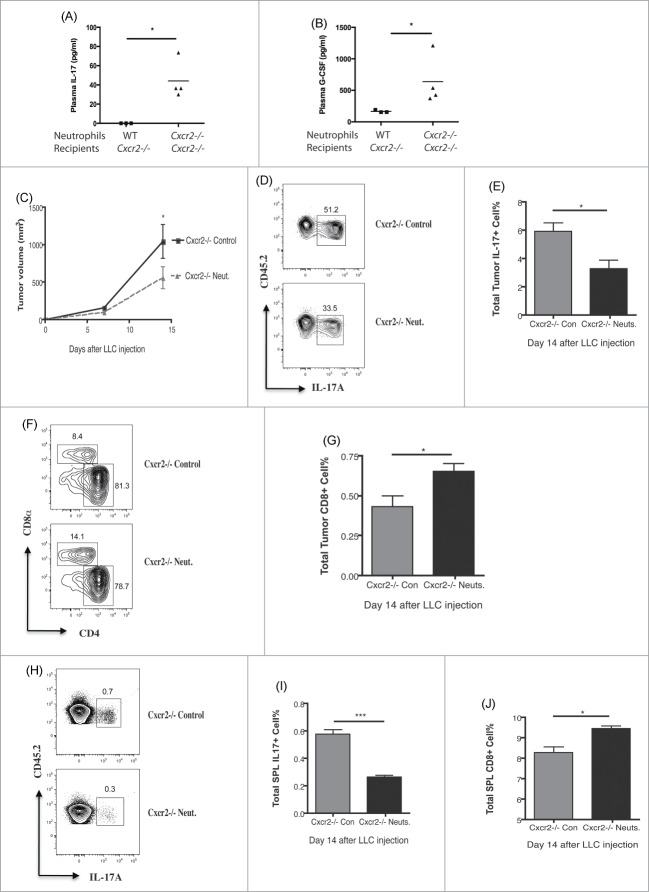
Our results from Cxcr2−/− mice and neutrophil-depleted WT mice suggested that neutrophils can condition the tumor microenvironment to limit IL-17A production and promote CD8+ T cell function. Therefore, we tested whether CXCR2 sufficient neutrophils could rescue the loss of tumor control observed in tumor-bearing Cxcr2−/− mice. We first compared the roles of normal neutrophils from C57BL/6 mice and chemotaxic-defective neutrophils from Cxcr2−/− mice by adoptively transferring 1×107 purified bone marrow neutrophils, either from WT or Cxcr2−/− mice, into Cxcr2−/− recipients. 24 h later, the Cxcr2−/− mice injected with WT neutrophils showed significantly reduced plasma levels of IL-17A and G-CSF compared with those injected with Cxcr2−/− neutrophils (Fig. 4A, B). These data indicate that only functional neutrophils can control the IL-17A/G-CSF axis, and that this is a systemic effect.
Figure 4.
For figure legend, see page 10.
To test whether functional neutrophils can limit tumor growth in vivo, we intravenously injected 1 × 107 bone marrow neutrophils isolated from naive C57BL/6 mice on day 1, 2, 8 and 11 after LLC tumor engraftment in Cxcr2−/− mice. Control tumor-bearing Cxcr2−/− mice were injected with PBS. As seen in Fig.4C, adoptive transfer of neutrophils limited tumor growth (Fig. 4C), led to decreased percentages of IL-17-producing cells (Fig. 4D, E) and increased CD8+ T cells in the tumors (Fig. 4F, G). Similar changes in cell populations were also found in the spleens (Fig. 4H–J). Surprisingly, neutrophil percentages were not significantly different in the tumors of control and neutrophil-replenished Cxcr2−/− mice (data not shown), suggesting that infused neutrophils did not contribute significantly to the tumor-associated neutrophils (TAN) population. Hence, the effects of these infused neutrophils on tumor control are likely indirect, due to the ability of functional neutrophils to limit IL-17A production systemically.
This suggests that increased IL-17A due to dysfunction or depletion of neutrophils will lead to loss of early tumor control independent of neutrophils directly infiltrating tumor. To test this, we injected Cxcr2−/− and C57BL/6 mice with a cell line that produces very little ELR+CXC neutrophil chemokine, the TC-1 cell line (Fig. 1A). As this tumor should not robustly recruit neutrophils via CXCL1/5, direct effects of neutrophils on limiting tumor growth should be minimal, allowing observation of indirect effects. We hypothesized that, similar to our LLC tumor observations, Cxcr2−/− mice would fail to control TC-1 tumors. Consistent with this, we observed that TC-1 injected Cxcr2−/− mice had increased tumor burden (Fig. S2A). TC-1 injected C57BL/6 mice did show some neutrophil infiltration into the tumor that was CXCR2-dependent, as this small population was further decreased in Cxcr2−/− mice (Fig. S2B, C). However, the neutrophil recruitment to TC-1 tumors was approximately five-fold lower than the recruitment observed in LLC tumors, suggesting a minimal role for direct cytotoxicity as an effective mechanism of neutrophil-mediated tumor control. Similar to our observations in the LLC tumor model, in TC-1 tumors from Cxcr2−/− mice we observed increased IL-17-producing cells, decreased CD8+ cells, and decreased IFNγ+ CD8 cells (Fig. S2D–F). Thus, in a model in which direct effects of neutrophils within the tumor are limited, dysregulation of IL-17 by defective neutrophils in Cxcr2−/− mice indirectly leads to loss of tumor control.
IL-17A suppresses proliferation and IFNγ production of CD8+ T cells in vitro
Our previous results indicate that neutrophils promote antitumor immunity by limiting IL-17A production systemically. However, it is unclear what role IL-17A plays in promoting tumor growth. To study mechanisms by which IL-17A affects tumor development, we first investigated whether IL-17A could directly promote tumor growth in vitro. IL-17A had no direct effect on LLC cell viability or growth (data not shown) after 24 h of stimulation. This indicates that IL-17A is unlikely to directly promote LLC tumor growth, but that IL-17 could lead to a pro-tumor microenvironment by affecting other cell types.
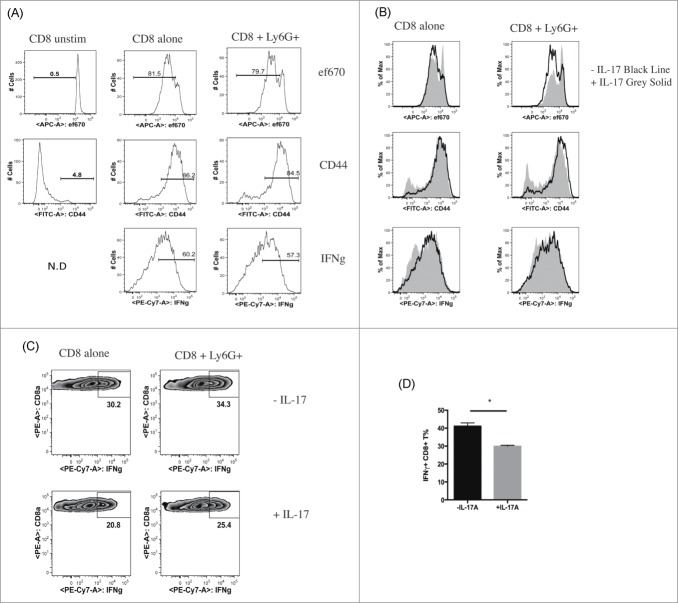
As our data indicated a correlation between IL-17A producing cells and CD8+ T cells, we focused on whether IL-17A might affect CD8+ T cells. We cocultured neutrophils from bone marrow of naive mice with anti-CD3/CD28 stimulated CD8+ T cells in the presence or absence of exogenous IL-17A with or without CD11b+Ly6G+ neutrophils. CD11b+Ly6G+ cells isolated from the bone marrow of naive C57BL/6 mice did not affect proliferation and IFNγ production of CD8+ T cells, consistent with CD11b+Ly6G+ neutrophils being functionally distinct from related MDSCs (Fig. 5A). Surprisingly, IL-17 alone decreased proliferation and IFNγ production of CD8+ T cells, in the presence or absence of neutrophils (Fig. 5B). To further examine the effect of IL-17A in the context of cells derived from tumor-bearing mice, we sorted CD11b+Ly6G+ cells from the spleens of 14 day LLC tumor-bearing mice, and cocultured them with CD8+ T cells from naive mice in the presence or absence of IL-17A. CD11b+Ly6G+ cells isolated from tumor-bearing mice did not show strong suppressive effects on T cell proliferation or IFNγ production (Fig. 5C and data not shown). We again observed that IL-17A suppressed IFNγ production of CD8+ T cells; this effect was not altered by coculture with CD11b+Ly6G+ cells from tumor bearing mice (Fig. 5C, D).
Figure 5.
IL-17A suppresses proliferation and IFNγ production of CD8+ T cells in vitro. (A) Representative flow cytometry histograms of CD8+ T cells from naive C57BL/6 mice cultured with or without CD11b+Ly6G+ cells isolated from the bone marrow of naive C57BL/6 mice. Proliferation, CD44 expression, IFNγ production of CD8+ T cells was measured by flow cytometry after anti-CD3/CD28 stimulation. (B) Representative flow cytometry histograms of CD8+ T cells from naive C57BL/6 mice cultured with or without CD11b+Ly6G+ cells, isolated from the bone marrow of naive C57BL/6 mice, in the presence or absence of IL-17A, and assayed by flow cytometry as in A. (C) Representative flow cytometry histograms of IFNγ production from CD8+ T cells cultured in the presence or absence of CD11b+Ly6G+ cells isolated from the spleens of LLC tumor-bearing C57BL/6 mice, with or without IL-17A. (D) Quantification of IFNγ production of CD8+ T cells cultured in the presence or absence of IL-17A. Values are mean ± SEM (n = 3).
Together with our neutrophil depletion and repletion experiments, in which IL-17A was correlated with tumor control, these results demonstrate that IL-17A could impair the antitumor immune response through suppressing proliferation and IFNγ production of tumor-infiltrating CD8+ T cells.
Discussion
A considerable body of evidence supports the importance of inflammatory cells in the control or progression of different types of cancer.36-39 Neutrophils are primary inflammatory cells and essential to protect the host during the early phases of microbial infection. However, the role of neutrophils in tumor development remains controversial.3,4,34,40-45 Here, we suggest that early control of the growth of syngeneic heterotopic tumors in mice is facilitated by neutrophils via an indirect mechanism that, to the best of our knowledge, has not heretofore been suggested.
Neutrophils are found within many human and murine tumors, and research thus far on the role of neutrophils in cancer has focused largely on these tumor associated neutrophils (TAN).3,6,46-50 The apparent function of these neutrophils may vary depending on the tumor context. Typically, the microenvironment surrounding a solid tumor possesses many of the characteristics of chronic inflammation, a condition considered favorable for tumor growth and spread. However, inducing a shift from this chronic inflammatory state toward an acute inflammatory response may convert neutrophils into anticancer effector cells.46 To date, a clear consensus on the relationship of neutrophil pro/antitumor function with the tumor microenvironment eludes the field.
The infiltration and effects of neutrophils within a tumor may be related to intrinsic properties of both the tumor and the host. In this regard, considerable interest has focused on the vascular supply to the tumor. CXCR2 on endothelial cells functions as an angiogenic receptor.15,35 Keane and coworkers reported that heterotopic LLC tumor growth was attenuated in Cxcr2−/− mice over a longer time course than that used in these studies, in which angiogenesis may indeed play a role.34 In our model, we observed less necrosis in tumors from day 14 LLC-injected Cxcr2−/− mice, but on day 21 somewhat more necrosis were found in tumors from Cxcr2−/− mice (Fig. S1A, B), suggesting that the angiogenic effects of CXCR2 are more important at later stages of tumor control. At both timepoints, regions of necrosis were associated with neutrophil infiltration in tumors of B6 mice, but not in Cxcr2−/− (Fig. 2D), further suggesting that direct cytotoxicity of neutrophils did not fully account for restricting tumor growth in CXCR2 sufficient mice.
To date, a systemic antitumor role for neutrophils has not been described. Rather, many groups have suggested that systemic MDSCs, including granulocytic MDSCS, function as a major protumor cellular compartment.4,10,51 Here, we show that functional CD11b+Ly6G+ cells with tissue-infiltrating potential, regardless of presence or absence in the tumor, possess not protumor, but antitumor ability at the early phases of tumorigenesis.
Direct cytotoxicity of neutrophils for cancer cells has been shown in a number of settings,2,52 and we have recapitulated these findings. We show here that bone marrow-derived neutrophils from naive C57BL/6 mice are capable of direct cytotoxicity, that neutrophils from tumor-bearing C57BL/6 mice are more directly toxic on cancer cells in vitro, and neutrophils from tumor-bearing mice are more readily stimulated by IL-1β/TNFα to enhance killing. These data are more consistent with a primed or partially activated neutrophil than with one that is suppressive. While these data suggest neutrophils can be cytotoxic against tumor cells, the artificial nature of these in vitro killing assays makes it questionable whether this is an important antitumor mechanism in vivo. However, for neutrophils, this may complement more robust indirect mechanisms by which they may promote an antitumor microenvironment.
It has been shown that IL-17A and F are capable of inducing neutrophil generation via induction of G-CSF and upregulating neutrophil chemokines.16-21 We have recently shown that IL-17 is part of a feedback control system to regulate neutrophil availability in tissues.17 Our data here show defective neutrophil trafficking induced more IL-17-producing cells in the tumors of Cxcr2−/− mice. Furthermore, TCRγδ+ T cells appear to become major IL-17-producing cells. Depletion of neutrophils, as we show here, acutely induces IL-17-producing cells (Fig. 3D). Thus, in both neutrophil dysfunction (Cxcr2−/− mice) and deficiency (neutrophil-depleted C57BL/6 mice), there is evidence of a compensatory increase in IL-17-producing cells, which can be detected in the tumor itself. Adoptive transfer of neutrophils induces a reciprocal change by decreasing numbers of IL-17-producing cells. By using two tumor cell lines, one which induces neutrophil chemotaxis into the tumor itself and one that does not, we have found that the conditioning effect of neutrophils on IL-17 production, systemically and in the tumor, is a primary driving force on early tumor control.
Availability of neutrophils directly affected local and systemic IL-17 production, however IL-17 had no direct effect on cancer cell viability. Since effector CD8+ T cells have been recognized as a main player in antitumor immune responses, we further examined the relationship of IL-17A and CD8+ T cells in vitro. IL-17 alone suppressed proliferation and IFNγ production of CD8+ T cells in the presence and absence of neutrophils. Neither CD11b+Ly6G+ cells isolated from the bone marrow of naive C57BL/6 mice, nor sorted CD11b+Ly6G+ cells from the spleens of 14 day LLC tumor-bearing mice affected CD8+ T cell function. These data indicate that the CD11b+Ly6G+ population, whether naive or primed, at early stages of tumorigenesis is functionally distinct from g-MDSCs, and that they play an antitumor role by regulating IL-17 production. In the absence of neutrophil-mediated control of IL-17 production, IL-17 exerts direct, negative effects on CD8+ T cell function.
This work raises several questions for further research. While focusing on early tumor growth allowed us to concentrate on the effects of neutrophils on tumor control, as opposed to angiogenesis, this required a larger inoculum which is a limitation of this study design. Specifically, in control mice this high inoculum may have supported a more pro-inflammatory, increased antitumor immune response than might be observed otherwise. Further, the suppressive effect of IL-17A on CD8+ T cells, which our in vitro work identified, could be occurring in peripheral lymphoid organs or in the tumor. Further work will be needed to clarify the role of IL-17A in CD8+ T cell dysfunction. Similarly, inferences of this study for cancer in humans is complex. However, the implication that chemotherapy-induced neutropenia might increase IL-17-producing cells in humans should be investigated as a confounding variable in the development of cancer immunotherapies.
In summary, neutrophil depletion and dysfunction, via deletion of CXCR2, are associated with enhanced tumor growth, increased IL-17-producing cells, and decreased CD8+T cells. Replenishment of Cxcr2−/− mice with functional neutrophils reverses these phenomenon. Our data suggest that availability of functional neutrophils orchestrates a complex immune response by regulating IL-17 production to modify tumor growth. Specifically, we determined that IL-17A suppresses proliferation and IFNγ production of CD8+ T cells in vitro, suggesting that neutrophils limit early tumor growth by indirectly promoting antitumor CD8+ T cell responses. Although MDSCs have been well recognized as playing a protumor role, our data indicate that CD11b+ Ly6G+ neutrophils are not suppressive on CD8+ T cell response. Moreover, they do not have to be found within the tumor to exert profound antitumor effects. This indirect effect of neutrophils on tumor control via limiting IL-17-producing cells fits well with data from several groups suggesting that IL-17-producing cells promote tumor growth.22,25, 53 Taken together, these data demonstrated that, while neutrophils could control tumor growth by direct cytotoxic effects, the primary mechanism by which neutrophils exert antitumor effects is to regulate IL-17 production, through which they indirectly promote CD8+ T cell responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Dmitry I. Gabrilovich and Dr. Thomas Condamine for providing technical direction in CD8+ T cell suppression experiments. We also thank Dr. Florin Tuluc and Dr. Eric Reidel for technical assistance in flow cytometry.
Funding
This study was supported by NIH grants R01HL068876 (to G.S. Worthen), P01CA66726 (to S.M. Albelda), and R01AI080765 (to P.M. Oliver).
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 2.Brandau S, Dumitru CA, Lang S. Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol 2013; 35(2):163-76; PMID:23007469; http://dx.doi.org/ 10.1007/s00281-012-0344-6 [DOI] [PubMed] [Google Scholar]
- 3.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 2011; 71:2411-6; PMID:21427354; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2583 [DOI] [PubMed] [Google Scholar]
- 4.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P et al.. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol 2011; 89:311-7; PMID:21106641; http://dx.doi.org/ 10.1189/jlb.0310162 [DOI] [PubMed] [Google Scholar]
- 5.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL et al.. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19(1):57-64; PMID:23202296 [DOI] [PubMed] [Google Scholar]
- 6.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012; 33:949-55; PMID:22425643; http://dx.doi.org/ 10.1093/carcin/bgs123 [DOI] [PubMed] [Google Scholar]
- 7.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011; 20:300-14; PMID:21907922; http://dx.doi.org/ 10.1016/j.ccr.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell 2009; 16:183-94; PMID:19732719; http://dx.doi.org/ 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res 2012; 18:5212-23; PMID:22837179; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava MK, Zhu L, Harris-White M, Kar U, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S et al.. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 2012; 7:e40677; PMID:22815789; http://dx.doi.org/ 10.1371/journal.pone.0040677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One 2011; 6:e27690; PMID:22110722; http://dx.doi.org/ 10.1371/journal.pone.0027690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawant A, Schafer CC, Jin TH, Zmijewski J, Tse HM, Roth J, Sun Z, Siegal GP, Thannickal VJ, Grant SC et al.. Enhancement of antitumor immunity in lung cancer by targeting myeloid-derived suppressor cell pathways. Cancer Res 2013; 73:6609-20; PMID:24085788; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004; 172:989-99; PMID:14707072; http://dx.doi.org/ 10.4049/jimmunol.172.2.989 [DOI] [PubMed] [Google Scholar]
- 14.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci U S A 1995; 92:6254-8; PMID:7603979; http://dx.doi.org/ 10.1073/pnas.92.14.6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett 2008; 267:226-44; PMID:18579287; http://dx.doi.org/ 10.1016/j.canlet.2008.04.050 [DOI] [PubMed] [Google Scholar]
- 16.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood 2001; 98:3309-14; PMID:11719368; http://dx.doi.org/ 10.1182/blood.V98.12.3309 [DOI] [PubMed] [Google Scholar]
- 17.Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD et al.. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest 2012; 122:974-86; PMID:22326959; http://dx.doi.org/ 10.1172/JCI60588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Mei J, Gonzales L, Yang G, Dai N, Wang P, Zhang P, Favara M, Malcolm KC, Guttentag S et al.. IL-17A and TNF-alpha exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. J Immunol 2011; 186:3197-205; PMID:21282514; http://dx.doi.org/ 10.4049/jimmunol.1002016 [DOI] [PubMed] [Google Scholar]
- 19.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005; 22:285-94; PMID:15780986; http://dx.doi.org/ 10.1016/j.immuni.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008; 28:454-67; PMID:18400188; http://dx.doi.org/ 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J et al.. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001; 194:519-27; PMID:11514607; http://dx.doi.org/ 10.1084/jem.194.4.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, Hu Q, Mao C, Jiao Z, Wang S, Yu L, Xu Y, Dai D, Yin L, Xu H. Increased IL-17-producing CD4(+) T cells in patients with esophageal cancer. Cell Immunol 2012; 272(2):166-74; http://dx.doi.org/ 10.1016/j.cellimm.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 23.Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, Yamano S, Kamada M, Aono T. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun 2001; 282:735-8; PMID:11401524; http://dx.doi.org/ 10.1006/bbrc.2001.4618 [DOI] [PubMed] [Google Scholar]
- 24.Lee JJ, Chang YL, Lai WL, Ko JY, Kuo MY, Chiang CP, Azuma M, Chen CW, Chia JS. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck 2011; 33:1301-8; PMID:21837700; http://dx.doi.org/ 10.1002/hed.21607 [DOI] [PubMed] [Google Scholar]
- 25.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol 2010; 184:2281-8; PMID:20118280; http://dx.doi.org/ 10.4049/jimmunol.0902574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reppert S, Boross I, Koslowski M, Tureci O, Koch S, Lehr HA, Finotto S. A role for T-bet-mediated tumour immune surveillance in anti-IL-17A treatment of lung cancer. Nat Commun 2011; 2:600; PMID:22186896; http://dx.doi.org/ 10.1038/ncomms1609 [DOI] [PubMed] [Google Scholar]
- 27.Reppert S, Koch S, Finotto S. IL-17A is a central regulator of lung tumor growth. Oncoimmunology 2012; 1:783-5; PMID:22934282; http://dx.doi.org/ 10.4161/onci.19735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challacombe JM, Suhrbier A, Parsons PG, Jones B, Hampson P, Kavanagh D, Rainger GE, Morris M, Lord JM, Le TT et al.. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol 2006; 177:8123-32; PMID:17114487; http://dx.doi.org/ 10.4049/jimmunol.177.11.8123 [DOI] [PubMed] [Google Scholar]
- 29.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC et al.. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996; 56:21-6; PMID:8548765 [PubMed] [Google Scholar]
- 30.Riese MJ, Wang LC, Moon EK, Joshi RP, Ranganathan A, June CH, Koretzky GA, Albelda SM. Enhanced effector responses in activated CD8+ T cells deficient in diacylglycerol kinases. Cancer Res 2013; 73:3566-77; PMID:23576561; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viola A, Sarukhan A, Bronte V, Molon B. The pros and cons of chemokines in tumor immunology. Trends Immunol 2012; 33:496-504; PMID:22726608; http://dx.doi.org/ 10.1016/j.it.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 32.Dubinett SM, Lee JM, Sharma S, Mule JJ. Chemokines: can effector cells be redirected to the site of the tumor? Cancer J 2010; 16:325-35; PMID:20693843; http://dx.doi.org/ 10.1097/PPO.0b013e3181eb33bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR et al.. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res 2005; 65:11752-61; PMID:16357188; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1658 [DOI] [PubMed] [Google Scholar]
- 34.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol 2004; 172:2853-60; PMID:14978086; http://dx.doi.org/ 10.4049/jimmunol.172.5.2853 [DOI] [PubMed] [Google Scholar]
- 35.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev 2005; 16:593-609; PMID:16046180; http://dx.doi.org/ 10.1016/j.cytogfr.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 36.de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol 2006; 13:118-37; PMID:16627962; http://dx.doi.org/ 10.1159/000092969 [DOI] [PubMed] [Google Scholar]
- 37.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD et al.. Inflammatory Monocyte Mobilization Decreases Patient Survival in Pancreatic Cancer: A Role for Targeting the CCL2/CCR2 Axis. Clin Cancer Res 2013; 19:3404-15; PMID:23653148; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86:1065-73; PMID:19741157; http://dx.doi.org/ 10.1189/jlb.0609385 [DOI] [PubMed] [Google Scholar]
- 39.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 2008; 84:623-30; PMID:18467655; http://dx.doi.org/ 10.1189/jlb.1107762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt H, Suciu S, Punt CJ, Gore M, Kruit W, Patel P, Lienard D, von der Maase H, Eggermont AM, Keilholz U et al.. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol 2007; 25:1562-9; PMID:17443000; http://dx.doi.org/ 10.1200/JCO.2006.09.0274 [DOI] [PubMed] [Google Scholar]
- 41.Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI. Regulatory myeloid suppressor cells in health and disease. Cancer Res 2009; 69:7503-6; PMID:19752086; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol 2011; 186:807-15; PMID:21148040; http://dx.doi.org/ 10.4049/jimmunol.1001483 [DOI] [PubMed] [Google Scholar]
- 43.Kish DD, Gorbachev AV, Parameswaran N, Gupta N, Fairchild RL. Neutrophil expression of Fas ligand and perforin directs effector CD8 T cell infiltration into antigen-challenged skin. J Immunol 2012; 189:2191-202; PMID:22815291; http://dx.doi.org/ 10.4049/jimmunol.1102729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jinesh GG, Kamat AM. Redirecting neutrophils against bladder cancer cells by BCG and Smac mimetic combination. Oncoimmunology 2012; 1:1161-2; PMID:23170264; http://dx.doi.org/ 10.4161/onci.20928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009; 27:4709-17; PMID:19720929; http://dx.doi.org/ 10.1200/JCO.2008.18.9498 [DOI] [PubMed] [Google Scholar]
- 46.Souto JC, Vila L, Bru A. Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Med Res Rev 2011; 31:311-63; PMID:19967776; http://dx.doi.org/ 10.1002/med.20185 [DOI] [PubMed] [Google Scholar]
- 47.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 2012; 82:296-309; PMID:21798756; http://dx.doi.org/ 10.1016/j.critrevonc.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 48.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother 2012; 61:1155-67; PMID:22692756; http://dx.doi.org/ 10.1007/s00262-012-1294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS et al.. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One 2012; 7:e31524; PMID:22348096; http://dx.doi.org/ 10.1371/journal.pone.0031524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother 2013; 62:1745-56; PMID:24092389; http://dx.doi.org/ 10.1007/s00262-013-1476-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 2001; 97:339-45; PMID:11154206; http://dx.doi.org/ 10.1182/blood.V97.2.339 [DOI] [PubMed] [Google Scholar]
- 53.Hayata K, Iwahashi M, Ojima T, Katsuda M, Iida T, Nakamori M, Ueda K, Nakamura M, Miyazawa M, Tsuji T et al.. Inhibition of IL-17A in tumor microenvironment augments cytotoxicity of tumor-infiltrating lymphocytes in tumor-bearing mice. PLoS One 2013; 8:e53131; PMID:23372655; http://dx.doi.org/ 10.1371/journal.pone.0053131 [DOI] [PMC free article] [PubMed] [Google Scholar]