Abstract
The immune microenvironment of the brain differs from that of other organs and the role of tumor-infiltrating lymphocytes (TILs) in brain metastases (BM), one of the most common and devastating complication of cancer, is unclear. We investigated TIL subsets and their prognostic impact in 116 BM specimens using immunohistochemistry for CD3, CD8, CD45RO, FOXP3, PD1 and PD-L1. The Immunoscore was calculated as published previously. Overall, we found TIL infiltration in 115/116 (99.1%) BM specimens. PD-L1 expression was evident in 19/67 (28.4%) BM specimens and showed no correlation with TIL density (p > 0.05). TIL density was not associated with corticosteroid administration (p > 0.05). A significant difference in infiltration density according to TIL subtype was present (p < 0.001; Chi Square); high infiltration was most frequently observed for CD3+ TILs (95/116; 81.9%) and least frequently for PD1+ TILs (18/116; 15.5%; p < 0.001). Highest TIL density was observed in melanoma, followed by renal cell cancer and lung cancer BM (p < 0.001). The density of CD8+ TILs correlated positively with the extent of peritumoral edema seen on pre-operative magnetic resonance imaging (p = 0.031). The density of CD3+ (15 vs. 6 mo; p = 0.015), CD8+ (15 vs. 11 mo; p = 0.030) and CD45RO+ TILs (18 vs. 8 mo; p = 0.006) showed a positive correlation with favorable median OS times. Immunoscore showed significant correlation with survival prognosis (27 vs. 10 mo; p < 0.001). The prognostic impact of Immunoscore was independent from established prognostic parameters at multivariable analysis (HR 0.612, p < 0.001). In conclusion, our data indicate that dense TILs infiltrates are common in BM and correlate with the amount of peritumoral brain edema and survival prognosis, thus identifying the immune system as potential biomarker for cancer patients with CNS affection. Further studies are needed to substantiate our findings.
Keywords: brain metastases, immunoscore, overall survival, prognosis, tumor infiltrating lymphocytes
Introduction
BM are common in cancer patients and are associated with high morbidity and mortality. Median overall survival (OS) times typically range from weeks to few months, although long-term survival can be observed in some patients.1 Current standard treatment approaches include neurosurgical resection, stereotactic radiosurgery (SRS) and whole-brain radiotherapy (WBRT). Some molecularly targeted medications have recently shown benefit in selected BM patients and offer the chance for improved outcome for selected patients.2 However, better understanding of the pathobiology of BM is urgently needed for development of better treatments with relevance for larger patient cohorts.
The interplay between cancer and immune cells is a major determinant in cancer progression and the immune system is emerging as powerful prognostic marker and therapeutic target in oncology.3 It is believed that a constantly ongoing process of immunologic tumor elimination and sculpting of the immunogenic cancer cell phenotype (“immune editing”) is associated with tumor formation and tumor maintenance in immuno-competent host organisms.4 The validity of this concept is supported by recent data showing a strong correlation of intratumoral immune cell infiltrates with survival times of cancer patients.5-8 Further, promising clinical data of therapeutic agents that inhibit tumor-associated immunosuppression such as immune-checkpoint inhibitors was reported.9 However, these data have been generated almost exclusively in patients with extracranial tumor manifestations of colorectal cancer, ovarian cancer, breast cancer, melanoma and non-small cell lung cancer, but not in patients with metastatic affection of the central nervous system (CNS).6,8,10
The CNS is recognized to have a specialized immunological microenvironment that differs from that of other organs.11 The influx of lymphocytes into the CNS is strictly regulated and lymphocytes are typically absent from the healthy brain parenchyma. Furthermore, most primary brain tumors are characterized by prominent immunosuppression and harbor only few TILs.12 However, there is little knowledge on the role of the immune system in BM. Therefore, we undertook the present study to characterize TIL infiltrates and their clinical relevance in BM. To this end, we compiled a well-characterized cohort of patients operated for a single BM of a solid cancer. We quantified TIL subsets, cytotoxic, memory as well as regulatory T cells, in the BM specimens using semiquantitative and automated quantification methods and performed statistical correlations with clinico-pathological parameters including extent of brain edema and survival times.
Results
Patients’ characteristics
BM specimens of 116 patients (Table 1) were included. All patients presented with a single BM and were treated with neurosurgical resection as first line treatment approach for newly diagnosed BM.
Table 1.
Patients characteristics
| Patient population (n = 116) |
||
|---|---|---|
| Characteristic | n | % |
| Median age at diagnosis of BM, years (range) | 58.5 (37–80) | |
| Primary tumor | ||
| Lung cancer | 61 | 52.6 |
| Breast cancer | 17 | 14.7 |
| Melanoma | 6 | 5.2 |
| Renal cell cancer | 10 | 8.6 |
| Other | 22 | 20.0 |
| Chemotherapy before diagnosis of BM | ||
| Yes | 49 | 42.2 |
| No | 67 | 57.8 |
| Extracranial metastases | ||
| Yes | 40 | 34.5 |
| No | 76 | 65.5 |
| Status of extracranial disease at diagnosis of BM | ||
| No evidence of disease | 32 | 27.6 |
| Stable disease | 28 | 24.1 |
| Progressive disease | 8 | 6.9 |
| Simultaneous diagnosis of primary tumor and BM | 48 | 41.4 |
| Karnofsky Performance status at diagnosis of BM | ||
| > 70 | 107 | 92.2 |
| < 70 | 9 | 7.8 |
| Number of BM | ||
| 1 | 116 | 100.0 |
| GPA | ||
| Class I | 40 | 34.5 |
| Class II | 30 | 25.9 |
| Class III | 41 | 35.3 |
| Class IV | 5 | 4.3 |
| Leucocyte count at diagnosis of BM | ||
| Normal | 67 | 57.8 |
| Above upper limit of normal | 49 | 42.2 |
| Localization of investigated BM | ||
| Supratentorial | 85 | 73.3 |
| Infratentorial | 31 | 26.7 |
| Preoperative corticosteroid treatment | ||
| Yes | 74 | 63.7 |
| No | 42 | 36.2 |
| Post-surgical WBRT | ||
| Yes | 81 | 69.8 |
| No | 35 | 30.2 |
| Chemotherapy after diagnosis of BM | ||
| Yes | 41 | 35.5 |
| No | 75 | 64.7 |
| Alive at last follow up | ||
| Yes | 9 | 7.8 |
| No | 107 | 92.2 |
| Median Overall survival from diagnosis of BM, months (range) | 13 (0–122) | |
Description of TIL infiltration and PD-L1 expression in BM specimens
TIL density
115/116 (99.1%) specimens presented with TIL infiltration. Table 2 lists further details on TIL density in the investigated areas. A significant difference in infiltration density according to TIL subtype was present (p < 0.001; Chi Square); thereby high infiltration was most frequently observed for CD3+ TILs (95/116; 81.9%) and least frequently for PD1+ TILs (18/116; 15.5%; p < 0.001). Fig. 1 shows frequency of density according to the TIL subtype.
Table 2.
Density of tumor infiltrating lymphocytes (TILs)
| CD3 + TILs | CD8+ TILs | CD45RO + TILs | FOXP3 + TILs | PD1 + TILs | |
|---|---|---|---|---|---|
| Overall impression | |||||
| Sparse infiltration | 20/116 (17.2%) | 42/116 (36.2%) | 25/116 (21.6%) | 45/116 (38.8%) | 35/116 (30.4%) |
| Moderate infiltration | 30/116 (25.9) | 34/116 (29.3%) | 30/116 (25.9%) | 26/116 (22.4) | 12/116 (10.4%) |
| Dense infiltration | 39/116 (33.6%) | 28/116 (42.1%) | 31/116 (26.7%) | 0/116 (0.0%) | 6/116 (5.2%) |
| Very dense infiltration | 26/116 (22.4%) | 8/116 (6.9%) | 11/116 (9.5%) | 0/116 (0.0%) | 0/116 (0.0%) |
| Total | 115/116 (99.1%) | 102/116 (96.6%) | 97/116 (83.6%) | 71/116 (61.2%) | 53/116 (46.1%) |
| Solid tumor | |||||
| Sparse infiltration | 38/116 (32.8%) | 64/116 (55.7%) | 29/116 (25.0%) | 33/116 (28.4%) | 25/116 (21.7%) |
| Moderate infiltration | 13/116 (11.2%) | 7/116 (6.1%) | 10/116 (8.6%) | 4/116 (3.5%) | 3/116 (2.6%) |
| Dense infiltration | 9/116 (7.8%) | 7/116 (6.1%) | 5/116 (4.3%) | 0/116 (0.0%) | 5/116 (4.3%) |
| Very dense infiltration | 5/116 (4.3%) | 4/116 (3.5%) | 4/116 (3.4%) | 0/116 (0.0%) | 0/116 (0.0%) |
| Total | 65/116 (56.0%) | 82/116 (71.3%) | 48/116 (41.4%) | 37/116 (31.9%) | 33/116 (28.7%) |
| Tumor stroma | |||||
| Sparse infiltration | 11/99 (11.1%) | 38/99 (38.4%) | 24/99 (24.2%) | 52/99 (52.5%) | 34/100 (34.0%) |
| Moderate infiltration | 28/99 (28.3%) | 26/99 (26.3%) | 27/99 (27.3%) | 21/99 (21.2%) | 13/100 (13.0%) |
| Dense infiltration | 29/99 (29.3%) | 23/99 (23.2%) | 24/99 (24.2%) | 0/99 (0.0%) | 0/100 (0.0%) |
| Very dense infiltration | 30/99 (30.3%) | 5/99 (5.1%) | 15/99 (15.2%) | 0/99 (0.0%) | 0/100 (0.0%) |
| Total | 98/99 (99.0%) | 92/99 (92.9%) | 90/99 (90.9%) | 73/99 (73.7%) | 47/100 (47.0%) |
| Border Region | |||||
| Sparse infiltration | 27/82 (32.9%) | 37/83 (44.6%) | 36/80 (45.0%) | 40/80 (50.0%) | 36/78 (46.2%) |
| Moderate infiltration | 23/82 (28.0%) | 27/83 (32.5%) | 19/80 (23.8%) | 5/80 (6.3%) | 13/78 (16.7%) |
| Dense infiltration | 13/82 (15.9%) | 9/83 (10.8%) | 11/80 (13.8%) | 0/80 (0.0%) | 0/78 (0.0%) |
| Very dense infiltration | 12/82 (14.6%) | 1/83 (1.2%) | 1/80 (1.3%) | 0/80 (0.0%) | 0/78 (0.0%) |
| Total | 75/82 (91.5%) | 74/83 (89.2%) | 67/80 (83.8%) | 45/80 (56.3%) | 49/78 (62.8%) |
Figure 1.
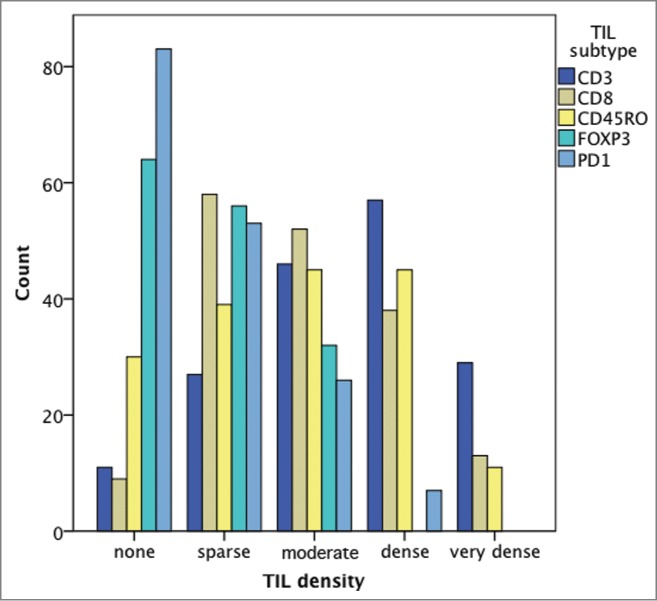
Frequency of density according to the TIL subtype.
Distribution of TIL infiltration
TIL infiltration was most prominent in the tumor stroma (CD3+ TILs; Fig. 2A, arrow a; Fig. 2C) and at the border region to the peritumoral brain parenchyma (CD3+ TILs; Fig. 2A, arrow c), while only sparse infiltration was observed within the solid tumor (CD3+ TILs; Fig. 2A, arrow b; Fig. 2B; Table 2). No TIL infiltration was evident in areas of necrosis or in areas of brain parenchyma other than the immediate peritumoral border region.
Figure 2.
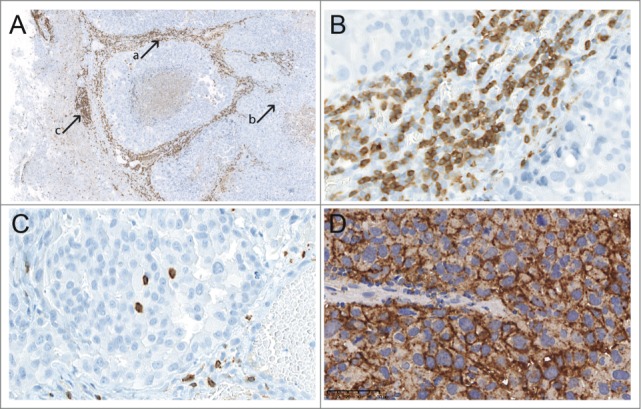
A TIL distribution between different areas (CD3+ TILs, magnification ×5); B TIL density within the tumor stroma (CD3+ TILs, magnification ×200); C TIL density within the solid tumor (CD3+ TILs, magnification ×200); D PD-L1 expression in a melanoma BM (magnification ×200).
PD-L1 expression
Overall, 19/67 (28.4%) BM specimens presented with membranous PD-L1 expression in over 5% of viable BM tumor cells. 16/61 (26.2%) lung cancer BM and 3/6 (50.0%) melanoma BM presented with PD-L1 expression in over 5% of viable BM tumor cells (Fig. 2D).
Correlation of TIL infiltration and PD-L1 expression
No correlation of CD3+ (p = 0.525; Chi Square test), CD8+ (p = 0.242; Chi Square test), CD45RO+ (p = 0.766; Chi Square test), FOXP3+ (p = 0.415; Chi Square test) or PD1+ (p = 0.216; Chi Square test) TIL density with PD-L1 expression was observed.
Correlation of TIL density with clinical parameters
Primary tumor subtype
Melanoma BM presented with the highest density of CD3+ TILs (p = 0.025; Chi Square test), CD8+ TILs (p = 0.029; Chi Square test) and PD1+ TILs (p < 0.001; Chi Square test) compared to the other primary tumor types (Fig. S1). No correlation was observed between FOXP3+ TIL (p = 0.615; Chi Square test) or CD45RO+ TIL (p = 0.521; Chi Square test) density and primary tumor subtype.
Blood leukocyte count
No correlation was observed between blood leucocyte count on the day of surgery and CD3+ (p = 0.478; Kruskal–Wallis test), CD8+ (p = 0.918; Kruskal–Wallis test), CD45RO+ (p = 0.402; Kruskal–Wallis test), FOXP3+ (p = 0.800; Kruskal–Wallis test) or PD1+ TIL (p = 0.398; Kruskal–Wallis test) density.
Graded prognostic assessment (GPA)
A low association was observed between GPA class and CD3+ TIL density (Spearman correlation coefficient −0.372; p < 0.001). No association was evident between GPA class and CD8+ TIL density (Spearman correlation coefficient −0.253; p = 0.006), CD45RO+ TIL density (Spearman correlation coefficient −0.259; p = 0.005), FOXP3+ TIL density (Spearman correlation coefficient −0.025; p = 0.792) or PD1+ TIL density (Spearman correlation coefficient 0.099; p = 0.293).
Preoperative corticosteroid treatment
No correlation was observed between preoperative corticosteroid treatment and CD3+ (p = 0.610; Chi Square test), CD8+ (p = 0.543; Chi Square test), CD45RO+ (p = 0.859; Chi Square test), FOXP3+ (p = 0.379; Chi Square test) or PD1+ TIL density (p = 0.323; Chi Square test).
Preoperative peritumoral edema
Seventeen/116 (14.7%) patients presented with grade I (<1 cm) peritumoral edema, 77/116 (66.4%) with grade II (>1 cm, not crossing the midline) and 22/116 (19.0%) with grade III (>1 cm and crossing the midline) peritumoral edema in the pre-surgical MRI. High density of CD8+ TIL was more frequently observed in patients with grade I peritumoral edema, as compared to patients with grade II or grade III peritumoral edema (p = 0.031; Chi Square test). No correlation of peritumoral edema and CD3+ TIL density (p = 0.820; Chi square test), CD45RO+ TIL density (p = 0.518; Chi Square test), FOXP3+ TIL density (p = 0.070; Chi Square test) or PD1+ TIL density (p = 0.356; Chi Square test) was observed.
Correlation of clinical characteristics with survival times
GPA score
GPA score showed significant correlation with OS. Patients with class I GPA score presented with a median OS of 19 mo compared to 18 mo in class II, 8 mo in class III and 22 d in patients with class IV GPA score (p < 0.001; log-rank test).
Primary tumor subtype
Primary tumor type showed a significant impact on OS as patients with breast cancer presented with a median OS of 19 mo, patients with RCC with 18 mo, patients with lung cancer with 13 mo, patients with melanoma with 4 mo and patients with other primary tumor with 6 mo OS (p = 0.024; log-rank test).
Postoperative treatment
81/116 (69.8%) patients received WBRT and 41/166 (35.3%) patients received chemotherapy after neurosurgical resection of BM. Chemotherapy had no impact on survival prognosis (no chemotherapy: 12 mo vs. chemotherapy 15 mo; p = 0.845; log-rank test). Patients treated with WBRT after neurosurgical resection presented with a significantly improved survival prognosis (median OS 15 mo) compared to patients not receiving postoperative WBRT (median OS 7 mo; p = 0.009; log-rank test).
Correlation of TIL density with survival
Semiquantitative analysis
Patients with high density of CD3+ TILs (median OS 15 mo) showed an improved OS prognosis compared to patients with low density (median OS 6 mo; p = 0.015; log-rank test; Fig. 3A). Similarly, high density of CD8+ TILs (median OS 15 mo) was associated with improved OS prognosis compared to low CD8+ TIL density (median OS 11 mo; p = 0.030; log-rank test; Fig. 3B). Further, high density of CD45RO+ TILs (median OS 18 mo) showed an association with favorable OS prognosis compared to low density (median OS 8 mo; p = 0.006; log-rank test; Fig. 3C). No impact on survival prognosis was observed for FOXP3+ TIL density (high density: median OS 18 mo vs. low density: median OS 11 mo; p = 0.134; log-rank test) and PD1+ TIL density (high density: median OS 18 mo vs. low density: median OS 13 mo; p = 0.314; log-rank test). No impact on survival according to TIL density within the different analyzed areas namely solid tumor, the tumor stroma or the border region to the peritumoral brain parenchyma was observed (Table 2). FOXP3+ to CD8+ TIL ratio had no statistically significant impact on survival prognosis (p = 0.357; log-rank test).
Figure 3.
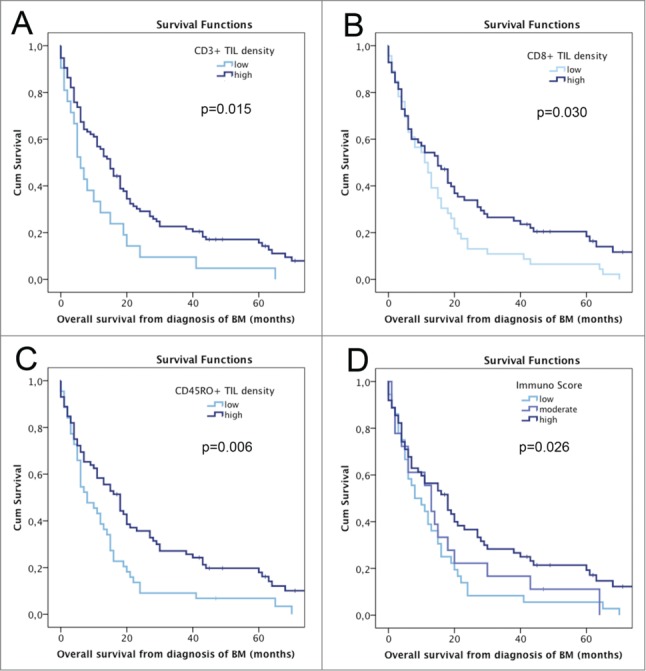
OS according to semiquantitatively assessed TIL density and Immunoscore; A OS according to CD3+ TIL density; B OS according to CD8+ TIL density; C OS according to CD45RO+ TIL density; D OS according to Immunoscore.
In multivariable analysis as co-variables including postoperative WBRT, primary tumor type, GPA class and density of CD3+, CD8+ and CD45RO+ TILS, postoperative WBRT (HR 0.583; 95% CI 0.379–0.898; p = 0.014; Cox regression model) and GPA class (HR 1.564; 95% CI 1.193–2.049; p = 0.001; Cox regression model) remained statistically significant.
Automated image analysis
High CD3+ TIL density (entered as decimal logarithm) in the tumor center (HR 0.557; 95% CI 0.364–0.852; p = 0.007; univariate Cox model) as well as in border region to the peritumoral brain parenchyma (HR 0.517; 95% CI 0.273–0.980; p = 0.043; univariate Cox model) presented with statistically significant favorable correlation with OS prognosis. Similarly, high CD8+ TIL density (entered as decimal logarithm) in the tumor center (HR 0.676; 95% CI 0.482–0.949; p = 0.024; univariate Cox model) as well as in the border region to the peritumoral brain parenchyma (HR 0.578; 95% CI 0.349–0.957; p = 0.033; univariate Cox model) showed statistically significant favorable correlation with OS prognosis.
Immunoscore
58/97 (59.8%) BM specimens presented with a low Immunoscore and 39/97 (40.2%) with a high Immunoscore. No correlation of Immunoscore and PD-L1 expression was observed (p = 0371; Chi Square test). A significant impact of the Immunoscore on survival prognosis was observed, as patients with low Immunoscore presented with a median OS of 10 mo compared to 27 mo in patients with high Immunoscore (HR 2.595; 95% CI 1.621–4.152; p < 0.001; Cox regression model; Fig. 3D).
Both Immunoscore and post-surgical WBRT showed an independent additive effect on OS prognosis. Median OS in patients with high Immunoscore receiving WBRT was 27 mo, median OS in patients with high Immunoscore not treated with post-surgical WBRT was 20 mo. Median OS in patients with low Immunoscore receiving WBRT was 12 mo and median OS in patients with low Immunoscore not treated with WBRT was 4 mo.
This is confirmed in multivariable analysis including postoperative WBRT, primary tumor type, GPA class and Immunoscore, postoperative WBRT (HR 0.585; 95% CI 0.361-0.950; p = 0.030; Cox regression model), GPA class (HR 1.640; 95% CI 1.225-2.196; p = 0.001; Cox regression model) and Immunoscore (HR 0.612; 95% CI 0.480–0780; p < 0.001; Cox regression model) remained significant.
Discussion
The interaction of the immune system and cancer has been identified as major determinant of disease course in several cancer types and novel highly effective immunomodulating cancer treatments are emerging in several tumor types including melanoma, lung cancer, renal cell cancer and others.4 However, so far there was a lack of data on the role of the immune system in BM, one of the most common and devastating complications of cancer. We show in the present study that dense TIL infiltrates are common in BM and correlate with the amount of peritumoral brain edema and survival prognosis. Thus, our data provide strong support of a clinically relevant role of the immune system in BM and identify the immune system as promising biomarker for cancer patients with CNS metastases.
The brain parenchyma and primary brain tumors have long been recognized as highly immunoregulatory environments that express immunosuppressive molecules such as IDO, TGF-β, PD-L1 and others.13,12 In line with this, lymphocytes are typically absent or only rarely found in the CNS parenchyma and most primary brain tumors.12 In contrast, we show in the present study that very dense TIL infiltrates are common in BM of different cancer types. In general, the lymphocytic infiltrates were of mixed composition and contained both immuno-activating TIL subsets such as CD3+ effector and CD8+ cytotoxic TILs, memory TIL subsets CD45R0+ and immunosuppressive TIL subsets such as FOXP3+, and PD1+ TILs. Further studies need to elaborate whether activation or suppression of these cell types, e.g. by monoclonal antibodies directed at co-stimulatory immune check-point molecules, may be an effective therapeutic opportunity against BM. Expression of PD-L1 was postulated to be associated with increased likelihood of response to PD-1 targeting immune checkpoint inhibitors like nivolumab or pembrolizumab.14,15 Here, we could demonstrate PD-L1 expression in BM, supporting the investigation of immune checkpoint inhibitors in patients with BM.16,17 Of note, however, proof of concept for this approach is available, as activity of the anti-CTLA4 antibody ipilimumab that activates the antitumor immune response has been shown to be effective against melanoma BM.18
We found a strong correlation between the density of TIL and patient´s survival times using several different quantification methodologies. In addition to manual and automated evaluation methods we also applied the Immunoscore methodology, which has been elaborated in colorectal cancer and provides a score based on the numeration of CD3+ and CD8+ lymphocytes in different tumor areas.5,19-21 The Immunoscore has been shown to have a strong correlation with patient outcome in colorectal cancer patients and is currently being developed as standardized clinical biomarker in a large international consortium.19,22 Here, we show that Immunoscore also carries strong prognostic information in BM, as patients with a low Immunoscore had median OS times of 10 mo compared to 27 mo in patients with a high Immunoscore. The prognostic impact of Immunoscore was independent from that of established prognostic parameters at multivariable survival analysis, thus further underscoring the important biological role of the immune system in brain-metastatic cancer. Our results need to be validated in independent, ideally prospectively studies, but strongly support a clinically relevant role of this novel biomarker for BM patients.
Interestingly, we found a positive correlation between the amount of perioperative peritumoral brain edema and the density of CD8+ TILs. This observation provides further evidence of a significant activation of the immune system, as edema is a well-recognized characteristic of inflammatory processes and is likely related to the release of cytokines such as interleukin 2.23,24 In line with our findings of the present study, we have already previously shown that the size for the peritumoral edema around BM correlates positively with favorable OS times.25 Thus, the size of the peritumoral edema could serve as surrogate marker of immune activation against BM and this concept should be validated in further studies including clinical studies of immune modulatory agents in patients with BM. If confirmed, this surrogate marker could be applied not only to operated BM as in our study, but potentially also to the larger group of patients with multiple BM of whom usually no tissue specimens are available.
Although we were able to investigate the TIL infiltration a well-characterized and large cohort of BM specimens, our study has several limitations. The study was performed in a retrospective manner and is inherently limited by some heterogeneity in patient baseline characteristic and therapy protocols. We tried to minimize the influence of this confounder by applying strict inclusion criteria that allowed only patients with single BM that were treated by neurosurgical resection as first-line therapy and for whom full clinical and follow-up data were available. Still, it must be noted that our study approach is exploratory and hypothesis-generating in nature and requires external validation. Another limitation concerns the lack of matched primary tumors for comparative analyses of TIL infiltrates, as many patients were operated for their primary tumor in other hospitals and were referred to our tertiary care center only for neurosurgery of BM. Such investigations would be of interest to gain insights into the evolution of the antitumor immune response over the disease course and might be subject to further research projects.
In conclusion, the presented data support the concept of immune surveillance and the critical role of the natural adaptive immune reaction within BM to prolong the life of the patients. Further investigations are needed to substantiate the findings of our retrospective and hypothesis-generating study.
Methods
Patients
We retrospectively identified patients with newly diagnosed BM treated the Medical University of Vienna between April 2002 and December 2010. The inclusion criteria for our study were as follows: (1) newly diagnosed, single BM treated by neurosurgical resection as 1st line treatment, (2) availability of pre-surgical cranial magnetic resonance images (MRI), (3) availability of at least one block of formalin fixed and paraffin embedded (FFPE) tumor tissue containing viable BM tumor tissue, and (4) availability of full clinical follow up data including survival times. The graded prognostic assessment (GPA) class was calculated as previously published based on age, status of extracranial disease, number of BM and Karnofsky performance score.1 Survival data were obtained from the database of National Cancer Registry of Austria and the Austrian Brain Tumor Registry.26,27 The study was approved by the ethics committee of the Medical University of Vienna (Vote 078/2004).
Neuroimaging analysis
Data on the extent of peritumoral brain edema on pre-surgical MRI were available from a previous study.25 In brief, the extent of peritumoral brain edema was measured from the tumor margin and defined as: grade 1: maximum diameter smaller than 1 cm; grade 2: maximum diameter larger than 1 cm, but not crossing the midline; grade 3 maximum larger than 1 cm and crossing the midline.25
Immunohistochemical analysis
Tissue blocks were cut into 3 μm slices with a microtome for further immunohistochemical processing according to standard laboratory practice.
Immunohistochemistry for CD3, CD8, CD45RO, FOXP3 and PD1 was performed on a Ventana Benchmark Ultra immunostaining system (Ventana, Tucson, AZ, USA). Immunostaining protocols and applied antibodies are listed in (Table S1). Tissue of human non-malignant lymph nodes was used as positive control.
PD-L1 immunostaining was performed as described previously in 67 BM specimens of lung cancer (n = 61) and melanoma (n = 3).28 In brief, immunohistochemistry for PD-L1 was performed using the monoclonal mouse antibody, Clone 5H1 (diluation1:100; kindly provided by Dr. Lieping Chen15) on a Dako AutostainerPlusLink immunostaining system (Dako, Glostrup, Denmark).
Evaluation of immunohistochemical analysis
Semiquantitative analysis
Semiquantiative analysis of TILs was performed on full slides. Immunohistochemical staining of CD3+, CD8+, CD45RO+, FOXP3+ and PD1+ TILs was evaluated according to overall impression at low microscopic magnification (100×) and in predefined areas namely within the solid tumor tissue, in the tumor stroma and within the border region of the BM tissue and the peritumoral brain parenchyma at higher magnification (200×–400×). The tumor stroma was only evaluated in BM specimens with clear-cut stroma component in the H&E staining. In specimens without stroma component in the H&E scan this area was not evaluated. Further, the border region of the BM tissue and the peritumoral brain parenchyma is not routinely included in neurosurgical BM specimens as BM are not routinely resected with an safety margin in dependence of brain area, infiltrative properties of the BM and surgeon. Therefore, the areas tumor stroma, border region of the BM tissue and peritumoral brain parenchyma were not evaluable in all specimens.
Previously published semiquantitative evaluation criteria were used and TILs were judged to be: absent (0), sparse (1+), moderately dense (2+), dense (3+) or very dense (4+).29 FOXP3+ to CD8+ TIL ratio was calculated by dividing FOXP3+ TIL density by CD8+ TIL density.
Automated image analysis
Automated image analysis of TILs was performed for CD3 and CD8 immunohistochemical staining and on full slides (Immunoscore assay). All slides were digitalized using an automatic slide scanner (Hamamatsu, Hersching am Ammersee, Germany). Then, TIL density was analyzed using Definiens software (Definiens AG, Munich, Germany). TIL density was separately evaluated within the tumor center and, if available, within the border region to the peritumoral brain parenchyma. The invasive margin (IM) was defined as previously published as the intersection between BM and surrounding parenchyma. TILs located in the perivascular spaces occurring in the area were included in the analysis.5 Measurements were recorded as the mean number of cells presenting with an immunohistochemical staining signal per tissue surface unit in square millimeters.
Statistical analysis
Primary tumor categories were defined as “lung cancer,” “breast cancer,” “melanoma,” “renal cell carcinoma” and “others.” The category “others” included rare entities with less than five cases. Chi square test and Kruskal–Wallis test were used as appropriate to assess group differences. Spearman correlation coefficient was used to correlate two scale variables. A Spearman correlation coefficient from (–)0.7 to (–)1 was regarded as a strong association, a correlation coefficient of (–)0.5 to (–)0.7 as a moderate and a correlation coefficient of (–)0.3 to (–)0.5 as a low association. A Spearman correlation coefficient of 0 to (–)0.3 was interpreted as no association. OS times of patients were estimated with the Kaplan–Meier product limit method and group differences were assessed with the log-rank test. Cox regression model was used to analyze survival impact of continues variables and to perform a multivariable analysis. A two-tailed significance level of 0.05 was applied.
Semiquantitatively evaluated TIL density was entered as ordinal variable divided in low density (no or sparse infiltration) and high density (moderately dense, dense and very dense infiltration) in all statistical analyses. For TIL density evaluated by automated image analysis, mean density per square millimeter was used for further statistical analysis. Decimal logarithm was performed of aslope distributed data in order to obtain a normal distribution.
The Immunoscore was calculated as previously published.19-21 In brief, the optimal cut-off, using the minimum p value approach, for CD3+ and CD8+ TIL density in each region (tumor center and border region to the peritumoral brain parenchyma) was assessed and recorded as a dichotomous (high vs. low) variable. Information of CD3+ and CD8+ TIL density within the tumor center and within the border region to the peritumoral brain parenchyma was added in order to retrieve a high Immunoscore (all high to at least three regions high) and low Immunoscore (all low to at least two regions low). To have a more complete Immunoscore dataset, missing immune density values due to a very small or absent IM were compiled to an Immunoscore I3–4 when both CD3 and CD8 where high (Hi) in the respective center of the metastasis (CT) and to an Immunoscore I0–2 otherwise. The minimum p value approach in order to generate the best separation of patients according to their survival time from diagnosis of BM was used. p values were corrected applying the method proposed by Altman et al.30 Hazard ratios were corrected as suggested by Holländer et al.31
Statistical analysis of the Immunoscore was performed with the statistical software R (survival package). All other statistical analysis was performed with statistical package for the social sciences (SPSS) 20.0 software (SPSS Inc.., Chicago, IL, USA).
Previous Presentations
The contents of this manuscript were partly presented during a Poster Highlight Session at the Annual Meeting of the American Society of Clinical Oncology on May 30th 2014 and during the Top Scoring Abstract Session at the Annual Meeting of the Society of Neuro-Oncology on November 14th 2014.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Elisabeth Dirnberger, Manuel Magerle, Orsolya Rajky, Benjamin Prascher and Cansu Ilhan for excellent technical assistance.
Funding
The costs for this project were covered by the research budget of the Medical University of Vienna and the grants “Initiative Krebsforschung” with the project title “Tumorimmunologie von Hirnmetastasen” and “Hochschuljubuläumsstiftung” with the project title “Das Immunsystem im Kampf gegen Krebs.” BM, GB, AB and the work were supported by grants from the National Cancer Institute of France (INCa), INSERM, the Cancer research for personalized medicine (CARPEM), Paris Alliance of Cancer Research Institutes (PACRI), and the LabEx Immuno-oncology.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol, Biol, Phys 2008; 70:510-4; PMID:17931798; http://dx.doi.org/ 10.1016/j.ijrobp.2007.06.074 [DOI] [PubMed] [Google Scholar]
- 2.Berghoff AS, Rajky O, Winkler F, Bartsch R, Furtner J, Hainfellner JA, Goodman SL, Weller M, Schittenhelm J, Preusser M. Invasion patterns in brain metastases of solid cancers. Neuro-oncology 2013; 15:1664-72; PMID:24084410; http://dx.doi.org/ 10.1093/neuonc/not112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 6.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol: Off J Am Soc Clin Oncol 2014;32:2959-66; PMID:25071121; http://dx.doi.org/ 10.1200/JCO.2013.55.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol: Off J Am Soc Clin Oncol 2013; 31:860-7; PMID:23341518; http://dx.doi.org/ 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 8.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New Engl J Med 2005; 353:2654-66; PMID:16371631; http://dx.doi.org/ 10.1056/NEJMoa051424 [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11-26; PMID:23890060; http://dx.doi.org/ 10.1016/j.immuni.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 10.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT et al. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res: Off J Am Assoc Cancer Res 2008; 14:3372-9; PMID:18519766; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol 2007; 28:12-8; PMID:17129764; http://dx.doi.org/ 10.1016/j.it.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 12.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M et al.. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-oncology 2014; 17(8):1064-75; PMID:25355681; http://dx.doi.org/ 10.1093/neuonc/nou307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platten M, Ochs K, Lemke D, Opitz C, Wick W. Microenvironmental clues for glioma immunotherapy. Curr Neurol Neurosci Rep 2014; 14:440; PMID:24604058; http://dx.doi.org/ 10.1007/s11910-014-0440-1 [DOI] [PubMed] [Google Scholar]
- 14.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res: Off J Am Assoc Cancer Res 2014; 20:5064-74; PMID:24714771; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berghoff A, Inan C, Ricken G, Widhalm G, Dieckmann K, Birner P, Oberndorfer F, Dome F, Bartsch R, Zielinski C et al.. Tumor-infiltrating lymphocytes (TILs) and PD-L1 expression in non-small cell lung cancer brain metastases (BM) and matched primary tumors (PT). ESMO 2014; 1324P [Google Scholar]
- 17.Berghoff AS, Ricken G, Widhalm G, Rajky O, Dieckmann K, Birner P, Bartsch R, Holler C, Preusser M. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology 2015; 66:289-99; PMID:25314639; http://dx.doi.org/ 10.1111/his.12537 [DOI] [PubMed] [Google Scholar]
- 18.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012; 13:459-65; PMID:22456429; http://dx.doi.org/ 10.1016/S1470-2045(12)70090-6 [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G et al. Cancer classification using the Immunoscore: a worldwide task force. J Trans Med 2012; 10:205; PMID:23034130; http://dx.doi.org/ 10.1186/1479-5876-10-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Trans Med 2012; 10:1; PMID:22214470; http://dx.doi.org/ 10.1186/1479-5876-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol: Off J Am Soc Clin Oncol 2011; 29:610-8; PMID:21245428; http://dx.doi.org/ 10.1200/JCO.2010.30.5425 [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232:199-209; PMID:24122236; http://dx.doi.org/ 10.1002/path.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 2010; 107:11906-11; PMID:20547866; http://dx.doi.org/ 10.1073/pnas.1002569107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tjuvajev J, Gansbacher B, Desai R, Beattie B, Kaplitt M, Matei C, Koutcher J, Gilboa E, Blasberg R. RG-2 glioma growth attenuation and severe brain edema caused by local production of interleukin-2 and interferon-gamma. Cancer Res 1995; 55:1902-10; PMID:7728757 [PubMed] [Google Scholar]
- 25.Spanberger T, Berghoff AS, Dinhof C, Ilhan-Mutlu A, Magerle M, Hutterer M, Pichler J, Wohrer A, Hackl M, Widhalm G et al. Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1 a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metast 2013; 30:357-68; PMID:23076770; http://dx.doi.org/ 10.1007/s10585-012-9542-9 [DOI] [PubMed] [Google Scholar]
- 26.Woehrer A. Brain tumor epidemiology in Austria and the Austrian Brain Tumor Registry. Clin Neuropathol 2013; 32:269-85; PMID:23611589; http://dx.doi.org/ 10.5414/NP300600 [DOI] [PubMed] [Google Scholar]
- 27.Wohrer A, Waldhor T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mosenbacher U, Kiefer A, Maier H, Motz R, Reiner-Concin A et al. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol 2009; 95:401-11; PMID:19562257; http://dx.doi.org/ 10.1007/s11060-009-9938-9 [DOI] [PubMed] [Google Scholar]
- 28.Berghoff AS, Ricken G, Widhalm G, Rajky O, Dieckmann K, Birner P, Bartsch R, Holler C, Preusser M. Tumor infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology 2014; 66:289-99; PMID:25314639; http://dx.doi.org/ 10.1111/his.12537 [DOI] [PubMed] [Google Scholar]
- 29.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Modern Pathol:Off J U S Can Acad Pathol, Inc 2011; 24:671-82; PMID:21240258; http://dx.doi.org/ 10.1038/modpathol.2010.234 [DOI] [PubMed] [Google Scholar]
- 30.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994; 86:829-35; PMID:8182763; http://dx.doi.org/ 10.1093/jnci/86.11.829 [DOI] [PubMed] [Google Scholar]
- 31.Holländer N, Sauerbrei W, Schumacher M. Confidence intervals for the effect of a prognostic factor after selection of an ‘optimal’ cutpoint. Stat Med 2004; 23:1701-13; PMID:15160403; http://dx.doi.org/ 10.1002/sim.1611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


