Abstract
Bohring-Opitz syndrome is a rare genetic condition characterized by distinctive facial features, variable microcephaly, hypertrichosis, nevus flammeus, severe myopia, unusual posture (flexion at the elbows with ulnar deviation, and flexion of the wrists and metacarpophalangeal joints), severe intellectual disability, and feeding issues. Nine patients with Bohring-Opitz syndrome have been identified as having a mutation in ASXL1. We report on eight previously unpublished patients with Bohring-Opitz syndrome caused by an apparent or confirmed de novo mutation in ASXL1. Of note, two patients developed bilateral Wilms tumors. Somatic mutations in ASXL1 are associated with myeloid malignancies, and these reports emphasize the need for Wilms tumor screening in patients with ASXL1 mutations. We discuss clinical management with a focus on their feeding issues, cyclic vomiting, respiratory infections, insomnia, and tumor predisposition. Many patients are noted to have distinctive personalities (interactive, happy, and curious) and rapid hair growth; features not previously reported.
Keywords: ASXL1, Bohring-Opitz syndrome, Wilms Tumor, Nevus Flammeus, Myopia, Failure to Thrive, Intellectual Disability, Hypertrichosis, Cyclic Vomiting
INTRODUCTION
Bohring-Opitz syndrome (BOS), previously reported as Oberklaid-Danks syndrome, is a rare genetic condition that was initially distinguished from Opitz Trigonocephaly C syndrome by Dr. Axel Bohring in 1999 [Oberklaid and Danks, 1975; Bohring et al., 1999]. To date, 43 patients with BOS have been published, and efforts have been made to define a set of clinical diagnostic criteria [Bohring et al., 2006]. Bohring-Opitz syndrome has previously been associated with high infant mortality (11 out of 43 reported patients (26%), intrauterine growth retardation, feeding difficulties often requiring a feeding tube, failure to thrive, severe to profound developmental delays, recurrent infections, nonspecific brain abnormalities, variable microcephaly, distinctive facial features (Fig. 1A–D), and an unusual posture cited in the literature as BOS posture (Fig. 1E–F) [Bohring et al., 1999]. Bohring-Opitz syndrome posture is characterized by flexion at the elbows with ulnar deviation, and flexion of the wrists and metacarpophalangeal joints. Phalangeal creases are normal and the positioning is not due to contractures. Patients with BOS have distinctive facial features including nevus flammeus typically over the glabella and eye lids, facial hypotonia, proptosis with high myopia, widely spaced eyes, flat and wide nasal bridge, anteverted nares, palatal anomalies including cleft palate and prominent palatine ridges, micro and retrognathia, low-set ears with increased posterior angulation, a low posterior hairline, and hypertrichosis (Fig. 1) [Bohring et al., 2006; Hastings et al., 2011; Magini et al., 2012].
Figure 1.
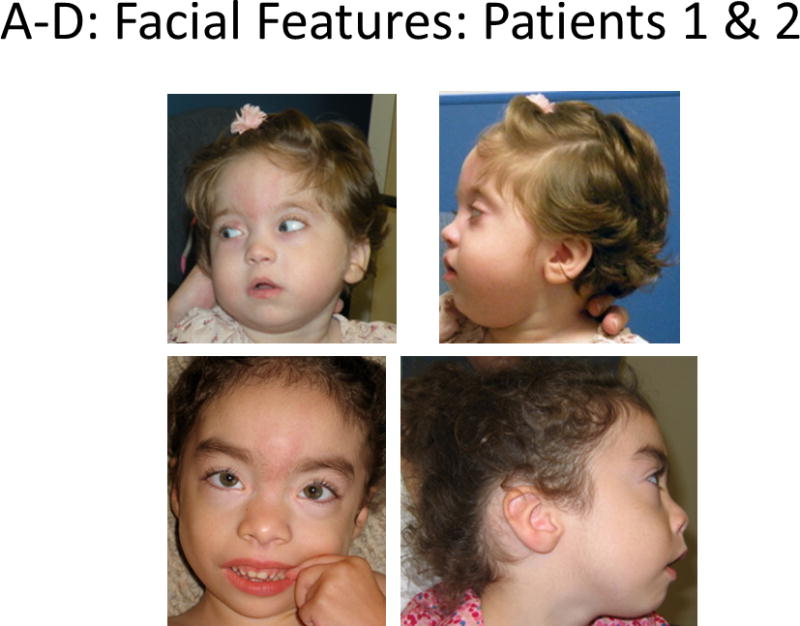
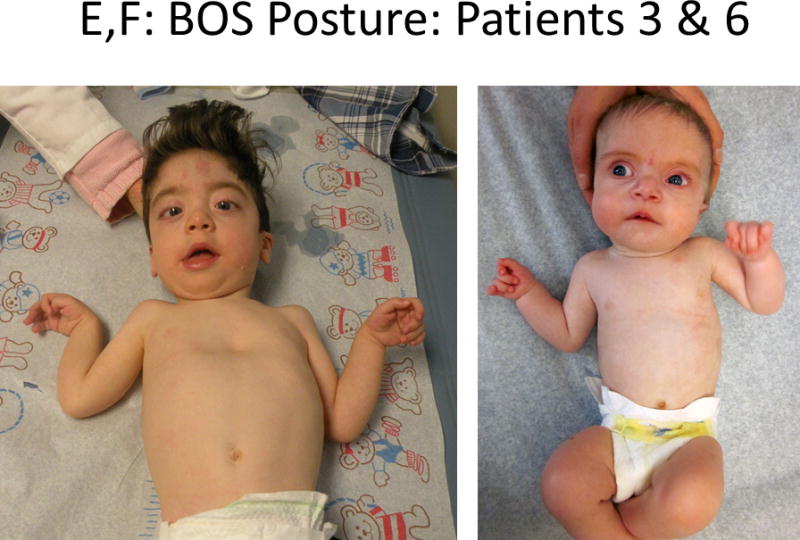
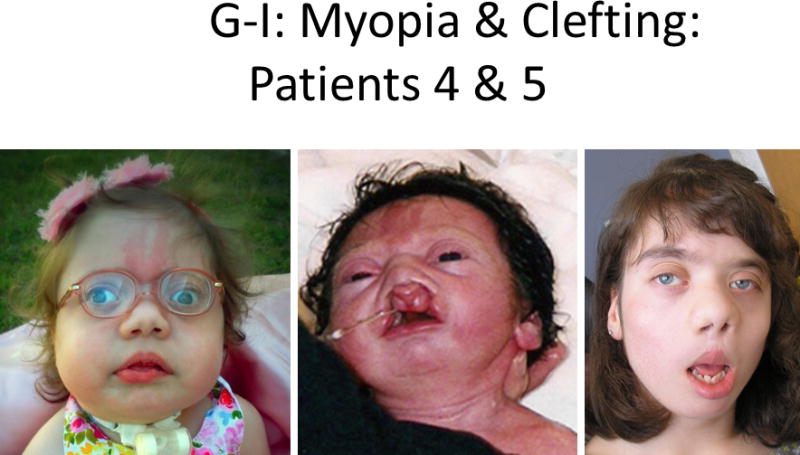
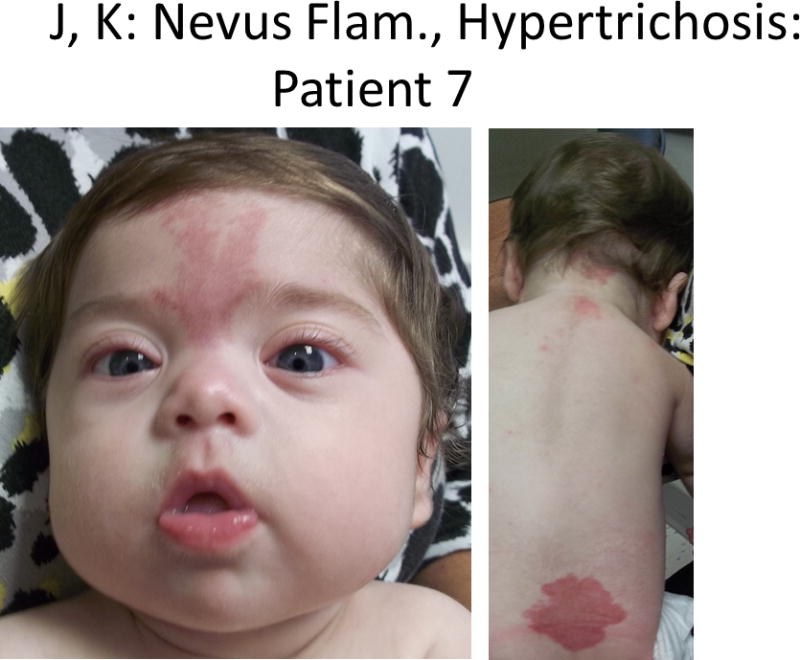
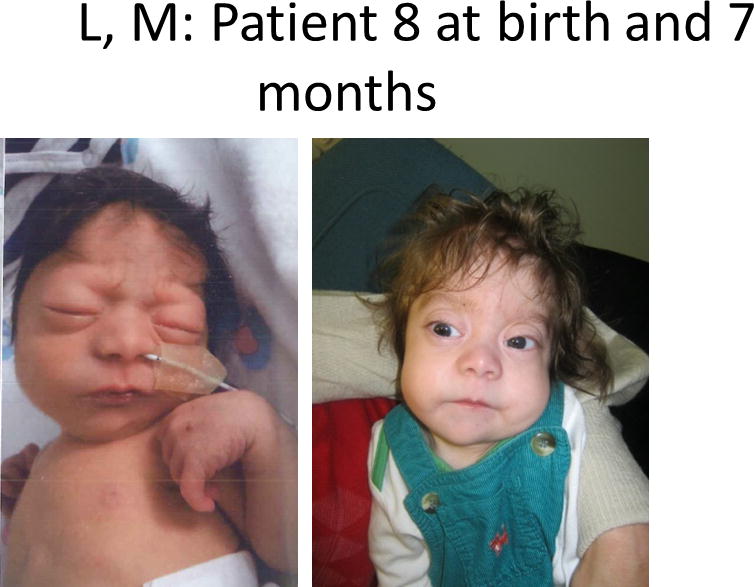
Comparison of photographs of patients with Bohring-Opitz syndrome (BOS) caused by ASXL1 mutations. Patient 1 at 9 years (panels A, B) and Patient 2 at 2 years (panels C, D) with classic facial features. Patient 3 at 18 months (panel E) and Patient 6 at 3 months (panel F) demonstrating the typical BOS posture. Patient 4 at 2 years (panel G) and Patient 5 as an infant and at 12 years (panels H, I) with myopia and clefting. Patient 7 at 11 months (panels J, K) with prominent nevus flammeus and hypertrichosis. Patient 8 at birth and 7 months demonstrating progression of features over time (panels L, M). Facial features include nevus flammeus that fades with age, facial hypotonia, proptosis, widely spaced eyes, depressed and wide nasal bridge, anteverted nares, palatal anomalies including cleft palate and prominent palatine ridges, micro and retrognathia, low-set ears with increased posterior angulation, a low posterior hairline, and hypertrichosis. The characteristic BOS posture can be seen in panels E and F with flexion at the elbows with ulnar deviation, and flexion of the wrists and metacarpophalangeal joints.
Through exome sequencing, de novo nonsense mutations in ASXL1 were found in nine patients with BOS [Hoischen et al., 2011; Magini et al., 2012]. However, ASXL1 mutations have only been found in a subset of patients clinically diagnosed with BOS [Hoischen et al., 2011]. Patients with an identifiable ASXL1 mutation have a higher incidence of myopia (87% versus 40%) and hypertrichosis (89% versus 17%) compared to those without identified mutations [Magini et al., 2012].
ASXL1 maps to 20q11.21 and is a member of the polycomb group (PcG) with trithorax complexes required for activation and silencing of Hox genes [Fisher et al., 2003; Fisher et al., 2010]. Hox genes are important for development, and in Drosophila, Asx regulation is highly variable and tightly controlled immediately following fertilization [Sinclair et al., 1998]. ASXL1 is involved in chromatin remodeling and is an epigenetic modifier. Somatic mutations in ASXL1 have been strongly associated with acute myeloid leukemia, ring sideroblastic anemia and mylodysplastic syndromes [Aravind and Iyer, 2012; Wang et al., 2014]. Somatic mutations in ASXL1 found in previously healthy individuals increase the risk of hematologic cancers [Jaiswal et al., 2014]. To date, the only published patients with a neoplastic condition and BOS include a patient with nephroblastomatosis diagnosed post-mortem [Brunner et al., 2000] and a patient with fatal medulloblastoma, both in ASXL1-negative patients [Hastings et al., 2010; Hoischen et al., 2011]. We report and address the clinical management of eight previously unpublished patients with BOS caused by apparent de novo mutations in ASXL1. Furthermore, two patients developed bilateral Wilms tumor at two and six years of age; these are the first reported patients with a neoplasm and an ASXL1mutation.
PATIENTS
Patient 1 was an 11-year-old female born via normal spontaneous vaginal delivery at 40 weeks gestation as the first pregnancy for her 28-year-old mother and 31-year-old father (Fig. 1A–B). A high palate was noted at birth, but no other anomalies were appreciated. The trajectory of her growth curve began to decrease in early infancy, and by nine months, she was well below the fifth centile. Feeding was consistently difficult, but she did not require tube feeds. Her mother, a lactation consultant, was able to breastfeed her until age three years. She required extensive feeding therapy to successfully feed her small, high calorie meals, and she showed a preference for crunchy, salty, and flavorful foods. She gagged and vomited easily, but she did not have regular emesis.
She had repeated upper respiratory infections as a young child and was subsequently diagnosed with reactive airway disease at 30 months of age. Once she received inhaled corticosteroids, these respiratory infections decreased in frequency, and she no longer required regular treatments for her respiratory symptoms. She had normal cranial magnetic resonance imaging (MRI) and a normal electroencephalogram (EEG) as an infant, but at four years of age, she had a clinical seizure, and cranial MRI at that time showed questionable subtle linear signal abnormalities in the white matter of the left frontal and temporal lobes that resembled gray matter in signal intensity and were suspicious for heterotopia. She had no further seizure-like activity, but she had difficulties initiating sleep, which improved with the use of melatonin. Her sleep issues were cyclic with several months of normal sleep between episodes. An overnight EEG showed an increased frequency of spike wave discharges at night concerning for electrical status epilepticus of sleep, not associated with difficulties falling sleep. At five years she was diagnosed with stage V bilateral Wilms tumor. She underwent 12 weeks of chemotherapy with vincristine, doxorubicin, and actinomycin D, as well as a right partial nephrectomy and left radical nephrectomy, followed by 9 more weeks of chemotherapy. She was in remission at time of publication.
Developmentally, she sat at 12 months of age and began walking at 3.5 years. She walked with ankle-foot orthotics but could not pull herself to stand from a seated position on the floor. She had significant fine motor delays. At the age 10 years, she had a few words but was mostly communicating through gestures, and she was learning to use an augmentative communication device. She became easily frustrated, resulting in behavioral problems. She successfully identified many objects, and her receptive skills were much better than her expressive language. She was noted to have a happy, pleasant, curious demeanor, and she lacked significant stranger anxiety.
On physical examination at the age 8.5 years, OFC was 52.2 cm (50th centile), height was 122 cm (5th centile) and weight was 22 kg (10th centile). Additional physical features noted in Table I and Fig. 1A–B. She had an everted lower lip, synophrys, and midface retrusion. She had absent deep tendon reflexes at her ankles and mild 2-3-4 partial cutaneous toe syndactyly with severe pronation of her feet. She had persistent fetal pads on all digits. Redundant skin on her hands and neck with wrinkled palmar surfaces was also noted. She had rapidly growing hair and nails. She had a benign heart murmur. Echocardiogram was not performed. At age 10 years, she developed breast buds and fine pubic hair. A pediatric endocrinology evaluation was negative for precocious puberty.
Table I.
Physical features seen in 8 previously unreported patients with Bohring Opitz syndrome.
| Patients: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Age/Gender | 11 y F | 3 y F | 3 y M | 3 y F | 12 y F | 3 y M | 1 y F | 7 m M |
| Birth Parameters | ||||||||
| BW in grams (%) | 3118 (20) | 2426 (25–50) | 2300 (3) | 2920 (25) | 2639 (5–10) | 2520 (2) | 2195 (10–25) | 2095 (<0.4) |
| Length in cm (%) | 48 (25) | 43 (<10) | 47 (10) | 36 (<1) | 45 (3) | 45.5 (<2) | 44 (10–25) | * |
| OFC in cm (%) | 35.5 (90–95) | 31 (10–25) | 31 (3) | 33 (10) | 33 (10) | 33.2 (10–25) | 29.5 (10) | 31.0 (<0.4) |
| Craniofacial | ||||||||
| Proptosis | + | + | + | + | + | + | + | + |
| Myopia in diopters (age diagnosed) | −7 (4 m) | −6.5/−7.5 (25 m) | −11/−10 (1 m) | −10.5/−8 (2 y) | −13.5/−17.5 (3 m) | −4 (13 m) | −11 (10 m) | −16/−16 (7m) |
| Upslanting palpebral fissures | − | + | − | − | + | + | + | + |
| Prominent forehead | + | − | + | − | + | − | − | − |
| Microcephaly | − | − | + | − | + | − | + | + |
| Micro/retrognathia | + | + | + | + | + | + | + | + |
| Depressed nasal bridge | + | + | + | + | + | + | + | + |
| Wide nasal bridge | + | − | + | + | + | + | + | + |
| Anteverted nares | + | + | − | + | + | − | + | + |
| Low-set ears, posterior angulation | + | + | − | − | + | − | + | + |
| Overfolding helices | + | − | + | − | + | − | + | − |
| High palate | + | + | + | + | + | + | + | + |
| Prominent palatine ridges | + | + | + | + | − | − | * | + |
| Hair/Skin | ||||||||
| Low hairline | + | + | + | − | + | − | + | + |
| Hypertrichosis | + | + | + | + | + | − | + | + |
| Rapid hair growth | + | + | + | + | + | + | + | + |
| Glabellar nevus flammeus | + | + | + | + | + | + | + | − |
| Neurological/Skeletal | ||||||||
| BOS posture | + | + | + | + | + | + | + | + |
| Pectus excavatum | + | + | + | − | − | − | − | − |
| Contractures | − | − | − | + | + | − | − | + |
| Hypotonia | + | + | + | + | + | + | + | − |
Unattainable information regarding patient 7 and 8.
Following the diagnosis of Wilms tumor, DNA from the tumor and normal kidney tissue was analyzed for loss of heterozygosity at the ASXL1 locus. As tissue was collected after chemotherapy, widespread necrosis limited the amount of tissue. SNaPshot analysis of three ASXL1 variants did not show loss of heterozygosity, ruling out conversion of the wild-type allele as well as deletion of the entire wild-type ASXL1 locus (data not shown).
Patient 2 was a 3-year-old female born at 36 weeks gestation as the third pregnancy for her 34-year-old mother and 38-year-old father (Fig. 1C–D). Her mother had one healthy child and an earlier miscarriage. She was noted to be dysmorphic at birth, and shortly after delivery she developed significant feeding difficulties. A bowel obstruction was diagnosed, and surgery at four days showed an annular pancreas. Her sucking reflex was intact, but she required supplemental nutrition and struggled with severe episodes of emesis, often associated with feeding and attributed to dysmotility, with negative testing for reflux. At age six months a gastrostomy tube was placed, followed by a gastro-jejunal tube. As she developed, her severe episodes of uncontrollable emesis were noted to be a response to specific insults, including infectious processes, constipation, vaccinations, and anesthesia. When daily cyproheptadine was started for cyclic vomiting, these episodes decreased. Her hospitalizations also lessened with the use of lorazapam, ondansetron, and acetaminophen as rescue medications at the onset of emesis.
She has had over 20 hospitalizations with multiple extended stays due to upper respiratory infections, pneumonia, urinary tract infections, and dehydration secondary to cyclic emesis. Multiple respiratory infections and airway compromise improved with inhaled steroids and beta agonist treatments. She had severe obstructive sleep apnea at age 6 months, requiring nightly oxygen, but by age 16 months, the obstructive apnea had resolved, with persistence of central apnea and occasional bradycardia at age 27 months. Recurrent urinary tract infections were a significant problem until she was diagnosed with nightly urinary retention requiring in-dwelling catheterization and prophylactic antibiotics. Aggressive treatment for severe constipation improved her urinary retention. She received regular renal ultrasounds for close monitoring and Wilms tumor surveillance. She had difficulty sleeping and episodes of severe fatigue of unknown etiology. Her insomnia was initially treated with melatonin, but significant improvement was noted when iron supplementation began for severe anemia. A cranial MRI showed a small corpus callosum and spinal MRI showed a small cyst over the conus, which was thought to be benign. A neonatal echocardiogram demonstrated normal anatomy with a small patent foramen ovale and moderate patent ductus arteriosus.
At 35 months she had no spoken words but babbled and followed one-step commands. She was able to sit upright independently for brief periods, roll, and ambulate in a walker. She had a happy, curious personality and lacked stranger anxiety. She had shown good growth with length of 86 cm (10th centile), weight of 12.3 kg (20th centile) and OFC of 47.5 cm (50th centile). She was noted to have rapid hair and nail growth. See Table I and Fig. 1 C–D for additional physical features. At age 3.5 years, her health improved to the extent that she was no longer hospitalized on a frequent basis, and she was making regular developmental progress.
Patient 3 was a 3-year-old-male born at 37 weeks gestation by normal vaginal delivery as the first pregnancy for his 30-year-old parents (Fig. 1 E). Pregnancy was complicated by maternal bleeding, and he was noted to have prenatal onset growth restriction. Dysmorphic features were noted at birth, and he developed mild respiratory distress requiring a 10-day intensive care stay for suspected sepsis and intrauterine growth restriction. Severe myopia was diagnosed in the first month of life (−11 OD and −10 OS diopters) and improved to −2, −3 diopters with normal optic nerves. A cranial MRI and hearing evaluation were normal. He had significant obstructive sleep apnea requiring the use of CPAP. Silent aspiration was identified via a swallow study and he had a gastrostomy tube placed at 20 months.
An extensive neonatal evaluation included a normal echocardiogram, renal ultrasound, and TORCH screen. At age 22 months he was unable to support his head or trunk, roll over or use words. He was responsive to external stimuli and made extended eye contact, with a pleasant and friendly demeanor. He remained delayed in his growth with a length of 76 cm (−4 SD below the mean), weight of 7.36 kg (−5 SD below mean for age) and OFC 44 cm (−4 SD below mean for age). He had downslanting palpebral fissures with small ears, a short nose, short smooth philtrum, and delayed dental eruption. He also had widely-spaced nipples, single palmar creases, redundant skin, proximally placed thumbs, and neuromuscular scoliosis. He had rapid growth of hair and nails. Additional clinical findings are in Table I and Fig. 1 E. He also had overproduction of saliva and mucous. At age 3 years his sleep apnea had improved, and his parents reported rapid growth of his hair and nails. He was making slow developmental progress, with improvement of his BOS posture.
Patient 4 was a 3-year-old female born at 39 weeks by repeat cesarean as the second pregnancy for her 24-year-old mother and 27-year-old father (Fig. 1 G). The pregnancy was complicated by black mold exposure and removal of an intrauterine device during the second trimester. Length could not be accurately determined at birth due to contractures (measured at 36 cm, <1st centile), but length was 50.5 cm at one month (25th centile). At birth she had severe hip flexion, clubbed feet, contracture of the right knee, flexion of the elbows and wrists with adducted thumbs. She was given a diagnosis of arthrogryposis multiplex, with no dislocations noted.
She had significant feeding issues requiring a gastrostomy tube, followed by a gastro-jejunostomy tube placement. A tracheostomy was placed and she underwent mandibular distraction for retrognathia, along with a tongue-lip adhesion, which resolved her obstructive sleep apnea. Heel cord releases were performed for her clubbed feet. A head CT showed enlarged subarachnoid spaces and agenesis of the corpus callosum, and she required treatment with levetiracetam for seizures. She required intermittent inhaled steroids and bronchodilators for reactive airway disease, polyethylene glycol for constipation, and melatonin for sleep.
At age 18 months she was unable to sit alone, lacked head control and was unable to visually track objects. Her weight was 10.1 kg (25th centile), length 73 cm (3rd centile) and OFC 43.5 cm (<3rd centile). She had a short nose, decreased muscle mass in her legs with hypermobility at the hips and knees, status post heel cord lengthening. She had strabismus. Rapidly growing hair and nails were noted. Additional physical features are in Table I and Figure 1 G.
Shortly after BOS was molecularly confirmed, a screening renal ultrasound showed bilateral multifocal Wilms tumor involving most of her left kidney and the upper pole of her right kidney. Biopsy of the left kidney showed histology that was between a nephrogenic rest and a Wilms tumor, with right kidney histology compatible with a nephrogenic rest. Immunohistochemical staining was strongly positive for WT1 and CD56. She was treated with vincristine, actinomycin and doxorubicin, followed by resection of her left kidney at age 30 months. Chemotherapy resulted in hair loss, with rapid regrowth after the completion of her therapy, and her renal function remained adequate. She was very irritable prior to treatment for Wilms tumor, but after completion of therapy, her irritability improved with emergence of her true happy and outgoing personality.
Patient 5 was a 12-year-old female born full term as the first child for her 28-year-old mother and 31-year-old father, with a prior spontaneous abortion at 9 weeks gestation and a subsequent healthy son. Pregnancy was uneventful with a vaginal delivery, which was complicated by the patient’s poor respiratory effort and bradycardia. She received continuous bag and mask ventilation with resolution of her poor cardiopulmonary output. At birth she was noted to have dysmorphic features, hypotonia, and bilateral cleft lip and palate with preservation of the premaxilla (Fig. 1H–I). A sacral hair patch was present and spinal ultrasound confirmed a sacral sub-arachnoid cyst.
She had a complex clinical course requiring multiple hospitalizations and surgeries, and she improved with age. During her multiple illnesses, marked leukocytosis was noted in response to stress. Laryngomalacia and subglottic stenosis were treated with a supraglottoplasty at age six weeks. She underwent repair of her cleft lip and palate. Severe gastrointestinal reflux with silent aspiration, poor intestinal motility, and poor gastric emptying necessitated gastrostomy tube placement at six weeks of age, followed by a Roux en Y jejeunostomy placement at seven months and a Nissen fundoplication at six years. Cyclic vomiting was diagnosed at seven years, and cyproheptadine treatment helped dramatically. Triggers such as anesthesia and illness were identified, and she took lorazapam and ondansetron at the start of an episode. A paraesophageal hernia was identified at age 10 years, which required 2 repairs. At age 12, she continued to be fed primarily through her gastrostomy tube.
Neonatal echocardiogram demonstrated pulmonary hypertension with subsequent identification of pulmonic stenosis, ventricular hypertrophy, and an atrial septal defect, which was repaired at age one year. Echocardiogram at age 12 demonstrated trivial pulmonary valve insufficiency and borderline left ventricular hypertrophy. Reactive airway disease was diagnosed at age three years and treated with albuterol. She was prone to recurrent upper respiratory infections, which improved over time. Her respiratory status quickly deteriorated with acute illness or surgery, resulting in poor oxygenation and need for mechanical ventilation or BiPap. She had obstructive apnea requiring CPAP, as well as significant insomnia that improved with treatment of her severe anemia. The patient began having generalized tonic-clonic seizures at age seven months, which continued to occur in association with acute illness. Cranial MRI in infancy showed prominent extra axial spaces but no other abnormalities. At age 12, cranial MRI demonstrated parenchymal volume loss, tortuous, prominent bilateral cavernous carotid arteries and vertebral arteries with enlargement of bilateral superior optic veins.
Extreme myopia (−13.5 OD and −17.5 OS diopters) was diagnosed at age three months. She had nystagmus, right exotropia, and thin, atrophic appearing retinas. She had small ear canals and recurrent otitis media requiring multiple sets of ventilation tubes. An ABR showed mild to moderate conductive hearing loss bilaterally. Skeletal abnormalities included neuromuscular scoliosis requiring posterior spinal fusion at age nine years, a history of hip and patellar subluxations, and acquired right sided pectus excavatum and left-sided pectus carinatum. Precocious puberty occurred with pubic hair development at age seven months, with breast development at age seven years. Bone age was advanced at (+4 SD, bone age 13 years 6 months at chronological age of 9 years 11 months).
She had global developmental delays and was wheelchair-bound. She was nonverbal but communicated with a few signs. She responded to family and friends and had a happy, pleasant personality. At 9 years her height was 129.4 cm (26th centile), weight was 23.1 kg (8th centile) and OFC was 50 cm (6th centile). On examination, she had epicanthal folds and small feet (toddler size 12 at age 12 years). She had rapidly growing hair and nails. All other physical findings are noted in Table I and Fig. 1 H–I.
Patient 6 was a 3 year-old boy born at full term with asymmetric intrauterine growth retardation (Fig. 1 F). He had meconium aspiration requiring intubation, but he was weaned off supplemental oxygen by two weeks of age. Due to a poor suck, he required tube feeding the first two weeks. Although he fed well using a regular bottle, by age 5 months, his weight and length were −5 to −6 SD.
Cranial MRI at six months showed reduced deep white matter, but relatively well-preserved subcortical white matter. He had a normal EEG at nine months with no clinical seizures. He had chronic constipation requiring laxative use and gastroesophageal reflux managed with a gastrostomy tube. Abdominal ultrasound to screen for intra-abdominal embryonal tumors was normal. He was first diagnosed with myopic astigmatism (−4 diopters OU) at 13 months and gradually became more myopic, with a prescription of −6 diopters OU by 3 years of age. Electroretinography (ERG) demonstrated attenuated responses to full field stimuli in both scotopic (dark; rod photoreceptor mediated) and photopic (light; cone photoreceptor mediated) conditions with concern for retinal dystrophy. In addition, he was thought to have reduced visual acuity due to difficulties with “visual processing”. His hearing was normal. He had congenital hypertonia, but over time he developed truncal hypotonia and weakness. He was otherwise in good health without respiratory concerns or multiple hospitalizations.
Developmentally, he started sitting with support at five months and he was able to sit without support and to feed himself with a bottle at age three years. He was unable to crawl. At age three years, he had two words that he used consistently and a few other words that he used occasionally, but he lost the use of those words. He had a happy personality, sometimes laughing on his own for no apparent reason. He enjoyed socializing with other children but became overwhelmed in large groups. He had a history of intentional breath holding spells but calmed with classical music. He was also sensitive to extreme temperatures including weather, food, and baths. He continued to mouth objects with excessive salivary production. His sleep latency was mildly increased (about one hour) and he would often laugh or giggle while asleep, but he typically slept through the night.
On physical examination at 3 years of age, his length was −5.2 SD below the mean, weight was −6.5 SD below the mean, and OFC was approximately 10th centile. He had facial asymmetry secondary to torticollis-plagiocephaly deformation sequence, with the right side of the face being more prominent than the left. He had left exotropia and his left pupil appeared slightly larger than his right. He had mild premaxillary prominence with a well-formed philtrum. He had a thin vermilion of the upper lip, full lower lip, and a slightly small mouth. Both ears were normally positioned with stenotic ear canals, an antihelical shelf in each ear, and a slightly wide incisura in the right ear. He had a prominent metopic ridge and bitemporal narrowing, but no trigonocephaly. There was dolichocephaly with flattening of the right occipito-parietal region related to left congenital muscular torticollis. He had weakness in all four limbs with brisk deep tendon reflexes. He had rapidly growing hair and nails. Additional physical findings are in Table I and Fig. 1F.
Patient 7 was a 1-year-old female born at 35-6/7 weeks gestation as the first pregnancy for her 23-year-old mother after a pregnancy complicated by maternal hip dysplasia (Fig. 1J–K). She was delivered by caesarean and noted to have respiratory distress soon after birth. She was discharged home at three and a half weeks of life on full oral feeds, but had significant difficulty feeding at home. She was diagnosed with gastrointestinal reflux and laryngomalacia, and swallowing studies showed aspiration of thin liquids. Nasogastric tube feeds were used for persistent vomiting and episodes of aspiration pneumonia, and symptoms improved following the placement of a gastrostomy tube and a Nissen procedure at age 11 months. Despite tube feedings, she continued to have recurrent pneumonia and respiratory infections. An initial echocardiogram demonstrated a moderate patent ductus arteriosis, patent foramen ovale, and tricuspid regurgitation with elevated right ventricular pressures. Spine ultrasound showed a low-lying conus medullaris and pilonidal tract cyst. Cranial MRI identified partial absence of the corpus callosum. Renal imaging showed no abnormalities. At 13 months of age she was diagnosed with myopia (−4 OU diopters). Other medical complications included obstructive sleep apnea, and EEG findings of centro-temporal spikes without seizures. At 11 months her weight, length, and OFC were below the 3rd centile. She had short palpebral fissures, small nose with a bulbous tip, small mouth, fifth digit clinodactyly, and a 3 × 5 cm vascular malformation over her sacral area. She had fast growing hair and nails. See Table I and Fig. 1J–K for additional physical findings. Developmentally, she was able to roll, reach for objects, and babbled. She had a happy and pleasant personality.
Patient 8 was a 7-month-old male born at full term as the first child to a 42-year-old-mother and 38-year-old father (Fig. 1L–M). Serum screening in pregnancy had indicated a high risk for Down syndrome, but detailed anomaly scans were normal and amniocentesis showed a normal male karyotype. At 36 weeks gestation, oligohydramnios, short femurs, and absent umbilical arterial end diastolic flow were noted. He was delivered with reassuring Apgar scores, but respiratory distress was noted after his initial feeding, leading to admission to the neonatal intensive care unit. He required supplemental oxygen for several days and had difficulties clearing oral secretions or demonstrating a gag reflex. He was discharged home with exclusive nasogastric tube feeding. Initial echocardiogram showed a small atrial septal defect and patent ductus arteriosus. A newborn renal ultrasound was normal.
At six months of age, he continued to have feeding difficulties and frequent emesis despite routine reflux management. A gastrojejunostomy tube was placed, with feeding through the gastrostomy port. Weight gain improved with tube feeds but continued to be well below the third centile at 7 months. A right inguinal hernia was repaired at 4 months of age, and he was found to have right-sided grade 3 vesicoureteric reflux with pelvicalyceal dilatation of his right kidney. A repeat renal ultrasound at age 6 months was normal. A cranial MRI scan demonstrated a thin corpus callosum with enlarged ventricular lateral horns. An immunology assessment demonstrated poor response to the pneumococcal vaccine, but normal immunoglobulins and lymphocyte subsets. He did not have recurrent respiratory infections. At 6 months of age he was diagnosed with myopia of −16 diopters OU and hypopigmented retinas.
Developmentally, at seven months of age he had improving head control. He put his hands to his mouth, used his arms to hold objects midline, and started to roll. He was unable to reach for objects or sit unsupported. His personality was not yet well defined. On examination, he demonstrated typical BOS posture and contractures of both third digits as well as ulnar deviation of second and third digits. He had single palmar creases. He had rapidly growing hair and nails. Additional physical features are listed in Table I and Fig. 1L–M.
METHODS AND RESULTS
ASXL1 Mutation Identification
Table III presents the ASXL1 mutations identified in Patients 1–8 as well as whether the mutation are de novo as well as other clinical and molecular testing that was done.
Table III.
ASXL1 mutations in 8 previously unreported patients.
| Patients | ASXL1 | De novo | Other Testing |
|---|---|---|---|
| 1 | c.2013_2014 del, p.Cys672Trpfsx41 | + | Karyotype, microarray, DHCR7, HRAS, KRAS, BRAF, MAP2K1, MAP2K2 |
| 2 | c.3077del, p.Gly1026fs2 | Untested | Karyotype |
| 3 | c.1924 G>T, p.Gly642X3 | Untested | Karyotype, microarray TCOF1, NIPBL |
| 4 | c.2324 T>G, p.Leu775X2 | Untested | Karyotype, microarray (168 kb dup 8q21.3) |
| 5 | c.2324T>G, p.Leu775X4 | Untested | Karyotype, extensive testing |
| 6 | c.2757_2758insCCAT, p.Ile1919fs5 | + | Karyotype, microarray (185 kb dup Xp22.2), DHCR7, breakage studies, metabolic |
| 7 | c.1272_1273delGT, p.Tyr425GlnfsX126 | Untested | Karyotype, FISH, microarray, DHCR7, ASXL2, ASXL3, CD96 |
| 8 | c.2535dup, p.Ser846fs2* | Pending | Karyotype |
Mutation previously reported in Hoischen et al 2011.
Exome sequencing at the National Institutes of Health Intramural Sequencing Center
University Medical Center St. Radboud Molecular Diagnostic Laboratory in Nijmegen, Netherlands
Exome sequencing at GeneDx
Dr. Axel Bohring on a research basis
Clinical exome sequencing
Targeted exome sequencing analysis of ASXL1, ASXL2, ASXL3 and CD96 at GeneDx
ASXL1 Loss of Heterozygosity for Patient 1
Patient slides of Wilms tumor tissue from Patient 1 were acquired and DNA was isolated from mounted sections either by scraping (normal kidney, tumor) or through laser capture microdissection (tumor). Peripheral blood DNA was isolated from whole blood using standard salting out methods. Heterozygosity at the ASXL1 locus (chr20:30,946,147-31,027,122) was determined for three variants in ASXL1 present in the patient, including the causative alteration. Two SNPs not at the ASXL1 locus were tested (IL7R, chr5; XDH, chr2). Samples tested included patient DNA from peripheral blood, two normal kidney sections, a scraped tumor sample, and a microdissected tumor sample. A DNA sample from a control individual was tested. PCR primers for all amplicons containing targeted variants were combined into a single PCR reaction. Variants were detected using the ABI PRISM SNaPshot Multiplex kit according to the standard protocol (Applied Biosystems, Foster City, CA).
DISCUSSION
Since its initial delineation and separation from Opitz Trigonocephaly C syndrome and the association of BOS with ASXL1 mutations, efforts have been made to further delineate the clinical features for this disorder. Patients tend to have a variable neonatal presentation, as demonstrated by minimal features seen in Patient 1 at birth versus Patient 4 with significant arthrogryposis at birth. The differential is broad for these patients with hypertrichosis and IUGR resembling Cornelia de Lange syndrome, coarse facial features resembling a storage disease or RASopathy, and micrognathia resembling Treacher Collins syndrome. With only 43 previously reported patients and a high incidence of infant mortality, many previous patients underwent extensive testing prior to diagnosis. Now, patients are living into adulthood, and little has been done to identify specific management strategies. The focus on the natural history and clinical management in the patients reported herein initiates this discussion on how to care for these complex patients. It is of interest that these patients reported herein have happy personalities as well as rapidly growing hair and nails, and two patients had early onset of pubertal changes, which are novel features.
The recent identification of ASXL1mutations in BOS led to increasing recognition of BOS. There appears to be a consistent BOS phenotype, yet six patients who met the original diagnostic criteria tested negative for ASXL1 mutations, suggesting possible genetic heterogeneity [Hoischen et al., 2011], possible somatic mosaicism, or a need to refine these initial diagnostic criteria. Bainbridge et al. [2013] recently identified four patients with ASXL3 mutations and phenotypes similar to, yet distinct from, BOS [Bainbridge et al., 2013]. A comparison of the features of patients with ASXL1 and ASXL3 mutations demonstrated overlap of some features including IUGR, severe to profound developmental delay, feeding difficulties, microcephaly, high palate, prominent eyes, similar but milder facial features, generalized hypotonia, and partial BOS posture with ulnar deviation of hands [Russell and Graham, 2013]. The patients with BOS published by Hoischen et al. [2011] who did not have an ASXL1 mutation were also negative for ASXL3 mutations [Bainbridge et al., 2013], suggesting that ASXL3 may not play a role in the genetic heterogeneity of BOS. Given the potentially lethal impact of mutations in ASXL1, somatic mosaicism should be considered in patients with a typical phenotype and no detectable mutation in lymphocyte DNA, as reported by Huisma et al. [2013] for Cornelia de Lange syndrome, where 10/44 (23%) of patients were diagnosed with buccal swab DNA. More questions remain regarding possible genetic heterogeneity versus somatic mosaicism and other conditions with overlapping phenotypes.
Clinical management of patients with BOS is challenging given the complexity and high mortality rate. Limited data have been published on the natural history of BOS. Table II summarizes the management of patients with BOS as presented in the medical literature. The histories of the eight patients described herein are relevant to the issues of feeding intolerance, emesis, sleep difficulties, respiratory issues, and tumor surveillance. All eight patients struggled with adequate caloric intake by mouth, and Patients 2 and 5 had significant episodes of cyclic emesis. These challenges are common among patients with BOS, since over half (53%) required gastrostomy tubes (Table II). The family of Patient 1 was able to overcome her feeding problems and prevent the need for a gastrostomy tube by extended breastfeeding, followed by small, high calorie meals and, subsequently offering flavorful, crunchy, textured foods. Patients 2 and 5 struggled with the most severe feeding difficulties including episodes of emesis that resulted in multiple episodes of hospitalizations for Patient 2. Their families noted that emesis was typically associated with an organic cause such as vaccines, anesthesia, or infections. They both required a gastrostomy tube for nutrition, and treatment for cyclic vomiting with cyproheptadine daily, as well as lorazepam and ondansetron at the start of an episode of emesis, which resulted in decreased hospitalizations and improved nutrition. Patients 2–6 required gastrostomy tubes due to aspiration and/or an inability to take adequate calories orally. We suggest that a treatment goal for these feeding difficulties should be to avoid unnecessary hospitalization and surgery if emesis can be controlled with alternative feeding strategies and treatment for cyclic vomiting. Many cited occurrences of severe feeding difficulties describe emesis with feeding without confirmation of gastroesophageal reflux [Bohring et al., 1999; Pierron et al. 2009; Hastings et al., 2010; Magini et al., 2012]. This led us to consider alternative mechanisms for chronic emesis including gastric dysmotility related to hypotonia. Regardless, previous reports and the clinical course of the patients herein suggest that feeding difficulties improve with age, which implies that gastrostomy tubes may not be required throughout life [Greenhalgh et al., 2003; Hastings et al., 2011].
Table II.
Clinical management concerns, causes of death and the most common procedures performed in patients with BOS.
| Clinical Management Concerns | ASXL1− (%) | ASXL1+ (%) | All BOS (%) |
|---|---|---|---|
| Failure to thrive | 6/6 (100%) | 17/17 (100%) | 43/43 (100%) |
| Severe/Profound ID | 6/6 (100%) | 17/17 (100%) | 39/39 (100%) |
| Feeding difficulties | 6/6 (100%) | 17/17 (100%) | 41/41 (100%) |
| Myopia | 1/3 (33%) | 15/16 (93%) | 22/24 (92%) |
| Frequent emesis | 4/4 (100%) | 9/11 (81%) | 23/25 (92%) |
| Recurrent infections | 4/5 (80%) | 8/13 (62%) | 18/35 (51%) |
| Seizures | 3/6 (50%) | 8/15 (53%) | 18/28 (64%) |
| Apnea | 1/5 (20%) | 6/12 (50%) | 16/25 (64%) |
| Bradycardia | 0/5 (0%) | 1/13 (8%) | 6/23 (26%) |
| Causes of Death* | |||
| Respiratory infections | 1/2 (50%) | 1/1 (100%) | 5/12 (42%) |
| Cardiovascular/Apnea/Bradycardia | 0/2 (0%) | 0/1 (0%) | 4/12 (33%) |
| Seizure | 0/2 (0%) | 0/1 (0%) | 1/12 (8%) |
| Medulloblastoma | 1/2 (50%) | 0/1 (0%) | 1/12 (8%) |
| Sepsis | 0/2 (0%) | 0/1 (0%) | 1/12 (8%) |
| Procedures | |||
| Nissen Fundoplication | 2/6 (33%) | 2/17 (12%) | 8/43 (19%) |
| G-Tube | 4/6 (67%) | 9/17 (53%) | 23/43 (53%) |
| Tracheostomy | 0/6 (0%) | 1/17 (6%) | 2/43 (5%) |
Table shows fraction and percent in ASXL1 negative (6 total), ASXL1 positive (17 total) and all patients which includes untested patients with the clinical diagnosis of BOS (43 total). When authors did not discuss a feature in their publication or the patient is still living, that patient is not included in the denominator.
17 of 43 (40%) BOS patients have reported deaths in the literature ages 23 hours to 6 years-of-age with 11 deaths (26%) at less than 1 year-of-age. 12 deaths have reported etiologies.
Respiratory infections and reactive airway disease are common in patients with BOS, accounting for 42% of deaths, particularly in the first two years of life (Table II). The early years of Patients 1, 2, 4, and 5 were centered on the management of multiple severe respiratory infections. Patient 1 endured many hospitalizations and chronic lung infections until she was treated for reactive airway disease with prophylactic inhaled corticosteroids at age two and a half, after which her infections diminished and she no longer required respiratory medications. Patient 2 spent most her first year in the hospital due to chronic emesis, dehydration, and urinary tract infections, further complicated by repeated severe respiratory infections. With control of her cyclic vomiting and the use of inhaled corticosteroids and beta agonists daily, her frequency and severity of respiratory illnesses dramatically decreased. This may be multifactorial given her decreased infectious exposure outside the hospital, decreased aspiration risk with control of her emesis, and the use of daily respiratory treatments. Patients 4 and 5 had mild respiratory symptoms, but benefited from treatment for reactive airway disease. Lessons learned from these patients led to treatment goals including reducing aspiration and treating possible reactive airway disease. Similarly to feeding difficulties, the occurrence of respiratory infections improves with age [Greenhalgh et al., 2003; Hastings et al., 2011].
Another challenging feature that five of the eight patients shared was sleep disturbances. This is a symptom that has not been described, but it had a dramatic effect on the families. Melatonin seemed to offer some benefit, but it is interesting that treatment for anemia in Patients 2 and 5 improved their sleep disturbances. Treatment for obstructive sleep apnea by mandibular distraction and CPAP may be beneficial.
Reports of malignancies in patients with BOS have been limited to a patient who died at five months and was found on autopsy to have bilateral nephroblastomatosis [Brunner et al., 2000], and a patient who developed medulloblastoma at age five years [Hastings et al., 2011]. Neither had a mutation in ASXL1 [Hoischen et al., 2011]. It is interesting to note that Patients 1 and 4 developed bilateral Wilms tumor at age six and age two years, respectively. Unlike the previously reported patients with BOS and malignancy, they had a mutation in ASXL1. ASXL1 mutations have been identified in patients with myelodysplastic syndromes [Aravind and Iyer, 2012; Wang et al., 2014]. Leukemia cells with heterozygous ASXL1 mutations have reduced or absent ASXL1 protein expression, suggesting a role for ASXL1 as a tumor suppressor [Abdel-Wahab et al., 2012]. In order to determine if loss of heterozygosity of ASXL1 might contribute to Wilms tumor in BOS, tumor DNA from Patient 1 was tested for loss of heterozygosity at the ASXL1 locus. Loss of heterozygosity was not appreciated; however, it is possible that functional loss of the wild-type copy of the gene occurred in the tumor through a mechanism other than gene deletion or conversion. Given the link between ASXL1 and myelodysplastic conditions [Aravind and Iyer, 2012, Wang et al., 2014], combined with the small number of reported patients with BOS and high infant mortality, predisposition for neoplasms may be higher than reported. Including the patients discussed herein, there are 43 published patients with BOS of which two had Wilms tumor and one had bilateral nephroblastomatosis in infancy, making the incidence of a renal neoplastic process 7% among published patients, although this may be inflated due to biased ascertainment. Screening protocols for Wilms tumor in patients with Beckwith-Wiedemann syndrome are based on an estimated lifelong incidence of 4% and include renal ultrasound scans every three to four months until eight years of age [Tan and Amor, 2006]. It is therefore reasonable to consider an abdominal ultrasound every three to four months in the first eight years old life for patients with BOS.
Ultimately, identification of ASXL1 as a causative mutation for BOS allowed for molecular diagnosis and led to concern about an increased neoplastic risk for patients with BOS as demonstrated by a relatively high rate of renal neoplastic conditions in a small sample size. Lack of an ASXL1 mutation in some patients with clinically-diagnosed BOS results in uncertainty as to the genetic heterogeneity of the syndrome versus somatic mosaicism. Diversity in phenotype makes clinical diagnosis challenging. Given the rarity of BOS, the natural history and clinical management of these patients need to be further explored.
Acknowledgments
The authors thank the patient’s families for their unwavering dedication to their children and for their willingness to contribute to our knowledge of Bohring Opitz syndrome. We appreciate our patients and their families for always asking the right questions and inspiring us to improve the care of these children.
We also thank Dr. Axel Bohring for performing mutation analysis on Patient 5. We gratefully acknowledge The Manton Center for Orphan Disease Research Gene Discovery Core and the Research Connection at Boston Children’s Hospital for facilitating patient DNA collection and financial support for exome sequencing, as well as Pankaj Agrawal and Meghan Towne for assistance in sample collection and analyses on Patient 6. We also acknowledge Codified Genomics for helping in variant analysis and access to their informatics pipeline for Patient 6. JJJ and LGB were supported by funding from the National Human Genome Research Institute Intramural Research Program of NIH.
References
- Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, Pandey S, Patel JP, Chung YR, Koche R, Perna F, Zhao X, Taylor JE, Park CY, Carroll M, Melnick A, Nimer SD, Jaffe JD, Aifantis I, Bernstein BE, Levine RL. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–193. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addor MC, Stefanutti D, Farron F, Meinecke P, Lacombe D, Sarlangue J, Prescia G, Schorderet DF. C Trigonocephaly syndrome with diaphragmatic hernia. Genet Counsel. 1995;6:113–120. [PubMed] [Google Scholar]
- Antley RM, Hwang DS, Theopold W, Gorlin RJ, Steeper T, Pitt D, Danks DM, McPherson E, Bartels H, Wiedemann HR, Opitz JM. Further delineation of the C (trigonocephaly) syndrome. Am J Med Genet. 1981;9:147–163. doi: 10.1002/ajmg.1320090209. [DOI] [PubMed] [Google Scholar]
- Aravind L, Iyer LM. The HARE-HTH and associated domains: novel modules in the coordination of epigenetic DNA and protein modifications. Cell Cycle. 2012;11:119–131. doi: 10.4161/cc.11.1.18475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge MN, Hao H, Muzny DM, Musante L, Lupski JR, Graham BH, Chen W, Gripp KW, Wienker TF, Yang Y, Sutton VR, Gibbs RA, Ropers HH. De novo truncating mutations in ASXL3 are associated with a novel clinical phenotype with similarities to Bohring-Opitz syndrome. Genome Med. 2013;5:11. doi: 10.1186/gm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard AM, Kirchhoff M, Nielsen JE, Brandt C, Hove H, Jepsen B, Jensen T, Ullmann R, Skovby F. Transmitted cytogenetic abnormalities in patients with mental retardation: pathogenic or normal variants? Eur J Med Genet. 2007;50:243–255. doi: 10.1016/j.ejmg.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Bohring A, Oudesluijs GG, Grange DK, Zampino G, Thierry P. New cases of Bohring-Opitz syndrome, update, and critical review of the literature. Am J Med Genet Part A. 2006;140A:1257–1263. doi: 10.1002/ajmg.a.31265. [DOI] [PubMed] [Google Scholar]
- Bohring A, Silengo M, Lerone M, Superneau DW, Spaich C, Braddock SR, Poss A, Opitz JM. Severe end of Opitz trigonocephaly (C) syndrome or new syndrome? Am J Med Genet. 1999;85:438–446. doi: 10.1002/(sici)1096-8628(19990827)85:5<438::aid-ajmg2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Van Tintelen JP, De Boer RJ. Bohring syndrome. Am J Med Genet. 2000;92:366–368. doi: 10.1002/1096-8628(20000619)92:5<366::aid-ajmg15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fisher CL, Berger J, Randazzo F, Brock HW. A human homolog of Additional sex combs, Additional Sex Combs-Like 1, maps to chromosome 20q11. Gene. 2003;306:115–126. doi: 10.1016/s0378-1119(03)00430-x. [DOI] [PubMed] [Google Scholar]
- Fisher CL, Lee1 I, Bloyer S, Bozza S, Chevalier J, Dahl A, Bodner C, Helgason CD, Hess JL, Humphries RK, Brock HW. Additional sex combs-like 1 belongs to the enhancer of trithorax and Polycomb Group and genetically interacts with Cbx2 in mice. Dev Biol. 2010;337:9–15. doi: 10.1016/j.ydbio.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh KL, Newbury-Ecob RA, Lunt PW, Dolling CL, Hargreaves H, Smithson SF. Siblings with Bohring-Opitz syndrome. Clin Dysmorphol. 2003;12:15–19. doi: 10.1097/00019605-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Hastings R, Cobben JM, Gillessen-Kaesbach G, Goodship J, Hove H, Kjaergaard S, Kemp H, Kingston H, Lunt P, Mansour S, McGowan R, Metcalfe K, Murdoch-Davis C, Ray M, Rio M, Smithson S, Tolmie J, Turnpenny P, van Bon B, Wieczorek D, Newbury-Ecob R. Bohring-Opitz (Oberklaid-Danks) syndrome: clinical study, review of the literature, and discussion of possible pathogenesis. Eur J Hum Genet. 2011;19:513–519. doi: 10.1038/ejhg.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings RW, Newbury-Ecob R, Lunt PW. A case of probable Bohring-Opitz syndrome with medulloblastoma. Clin Dysmorphol. 2010;19:202–205. doi: 10.1097/MCD.0b013e32833e07de. [DOI] [PubMed] [Google Scholar]
- Hoischen A, van Bon BW, Rodriguez-Santiago B, Gilissen C, Vissers LE, de Vries P, Janssen I, van Lier B, Hastings R, Smithson SF, Newbury-Ecob R, Kjaergaard S, Goodship J, McGowan R, Bartholdi D, Rauch A, Peippo M, Cobben JM, Wieczorek D, Gillessen-Kaesbach G, Veltman JA, Brunner HG, de Vries BBBA. De novo nonsense mutations in ASXL1 cause Bohring-Opitz syndrome. Nat Genet. 43:729–731. doi: 10.1038/ng.868. [DOI] [PubMed] [Google Scholar]
- Huisman SA, Redeker EJW, Maas SM, Mannens MM, Hennekam RCM. High rate of mosaicism in individuals with Cornelia de Lange syndrome. J Med Genet. 2013;50:339–344. doi: 10.1136/jmedgenet-2012-101477. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JJ, Wen KK, Keppler-Noreuil K, McKane M, Maiers JL, Greiner A, Sapp JC, Demali KA, Rubenstein PA, Biesecker LG. Functional analysis of a de novo ACTB mutation in a patient with atypical Baraitser-Winter syndrome. Hum Mutat. 2013;34:1242–1249. doi: 10.1002/humu.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor NM. Severe end of Opitz Trigonocephaly C Syndrome. Am J Med Genet. 2000;92:363–365. doi: 10.1002/1096-8628(20000619)92:5<363::aid-ajmg14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Magini P, Della Monica M, Uzielli ML, Mongelli P, Scarselli G, Gambineri E, Scarano G, Seri M. Two novel patients with Bohring-Opitz syndrome caused by de novo ASXL1 mutations. Am J Med Genet Part A. 2012;158A:917–921. doi: 10.1002/ajmg.a.35265. [DOI] [PubMed] [Google Scholar]
- McGaughran J, Aftimos S, Oei P. Trisomy of 3pter in a patient with apparent C (trigonocephaly) syndrome. Am J Med Genet. 2000;94:311–315. doi: 10.1002/1096-8628(20001002)94:4<311::aid-ajmg9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Nakane T, Kubota T, Fukushima Y, Hata Y, Ishii J, Komiyama A. Opitz trigonocephaly (C)-like syndrome, or Bohring-Opitz syndrome: another example. Am J Med Genet. 2000;92:361–362. doi: 10.1002/1096-8628(20000619)92:5<361::aid-ajmg13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Oberklaid F, Danks D. The Opitz trigonocephaly syndrome. Am J Dis Child. 1975;129:1348–1349. doi: 10.1001/archpedi.1975.02120480062016. [DOI] [PubMed] [Google Scholar]
- Osaki M, Makita Y, Miura J, Abe N, Noguchi S, Miyamoto A. A Japanese boy with apparent Bohring-Opitz or “C-like” syndrome. Am J Med Genet Part A. 2006;140A:897–899. doi: 10.1002/ajmg.a.31164. [DOI] [PubMed] [Google Scholar]
- Pierron S, Richelme C, Triolo V, Mas JC, Griffet J, Karmous-Benailly H, Quere M, Kaname T, Lambert JC, Giuliano F. Evolution of a patient with Bohring-Opitz syndrome. Am J Med Genet Part A. 2009;149A:1754–1757. doi: 10.1002/ajmg.a.32910. [DOI] [PubMed] [Google Scholar]
- Russell B, Graham JM. Expanding our knowledge of conditions associated with the ASXL gene family. Genome Med. 2013;5:16. doi: 10.1186/gm420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA, Milne TA, Hodgson JW, Shellard J, Salinas CA, Kyba M, Randazzo F, Brock HW. The additional sex combs gene of Drosophila encodes a chromatin protein that binds to shared and unique Polycomb group sites on polytene chromosomes. Development (Cambridge, England) 1998;125:1207–1216. doi: 10.1242/dev.125.7.1207. [DOI] [PubMed] [Google Scholar]
- Simpson AR, Gibbon CE, Quinn AG, Turnpenny PD. Infantile high myopia in Bohring-Opitz syndrome. J AAPOS. 2007;11:524–525. doi: 10.1016/j.jaapos.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Tan TY, Amor DJ. Tumor surveillance in Beckwith-Wiedemann syndrome and hemihyperplasia: A critical review of the evidence and suggested guidelines for local practice. J Paediatr Child Health. 2006;42:486–490. doi: 10.1111/j.1440-1754.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Li Z, He Y, Pan F, Chen S, Rhodes S, Nguyen L, Yuan J, Jiang L, Yang X, Weeks O, Liu Z, Zhou J, Ni H, Cai CL, Xu M, Yang FC. Loss of Asxl1 leads to myelodysplastic syndrome-like disease in mice. Blood. 2014;123:541–553. doi: 10.1182/blood-2013-05-500272. [DOI] [PMC free article] [PubMed] [Google Scholar]


