Abstract
Suppression of ovarian hormones in premenopausal women with gonadotropin releasing hormone agonist therapy (GnRHAG) can cause fat mass (FM) gain and fat-free mass (FFM) loss. It is unknown if this is specifically due to the decline in serum estradiol (E2).
Objective
To evaluate the effects of GnRHAG with add-back of placebo (PL) or E2 on FM, FFM, and bone mineral density (BMD). An exploratory aim evaluated the effects of resistance exercise on body composition during the drug intervention.
Methods
Seventy healthy, premenopausal women underwent 5 months of GnRHAG and were randomized to add-back of transdermal E2 (GnRHAG+E2, n=35) or placebo (GnRHAG+PL, n=35). As part of an exploratory aim to evaluate whether exercise can minimize effects of hormone suppression, some women within each drug arm were randomized to a resistance exercise program (GnRHAG+E2+Ex, n=12; GnRHAG+PL+Ex, n=12).
Results
The groups did not differ in age (mean±SD) (36±8yr, 35±9yr) or BMI (both 28±6kg/m2). FFM declined in response to GnRHAG+PL (mean; 95% CI) (−0.6kg; −1.0, −0.3) but not GnRHAG+E2 (0.3kg; −0.2, 0.8) or GnRHAG+PL+Ex (0.1kg;−0.6, 0.7). Although FM did not change in either group, visceral fat area increased in response to GnRHAG+PL but not GnRHAG+E2. GnRHAG+PL caused decreased BMD at the lumbar spine and proximal femur that were prevented by E2. Preliminary data suggest that exercise may have favorable effects on FM, FFM, and hip BMD.
Conclusions
Suppression of ovarian E2 resulted in loss of bone and FFM and expansion of abdominal adipose depots. Failure of hormone suppression to increase total FM conflicted with previous studies of the effects of GnRHAG. Further research is necessary to understand the role of estrogen in the regulation of energy balance and fat distribution.
Keywords: body composition, bone mineral density, fat mass, fat-free mass, resistance exercise, menopause
INTRODUCTION
Obesity is a major public health problem in the U.S. and in developed countries worldwide. Although there are many behavioral, environmental and biological factors that contribute to the development of obesity,1 there is evidence that the loss of ovarian hormones increases the propensity for weight gain in women. For example, fat mass (FM) increases in response to ovarian hormone suppression in premenopausal women 2-6 and estrogen-based hormone therapy (HT) attenuates weight gain in postmenopausal women.7-18 Ovarian hormone deficiency may also alter fat distribution, resulting in a disproportionate increase in abdominal adiposity.2,6,7,12,13,15 Because accumulation of abdominal fat increases the risk of obesity-related diseases (i.e., type 2 diabetes mellitus, coronary artery disease, hypertension),19 it is important to understand how the loss of gonadal function influences regional adiposity and whether this is specifically related to the decline in serum estradiol (E2).
There are well-known adverse effects of E2 deficiency on bone mineral density (BMD).20,21 Less well known is whether E2 deficiency triggers a decline in lean tissue other than bone, such as skeletal muscle. The suppression of ovarian function in premenopausal women has been observed to result in a decline in fat-free mass (FFM) in some studies,2,3,5,6 but it is not clear whether this is specifically related to the decline in serum E2 concentration.
Collectively, evidence suggests that the menopausal transition is associated with unfavorable changes in body composition that include an increase in FM, particularly in the abdominal region, and decreases in FFM and BMD. Because the menopause is inextricably associated with age, it is challenging to isolate the independent effects of age or menopause from those of sex hormone deficiency per se in observational studies and this underscores the need for controlled studies of the effects of sex hormones on body composition. In this context, the aim of the present study was to evaluate the effects of 5 months of ovarian hormone suppression (gonadotropin releasing hormone agonist; GnRHAG) on body composition (i.e., FM, FFM, BMD) in healthy, premenopausal women. To evaluate the mechanistic role of E2, women were randomized to GnRHAG+placebo (PL) or GnRHAG+E2. Our hypothesis was that GnRHAG+PL would increase FM and decrease FFM and BMD and that these changes would be attenuated by E2 add back.
If ovarian hormone suppression does result in adverse changes in body composition and BMD, there is little knowledge as to whether such changes can be prevented by exercise training. Therefore, an exploratory aim was to determine if resistance exercise attenuates the effects of ovarian hormone suppression on body composition and BMD.
METHODS
This was a randomized controlled trial in which premenopausal women were randomized to undergo 5 months of GnRHAG+PL or GnRHAG+E2. The study was approved by the Colorado Multiple Institutional Review Board (COMIRB) and all volunteers provided written informed consent to participate.
Study participants
Participants were healthy, premenopausal women aged 20 to 49 y with normal menstrual cycle function, defined as no missed cycles in the previous year and cycle length 28±5 days. Only non-smokers were enrolled. Volunteers were screened for eligibility through a review of medical and menstrual cycle history, physical examination, assessment of depressive symptoms, blood chemistries (metabolic panel, complete blood count), measurement of BMD, and a graded exercise test (GXT). Exclusion criteria included: use of hormonal contraception, oral glucocorticoids, or diabetes medications; history of cardiovascular, renal, or hepatobiliary disease; history of breast cancer, other estrogen-dependent neoplasms, or venous thromboembolic events; uncontrolled thyroid disease (ultrasensitive thyroid stimulating hormone <5 mU/L or >10 mU/L); uncontrolled hypertension (systolic >150 mmHg or diastolic >90 mmHg); symptoms of depression (CES-D score ≥16 or BDI-II score >18); lactation, pregnancy, or intent to become pregnant; renal (serum creatinine >1.3 mg/dL) or hepatic dysfunction (ALT, AST >1.5x upper limits of normal); hematocrit <33%; proximal femur or lumbar spine BMD T score <−2.0; body mass index >39 kg/m2; and abnormal ECG responses to exercise, confirmed by follow-up evaluation by a cardiologist, that contraindicated vigorous exercise.
Intervention and procedures
Participants underwent baseline testing during days 2 to 6 of the menstrual cycle, although some women were tested later in the follicular phase due to scheduling challenges. GnRHAG therapy (leuprolide acetate 3.75 mg; TAP Pharmaceutical Products, Inc; Lake Forest, IL) was initiated at the beginning of the menstrual cycle; subsequent injections were delivered at 4-week intervals for 20 weeks. Absence of pregnancy was confirmed by a urine pregnancy test before each injection.
Participants were randomized to receive transdermal E2 (GnRHAG+E2; Bayer HealthCare Pharmaceuticals, Berkeley, CA) 0.075 mg/d or placebo patches (GnRHAG+PL). The E2 regimen was expected to maintain serum E2 concentration in the mid-follicular phase range. Some participants in each drug group were also randomized to progressive resistance exercise training (GnRHAG+E2+Ex, n=12; GnRHAG+PL+Ex, n=12). The goal of this exploratory aspect of the study was to generate preliminary data on the effectiveness of exercise to prevent changes in body composition and BMD during ovarian hormone suppression.
Exercise intervention
The progressive resistance exercise intervention included 4 d/wk of supervised exercise for 18 weeks (i.e., ended 2 weeks before the completion of the GnRHAG intervention), with 2 sessions per week focused on upper-body exercises (chest press, lat pulldown, overhead press, seated row, chin-ups/dips on a weight-assisted machine) and 2 on lower-body exercises (leg press, knee extension and flexion, hip abduction and adduction, squats on a Smith machine). Participants performed 3 sets of 12 repetitions of each exercise for the first 2 weeks at a light intensity to learn how to perform the exercises. Over weeks 3 to 6 the intensity was increased to ~70% of 1 repetition maximum (RM), such that muscle fatigue occurred in 8-12 repetitions. Thereafter, intensity was increased to ~80% of 1RM (5-8 repetitions per set).
Body composition and BMD
Body composition (total mass, FM, FFM) and total body, lumbar spine (L1-L4), and proximal femur (total hip, femoral neck, trochanter, subtrochanteric region) BMD were measured by DXA at baseline and week 18 of the intervention using a Hologic Discovery-W instrument (software v11.2, Waltham, MA). Regional FM and FFM (i.e., trunk and leg) measurements were obtained from the total body DXA scan. Intra-instrument CVs for scans completed on women <50 yr of age are: − 0.8% total mass, 2.6% FM, 1.1% FFM; 0.8% lumbar spine BMD, 0.9% total hip BMD, 1.9% femoral neck BMD, 1.1% trochanter BMD, and 0.99% subtrochanteric BMD. Scans in the current study were completed by two trained and experienced technicians and reviewed by one of the investigators to insure appropriate data acquisition and image analysis.
Axial CT images were obtained through the center of the L2-L3 and L4-L5 inter-vertebral disc spaces for measurement of abdominal fat areas and 20 cm superior to the distal edge of the lateral condyle of the right femur for measurement of thigh muscle and fat areas (120 kVp, 200-300 maS, and 10 mm slice thickness; General Electric instrument; Waukesha, WI). Images were analyzed by the technicians at the CT Scan Reading Center. Adipose tissue areas were determined using a CT intensity range (−190 to −20 Hounsfield units) that was defined by image-generated histograms of adipose and soft tissue regions. The visceral fat areas (cm2) were manually outlined by tracing the muscles of the abdominal wall. Fat in the bowel was subtracted from the visceral fat area. The subcutaneous fat areas (cm2) were calculated by subtracting the visceral and bowel fat areas from the total abdominal fat area. Thigh muscle area was separated from subcutaneous fat area by manually tracing along the deep fascial plane surrounding the muscles. Abdominal fat areas were averaged over the two abdominal slices and thigh muscle over the right and left thigh slices. Threshold for inclusion of repeat thigh scans was ±1 cm of baseline scan location. Analysis programs were developed by the University of Colorado CT Reading Center using IDL software (RSI, Inc., Boulder, CO) on a Sparc 20 workstation (Sun Microsystems, Sunnyvale, CA). Scans in the current study were completed by two trained and experienced technicians and reviewed by one of the investigators to insure appropriate data acquisition and image analysis.
Sex hormones
Blood samples for sex hormones were collected during baseline testing and during week 20 of the intervention. Collection samples were stored at −80°C until analysis. Estrone (E1), E2, and progesterone (P) were determined by radioimmunoassay (RIA, Diagnostic Systems Lab, Webster, TX). Respective intra-and inter-assay CVs were 8.7% and 8.6% for E1, 6% and 11% for E2, and 7.5% and 10.2% for P. Total testosterone (T) was analyzed by chemiluminescence immunoassay (Beckman Coulter, Inc. Fullerton, CA; 2.1% and 5.1%) and sex hormone-binding globulin (SHBG) by immunoradiometric assay (Diagnostic Systems Laboratory; 5.1% and 12%).
Statistical methods
The primary analysis compared the GnRHAG+E2 and GnRHAG+PL groups, pooled across exercise status. It was acknowledged that the inclusion of exercisers could minimize the effects of GnRHAG, but would be reflective of the effects of ovarian hormone suppression in sedentary and active women. Baseline differences in all variables between the GnRHAG+E2 and GnRHAG+PL groups were evaluated using two-group t tests and changes within each group in response to intervention were evaluated with paired t tests. Differences in change over time between groups were tested using an ANCOVA model conditioned on baseline. The study was not powered to detect differences among the 4 treatment groups for the exploratory exercise intervention aim. Therefore, only descriptive statistics and within-group changes are presented. All analyses were done using SAS 9.2 (SAS Institute, Cary, NC). Data are reported as mean and 95% CI unless otherwise specified.
RESULTS
Seventy-nine women were randomized and 9 participants were lost to follow-up (personal reasons, 4; lack of time, 3; side effects of GnRHAG, 1; uncontrolled hypertension; 1). Of the 70 women who completed the intervention, 35 were randomized to GnRHAG+E2 and 35 to GnRHAG+PL. There were no significant differences in the characteristics of the drug groups at baseline (Table 1).
Table 1.
Baseline characteristics of participants randomized to gonadotropin releasing hormone agonist (GnRHAG) therapy plus placebo (PL) or estradiol (E2) treatment. Values are n (%) or mean ± SD.
| GnRHAG+PL (n = 35) |
GnRHAG+E2
(n = 35) |
p value | |
|---|---|---|---|
| Ethnicity | 0.44 | ||
| Hispanic or Latino | 6 (17.1) | 3 (8.6) | |
| Not Hispanic or Latino | 27 (77.1) | 31 (88.6) | |
| Unknown | 2 (5.7) | 1 (2.9) | |
| Race | 0.06 | ||
| White | 24 (68.6) | 25 (71.4) | |
| American Indian or Alaska Native | 1 (2.9) | 0 (0.0) | |
| Asian | 2 (5.7) | 0 (0.0) | |
| Black or African American | 3 (8.6) | 6 (17.1) | |
| Native Hawaiian or Pacific Islander | 0 (0.0) | 1 (2.9) | |
| More than one race | 5 (14.3) | 1 (2.9) | |
| Refused to answer | 0 (0.0) | 2 (5.7) | |
| Age (yr) | 36 ± 8 | 35 ± 9 | 0.61 |
| BMI (kg/m2) | 28 ± 6 | 28 ± 6 | 0.86 |
| Body Composition (kg) | |||
| Total mass | 74.4 ± 15.8 | 76.6 ± 17.7 | 0.59 |
| Fat mass | 27.8 ± 11.1 | 28.2 ± 11.8 | 0.86 |
| Fat-free mass | 46.6 ± 6.2 | 48.3 ± 6.6 | 0.27 |
| BMD (g/cm2) | |||
| Lumbar Spine | 1.053 ± 0.109 | 1.083 ± 0.077 | 0.19 |
| Total Hip | 0.996 ± 0.117 | 0.984 ± 0.093 | 0.64 |
| Femoral Neck | 0.868 ± 0.107 | 0.871 ± 0.097 | 0.91 |
| Trochanter | 0.747 ± 0.105 | 0.738 ± 0.076 | 0.67 |
| Subtrochanter | 1.176 ± 0.142 | 1.157 ± 0.114 | 0.54 |
| Serum Sex Hormones | |||
| Estradiol (pg/mL) | 85.8 ± 78.3 | 62.1 ± 45.5 | 0.17 |
| Estrone (pg/mL) | 56.7 ± 21.7 | 56.4 ± 18.5 | 0.95 |
| Progesterone (ng/mL) | 1.2 ± 1.6 | 0.8 ± 1.1 | 0.21 |
| Testosterone (ng/dL) | 32.1 ± 16.8 | 37.9 ± 13.2 | 0.19 |
| SHBG (nmol/L) | 49.2 ± 23.6 | 41.9 ± 16.3 | 0.18 |
Sex hormones
There were significant decreases in serum E1, E2, P, T, and SHBG in response to GnRHAG+PL. GnRHAG+E2 resulted in significant decreases in P and T, non-significant increases in E1 and E2, and no change in SHBG. The changes in E1, E2, and SHBG were significantly different between the groups (Table 2).
Table 2.
Serum sex hormone concentrations before and after 5 months of gonadotropin releasing hormone agonist plus placebo (GnRHAG+PL) or estradiol (GnRHAG+E2) treatment. Values are mean (SEM or 95% CI).
| GnRHAG+PL (n = 35) |
GnRHAG+E2
(n = 35) |
||||||
|---|---|---|---|---|---|---|---|
| Before | After | Within-group Change (95% CI) |
Before | After | Within-group Change (95% CI) |
Between-group Difference in Changea (95% CI) |
|
| Estradiol, pg/mL |
85.8 (14.3) |
21.4 (1.5) |
−64.4 (−94.1, −34.6) |
62.1 (8.6) |
93.6 (17.1) |
32.1 (−8.7, 72.8) |
−72.4 (−108, −37.0) |
| Estrone, pg/mL |
56.7 (3.9) |
37.0 (4.1) |
−19.7 (−28.1, −11.4) |
56.4 (3.4) |
68.3 (6.1) |
11.9 (−0.9, 24.7) |
−31.5 (−45.3, −17.6) |
| Progesterone, ng/mL |
1.2 (0.3) |
0.3 (0.0) |
−0.9 (−1.5, −0.3) |
0.8 (0.2) |
0.3 (0.0) |
−0.5 (−0.9, −0.1) |
0.0 (−0.1, 0.1) |
| Testosterone, ng/dL |
32.1 (3.1) |
27.1 (3.5) |
−5.0 (−8.2, −1.8) |
37.9 (2.8) |
30.9 (2.6) |
−7.0 (−10.5, −3.5) |
1.3 (−3.3, 5.9) |
| SHBG, nmol/L |
49.2 (4.2) |
41.0 (3.8) |
−8.2 (−12.9, −3.5) |
41.9 (3.4) |
43.1 (3.3) |
1.2 (−3.4, 5.8) |
−7.8 (−13.9, −1.7) |
Adjusted for baseline values
Body composition and BMD
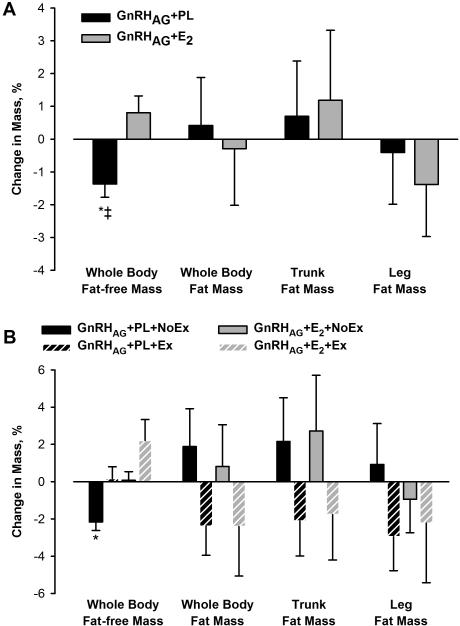
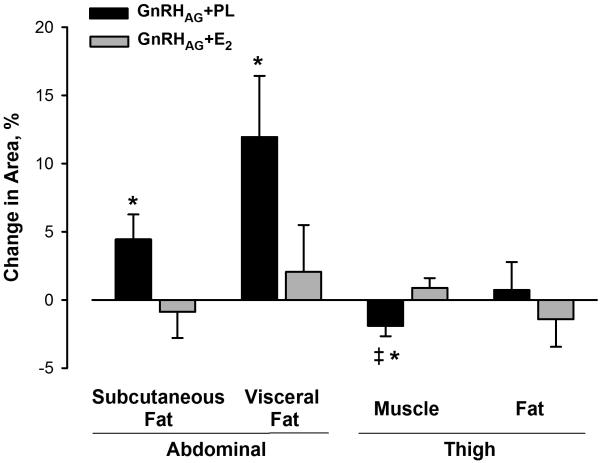
There was a decline in FFM in response to GnRHAG+PL that was significantly different from the gain in response to GnRHAG+E2; the loss was distributed across the trunk and leg regions (Table 3, Figure 1A). Thigh muscle area, as measured by CT, decreased after GnRHAG+PL, but not GnRHAG+E2 (Figure 2). After adjustment for baseline value, there was a difference between the add-back groups in the change in thigh muscle area (−3.27 cm2 (95% CI, −5.86, −0.68); p=0.01).
Table 3.
Body composition and BMD before and after 5 months of gonadotropin releasing hormone agonist plus placebo (GnRHAG+PL) or estradiol (GnRHAG+E2) treatment. Values are mean (SEM or 95% CI).
| GnRHAG+PL (n = 35) |
GnRHAG+E2
(n = 35) |
||||||
|---|---|---|---|---|---|---|---|
| Before | After | Within-group Change (95% CI) |
Before | After | Within-group Change (95% CI) |
Between-group Difference in Changea (95% CI) |
|
| Total mass (kg) | 74.4 (2.7) |
73.8 (2.7) |
−0.6 (−1.3, 0.2) |
76.6 (3.0) |
76.9 (3.0) |
0.3 (−0.9, 1.5) |
−0.9 (−2.3, 0.5) |
| FFM (kg) | 46.6 (1.0) |
46.0 (1.0) |
−0.6 (−1.0, −0.3) |
48.3 (1.1) |
48.7 (1.1) |
0.3 (−0.2, 0.8) |
−1.1 (−1.7,- 0.4) |
| Trunk FFM (kg) | 23.3 (0.5) |
23.0 (0.5) |
−0.3 (−0.5, 0.0) |
23.9 (0.6) |
24.0 (0.5) |
0.2 (−0.2, 0.5) |
−0.5 (−0.9, −0.1) |
| Leg FFM (kg) | 15.2 (0.5) |
14.9 (0.4) |
−0.3 (−0.5, −0.1) |
16.1 (0.5) |
16.2 (0.5) |
0.1 (−0.1, 0.3) |
−0.5 (−0.8, −0.2) |
| FM (kg) | 27.8 (1.9) |
27.8 (1.9) |
0.1 (−0.6, 0.8) |
28.2 (2.0) |
28.2 (2.1) |
0.0 (−1.0, 0.9) |
0.1 (−1.1, 1.3) |
| Trunk FM (kg) | 13.6 (1.0) |
13.7 (1.1) |
0.1 (−0.3, 0.5) |
13.4 (1.1) |
13.5 (1.1) |
0.1 (−0.4, 0.7) |
0.0 (−0.7, 0.6) |
| Leg FM (kg) | 10.4 (0.7) |
10.3 (0.7) |
0.0 (−0.3, 0.2) |
11.0 (0.8) |
10.9 (0.8) |
−0.1 (−0.5, 0.2) |
0.1 (−0.4, 0.5) |
| BMD (g/cm2) | |||||||
| Lumbar spine | 1.053 (0.018) |
1.021 (0.019) |
−0.032 (−0.041, −0.023) |
1.083 (0.013) |
1.076 (0.012) |
−0.007 (−0.015, 0.001) |
−0.026 (−0.038, −0.014) |
| Total hip | 0.996 (0.020) |
0.984 (0.020) |
−0.012 (−0.022, −0.003) |
0.984 (0.016) |
0.988 (0.016) |
0.003 (0.002, 0.008) |
−0.015 (−0.026, −0.005) |
| Femoral neck | 0.868 (0.018) |
0.850 (0.018) |
−0.018 (−0.030, −.0006) |
0.871 (0.016) |
0.873 (0.017) |
0.002 (−0.007, 0.011) |
−0.020 (−0.035, −0.005) |
| Trochanter | 0.747 (0.018) |
0.738 (0.018) |
−0.009 (−0.018, −0.001) |
0.738 (0.013) |
0.741 (0.014) |
0.003 (0.002, 0.008) |
−0.013 (−0.022, −0.003) |
| Subtrochanter | 1.176 (0.024) |
1.168 (0.024) |
−0.008 (−0.019, 0.002) |
1.157 (0.019) |
1.160 (0.019) |
0.003 (−0.005, 0.011) |
−0.011 (−0.024, 0.002) |
Adjusted for baseline values; fat-free mass (FFM), fat mass (FM), bone mineral density (BMD)
Figure 1.
Percent change from baseline in fat-free mass and fat mass measured by DXA in response to 5 months of GnRHAG therapy with placebo or estradiol treatment (Panel A) and resistance exercise training or no exercise (Panel B). * p<0.05, within-group change; ‡ p <0.05, between-group difference.
Figure 2.
Percent change in area of abdominal subcutaneous and visceral fat and thigh muscle and fat measured by CT in response to 5 months of GnRHAG therapy with placebo or estradiol treatment. * p<0.05, within-group change; ‡ p <0.01, between-group difference.
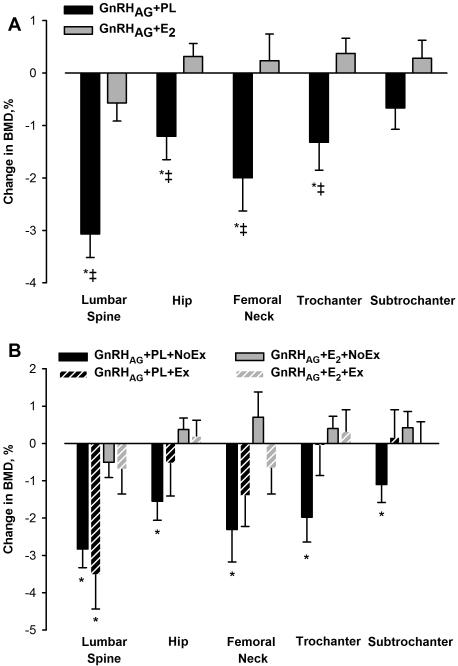
There were no significant changes in total FM in either drug group (Table 3, Figure 1A). However, there were significant increases in both subcutaneous and visceral abdominal fat areas by CT in the GnRHAG+PL group but not in the GnRHAG+E2 group (Figure 2). Leg fat, as measured by DXA (Table 3, Figure 1A) and thigh fat as measured by CT (Figure 2), did not change (Figure 2). The decreases in spine and hip BMD in response to GnRHAG+PL were significantly different (except at the subtrochanteric region) from the changes in response to GnRHAG+E2 (Table 3, Figure 3A).
Figure 3.
Percent change from baseline in bone mineral density in response to 5 months of GnRHAG therapy with placebo or estradiol treatment (Panel A) and resistance exercise training or no exercise (Panel B). * p <0.05, within-group change; ‡ p <0.05, between-group difference.
Effects of resistance exercise training
FFM decreased in the GnRHAG+PL+NoEx group, was preserved in the GnRHAG+PL+Ex and GnRHAG+E2+NoEx groups, and increased non-significantly in the GnRHAG+E2+Ex group (Table 4, Figure 1B). There were no significant changes in FM in any of the groups, but FM tended to increase in non-exercisers and decrease in exercisers.
Table 4.
Changes in body composition and BMD in response to 5 months of gonadotropin releasing hormone agonist plus placebo (GnRHAG+PL) or estradiol (GnRHAG+E2) treatment and resistance exercise training or no exercise. Values are mean (95% CI).
| GnRHAG+PL | GnRHAG+E2 | |||
|---|---|---|---|---|
|
| ||||
| No Exercise n = 23 |
Exercise n = 12 |
No Exercise n = 23 |
Exercise n = 12 |
|
| Total Mass (kg) | −0.6 (−1.6, 0.4) | −0.5 (−2.0, 0.9) | 0.4 (−0.7, 1.4) | 0.1 (−2.9, 3.2) |
| FFM (kg) | −1.0 (−1.5, −0.6) | 0.1 (−0.6, 0.7) | −0.0 (−0.5, 0.4) | 1.0 (−0.3, 2.2) |
| Trunk FFM (kg) | −0.5 (−0.8, −0.2) | 0.2 (−0.2, 0.7) | 0.1 (−0.2, 0.4) | 0.2 (−0.7, 1.2) |
| Leg FFM (kg) | −0.4 (−0.7, −0.2) | −0.1 (−0.4, 0.3) | −0.0 (−0.3, 0.2) | 0.4 ( 0.1, 0.8) |
| FM (kg) | 0.5 (−0.5, 1.4) | −0.6 (−1.7, 0.5) | 0.4 (−0.7, 1.5) | −0.8 (−2.9, 1.2) |
| Trunk FM (kg) | 0.3 (−0.2, 0.8) | −0.3 (−0.9, 0.3) | 0.4 (−0.3, 1.1) | −0.4 (−1.4, 0.7) |
| Leg FM (kg) | 0.1 (−0.3, 0.5) | −0.3 (−0.7, 0.2) | 0.0 (−0.3, 0.4) | −0.4 (−1.3, 0.5) |
| BMD (g/cm2) | ||||
| Lumbar spine | −0.029 (−0.039, −0.019) | −0.037 (−0.058, −0.016) | −0.006 (−0.015, 0.004) | −0.008 (−0.024, 0.008) |
| Total hip | −0.015 (−0.026, −0.005) | −0.006 (−0.026, 0.014) | 0.004 (−0.003, 0.010) | 0.002 (−0.007, 0.011) |
| Femoral neck | −0.021 (−0.038, −0.004) | −0.012 (−0.027, 0.003) | 0.006 (−0.006, 0.019) | −0.006 (−0.018, 0.006) |
| Trochanter | −0.014 (−0.024, −0.004) | −0.000 (−0.015, 0.014) | 0.003 (−0.002, 0.008) | 0.003 (−0.007, 0.013) |
| Subtrochanter | −0.013 (−0.026, −0.001) | 0.001 (−0.019, 0.021) | 0.005 (−0.006, 0.015) | 0.000 (−0.014, 0.014) |
Fat-free mass (FFM), fat mass (FM), bone mineral density (BMD)
BMD decreased at all sites in the GnRHAG+PL+NoEx group, but only at the lumbar spine in the GnRHAG+PL+Ex group (Table 4, Figure 3B). BMD was preserved in all regions in both the GnRHAG+E2+NoEx and the GnRHAG+E2+Ex groups.
DISCUSSION
The primary aims of this study were to determine whether suppression of ovarian function with GnRHAG adversely affected body composition and BMD and whether changes were specifically related to the suppression of E2. In support of our hypotheses, we observed that ovarian hormone suppression resulted in decreases in FFM and lumbar spine and proximal femur BMD that were prevented by E2 add-back therapy. Unexpectedly, we did not find an increase in FM in response to GnRHAG+PL, as has been observed by others,2,3,5,6 but there was an increase in abdominal adiposity as measured by CT.
Effects of the drug intervention on FFM
To our knowledge, there have been 5 previous studies of the effects of GnRHAG therapy of at least 4 months in duration on body composition in premenopausal women.2,3,5,6,22 Previous studies included women with uterine leiomyoma,2,3,5,6,22 or endometriosis.5 All of these studies found increases in FM and decreases in FFM although not all changes were statistically significant. The current study was the first to isolate the role of E2 in mediating these changes in body composition by randomizing women to GnRHAG+PL versus GnRHAG+E2.
The finding that ovarian hormone suppression caused a decrease in FFM was consistent with previous observations.2,3,5,6 However, because none of the previous studies included E2 add-back therapy, it was not clear whether the decline in FFM was related to the suppression of estrogens or androgens by GnRHAG. In the current study, there were similar nonsignificant decreases in serum testosterone concentration in both the GnRHAG+PL and GnRHAG+E2 groups, but FFM was decreased only in the former. This suggests that the loss of FFM in the GnRHAG+PL group was mechanistically linked with the suppression of E2, either through direct actions of E2 on skeletal muscle or indirect effects of E2 on other anabolic factors (e.g., insulin-like growth factor I). In laboratory animals, estrogens play a key role in the maintenance of skeletal muscle mass and function.23,24 There is also growing evidence that estrogen-based hormone therapy helps to preserve muscle mass, strength, and function in postmenopausal women.23,25 Muscle mass, per se, was not measured in the current study, but the changes in thigh muscle area were consistent with the changes in FFM. These findings suggest that the loss of E2 in women accelerates the loss of muscle mass. Importantly, the use of transdermal E2 in the current study likely resulted in a smaller decrease in bioavailable androgens than if oral therapy had been used. Oral estrogens were found to increase SHBG and decrease free testosterone, whereas transdermal E2 did not.26 It is not clear whether oral estrogens would be as effective in preserving FFM as transdermal E2. Effects of the drug intervention on FM
Multiple studies of premenopausal women treated with GnRHAG therapy found increases in FM.2,3,5,6 In these studies, fat mass increased by 0.9 to 1.9 kg in response to 16 to 24 weeks of GnRHAG therapy. There was also a shift in fat distribution toward abdominal accumulation in response to ovarian hormone suppression, as evidenced by an increase in trunk-to-leg fat ratio after 4 months of GnRHAG therapy.2,6 Based on the energy content of the fat gains in the four previous studies that involved 16 or 20 weeks of GnRHAG therapy,2,3,5,6 the magnitude of disruption in energy balance was roughly equivalent to +90 to +130 kcal/d.
Suppression of gonadal hormones also promotes fat gain in men. In healthy men aged 20 to 50 years who underwent 16 weeks of GnRHAG and add-back placebo or testosterone therapy, with or without an aromatase inhibitor to block the conversion of testosterone to estrogens, FM increased in men on placebo add-back and this was exaggerated by aromatase inhibition.27 These findings suggest that E2 is mechanistically linked with the regulation of energy balance and fat gain in women and men. This is consistent with the observation that estrogen receptor alpha deficiency causes excess fat gain in both female and male mice.28
In the current study, we did not observe the increase in total FM that has been observed in other studies of gonadal hormone suppression.2,3,5,6,22 A potential factor that may have contributed to the minimal fat gain in the current study was that the consenting process included a discussion of weight gain as a risk of the study. Thus, participants may have been sensitized to the possibility of weight gain and compensated by making behavioral changes. This may not have been the case in previous studies of the effects of GnRHAG therapy on body composition because they were observational studies of patients undergoing treatment for endometriosis5 or uterine leiomyomas.2,3,5,6,22 The exercise intervention in a subset of participants in the current study also attenuated the magnitude of fat gain. It was not clear whether the previous studies of the effects of GnRHAG therapy on body composition included active and/or sedentary women.
Despite the lack of change in FM in the current study, there were significant increases in both subcutaneous and visceral abdominal fat areas in the GnRHAG+PL group but not in the GnRHAG+E2 group. This finding was consistent with the observation that abdominal fat areas, particularly visceral, increase dramatically during the menopausal transition.29 It has been observed that the level of abdominal visceral adiposity in women aged 42 to 52 y is linked more closely with androgens than estrogens,30 but the current observations suggest that it is the loss of E2 that triggers the expansion of abdominal fat depots in women.
Effects of the intervention on BMD
As expected, BMD decreased at most skeletal sites in the GnRHAG+PL group and this was prevented by E2. GnRHAG therapy has been observed to cause decreases in BMD of 4 to 5% at the lumbar spine after 6 months of treatment.31-33 The severity of bone loss depends on the GnRHAG dose, length of treatment, and participant characteristics.34 When duration of treatment is less than 6 months, the bone loss has been found to be reversible.35-38
Effects of the exercise intervention
Subsets of women in each drug treatment arm also underwent concurrent resistance training. The intent was to generate preliminary evidence regarding whether exercise can prevent or attenuate body composition changes that occur in response to ovarian hormone suppression. Because the study was not powered for between-group comparisons, only within-group changes were evaluated. The findings suggest that resistance exercise may be helpful in attenuating the decline in FFM that occurred in response to the suppression of ovarian hormones. Resistance exercise also appeared to mitigate at least some of the decline in BMD at the proximal femur, but not lumbar spine. These encouraging preliminary data suggest that regular exercise training may be particularly important during the menopausal transition to minimize bone loss and unfavorable changes in body composition.
A strength of this study was the randomized controlled design that provided robust experimental control over the sex hormone environment by using pharmacologic suppression of endogenous sex hormones (“medical menopause”) and add-back E2 or placebo. The potential confounding effects of age were minimized by studying premenopausal women. However, the use of the “medical menopause” model was also a limitation of the study because it does not simulate all the aspects of “natural menopause” (e.g., more abrupt hormone withdrawal, suppression rather than elevation of gonadotropins). Other limitations were that potential effects of route of E2 delivery (oral vs. transdermal), E2 dose, or combined E2+P add-back were not evaluated.
CONCLUSIONS
Large randomized controlled trials of the risks and benefits of HT in postmenopausal women39-41 have increased awareness that benefits of HT may not outweigh the risks for some women. The current study demonstrated that the suppression of ovarian hormones in premenopausal women caused decreases in FFM and BMD and that these changes were specifically related to the suppression of E2. Our preliminary data further suggests that resistance exercise may help to maintain FFM and BMD during ovarian hormone suppression, but further research will be needed to confirm this potential benefit. It is not clear whether the findings from this study of ovarian hormone suppression in premenopausal women reflect changes that occur in response to the natural menopause transition. Future studies using this methodologic approach should be carried out in women who are approaching menopause to determine if there is an independent effect of age.
ACKNOWLEDGMENTS
We are grateful to the nursing, bionutrition, core laboratory, information systems, and administrative staffs of the Clinical and Translational Research Center (CTRC) and Energy Balance Core of the Nutrition and Obesity Research Center for their support of the study. We also acknowledge the members of our research group who helped with initiation of the study and carried out day-to-day activities for the project, specifically Wendolyn S. Gozansky, MD, MPH. Finally, we thank the women who volunteered to participate in the study for their time and efforts.
Grant support: Supported by NIH grants R01 AG018198, P30 DK048520, UL1 TR001082, P50 HD073063, and T32 AG000279
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Study design: WK, JK, RS, SV. Study conduct and data collection: KS, EG, AS. Data analysis: PW. Data interpretation: WK, KS, EM, PW, KG. Drafting manuscript: KS, KG, WK. Revising manuscript content: WK, KS, EM, KG, MW, PW.
REFERENCES
- 1.Stein CJ, Colditz GA. The epidemic of obesity. J. Clin. Endocrinol. Metab. 2004 Jun;89(6):2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 2.Douchi T, Kuwahata R, Yamasaki H, et al. Inverse relationship between the changes in trunk lean and fat mass during gonadotropin-releasing hormone agonist therapy. Maturitas. 2002 May 20;42(1):31–35. doi: 10.1016/s0378-5122(02)00004-x. [DOI] [PubMed] [Google Scholar]
- 3.Douchi T, Kuwahata T, Yoshimitsu N, Iwamoto I, Yamasaki H, Nagata Y. Changes in serum leptin levels during GnRH agonist therapy. Endocr. J. 2003 Jun;50(3):355–359. doi: 10.1507/endocrj.50.355. [DOI] [PubMed] [Google Scholar]
- 4.Dumesic DA, Abbott DH, Eisner JR, et al. Pituitary desensitization to gonadotropin-releasing hormone increases abdominal adiposity in hyperandrogenic anovulatory women. Fertil. Steril. 1998 Jul;70(1):94–101. doi: 10.1016/s0015-0282(98)00098-3. [DOI] [PubMed] [Google Scholar]
- 5.Revilla R, Revilla M, Villa LF, Cortes J, Arribas I, Rico H. Changes in body composition in women treated with gonadotropin-releasing hormone agonists. Maturitas. 1998 Nov 30;31(1):63–68. doi: 10.1016/s0378-5122(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 6.Yamasaki H, Douchi T, Yamamoto S, Oki T, Kuwahata R, Nagata Y. Body fat distribution and body composition during GnRH agonist therapy. Obstet. Gynecol. 2001 Mar;97(3):338–342. doi: 10.1016/s0029-7844(00)01181-9. [DOI] [PubMed] [Google Scholar]
- 7.Espeland MA, Stefanick ML, Kritz-Silverstein D, et al. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J. Clin. Endocrinol. Metab. 1997 May;82(5):1549–1556. doi: 10.1210/jcem.82.5.3925. [DOI] [PubMed] [Google Scholar]
- 8.Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, Genazzani AR. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas. 2001 Aug 25;39(2):125–132. doi: 10.1016/s0378-5122(01)00194-3. [DOI] [PubMed] [Google Scholar]
- 9.Gambacciani M, Ciaponi M, Cappagli B, Genazzani AR. Effects of low-dose continuous combined conjugated estrogens and medroxyprogesterone acetate on menopausal symptoms, body weight, bone density, and metabolism in postmenopausal women. Am. J. Obstet. Gynecol. 2001 Nov;185(5):1180–1185. doi: 10.1067/mob.2001.117669. [DOI] [PubMed] [Google Scholar]
- 10.Hassager C, Christiansen C. Estrogen/gestagen therapy changes soft tissue body composition in postmenopausal women. Metabolism. 1989 Jul;38(7):662–665. doi: 10.1016/0026-0495(89)90104-2. [DOI] [PubMed] [Google Scholar]
- 11.Jensen LB, Vestergaard P, Hermann AP, et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J. Bone Miner. Res. 2003 Feb;18(2):333–342. doi: 10.1359/jbmr.2003.18.2.333. [DOI] [PubMed] [Google Scholar]
- 12.Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2003 Jan 7;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kohrt WM, Ehsani AA, Birge SJ., Jr HRT preserves increases in bone mineral density and reductions in body fat after a supervised exercise program. J. Appl. Physiol. 1998 May;84(5):1506–1512. doi: 10.1152/jappl.1998.84.5.1506. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen K, Pedersen SB, Vestergaard P, Mosekilde L, Richelsen B. Hormone replacement therapy affects body composition and leptin differently in obese and non-obese postmenopausal women. J. Endocrinol. 1999 Oct;163(1):55–62. doi: 10.1677/joe.0.1630055. [DOI] [PubMed] [Google Scholar]
- 15.Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004 Jul;47(7):1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 16.Mattiasson I, Rendell M, Tornquist C, Jeppsson S, Hulthen UL. Effects of estrogen replacement therapy on abdominal fat compartments as related to glucose and lipid metabolism in early postmenopausal women. Horm. Metab. Res. 2002 Oct;34(10):583–588. doi: 10.1055/s-2002-35420. [DOI] [PubMed] [Google Scholar]
- 17.Utian WH, Gass ML, Pickar JH. Body mass index does not influence response to treatment, nor does body weight change with lower doses of conjugated estrogens and medroxyprogesterone acetate in early postmenopausal women. Menopause. 2004 May-Jun;11(3):306–314. doi: 10.1097/01.gme.0000117062.54779.bd. [DOI] [PubMed] [Google Scholar]
- 18.Walker RJ, Lewis-Barned NJ, Sutherland WH, et al. The effects of sequential combined oral 17beta-estradiol norethisterone acetate on insulin sensitivity and body composition in healthy postmenopausal women: a randomized single blind placebo-controlled study. Menopause. 2001 Jan-Feb;8(1):27–32. doi: 10.1097/00042192-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lamarche B. Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron. Artery Dis. 1998;9(8):473–481. doi: 10.1097/00019501-199809080-00002. [DOI] [PubMed] [Google Scholar]
- 20.Sirola J, Kroger H, Honkanen R, et al. Factors affecting bone loss around menopause in women without HRT: a prospective study. Maturitas. 2003 Jul 25;45(3):159–167. doi: 10.1016/s0378-5122(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 21.Wu F, Ames R, Clearwater J, Evans MC, Gamble G, Reid IR. Prospective 10-year study of the determinants of bone density and bone loss in normal postmenopausal women, including the effect of hormone replacement therapy. Clin. Endocrinol. (Oxf.) 2002 Jun;56(6):703–711. doi: 10.1046/j.1365-2265.2002.01534.x. [DOI] [PubMed] [Google Scholar]
- 22.Nowicki M, Adamkiewicz G, Bryc W, Kokot F. The influence of luteinizing hormone-releasing hormone analog on serum leptin and body composition in women with solitary uterine myoma. Am. J. Obstet. Gynecol. 2002 Mar;186(3):340–344. doi: 10.1067/mob.2002.120485. [DOI] [PubMed] [Google Scholar]
- 23.Tiidus PM, Lowe DA, Brown M. Estrogen replacement and skeletal muscle: mechanisms and population health. J Appl Physiol (1985) 2013 Sep 1;115(5):569–578. doi: 10.1152/japplphysiol.00629.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol (1985) 2007 Apr;102(4):1387–1393. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 25.Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogen's beneficial effect on muscle strength in females. Exerc. Sport Sci. Rev. 2010 Apr;38(2):61–67. doi: 10.1097/JES.0b013e3181d496bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shifren JL, Desindes S, McIlwain M, Doros G, Mazer NA. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007 Nov-Dec;14(6):985–994. doi: 10.1097/gme.0b013e31803867a. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013 Sep 12;369(11):1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U. S. A. 2000 Nov;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008 Jun;32(6):949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women's Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 2010 Mar;18(3):604–610. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franke HR, van de Weijer PH, Pennings TM, van der Mooren MJ. Gonadotropin-releasing hormone agonist plus "add-back" hormone replacement therapy for treatment of endometriosis: a prospective, randomized, placebo-controlled, double-blind trial. Fertil. Steril. 2000 Sep;74(3):534–539. doi: 10.1016/s0015-0282(00)00690-7. [DOI] [PubMed] [Google Scholar]
- 32.Moghissi KS, Schlaff WD, Olive DL, Skinner MA, Yin H. Goserelin acetate (Zoladex) with or without hormone replacement therapy for the treatment of endometriosis. Fertil. Steril. 1998 Jun;69(6):1056–1062. doi: 10.1016/s0015-0282(98)00086-7. [DOI] [PubMed] [Google Scholar]
- 33.Roux C, Pelissier C, Listrat V, et al. Bone loss during gonadotropin releasing hormone agonist treatment and use of nasal calcitonin. Osteoporos. Int. 1995 May;5(3):185–190. doi: 10.1007/BF02106098. [DOI] [PubMed] [Google Scholar]
- 34.Palomba S, Morelli M, Di Carlo C, Noia R, Pellicano M, Zullo F. Bone metabolism in postmenopausal women who were treated with a gonadotropin-releasing hormone agonist and tibolone. Fertil. Steril. 2002 Jul;78(1):63–68. doi: 10.1016/s0015-0282(02)03149-7. [DOI] [PubMed] [Google Scholar]
- 35.Johansen JS, Riis BJ, Hassager C, Moen M, Jacobson J, Christiansen C. The effect of a gonadotropin-releasing hormone agonist analog (nafarelin) on bone metabolism. J. Clin. Endocrinol. Metab. 1988 Oct;67(4):701–706. doi: 10.1210/jcem-67-4-701. [DOI] [PubMed] [Google Scholar]
- 36.Matta WH, Shaw RW, Hesp R, Evans R. Reversible trabecular bone density loss following induced hypo-oestrogenism with the GnRH analogue buserelin in premenopausal women. Clin. Endocrinol. (Oxf.) 1988 Jul;29(1):45–51. doi: 10.1111/j.1365-2265.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 37.Paoletti AM, Serra GG, Cagnacci A, et al. Spontaneous reversibility of bone loss induced by gonadotropin-releasing hormone analog treatment. Fertil. Steril. 1996 Apr;65(4):707–710. [PubMed] [Google Scholar]
- 38.Surrey ES. Gonadotropin-releasing hormone agonist and add-back therapy: what do the data show? Curr. Opin. Obstet. Gynecol. 2010 Aug;22(4):283–288. doi: 10.1097/GCO.0b013e32833b35a7. [DOI] [PubMed] [Google Scholar]
- 39.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998 Aug 19;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 40.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 41.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]