ABSTRACT
How mechanical cues from the extracellular environment are translated biochemically to modulate the effects of TGF-β on myofibroblast differentiation remains a crucial area of investigation. We report here that the focal adhesion protein, Hic-5 (also known as TGFB1I1), is required for the mechanically dependent generation of stress fibers in response to TGF-β. Successful generation of stress fibers promotes the nuclear localization of the transcriptional co-factor MRTF-A (also known as MKL1), and this correlates with the mechanically dependent induction of α smooth muscle actin (α-SMA) and Hic-5 in response to TGF-β. As a consequence of regulating stress fiber assembly, Hic-5 is required for the nuclear accumulation of MRTF-A and the induction of α-SMA as well as cellular contractility, suggesting a crucial role for Hic-5 in myofibroblast differentiation. Indeed, the expression of Hic-5 was transient in acute wounds and persistent in pathogenic scars, and Hic-5 colocalized with α-SMA expression in vivo. Taken together, these data suggest that a mechanically dependent feed-forward loop, elaborated by the reciprocal regulation of MRTF-A localization by Hic-5 and Hic-5 expression by MRTF-A, plays a crucial role in myofibroblast differentiation in response to TGF-β.
KEY WORDS: Hic-5, MRTF-A, Mechanotransduction, Myofibroblast, Fibrosis, Wound healing
Summary: The focal adhesion protein Hic-5 plays a crucial role in myofibroblast differentiation in response to TGF-β by facilitating tension-dependent stress fiber assembly and MRTF-A nuclear accumulation.
INTRODUCTION
In normal cutaneous wounds, myofibroblasts are present transiently – lost through apoptosis, phenotypic reversion or senescence. By contrast, in pathogenic settings, myofibroblasts remain in an activated state, continuing to generate and remodel collagen-rich scar tissue, resulting in the excessive tissue stiffening that is characteristic of organ fibrosis, tumor stroma and pathogenic scarring (Hinz, 2010; Tomasek et al., 2002; Van De Water et al., 2013). Importantly, although myofibroblasts are responsible for the increased extracellular matrix (ECM) stiffness (decreased compliance) of scars, ECM rigidity and intracellular tension are also required for myofibroblast differentiation (Chan et al., 2010; Hinz, 2010; Tomasek et al., 2002; Van De Water et al., 2013). Active TGF-β is also required for myofibroblast differentiation and is derived from a latent TGF-β precursor that is associated with the ECM (Robertson et al., 2015). A principal mechanism through which active TGF-β is generated involves integrin-mediated mechanical ‘tugging’ on latent TGF-β to release the active form (Klingberg et al., 2014; Wipff et al., 2007). This dependence on, and generation of, tissue stiffness suggests that feed-forward loops drive myofibroblast differentiation as well as their persistence.
Myocardin-related transcription factor A (MRTF-A, also termed MKL1 or MAL) is a transcriptional co-factor that potentiates the activity of the ubiquitously expressed transcription factor serum response factor (SRF). MRTF-A and SRF bind to a promoter element (i.e. CArG box), and this complex is required for the expression of several contractile genes, including α-SMA (ACTA2), and plays a crucial role in myofibroblast differentiation (Crider et al., 2011; Sun et al., 2006; Wang et al., 2002). Under conditions in which actin is polymerized (F-actin), the monomeric actin (G-actin) pool decreases, and the G-actin-dependent cytoplasmic sequestration of MRTF-A is relieved, thereby promoting the nuclear localization and transcriptional functions of MRTF-A (Miralles et al., 2003; Vartiainen et al., 2007).
Actin-rich contractile stress fibers that are assembled in response to sufficient ECM stiffness provide a tension-dependent intracellular reservoir for F-actin that supports MRTF-A nuclear localization (Huang et al., 2012; Johnson et al., 2013; O'Connor et al., 2015). TGF-β is known to promote the assembly of stress fibers in a Rho GTPase A (Rho-A)- and Rho Kinase (ROCK)-dependent manner, which leads to the nuclear localization of MRTF-A, and subsequent induction of focal adhesion and contractile genes, such as α-SMA (Cen et al., 2003; Crider et al., 2011; Sandbo et al., 2011; Small et al., 2010; Trembley et al., 2015; Zhao et al., 2007). These data support a model in which stress fiber formation is not only a consequence but also a crucial regulator of myofibroblast differentiation.
We have previously reported that hypertrophic scar (HTS) myofibroblasts exhibit a stably differentiated and contractile phenotype in culture by elucidating a TGF-β-dependent autocrine loop in which the focal adhesion protein hydrogen peroxide inducible clone-5 (Hic-5; also termed TGFB1I1 or ARA55) is essential (Dabiri et al., 2006, 2008a). Hic-5 is a member of the LIM-protein family with homology (72% sequence similarity) to paxillin (Shibanuma et al., 1994; Thomas et al., 1999). Like paxillin, Hic-5 is localized to focal adhesions through LIM domains and serves as a scaffolding protein that interacts with focal adhesion proteins FAK, Pyk2, FRNK, GIT1 and vinculin (Matsuya et al., 1998; Nishiya et al., 2002; Thomas et al., 1999). However, Hic-5 and paxillin are differentially recruited to focal adhesions (Deakin and Turner, 2011). Hic-5 has also been reported to translocate from focal adhesions to stress fibers in cells undergoing cyclic stretching (Kim-Kaneyama et al., 2005; Nishiya et al., 1999; Yund et al., 2009). Importantly, treating HTS myofibroblasts with small interfering (si)RNA against Hic-5 reduces p21cip1 levels, thereby promoting cell cycle progression, and reduces latent TGF-β production, α-SMA expression, type I collagen production and collagen gel contraction (Dabiri et al., 2008a,b; Mori et al., 2012). These data suggest a key functional role for Hic-5 in myofibroblasts.
Building on these previous findings, we hypothesized that Hic-5 plays a crucial regulatory role in myofibroblast differentiation. In vivo, wound healing experiments and staining of human hypertrophic scars revealed that Hic-5 expression often colocalized with that of α-SMA and was, like α-SMA, upregulated upon increasing mechanical tension. In vitro, TGF-β-dependent Hic-5 expression preceded induction of α-SMA, was mechanically sensitive and required both the canonical SMAD3 pathway as well as the non-canonical SRF–MRTF-A pathway in normal human dermal fibroblasts (NHDFs). Importantly, Hic-5 expression was required for TGF-β to synergize with extracellular stiffness in promoting stress fiber growth and myofibroblast differentiation, and for TGF-β-dependent MRTF-A nuclear translocation and α-SMA induction. These data indicate that the reciprocal regulation of MRTF-A localization by Hic-5 and Hic-5 expression by MRTF-A defines a new mechanically sensitive positive feed-forward loop that promotes the myofibroblast phenotype.
RESULTS
Hic-5 expression in myofibroblasts in vivo
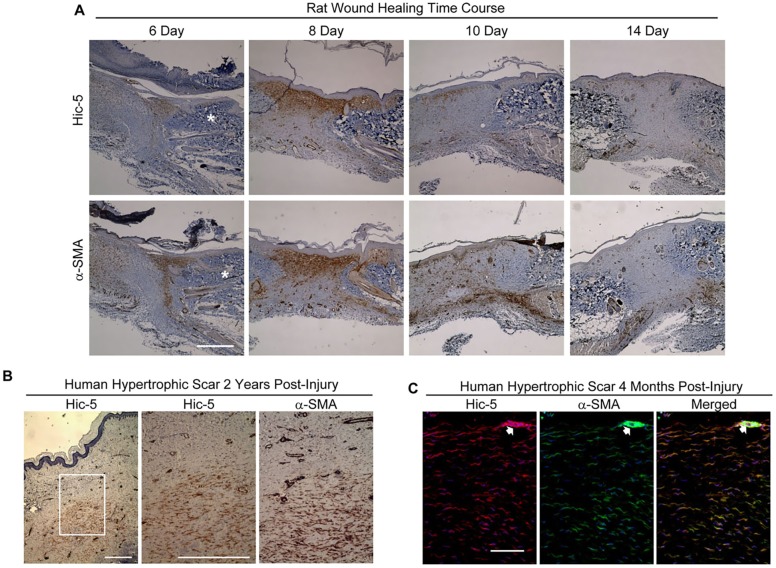
Hic-5 expression, in vivo, has been observed in contractile cell types – including myoepithelial cells and smooth muscle cells – and in contractile tissues such as the bladder and uterus, suggesting a role for Hic-5 in establishing or maintaining a contractile state (Wang et al., 2011; Yuminamochi et al., 2003). We now report that Hic-5 is also expressed in myofibroblasts during normal cutaneous rat wound healing, as is α-SMA (days 6-10, Fig. 1A). Neither Hic-5 nor α-SMA expression was detectable in the granulation tissue 14 days post wounding, which is consistent with the transient appearance of myofibroblasts during normal acute wound healing. In contrast to normal wound tissue, Hic-5 expression persists in fibrotic scar tissue, such as human HTS tissues resected 2 years or 4 months post injury (Fig. 1B and C, respectively). Staining of Hic-5 and α-SMA overlapped in adjacent tissue sections (Fig. 1B) and colocalized within HTS tissue (Fig. 1C). The pattern of Hic-5 expression observed in acute wounds and pathogenic scars suggests a functional role for Hic-5 in the contractile myofibroblast phenotype.
Fig. 1.
Transient Hic-5 and α-SMA expression in rat wounds, and persistent expression in human HTS tissue. (A) Closely adjacent rat wound sections were immunostained for Hic-5 or α-SMA (bottom) at 6, 8, 10, 14 days after injury (epidermis at top of section). Hic-5 and α-SMA levels were elevated in the wound edge at 6 days and reached a maximum at 8 days relative to adjacent unwounded dermis (marked *). Staining for Hic-5 and α-SMA decreased by day 10 and was almost entirely absent from the scar by day 14 post injury. (B) Closely adjacent sections of human HTS that had been resected 2 years post injury were immunostained for Hic-5 (left and middle panels) or α-SMA (right panel) (epidermis at top of section, box in left panel indicates areas shown middle and right). (C) Cryostat sections of human hypertrophic scar tissue (4 months post injury) were double-stained for Hic-5 (red) and α-SMA (green) with directly conjugated antibodies. The analysis of Hic-5 and α-SMA proteins shows extensive colocalization deep within the scar tissue (right panel) (epidermis to the top of section but not visible). Scale bars: 500 µm (A,B); 100 µM (C). The gain was altered in images for α-SMA and Hic-5 to reduce the intense staining for both proteins within arterioles (arrow) (C).
Hic-5 expression in myofibroblasts is modulated by extracellular stiffness in vivo
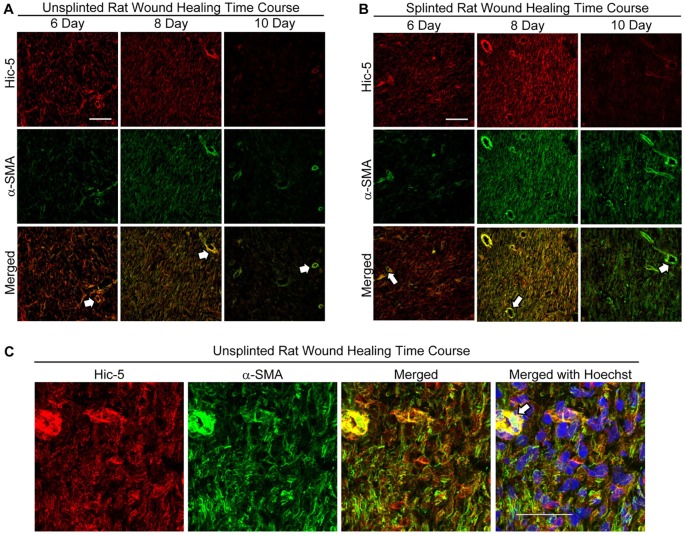
Increased mechanical tension within healing wounds promotes early and prolonged expression of myofibroblast markers, including α-SMA, and is a contributing factor to the persistence of pathogenic myofibroblasts in fibrotic tissues such as HTS (Hinz, 2015; Van De Water et al., 2013). We evaluated the expression of α-SMA and Hic-5 in full-thickness rat wounds with and without the increased mechanical tension induced by wound splinting. Significant expression of Hic-5 and α-SMA was observed at 6 and 8 days post wounding in unsplinted rat wounds but was significantly reduced by day 10 (Fig. 2A). Expression of Hic-5 and α-SMA within splinted wounds was similar to that in unsplinted wounds at day 6. However, in splinted wounds, both markers were upregulated at day 8 and persisted through day 10 (Fig. 2B), demonstrating that, during wound healing, levels of Hic-5, like those of α-SMA, are augmented by increased mechanical tension. Importantly, Hic-5 and α-SMA expression was observed within many of the same cells and, in some cases, colocalized in similar structures (Fig. 2C).
Fig. 2.
Wound splinting promotes increased and prolonged expression of Hic-5 and α-SMA expression within granulation tissue. Cryostat sections from 6-, 8- and 10-day unsplinted (A) or splinted (B) rat wounds were double-label immunostained for Hic-5 (red) and α-SMA (green) directly conjugated antibodies. (A) In unsplinted wounds, Hic-5 and α-SMA immunostaining was observed at 6 days, increased at 8 days post wounding and was significantly reduced by 10 days. (B) Splinted wounds showed stronger Hic-5 and α-SMA immunostaining at 8 days post wounding, and staining persisted through to 10 days post injury. The gain was altered in images for α-SMA and Hic-5 to reduce the intense staining for both proteins within arterioles (arrows) (A,B) (epidermis to the left of sections but not visible). (C) Confocal images of cryostat sections from an unsplinted day 8 wound immunostained as described in A show Hic-5 and α-SMA expression within individual cells. Scale bars: 100 µM (A,B), 50 μM (C).
Induction of Hic-5 requires both the canonical SMAD3 and the non-canonical TGF-β-dependent MRTF-A–SRF pathways
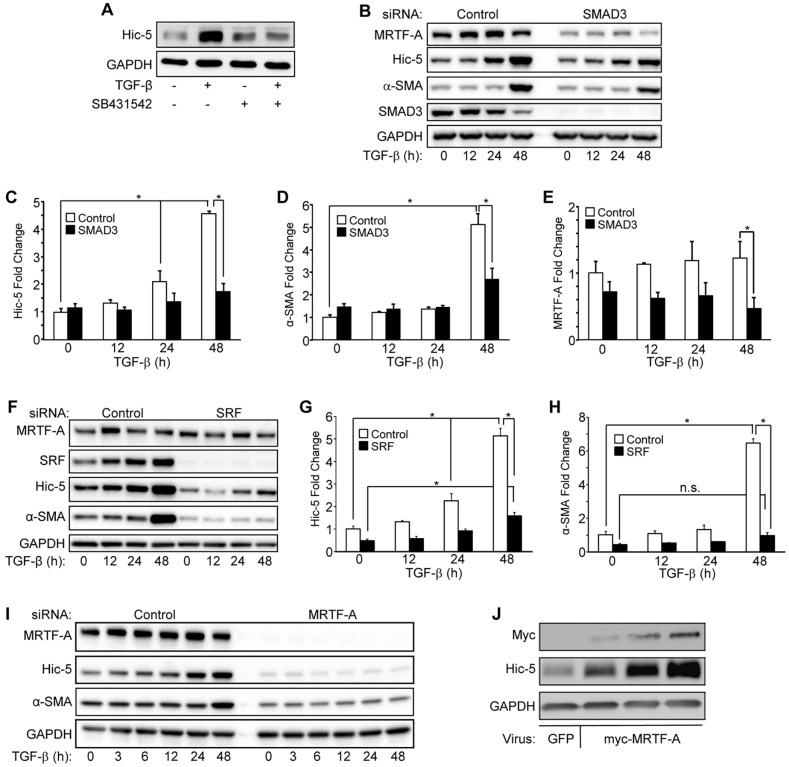
Our observations in vivo prompted us to identify the mechanism by which Hic-5 is induced by TGF-β in primary NHDFs in vitro. We found that the TGF-β-dependent induction of Hic-5 was markedly reduced by inhibition of the TGF-β receptor kinases with SB431542 (Fig. 3A). Hic-5 induction by TGF-β occurred as early as 24 h in control NHDFs and further increased after 48 h of stimulation, whereas α-SMA induction by TGF-β was only significant after 48 h of stimulation (Fig. 3C,D). SMAD3 knockdown (Fig. 3B) significantly reduced the induction of both Hic-5 (Fig. 3C) and α-SMA (Fig. 3D) after 48 h of TGF-β stimulation.
Fig. 3.
Induction of Hic-5 by TGF-β requires the SMAD3 and SRF–MRTF-A pathways. (A) NHDFs that had been treated with either 10 µM SB431542 (TGF-β receptor kinase inhibitor) or vehicle control with or without 10 ng/ml of TGF-β for 24 h were lysed and subjected to western blotting for Hic-5 and GAPDH (loading control) levels (representative blot, n=3 experiments). (B–E) NHDFs that had been treated with either non-targeting control or SMAD3-targeting (‘SMAD3’ on top of image) siRNAs were treated with TGF-β for the indicated times and analyzed by western blotting (B, representative blot) for Hic-5, α-SMA, SMAD3, MRTF-A and GAPDH. Data from three independent experiments were pooled, and the induction by TGF-β of Hic-5 (C) α-SMA (D) and MRTF-A (E) was quantified. (F–H) NHDFs that had been transfected with either SRF-targeting (‘SRF’ on top of image) or non-targeting siRNAs were stimulated with TGF-β for the indicated times and analyzed by western blotting (F, representative blot) for SRF, Hic-5, α-SMA, MRTF-A and GAPDH. (G,H, pooled data, n=3). (I) NHDFs that had been transduced with lentiviruses expressing MRTF-A-targeting (‘MRTF-A’ on top of blot) or non-targeting shRNAs and stimulated with TGF-β for 0, 3, 6, 12, 24 and 48 h (representative blot, n=3). NHDFs expressing MRTF-A-targeting shRNA exhibited reduced basal levels of Hic-5 (compare to t=0), and Hic-5 protein levels failed to respond to TGF-β stimulation at any time point. (J) NDHFs that had been infected with three different concentrations of adenovirus encoding MRTF-A or a high dose of adenoviral GFP. Statistical significance was determined by two-way ANOVA and Tukey's post-hoc analysis (*P<0.05). Error bars are +s.e.m. n.s., not significant.
One way in which SMADs are thought to regulate the induction of contractile genes important for myofibroblast differentiation is by regulating the expression and function of MRTF-A (Charbonney et al., 2011; Masszi et al., 2010; Mihira et al., 2012; Morita et al., 2007; Scharenberg et al., 2014). We observed that MRTF-A levels were significantly lower in SMAD3-depleted NHDFs at 48 h post treatment (Fig. 3B,E), suggesting that the SMAD3 knockdown could impact Hic-5 and α-SMA induction through regulation of MRTF-A levels. MRTF-A is known to promote transcription by binding to SRF, and the Hic-5 promoter contains the CArG box that is utilized in smooth muscle cells by SRF and the smooth-muscle-specific SRF co-factor myocardin (Sun et al., 2006; Wang et al., 2011). We found that knockdown of SRF by using siRNA resulted in some but not complete inhibition of Hic-5 induction by TGF-β at 24 h and 48 h (Fig. 3F,G). By contrast, TGF-β stimulation failed to induce α-SMA at any time point in SRF-depleted NHDFs (Fig. 3H).
We next hypothesized that MRTF-A is also required for TGF-β-dependent induction of Hic-5. Therefore, we infected NHDFs with lentivirus expressing non-targeting or MRTF-A-targeting small hairpin (sh)RNA and performed a TGF-β stimulation time course (0–48 h). Under control conditions (non-targeting shRNA), we found that TGF-β-stimulated Hic-5 induction occurred at 24 h and further increased at 48 h in NHDFs (Fig. 3I). By contrast, NHDFs expressing an shRNA targeting MRTF-A failed to show TGF-β-induced expression of Hic-5 or α-SMA at any time point. Moreover, unstimulated basal levels of Hic-5 and α-SMA were reduced in NHDFs that expressed MRTF-A-targeting shRNA (Fig. 3I). To confirm the role of MRTF-A in Hic-5 expression, we overexpressed MRTF-A by infecting NHDFs with differing concentrations of an adenovirus expressing either GFP or MRTF-A. We observed a dose-dependent increase in Hic-5 expression in response to MRTF-A overexpression when compared to that in cells infected with GFP-expressing control adenovirus, demonstrating that MRTF-A has the capacity to regulate Hic-5 expression (Fig. 3J). These data demonstrate that MRTF-A is both required for the induction of Hic-5 by TGF-β and is sufficient to stimulate Hic-5 expression when overexpressed.
Induction of Hic-5 is mechanically sensitive in vitro
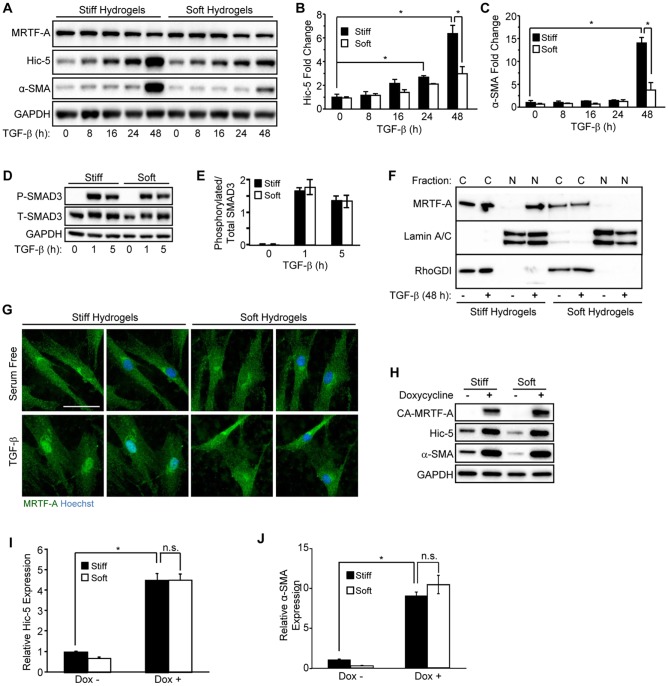
Given the fact that Hic-5 expression in vivo is augmented by mechanical tension (Fig. 2), we sought to determine if Hic-5 induction by TGF-β is mechanically sensitive in NHDFs in vitro, and if so, through which mechanisms this occurs. We cultured NHDFs on polyacrylamide hydrogels (PAHGs) with Young's moduli that approximated the stiffness of wounds – the provisional matrix and early granulation tissue, ∼0.5 kPa (soft) and ∼8.5 kPa (stiff), respectively (Hinz, 2010; Tse and Engler, 2010). We observed a TGF-β-dependent increase in Hic-5 protein on stiff PAHG as early as 16 h (P=0.18, two-way ANOVA, Tukey post-hoc analysis), which became statistically significant at 24 h and was increased further at 48 h post TGF-β stimulation (Fig. 4A,B); α-SMA was induced only at the 48-h time point on stiff PAHG (Fig. 4A,C). By contrast, NHDFs that had been cultured on soft PAHG showed significantly reduced Hic-5 and α-SMA induction by TGF-β at the 48-h time point (Fig. 4B,C).
Fig. 4.
Extracellular stiffness regulates induction of Hic-5 and α-SMA through nuclear accumulation of MRTF-A in response to TGF-β. (A–C) NHDFs were cultured on stiff or soft PAHGs with 10 ng/ml of TGF-β for the indicated time points, lysed and analyzed by western blotting (representative blot, n=3). (B,C) Pooled data are shown for (B) Hic-5 and (C) α-SMA. (D) NHDFs cultured on stiff or soft PAHGs and incubated (0, 1 and 5 h) with TGF-β were lysed and subjected to western blotting for phosphorylated SMAD3 (P-SMAD3) and total SMAD3 (T-SMAD3). (E) Pooled data (n=3) are shown expressing the relative phosphorylation of SMAD3 as a ratio of the phosphorylated to total SMAD3 levels. (F) NHDFs that had been cultured (48 h) on soft or stiff PAHGs (with or without TGF-β) were extracted (see Materials and Methods section) and analyzed by western blotting for nuclear (‘N’) and cytoplasmic (‘C’) MRTF-A. Lamin A/C and RhoGDI and were used as nuclear and cytoplasmic loading controls, respectively. (G) NHDFs that had been treated as described in panel F were immunostained for MRTF-A (green) and co-stained with Hoechst 33342 (blue). Scale bar: 50 µm. (H) NHDFs that had been infected with a lentivirus encoding a doxycycline-inducible constitutively active MRTF-A construct (CA-MRTF-A) were cultured on soft or stiff PAHGs in serum-free medium with or without doxycycline (0.5 µg/ml) for 24 h. (I,J) Pooled data taken from the blot in H for (I) Hic-5 and (J) α-SMA (n=3). Statistical significance was determined by two-way ANOVA followed by Tukey's post-hoc analysis (*P<0.05) for panels B,C,E,I,J. Error bars are ±s.e.m. n.s., not significant.
We next evaluated the relative activation of SMAD3 by TGF-β on stiff and soft PAHGs and found that activation of SMAD3 was unaffected by ECM stiffness, as measured by the level of phosphorylation of SMAD3 (Fig. 4D,E). However, the nuclear localization of MRTF-A has recently been shown to be mechanically sensitive owing to a deficit in actin polymerization in cells cultured on soft PAHGs (Huang et al., 2012; Johnson et al., 2014; O'Connor et al., 2015). We cultured NHDFs on either stiff or soft PAHGs, and treated them with TGF-β for 48 h before cell lysates were separated into cytoplasmic and nuclear fractions (Fig. 4F). Although total expression levels of MRTF-A were unaltered by TGF-β and substrate stiffness (Fig. 4A), MRTF-A nuclear accumulation occurred in response to TGF-β stimulation on stiff PAHGs. Nuclear accumulation of MRTF-A on soft PAHGs was substantially reduced (Fig. 4F,G).
We next determined whether or not rescuing the nuclear localization of MRTF-A on soft PAHGs would be sufficient to stimulate Hic-5 expression, as others have reported for α-SMA (Huang et al., 2012). We infected NHDFs with a lentivirus containing a doxycycline-inducible promoter with a constitutively nuclear MRTF-A cDNA (CA-MRTF-A) that does not encode the N-terminal RPEL domains, which facilitate binding to G-actin and nuclear export (Muehlich et al., 2008). These NHDFs were then cultured on PAHGs for 48 h and serum starved overnight before stimulating expression of CA-MRTF-A with 0.5 µg/ml doxycycline for 24 h (Fig. 4H). Expression of CA-MRTF-A was sufficient to stimulate expression of Hic-5 (Fig. 4I) and α-SMA (Fig. 4J) to similar levels, regardless of PAHG stiffness, in the absence of TGF-β. Taken together, these data demonstrate that TGF-β-mediated induction of Hic-5 precedes the induction of α-SMA on stiff PAHGs and correlates with the nuclear localization of MRTF-A. In contrast, MRTF-A was mostly cytoplasmic in NHDFs that had been cultured on soft PAHGs, and Hic-5 and α-SMA levels remained low in the presence of TGF-β. Our data suggest that the failure of sufficient levels of MRTF-A to accumulate in the nucleus of NHDFs on soft PAHGs is responsible for the reduced induction of Hic-5 and α-SMA in response to TGF-β.
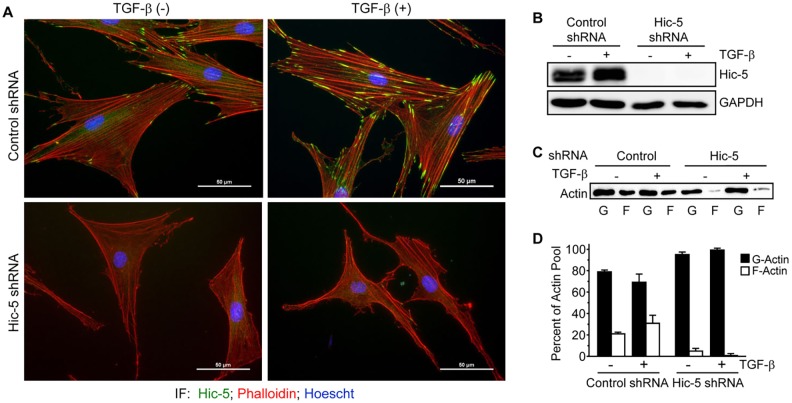
Hic-5 is required for stress fiber formation in response to TGF-β
MRTF-A translocation to, and retention in, the nucleus is dependent upon the actin polymerization state, and Hic-5 has been shown to regulate the formation of supermature focal adhesions, to interact with vinculin when RhoA is active and to bind to stress fibers in cells under cyclical strain (Dabiri et al., 2006, 2008a; Deakin and Turner, 2011; Kim-Kaneyama et al., 2005). We next hypothesized that Hic-5 could regulate stress fiber formation, thereby influencing myofibroblast differentiation. NHDFs that had been grown on plastic, transduced with non-targeting shRNA-expressing lentivirus and stimulated with TGF-β showed increases in F-actin (phalloidin) staining after 24 h, whereas NHDFs that expressed a Hic-5-targeting shRNA showed dramatically decreased phalloidin staining under both basal and TGF-β-stimulated conditions (Fig. 5A,B). TGF-β promoted a modest but statistically insignificant shift from G-actin to F-actin in control cells; however, Hic-5-knockdown cells showed substantially less F-actin, even in the presence of TGF-β, as determined by ultracentrifugation (Fig. 5C,D). These data demonstrate a crucial role for Hic-5 in the formation of the contractile actin cytoskeleton. Importantly, actin polymerization in response to TGF-β was gradual and continued to occur at 48 and 72 h after stimulation, becoming statistically significant at 48 h (Fig. S1). Therefore, for the remainder of the study, stimulation with TGF-β was performed for 48 h.
Fig. 5.
Hic-5 is required for TGF-β-stimulated stress fiber maturation. (A,B) NHDFs were infected with lentiviruses expressing non-targeting or Hic-5-targeting shRNAs, and incubated (24 h) with TGF-β (10 ng/ml). Samples were either (A) fixed and immunostained for Hic-5 (green), and stained for DNA (blue) and F-actin (phalloidin, red) or (B) lysed for western blotting (representative, n=3). Scale bars: 50 µm. (C) NHDFs that had been treated as described in A and B were lysed and fractioned into Triton-soluble and -insoluble fractions by ultracentrifugation (see Materials and Methods). A representative (n=3) western blot for actin showing soluble (G-actin, ‘G’) and pelleted (F-actin, ‘F’) fractions. (D) Pooled data showing that Hic-5-depleted NHDFs had dramatically less F-actin, even in the presence of TGF-β (two-way ANOVA, the main effect due to shRNA had a significance of P<0.05). Error bars are +s.e.m.
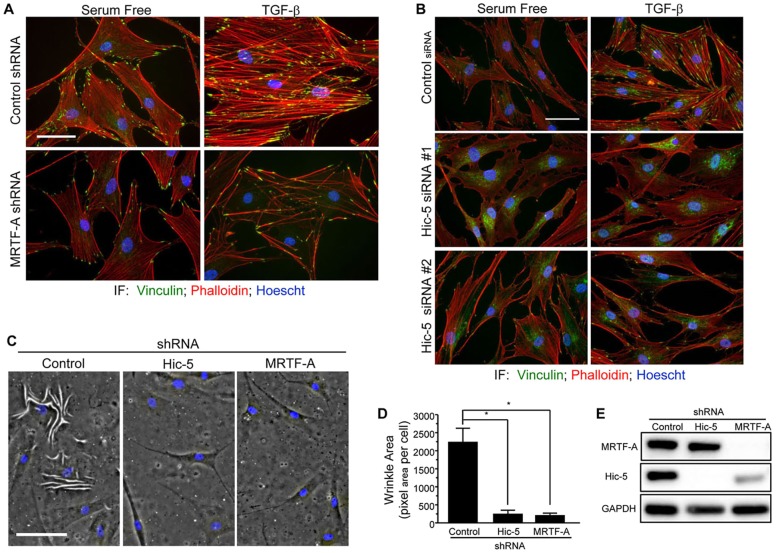
MRTF-A and Hic-5 expression are required for the contractile phenotype
Cellular contraction is a crucial function of myofibroblasts, requiring the formation of a robust actin cytoskeleton. We hypothesized that MRTF-A, like Hic-5, is required for normal stress fiber formation and cellular contractility. To test this hypothesis, we analyzed the capacity of NHDFs that either expressed MRTF-A-targeting shRNA or had been transfected with Hic-5-targeting siRNAs to generate stress fibers in response to TGF-β after 48 h of stimulation (Fig. 6A,B). We conclude that both MRTF-A and Hic-5 are required for stress fiber assembly in response to TGF-β. We next cultured NHDFs on thin soft silicone substrates and observed significant defects in the ability of the cells to generate wrinkles in the substrate when either Hic-5 or MRTF-A had been knocked down by using shRNA interference (Fig. 6C–E). Taken together, these data demonstrate that MRTF-A and Hic-5 are required for cellular contraction in NHDFs by regulating stress fibers.
Fig. 6.
Hic-5 and MRTF-A regulate cellular contraction in primary human dermal fibroblasts. (A,B) NHDFs that had been infected with lentiviruses expressing a non-targeting or MRTF-A-targeting shRNA (A), or transfected with non-targeting or Hic-5-targeting siRNA (B) were stimulated with TGF-β for 48 h before fixation and immunostaining for vinculin (green) and co-staining with Hoechst 33342 (blue) and phalloidin (red). (C–E) NHDFs that had been infected with lentiviruses expressing non-targeting, Hic-5-targeting or MRTF-A- and MRTF-B-targeting shRNAs (see Materials and Methods for explanation of MRTF-A-targeting shRNA) were cultured on soft silicone substrate (PDMS) that was capable of deformation under cellular contraction (C) or lysed to asses knockdown (E). Quantification of the area underneath the wrinkled PDMS was performed by drawing a region of interest around the wrinkles and dividing the pixel area by the number of cells in each field (D). A one-way ANOVA with Tukey's post-hoc analysis was used to determine statistical significance (*P<0.05). Scale bars: 50 µm (A–C). IF, immunofluorescence. Error bars are +s.e.m.
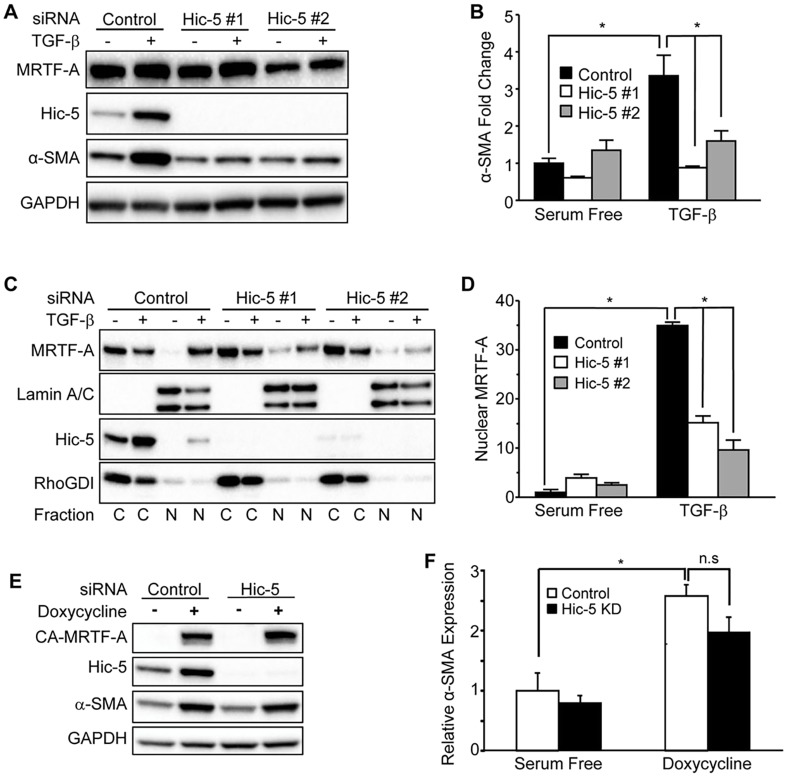
Hic-5 regulates induction of α-SMA in response to TGF-β stimulation by facilitating nuclear localization of MRTF-A
We next tested the requirement for Hic-5 in the induction of α-SMA in response to TGF-β. NDHFs were transfected with Hic-5-targeting or non-targeting siRNAs before stimulation with TGF-β (48 h). Indeed, knockdown with two independent siRNA oligonucleotides demonstrated a requirement for Hic-5 in the induction of α-SMA after 48 h of TGF-β stimulation (Fig. 7A,B). We then fractioned the lysates into cytoplasmic and nuclear compartments and observed a marked redistribution of MRTF-A to the nuclear fraction in NHDFs that had been transfected with the non-targeting siRNA. NHDFs that had been transfected with Hic-5-targeting siRNAs had significantly less MRTF-A in nuclear fractions at the 48-h time point (Fig. 7C,D). This effect was also observed at 24 h when NHDFs were transduced with shRNA-expressing lentivirus (Fig. S2). These data suggest that Hic-5 regulates the induction of α-SMA in response to TGF-β by facilitating sufficient nuclear accumulation of MRTF-A.
Fig. 7.
Hic-5 regulates induction of α-SMA in response to TGF-β stimulation by facilitating nuclear localization of MRTF-A. (A) NHDFs that had been transfected with non-targeting or Hic-5-targeting siRNAs were incubated (48 h) with TGF-β (10 ng/ml), lysed and analyzed by western blotting (representative, n=4). (B) Pooled data (n=4) depicting the fold change in α-SMA expression. (C) NHDFs that had been treated as described in panel A were fractioned into cytoplasmic (‘C’) or nuclear (‘N’) lysates before western blot analysis. RhoGDI and lamin A/C were used as cytoplasmic and nuclear loading controls, respectively. (D) Pooled data depicting nuclear MRTF-A levels, normalized to the levels of lamin A/C. (E) NHDFs that had been infected with a lentivirus encoding the doxycycline-inducible CA-MRTF-A construct were transfected with non-targeting or pooled Hic-5-targeting siRNAs and stimulated with doxycycline (0.5 μg/ml) for 24 h (representative, n=3). (F) Expression of CA-MRTF-A stimulated α-SMA expression to statistically similar levels, even when Hic-5 was knocked down (‘KD’). Statistical significance was determined by two-way ANOVA and Tukey's post-hoc analysis (*P<0.05) for panels B,D,F. Error bars are +s.e.m. n.s., not significant.
Interestingly, we also observed TGF-β-dependent nuclear accumulation of Hic-5 at 48 h (Fig. 7C, left). Hic-5 has been shown to serve as a transcriptional co-factor for some genes (Heitzer and DeFranco, 2006; Kim-Kaneyama, 2012). Therefore, we tested the hypothesis that nuclear Hic-5 is required for α-SMA expression. To do so, we knocked down Hic-5 with pooled siRNA duplexes (Fig. 7A–D) in fibroblasts that expressed the doxycycline-inducible CA-MRTF-A construct, in order to bypass the requirement for Hic-5 in the nuclear localization of MRTF-A. These cells were serum starved overnight before stimulation with 0.5 µg/ml doxycycline for 24 h (Fig. 7E). The increase of α-SMA that was induced by CA-MRTF-A was not significantly different in cells that either did (control siRNA) or did not (pooled siRNAs against Hic-5) express Hic-5 (Fig. 7E,F). These data demonstrate that a role for Hic-5 as a transcriptional co-regulator is not required for the MRTF-A–SRF-dependent induction of α-SMA. Taken with our earlier data, we conclude that Hic-5 regulates TGF-β-dependent induction of α-SMA by supporting the formation of stress fibers and promoting MRTF-A translocation to the nucleus.
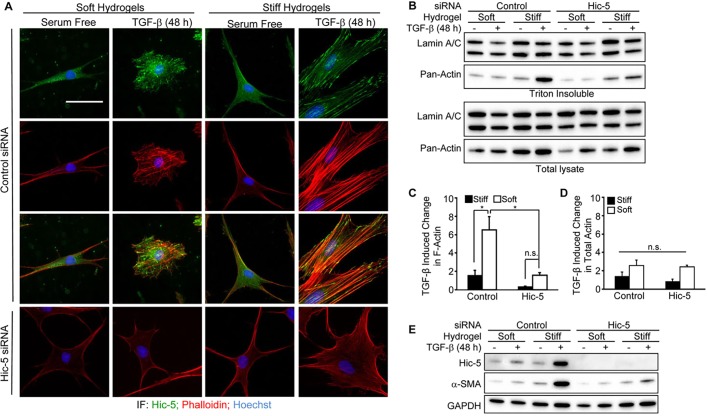
Hic-5 potentiates the synergy between TGF-β and extracellular stiffness by regulating stress fiber maturation
Because Hic-5 regulates stress fibers as well as the induction of α-SMA in response to TGF-β on plastic, we hypothesized that Hic-5 expression is required for the synergy between extracellular stiffness and TGF-β that is necessary for myofibroblast differentiation. NHDFs were transfected with non-targeting control or pooled Hic-5-targeting siRNA duplexes, and cultured on soft or stiff PAHGs. After 48 h of TGF-β stimulation, we observed that NHDFs that had been transfected with control siRNAs and cultured on soft PAHGs responded to TGF-β by producing short and disorganized stress fibers, simultaneously adopting a rounded morphology (Fig. 8A). The staining pattern of Hic-5 in NHDFs that had been cultured on soft PAHGs was diffuse and cytoplasmic, whereas TGF-β-stimulated samples showed concentrated Hic-5 staining that partially overlapped with that of F-actin. Hic-5-depleted fibroblasts that had been cultured on soft PAHGs failed to generate noticeable stress fibers after TGF-β stimulation, and their morphology remained similar to that of serum-free treated samples without TGF-β (Fig. 8A). NHDFs that had been transfected with control siRNAs and cultured on stiff PAHGs in serum-free medium contained few prominent stress fibers, and Hic-5 staining was predominately diffuse. After exposure to TGF-β, these NHDFs developed prominent stress fibers and Hic-5 staining that was concentrated in focal adhesions and appeared to partially colocalize with stress fibers. By contrast, Hic-5-depleted NHDFs that had been cultured on stiff PAHGs showed very few stress fibers after TGF-β treatment, and phalloidin staining was mostly diffuse and disorganized relative to that of control NHDFs (Fig. 8A). These data suggest that Hic-5 expression is required for extracellular stiffness to support the stress-fiber-stimulating effects of TGF-β.
Fig. 8.
Hic-5 potentiates the synergy between TGF-β and extracellular stiffness by regulating stress fiber maturation and myofibroblast differentiation. (A) NHDFs that had been transfected with non-targeting or pooled Hic-5-targeting siRNAs were cultured (48 h) with TGF-β (10 ng/ml) on soft or stiff PAHGs, fixed, permeabilized and immunostained for Hic-5 (green), and stained for DNA (blue) and F-actin (phalloidin, red). (B) Triton X-100-extracted lysates or total cell lysates of NHDFs that had been cultured as described in panel A were analyzed by western blotting for pan-actin and lamin A/C. (C) Fold changes in F-actin were determined by calculating the increase in pan-actin band intensity in response to TGF-β within Triton-insoluble lysates and then normalizing to lamin A/C expression (pooled data, n=3). (D) TGF-β-induced changes in total actin were determined by calculating the increase in pan-actin band intensity in response to TGF-β within total lysates and normalizing to lamin A/C levels from total lysates (pooled data, n=3). (E) A representative western blot (n=3) of the total lysates of samples shown in B, which were probed for Hic-5, α-SMA and GAPDH. Statistical significance was determined by two-way ANOVA and Tukey's post-hoc analysis (*P<0.05). Scale bar: 50 µm (A). Error bars are +s.e.m. n.s., not significant.
We then sought to quantify actin polymerization in response to TGF-β within these samples. However, challenges arising from the delicate nature of PAHGs prevented us from utilizing the ultracentrifugation techniques that we had successfully employed previously (above) (Fig. 5). We, therefore, took advantage of the fact that F-actin is insoluble in Triton X-100 and lysed cells either before or after extraction, which we found to be a suitable method for quantifying changes in F-actin in response to TGF-β (Fig. S1). Levels of Triton-X-100-insoluble actin (F-actin) dramatically increased in response to TGF-β in Hic-5-expressing NHDFs that had been cultured on stiff PAHGs when compared with those in NHDFs that had been cultured on soft PAHGs. However, TGF-β-dependent increases in F-actin were modest in Hic-5-depleted NHDFs, regardless of PAHG stiffness (Fig. 8B,C). Importantly, although TGF-β did induce total actin to some degree in all samples, none of these increases were significantly different among the tested groups (Fig. 8D). These data demonstrate that the ability of cells to generate F-actin in response to TGF-β is facilitated by the expression of Hic-5 and sufficient extracellular stiffness. Finally, we probed the capacity of Hic-5 to promote the TGF-β-dependent induction of α-SMA in NHDFs that had been cultured on soft and stiff PAHGs and treated with TGF-β. The induction of both Hic-5 and α-SMA levels by TGF-β were significantly augmented when control NHDFs were cultured on stiff PAHGs (see Fig. 4A–C). By contrast, extracellular stiffness failed to support the induction of α-SMA in response to treatment with TGF-β in Hic-5-depleted fibroblasts (Fig. 8E). Taken together, these data demonstrate a crucial role for Hic-5 in potentiating the synergy between TGF-β and extracellular stiffness with respect to stress fiber assembly and myofibroblast differentiation.
DISCUSSION
The impact of the mechanical properties of the ECM on disease pathology and myofibroblast function is well recognized, but the mechanisms through which mechanical and biochemical signals converge to support pathogenesis remain unclear. In this study, we report that the focal adhesion protein Hic-5 is expressed by myofibroblasts in vivo. Mechanically splinted rat wounds and human HTS tissue exhibited higher and prolonged expression of Hic-5 and α-SMA than that found in normal healing wounds, suggesting that, like α-SMA, Hic-5 expression is promoted and extended temporally through increased mechanical challenge (Hinz, 2010). We also found that Hic-5 and α-SMA induction by TGF-β requires a stiff microenvironment in vitro and that Hic-5 induction precedes that of α-SMA in NHDFs. The induction of Hic-5 by TGF-β required both the canonical SMAD3 and non-canonical MRTF-A–SRF pathways. We observed normal phosphorylation of SMAD3 but reduced nuclear localization of MRTF-A in cells that had been cultured on soft PAHGs, which correlated with blunted induction of Hic-5 expression by TGF-β. These data indicate that the mechanical sensitivity of the MRTF-A pathway is responsible for the reduced induction of Hic-5 in NHDFs cultured on soft PAHGs. Indeed, when a constitutively nuclear mutant of MRTF-A was expressed in NHDFs, Hic-5 and α-SMA were expressed to equal extents, regardless of extracellular stiffness. Notably, we also established that Hic-5 and MRTF-A expression are both required for the prominent assembly of stress fibers in response to TGF-β and cell-derived contractile forces. Our data illuminate a crucial role for Hic-5 in the differentiation and perpetuation of the contractile myofibroblast phenotype in pathogenic settings.
Increased mechanical stiffness of the ECM is required for myofibroblast differentiation and contributes to fibrosis. Changes in ECM stiffness alter how cells interact with molecules in the extracellular space as well as how molecules interact intracellularly. As an example of the former, increased stiffness in the ECM is known to promote the integrin-dependent mechanical activation of TGF-β (Klingberg et al., 2014; Wipff et al., 2007). As an example of the latter, the cellular localization of a transcriptional co-factor (MRTF-A) can alter gene expression as a consequence of changes in the actin cytoskeleton driven by mechanical properties of the extracellular environment (Huang et al., 2012; Johnson et al., 2013; O'Connor et al., 2015). We found that in serum-starved NHDFs, MRTF-A was primarily localized in the cytoplasm, regardless of the mechanical stiffness of the culture milieu. However, upon TGF-β stimulation, nuclear accumulation of MRTF-A occurred more readily in NHDFs that had been cultured on stiff, compared to soft, PAHG substrates. We now observe a direct link between the mechanical sensitivity of MRTF-A localization in response to TGF-β and the mechanical sensitivity of actin polymerization during stress fiber assembly, and we report that Hic-5 is an essential component of this link. We infer from our data that Hic-5-dependent sustained development of stress fibers in pathogenic myofibroblasts supports persistent MRTF-A localization, thereby perpetuating the myofibroblast phenotype. Consistent with this notion is the observation that small molecules that promote MRTF-A function result in quicker wound closure (Velasquez et al., 2013), whereas MRTF-A inhibitors can reduce fibrotic markers in scleroderma mouse models (Haak et al., 2014; Shiwen et al., 2015).
Stress fibers are unique cytoskeletal structures that generate force and bear mechanical loads. Stress fiber formation is known to require a certain level of extracellular stiffness from the ECM (Burridge and Wittchen, 2013; Hinz, 2010). Activation of RhoA is a crucial step in stress fiber assembly, and RhoA activation is augmented when tension is applied to integrins (Guilluy et al., 2011; Zhao et al., 2007). A stiff ECM is likely to support this process by resisting cellular contractile forces and allowing build-up of isometric tension within the engaged integrins. Isometric tension across actin filaments has also been shown to stabilize F-actin through reduced cofilin binding, in addition to decreased spontaneous depolymerization rates (Hayakawa et al., 2011; Lee et al., 2013). The capacity of extracellular tension conveyed by the ECM to regulate the growth or stability of stress fibers and to evoke changes in the expression of genes involved in regulating contractility highlights the importance of MRTF-A as a crucial link between ECM stiffness and myofibroblast differentiation. Importantly, these findings suggest that disrupting the capacity of TGF-β to promote the generation of stress fibers would decouple ECM stiffness from myofibroblast differentiation by preventing nuclear accumulation of MRTF-A, resulting in decreased cellular contractility (Fig. 6). The development of stress fibers not only promotes the nuclear localization of MRTF-A but is also reinforced through MRTF-A function by inducing genes such as Hic-5, which potentiate this mechanically sensitive feed-forward loop.
Our data reveal a potentially important regulatory difference between the TGF-β-dependent induction of Hic-5 and α-SMA. Hic-5 and α-SMA induction are absolutely dependent upon MRTF-A, whereas Hic-5 can be partially induced in the absence of SRF, suggesting that MRTF-A might have the capacity to regulate Hic-5 through an SRF-independent mechanism (Fig. 3). Moreover, silencing SMAD3 also impacted the TGF-β-dependent induction of Hic-5, suggesting that a putative MRTF-A- and SMAD3-dependent mechanism induces Hic-5 during the earlier phases following TGF-β induction, thereby initiating a new pool of Hic-5 to support the growth of stress fibers and to stabilize nuclear MRTF-A. In fact, SMAD3 and MRTF-A are known to interact as a transcriptionally active complex during epithelial–mesenchymal transition (EMT) (Morita et al., 2007). Indeed, it has been suggested that myofibroblast differentiation is biphasic with an early SMAD-dependent phase that induces genes, which aid in stress fiber growth (Sandbo et al., 2011), and then a later contractile phase governed by MRTF-A and SRF (Charbonney et al., 2011; Masszi et al., 2010).
Importantly, there are other mechanisms through which Hic-5 might regulate myofibroblast differentiation. Hic-5 interacts with SMAD-family members, altering their stability, and could regulate the biphasic differentiation by terminating this early SMAD-dependent phase and enabling the later MRTF-A–SRF phase (Charbonney et al., 2011; Masszi et al., 2010; Wang et al., 2008, 2005). The reported negative regulation of SMAD7 by Hic-5 might also partially explain the recent finding that Hic-5-knockout mice are resistant to the development of liver fibrosis (Lei et al., 2016). Hic-5 has also been reported to serve as a transcriptional co-regulator for specific genes [e.g. p21cip1 (Mori et al., 2012)], raising the possibility that Hic-5 might also regulate myofibroblast differentiation in this manner. Intriguingly, we did observe TGF-β-dependent nuclear translocation of Hic-5 at 48 h (Fig. 7C) but not at 24 h after treatment (Fig. S2). However, we also showed that a constitutively nuclear mutant of MRTF-A successfully induces α-SMA, even in the absence of Hic-5, indicating that MRTF-A-dependent induction of α-SMA is not strictly dependent on the transcription co-factor activities of Hic-5 (Fig. 7E,F). Interestingly, our data also demonstrate that although a modest level of MRTF-A was present in the nucleus of Hic-5-depleted cells (Fig. 7C,D), it was insufficient to promote induction of α-SMA (Fig. 7A,B), suggesting that the induction of α-SMA requires a higher threshold level of nuclear MRTF-A than can be generated in the absence of Hic-5.
We also observed that SMAD3 knockdown significantly reduced expression of MRTF-A and, thus, could influence Hic-5 and α-SMA expression indirectly (Fig. 3B,E). By contrast, it has been reported that during EMT, a SMAD3-dependent loss of MRTF-A occurs in the absence of stabilizing levels of β-catenin (Charbonney, 2011). Others have shown that SMADs participate in the transcription of MRTF-A downstream of TGF-β (Mihira et al., 2012; Scharenberg et al., 2014). However, we did not observe significant induction of MRTF-A in response to TGF-β, despite observing an overall SMAD3-dependence of MRTF-A expression in NHDFs, suggesting that the relationship between these proteins might be different in fibroblasts (Fig. 3B,E). Experiments are currently underway in our laboratory to test how SMAD3 and MRTF-A participate in Hic-5 induction and the extent to which Hic-5 modulates SMAD–MRTF-A signaling. Taken together, our data support our conclusion that the main function of Hic-5 in the early stages of myofibroblast differentiation is to facilitate stress fiber growth, thereby promoting nuclear accumulation of MRTF-A.
In summary, our data provide new evidence that demonstrates a crucial role for Hic-5 in the TGF-β-dependent commitment to the differentiated myofibroblast phenotype. The dependence on Hic-5 for stress fiber assembly in response to TGF-β, a mechanically dependent process, suggests that there is a crucial role for Hic-5 in translating mechanical cues from the ECM into gene expression through the action of MRTF-A. The reciprocity in the regulation of MRTF-A localization and Hic-5 expression implies the existence of a mechanically sensitive feed-forward loop that is initiated when the ECM is sufficiently stiff to support the Hic-5-dependent assembly of actin-rich stress fibers in response to TGF-β, and is enforced with the induction of Hic-5 by MRTF-A. By contrast, TGF-β fails to induce stress fiber assembly when the ECM is too soft, resulting in the failure to sustain a pool of nuclear MRTF-A. This model suggests that disruption of isometric tension could be an important therapeutic strategy for fibrotic diseases that occur in skin, as well as in parenchymal organs such as the lung and liver. Our data differ from those of a recent study on pulmonary fibroblasts, in which depletion of Hic-5 augmented both basal α-SMA expression and TGF-β induction of α-SMA after 24 h (Desai et al., 2014). However, when we knocked down Hic-5 in IMR-90 cells with two different siRNA duplexes, we observed the opposite result, namely that the induction of α-SMA after 24 h and 48 h of TGF-β stimulation (2 ng/ml) was reduced in Hic-5-deficient cells. In addition, we did not detect increases in baseline α-SMA expression as a result of Hic-5 knockdown, suggesting that the pro-fibrotic feed-forward loop proposed here could also apply to pulmonary fibrosis (Fig. S3). Indeed, others have now shown a requirement for Hic-5 in the TGF-β-dependent induction of α-SMA in glomerular mesangial cells, suggesting that this relationship could be conserved in other cell types (Pattabiraman and Rao, 2015).
Indeed, therapeutic approaches have sought to increase the compliance of the ECM in fibrotic tissues or tumor stroma by mechanical means (Wong et al., 2013), or by inhibiting collagen crosslinking enzymes such as lysyl oxidases (Levental et al., 2009). Targeting TGF-β or MRTF-A therapeutically has the potential for unintended pleotropic consequences, given the wide range of cells that respond to or express these proteins (Derynck and Miyazono, 2008; Wang et al., 2002). We speculate that targeting the function of Hic-5 might provide an important therapeutic approach in which ECM stiffness is decoupled from changes in gene expression, thereby limiting myofibroblast pathogenesis. Finally, we have demonstrated previously a crucial role for Hic-5 in regulating hypertrophic scar myofibroblast function in vitro by establishing an autocrine feed-forward loop in which Hic-5 is induced by TGF-β and is required for synthesis of TGF-β and type I collagen (Dabiri et al., 2006, 2008a,b). The feed-forward loops described previously and reported here might not be mutually exclusive and could work in concert to regulate myofibroblast differentiation as well as to perpetuate the pathogenic myofibroblast phenotype.
MATERIALS AND METHODS
Materials
SB431542, HEPES buffer, sodium pyruvate, 3-aminopropyl trimethoxysilane, TEMED, NaOH and ammonium persulfate were purchased from Sigma-Aldrich. Collagenase (catalog number 4197) and DNAse (catalog number 2139) were purchased from Worthington Biochemical Corporation (Lakewood, NJ). Recombinant human active TGF-β1 was purchased from R&D Systems. Coverslips (catalog number 12-546-2-25CIR-2), surfasil, acrylamide and bis-acrylamide were purchased from Fisher (Pittsburgh, PA). Antibodies against the following proteins were used: SRF (clone G20), Myc and RhoGDI (Santa Cruz); Hic-5 (clone 34; BD Biosciences); GAPDH (Ambion - Applied Biosystems); α-SMA (clone 1A4; Sigma-Aldrich); phosphorylated SMAD3 (catalog number EP823Y), total SMAD3 (catalog number EP568Y) and V5 (catalog number ab27671; Abcam); lamin A/C (catalog number 2032; Cell Signaling); pan-actin (Cytoskeleton, Denver, CO); MRTF-A (MKL1, catalog number A302-201A; Bethyl Laboratories).
RNAimax, Opti-mem, antibiotic-antimycotic (100× liquid), RPMI Medium 1640 and Alexa-Fluor-594–phalloidin were purchased from Invitrogen. An F-actin–G-actin fractionation kit was purchased from Cytoskeleton. Dicer-substrate RNAs were purchased from Integrated DNA Technologies (Coralville, IA). Collagen type 1 was purchased from Advanced Biomatrix (catalog number 5005-B; San Diego, CA). Polydimethylsiloxane (PDMS; 184 silicone elastomer) was purchased from Dow Corning (Midland, MI).
Cell culture
Primary NHDFs were purified from tissue obtained at Albany Medical Center under the auspices of protocols approved by the Institutional Review Board (IRB) at Albany Medical Center. Tissue was enzymatically digested (2225 U/ml collagenase and 200 U/ml DNAse) in RPMI buffer (4 ml/g of tissue), strained (60 µM filter) and cultured in complete medium (see below). Cells were immunostained with an anti-vimentin antibody to confirm their mesenchymal lineage. Cells were maintained in a humidified incubator at 37°C under 10% CO2 in serum-containing medium (McCarthy et al., 1997) comprising DMEM, 1% penicillin–streptomycin and 10% fetal bovine serum. Serum-free medium comprised DMEM and 1% penicillin–streptomycin. IMR 90 fibroblasts, purchased from American Type Culture Collection (ATCC; lot: 60286830), were cultured as described above for NHDFs. NHDFs and IMR 90 fibroblasts were serum starved overnight before treatment with TGF-β.
Immunohistology
Rat wound tissue (4-mm diameter, full thickness; approved by Albany Medical College Institutional Animal Care and Use Committee) was obtained from female Sprague-Dawley rats at the indicated time points and processed as described previously (Singh et al., 2009). Resected HTS tissue was obtained without individual identifiers with approval of the Institutional Review Board (Massachusetts General Hospital; MGH) while Dr Van De Water was at Shriners Hospital, MGH and Harvard Medical School, Boston, MA. Immunostaining was performed on 10-μm paraffin sections using directly labeled primary antibodies against Hic-5 (clone 34, BD Biosciences; dilution 1:500) and α-SMA (clone 1A4, Sigma-Aldrich; dilution 1:500) or visualized with horse radish peroxidase (HRP)-conjugated secondary reagents (ABC method, Vector Labs; dilutions 1:2000). Slides were examined, and images were taken on a SPOT color mosaic camera and associated software.
Immunofluorescence
Samples were fixed in 4% paraformaldehyde in PBS for 10 min, washed with PBS and quenched with 0.1 M glycine before permeabilization with 0.5% Triton X-100 or 0.02% saponin in PBS. Samples were then blocked in 5% BSA and incubated with primary antibody overnight (dilutions 1:500 Hic-5; 1:500 MRTF-A; 1:1000 vinculin), washed with TBST or PBS with 0.5% BSA and 0.02% saponin before incubation with secondary antibodies, phalloidin and Hoechst 33342 for 1 h. Slides were examined and images taken on a Roper/Photometrics Coolsnap ES camera. Confocal images were taken on an Olympus 1X81 microscope with a Fluoview FV1000 laser and analyzed using Olympus Fluoview FV10-ASW software (Fig. 2C). Images were processed using Nikon Elements software. In some cases (see figure legends), the images were altered by adjusting the gain function; however, these manipulations were applied equally to all images for a given channel within a given experiment.
Western blot analysis
Protein lysates from NHDFs and HTS fibroblasts were prepared in Laemmli buffer, separated by SDS-PAGE, transferred to nitrocellulose and analyzed with antibodies against the following proteins: Hic-5, GAPDH, Erk1 and Erk2 (all at 1:1000 dilution); Myc (1:200 dilution); SRF (1:5000 dilution); MRTF-A (1:500 dilution); α-SMA (1:2000 dilution). Relative protein expression was normalized to that of an internal loading control (GAPDH or lamin A/C) and expressed as a fold change relative to the average ratio (n=3 or more) in control lysates.
RNA interference
MRTF-A and Hic-5 knockdown and non-targeting shRNA sequences were as follows: MRTF-A sense 5ʹ-CATGGAGCTGGTGGAGAAGAA-3ʹ [it is important to note that this targeting sequence targets MRTF-A as well as MRTF-B in mice but is specific to MRTF-A in humans (Lee et al., 2010)]; Hic-5 sense 5ʹ-CGGTTGCTTCAGGAACTTAAT-3ʹ; non-targeting sense 5ʹ-CAACAAGATGAAGAGCACCAA-3ʹ.
Dicer-substrate siRNAs targeting Hic-5 were as follows: duplex 1 sense 5ʹ-GGAACUUAAUGCCACUCAGUUCAAC-3ʹ; duplex 2 sense 5ʹ-GGUGGCUUCAGGAGAGCAGAAGGAG-3ʹ. Other siRNAs targeting the following genes were: SRF duplex 1 sense 5ʹ-ACAGCACCAAGAGUGAAUGAUCCGC-3ʹ; SRF duplex 2 sense 5ʹ-AGAUGGAGUUCAUCGACAACAAGCT-3ʹ (data not shown); SMAD3 duplex 1 sense 5ʹ-GCUCAAAUGUGAUGAGAUAUCAGAAAUCUCAUCACA-3ʹ; SMAD3 duplex 2 sense 5ʹ-AUCUCAAAGAGAUUCGAAACCGUCAUUCGAAUCUCU-3ʹ (data not shown); non-targeting duplex sense 5ʹ-CGUUAAUCGCGUAUAAUACGCGUAT-3ʹ. Each siRNA was used at a 10 nM concentration and was reverse transfected into NDHFs with RNAimax using the manufacturer's protocol. NHDFs were trypsinized and replated after an overnight or a 72-h incubation with the dicer substrate siRNAs and allowed to recover in serum-containing medium for 24 h before serum starvation and TGF-β stimulation.
Adenovirus infection
Adenovirus encoding Myc-tagged MRTF-A (CMV promoter) has been described previously (Zhang et al., 2007). Three dilutions of virus (in Opti-MEM) were added to NHDFs for 16 h. A GFP-expressing adenovirus was used as a control at a comparable concentration to the highest concentration used for the MRTF-A-expressing adenovirus. Cells were allowed to recover in conditioned medium for 48 h before protein was harvested and analyzed by western blotting, as previously described.
Lentivirus infection
Lentiviruses were produced in HEK293FT cells according to the manufacturer's instructions (Invitrogen). Viruses were added to NHDFs for 24 h with polybrene (final 6 µg/ml). Virus was removed, and cells were replated and allowed to recover for 48 h before the start of the experiment.
Quantification of actin polymerization
The ratio of F-actin:G-actin was quantified using a specific fractionation kit as per the manufacturer's instructions (Cytoskeleton) (Fig. 5). Alternatively, an adapted protocol for isolating F-actin using Triton extraction was employed (Brown et al., 1976; Sandbo et al., 2011). Briefly, NHDFs were washed with ice-cold PBS without Ca2+ or Mg2+ and then extracted with five buffer exchanges of ice-cold 0.1% Triton X-100, 0.1 mM EGTA and 0.1 mM EDTA in PBS without Ca2+ or Mg2+ before lysis with 1× SDS Laemmli buffer containing protease inhibitor cocktail and 0.1 M DTT (Fig. 8 and Fig. S1).
Preparation of PAHGs
A protocol was adapted from Cretu et al., 2010; Tse and Engler, 2010; Yeung et al., 2005. In brief, coverslips were prepared for PAHG crosslinking by incubating cells with NaOH (0.1 M, 3 min) and 3-aminopropyltrimethoxysilane (97%, 3 min), followed by treatment with glutaraldehyde (0.5%, 30 min).
PAHGs were prepared by mixing different concentrations of acrylamide and bis-acrylamide [acrylamide 5% and bis-acrylamide 0.225% for ‘stiff’ PAHGs (∼8.5 kPa) and acrylamide 3% and bis-acrylamide 0.06% for ‘soft’ PAHGs (∼0.5 kPa) (Tse and Engler, 2010)]. PAHGs were allowed to polymerize for 2 h in a humidified chamber before functionalizing the surface with 0.5 mg/ml Sulpho-SANPAH in 10 mM HEPES, pH 6, using the spectrolinker XL-1500 (Spectroline, Westbury, NY) set to the highest setting for 3 min (UV 365 nm). PAHGs were washed with PBS (pH 7) before crosslinking type 1 collagen to the surface at 50 µg/ml, diluted in ice-cold PBS just before addition to the PAHGs, and the PAHGs were then incubated on ice. After 15 min, excess collagen was washed away with PBS before cell seeding.
Preparation of PDMS-coated plates for wrinkle assays
PDMS was mixed at an 80:1 base to curing agent ratio and poured into the wells of a 6-well plate. The PDMS was then degassed and then cured at 65°C overnight. PDMS surfaces were treated with plasma to promote cell attachment.
Statistical analysis
Western blots were imaged using a Bio-Rad ChemiDoc MP imaging system, and densitometry was performed using Image Lab 4.1 software. Changes in the levels of the proteins of interest were calculated by dividing the protein-to-loading-control ratio of a lane by the protein-to-loading-control ratio of the blot, which was subsequently normalized to the average control value from at least three independent experiments. Statistical analysis was performed on the normalized data using Prism 6 software (see figure legends for statistical tests performed).
Acknowledgements
The authors thank Dr Günther Machens (Klinikum Rechts der Isar at Technical University, Munich, Germany) for providing hypertrophic scar tissue. We are grateful to Debbie Moran for her help with the preparation of this manuscript and to Tessa Simone for initiating, and Chrissy Rotondi (Histology Core) for performing, the histology and to Danielle Hunt for help with confocal microscopy. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
S.D.V. conducted most of the experiments, provided intellectual input and drafted the manuscript. C.B.B. initiated this project, provided data for the manuscript as well as intellectual input and editorial help on the manuscript. R.Z. and L.W. conducted experiments and provided intellectual input. B.H. provided tissue, intellectual input and a critical reading of the manuscript. J.Z. provided valuable advice at all stages of the research and editorial help on the manuscript. L.V.D.W. directed the research and assisted in writing and editing the manuscript.
Funding
This work was supported by National Institutes of Health General Medical Sciences grant [grant number GM 056442]; including funding from the American Recovery and Reinvestment Act (ARRA) to L.V.D.W.; and by the Albany Medical College Bridge Funding Program (to L.V.D.W.). The research of B.H. is supported by Canadian Institutes of Health Research [grant numbers 210820, 286920, 286720 and 497202); the Collaborative Health Research Programme (between CIHR and Natural Sciences and Engineering Research Council of Canada) [grant numbers 1004005 and 413783]; and the Canada Foundation for Innovation and Ontario Research Fund [grant number 26653]. The research of J.Z. is supported by a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health [grant number R01HL109605]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.170589/-/DC1
References
- Brown S., Levinson W. and Spudich J. A. (1976). Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J. Supramol. Struct. 5, 119-130. 10.1002/jss.400050203 [DOI] [PubMed] [Google Scholar]
- Burridge K. and Wittchen E. S. (2013). The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 200, 9-19. 10.1083/jcb.201210090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen B., Selvaraj A., Burgess R. C., Hitzler J. K., Ma Z., Morris S. W. and Prywes R. (2003). Megakaryoblastic leukemia 1, a potent transcriptional coactivator for Serum Response Factor (SRF), is required for serum induction of SRF target genes. Mol. Cell. Biol. 23, 6597-6608. 10.1128/MCB.23.18.6597-6608.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M. W. C., Chaudary F., Lee W., Copeland J. W. and McCulloch C. A. (2010). Force-induced myofibroblast differentiation through collagen receptors is dependent on mammalian diaphanous (mDia). J. Biol. Chem. 285, 9273-9281. 10.1074/jbc.M109.075218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonney E., Speight P., Masszi A., Nakano H. and Kapus A. (2011). beta-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition. Mol. Biol. Cell 22, 4472-4485. 10.1091/mbc.E11-04-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretu A., Castagnino P. and Assoian R. (2010). Studying the effects of matrix stiffness on cellular function using acrylamide-based hydrogels. J. Vis. Exp. 42, e2089 10.3791/2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider B. J., Risinger G. M. Jr, Haaksma C. J., Howard E. W. and Tomasek J. J. (2011). Myocardin-related transcription factors A and B are key regulators of TGF-beta1-induced fibroblast to myofibroblast differentiation. J. Invest. Dermatol. 131, 2378-2385. 10.1038/jid.2011.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri G., Campaner A., Morgan J. R. and Van De Water L. (2006). A TGF-beta1-dependent autocrine loop regulates the structure of focal adhesions in hypertrophic scar fibroblasts. J. Invest. Dermatol. 126, 963-970. 10.1038/sj.jid.5700187 [DOI] [PubMed] [Google Scholar]
- Dabiri G., Tumbarello D. A., Turner C. E. and Van De Water L. (2008a). Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. J. Invest. Dermatol. 128, 2518-2525. 10.1038/jid.2008.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri G., Tumbarello D. A., Turner C. E. and Van De Water L. (2008b). TGF-beta1 slows the growth of pathogenic myofibroblasts through a mechanism requiring the focal adhesion protein, Hic-5. J. Invest. Dermatol. 128, 280-291. 10.1038/sj.jid.5700975 [DOI] [PubMed] [Google Scholar]
- Deakin N. O. and Turner C. E. (2011). Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol. Biol. Cell 22, 327-341. 10.1091/mbc.E10-09-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R. and Miyazono K. (2008). The TGF-Beta Family. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Desai L. P., Zhou Y., Estrada A. V., Ding Q., Cheng G., Collawn J. F. and Thannickal V. J. (2014). Negative regulation of NADPH oxidase 4 by hydrogen peroxide-inducible clone 5 (Hic-5) protein. J. Biol. Chem. 289, 18270-18278. 10.1074/jbc.M114.562249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C., Swaminathan V., Garcia-Mata R., O'Brien E. T., Superfine R. and Burridge K. (2011). The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 724-729. 10.1038/ncb2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak A. J., Tsou P.-S., Amin M. A., Ruth J. H., Campbell P., Fox D. A., Khanna D., Larsen S. D. and Neubig R. R. (2014). Targeting the myofibroblast genetic switch: inhibitors of myocardin-related transcription factor/serum response factor-regulated gene transcription prevent fibrosis in a murine model of skin injury. J. Pharmacol. Exp. Ther. 349, 480-486. 10.1124/jpet.114.213520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Tatsumi H. and Sokabe M. (2011). Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J. Cell Biol. 195, 721-727. 10.1083/jcb.201102039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer M. D. and DeFranco D. B. (2006). Hic-5/ARA55, a LIM domain-containing nuclear receptor coactivator expressed in prostate stromal cells. Cancer Res. 66, 7326-7333. 10.1158/0008-5472.CAN-05-2379 [DOI] [PubMed] [Google Scholar]
- Hinz B. (2010). The myofibroblast: paradigm for a mechanically active cell. J. Biomech. 43, 146-155. 10.1016/j.jbiomech.2009.09.020 [DOI] [PubMed] [Google Scholar]
- Hinz B. (2015). The extracellular matrix and transforming growth factor-beta1: tale of a strained relationship. Matrix Biol. 47, 54-65. 10.1016/j.matbio.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Huang X., Yang N., Fiore V. F., Barker T. H., Sun Y., Morris S. W., Ding Q., Thannickal V. J. and Zhou Y. (2012). Matrix stiffness–induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol. 47, 340-348. 10.1165/rcmb.2012-0050OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. A., Rodansky E. S., Sauder K. L., Horowitz J. C., Mih J. D., Tschumperlin D. J. and Higgins P. D. (2013). Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm. Bowel Dis. 19, 891-903. 10.1097/MIB.0b013e3182813297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. A., Rodansky E. S., Haak A. J., Larsen S. D., Neubig R. R. and Higgins P. D. (2014). Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-beta–induced fibrogenesis in human colonic myofibroblasts. Inflamm. Bowel Dis. 20, 154-165. 10.1097/01.MIB.0000437615.98881.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Kaneyama J. R. (2012). [Hydrogen peroxide-inducible clone 5 (Hic-5) as a potential therapeutic target]. Seikagaku 84, 261-265. [PubMed] [Google Scholar]
- Kim-Kaneyama J.-r., Suzuki W., Ichikawa K., Ohki T., Kohno Y., Sata M., Nose K. and Shibanuma M. (2005). Uni-axial stretching regulates intracellular localization of Hic-5 expressed in smooth-muscle cells in vivo. J. Cell Sci. 118, 937-949. 10.1242/jcs.01683 [DOI] [PubMed] [Google Scholar]
- Klingberg F., Chow M. L., Koehler A., Boo S., Buscemi L., Quinn T. M., Costell M., Alman B. A., Genot E. and Hinz B. (2014). Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J. Cell Biol. 207, 283-297. 10.1083/jcb.201402006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-M., Vasishtha M. and Prywes R. (2010). Activation and repression of cellular immediate early genes by serum response factor cofactors. J. Biol. Chem. 285, 22036-22049. 10.1074/jbc.M110.108878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-y., Lou J., Wen K.-k., McKane M., Eskin S. G., Ono S., Chien S., Rubenstein P. A., Zhu C. and McIntire L. V. (2013). Actin depolymerization under force is governed by lysine 113:glutamic acid 195-mediated catch-slip bonds. Proc. Natl. Acad. Sci. USA 110, 5022-5027. 10.1073/pnas.1218407110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X.-F., Fu W., Kim-Kaneyama J.-r., Omoto T., Miyazaki T., Li B. and Miyazaki A. (2016). Hic-5 deficiency attenuates the activation of hepatic stellate cells and liver fibrosis through upregulation of Smad7 in mice. J. Hepatol. 64, 110-117. 10.1016/j.jhep.2015.08.026 [DOI] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F. T., Csiszar K., Giaccia A., Weninger W. et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891-906. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masszi A., Speight P., Charbonney E., Lodyga M., Nakano H., Szaszi K. and Kapus A. (2010). Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J. Cell Biol. 188, 383-399. 10.1083/jcb.200906155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuya M., Sasaki H., Aoto H., Mitaka T., Nagura K., Ohba T., Ishino M., Takahashi S., Suzuki R. and Sasaki T. (1998). Cell adhesion kinase beta forms a complex with a new member, Hic-5, of proteins localized at focal adhesions. J. Biol. Chem. 273, 1003-1014. 10.1074/jbc.273.2.1003 [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Chen D., Yang B. S., Garcia Ramirez J. J., Cherwinski H., Chen X. R., Klagsbrun M., Hauser C. A., Ostrowski M. C. and McMahon M. (1997). Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol. Cell. Biol. 17, 2401-2412. 10.1128/MCB.17.5.2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihira H., Suzuki H. I., Akatsu Y., Yoshimatsu Y., Igarashi T., Miyazono K. and Watabe T. (2012). TGF-beta-induced mesenchymal transition of MS-1 endothelial cells requires Smad-dependent cooperative activation of Rho signals and MRTF-A. J. Biochem. 151, 145-156. 10.1093/jb/mvr121 [DOI] [PubMed] [Google Scholar]
- Miralles F., Posern G., Zaromytidou A.-I. and Treisman R. (2003). Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329-342. 10.1016/S0092-8674(03)00278-2 [DOI] [PubMed] [Google Scholar]
- Mori K., Hamanaka H., Oshima Y., Araki Y., Ishikawa F., Nose K. and Shibanuma M. (2012). A HIC-5- and KLF4-dependent mechanism transactivates p21(Cip1) in response to anchorage loss. J. Biol. Chem. 287, 38854-38865. 10.1074/jbc.M112.377721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Mayanagi T. and Sobue K. (2007). Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J. Cell Biol. 179, 1027-1042. 10.1083/jcb.200708174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlich S., Wang R., Lee S.-M., Lewis T. C., Dai C. and Prywes R. (2008). Serum-induced phosphorylation of the serum response factor coactivator MKL1 by the extracellular signal-regulated kinase 1/2 pathway inhibits its nuclear localization. Mol. Cell. Biol. 28, 6302-6313. 10.1128/MCB.00427-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiya N., Iwabuchi Y., Shibanuma M., Cote J.-F., Tremblay M. L. and Nose K. (1999). Hic-5, a paxillin homologue, binds to the protein-tyrosine phosphatase PEST (PTP-PEST) through its LIM 3 domain. J. Biol. Chem. 274, 9847-9853. 10.1074/jbc.274.14.9847 [DOI] [PubMed] [Google Scholar]
- Nishiya N., Shirai T., Suzuki W. and Nose K. (2002). Hic-5 interacts with GIT1 with a different binding mode from paxillin. J. Biochem. 132, 279-289. 10.1093/oxfordjournals.jbchem.a003222 [DOI] [PubMed] [Google Scholar]
- O'Connor J. W., Riley P. N., Nalluri S. M., Ashar P. K. and Gomez E. W. (2015). Matrix rigidity mediates TGFbeta1-induced epithelial-myofibroblast transition by controlling cytoskeletal organization and MRTF-A localization. J. Cell Physiol. 230, 1829-1839. 10.1002/jcp.24895 [DOI] [PubMed] [Google Scholar]
- Pattabiraman P. P. and Rao P. V. (2015). Hic-5 regulates actin cytoskeletal reorganization and expression of fibrogenic markers and myocilin in trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 56, 5656-5669. 10.1167/iovs.15-17204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I. B., Horiguchi M., Zilberberg L., Dabovic B., Hadjiolova K. and Rifkin D. B. (2015). Latent TGF-beta-binding proteins. Matrix Biol. 47, 44-53. 10.1016/j.matbio.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbo N., Lau A., Kach J., Ngam C., Yau D. and Dulin N. O. (2011). Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-beta. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L656-L666. 10.1152/ajplung.00166.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg M. A., Pippenger B. E., Sack R., Zingg D., Ferralli J., Schenk S., Martin I. and Chiquet-Ehrismann R. (2014). TGF-beta-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J. Cell Sci. 127, 1079-1091. 10.1242/jcs.142075 [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Mashimo J., Kuroki T. and Nose K. (1994). Characterization of the TGF beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J. Biol. Chem. 269, 26767-26774. [PubMed] [Google Scholar]
- Shiwen X., Stratton R., Nikitorowicz-Buniak J., Ahmed-Abdi B., Ponticos M., Denton C., Abraham D., Takahashi A., Suki B., Layne M. D. et al. (2015). A role of myocardin related transcription factor-A (MRTF-A) in scleroderma related fibrosis. PLoS ONE 10, e0126015 10.1371/journal.pone.0126015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Chen C., Pal-Ghosh S., Stepp M. A., Sheppard D. and Van De Water L. (2009). Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J. Invest. Dermatol. 129, 217-228. 10.1038/jid.2008.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. M., Thatcher J. E., Sutherland L. B., Kinoshita H., Gerard R. D., Richardson J. A., DiMaio J. M., Sadek H., Kuwahara K. and Olson E. N. (2010). Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 107, 294-304. 10.1161/CIRCRESAHA.110.223172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Chen G., Streb J. W., Long X., Yang Y., Stoeckert C. J. Jr and Miano J. M. (2006). Defining the mammalian CArGome. Genome Res. 16, 197-207. 10.1101/gr.4108706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Hagel M. and Turner C. E. (1999). Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 112, 181-190. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C. and Brown R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349-363. 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- Trembley M. A., Velasquez L. S., de Mesy Bentley K. L. and Small E. M. (2015). Myocardin-related transcription factors control the motility of epicardium-derived cells and the maturation of coronary vessels. Development 142, 21-30. 10.1242/dev.116418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse J. R. and Engler A. J. (2010). Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 47:10.16:10.16.1-10.16.16 10.1002/0471143030.cb1016s47 [DOI] [PubMed] [Google Scholar]
- Van De Water L., Varney S. and Tomasek J. J. (2013). Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: opportunities for new therapeutic intervention. Adv. Wound Care 2, 122-141. 10.1089/wound.2012.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen M. K., Guettler S., Larijani B. and Treisman R. (2007). Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749-1752. 10.1126/science.1141084 [DOI] [PubMed] [Google Scholar]
- Velasquez L. S., Sutherland L. B., Liu Z., Grinnell F., Kamm K. E., Schneider J. W., Olson E. N. and Small E. M. (2013). Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proc. Natl. Acad. Sci. USA 110, 16850-16855. 10.1073/pnas.1316764110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.-Z., Li S., Hockemeyer D., Sutherland L., Wang Z., Schratt G., Richardson J. A., Nordheim A. and Olson E. N. (2002). Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl. Acad. Sci. USA 99, 14855-14860. 10.1073/pnas.222561499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Song K., Sponseller T. L. and Danielpour D. (2005). Novel function of androgen receptor-associated protein 55/Hic-5 as a negative regulator of Smad3 signaling. J. Biol. Chem. 280, 5154-5162. 10.1074/jbc.M411575200 [DOI] [PubMed] [Google Scholar]
- Wang H., Song K., Krebs T. L., Yang J. and Danielpour D. (2008). Smad7 is inactivated through a direct physical interaction with the LIM protein Hic-5/ARA55. Oncogene 27, 6791-6805. 10.1038/onc.2008.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu G., Betts C., Harmon E. Y., Keller R. S., Van De Water L. and Zhou J. (2011). Transforming growth factor-beta1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. J. Biol. Chem. 286, 41589-41599. 10.1074/jbc.M111.250878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff P.-J., Rifkin D. B., Meister J.-J. and Hinz B. (2007). Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 179, 1311-1323. 10.1083/jcb.200704042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V. W., Beasley B., Zepeda J., Dauskardt R. H., Yock P. G., Longaker M. T. and Gurtner G. C. (2013). A mechanomodulatory device to minimize incisional scar formation. Adv. Wound Care 2, 185-194. 10.1089/wound.2012.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T., Georges P. C., Flanagan L. A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V. and Janmey P. A. (2005). Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell. Motil. Cytoskeleton 60, 24-34. 10.1002/cm.20041 [DOI] [PubMed] [Google Scholar]
- Yuminamochi T., Yatomi Y., Osada M., Ohmori T., Ishii Y., Nakazawa K., Hosogaya S. and Ozaki Y. (2003). Expression of the LIM proteins paxillin and Hic-5 in human tissues. J. Histochem. Cytochem. 51, 513-521. 10.1177/002215540305100413 [DOI] [PubMed] [Google Scholar]
- Yund E. E., Hill J. A. and Keller R. S. (2009). Hic-5 is required for fetal gene expression and cytoskeletal organization of neonatal cardiac myocytes. J. Mol. Cell Cardiol. 47, 520-527. 10.1016/j.yjmcc.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Fang H., Zhou J. and Herring B. P. (2007). A novel role of Brg1 in the regulation of SRF/MRTFA-dependent smooth muscle-specific gene expression. J. Biol. Chem. 282, 25708-25716. 10.1074/jbc.M701925200 [DOI] [PubMed] [Google Scholar]
- Zhao X.-H., Laschinger C., Arora P., Szaszi K., Kapus A. and McCulloch C. A. (2007). Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801-1809. 10.1242/jcs.001586 [DOI] [PubMed] [Google Scholar]