Background and Purpose—
Although the incidence of stroke is on the decline worldwide, this is not the case for early stroke. We aimed to determine whether nonpsychotic mental disorder at the age of 18 years is a risk factor for early stroke, and if adolescent cardiovascular fitness and intelligence quotient might attenuate the risk.
Method—
Population-based Swedish cohort study of conscripts (n=1 163 845) who enlisted during 1968 to 2005. At conscription, 45 064 males were diagnosed with nonpsychotic mental disorder. Risk of stroke during follow-up (5–42 years) was calculated with Cox proportional hazards models. Objective baseline measures of fitness and cognition were included in the models in a second set of analyses.
Results—
There were 7770 first-time stroke events. In adjusted models, increased risk for stroke was observed in men diagnosed with depressive/neurotic disorders (hazard ratio [HR], 1.23; 95% confidence interval [CI], 1.11–1.37), personality disorders (HR, 1.52; 95% CI, 1.29–1.78), and alcohol/substance use disorders (HR, 1.61; 95% CI, 1.41–1.83) at conscription. Corresponding figures for fatal stroke were HR, 1.38; 95% CI, 1.06 to 1.79; HR, 2.26; 95% CI, 1.60 to 3.19; and HR, 2.20; 95% CI, 1.63 to 2.96. HRs for stroke were attenuated when fitness level and intelligence quotient were introduced. Associations remained significant for personality disorders and alcohol/substance use in the fully adjusted models. The interaction term was statistically significant for fitness but not for intelligence quotient.
Conclusions—
Our findings suggest that fitness may modify associations between nonpsychotic disorders and stroke. It remains to be clarified whether interventions designed to improve fitness in mentally ill youth can influence future risk of early stroke.
Keywords: adolescent, exercise, mental disorders, population, stroke
There are indications that mental well-being is declining in young people.1 Consequences of mental ill-health in this age group are pernicious and include poorer quality of life, marginalization, and suicidal behavior, as well as increased risk of coronary heart disease.2,3 As the latter shares many pathogenic factors with stroke, it could be anticipated that mental disorder in adolescence might also be associated with increased risk of stroke. Schizophrenia4 and bipolar disorder have, in meta-analyses, been associated with increased incidence of stroke.5 About nonpsychotic disorders, an association between panic disorder and stroke was observed in 1 study.6 Another study showed a possible link between depressive symptoms and future stroke in older males.7 In teenage males, low stress resilience was associated with increased risk of early stroke.8 The potential risk of stroke in young adults diagnosed with nonpsychotic mental disorders has yet to be investigated.
Although age-standardized rates of stroke have decreased worldwide,9 no such trend has been observed for early stroke (before the age of 65 years).10 In Sweden, early stroke is on the rise, now comprising one fifth of all stroke cases.11 Early stroke often leads to a long-term disability, including reduced work capacity. The personal, societal, and economic burden of the disease makes it important to identify modifiable risk factors.12,13 In this study, we hypothesized that nonpsychotic mental disorder in adolescence would be associated with increased risk of early stroke. Because previous research suggests that high intelligence,14 as well as high fitness15 might constitute protective factors in this context, we hypothesized that these factors would attenuate stroke risk in persons with nonpsychotic mental disorders. We performed a prospective cohort study of all Swedish men born in 1950 to 1987 who were enlisted for mandatory military service at the age of 18 years and followed for at least 5 and ≤42 years. Because the oldest individuals in this study were 60 years old at the time of diagnosis, all cases can be considered early stroke cases. The main aim was to determine whether nonpsychotic mental disorders were associated with risk of early stroke and death by stroke. The second aim was to examine how fitness and intelligence quotient (IQ) at the age of 18 years might affect on such associations.
Methods
Participants
A cohort of 18-year-old Swedish males who enlisted for military service between 1968 and 2005 (ie, born between 1950 and 1987, n=1 694 121) was compiled from the Swedish Military Service Conscription Register. During that time, Swedish law required all 18-year-old Swedish men to enlist, with exemptions granted only for those who were incarcerated or had severe chronic medical or mental conditions or functional disabilities documented by a medical certificate (≈2% to 3% each year). All Swedes have a unique personal identification number making linkage to other registers possible. The Ethics Committee of the University of Gothenburg and Confidentiality Clearance at Statistics Sweden approved the study.
Conscription Register Data
During a 2-day examination, all men in the cohort underwent standardized physical and cognitive examinations before being assigned to service in the Swedish armed forces. All conscripts were seen by a psychologist and a physician. Weight, height, and blood pressure were measured.
Psychiatric Diagnoses Recorded at Conscription
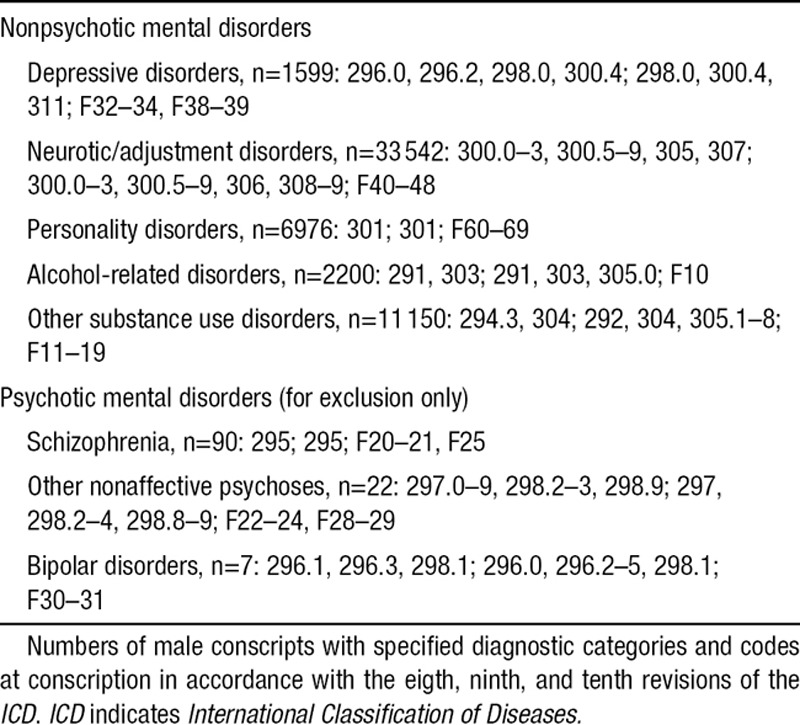
Psychiatric symptoms were assessed during a structured interview with a psychologist. Symptoms, when present, were further evaluated by a physician, and psychiatric disorders were diagnosed according to the International Classification of Diseases (ICD). Diagnostic codes of interest for this article (hitherto referred to as nonpsychotic mental disorders) are shown in Table 1. To avoid misclassification of conscripts with prodromal episodes of psychotic disorder, individuals with hospital admissions (see below) for schizophrenia, other nonaffective psychoses, and bipolar disorder with onset any time after conscription were excluded.
Table 1.
Diagnostic Categories and ICD Codes
Cardiovascular Fitness Test
Cardiovascular fitness was assessed using the cycle ergometric test. The procedure, including elements of validity and reliability, has been described in detail previously.16–18
Intelligence Quotient
The cognitive performance tests are described in detail elsewhere; data have been used in other studies.19,20 For this study, results of 4 tests (logical performance, verbal test of synonyms and opposites, test of visuospatial/geometric perception, and technical/mechanical skills including mathematical/physics problems) were summed yielding a measure of combined intelligence (henceforth IQ). Test results were standardized against data from previous years to give scores from 1 (low) to 9 (high) with a Gaussian distribution. The standardization to a stanine scale provided long-term stability of the data sets. Before 1996, raw data were not electronically recorded and only stanine scores could be accessed for statistical analysis. Therefore, only stanine scores were assessed in our analysis.
Stress Resilience
The psychological examination included also an assessment of the conscript’s potential ability to cope with wartime stress. During this assessment, the conscripts met the psychologist for a semistructured interview with an average duration of 20 to 25 minutes. The interview included questions about predisposition to anxiety, ability to control and channel nervousness, and stress tolerance. It also covered areas relevant to everyday life, including psychosocial dimensions (interests, recreational activities, psychological motivation, social maturity, and emotional stability). Responses were summarized in a global stress resilience score (1–9, with higher values indicating greater resilience). To ensure consistent evaluation over time and between different test centers, a central authority supervised the instruction and training of participating psychologists, supported by a written manual. Test data have been used in published research.8,21
Muscle Strength
Isometric muscle strength was measured by knee extension (weighted 1.3×), elbow flexion (weighted 0.8×), and hand grip (tested with a tensiometer; weighted 1.7×).16 Weighted values were integrated into 1 estimate in kilopond (before 1979) or Newton (after 1979), and divided into stanines.
Outcomes
Stroke
Stroke cases were identified by the national Hospital Discharge Register.22 Sweden has a universal healthcare system that provides low-cost healthcare, including hospital care. Registration of 1 principal discharge diagnosis and ≤5 contributory diagnoses is mandatory in the Hospital Discharge Register. Register coverage increased gradually during 1968 to 1986, and is complete since 1987. Stroke cases were classified according to the ICD-8 (1968–1988), ICD-9 (1989–1996), or ICD-10 (1997–ongoing). The reliability of stroke diagnoses in the Hospital Discharge Register has been shown to be solid.22,23 Stroke cases were categorized as ischemic, intracerebral hemorrhagic, and subarachnoidal hemorrhagic as follows: ischemic stroke, 433 and 434 (ICD-8–9) and I63 (ICD-10); hemorrhagic stroke, 431 (ICD-9) and I61 (ICD-10); and subarachnoid bleeding, 430 (ICD-8 and ICD-9) and I60 (ICD-10). Both principal and secondary diagnoses were included. In the analyses of stroke types, first event by each type was used, resulting in a larger sum than for any stroke because an individual could have >1 type of stroke during the observation period.
Fatal Stroke
Stroke deaths were identified by linkage with the Swedish Cause of Death Register, which is maintained at the National Board of Health and Welfare. This register is annually updated based on death certificate diagnoses, covering virtually all deaths since 1961. Fatal strokes were defined as (1) stroke deaths identified by the Cause of Death Register or (2) patients who were hospitalized for stroke and died from any cause within 28 days of stroke onset as identified by the Cause of Death Register. All other strokes registered in the Hospital Discharge Register were classified as nonfatal.
Covariates From Other Data Sources
LISA
Information on parental education was obtained from the longitudinal integration database for health insurance and labor market studies (Swedish acronym LISA, 80% coverage). The LISA database at Statistics Sweden was initiated in 1990 and includes all registered residents aged ≥16 years. The database, which is annually updated, integrates data from the labor market, as well as educational and social sectors. Parental education was rated in 7 levels: pre-high school education <9 years, pre-high school education 9 years (ie, mandatory education only in Table 2), high school education, university (<2 years), university (≥2 years), postgraduate education, and postgraduate research training.
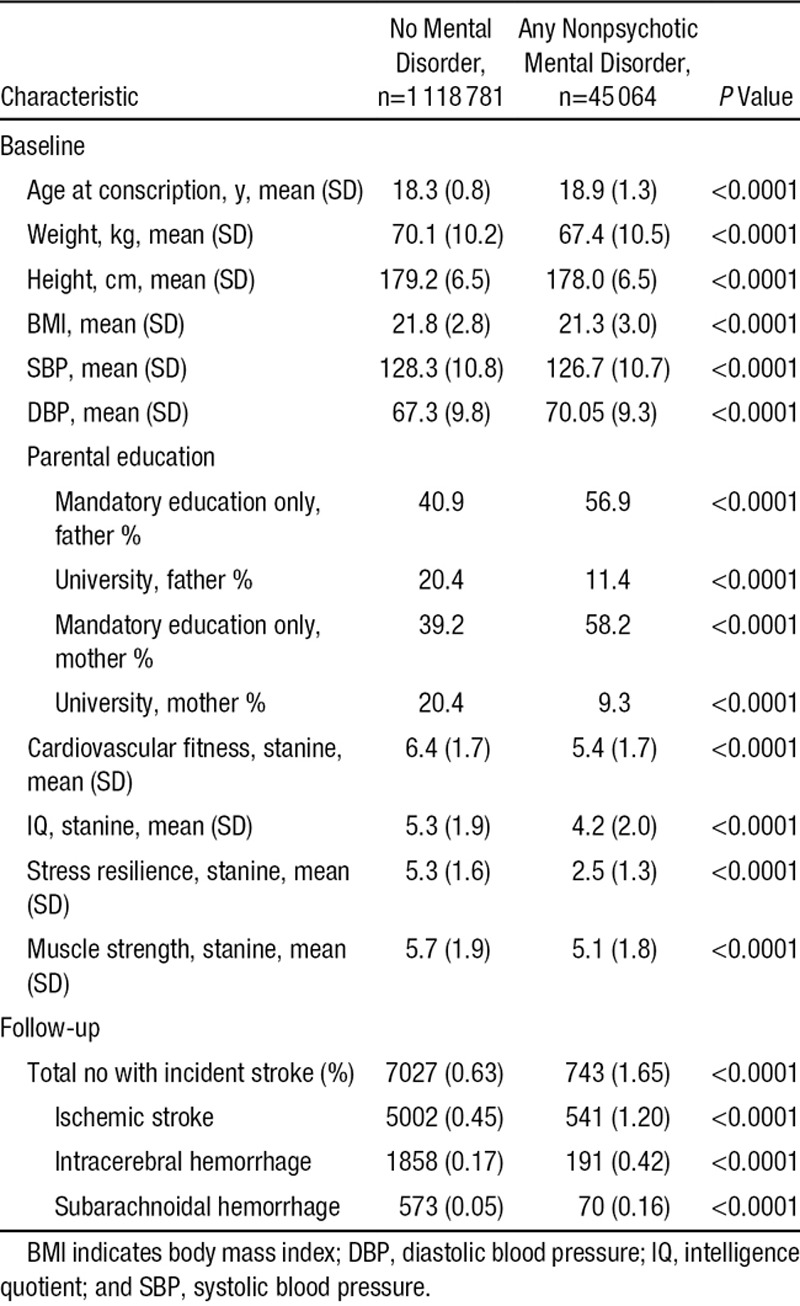
Table 2.
Population Characteristics in a National Cohort of 18-Year-Old Male Conscripts and Numbers and Proportions (%) With Incident Stroke During 5 to 42 Years of Follow-Up
Statistical Analysis
All statistical calculations were performed with SAS version 8.1 (SAS Institute, NC). The follow-up period began at the date of conscription (baseline) and subjects were censored at time of (1) first stroke event or (2) death from other causes or (3) emigration or (4) at the end of follow-up, that is, on December 31, 2010.
Fisher Exact Test was used for expression of the difference in the distribution of stroke between conscripts with or without a nonpsychotic mental disorder. For comparison of the population characteristics a 1-way analysis of variance was used.
We used Cox proportional hazards models to assess the influence of a nonpsychotic mental disorder at the age of 18 years and potential confounders on the occurrence of first onset of stroke during the observation period. To assess effects of secular variation in rates of stroke outcome and differences in conscription procedures over time, we adjusted for calendar years by stratifying the Cox model by conscription decade (60s, 70s, etc.). Because differences among regions and test centers could introduce bias, conscription test center was considered a possible confounder and adjusted for. Early obesity and hypertension could be risk factors for stroke.12,13 Therefore, adjustments for the continuous variables body mass index, systolic and diastolic blood pressures were performed. Because alcohol/substance use disorders are known risk factors for stroke,24 and these conditions may co-occur with depressive, neurotic, and personality disorders, models were constructed including alcohol/substance use disorders as a potential confounder.
To examine effect modification of fitness and IQ at the age of 18 years on the associations between mental disorder at the age of 18 years and stroke risk in adulthood, we included fitness and IQ as interaction terms. The interaction terms (IQ×specified disorders and fitness×specified disorders) were introduced together in the fully adjusted models for each disorder category. Separate stratifications were also performed: fitness stanines were trichotomized as low (stanine score, 1–4), medium (stanine score, 5–7), and high (stanine score, 8–9).
Population-attributable risk—the association of a specific risk factor with a specific disease as a proportion of all risk factors for that disease—was calculated by the method of Natarajan et al,25 using the hazard ratios (HRs) from the Cox proportional hazard regression models.
Results
Participant Characteristics
Of conscripts who enlisted for mandatory military service from 1968 to 2005, our analyses are based on the 1 163 845 with complete information. Of these, after exclusion of conscripts diagnosed with a psychotic mental disorder (n=119) and also those who later developed psychosis (n=1900), 45 064 (3.9% of the cohort) were found to have any of the mental disorders listed in Table 1 at baseline. Of those, 78.2% had 1 diagnosis, 20.5% had 2 diagnoses, 1.2% had 3 diagnoses, and 0.1% had 4 diagnoses. Among those with depressive/neurotic disorders, 25.1% (n=8829) had concurrent alcohol/substance use disorders. The corresponding figure for those with personality disorders was 9.6% (n=667).
Baseline characteristics are shown in Table 2. There were differences in age, weight, height, body mass index, or blood pressure between those with and without nonpsychotic mental disorder. These differences were statistically significant because of the large numbers of observations, but they were marginal (<4%) in magnitude. Larger differences in magnitude were found, however, for parental education in the mental disorder group, as well as for lower fitness, IQ, stress resilience, and muscle strength (P<0.0001 for each variable).
Nonpsychotic Mental Disorder at the Age of 18 Years and Future Early Stroke
In total, the study encompassed 29 132 278 person-years of follow-up. There were 7770 first-time stroke events. The mean interval from conscription to first admission to hospital inpatient care for stroke was 26.6 years (SD, 9.0). The proportion of strokes was 2.8× greater in the mental disorder group compared with the rest of the population (Table 2; 1.65 versus 0.63%, P<0.0001). The Figure shows the cumulative incidence (%) of any stroke during the follow-up period.
Figure.
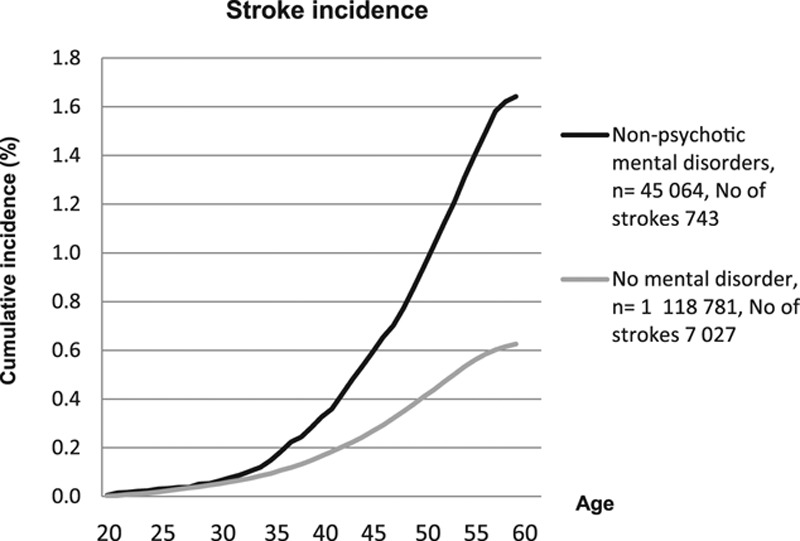
Cumulative incidence (%) of early stroke of any type in men with and without nonpsychotic mental disorder at the age of 18 years.
All nonpsychotic mental disorders were considered together in the first analyses. In line with our first hypothesis, the presence of a nonpsychotic mental disorder diagnosis at the age of 18 years was a strong risk factor for all stroke (any subtype) as well as nonfatal stroke (Table 3). Performing separate analyses for the different diagnostic categories (depressive/neurotic, personality disorders, and substance use disorders) showed that all these categories were associated with all stroke and nonfatal stroke (Table 3). The strength of the associations changed little in models that controlled for alcohol/substance use disorders or systolic and diastolic blood pressures.
Table 3.
Nonpsychotic Mental Disorder at the Age of 18 Years and Future Early Stroke
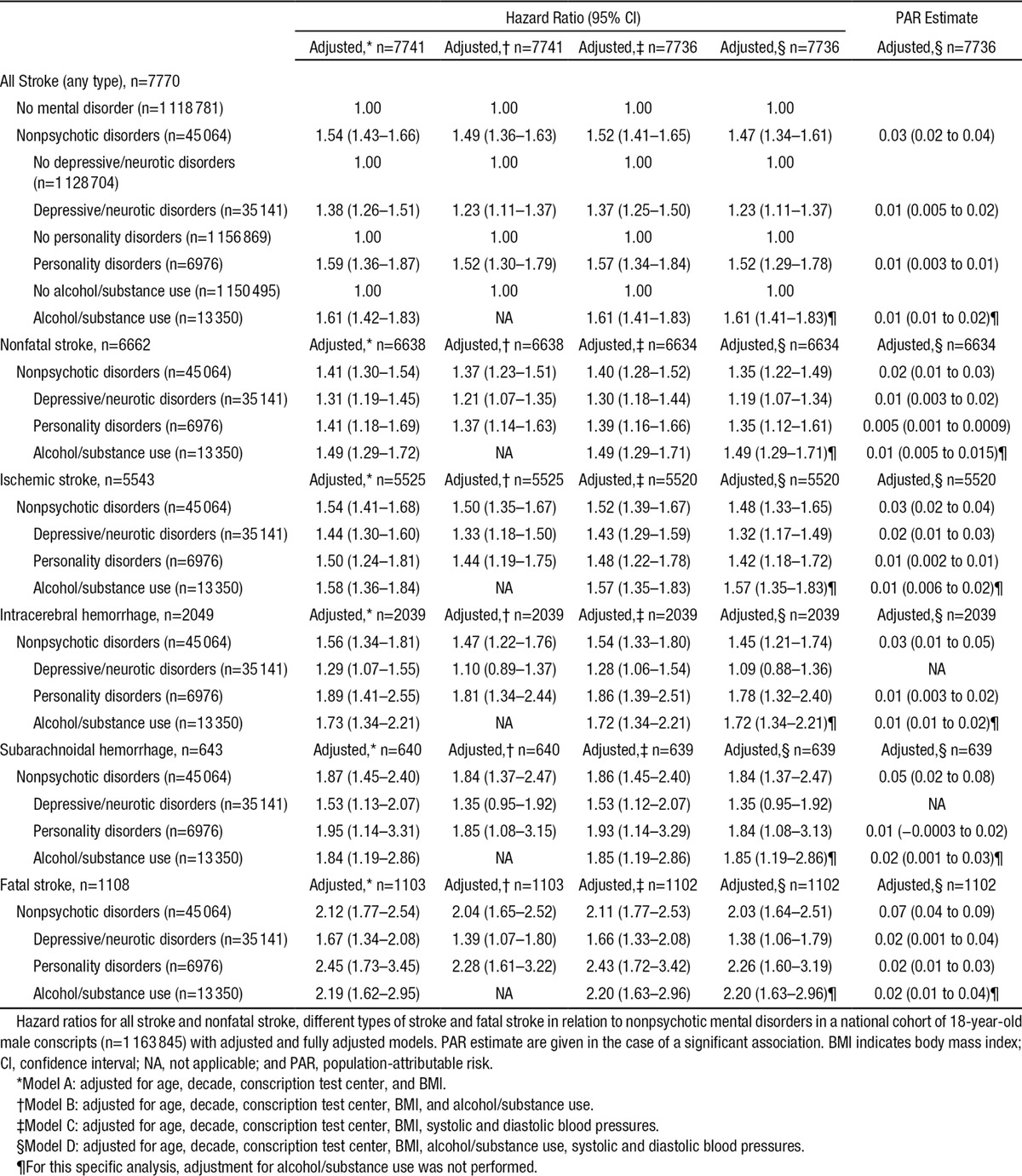
We examined whether the associations differed with respect to different stroke types (Table 3). Similar HRs were found for all 3 diagnostic categories regarding ischemic stroke. For intracerebral hemorrhage and subarachnoidal hemorrhage, similar HRs were found for personality disorders and substance use disorders. For these 2 stroke types, the association with depressive/neurotic disorders became nonsignificant in models that controlled for alcohol/substance use disorders.
Fatal outcome was more common in those with nonpsychotic mental disorders (19.1 versus 13.7% in all others, P<0.0001). In the analysis adjusted for age, decade, conscription test center, body mass index, alcohol/substance use, systolic and diastolic blood pressures, nonpsychotic mental disorder was associated with a 2-fold increase in the HR (2.03) and a population-attributable risk estimate of 0.07 for death caused by stroke (Table 3). When the diagnostic categories were analyzed separately, significant associations with fatal stroke were observed for depressive/neurotic disorders, personality disorders, and alcohol/substance use disorders. The numerically highest risk increase was seen in personality disorders but there was considerable overlap in confidence intervals for the 3 mental illness categories.
Importance of IQ and Fitness and Future Stroke Risk Among Young Men With Nonpsychotic Mental Disorders
In accordance with our second aim, we explored how IQ and fitness at the age of 18 years would affect on the early stroke risk in adulthood among young men diagnosed with nonpsychotic mental disorder. Descriptive information on the distribution of nonpsychotic mental disorder and future stroke among the fitness and IQ stanine scores is shown in Table 4. After adjustments, the inclusion of IQ as a covariate attenuated the association between depressive/neurotic disorders, personality disorders and alcohol/substance use and incident stroke (Table 5A, model B). When fitness was added as a covariate in the analyses (model C), the associations with incident stroke decreased similarly. When both IQ and fitness were included in the fully adjusted model (model D), the HRs were further decreased. Although associations for personality disorders and alcohol/substance use disorders remained significant, the association for depressive/neurotic disorders did not.
Table 4.
Descriptive Data, Fitness, and IQ Stanine Scores in a National Cohort of 18-Year-Old Male Conscripts
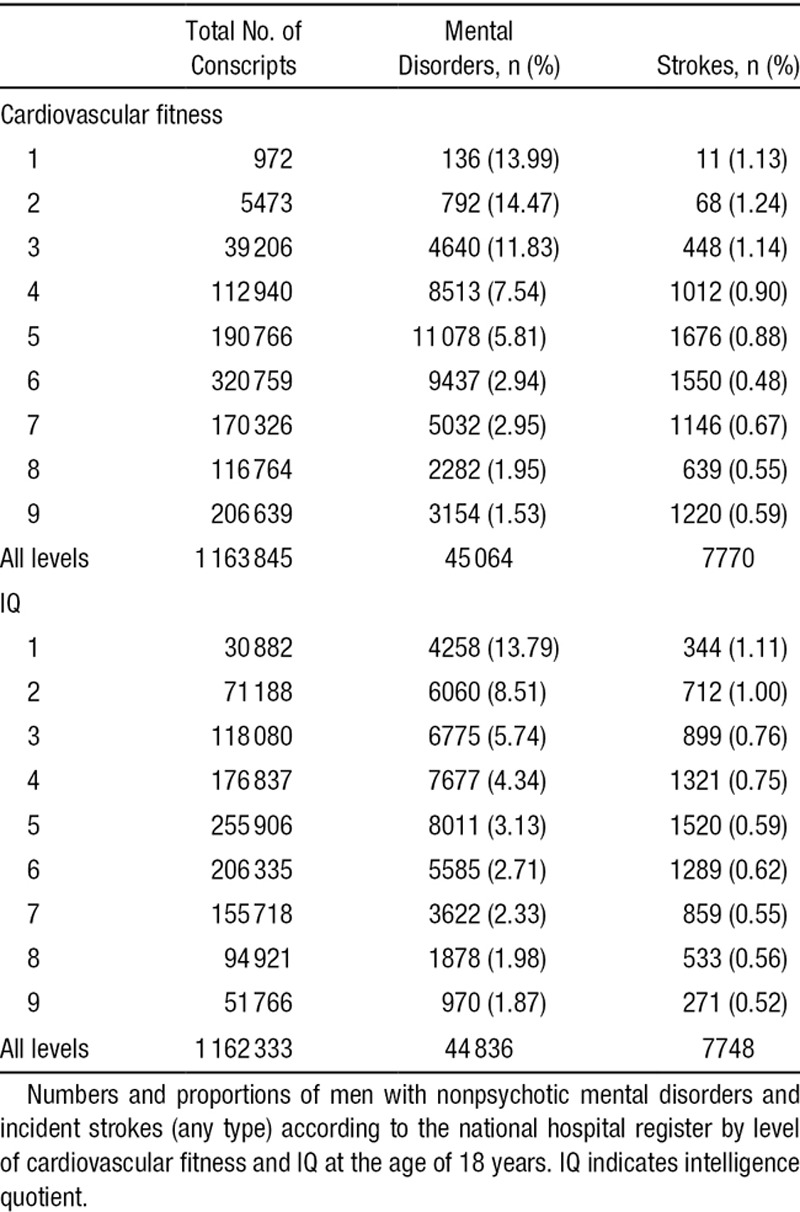
Table 5.
Importance of IQ and Fitness for Future Stroke Risk Among Young Men Diagnosed With Nonpsychotic Mental Disorder at the Age of 18 Years
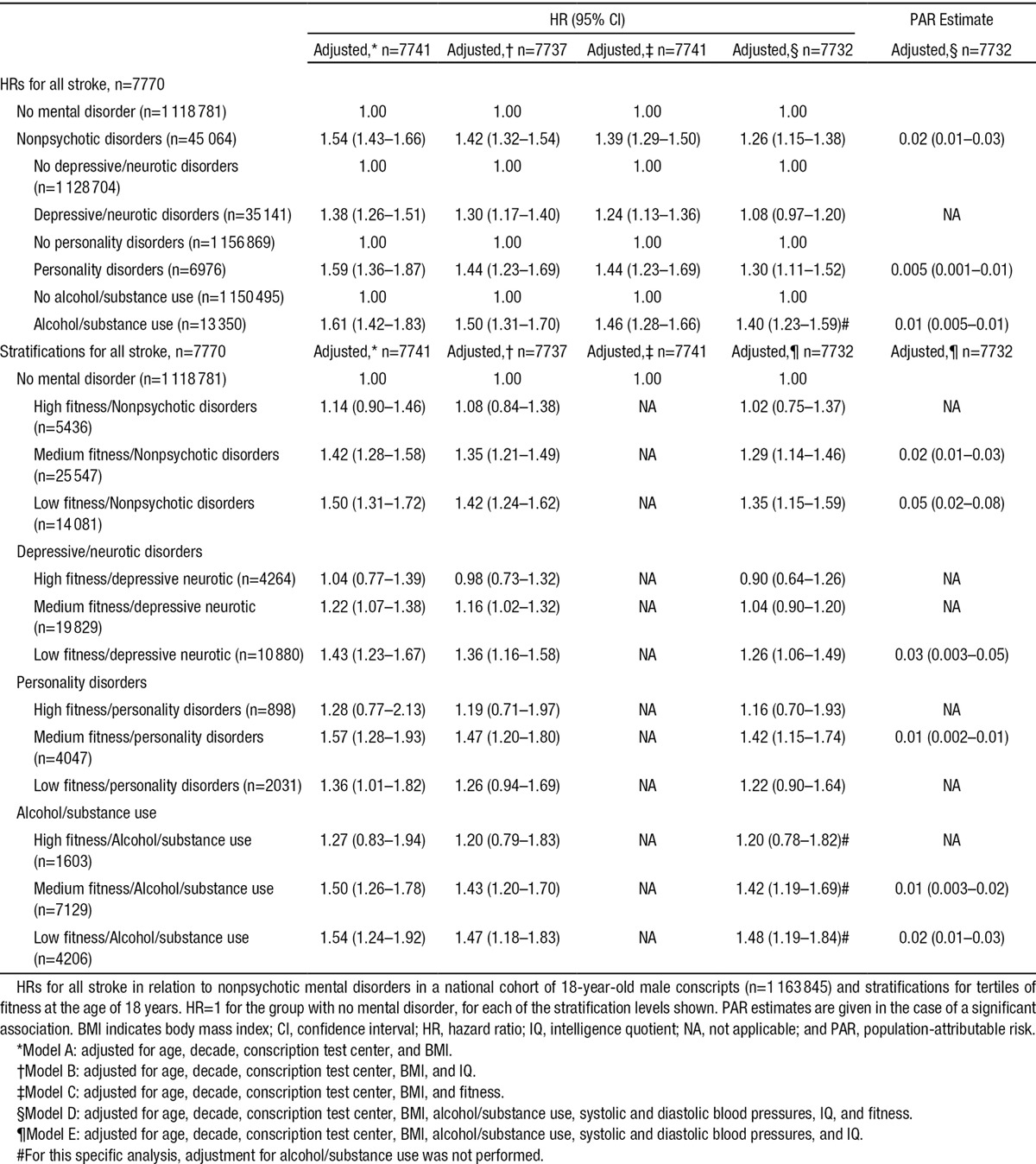
To examine effect modification of fitness and IQ at the age of 18 years on the associations between the subtypes of mental disorder at the age of 18 years and stroke risk in adulthood, we included fitness and IQ as interaction terms. The interaction terms (IQ×specified disorders and fitness×specified disorders) were introduced together in the fully adjusted models (model D) for each disorder category. The interaction term for IQ for men with depressive/neurotic disorders was nonsignificant (P=0.89). However, the interaction term for fitness and depressive/neurotic disorders was significant (P=0.001). Corresponding data were found for personality disorders (IQ P=0.67; fitness P=0.03) and alcohol/substance (IQ P=0.71; fitness P=0.008).
Table 5B shows data for men with depressive/neurotic disorders, personality disorders, and alcohol/substance use stratified by tertiles of fitness at the age of 18 years revealing relationships in a graded fashion with the highest risk increases for incident stroke in the low fitness groups. For depressive/neurotic disorders, associations with incident stroke were nonsignificant in the high fitness category, attenuated in the medium fitness category, and nonsignificant in the fully adjusted model. The HR remained significant, however, in the low fitness/depression neurotic group. Having both low fitness and personality disorder at the age of 18 years was not associated with incident stroke, but medium fitness and personality disorder was. About alcohol/substance use disorders, the association with incident stroke became nonsignificant in the high fitness category, but remained in both the medium and the low fitness categories.
Discussion
In this national cohort study, we demonstrate a relationship between nonpsychotic mental disorder in young adult males and risk of early stroke. Relationships were observed for both depressive/neurotic disorders and personality disorders also after adjustment for alcohol/substance use disorders. In line with our second hypothesis, HRs for stroke were attenuated when fitness level and IQ were introduced. Associations remained significant for personality disorders and alcohol/substance use but not for depressive/neurotic disorders in the fully adjusted models. Statistically significant interactions were shown for fitness suggesting that this modifiable risk factor may constitute a target for interventions for young men with nonpsychotic mental health issues.
Important strengths of this study are the size (>1.1 million individuals), the prospective population-based design, and the long follow-up time that increase both the validity and the reliability. The reliance on psychologists and physicians for baseline assessment of mental health status allowed the identification of individuals with current illness episodes. The Swedish National Hospital Discharge Register enabled us to cover virtually all inpatient care for stroke during 1987 to 2010. An additional strength of this study is the inclusion of objective measures of both fitness and cognitive performance.
However, there are also drawbacks of the current design. It has previously been reported that 0.7% of conscripts had a history of psychiatric hospitalization before conscription, yet only 19% of these were diagnosed with a mental disorder at conscription.3 Coverage on neuropsychiatric disorders was insufficient; these disorders could not be included in the analyses. Failure to diagnose mental disorders at baseline would probably result in more conservative estimates. A further consideration is that cases of early stroke that occurred between the ages 61 and 65 years were missed, and not all men had a follow-up time until the age of 60 years. Results from our study cannot be directly extrapolated to women. Early stroke is more common in men,11 with onset occurring 5 to 10 years earlier, and sex differences about subtypes of early stroke have been reported.26 We were not able to adjust for mental ill-health in parents that also might have affected the results. There are possible confounders that may increase the risk for both mental disorder and stroke, which we were not able to control for such as genetic vulnerability and early developmental influences. Furthermore, data were lacking about potential mediating confounders in adulthood, such as combined effects of socioeconomic position, smoking, alcohol consumption, type 2 diabetes mellitus, and hypertension.27,28
The strongest HRs and population-attributable risk estimates were found for the association between nonpsychotic mental disorder and fatal stroke. The explanation for this may be that a mental vulnerability impairs the brain’s ability to recover after a stroke. There could also be shared vulnerability for both mental illness and stroke. Depression is common after stroke29 and persons with mental ill-health in early adulthood might be at particular risk. Reduced motivation, compromised coping skills and nonadherence to rehabilitation programs may lead to poorer poststroke prognosis. Furthermore, obesity, lower level of physical activity, diabetes mellitus, hypertension, and dyslipidemia/poorer diet all contribute to poorer outcome after stroke.30
We aimed to examine if adolescent cardiovascular fitness and IQ might modulate the relationship between nonpsychotic disorders and stroke. The effect modification was tested in statistical models with interaction terms for IQ and fitness. Fitness, in contrast to IQ, showed a significant interaction, that is, one of the 3 HRs (low, medium, and high fitness) is different from the other 2 and seems to modify the associations between nonpsychotic disorders and stroke. Our stratification analyses revealed relationships in a graded fashion with the highest risk increases for incident stroke in the low and medium fitness groups compared with the high fitness group in the fully adjusted models. A conceivable interpretation coherent with other studies is that high fitness may ameliorate a negative impact of mental disorder on stroke. Self-reported low frequency of physical activity is associated with increased risk of incident stroke31 and regular physical activity is an important recommendation for stroke prevention.30 The effect of physical activity is likely to be mediated through reducing traditional vascular risk factors.32 Several potential mechanisms exist through which fitness could affect brain health later in life.33,34 By enhancing neuroplasticity in young men with mental disorders, increased physical exercise might have a protective effect on pathogenic processes that lead up to the stroke. Evaluating new strategies could prove to be clinically important, considering challenges in treating mental illness among young people.35
Conclusions
This is, to our knowledge, the first study to explore relationships between different types of nonpsychotic mental disorders in adolescents and risk for early stroke. Future intervention studies have to clarify whether improvement of fitness in mentally ill youth can influence future risk of early stroke.
Acknowledgments
We thank Dr Tommy Johnson for statistical help and advice.
Sources of Funding
This study was supported by grants from the Märtha Lundqvists Stiftelse, Sahlgrenska University Hospital (ALF) and the Swedish Research Council 2013–2699.
Disclosures
None.
Supplementary Material
References
- 1.Lager A, Berlin M, Heimerson I, Danielsson M. Young people’s health: Health in Sweden: The National Public Health Report 2012. Chapter 3. Scand J Public Health. 2012;40(suppl 9):42–71. doi: 10.1177/1403494812459459. doi: 10.1177/1403494812459459. [DOI] [PubMed] [Google Scholar]
- 2.Gravseth HM, Bjerkedal T, Irgens LM, Aalen OO, Selmer R, Kristensen P. Influence of physical, mental and intellectual development on disability in young Norwegian men. Eur J Public Health. 2008;18:650–655. doi: 10.1093/eurpub/ckn055. doi: 10.1093/eurpub/ckn055. [DOI] [PubMed] [Google Scholar]
- 3.Gale CR, Batty GD, Osborn DP, Tynelius P, Rasmussen F. Mental disorders across the adult life course and future coronary heart disease: evidence for general susceptibility. Circulation. 2014;129:186–193. doi: 10.1161/CIRCULATIONAHA.113.002065. doi: 10.1161/CIRCULATIONAHA.113.002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Z, Wu Y, Shen J, Ji T, Zhan R. Schizophrenia and the risk of cardiovascular diseases: a meta-analysis of thirteen cohort studies. J Psychiatr Res. 2013;47:1549–1556. doi: 10.1016/j.jpsychires.2013.07.011. doi: 10.1016/j.jpsychires.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Prieto ML, Cuéllar-Barboza AB, Bobo WV, Roger VL, Bellivier F, Leboyer M, et al. Risk of myocardial infarction and stroke in bipolar disorder: a systematic review and exploratory meta-analysis. Acta Psychiatr Scand. 2014;130:342–353. doi: 10.1111/acps.12293. doi: 10.1111/acps.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou PH, Lin CH, Loh el-W, Chan CH, Lan TH. Panic disorder and risk of stroke: a population-based study. Psychosomatics. 2012;53:463–469. doi: 10.1016/j.psym.2012.03.007. doi: 10.1016/j.psym.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Bos MJ, Lindén T, Koudstaal PJ, Hofman A, Skoog I, Breteler MM, et al. Depressive symptoms and risk of stroke: the Rotterdam Study. J Neurol Neurosurg Psychiatry. 2008;79:997–1001. doi: 10.1136/jnnp.2007.134965. doi: 10.1136/jnnp.2007.134965. [DOI] [PubMed] [Google Scholar]
- 8.Bergh C, Udumyan R, Fall K, Nilsagård Y, Appelros P, Montgomery S. Stress resilience in male adolescents and subsequent stroke risk: cohort study. J Neurol Neurosurg Psychiatry. 2014;85:1331–1336. doi: 10.1136/jnnp-2013-307485. doi: 10.1136/jnnp-2013-307485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. doi: 10.1001/jama.2014.7692. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 11.Rosengren A, Giang KW, Lappas G, Jern C, Torén K, Björck L. Twenty-four-year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013;44:2388–2393. doi: 10.1161/STROKEAHA.113.001170. doi: 10.1161/STROKEAHA.113.001170. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. American Heart Association/American Stroke Association Stroke Council; Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Academy of Neurology. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 13.Hankey GJ. Potential new risk factors for ischemic stroke: what is their potential? Stroke. 2006;37:2181–2188. doi: 10.1161/01.STR.0000229883.72010.e4. doi: 10.1161/01.STR.0000229883.72010.e4. [DOI] [PubMed] [Google Scholar]
- 14.Modig Wennerstad K, Silventoinen K, Tynelius P, Bergman L, Rasmussen F. Association between intelligence and type-specific stroke: a population-based cohort study of early fatal and non-fatal stroke in one million Swedish men. J Epidemiol Community Health. 2010;64:908–912. doi: 10.1136/jech.2008.084020. doi: 10.1136/jech.2008.084020. [DOI] [PubMed] [Google Scholar]
- 15.Åberg ND, Kuhn HG, Nyberg J, Waern M, Friberg P, Svensson J, et al. Influence of cardiovascular fitness and muscle strength in early adulthood on long-term risk of stroke in Swedish men. Stroke. 2015;46:1769–1776. doi: 10.1161/STROKEAHA.115.009008. doi: 10.1161/STROKEAHA.115.009008. [DOI] [PubMed] [Google Scholar]
- 16.Nordesjö LO, Schéle R. Validity of an ergometer cycle test and measures of isometric muscle strength when prediction some aspects of military performance. Swedish J Defence Med. 1974;10:11–23. [Google Scholar]
- 17.Åberg MA, Nyberg J, Torén K, Sörberg A, Kuhn HG, Waern M. Cardiovascular fitness in early adulthood and future suicidal behaviour in men followed for up to 42 years. Psychol Med. 2014;44:779–788. doi: 10.1017/S0033291713001207. doi: 10.1017/S0033291713001207. [DOI] [PubMed] [Google Scholar]
- 18.Nyberg J, Åberg MA, Schiöler L, Nilsson M, Wallin A, Torén K, et al. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain. 2014;137(pt 5):1514–1523. doi: 10.1093/brain/awu041. doi: 10.1093/brain/awu041. [DOI] [PubMed] [Google Scholar]
- 19.Åberg MA, Pedersen NL, Torén K, Svartengren M, Bäckstrand B, Johnsson T, et al. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci U S A. 2009;106:20906–20911. doi: 10.1073/pnas.0905307106. doi: 10.1073/pnas.0905307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sörberg A, Allebeck P, Melin B, Gunnell D, Hemmingsson T. Cognitive ability in early adulthood is associated with later suicide and suicide attempt: the role of risk factors over the life course. Psychol Med. 2013;43:49–60. doi: 10.1017/S0033291712001043. doi: 10.1017/S0033291712001043. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery S, Udumyan R, Magnuson A, Osika W, Sundin PO, Blane D. Mortality following unemployment during an economic downturn: Swedish register-based cohort study. BMJ Open. 2013;3:e003031. doi: 10.1136/bmjopen-2013-003031. doi: 10.1136/bmjopen-2013-003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulsvik AK, Gulsvik A, Svendsen E, Mæhle BO, Thelle DS, Wyller TB. Diagnostic validity of fatal cerebral strokes and coronary deaths in mortality statistics: an autopsy study. Eur J Epidemiol. 2011;26:221–228. doi: 10.1007/s10654-010-9535-4. doi: 10.1007/s10654-010-9535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boden-Albala B, Sacco RL. Lifestyle factors and stroke risk: Exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2:160–166. doi: 10.1007/s11883-000-0111-3. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan S, Lipsitz SR, Rimm E. A simple method of determining confidence intervals for population attributable risk from complex surveys. Stat Med. 2007;26:3229–3239. doi: 10.1002/sim.2779. doi: 10.1002/sim.2779. [DOI] [PubMed] [Google Scholar]
- 26.Yesilot Barlas N, Putaala J, Waje-Andreassen U, Vassilopoulou S, Nardi K, Odier C, et al. Etiology of first-ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol. 2013;20:1431–1439. doi: 10.1111/ene.12228. doi: 10.1111/ene.12228. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Ostenson CG. Psychological distress and risk of pre-diabetes and type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet Med. 2008;25:834–842. doi: 10.1111/j.1464-5491.2008.02463.x. doi: 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- 28.Nordahl H, Osler M, Frederiksen BL, Andersen I, Prescott E, Overvad K, et al. Combined effects of socioeconomic position, smoking, and hypertension on risk of ischemic and hemorrhagic stroke. Stroke. 2014;45:2582–2587. doi: 10.1161/STROKEAHA.114.005252. doi: 10.1161/STROKEAHA.114.005252. [DOI] [PubMed] [Google Scholar]
- 29.De Ryck A, Fransen E, Brouns R, Geurden M, Peij D, Mariën P, et al. Poststroke depression and its multifactorial nature: results from a prospective longitudinal study. J Neurol Sci. 2014;347:159–166. doi: 10.1016/j.jns.2014.09.038. doi: 10.1016/j.jns.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 30.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 31.McDonnell MN, Hillier SL, Hooker SP, Le A, Judd SE, Howard VJ. Physical activity frequency and risk of incident stroke in a national US study of blacks and whites. Stroke. 2013;44:2519–2524. doi: 10.1161/STROKEAHA.113.001538. doi: 10.1161/STROKEAHA.113.001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faulkner J, Lambrick D, Woolley B, Stoner L, Wong LK, McGonigal G. Effects of early exercise engagement on vascular risk in patients with transient ischemic attack and nondisabling stroke. J Stroke Cerebrovasc Dis. 2013;22:e388–e396. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.014. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 34.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibbons RD, Brown CH, Hur K, Davis J, Mann JJ. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69:580–587. doi: 10.1001/archgenpsychiatry.2011.2048. doi: 10.1001/archgenpsychiatry.2011.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]


