Abstract
Orai1, a specific nonvoltage‐gated Ca2+ channel, has been found to be one of key molecules involved in store‐operated Ca2+ entry (SOCE). Orai1 may associate with other proteins to form a signaling complex, which is essential for regulating a variety of physiological functions. In this study, we studied the possible interaction between Orai1 and large conductance Ca2+‐activated potassium channel (BKC a). Using RNA interference technique, we demonstrated that the SOCE and its associated membrane hyperpolarization were markedly suppressed after knockdown of Orai1 with a specific Orai1 siRNA in rat mesenteric artery smooth muscle. Moreover, isometric tension measurements showed that agonist‐induced vasocontraction was increased after Orai1 was knocked down or the tissue was incubated with BKC a blocker iberiotoxin. Coimmunoprecipitation data revealed that BKC a and Orai1 could reciprocally pull down each other. In situ proximity ligation assay further demonstrated that Orai1 and BKC a are in close proximity. Taken together, these results indicate that Orai1 physically associates with BKC a to form a signaling complex in the rat mesenteric artery smooth muscle. Ca2+ influx via Orai1 stimulates BKC a, leading to membrane hyperpolarization. This hyperpolarizing effect of Orai1‐BKC a coupling could contribute to reduce agonist‐induced membrane depolarization, therefore preventing excessive contraction of the rat mesenteric artery smooth muscle in response to contractile agonists.
Keywords: BKCa, mesenteric artery, Orai1, store‐operated Ca2+ entry, vascular smooth muscle cells
Introduction
Ca2+ ion is an important intracellular second messenger, regulating a plethora of cell functions such as secretion, transcription, growth, and apoptosis (Bootman and Berridge 1995; Berridge 1998, 2013; Genazzani and Thorn 2002; Lipskaia and Lompre 2004). One of the most common and ubiquitous pathways involved in modulating Ca2+ influx into cells is store‐operated Ca2+ entry (SOCE), which is mediated via specific plasma membrane ion channels in response to the depletion of Ca2+ content of intracellular Ca2+ stores (Gwack et al. 2007; Dominguez‐Rodriguez et al. 2012). The previous studies showed that knockdown of Orai1, one of the key components of SOCE, reduced SOCE activity (Li et al. 2011; Yang et al. 2012).
The Orai1 is a plasma membrane protein predicted to contain four TM segments (TM1 to TM4) with both N‐ and C‐termini located in the cytosol. Orai1 is widely expressed in many cell types, including vascular smooth muscle cells (VSMCs) and endothelial cells (Beech 2012; Berna‐Erro et al. 2012; Trebak 2012). Functionally, Orai1 activity is associated with vascular remodeling that relative to neointimal hyperplasia and angiogenesis. For example, Orai1 plays significant positive roles in migrating and proliferating behaviors of VSMCs. Inhibition of migration and proliferation has been observed after Orai1 knockdown by siRNA (Potier et al. 2009; Zhang et al. 2011).
Accumulated evidence suggests that Orai1 could interact with Ca2+‐activated potassium (KCa) channels in modulating SOCE (Clarysse et al. 2014; Lin et al. 2014). Recently, one study showed that Orai1 forms complex with SK3 in lipid rafts to control constitutive Ca2+ entry and cancer cell migration, as well as bone metastasis (Chantome et al. 2013). Furthermore, our study also demonstrated that Orai1 could form a signaling complex with SK3, modulating SOCE and its associated membrane hyperpolarization in gallbladder smooth muscle (Song et al. 2015). However, whether Orai1 interacts with BKCa in VSMCs is still unknown. In this study, we investigated possible interaction between Orai1 and BKCa in VSMCs of rat mesenteric arteries. Our results showed that Orai1 physically associates with BKCa to form a signaling complex and that Ca2+ influx through Orai1 activates BKCa to induce membrane hyperpolarization. This hyperpolarizing effect of Orai1‐BKCa coupling may contribute to prevent excessive contraction of smooth muscle in response to contractile agonists.
Materials and Methods
Materials
Phenylephrine (Phe) and iberiotoxin (IbTX) were purchased from Sigma‐Aldrich. Thapsigargin (TG) and endothelin 1 (ET‐1) were obtained from Calbiochem. Anti‐Orai1 (sc‐74778) primary antibody was purchased from Santa Cruz. Anti‐BKCa (APC‐107) primary antibody was from Alomone Lab. Orai1 specific siRNA for rat was obtained from Invitrogen. The sequence is as follows: CAACAGCAAUCCGGAGCUU (Potier et al. 2009).
Cell culture
All animal experiments were conducted in accordance with NIH publication no. 8523 and were approved by the Animal Experimentation Ethics Committee of Anhui Medical University. Primary cultured VSMCs were isolated from Sprague–Dawley rats. Briefly, mesenteric artery was dissected. After rubbing off endothelial layer, smooth muscle layer was peeled off and then digested with 0.2% collagenase type IA and 0.9% papain for 1 h. The dispersed VSMCs were cultured in Dulbecco modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin for 5 to 7 days before experimental use. Cells were grown at 37°C in a 5% CO2 humidified incubator. The first passage was used in all experiments.
Membrane potential measurement
Membrane potential was measured as previously described (Kwan et al. 2009). Briefly, primary cultured VSMCs were loaded with 100 nmol/L of potentiometric fluorescence dye bis‐oxonol [DiBAC4(3)] at 37°C for 10 min. Cells were incubated with/without IbTX (50 nmol/L) for 10 min, or with scrambled siRNA or Orai1 siRNA for 24 h. Cells were treated with 4 μmol/L TG in the dark for 8 to 15 min in a Ca2+‐free physiological saline solution (0Ca2+‐PSS), which contained (in mmol/L): 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, 0.2 EGTA, 5 Hepes, pH 7.4. SOCE was then initiated by applying 1 mmol/L extracellular Ca2+, resulting in a marked membrane hyperpolarization. Changes in fluorescence were measured by Nikon Diaphot inverted microscope.
Immunoprecipitation and immunoblots
Immunoprecipitation and immunoblots were performed as previously described (Kwan et al. 2004). In brief, smooth muscle layer was peeled off from the adventitial layer with forceps, followed by homogenization. The proteins were extracted from smooth muscle cell lysates with detergent extracted buffer, which contained 1% (vol/vol) Nonidet P‐40, 150 mmol/L NaCl, 20 mmol/L Tris‐HCl, pH 8.0, with the addition of protease inhibitor cocktail tablets. 800 μg of extracted proteins was then incubated with 3 μg of anti‐Orai1 or anti‐BKCa antibody on a rocking platform overnight at 4°C. Protein A agarose was then applied, followed by a further incubation at 4°C for 3 h. The immunoprecipitates were washed with saline for three times and then resolved on 8% SDS‐PAGE gel. The proteins were then transferred to a PVDF membrane using a semidry transfer system (Bio‐Rad). The membrane carrying the transferred proteins was incubated at 4°C overnight with the primary antibody at 1:250 dilution in PBST buffer containing 0.1% Tween 20 and 5% nonfat dry milk. Immunodetection was accomplished using horseradish peroxidase‐conjugated secondary antibody. Antibody binding was detected by the ECL system.
Mesenteric artery tension measurement
Mesenteric artery tension measurement was performed as previously reported (Kwan et al. 2009). Briefly, segments of the tertiary branches of rat mesenteric artery (2 mm long) were dissected in a Petri dish filled with ice‐cold Krebs solution (composition in mM: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4 (7 H2O), 25.2 NaHCO3, and 11.1 glucose) oxygenated with a gas mixture of 95% O2 and 5% CO2. The segments were mounted in a DMT myograph (model 610M; Danish Myo Technology, Aarhus, Denmark) under a normalized tension as previously described (Cheng et al. 2008). After the equilibration period, the contractile function of the vessel was tested by replacing the Krebs solution with 60 mmol/L K+ solution (60 mmol/L high K+ solution was prepared by substituting NaCl with an equimolar amount of KCl). After the washout, the rings were challenged with 1 μmol/L Phe to test their contractile responses and subsequently exposed to 1 μmol/L acetylcholine to verify endothelial integrity. The contractile response to Phe (10−7.5–10−5 mol/L) or ET‐1 (10‐10‐10‐8) were obtained by cumulatively adding agonists into the bath with or without the pretreatment of IbTX (50 μmol/L) for 10 min. For the Orai1 knockdown experiment, mesenteric artery was incubated with Orai1 siRNA or scrambled siRNA for 24 h before experiments.
[Ca2+]i measurement
Cytosolic Ca2+ ([Ca2+]i) was measured as previously described (Shen et al. 2011). In brief, cells were incubated with 10 μmol/L Fluo‐8/AM and 0.02% pluronic F‐127 (Invitrogen, Carlsbad, CA) for 40 min in the dark at 37°C. Ca2+ stores were depleted by treating cells with 4 μmol/L TG for 10 min in 0Ca2+‐PSS. Ca2+ influx was initiated by applying 1 mmol/L extracellular Ca2+. Cells were pretreated with scrambled siRNA or Orai1 siRNA for 24 h before experiments. Fluorescence signal was recorded by Leica TCS SP5 confocal laser system. Changes in [Ca2+]i were displayed as the ratio of fluorescence relative to the intensity before applying extracellular Ca2+ (F1/F0).
In situ proximity ligation assay (PLA)
Interaction of Orai1 with BKCa was detected by using in situ PLA kit Duolink (Sigma‐Aldrich, St. Louis, MO), following the manufacturer's instructions. Briefly, VSMCs were freshly isolated from mesenteric arteries and attached on coverslips. The cells were fixed and permeabilized. After blocked with Duolink blocking solution, VSMCs were incubated with anti‐Orai1 and anti‐BKCa (1:40, each) antibodies overnight at 4°C in Duolink antibody diluent. Negative control slides were incubated with anti‐Orai1 antibody alone. Then cells were washed with physiological saline solution and incubated with Duolink secondary antibodies conjugated with oligonucleotides (anti‐goat PLA probe Plus, Cat. DUO92003 and anti‐rabbit PLA probe Minus, Cat. DUO92005) in a preheated humidity chamber for 1 h at 37°C. Subsequently, cells were incubated with a ligation solution containing two oligonucleotides and one ligase. The oligonucleotides hybridize to the two PLA probes only if they are in close proximity (<40 nm separation), whereas the ligase joins the two hybridized oligonucleotides to form a close circle. Ligation of the oligonucleotides was followed by a rolling‐circle amplification reaction using the ligated circle as a template, resulting in a repeated sequence product. The amplification products were then detected by a fluorescence (Texas Red channel)‐labeled complementary oligonucleotide detection probes. Slides were mounted with Duolink mounting medium containing DAPI nuclear stain. PLA signals (red fluorescent dots) were visualized and imaged using a Leica TCS SP5 confocal microscope.
Statistical analysis
Collected data were presented as means ± SE. The significance was analyzed using two‐tailed Student's t test or two‐way analysis of variance (ANOVA) followed by the Bonferroni post hoc test when more than two treatments were compared. A value of P < 0.05 was considered statistically significant.
Results
The role of Orai1 in SOCE and its associated membrane hyperpolarization in VSMCs
We first investigated SOCE in the primary cultured VSMCs of rat mesenteric arteries. Preincubation of VSMCs with 4 μmol/L TG for 8–10 min in 0Ca2+‐PSS resulted in a rise in [Ca2+]i, which indicated the Ca2+ release from intracellular Ca2+ stores. Subsequent addition of extracellular Ca2+ (1 mmol/L) initiated SOCE (Figure 1A). The role of SOCE in regulating membrane potential was then examined with a potentiometric fluorescence dye DiBAC4(3) (Baczko et al. 2004). Stimulation of SOCE by adding 1 mmol/L extracellular Ca2+ resulted in smooth muscle cell membrane hyperpolarization, which was indicated by a significant decrease in DiBAC4(3) fluorescence (Figure 2A and 2C). Taken together, our results indicate that SOCE induces membrane hyperpolarization in VSMCs of rat mesenteric arteries.
Figure 1.
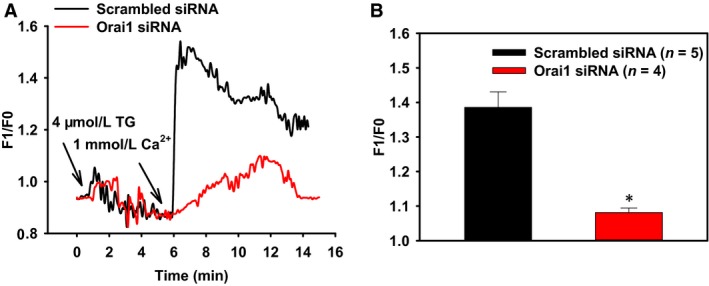
The role of Orai1 in store‐operated Ca2+ entry of rat mesenteric artery vascular smooth muscle cells. (A) Representative traces for changes in [Ca2+]i in response to thapsigargin (TG) and extracellular Ca2+ with the pretreatment of scrambled siRNA or Orai1 siRNA. (B) Summary of data showing changes in [Ca2+]i increase in response to extracellular Ca2+. Values are means ± SE (n = 4–5 samples). *P < 0.05 versus scrambled siRNA.
Figure 2.
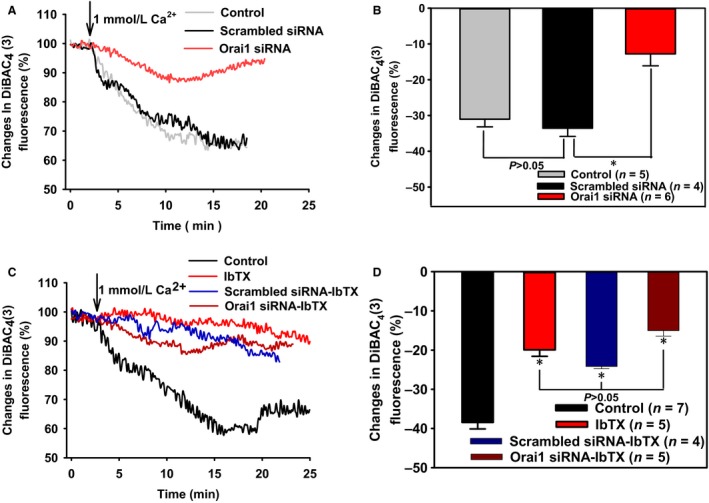
The role of Orai1 and BKC a in store‐operated Ca2+ entry‐induced membrane hyperpolarization of rat mesenteric artery vascular smooth muscle cells (VSMCs). A and C, Representative traces showing after treated with 4 μmol/L thapsigargin for 10 min in 0Ca2+‐PSS, membrane hyperpolarization was evoked by 1 mmol/L extracellular Ca2+ in VSMCs pretreated with scrambled siRNA or Orai1 siRNA, or without siRNA (control) (A), or with/without 50 nmol/L iberiotoxin (IbTX) (C). B and D, Summary of data showing changes in membrane hyperpolarization in response to extracellular Ca2+. Values are means ± SE (n = 4–7 samples). *P < 0.05 versus scrambled siRNA or Control.
To explore the functional role of Orai1 in SOCE, we employed RNA interference technique. Orai1 specific siRNA was designed and transfected into primary cultured VSMCs of rat mesenteric arteries. Immunoblotting data confirmed that Orai1 siRNA significantly suppressed Orai1 expression in VSMCs (data not shown). We subsequently used Orai1 siRNA to test the role of Orai1 in [Ca2+]i change in VSMCs. Knockdown of Orai1 markedly reduced SOCE compared with the treatment of scrambled siRNA (Figure 1). This result indicates that Orai1 plays an important role in the regulation of SOCE in VSMCs of rat mesenteric arteries.
The effect of Orai1 siRNA on SOCE‐induced membrane hyperpolarization was then examined. Incubation of VSMCs with Orai1 siRNA (1:250) for 24 h significantly inhibited this hyperpolarization compared with the treatment of scrambled siRNA (Figure 2A and 2B), suggesting the pivotal role of Orai1 in modulating SOCE‐associated membrane hyperpolarization. Additionally, scrambled siRNA transfection did not affect SOCE‐induced membrane hyperpolarization compared with control group (Figure 2A and 2B).
The role of BKCa in SOCE‐induced membrane hyperpolarization of VSMCs
Presumably, Ca2+ influx via Orai1 should depolarize plasma membrane potential instead of hyperpolarization. Therefore, we hypothesized that Ca2+ influx via Orai1 may activate BKCa, causing membrane hyperpolarization. A BKCa‐specific blocker IbTX was used to examine this hypothesis. Preincubation of IbTX at 50 nmol/L markedly reduced SOCE‐induced membrane hyperpolarization (Figure 2C and 2D). Note that in the presence of IbTX, Orai1 or scrambled siRNA had no additional effect on SOCE‐induced membrane hyperpolarization, compared with IbTX preincubation alone (Figure 2C and 2D). Taken together, there data strongly suggest that Orai1 is functionally coupled with BKCa in VSMCs.
The role of Orai1‐BKCa coupling in agonist‐induced vasocontraction in rat mesenteric arteries
We further determined the role of Orai1‐BKCa coupling in the regulation of agonist‐induced vasocontraction. Our previous study had demonstrated that α1‐adrenoceptor agonist Phe and endothelin receptor agonist ET‐1 induced smooth muscle membrane depolarization in isolated rat aortic arteries, accompanied with vasocontraction (Kwan et al. 2009). In this study, our isometric tension results showed that Phe and ET‐1 caused a dose‐dependent vasocontraction in isolated rat mesenteric arteries. Importantly, preincubation of the vessels with Orai1 siRNA (1:250, 24 h) or IbTX (50 nmol/L, 10 min) significantly increased vasocontraction in response to Phe and ET‐1 (Figure 3). These data indicate a role of Orai1‐BKCa coupling in agonist‐induced vasocontraction in rat mesenteric arteries.
Figure 3.
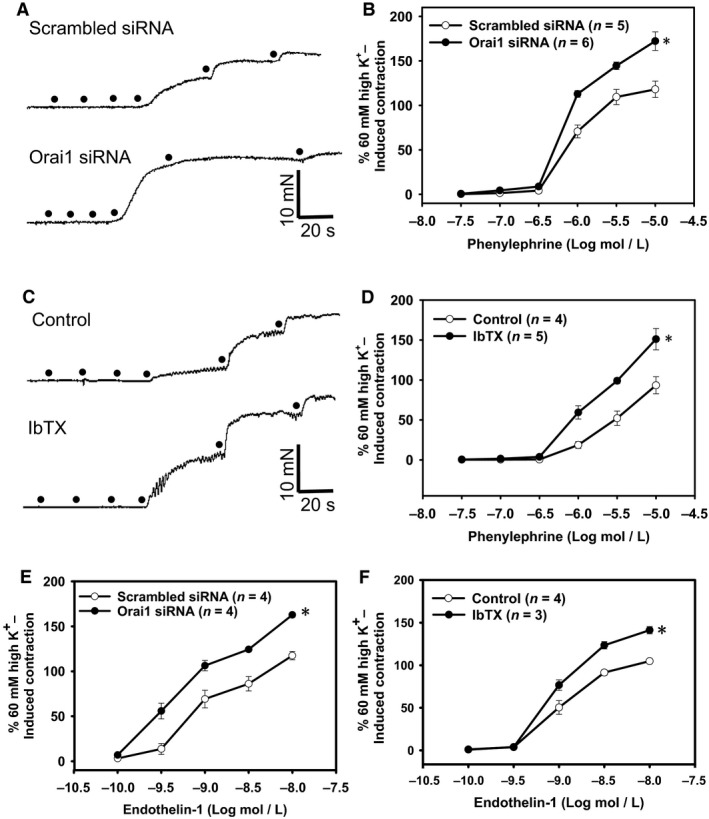
The role of Orai1 and BKC a in agonist‐induced vasocontraction in rat mesenteric arteries. A and C, Representative traces for phenylephrine (Phe)‐induced concentration‐dependent contraction in rat mesenteric arteries pretreated with scrambled siRNA or Orai1 siRNA (A), or with/without 50 nmol/L iberiotoxin (IbTX) (C). B and D–F, Summarized data showing the effects of Orai1 siRNA (B and E) and IbTX (D and F) on Phe (B and D)‐ or endothelin 1 (E and F)‐induced concentration‐dependent contraction of rat mesenteric arteries. Values are means ± SE(n = 3–7 samples). *P < 0.05 versus scrambled siRNA or Control.
Orai1 physically associates with BKCa in smooth muscle of rat mesenteric artery
The above results suggest that Orai1 and BKCa are functionally coupled. We next tested whether these two proteins are physically associated using a coimmunoprecipitation method. Two antibodies anti‐Orai1 and anti‐BKCa, which are highly specific to their targets respectively, were employed for this experiment. The immunoblot results verified that these two antibodies recognized a single band of Orai1 and BKCa, respectively (Figure 4A). Importantly, coimmunoprecipitation data showed that anti‐BKCa antibody was able to pull down Orai1 in the protein lysates freshly prepared from smooth muscle layer (Figure 4A, left panel). Moreover, anti‐Orai1 antibody was able to reciprocally pull down BKCa (Figure 4A, right panel). In control experiments (labeled as IP(‐) in Figure 4A), the pull‐down experiments were conducted using pre‐immune IgG. As expected, no bands were detected (IP(‐) in Figure 4A). Taken together, these results indicate that Orai1 is physically associated with BKCa to form a signaling complex in smooth muscle of rat mesenteric arteries.
Figure 4.
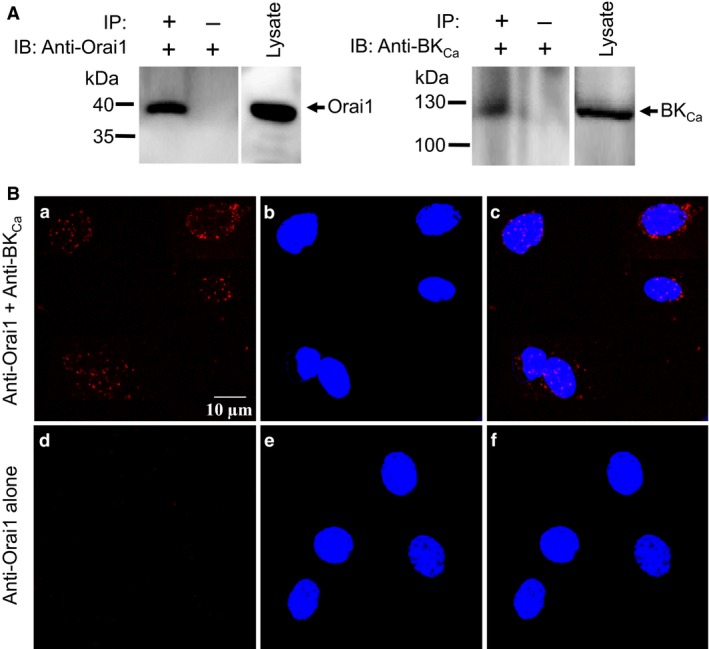
Coimmunoprecipitation and in situ proximity ligation assay of Orai1 and BKC a in fresh‐isolated rat mesenteric artery smooth muscle cells. A, Representative images showing coimmunoprecipitation followed by immunoblots [left, immunoblot with goat anti‐Orai1 antibody; right, immunoblot with rabbit anti‐BKC a antibody]. Proteins from smooth muscle layers of rat mesenteric arteries were immunoprecipitated with indicated antibody (+) or preimmune IgG (−). n = 3 experiments. B, In situ proximity ligation assay (PLA) analysis to detect the interaction between Orai1 and BKC a. PLA results were displayed in the presence of both goat anti‐Orai1 and rabbit anti‐BKC a primary antibodies (a–c), or in the presence of goat anti‐Orai1 primary antibody alone (d–f). Duolink secondary antibodies conjugated with oligonucleotides (anti‐goat PLA probe Plus and anti‐rabbit PLA probe Minus) were used to detect primary antibodies. Nuclei (blue) were marked by DAPI staining. Scale bar represents 10 μm.
To further confirm that Orai1 and BKCa indeed have physical interaction, we applied PLA analysis, which detects proteins located within a radius of <40 nm. PLA results in easily detectable fluorescent dots in the presence of both anti‐Orai1 and anti‐BKCa antibodies in fixed primary cultured VSMCs (Figure 4B: a–c). Moreover, a negative control consisting of incubation with anti‐Orai1 antibody alone displayed a negligible number of fluorescent dots (Figure 4B: d–f). These results suggest that Orai1 indeed physically interacts with BKCa in rat mesenteric artery VSMCs.
Discussion
This study demonstrated that in rat mesenteric arteries, (1) SOCE was mediated by Orai1, and Ca2+ influx via Orai1 induced membrane hyperpolarization of VSMCs; (2) this Orai1‐mediated membrane hyperpolarization was decreased by BKCa blocker; (3) inhibition of Orai1 activity by transfection with Orai1‐specific siRNA or preincubation of BKCa blocker markedly enhanced vasocontraction of rat mesenteric arteries in response to contractile agonists; (4) coimmunoprecipitation data revealed that anti‐Orai1 antibody could pull down BKCa, and anti‐BKCa antibody could inversely pull down Orai1;(5) PLA analysis showed that Orai1 and BKCa were physically interacted in VSMCs. Taken together, these results indicate that Orai1 physically interacts with BKCa to form a signaling complex in rat mesenteric artery VSMCs, and that Ca2+ influx via Orai1 activates BKCa, causing membrane hyperpolarization. Furthermore, this hyperpolarizing effect of Orai1‐BKCa coupling could contribute to suppress contractile agonist‐induced membrane depolarization, preventing excessive contraction of smooth muscle in response to contractile agonists.
Orai1 channel plays an essential role in SOCE. To elucidate the role of Orai1 in smooth muscle of rat mesenteric artery, Orai1 expression was knocked down using specific siRNA. Interestingly, Orai1 siRNA not only effectively suppressed Orai1 protein expression, but also reduced SOCE as well as membrane hyperpolarization. Presumably, suppressing Ca2+ influx through Orai1 should result in a decrease in smooth muscle contraction, instead of contraction increase. However, in isometric tension experiments, our data showed that smooth muscle contractility was significantly enhanced after Orai1 proteins were knocked down. Therefore, we hypothesized that Ca2+ influx via Orai1 may activate BKCa, leading to a decrease of smooth muscle contraction. BKCa blocker was used to test this hypothesis. Our data showed that Orai1‐mediated membrane hyperpolarization was decreased by BKCa blocker, indicating that Orai1‐BKCa coupling plays an important functional role in regulating SOCE and its associated membrane hyperpolarization in VSMCs of rat mesenteric arteries. Our coimmunoprecipitation and PLA results further demonstrated that Orai1 and BKCa indeed have physical interaction, which would allow an efficient signal transduction between Orai1 and BKCa.
Since both Orai1 and BKCa. are abundantly expressed in many types of smooth muscle cells, we hypothesized that the membrane hyperpolarization initiated by Orai1‐BKCa coupling may inactivate voltage‐gated Ca2+ channels (VGCCs), which dominantly regulate Ca2+ influx in VSMCs in response to contractile agonists, thereby contribute to reduce vascular contraction. To test this hypothesis, endogenous contractile agonists Phe and ET‐1 were used, which bind to α1‐adrenoceptor and endothelin receptor and induce Ca2+ influx via VGCCs, resulting in membrane depolarization and vascular contraction of VSMCs. Additionally, the store Ca2+ release was induced by both agonists, which could initiate SOCE. According to the Orai1‐BKCa coupling model of this study, we suggest that this SOCE may exert its effect to hyperpolarize the plasma membrane and thereby reduce agonist‐mediated membrane depolarization and vasocontraction. Our isometric tension data showed that treatment of the smooth muscle with Orai1 siRNA or BKCa blocker increased vasocontraction to Phe, indicating that Orai1‐BKCa coupling is functionally involved in agonist‐induced contraction in VSMCs of rat mesenteric arteries.
Some evidence suggests that KCa channels could interact with nonvoltage‐gated Ca2+ channels to produce a signal transduction between these proteins. TRPC1‐BKCa coupling contributes to reduce membrane depolarization in response to agonist‐induced vascular contraction, thereby preventing excessive contraction of aortic smooth muscle cells (Kwan et al. 2009). IKCa physically associates with Orai1 to mediate Ca2+ signaling, store refilling and migration in microglia (Ferreira and Schlichter 2013). Our recent study also demonstrated that Orai1 could form a signaling complex with SK3 to control SOCE and its associated membrane hyperpolarization in gallbladder smooth muscle (Song et al. 2015). In this study, we for the first time demonstrated that Orai1 physically interacts with BKCa in VSMCs of rat mesenteric arteries to regulate muscle contraction. It is possible that live cell may use different signaling complexes such as TRPC1‐BKCa and Orai1‐BKCa to response to different agonists. However, even we demonstrated that Orai1 and BKCa form a signal complex to regulate vascular tone, the role of Orai1‐BKCa coupling in human disease still remained. The functional change in Orai1‐BKCa interaction may be linked with some vascular diseases, such as hypertension, diabetes, and atherosclerosis. Therefore, the future study will benefit the understanding of pathological relevance of Orai1‐BKCa coupling.
In conclusion, we verified that Orai1 physically interacted with BKCa to form a signaling complex in VSMCs of rat mesenteric arteries. Ca2+ influx via Orai1 activates BKCa, causing membrane hyperpolarization. This hyperpolarizing effect of Orai1‐BKCa could contribute to prevent excessive contraction of smooth muscle in response to contractile agonists.
Conflict of Interest
None declared.
Acknowledgment
We thank Mr. Dake Huang in the comprehensive Laboratory of Basic Medical School Anhui Medical University for technique support in confocal microscopy.
Chen M., Li J., Jiang F., Fu J., Xia X., Du J., Hu M., Huang J., Shen B.. Orai1 forms a signal complex with BKC a channel in mesenteric artery smooth muscle cells. Physiol Rep, 4 (1), 2016, e12682, doi: 10.14814/phy2.12682
Funding Information
This work was supported by grants from the Natural Science Foundation of China (Grant No. 81570403, 81371284, 81270454); Young Prominent Investigator Supporting Program from Anhui Medical University; Outstanding Young Investigator of Anhui Medical University; and Supporting Program for Excellent Young Talents in Universities of Anhui Province. Pearl River Scholar Program in Guangdong Province.
References
- Baczko, I. , Giles W. R., and Light P. E.. 2004. Pharmacological activation of plasma‐membrane KATP channels reduces reoxygenation‐induced Ca(2 + ) overload in cardiac myocytes via modulation of the diastolic membrane potential. Br. J. Pharmacol. 141:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech, D. J. 2012. Orai1 calcium channels in the vasculature. Pflugers Archiv: European Journal of Physiology 463:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna‐Erro, A. , Woodard G. E., and Rosado J. A.. 2012. Orais and STIMs: physiological mechanisms and disease. J. Cell Mol. Med. 16:407–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, M. J. 1998. Neuronal calcium signaling. Neuron 21:13–26. [DOI] [PubMed] [Google Scholar]
- Berridge, M. J. 2013. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion 7:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman, M. D. , and Berridge M. J.. 1995. The elemental principles of calcium signaling. Cell 83:675–678. [DOI] [PubMed] [Google Scholar]
- Chantome, A. , Potier‐Cartereau M., Clarysse L., Fromont G., Marionneau‐Lambot S., Gueguinou M., et al. 2013. Pivotal role of the lipid Raft SK3‐Orai1 complex in human cancer cell migration and bone metastases. Cancer Res. 73:4852–4861. [DOI] [PubMed] [Google Scholar]
- Cheng, K. T. , Leung Y. K., Shen B., Kwok Y. C., Wong C. O., Kwan H. Y., et al. 2008. CNGA2 channels mediate adenosine‐induced Ca2 + influx in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28:913–918. [DOI] [PubMed] [Google Scholar]
- Clarysse, L. , Gueguinou M., Potier‐Cartereau M., Vandecasteele G., Bougnoux P., Chevalier S., et al. 2014. cAMP‐PKA inhibition of SK3 channel reduced both Ca2 + entry and cancer cell migration by regulation of SK3‐Orai1 complex. Pflugers Archiv: European Journal of Physiology 466:1921–1932. [DOI] [PubMed] [Google Scholar]
- Dominguez‐Rodriguez, A. , Diaz I., Rodriguez‐Moyano M., Calderon‐Sanchez E., Rosado J. A., Ordonez A., et al. 2012. Urotensin‐II signaling mechanism in rat coronary artery: role of STIM1 and Orai1‐dependent store operated calcium influx in vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 32:1325–1332. [DOI] [PubMed] [Google Scholar]
- Ferreira, R. , and Schlichter L. C.. 2013. Selective activation of KCa3.1 and CRAC channels by P2Y2 receptors promotes Ca(2 + ) signaling, store refilling and migration of rat microglial cells. PLoS ONE 8:e62345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani, A. A. , and Thorn P.. 2002. Calcium signalling: calcium goes global. Curr. Biol. 12:R432–R433. [DOI] [PubMed] [Google Scholar]
- Gwack, Y. , Srikanth S., Feske S., Cruz‐Guilloty F., Oh‐hora M., Neems D. S., et al. 2007. Biochemical and functional characterization of Orai proteins. J. Biol. Chem. 282:16232–16243. [DOI] [PubMed] [Google Scholar]
- Kwan, H. Y. , Huang Y., and Yao X.. 2004. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc. Natl Acad. Sci. USA 101:2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, H. Y. , Shen B., Ma X., Kwok Y. C., Huang Y., Man Y. B., et al. 2009. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ. Res. 104:670–678. [DOI] [PubMed] [Google Scholar]
- Li, J. , McKeown L., Ojelabi O., Stacey M., Foster R., O'Regan D., et al. 2011. Nanomolar potency and selectivity of a Ca(2)(+) release‐activated Ca(2)(+) channel inhibitor against store‐operated Ca(2)(+) entry and migration of vascular smooth muscle cells. Br. J. Pharmacol. 164:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H. , Zheng C., Li J., Yang C., and Hu L.. 2014. Ca2 + ‐activated K+ channel‐3.1 blocker TRAM‐34 alleviates murine allergic rhinitis. Int. Immunopharmacol. 23:642–648. [DOI] [PubMed] [Google Scholar]
- Lipskaia, L. , and Lompre A. M.. 2004. Alteration in temporal kinetics of Ca2 + signaling and control of growth and proliferation. Biol. Cell 96:55–68. [DOI] [PubMed] [Google Scholar]
- Potier, M. , Gonzalez J. C., Motiani R. K., Abdullaev I. F., Bisaillon J. M., Singer H. A., et al. 2009. Evidence for STIM1‐ and Orai1‐dependent store‐operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 23:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, B. , Kwan H. Y., Ma X., Wong C. O., Du J., Huang Y., et al. 2011. cAMP activates TRPC6 channels via the phosphatidylinositol 3‐kinase (PI3K)‐protein kinase B (PKB)‐mitogen‐activated protein kinase kinase (MEK)‐ERK1/2 signaling pathway. J. Biol. Chem. 286:19439–19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, K. , X. G., Zhong , Xia X. M., Huang J. H., Fan Y. F., Yuan R. X., et al. 2015. Orai1 forms a signal complex with SK3 channel in gallbladder smooth muscle. Biochem. Biophys. Res. Commun. 466:456–462. [DOI] [PubMed] [Google Scholar]
- Trebak, M. 2012. STIM/Orai signalling complexes in vascular smooth muscle. J. Physiol. 590:4201–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Gwozdz T., Dutko‐Gwozdz J., and Bolotina V. M.. 2012. Orai1 and Ca2 + ‐independent phospholipase A2 are required for store‐operated Icat‐SOC current, Ca2 + entry, and proliferation of primary vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 302:C748–C756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Halligan K. E., Zhang X., Bisaillon J. M., Gonzalez‐Cobos J. C., Motiani R. K., et al. 2011. Orai1‐mediated I (CRAC) is essential for neointima formation after vascular injury. Circ. Res. 109:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]


