Abstract
We studied the therapeutic potential of favipiravir (T-705) for Lassa fever, both alone and in combination with ribavirin. Favipiravir suppressed Lassa virus replication in cell culture by 5 log10 units. In a novel lethal mouse model, it lowered the viremia level and the virus load in organs and normalized levels of cell-damage markers. Treatment with 300 mg/kg per day, commenced 4 days after infection, when the viremia level had reached 4 log10 virus particles/mL, rescued 100% of Lassa virus–infected mice. We found a synergistic interaction between favipiravir and ribavirin in vitro and an increased survival rate and extended survival time when combining suboptimal doses in vivo.
Keywords: Lassa fever, favipiravir, ribavirin, mouse model, drug interaction
Lassa fever (LF) is a viral hemorrhagic fever caused by Lassa virus (LASV), an arenavirus endemic in West Africa. Standard of care for LF is the nucleoside analogue ribavirin [1]. However, because of the small therapeutic window [1] and the fact that still one third of patients with LF die despite ribavirin treatment [2], there is a need for more-effective drug therapies against LF. A promising candidate is the broad-spectrum antiviral drug favipiravir (Toyama Chemical) [3], which is approved for emerging influenza virus in Japan. Besides influenza virus, favipiravir shows potent antiviral activity in vitro and in vivo against a wide range of negative-strand RNA viruses, including arenaviruses [3, 4]. We have tested the efficacy of favipiravir alone and in combination with ribavirin in a novel, chimeric mouse model, which is based on lethally irradiated type I interferon receptor knockout (Ifnar–/–) mice transplanted with wild-type bone marrow progenitor cells (termed “Ifnar–/–B6 mice”). Ifnar–/–B6 mice feature a fully functional immune system and are susceptible to wild-type LASV infection (Oestereich et al, unpublished data).
METHODS
Antiviral and Toxicity Testing In Vitro
Vero E6 cells (4 × 104 cells per well in a 24-well plate) were inoculated with LASV Ba366 at a multiplicity of infection of 0.01, and compound was added 1 hour after infection. Concentration in cell culture supernatant of infectious virus particles was measured 3 days after infection, using an immunofocus assay. Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazoliumbromide (MTT) method. The concentrations that reduced the virus titer by 50% (IC50), 90% (IC90), and 99% were calculated from dose-response curves, using Prism GraphPad 6.0 (GraphPad Software). Drug interaction was tested in a 8 × 8 matrix; both favipiravir and ribavirin were tested at concentration of 0, IC90/4, IC90/2, IC90/1.5, IC90, IC90 × 1.5, IC90 × 2, and IC90 × 4 in all possible combinations. The data were analyzed according to the Bliss independence model [5]. Effects of compounds on the LASV replication complex were tested in the context of the cellular RNA polymerase I–based LASV replicon system.
Antiviral Testing In Vivo
Chimeric Ifnar–/–B6 C57BL/6 mice and Ifnar–/– A129 mice were infected intraperitoneally with 1000 focus-forming units of LASV Ba366 and monitored daily for signs of disease, body weight, and body temperature. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in blood were analyzed using a colorimetric assay (Reflotron, Roche Diagnostics). Organs were collected from randomly chosen mice 7 or 8 days after infection and from mice that died from the infection. Infectious virus particles in blood and organs were determined by immunofocus assay. Organs were fixed in 4% formaldehyde–phosphate-buffered saline (PBS), embedded in paraffin, and stained with hematoxylin-eosin. Ribavirin in 0.9% NaCl was administered intraperitoneally; 0.9% NaCl served as placebo. Favipiravir was suspended in methylcellulose-PBS and was administered twice daily per os, using a stomach probe; 0.5% methylcellulose-PBS served as placebo.
See Supplementary Methods for an extended description of methods.
RESULTS
Favipiravir and Ribavirin Show Comparable Antiviral Activity Against LASV In Vitro
Favipiravir suppressed LASV replication by ≥5 log10 units with IC50 of 29 µM and IC90 of 43 µM (Supplementary Figure 1). Ribavirin displayed comparable inhibitory activity with IC50 of 26 µM and IC90 of 33 µM. Neither drug significantly affected cell viability. The antiviral activity of favipiravir was confirmed in the LASV replicon system; the IC50 was approximately 15 µM.
Favipiravir Increases Survival Rate Among LASV-Infected Mice
All LASV-infected and placebo-treated Ifnar–/–B6 chimeras died within 10 days after infection (Figure 1 and Supplementary Figure 2A). They showed high levels of virus in blood and organs, rapidly lost weight, and displayed features of severe human LF, such as elevated AST and ALT levels, with AST levels increasing by a greater percentage than ALT levels, and hypothermia [2, 6]. To test the therapeutic efficacy of favipiravir, treatment was commenced at day 4 after infection, when viremia had reached a level of about 104 infectious virus particles/mL. Oral doses of 75 mg/kg per day and 150 mg/kg per day did not prevent or significantly delay death (Figure 1). However, treatment suppressed the viremia level by 2 log units between days 4 and 8, and it reduced virus levels in organs around the time of death. In addition, AST and ALT levels were significantly lower and terminal hypothermia was absent. Increasing the favipiravir dose to 300 mg/kg per day from days 4 to 11 after infection rescued 100% of LASV-infected mice (Figure 1). Viremia decreased within the first 3 days of treatment to levels below the limit of detection of the assay, and after 4 days of treatment the virus levels in organs were up to 6 log units lower than those in organs from placebo-treated animals. Decrease in body weight stopped after 4 days of treatment, hyperthermia was absent, and transaminase levels were hardly elevated or returned to normal during treatment. However, histopathological analysis revealed that widespread liver tissue damage remained even after 4 days of treatment with the high dose of favipiravir (Supplementary Figure 3A).
Figure 1.
Treatment of Lassa virus (LASV)–infected chimeric Ifnar–/–B6 mice with different doses of favipiravir. Chimeric Ifnar–/–B6 mice were inoculated intraperitoneally with 1000 focus-forming units (FFU) of LASV. Favipiravir was administered twice daily per os, using a stomach probe. Treatment was commenced 4 days after infection and continued until death or day 11. Organs were collected from 2 randomly chosen mice per group at day 7 after infection and from mice that died from infection between days 7 and 9 after infection and analyzed for infectious virus titers. The duration of treatment in the survival plots, the range of the viremia level below the detection limit of the immunofocus assay, and the normal reference range of the aspartate aminotransferase level (AST) in mice are shaded in gray. The corresponding values for weight, body temperature, and alanine aminotransferase level of the animals are shown in Supplementary Figure 2A. Mean values and standard deviations are shown. Favipiravir treatment groups were compared with the placebo group after 3 days of treatment (day 7 after infection), using statistical tests as indicated in the Supplementary Methods. *P ≤ .05, **P ≤ .01, and ***P ≤ .001. Abbreviation: NS, not significant.
To confirm the antiviral activity of favipiravir in a nontransplant model, we performed the same set of experiments in Ifnar–/– mice on the A129 genetic background (Supplementary Figure 4). These mice represent a nonlethal model for LASV infection [7]. As in the chimeric mice, favipiravir showed a dose-dependent beneficial effect on body weight, transaminase levels, viremia, and virus load in organs.
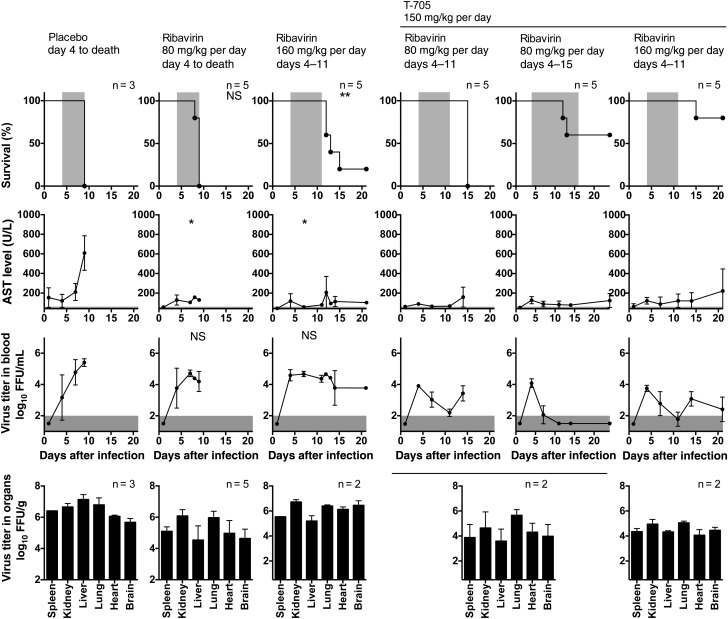
Moderate Effect of Ribavirin In Vivo
Ribavirin was tested at a dose of 80 mg/kg per day, with commencement of treatment at day 0 (Supplementary Figure 5A) and day 4 (Figure 2 and Supplementary Figure 2B) after infection. None of the treatment regimens increased the survival rate, compared with the rates in the respective placebo groups, and only treatment starting at day 0 after infection prolonged the survival duration. No significant effect was seen on viremia, although the virus levels in organs were somewhat decreased. However, both treatment regimens prevented increases in AST and ALT levels. Treatment from days 4 to 11 with a high dose of 160 mg/kg per day significantly prolonged the survival duration, and 1 of 5 animals (20%) survived (Figure 2). No reduction in levels of viremia or virus in organs was seen, while transaminase levels were suppressed as in the low-dose treatment groups. At day 8 after infection, widespread liver tissue damage was present in mice treated with ribavirin, although this finding seemed less pronounced than that for animals treated with placebo or favipiravir, suggesting a cell-protective effect of ribavirin (Supplementary Figure 3B).
Figure 2.
Treatment of Lassa virus (LASV)–infected chimeric Ifnar–/–B6 mice with different doses of ribavirin or a combination of favipiravir and ribavirin. Chimeric Ifnar–/–B6 mice were inoculated intraperitoneally with 1000 focus-forming units (FFU) of LASV. Ribavirin was administered once daily (80 mg/kg per day) or twice daily (160 mg/kg per day) by the intraperitoneal route, and favipiravir was administered twice daily per os, using a stomach probe. Treatment was commenced 4 days after infection and continued until death, day 11, or day 15. Organs were collected from 2 randomly chosen mice per group at day 7 after infection and from mice that died from infection between days 7 and 9 after infection and were analyzed for infectious virus titers. The duration of treatment in the survival plots, the range of the viremia level below the detection limit of the immunofocus assay, and the normal reference range of the aspartate aminotransferase level (AST) in mice are shaded in gray. The corresponding values for weight, body temperature, and alanine aminotransferase level among the animals are shown in Supplementary Figure 2B. Mean values and standard deviations are shown. Ribavirin treatment groups were compared with the placebo group after 3 days of treatment (day 7 after infection), using statistical tests as indicated in Supplementary Methods. Favipiravir-ribavirin combination treatment groups were compared with the 2 respective single-drug groups after 3 days of treatment (day 7 after infection). The significance levels are shown in Supplementary Table 1. *P ≤ .05 and **P ≤ .01. Abbreviation: NS, not significant.
In analogy to the favipiravir experiments, we tested ribavirin in Ifnar–/– A129 mice (80 mg/kg per day during days 0–7 after infection; Supplementary Figure 5B). Consistent with the findings in the chimeric mice, the drug reduced signs of LASV-associated disease such as weight loss and elevated aminotransferase levels, while it failed to influence the levels of viremia and virus in organs.
Beneficial Effect of Combination Treatment of Favipiravir With Ribavirin In Vitro and In Vivo
As ribavirin is the standard of care for LF, clinical trails may consider a combination of both drugs. Therefore, we first tested the antiviral activity of 64 combinations of ribavirin and favipiravir in cell culture (Supplementary Figure 6). The 8 × 8 concentration matrix was designed around the IC90 values of both drugs. The dose-response surface demonstrated that all combinations of ribavirin and favipiravir exhibited strong antiviral effects, with suppression of virus replication by ≥5 log units. Possible antagonistic or synergistic effects were evaluated according to the Bliss independence model [5]. This analysis revealed clear synergistic effects when the drugs were combined in concentrations of around IC90/2 to IC90. In this area of the matrix, the experimental virus titer was 1–2 log units lower than the titer predicted by the Bliss independence model for additive effect. The MTT test did not reveal drug toxicity over the whole matrix.
To explore whether combination of 2 subeffective doses may result in effective treatment in the lethal mouse model, we administered a ribavirin dose of 80 mg/kg per day plus a favipiravir dose of 150 mg/kg per day from days 4 to 11 after infection (Figure 2 and Supplementary Figure 2B). While none of the single-dose treatments improved the survival rate or prolonged the survival duration (Figure 1 and Figure 2), the combination treatment prolonged the survival duration by 6 days, compared with both single-drug treatments (P < .01; see Supplementary Table 1 for all statistical comparisons of combination vs single-drug treatments). The combination treatment led to a decline in viremia level until end of the treatment at day 11. However, a rebound in viremia level occurred following cessation of therapy, which may have caused the death of the animals around day 15 after infection Therefore, the combination treatment was extended until day 15 after infection (Figure 2), which resulted in survival of 3 of 5 mice (60%), with surviving mice effectively clearing virus from blood and regaining weight. Alternatively, the ribavirin dose was doubled to 160 mg/kg per day (for the single-drug experiment, see Figure 2) and administered in combination with a favipiravir dose of 150 mg/kg per day from days 4 to 11 after infection (Figure 2). This treatment led to survival of 4 of 5 mice (80%) during the observation period. However, a rebound in viremia level was not prevented. Surviving animals did not completely clear the virus from blood and still showed weight loss with biochemical evidence of disease at day 21 after infection. Increased AST and ALT levels, as seen in placebo controls, were effectively suppressed by all combination treatments. Histological analysis at day 8 after infection supported again a cell-protective effect of ribavirin (Supplementary Figure 3C). Liver tissue damage was still abundant but was slightly ameliorated in mice that received combined treatment, compared with those treated with favipiravir alone.
DISCUSSION
Favipiravir strongly suppressed LASV replication in vitro and in vivo, consistent with its potent antiviral effect against other negative-strand RNA viruses [3]. Even if treatment was commenced 4 days after infection, the drug led to a rapid decline in viremia level, ameliorated disease, and increased survival rate. The replicon data indicate that the drug is targeting the LASV polymerase complex, consistent with previous findings for influenza virus and lymphocytic choriomeningitis virus [4, 8]. We found a synergistic interaction between favipiravir and ribavirin in vitro and increased protection from mortality and extended survival durations when combining suboptimal doses as compared to treatment with each drug alone.
Despite the marginal antiviral effect of ribavirin in the mouse model, which may not be explained by insufficient metabolization of the drug in mouse cells [9], we observed a clear cell-protective effect. This resembles observations in influenza virus–infected mice [10]. Here, ribavirin improved survival rate and lung pathology, while it hardly reduced the virus titer in lungs. These findings led the authors to conclude that “it seems unlikely that the activity of ribavirin (in vivo) is based primarily upon its antiviral properties” [10, p. 1070]. Several hypotheses have been put forward to explain in vivo effects of ribavirin, including inhibition of induction of proinflammatory cytokines in virus-infected macrophages [11] and induction of interferon-stimulated genes [12].
An important finding of our study is the synergistic interaction of favipiravir and ribavirin in vitro and in vivo. Various scenarios may explain the synergism. First, depletion of the intracellular GTP pool by ribavirin [9] may facilitate the incorporation of favipiravir into viral RNA, as the favipiravir-nucleotide complex competes against GTP and ATP during RNA synthesis and exogenous elevation of the GTP and ATP levels reverses the inhibitory effect of favipiravir [4, 8, 13]. Second, it may be that both ribavirin and favipiravir are incorporated into the viral RNA and hamper progeny RNA synthesis and/or cause lethal mutagenesis in a synergistic manner [14, 15]. Third, the synergistic effect in vivo may also stem from combination of direct suppression of virus replication by favipiravir with modulation of pathophysiological or immunological host pathways by ribavirin [11, 12].
In conclusion, our data hold promise that favipiravir is of value in the treatment of LF and provide a rationale for clinical trials evaluating favipiravir in combination with ribavirin.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the animal caretakers at Heinrich Pette Institute and Bernhard Nocht Institute for their excellent technical assistance.
L. O., C. M.-F., and S. G. conceived and designed the experiments. L. O., T. R., A. L., P. R., S. W., E. P., S. B., S. K., and C. M.-F. performed experiments. L. O., C. M.-F., and S. G. analyzed the results and wrote the manuscript. All authors reviewed the manuscript.
Financial support. This work was supported by the Leibniz Center of Infection (grant LCI/HPI/2012/2) and the European Commission (European Union Seventh Framework Programme grant agreement 228292 “European Virus Archive”).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McCormick JB, King IJ, Webb PA et al. Lassa fever. Effective therapy with ribavirin. N Engl J Med 1986; 314:20–6. [DOI] [PubMed] [Google Scholar]
- 2.Asogun DA, Adomeh DI, Ehimuan J et al. Molecular diagnostics for Lassa fever at Irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoS Negl Trop Dis 2012; 6:e1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013; 100:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendenhall M, Russell A, Juelich T et al. T-705 (favipiravir) inhibition of arenavirus replication in cell culture. Antimicrob Agents Chemother 2011; 55:782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 1995; 47:331–85. [PubMed] [Google Scholar]
- 6.McCormick JB, Walker DH, King IJ et al. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am J Trop Med Hyg 1986; 35:401–7. [DOI] [PubMed] [Google Scholar]
- 7.Rieger T, Merkler D, Gunther S. Infection of type I interferon receptor-deficient mice with various old world arenaviruses: a model for studying virulence and host species barriers. PLoS One 2013; 8:e72290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuta Y, Takahashi K, Kuno-Maekawa M et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother 2005; 49:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smee DF, Bray M, Huggins JW. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir Chem Chemother 2001; 12:327–35. [DOI] [PubMed] [Google Scholar]
- 10.Berendt RF, Walker JS, Dominik JW, Stephen EL. Response of influenza virus-infected mice to selected doses of ribavirin administered intraperitoneally or by aerosol. Antimicrob Agents Chemother 1977; 11:1069–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ning Q, Brown D, Parodo J et al. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol 1998; 160:3487–93. [PubMed] [Google Scholar]
- 12.Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology 2011; 53:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS One 2013; 8:e68347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno H, Gallego I, Sevilla N, de la Torre JC, Domingo E, Martin V. Ribavirin can be mutagenic for arenaviruses. J Virol 2011; 85:7246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baranovich T, Wong SS, Armstrong J et al. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 2013; 87:3741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.