Abstract
Endemic Burkitt lymphoma is associated with Epstein-Barr virus (EBV) and Plasmodium falciparum coinfection, although how P. falciparum exposure affects the dynamics of EBV infection is unclear. We have used a modeling approach to study EBV infection kinetics in a longitudinal cohort of children living in regions of high and low malaria transmission in Kenya. Residence in an area of high malaria transmission was associated with a higher rate of EBV expansion during primary EBV infection in infants and during subsequent episodes of EBV DNA detection, as well as with longer episodes of EBV DNA detection and shorter intervals between subsequent episodes of EBV DNA detection. In addition, we found that concurrent P. falciparum parasitemia also increases the likelihood of the first and subsequent peaks of EBV in peripheral blood. This suggests that P. falciparum infection is associated with increased EBV growth and contributes to endemic Burkitt lymphoma pathogenesis.
Keywords: Epstein-Barr virus, P. falciparum malaria, Burkitt lymphoma, chronic infection, infection dynamics, co-infection
Burkitt lymphoma was first discovered in 1958 and is the most common childhood cancer in equatorial Africa [1]. Exposure to both Epstein-Barr virus (EBV) and Plasmodium falciparum malaria is a major risk factor in the development of endemic Burkitt lymphoma [2, 3]. Most African children are seropositive for EBV by 3 years of age [4, 5], with some infected before 6 months of age [6]. EBV infection persists at very low levels in adults from the United Kingdom and United States and at variable but increased levels in Kenyan and Gambian populations [7, 8]. Chronic or repeated P. falciparum coinfection results in EBV reactivation and an increased number of latently infected B cells [9, 10]. Children living in a malaria-holoendemic region have serological evidence of EBV reactivation [11], and this dysregulation of EBV persistence may contribute to the increased risk for Burkitt lymphoma [12]. Malaria transmission intensity can influence the age of primary EBV infection and contributes to a higher EBV load in infected children [5, 6].
In a recent study, we found that the age of primary EBV infection is younger for children in a region of high malaria transmission in Kenya. In addition, the presence of ongoing P. falciparum infection and younger age of infection are significant predictors for higher total EBV load within the first 24 months of life [6]. This suggests that early EBV infection may program a long-term lack of control of the virus, leading to higher viral loads. However, an earlier onset of primary EBV infection in regions of high malaria transmission may not be the sole factor driving high EBV loads and the increased incidence of Burkitt lymphoma, since it is also known that adults moving to regions of high malaria transmission are at increased risk for Burkitt lymphoma in adulthood [13]. Therefore, the presence of ongoing P. falciparum infections may also play a syndemic role in driving elevated viral loads. Understanding the mechanism by which P. falciparum exposure increases EBV load is important for further study between EBV infection, malaria, and Burkitt lymphoma.
In this study, we used a modeling approach to investigate how malaria exposure (either assessed as an ecological variable or as a biological variable) and the time of primary EBV infection determined the subsequent dynamics of EBV infection. Our results revealed that P. falciparum malaria is associated with both an increased risk of and more-rapid EBV increase in primary EBV infection and acts independent of but complementary to the age of EBV infection to increase the frequency of detection of EBV DNA following the initial resolution of primary EBV infection.
METHODS
Patients
Detailed methods associated with the cohort and analysis of viral loads from birth to 24 months have been described previously [6]. Additional data have been generated on the same cohort from 3 to 7 years of age (unpublished data). Briefly, 2 cohorts of infants enrolled within 1 month of birth were monitored in Kisumu (a region of high malaria transmission) and Nandi (a region of low malaria transmission), Kenya. Blood specimens were collected approximately monthly during the first 12 months and then every 4–10 months after that, up to 7 years of age. Quantitative polymerase chain reaction (qPCR) was used to measure EBV and P. falciparum DNA in whole-blood specimens, with a limit of detection of 2 copies/µg of human DNA [6], and antibodies to EBV viral capsid antigen and ENV nuclear antigen 1 were used in conjunction with detection of EBV load to determine age of primary EBV infection [6]. This assay detects total EBV DNA, including EBV DNA, in latently infected B cells and EBV virions and/or DNA fragments in the plasma. A recent study in children in western Kenya [14] demonstrated that EBV DNA levels in plasma and cellular fractions were significantly correlated.
This study was approved by the Kenya Medical Research Institute Ethical Review Committee (Kisumu) and the institutional review boards at SUNY Upstate Medical University (Syracuse, New York) and the University of Massachusetts Medical School (Worcester).
Survival and Statistical Analysis
To compare time to an event between groups (ie, children in regions of high and low malaria transmission), we used the log-rank test. If we observed a significant difference, then we modeled infection rates in the 2 populations, using exponential decay and a constant rate of infection. We included a delay to the exponential decay, as follows:
| (1) |
with k denoting the infection rate and Ton denoting the time delay. We used the Cox proportional hazard regression to investigate whether the time to the second episode of EBV detection was associated with age at first EBV detection, geographic location (Kisumu vs Nandi), P. falciparum parasite level, and EBV load. For this analysis, the time to the second episode was defined as the time from the last detection of EBV DNA (end of first episode) to the time of first detection of EBV DNA in the second episode. The change in parasite level with time was accounted for by using time-dependent covariates. To test the validity of the proportional hazard assumption, we did a formal test, using the cox.zph function in R, version 3.0.2, from the library survival. We used the Mann–Whitney U test to compare a single continuous variable between groups. To determine the effect of the time to first EBV detection (either by DNA testing or serologic analysis) on the rate of amplification of the EBV load (referred to as the “viral growth rate”), we used multivariable linear regression. Poisson regression was used to determine the relationship between the number of viral reactivation events, the age of EBV detection, and malaria exposure (as an ecological variable representing geographical region). The analysis was performed in R, version 3.0.2. To quantify the viral growth rate, we used a method for evaluating viral growth from regularly sampled data, in which only the first viral load measurement is required (Supplementary Text) [15].
RESULTS
Comparing Rates of Seroconversion and Viral Load Detection in Infants Living in Areas With High Versus Low Malaria Transmission
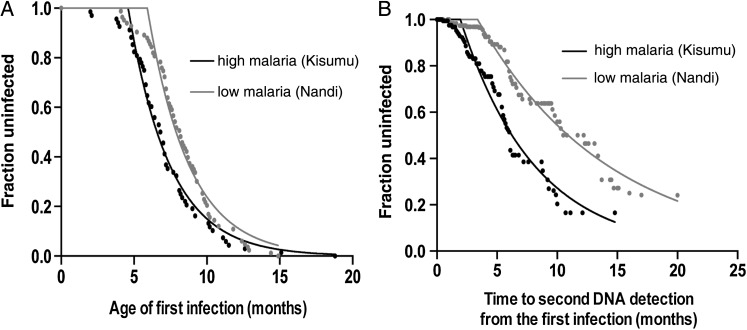
The time of initial infection with EBV was measured as the time from birth to the time of any evidence of EBV infection (seroconversion or EBV DNA detection). A recent study on the same cohort demonstrated evidence of an earlier age of EBV infection in the cohort from the area of high malaria transmission [6]. However, it is not clear whether the actual force of infection (ie, the risk of infection per day) was higher in the region of high malaria transmission or whether the population became susceptible or exposed to malaria earlier (but experienced the same force of infection once susceptible). We fitted an exponential survival curve with delay and used an F test to determine whether the main difference between groups was due to a longer delay to exposure or to a different force of infection (see Equation 1 in “Methods” section). The delay until infection commenced was significantly different between the groups (Ton, 4.6 months in the high-malaria-transmission group and 5.9 months in the low-malaria-transmission group; P < .001). However, there was no difference between the force of infection between groups (shared k, 0.35 per month, giving a median time to infection of approximately 2 months in both groups; P = .13). A Weibull with delay distribution model of survival was also tested against our data but did not fit significantly better (P = .71 for the high-malaria-transmission group and P = .12 for the low-malaria-transmission group)
EBV Load During Primary Infection in Infants
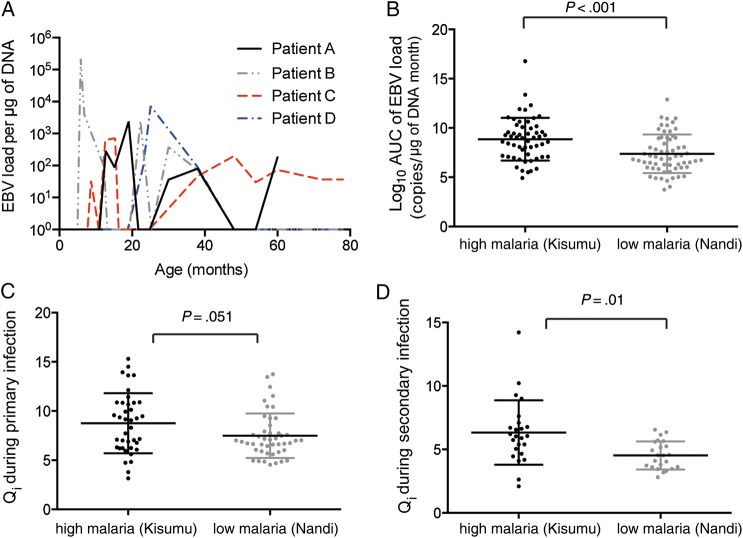
It has been reported previously that children in malaria-endemic regions show a higher median EBV load, either when sampled cross-sectionally [5] or when the total EBV load over time (ie, the area under the curve [AUC] of viral load vs time) is calculated [6]. However, this could occur either because of higher EBV levels during each episode of EBV DNA detection, longer duration of episodes, or more-frequent episodes of DNA detection. To dissect these possibilities, we aimed to compare viral dynamics in the first EBV episode (defined as the time of first detection of EBV DNA until the time the EBV load becomes undetectable; Figure 2A). First, we compared the total AUC of the EBV load during the first episode of EBV DNA detection between the 2 groups. We found a significantly higher total EBV load in children from the region of high malaria transmission during the first episode of infection (median AUC, 8.98 vs 6.97 log10 EBV copies per µg of DNA per month; P < .001; Figure 2B).
Figure 2.
Kinetics of the primary Epstein-Barr virus (EBV) episode. A, Example of the time course of EBV DNA detection in 4 subjects. B, Subjects from the area of high malaria transmission region (Kisumu, Kenya) show a significantly higher total of viral load during the first infection, compared with those from the area of low malaria transmission (Nandi, Kenya). C, Higher EBV growth rate is seen in children from Kisumu during primary EBV DNA detection. D, Higher EBV expansion rate could also be seen during secondary EBV DNA detection in children from Kisumu. Qi, a measure of viral expansion, corrected for testing gap.
The higher total EBV load during the first episode could have arisen either from a longer persistence of the first episode or from a higher initial growth rate of infection. The growth rate is typically measured between 2 closely spaced viral load measurements. However, in our data, even though samples were fairly regular, we often failed to sample 2 successive EBV measurements that were continuously rising (ie, 2 measurements in the expansion phase of infection). We have recently developed and validated a method of estimating viral growth in regularly sampled data, using the last negative reading and the first positive reading [15]. This approach incorporates the duration of the testing gap (ie, the time between the last negative and first positive readings), as well as the EBV load at detection (Supplementary Text). To obtain an accurate estimate, we used a narrow testing gap because longer testing gaps will tend to underestimate the growth rate (Supplementary Text).
Using this approach, we found that the Qi (a measure of viral expansion, corrected for testing gap) was higher in residents of the region of high malaria transmission (P = .051; Figure 2C). The doubling time of EBV load in the 2 populations can also be estimated directly from the Qi information [15]. We found that the doubling time of the EBV DNA level is around 1.6 days for children in the region of high malaria transmission and around 2.1 days for children in the region of low malaria transmission. Our calculation of the EBV doubling time is comparable to the previous estimation of the EBV doubling time of 2.7 days during EBV reactivation after hematopoietic stem cell transplantation [16]. When we analyzed the duration of EBV DNA detection, we found prolonged DNA detection in the region of high malaria transmission (median, 6.3 months vs 4.9 months; P = .042). Thus, the higher AUC of the EBV DNA load stemmed from both a higher EBV growth rate and prolonged detection of EBV DNA.
Malaria Exposure but Not Age of Seroconversion Predicts EBV Dynamics in Primary Infection
The higher rate of expansion of EBV and the higher AUC of the EBV load under conditions of high malaria exposure could arise either because of the earlier time of EBV exposure in this population or simply because higher rates of P. falciparum infection drive higher EBV expansion at the time of primary EBV infection that is independent of age. To investigate the role of age at first EBV detection and malaria exposure on viral kinetics, we used multivariable linear regression to study the EBV expansion rate and the AUC of the EBV load. The results suggest that malaria exposure is a strong determinant of the early EBV expansion rate (P = .05; Table 1) and also a major predictor of the total EBV load in the first episode (defined as the AUC of the EBV DNA load; P = .001; Table 2). However, the age at first EBV detection was not a significant predictor of either higher EBV expansion rate or AUC in the first EBV episode. The finding that the age at first EBV detection does not affect the dynamics of primary infection seems at first sight to contradict earlier findings of an association between younger age of infection and a higher total viral load over time [6]. However, an important difference in the 2 analyses is that, in our previous study, we considered the total AUC of the EBV DNA load from birth up to 24 months of age, which was associated with the age at first EBV detection. Here, we found that the total AUC of the EBV DNA load in the first episode was not associated with the age at first detection. Thus, it seems likely that if it is not the EBV levels in the first episode that change with age, then the age at first EBV detection might be affecting the frequency of subsequent episodes of EBV DNA detection.
Table 1.
Predictors of the Epstein-Barr Virus (EBV) DNA Expansion Rate per Month During the First Infection
| Explanatory Variable | All Testing Gaps (n = 150) |
Narrow Testing Gapa (n = 111) |
||
|---|---|---|---|---|
| Coefficient (95% CI) |
P Value | Coefficient (95% CI) |
P Value | |
| Age at first EBV detection, mo | −0.01 [−.1 to .07] | .74 | −0.16 [−.52 to .21] | .39 |
| Area of high vs low malaria transmission | 1.16 [.59 to 1.73] | <.001 | 1.43 [.72 to 2.13] | .05 |
| Testing gap, mo | −0.83 [−.1 to −.66] | <.001 | −0.03 [−.36 to .43] | .87 |
Abbreviation: CI, confidence interval.
a To reduce the effect of testing gap, we also restricted the analysis to subjects with a narrow testing gap. During the first infection, this was limited to 0.8–1.1 months.
Table 2.
Predictors of the Total Epstein-Barr Virus (EBV) Load During the First Infection
| Explanatory Variable | Coefficient (95% CI) | P Value |
|---|---|---|
| Area of high vs low malaria transmission | 1.38 (.57 to 2.19) | .001 |
| Age at first EBV detection, mo | −0.06 (−.22 to .09) | .42 |
| Testing gap, mo | 0.06 (−.18 to .31) | .61 |
The EBV load was calculated as the area under the viral load curve. The analysis was done using all patients, with no restriction on testing gap.
Abbreviation: CI, confidence interval.
Malaria Exposure Predicts a Short Time to the Second EBV Detection
Our understanding of primary EBV infection is based on studies of immunocompetent young adults with infectious mononucleosis [17]. In these studies, viral load measured over time following primary infection results in a consistent decline in EBV load to undetectable levels by around 8–15 months after infection, as assessed by PCR-based methods. However, we observed a much more dynamic pattern of EBV DNA detection in the Kenyan infant cohort, in which, after resolution of the initial EBV infection, there were subsequent episodes of DNA detection over time. Thus, having analyzed the first episode of EBV DNA detection (ie, the time from first detection of EBV DNA to the time it becomes undetectable by qPCR; eg, the period of EBV primary infection), we then wanted to understand the timing of subsequent episodes of EBV detection. We defined an episode of EBV DNA detection as any period of continuous EBV DNA detection, separated from other episodes by periods of undetectable EBV DNA. Children from the region of high malaria transmission were on average more likely to experience at least 1 episode of subsequent EBV DNA detection, compared with children from the area of low malaria transmission (84% vs 76%; P = .074, by the Fisher exact test). We analyzed the survival curve from the time of first negative EBV DNA test result after the first episode of infection to the time of the next positive EBV DNA test result. Children from the region of high malaria transmission had a significantly shorter time to the second episode of EBV DNA detection (P < .001, by the log-rank test; Figure 1B). We found that the rate of EBV DNA detection was significantly higher in the region of high malaria transmission, compared with the area of low malaria transmission (0.16 vs 0.09 detections per month; P < .001, by the F test; Figure 1B). Both rates were reduced as compared to the rate of EBV detection during primary infection (detection rate of 0.35 per month in both groups), consistent with the development of immune control.
Figure 1.
Time to Epstein-Barr virus (EBV) infection. Time to infection (first detection) for children in areas of high malaria transmission (Kisumu, Kenya) and low malaria transmission (Nandi, Kenya). Fitting of an exponential survival curve (see “Methods” section) shows different starting points but the same rate of infection in the 2 groups. B, Analysis of the time from the end of the primary episode to the start of the second episode of EBV DNA detection. Fitting of an exponential model shows a more rapid rate of onset of the second EBV episode in the cohort from Kisumu.
To investigate the factors contributing to the more rapid onset of the second episode of EBV detection in the group with high malaria exposure, we used Cox proportional hazard regression with multiple covariates. Younger age at primary EBV infection predicted an increased risk of a second episode of EBV DNA detection (hazard ratio [HR], 0.89; P = .007). Somewhat surprisingly, a higher viral load (calculated as the AUC of the EBV DNA load) during the first infection predicted a reduced risk of a second episode of EBV DNA detection (perhaps because it induced more efficient immunity; HR, 0.83; P = .048). The results are summarized in Table 3.
Table 3.
Cox Proportional Hazard Analysis of Predictors of the Time to the Second Episode of Epstein-Barr Virus (EBV) Detection
| Explanatory Variable | HR (95% CI) | P Value |
|---|---|---|
| Age at first EBV detection, mo | 0.89 (.83 to .97) | .007 |
| Total viral load (AUC at first infection), log10 copies/µg DNA mo | 0.83 (.76 to .91) | .048 |
| Area of high vs low malaria transmission | 2.26 (1.47 to 3.48) | <.001 |
There was no violation of the proportionate hazard assumption, based on the test of the proportionate hazard assumption (see “Methods” section).
Abbreviations: AUC, area under the curve; CI, confidence interval; HR, hazard ratio.
In many patients we observed a number of peaks in EBV DNA detection over time. We used Poisson regression to analyze the effects of age at first EBV infection and malaria endemicity on the number of EBV detection episodes. Our analysis revealed that younger age at initial EBV detection predicted more episodes of EBV DNA detection (P = .031) and that the number of episodes was also higher in the group with high malaria exposure (P = .03).
Analysis of EBV Growth Rate During Secondary EBV DNA Detection
We also analyzed the EBV expansion rate and the total EBV load (calculated as the AUC) during the second episode of viral detection. Since the testing gap increased with age (Supplementary Text), we used a narrow testing gap to obtain an accurate estimate. We observed a higher distribution of Qi (ie, EBV growth rate) for children from the region of high malaria transmission (P = .01; Figure 2D), with a mean doubling time of around 2.2 days, compared with 3.17 days for children in the region of low malaria transmission. For each group, this was slower than that in the primary episode (1.6 days vs 2.05 days, respectively), suggesting that both groups may have improved control of viral load during the second episode of viral detection. We also found that the region of malaria exposure is the only significant predictor for both the EBV expansion rate and the total EBV load during second episode (Supplementary Text). The duration of the second episode was also longer for children in region of high malaria transmission, compared with the region of low malaria transmission (median, 6 months vs 4.6 months; P = .009).
Effect of Concurrent P. falciparum Infection(s) on EBV Dynamics
The analysis above considers the role of malaria as an ecological variable; that is, it considers individuals living in areas of high or low malaria transmission. However, it also seems likely that the presence of P. falciparum infection at any given time may also play a role in the natural history of EBV in individuals. Therefore, we also investigated links between the level of P. falciparum parasitemia at the time of sampling (as detected by PCR) and the detection of EBV DNA.
First, we tested whether the time to first detection of EBV DNA is influenced by the level of P. falciparum parasitemia. We used Cox regression with a binary group variable (high and low malaria transmission) and P. falciparum DNA level as a time-dependent covariate. We found that the actual presence of P. falciparum at a sampling time point also increases the risk of first detection of EBV DNA at that time point (hazard ratio, 1.84; 95% confidence interval [CI], 1.4–2.3; P = .02), independent of malaria exposure region (HR, 2.11; 95% CI, 2.11–3; P < .001).
Then, we modified the Cox proportional hazard regression analysis described above by incorporating P. falciparum parasitemia level as a time-dependent covariate to determine whether P. falciparum parasitemia level also influenced the second EBV episode. First, we found evidence of the violation in the constant HR assumption (test on the proportional hazard assumption, P = .0148 for the malaria region binary variable and P = .032 for the log-transformed P. falciparum load). To deal with this, we fitted a stratified model that split the data into 2 periods—occurrence of second episode of EBV DNA detection in <10 months and ≥10 months after the primary EBV infection—so that the proportional hazards assumption was satisfied during each interval (Table 4). We found a similar trend in the analysis above for the influence of age at first EBV infection, total EBV load during the first detection episode, and malaria exposure group on the prediction of the time to the second EBV episode (Table 4). We demonstrated that concurrent P. falciparum parasitemia level is associated with a higher risk of second EBV DNA detection at any age (HR, 1.53 for the group with detection <10 months after primary infection [P = .002] and 1.24 for the group with detection ≥10 months after primary infection [P = .03]). This is consistent with the hypothesis that P. falciparum induces reactivation of latent EBV.
Table 4.
Predictors, Including Plasmodium falciparum Level, of the Time to the Second Episode of Epstein-Barr Virus (EBV) Detection
| Time, Explanatory Variable | HR (95% CI) | P Value |
|---|---|---|
| <10 mo from the end of primary EBV detection | ||
| P. falciparum level, log10 copies/mL blood | 1.53 (1.23 to 1.91) | .002 |
| Total viral load (AUC at first infection), log10 copies/µg DNA mo | 0.57 (.38 to .87) | .009 |
| Area of high vs low malaria transmission | 2.92 (1.98 to 4.3) | .014 |
| Age at first infection, mo | 0.61 (.42 to .89) | .01 |
| ≥10 mo from the end of primary EBV detection | ||
| P. falciparum level, log10 copies/mL blood | 1.24 (1.12 to 1.37) | .03 |
| Total viral load (AUC at first infection), log10 copies/µg DNA mo | 0.53 (.37 to .76) | <.001 |
| Area of high vs low malaria transmission | 1.87 (1.15 to 3.02) | .01 |
| Age at first infection, mo | 0.80 (.66 to .98) | <.001 |
Because of the violation of the proportion hazard assumption, we divided the time of risk into <10 months and ≥10 months after the end of the primary EBV infection, such that the proportion hazard assumption is upheld within each interval (based on a test of the proportion hazard assumption; see “Methods” section)
Abbreviations: AUC, area under the curve; CI, confidence interval; HR, hazard ratio.
DISCUSSION
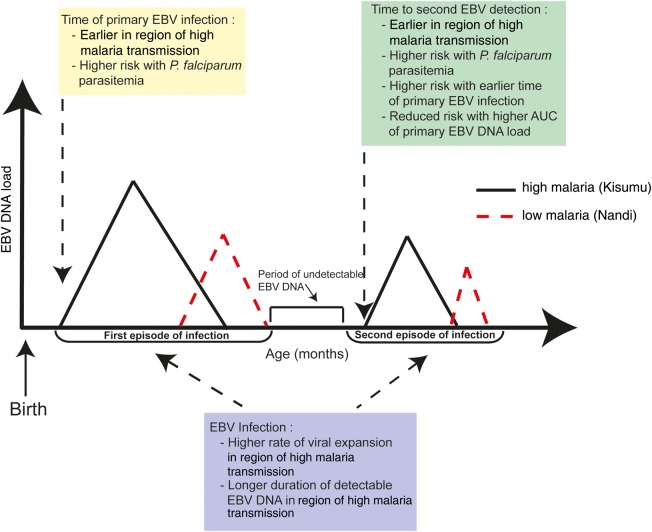
EBV and P. falciparum coinfection is a risk factor for endemic Burkitt lymphoma, although the mechanisms of this interaction are difficult to dissect. Previous work has determined that both the degree of malaria exposure [5] and the age at primary EBV infection are significant determinants of the total EBV load detected from birth up to 2 years of age [6]. We have analyzed the viral load kinetics of EBV in 2 cohorts of children with divergent levels of malaria exposure and made several novel observations. First, we found that the key determinant of the kinetics of primary EBV infection in children is the degree of malaria exposure and that this is independent of the age of primary EBV infection (Figure 3). This higher EBV expansion rate and longer duration of detectable EBV DNA combine to produce a higher total EBV load in the region of high malaria transmission. This also appears to be observed in subsequent episodes of EBV DNA detection in children residing in areas of high malaria transmission.
Figure 3.
Schematic of the role of malaria exposure and age of primary Epstein-Barr virus (EBV) infection. The analysis suggests that high malaria exposure leads to a higher rate of expansion of EBV, a higher total viral load (calculated as the area under the viral load curve [AUC]), and longer durations of detectable EBV DNA during primary infection and subsequent episodes of EBV DNA detection. The presence of Plasmodium falciparum parasitemia will increase the risk of having the first and second EBV infection. Early age at primary EBV infection is independently associated with a shorter time to the second episode of EBV DNA detection.
A second key observation involved the factors affecting the subsequent peaks of detectable EBV DNA seen in peripheral whole-blood samples in many individuals. Both earlier age at EBV infection and residence in a region of high malaria transmission decrease the time between the first peak in the EBV load and the second peak and increase the frequency of observing a second peak in the EBV load (Figure 3). The oscillations we observed in infants are different from those reported following EBV infection of young adults that resulted in acute infectious mononucleosis (AIM). In AIM, the EBV load resolves over a 1-year period and reaches a steady-state level [17], although an elevated EBV load is correlated with EBV reactivation in immunosuppressed transplant patients [18, 19]. Our studies cannot distinguish whether subsequent episodes of EBV detection represent reactivation of the primary infection or whether they are a true secondary infection (or superinfection). The fact that the rate of EBV expansion during secondary detection of EBV is slower than that during primary infection does not help us discriminate these mechanisms. Further work is clearly required to identify the molecular and cellular factors that promote the high levels of EBV in malaria-exposed individuals and those that are involved the immune responses to EBV during primary and subsequent episodes of viremia.
A final key observation was the role of concurrent P. falciparum infection, as compared to residence in a region of high malaria exposure. We observed that concurrent P. falciparum infection increased the risk for first EBV detection, as well as the risk for second EBV detection.
One model that has been proposed suggests that increased susceptibility of B cells to EBV infection may combine with increased mutation and reduced apoptosis to drive oncogenesis as a risk for Burkitt lymphoma in malaria-endemic regions [20]. If this model is correct, then malaria may have an additional contribution by expanding the pool of latently infected B cells that ultimately are susceptible to a second oncogenic hit.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgment. This manuscript was submitted with permission from the director of KEMRI.
Financial support. This work was supported by the National Institutes of Health (R01 CA102667 to R. R. and R01 CA134051 to A. M. M.) and the National Health and Medical Research Council, Australia (senior research fellowship APP1080001 to M. P. D.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Burkitt DP. The discovery of Burkitt's lymphoma. Cancer 1983; 51:1777–86. [DOI] [PubMed] [Google Scholar]
- 2.Morrow RH, Gutensohn N, Smith PG. Epstein-Barr virus-malaria interaction models for Burkitt's lymphoma: implications for preventive trials. Cancer Res 1976; 36:667–9. [PubMed] [Google Scholar]
- 3.Facer CA, Playfair JH. Malaria, Epstein-Barr virus, and the genesis of lymphomas. Adv Cancer Res 1989; 53:33–72. [DOI] [PubMed] [Google Scholar]
- 4.Biggar RJ, Henle W, Fleisher G, Bocker J, Lennette ET, Henle G. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer 1978; 22:239–43. [DOI] [PubMed] [Google Scholar]
- 5.Moormann AM, Chelimo K, Sumba OP et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis 2005; 191:1233–8. [DOI] [PubMed] [Google Scholar]
- 6.Piriou E, Asito AS, Sumba PO et al. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis 2012; 205:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorley-Lawson DA, Hawkins JB, Tracy SI, Shapiro M. The pathogenesis of Epstein-Barr virus persistent infection. Curr Opin Virol 2013; 3:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Njie R, Bell AI, Jia H et al. The effects of acute malaria on Epstein-Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J Infect Dis 2009; 199:31–8. [DOI] [PubMed] [Google Scholar]
- 9.Lam KM, Syed N, Whittle H, Crawford DH. Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet 1991; 337:876–8. [DOI] [PubMed] [Google Scholar]
- 10.Rasti N, Falk KI, Donati D et al. Circulating epstein-barr virus in children living in malaria-endemic areas. Scand J Immunol 2005; 61:461–5. [DOI] [PubMed] [Google Scholar]
- 11.Piriou E, Kimmel R, Chelimo K et al. Serological evidence for long-term Epstein-Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J Med Virol 2009; 81:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt's lymphoma: a polymicrobial disease? Nat Rev Microbiol 2005; 3:182–7. [DOI] [PubMed] [Google Scholar]
- 13.de-The G. Is Burkitt's lymphoma related to perinatal infection by Epstein-Barr virus? Lancet 1977; 1:335–8. [DOI] [PubMed] [Google Scholar]
- 14.Mulama DH, Bailey JA, Foley J et al. Sickle cell trait is not associated with endemic Burkitt lymphoma: an ethnicity and malaria endemicity-matched case-control study suggests factors controlling EBV may serve as a predictive biomarker for this pediatric cancer. Int J Cancer 2014; 134:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cromer D, Tey SK, Khanna R, Davenport MP. Estimating cytomegalovirus growth rates by using only a single point. J Virol 2013; 87:3376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bitan M, Or R, Shapira MY et al. Early-onset Guillain-Barre syndrome associated with reactivation of Epstein-Barr virus infection after nonmyeloablative stem cell transplantation. Clin Infect Dis 2004; 39:1076–8. [DOI] [PubMed] [Google Scholar]
- 17.Hadinoto V, Shapiro M, Greenough TC, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. On the dynamics of acute EBV infection and the pathogenesis of infectious mononucleosis. Blood 2008; 111:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inazawa N, Hori T, Hatakeyama N et al. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol 2015; 87:1427–35. [DOI] [PubMed] [Google Scholar]
- 19.Chiusolo P, Metafuni E, Cattani P et al. Prospective evaluation of epstein-barr virus reactivation after stem cell transplantation: association with monoclonal gammopathy. J Clin Immunol 2010; 30:894–902. [DOI] [PubMed] [Google Scholar]
- 20.Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA. A multifactorial role for P. falciparum malaria in endemic Burkitt's lymphoma pathogenesis. PLoS Pathog 2014; 10:e1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.