Abstract
Wheat is a staple food throughout the temperate world and an important source of nutrients for many millions of people. However, the last few years have seen increasing concerns about adverse effects of wheat on health, particularly in North America and Europe, with the increasing adoption of wheat‐free or gluten‐free diets. This relates to two concerns: that wheat products are disproportionally responsible for increases in obesity and type 2 diabetes and that wheat gluten proteins cause a range of adverse reactions, including allergies, coeliac disease and ‘non‐coeliac gluten sensitivity’. The first concern has been refuted in previous publications, and we therefore focus on the second here. Current evidence indicates that allergy to ingested wheat and coeliac disease (and related intolerances) each occur in up to 1% of the population. The extent to which their prevalence has increased is difficult to quantify due to improved diagnosis and increased awareness. However, neither appears to be increasing disproportionally when compared with other immunologically mediated adverse reactions to food. Other adverse reactions to wheat are more difficult to define as their mechanisms are not understood and they are therefore difficult to diagnose. In particular, ‘non‐coeliac wheat sensitivity’ has been reported to occur in 6% or more of the population in the US. However, the application of more rigorous diagnostic criteria is likely to give substantially lower estimates of prevalence. It is therefore unlikely that the health of more than a small proportion of the population will be improved by eliminating wheat or gluten from the diet. In fact, the opposite may occur as wheat is an important source of protein, B vitamins, minerals and bioactive components.
Keywords: coeliac disease, FODMAPs, food allergy, gluten, intolerance, wheat
Background
The last few years have seen increasing concerns, particularly in the media and lay press, about the effects of wheat‐based foods on health, with the increasing adoption of wheat‐free or gluten‐free diets. These concerns have largely been propagated through the media, particularly the popular press, Internet and social media, rather than conventional medical and public health channels, and the evidence base is often obscure. Nevertheless, the impact has been dramatic and of concern not only for wheat producers and the food industry but also for public health due to the impact on the intake of components, which are conventionally consumed in wheat products, such as dietary fibre, B vitamins and minerals (Steer et al. 2008).
The concerns can broadly be divided into two types: that wheat products are disproportionally responsible for increases in obesity and type 2 diabetes and that wheat gluten proteins cause a range of adverse reactions, including allergies, coeliac disease and a range of less well‐defined conditions. The role of wheat products in the increasing levels of obesity and associated conditions was promoted by the best‐selling book Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health (Davis 2011), which led to a proliferation of publications on wheat‐free diets and recipes. The scientific flaws in the arguments have been discussed in the scientific literature (Jones 2012; Brouns et al. 2013) but these scientific publications have had little or no effect on the public perception that wheat is bad for you!
We therefore focus here on the second topic, considering the evidence for increases in adverse reactions to the consumption of wheat gluten (and possibly also other wheat components). The spectrum of conditions is summarised in Figure 1, which is based on the outcome of an international panel of experts which met in 2011 (Sapone et al. 2012). However, it should be borne in mind that this is a simplification and some conditions may occur together in the same patients. Finally, it is important to note that methods of diagnosis have improved greatly over the past few decades, together with increased awareness of food‐related conditions among clinicians and consumers. Hence, it is important to consider whether these changes have affected our estimates of prevalence.
Figure 1.
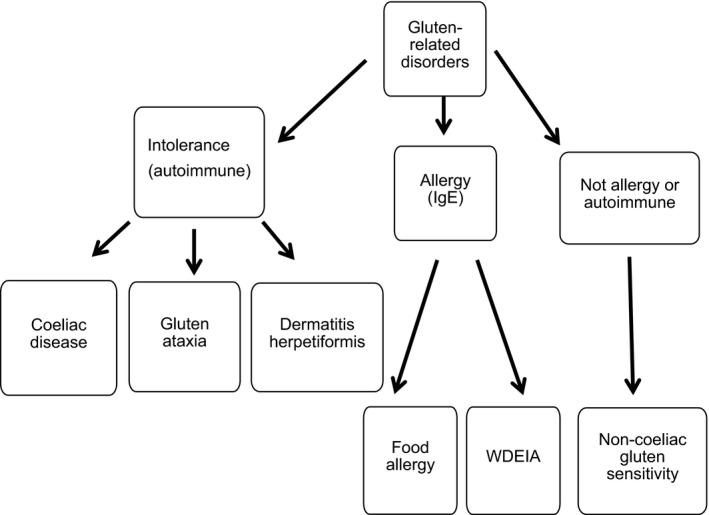
Nomenclature and classification of disorders proposed to be related to gluten in food. Modified from Sapone et al. (2012). Wheat‐dependent exercise‐induced anaphylaxis (WDEIA).
Wheat allergy
Allergies are hypersensitive responses to foreign components, most commonly proteins, and are usually associated with the production of a specific class of antibody called IgE (in contrast to the IgG antibodies which are produced in response to most invading pathogens). Symptoms of allergy to ingested wheat products include atopic dermatitis, urticaria (also called hives or nettle rash), and respiratory and gastrointestinal symptoms. A range of proteins has been implicated, notably α‐amylase inhibitors and gluten (gliadin and glutenin) proteins (reviewed by Tatham & Shewry 2008; Matsuo et al. 2015).
Zuidmeer et al. (2008) reported a detailed meta‐analysis of the prevalence of plant food allergies, which included data from 15 studies of wheat allergy. These comprised cohorts ranging between about 500 and 10 000 subjects with diagnosis by food challenge (but not always double‐blind), skin prick test and serum IgE analysis, and perception of wheat allergy measured by questionnaires. Four studies in which children (aged 3–14 years) were challenged with wheat in the diet showed a mean prevalence of 0.25% (7/2807) with a range from 0–0.5%. Two UK cohorts showed prevalences in children of 0.2% at 9–12 years and 0.3% at 6 years (Venter et al. 2006a, 2006b), but challenges of adults were not reported. Sensitisation to wheat, determined by reaction in a skin prick test, was higher in UK adolescents (15 years) (1.2%) (Pereira et al. 2005) than in UK children (4–11 years) (0.2–0.6%) (Arshad et al. 2001; Pereira et al. 2005; Venter et al. 2006a, 2006b) and a high level of sensitisation in adults (up to 3.6%) has also been reported for other countries based on the determination of IgE to wheat in the serum. It is often stated that wheat allergy is most common in infants and then disappears. This is consistent with the data on perception of wheat allergy, which tends to be higher than sensitisation or response to dietary challenge in children, but lower in adults. Of the 36 studies included in the meta‐analysis, only six used double‐blind food challenges and these showed that the prevalence of wheat allergy was within the ranges reported for allergies to other plant foods (fruit, nuts, vegetables, soya and sesame) (Zuidmeer et al. 2008).
Wheat‐dependent exercise‐induced anaphylaxis (WDEIA)
The best characterised form of wheat allergy is wheat‐dependent exercise‐induced anaphylaxis (WDEIA). This is a type of allergic response, which is triggered by the ingestion of food followed by physical exercise, with wheat and crustaceans being the commonest causes (Beaudouin et al. 2006). WDEIA has been studied in most detail by Japanese workers, who recognise two forms (Yokooji et al. 2015). Conventional (CO) WDEIA is the dominant form and is considered to be sensitised via the gastrointestinal tract, with the major allergen being ω‐5 gliadin (Palosuo et al. 2001; Morita et al. 2003). However, a second form has also been defined in Japan, which appears to be sensitised via the skin and/or mucosa by hydrolysed wheat protein (HWP) present in soap. The major sensitising agent in HWP‐WDEIA appears to be γ‐gliadin and reactions can occur after exposure to soap or consumption of wheat (Yokooji et al. 2013).
The prevalence of food‐dependent exercise‐induced anaphylaxis (including WDEIA) has been reported as 0.017% in Japanese children (Aihara et al. 2001), while screening of 935 Japanese adults for wheat allergy (also including WDEIA) using questionnaires, skin prick tests and determination of ω‐5 gliadin‐specific IgE identified only two allergic subjects (0.21%) (Morita et al. 2012).
Coeliac disease
Coeliac disease (CD) is an autoimmune condition, which affects the small intestine, resulting in malabsorption, weight loss, fatigue, abdominal pain, vomiting and diarrhoea. Consequently, patients with CD suffer from nutrient deficiencies including iron anaemia and folate deficiency. However, individuals may also be asymptomatic or present only mild symptoms.
The role of wheat gluten proteins in triggering CD is well‐established, with gliadin and glutenin proteins being the major cause (reviewed by Gilissen et al. 2014). Currently, 31 short peptide sequences in wheat gluten proteins, and related proteins in barley and rye, have been defined as being coeliac toxic: these are often referred to as coeliac ‘epitopes’. However, mapping is incomplete and the number of distinct epitopes is a matter of on‐going discussion (Sollid et al. 2012).
Although CD was historically considered as a paediatric condition, it is now recognised that it can present at any age, and large‐scale screening has revealed a substantial level of undiagnosed CD in adults. For example, a study of 7550 participants carried out in Cambridge (UK) showed that 1.2% of adults aged 45–76 years were serologically positive (West et al. 2003). Similarly, analysis of 16 847 adults aged 50 years or more in Minnesota showed 0.8% undiagnosed CD (Godfrey et al. 2010). Hence, the prevalence of CD in Europe and countries with high proportions of populations of European ancestry (e.g. the US, Australia) is now widely estimated as about 1% of the population, although substantial variation occurs between countries, from as low as 0.2% to over 5%. Within Europe, Finland has a particularly high incidence, reported as 1–2.4% (Maki et al. 2003; Godfrey et al. 2010; Mustalahti et al. 2010; Walker et al. 2010; Rubio‐Tapia et al. 2012).
There is a perception that the prevalence of CD is increasing, although this may be the result, at least in part, of increased awareness and improved diagnosis (with screening for the presence of antibodies to the enzyme tissue transglutaminase in the serum being used for initial diagnosis) (Ludvigsson et al. 2015). An increased prevalence in Sweden has been attributed to changes in infant feeding (Olsson et al. 2008; Myléus et al. 2009) while Lohi et al. (2007) reported a two‐fold increase in CD in Finnish adults between 1978–1980 and 2000–2001 (from 1.05% to 1.99%), after adjusting the data for improved diagnosis over the same period.
Green and Cellier (2007) note that adult CD is about twice as prevalent in women as in men, in common with higher prevalences of other autoimmune diseases. However, they also note that women are more likely to suffer from iron deficiency anaemia and osteoporosis, symptoms which may lead to investigations by health professionals. The prevalence in women has also been reported to decrease after about age 65 years (Green et al. 2001).
Even when the initial serological screening is confirmed by small bowel biopsy, patients may not experience changes in bodyweight or other symptoms. Nevertheless, the association of CD with increased risk of a range of other disorders (Corrao et al. 2001; Green et al. 2003; West et al. 2004; Green & Cellier 2007; Solaymani‐Dodaran et al. 2007; Godfrey et al. 2010) means that treatment is required even in the absence of symptoms.
Conditions related to coeliac disease
Coeliac disease may be associated with neurological conditions, with peripheral neuropathy and gluten ataxia (GA), in which the cerebellum is damaged, being the most common. Their prevalence has not been established but Hadjivassiliou et al. (2002) have estimated that neurological dysfunction may occur in about 6–10% of patients presenting with gastrointestinal symptoms. These authors reviewed 35 publications in which ataxia and peripheral neuropathy were each present in 29 of 83 patients. However, similar symptoms are also observed in patients defined as suffering from ‘gluten sensitivity’, in the absence of diagnosed CD (Hadjivassiliou et al. 2010). More recently, Hadjivassiliou et al. (2015) have reported that GA has a prevalence of 15% among all ataxias.
Dermatitis herpetiformis (DH) is a form of CD, which presents as a chronic skin disease. Its prevalence is much lower than typical CD, estimated at 0.001– 0.04%, and in common with CD, it is higher in populations of European descent and low in Asian and African‐American populations (Gawkrodger et al. 1984; Mobacken et al. 1984; Smith et al. 1992; Bolotin & Petronic‐Rosic 2011). In contrast to CD, the prevalence of DH is from 1.5–2 times higher in men than in women (Smith et al. 1992)
Schizophrenia and autism spectrum disorder
Associations between the consumption of wheat and milk and schizophrenia and autism spectrum disorder have been studied in some detail, with both conditions being improved in some patients by interventions with gluten‐free, casein‐free or gluten and casein‐free diets (Singh & Roy 1975; Christison & Ivany 2006; Kalaydiian et al. 2006; Whiteley et al. 2010, 2013).
It has been hypothesised that neuroactive peptides released by digestion of wheat gluten are responsible for neurological effects (Dohan 1979; Dohan et al. 1984), which has given rise to the concept of gluteomorphins. These are proposed to be opioid peptides that are released by digestion of gluten in the gastrointestinal tract and taken up into the bloodstream, resulting in neurological effects and ‘addictive’ properties. Similarly, casomorphins have been suggested to be responsible for similar symptoms associated with milk consumption. However, there is little experimental evidence for this hypothesis.
Non‐coeliac gluten sensitivity (NCGS)
In recent years, an increasing number of patients have reported symptoms related to wheat consumption, which are not classical allergic or autoimmune responses. This has led to the definition of a new condition called ‘non‐coeliac gluten sensitivity’ (NCGS) (Sapone et al. 2012). The range of symptoms varies widely, including gastrointestinal symptoms, tiredness, headache, dermatitis, pains in muscles and joints, depression, anxiety and anaemia, and it is not clear whether NCGS represents a single syndrome or a range of conditions (Sapone et al. 2012). It is therefore best defined in negative terms: as a reaction to gluten (or wheat) when both CD and allergy have been excluded (Aziz et al. 2012; Sapone et al. 2012). Furthermore, the role of gluten has not been clearly established and the symptoms could relate to other grain components. Hence, the term ‘non‐coeliac wheat sensitivity’ (NCWS) may be more appropriate (Carroccio et al. 2014; Catassi et al. 2015).
The pathogenesis of NCGS/NCWS is not understood but is likely to feature a mixture of factors including the stimulation of the innate immune system. This lack of understanding poses a challenge for diagnosis but the recent report of an expert group recommends a gluten‐free diet followed by a double‐blind, placebo‐controlled gluten challenge, with variation of 30% or more in one to three main symptoms being a positive result in both phases (Catassi et al. 2015).
The true prevalence of NCGS/NCWS will not be clear until these criteria are rigorously applied. The prevalences reported in previously published studies are therefore likely to be higher than the true values. For example, NCGS was diagnosed in 6% of 5896 patients seen at the Center for Celiac Research in Maryland, USA (Sapone et al. 2012) while Volta et al. (2014) identified 3% of 12 000 patients with suspected NCGS in a multisite study of outpatients in Italian health centres. A survey of 1002 adults in the Sheffield area of the UK identified 13% with self‐reported gluten sensitivity (GS) while a further study of 200 GS patients showed that 7% had CD and 93% NCGS (Aziz et al. 2014). The ratio of females to males in the latter two studies was about 4:1 (Aziz et al. 2014; Volta et al. 2014).
FODMAPs and gastrointestinal disorders
Wheat, in common with other plant foods, contains small fermentable carbohydrates, which have been termed FODMAPs (Fermentable, Oligo‐, Di‐, Mono‐saccharides And Polyols). The most abundant of these are fructo‐oligosaccharides (fructans) (up to about 2% dry weight), sucrose (0.5‐1.5% dry weight) and raffinose (0.2–0.7% dry weight) (reviewed by Shewry & Hey 2015). A low FODMAP diet may improve the management of irritable bowel disease (IBS) and inflammatory bowel disease (Crohn's disease and ulcerative colitis), by reducing fermentation in the colon (Gibson & Shepherd 2010; Staudacher et al. 2011; Muir & Gibson 2013). Furthermore, a tightly controlled intervention trial with self‐reported NCGS patients showed a significant improvement of symptoms during a low FODMAP run‐in period, but no effect of gluten challenge (Biesiekierski et al. 2013). The low contents of FODMAPs in gluten‐free products may therefore account for improvements experienced by IBS and NCGS patients on gluten‐free diets (Muir & Gibson 2013; Biesiekierski & Iven 2015).
Wider impact of gluten‐free diets on nutrition and health
Concern among the general public about the impact of wheat on health is increasing dramatically, particularly in the US where a third of adults have stated their wish to cut down or eliminate gluten consumption. This has created a valuable market for gluten‐free foods, estimated as worth $1.77 billion in the US ($3.42 billion globally) in 2013 and forecast to increase to almost $24 billion in the US by 2020 (Statista 2015). The numbers who are consuming gluten‐free diets now greatly exceed even the highest estimates for the prevalence of gluten‐related effects on health, which raises the question of how the changes in diet are affecting the dietary intake of nutrients and bioactive compounds. In the heat of the debate about the adverse effects of gluten, it is often forgotten that wheat, and other cereals, make a much broader contribution to diets. For example, the UK National Diet and Nutrition Survey (NDNS) showed that bread alone contributes 11% of the daily intake of protein, 18–21% of dietary fibre (non‐starch polysaccharides), 15–16% of thiamine (vitamin B1), 10–11% of niacin (vitamin B3), 12% of folates (vitamin B9), 15–16% of iron, 15–19% of calcium, and substantial proportions of a number of other essential micronutrients to the diets of UK adults (Bates et al. 2014). In addition, wheat (particularly wheat bran) is rich in a range of phytochemicals, including phenolic acids and betaine, which may have health benefits (reviewed by Shewry & Hey 2015). Several studies have shown that gluten‐free foods may be depleted in protein and micronutrients compared to conventional diets (Thompson 1999, 2000; Kinsey et al. 2008; Pellegrini & Agostini 2015; Wu et al. 2015) and food scientists have identified the challenge of improving the nutritional quality and health benefits of gluten‐free breads (Capriles et al. 2016).
Conclusions
Whereas adverse reactions to wheat could be considered to be well understood only a decade ago, the landscape has since become immensely more complicated. Wheat allergy remains the best understood condition, and the most readily diagnosed. The prevalence appears to be below 1% with WDEIA (which can result in anaphylaxis) being much rarer. There is no evidence that the prevalence is increasing disproportionally compared with other food allergies or that the prevalence is related to the types of wheat or wheat products that are consumed. The current prevalence of CD in the UK is also probably about 1%, but it is not clear whether the increases that have been observed in many countries reflect true increases in prevalence or result from greater awareness and improved diagnosis.
Other conditions related to wheat gluten, or other components of the wheat grain, are less well understood and diagnosis is problematic. However, there is no doubt that the prevalence is much lower than the proportions of consumers in North America and Western Europe who prefer gluten‐free diets, or the numbers who self‐report for NCGS, perhaps of the same order as allergies and CD. The agreement of diagnostic criteria for NCGS is therefore an important step for determining true prevalence while controlled interventions are also needed to identify whether wheat gluten, FODMAPs or other grain components are responsible.
It is therefore an over‐reaction to assume that the health of more than a small proportion of the population will be improved by eliminating wheat or gluten from the diet. In fact, the opposite may occur as wheat is an important source of protein, B vitamins, minerals and bioactive components.
Finally, it is important to note that wheat is the major staple food in much of the temperate world, including developing countries in North Africa and West and Central Asia, where it may contribute between 50–70% of total food intake, and parts of China and India. It is also contributing increasingly to the diet in Sub‐Saharan Africa. Although data are limited, there is little evidence that these countries are experiencing increases in adverse reactions to wheat consumption, and decreased wheat production due to concerns emanating from prosperous western countries would have a disastrous impact on food security.
Conflict of interest
The authors have no conflict of interest to disclose.
Acknowledgements
We are grateful to CGIAR and the CGIAR WHEAT programme for financial support to prepare a review (Proiect No. A403 1.09.47) on which this article is based. Rothamsted Research receives strategic funding from the Biotechnological and Biological Sciences Research Council (BBSRC) of the UK.
References
- Aihara Y, Takahashi Y, Kotoyori T et al (2001) Frequency of food‐dependent, exercise‐induced anaphylaxis in Japanese junior‐high‐school students. Journal of Allergy and Clinical Immunology 108: 1035–9. [DOI] [PubMed] [Google Scholar]
- Arshad SH, Tariq SM, Matthews S et al (2001) Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics 108: 33–40. [DOI] [PubMed] [Google Scholar]
- Aziz I, Hadjivassiliou M & Sanders DS (2012) Does gluten sensitivity in the absence of coeliac disease exist? British Medical Journal 345: e7907. [DOI] [PubMed] [Google Scholar]
- Aziz I, Lewis NR, Hadjivassiliou M et al (2014) A UK study assessing the population prevalence of self‐reported gluten sensitivity and referral characteristics to secondary care. European Journal of Gastroenterology and Hepatology 26: 33–9. [DOI] [PubMed] [Google Scholar]
- Bates BLA, Lennox A, Prentice A et al (2014) National Diet and Nutrition Survey. Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme (2008/2009 – 2011/2012). Department of Health: London. [Google Scholar]
- Beaudouin E, Renaudin J‐M, Morisset M et al (2006) Food‐dependent exercise‐induced anaphylaxis – update and current data. European Annals of Allergy and Clinical Immunology 38: 45–51. [PubMed] [Google Scholar]
- Biesiekierski JR & Iven J (2015) Non‐coeliac gluten sensitivity: piecing the puzzle together. United European Gastroenterology Journal 3: 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesiekierski JR, Peters SL, Newnham ED et al (2013) No effects of gluten in patients with self‐reported non‐celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short‐chain carbohydrates. Gastroenterology 145: 320–8. [DOI] [PubMed] [Google Scholar]
- Bolotin D & Petronic‐Rosic V (2011) Dermatitis herpetiformis Part I. Epidemiology, pathogenesis, and clinical presentation. Journal of the American Academy of Dermatology 64: 1017–24. [DOI] [PubMed] [Google Scholar]
- Brouns JPH, van Buel VJ & Shewry PR (2013) Does wheat make us fat and sick? Journal of Cereal Science 58: 209–15. [Google Scholar]
- Capriles VD, dos Santos FG, Areas JAG (2016) Gluten‐free breadmaking: improving nutritional and bioactive compounds. Journal of Cereal Science. In press. [Google Scholar]
- Carroccio A, Rini G & Mansueto P (2014) Non‐celiac wheat sensitivity is a more appropriate label than non‐celiac gluten sensitivity. Gastroenterology 146: 320–1. [DOI] [PubMed] [Google Scholar]
- Catassi C, Elli L, Bonaz B et al (2015) Diagnosis of non‐celiac gluten sensitivity (NCGS): the Salerno experts’ criteria. Nutrients 7: 4966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christison GW & Ivany K (2006) Elimination diets in autism spectrum disorders: any wheat amidst the chaff? Developmental and Behavioural Pediatrics 27: 162–71. [DOI] [PubMed] [Google Scholar]
- Corrao G, Corazza GR, Bagnardi V et al (2001) Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet 358: 356–61. [DOI] [PubMed] [Google Scholar]
- Davis WR (2011) Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health. Rodale Books: Emmaus, PA, USA. [Google Scholar]
- Dohan FC (1979) Schizophrenia and neuroactive peptides from food. Lancet 313: 1031. [DOI] [PubMed] [Google Scholar]
- Dohan FC, Harper EH, Clark MH et al (1984) Is schizophrenia rare if grain is rare? Biological Psychiatry 19: 385–99. [PubMed] [Google Scholar]
- Gawkrodger DJ, Blackwell JN, Gilmour HM et al (1984) Dermatitis herpetiformis: diagnosis, diet and demography. Gut 25: 151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson PR & Shepherd SJ (2010) Evidence‐based dietary management of functional gastrointestinal symptoms: the FODMAP approach. Journal of Gastroenterology and Hepatology 25: 252–8. [DOI] [PubMed] [Google Scholar]
- Gilissen LJWJ, van der Meer IM & Smulders MJM (2014) Reducing the incidence of allergy and intolerance to cereals. Journal of Cereal Science 59: 337–53. [Google Scholar]
- Godfrey JD, Brantner TL, Brinjikji W et al (2010) Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology 139: 763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PHR & Cellier C (2007) Celiac disease. New England Journal of Medicine 357: 1731–43. [DOI] [PubMed] [Google Scholar]
- Green PHR, Stavropoulos SN, Panagi SG et al (2001) Characteristics of adult celiac disease in the USA: results of a national survey. American Journal of Gastroenterology 96: 126–31. [DOI] [PubMed] [Google Scholar]
- Green PH, Fleischauer AT, Bhagat G et al (2003) Risk of malignancy in patients with celiac disease. American Journal of Medicine 115: 191–5. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Grünewald RA & Davies‐Jones GAB (2002) Gluten sensitivity as a neurological illness. Journal of Neurology, Neurosurgery and Psychiatry 72: 560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjivassiliou M, Sanders DS, Gruenewald RA et al (2010) Gluten sensitivity: from gut to brain. Lancet Neurology 9: 318–30. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Sanders DD & Aeschlimann DP (2015) Gluten‐related disorders: gluten ataxia. Digestive Diseases 33: 264–8. [DOI] [PubMed] [Google Scholar]
- Jones JM (2012) Wheat belly an analysis of selected statements and basic theses from the book. Cereal Foods World 57: 177–89. [Google Scholar]
- Kalaydiian AE, Eaton W, Cascella N et al (2006) The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatrica Scandinavica 113: 82–90. [DOI] [PubMed] [Google Scholar]
- Kinsey L, Burden ST & Bannerman E (2008) A dietary survey to determine if patients with coeliac disease are meeting current healthy eating guidelines and how their diet compares to that of the British general population. European Journal of Clinical Nutrition 62: 1333–42. [DOI] [PubMed] [Google Scholar]
- Lohi S, Mustalahti K, Kaukinen K et al (2007) Increasing prevalence of celiac disease over time. Alimentary Phamacology and Therapeutics 26: 1217–25. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Card TR, Kaukinen K et al (2015) Screening for celiac disease in the general population and in high‐risk groups. United European Gastroenterology Journal 3: 106–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M, Mustalahti K, Kokkenen J et al (2003) Prevalence of celiac disease among children in Finland. New England Journal of Medicine 348: 2517–24. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Yokooji T, Taogoshi T (2015) Common food allergens and their IgE‐binding epitopes. Allergology International 64: 332–43. [DOI] [PubMed] [Google Scholar]
- Mobacken H, Kastrup W & Nilsson LA (1984) Incidence and prevalence of dermatitis herpetiformis in western Sweden. Acta Dermato Venereologica 64: 400–4. [PubMed] [Google Scholar]
- Morita E, Matsuo H, Mihara S et al (2003) Fast ω‐5 gliadin, is a major allergen in wheat‐dependent exercise‐induced anaphylaxis. Journal of Dermatological Science 33: 99–104. [DOI] [PubMed] [Google Scholar]
- Morita E, Chinuki Y, Takahashi H et al (2012) Prevalence of wheat allergy in Japanese adults. Allergology International 61: 101–5. [DOI] [PubMed] [Google Scholar]
- Muir JG & Gibson PR (2013) The low FODMAP diet for treatment of irritable bowel syndrome and other gastrointestinal disorders. Gastroenterology and Hepatology 9: 450–2. [PMC free article] [PubMed] [Google Scholar]
- Mustalahti K, Catassi C, Reunanen A et al (2010) The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Annals of Medicine 42: 587–95. [DOI] [PubMed] [Google Scholar]
- Myléus A, Ivarsson A, Webb C et al (2009) Celiac disease revealed in 3% of Swedish 12‐year‐olds born during an epidemic. Journal of Pediatric Gastroenterology and Nutrition 49: 170–6. [DOI] [PubMed] [Google Scholar]
- Olsson C, Hernell O, Hornell A et al (2008) Difference in celiac disease risk between Swedish birth cohorts suggests an opportunity for primary prevention. Pediatrics 122: 528–34. [DOI] [PubMed] [Google Scholar]
- Palosuo K, Varjonen E, Kekki OM et al (2001) Wheat omega‐five gliadin is a major allergen in children with immediate allergy to ingested wheat. Journal of Allergy and Clinical Immunology 108: 634–8. [DOI] [PubMed] [Google Scholar]
- Pellegrini N & Agostini A (2015) Nutritional aspects of gluten‐free products. Journal of the Science of Food and Agriculture 95: 2380–5. [DOI] [PubMed] [Google Scholar]
- Pereira B, Venter C, Grundy J et al (2005) Prevalence of sensitisation to food allergens, reported adverse reactions to food, food avoidance, and food hypersensitivity among teenagers. Journal of Allergy and Clinical Immunology 116: 884–92. [DOI] [PubMed] [Google Scholar]
- Rubio‐Tapia A, Ludvigsson JF, Brantner TL et al (2012) The prevalence of celiac disease in the United States. The American Journal of Gastroenterology 107: 1538–44. [DOI] [PubMed] [Google Scholar]
- Sapone A, Bai JC, Ciacci C et al (2012) Spectrum of gluten‐related disorders: consensus on new nomenclature and classification. BMC Medicine 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry PR & Hey SJ (2015) The contribution of wheat to human diet and health. Food and Energy Security 4: 178–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MM & Roy SR (1975) Wheat gluten as a pathogenic factor in schizophrenia. Science 191: 401–2. [DOI] [PubMed] [Google Scholar]
- Smith JB, Tulloch JE, Meyer LJ et al (1992) The incidence and prevalence of dermatitis herpetiformis in Utah. Archives of Dermatology 128: 1608–10. [PubMed] [Google Scholar]
- Solaymani‐Dodaran M, West J & Logan RF (2007) Long‐term mortality in people with celiac disease diagnosed in childhood compared with adulthood: a population‐based cohort study. American Journal of Gastroenterology 102: 864–70. [DOI] [PubMed] [Google Scholar]
- Sollid LM, Qiao S‐W, Anderson RP et al (2012) Nomenclature and listing of celiac disease relevant gluten T‐cell epitopes restricted by HLA‐DQ molecules. Immunogenetics 64: 455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statista (2015). Global gluten‐free retail packaged food market size from 2009 to 2014 (in billion U.S. dollars). http://www.statista.com/topics/2067/gluten-free-foods-market/ (accessed 1 October 2015)
- Staudacher HM, Whelan K, Irving PM et al (2011) Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. Journal of Human Nutrition and Dietetics 24: 487–95. [DOI] [PubMed] [Google Scholar]
- Steer T, Thane C, Stephen A et al (2008) Bread in the diet: consumption and contribution to nutrient intakes of British adults. Proceedings of the Nutrition Society 67: E363. [Google Scholar]
- Tatham AS & Shewry PR (2008) Allergy to wheat and related cereals. Clinical and Experimental Allergy 38: 1712–26. [DOI] [PubMed] [Google Scholar]
- Thompson T (1999) Thiamin, riboflavin, and niacin contents of the gluten‐free diet: is there cause for concern? Journal of the American Dietetic Association 99: 858–62. [DOI] [PubMed] [Google Scholar]
- Thompson T (2000) Folate, iron, and dietary fiber contents of the gluten‐free diet. Journal of the American Dietetic Association 100: 1389–96. [DOI] [PubMed] [Google Scholar]
- Venter C, Pereira B, Grundy J et al (2006a) Prevalence of sensitization reported and objectively assessed hypersensitivity amongst six‐year‐old children: a population‐based study. Peditaric Allergy and Immunology 17: 356–63. [DOI] [PubMed] [Google Scholar]
- Venter C, Pereira B, Grundy J et al (2006b) Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. Journal of Allergy and Clinical Immunology 117: 1118–24. [DOI] [PubMed] [Google Scholar]
- Volta U, Bardella MT, Calabro A et al (2014) An Italian prospective multicentre survey on patients suspected of having non‐celiac gluten sensitivity. BMC Medicine 12: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MM, Murray JA, Ronkainen J et al (2010) Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population‐based study. Gastroenterology 139: 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Logan RF, Hill PG et al (2003) Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut 52: 960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Logan RF, Smith CJ et al (2004) Malignancy and mortality in people with coeliac disease: population based cohort study. British Medical Journal 329: 716–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley P, Haracopos D, Knivsberg A‐M et al (2010) The ScanBrit randomised, controlled, single‐blind study of a gluten‐ and casein‐free dietary intervention for children with autism spectrum conditions. Nutritional Neuroscience 13: 87–100. [DOI] [PubMed] [Google Scholar]
- Whiteley P, Shattock P, Knivsberg A‐M et al (2013) Gluten‐ and casein‐free dietary intervention for autism spectrum conditions. Frontiers in Human Neuroscience 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JHY, Neal B, Trevena H et al (2015) Are gluten‐free foods healthier than non‐gluten‐free foods? An evaluation of supermarket products in Australia. British Journal of Nutrition 74: 221–6. [DOI] [PubMed] [Google Scholar]
- Yokooji T, Kurihara S, Murakami T et al (2013) Characterization of causative allergens for wheat‐dependent exercise‐induced anaphylaxis sensitized with hydrolysed wheat proteins in facial soap. Allergology International 62: 435–45. [DOI] [PubMed] [Google Scholar]
- Yokooji T, Okamura Y, Chinuki Y et al (2015) Prevalences of specific IgE to wheat gliadin components in patients with wheat‐dependent exercise‐induced anaphylaxis. Allergology International 64: 206–8. [DOI] [PubMed] [Google Scholar]
- Zuidmeer L, Goldhahn K, Rona RJ et al (2008) The prevalence of plant food allergies: a systematic review. Journal of Allergy and Clinical Immunology 121: 1210–8. [DOI] [PubMed] [Google Scholar]


