Abstract
Aims
Urethral pathophysiology is often neglected in discussions of bladder dysfunction. It has been debated whether “urethral sphincter instability,” referred to based on observed “urethral pressure variations,” is an important aspect of overactive bladder syndrome (OAB). The purpose of this report is to summarize current urethral pathophysiology evidence and outline directions for future research based on a literature review and discussions during the ICI‐RS meeting in Bristol in 2014.
Methods
Urethral pathophysiology with a focus on urethral pressure variation (UPV) was presented and discussed in a multidisciplinary think tank session at the ICI_R meeting in Bristol 2014. This think tank session was based on collaboration between physicians and basic science researchers.
Results
Experimental animal studies or studies performed in clinical series (predominantly symptomatic women) provided insights into UPV, but the findings were inconsistent and incomplete. However, UPV is certainly associated with lower urinary tract symptoms (likely OAB), and thus, future research on this topic is relevant.
Conclusions
Future research based on adequately defined clinical (and urodynamic) parameters with precisely defined patient groups might shed better light on the cause of OAB symptoms. Further fundamental investigation of urethral epithelial–neural interactions via the release of mediators should enhance our knowledge and improve the management of patients with OAB. Neurourol. Urodynam. 35:318–323, 2016. © 2016 The Authors. Neurourology and Urodynamics published by Wiley Periodicals, Inc.
Keywords: overactive bladder syndrome, sensation, urethral pressure measurement, urethral pressure variations urethral instability
Abbreviations
- DO
detrusor overactivity
- OAB
overactive bladder syndrome
- UPV
urethral pressure variation
1. Take home messages.
‐ Urethral pressure variations during bladder filling (urethral instability) occur in a proportion of women, can be reliably demonstrated and are not artifacts
‐ Urethral pressure variations may be associated with OAB and DO
‐ The underlying mechanism(s) of urethral pressure variations are not known but may involve somatic (pudendal) and autonomic nerves
‐ Further urethral pathophysiology studies may enhance our knowledge of not only the unstable urethra but also the underlying causes of OAB and other bladder disorders
INTRODUCTION
The pathophysiology of the overactive bladder syndrome (OAB), urodynamically associated with detrusor overactivity (DO), is multifactorial1, 2, 3 and may include neurogenic, myogenic, or idiopathic factors. The recognition of the functional contribution of the urothelium,4, 5 spontaneous myocyte activity during bladder filling,6, 7, 8 and the multiplicity of nerve transmitters involved in bladder muscle activation has sparked interest in both the peripheral and central modulation of OAB/DO pathophysiology. Research on OAB has focused on detrusor dysfunction, typically without considering urethral muscle function as a potential contributor to OAB or DO. The pathophysiology of stress urinary incontinence refers predominantly to the passive mechanisms that produce urinary continence; however, in the pathophysiology of OAB, a muscle “activity” element of the closure mechanism could play a role.
The primary defect in DO may occur in the urethra.9 For example, in females, the pattern of urethral rapid pressure variation is associated with urgency or DO.10, 11, 12 How (and if) these urethral pressure variations (UPV) may be linked to the perception of urgency, OAB or the origin (or consequence) of DO has been a matter of debate since the earliest observations in the 1970s.29 Because the clinical significance of these pressure variations is still not fully understood, they have often been neglected or considered to be an artifact.13 However, Groenendijk et al.14 reviewed previous findings and considered the existence of UPV defined as urethral pressure decreased of >15 cm H2O during continuous fluid filling cystometry. They concluded that “the observation and recognition of UPV could be valuable in the diagnosis and evaluation of therapy in functional lower urinary tract disorders.”
The definition of urethral pressure instabilities (URI) has been discussed in the ICS since the late 1970s, and in 1981, the ICS defined URI as a “condition in which there is involuntary fall in urethral pressure during filling, resulting in urinary leakage in the absence of detrusor activity.” The term URI was not redefined in the subsequent standard document and has been deemed to be clinically irrelevant or insignificant. This paper will utilize the term UPV, as it is the most descriptive.
This paper reflects the ICI‐RS2014 Think‐Tank's discussion of the clinical and fundamental research evidence on UPV. The aim of this paper is to summarize what relatively recent novel fundamental research in the field of urethral pathophysiology may add to the earlier clinical studies and how such developments may guide further research.
MATERIALS AND METHODS
Prior to the multidisciplinary Think‐Tank in Bristol in September 2014, an online literature review was performed. The Medical Literature Analysis and Retrieval System Online (U.S. National Library of Medicine's life science database; MEDLINE; PubMed) was used for the literature review in August 2014. Searches were performed with the use of combined terms: “urethral pressure AND variation” and “urethral pressure AND instabilities.”
RESULTS
Literature Review
All retrieved manuscripts were screened manually based on the following criteria: interventions, other techniques, hypospadias, children, and therapeutic approaches. The next section reports the conclusions of the literature research and the discussion of the multidisciplinary Think‐Tank session.
Urethral Pressure Variations: Fact or Artifact?
Voiding begins with a decrease in urethral pressure, followed by a detrusor contraction that increases bladder pressure and opens the urethra, resulting in urine flow. Continuous pressure measurements in the urethra during the bladder storage phase allow for observations of (smooth) muscle closure activity. However, potential artifacts may be induced by the insertion of a catheter of a certain dimension that changes the basic closure conditions of the urethra.15 The muscle dynamic activity cannot be reliably observed or measured with urethral pressure profile measurements when the catheter is pulled through the urethra because the friction of the catheter inside the urethra will likely cause nonphysiological activity.16
However, if the catheter is kept steady in the urethra, quite pronounced pressure variations are revealed in a proportion of patients with symptoms of lower tract dysfunction; however, this phenomenon is also observable in normal volunteers. If the measurement is performed with a sufficiently high quality, the vascular pulsations and respirations become visible; this may be regarded as evidence that UPV do not measure a catheter artifact. The genuine urethral smooth muscle activity is presented with lower frequencies. Visual analysis suggests that UPV occur with frequencies of 3–5 per minute, but lower frequencies of up to 2–3 per hour can also be observed. However, attempts to identify the most relevant frequencies, for example, via Fast Fourier transformation, have not produced conclusive results.
UPV are identified in both healthy, fertile, and postmenopausal females17, 18 and in patients with stress urinary incontinence.17 Similar “sphincter dynamical” activity has been observed in the anal canal, where pressure variations (declines) have been referred to as slow waves and ultra‐slow waves.19 These observations demonstrate that urethral muscle function cannot be regarded as a fixed entity, even in physiologically normal cases. The bladder closure mechanism exhibits a certain amount of variation in activity that may lead to symptoms or dysfunction if an absolute or individual threshold is exceeded.
Urethral Pressure Variations and Uncontrolled Initiation of Voiding Reflex
The origin of UPV may be attributed to peri‐urethral smooth muscle activity. Because both pre and postmenopausal women develop UPV, it does not appear to be related to hormonal status. During night‐time, the urethral pressure is lower following the decreases in blood pressure. With this pressure decrease, there is also a decrease in the amplitude of variations. This decrease is most pronounced in the beginning of the night during stage‐4 sleep.20 There is a positive correlation between the maximum urethral closure pressure and amplitude of UPV. Two urodynamic patterns may be identified in patients with OAB; one is the traditional DO pattern when the detrusor pressure builds up to a threshold at which voiding cannot be deferred. However, there is also a second pattern where the primary cause of the urgency appears to be in the urethra (Fig. 1, Fig. 2). Continuous urethral pressure measurement with cystometry has shown that patients with OAB may display a sudden decrease in urethral pressure leading to the perception of urgency, likely via the activation of urethral mechano‐ and chemo‐receptors (Fig. 3).10, 11, 12 One single‐center study suggested that no subsequent detrusor contraction was evident when the guarding reflex is initiated (when using urethral pressure as a feedback for the patient). If, however, there is some latency, then a detrusor contraction developed and with an even more delayed reaction, along with leakage.21 The decrease in urethral pressure during UPV is clearly different from the other pressure variations. However, there are similarities with the initiation of normal voiding, but the process is uncontrolled. Therefore, it might be defined as an uncontrolled or premature micturition reflex .
Figure 1.
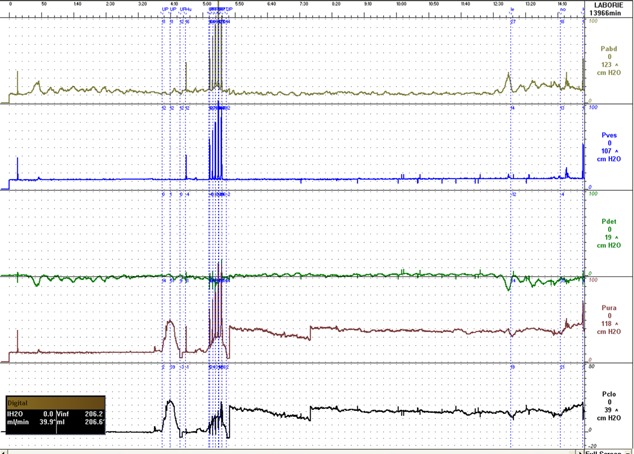
Typical pattern in patients with stress incontinence.
Figure 2.
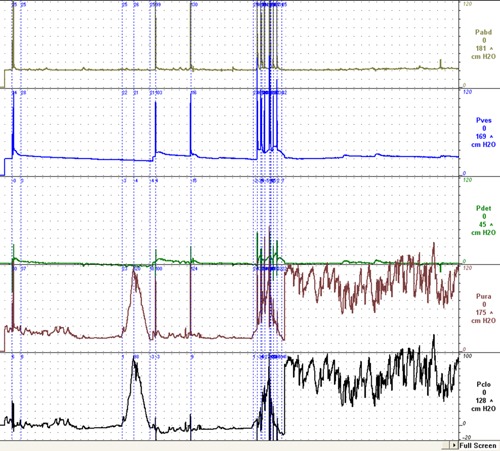
Typical pattern in patients with OAB.
Figure 3.
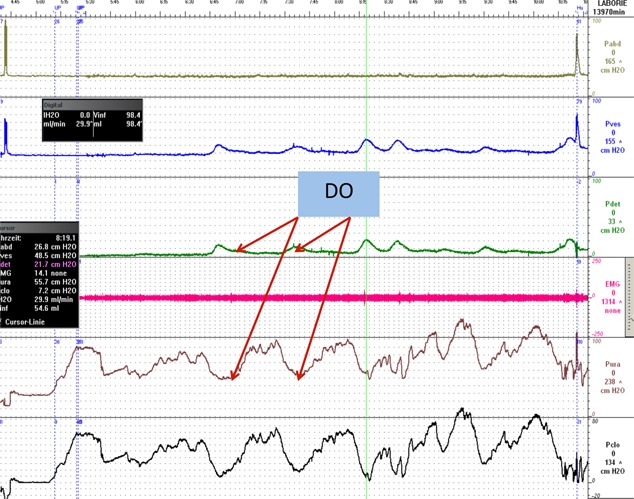
Detrusor over activity corresponding to decrease in urethral pressure.
If this hypothesis is accepted, it opens an entire new avenue both for further investigations into the etiology and pathogenesis of OAB as well as its treatment. The differentiation between physiological and nonphysiological urethral pressure variations must be evaluated within the context of symptoms and cystometrical findings in individuals. Pressure variations larger than 15 cm H2O have appeared to be most clinically relevant to date.14
Reflex Control of the Urethra
Reflexes within the bladder, between outlet and detrusor smooth muscle and between bladder and pelvic striated muscle play integral roles in the neural control of the lower urinary tract. Based on experiments in a decerebrated cat, Barrington22 summarized the following: “From these results it was concluded that in micturition, there are two reflexes leading to a powerful contraction of the bladder; one arises from distension of the bladder and the other from urine running through the urethra.” The finding that mechanical stimulation of the urethra, especially the flow of urine, may facilitate the micturition reflex has been confirmed by other investigators.23, 24 The importance and even the existence of such a reflex in humans have been questioned.25, 26, 27 Geirsson and Fall have evaluated both cold and tension‐mediated reflexes in an experimental setting and found that those reflexes were more difficult to evoke from the urethra than from the bladder. Nevertheless, they concluded that their findings support the existence of a cold‐sensitive reflex system in the human urethra.26 Shaffik et al. concluded that the urethrovesical reflex acts as a secondary micturition reflex that is responsible for the continuation of detrusor contraction and urination.27 Studies in animals have demonstrated the basis for an excitatory urethra‐to‐bladder reflex, showing that sensory nerves in the wall of the urethra fire in response to urethral urine flow and electrical stimulation and that this activity can initiate bladder contraction in a quiescent bladder and augment ongoing contraction in an active bladder. It may be assumed that the afferent activity evoked by mechanical urethral stimulation conveys both mechano‐afferent information (micturition reflex) and sensory information (sensation of bladder filling, nociception). Just before detrusor contraction that leads to bladder emptying, there is a decreased in urethral pressure.28, 29 In the presence of DO, an identical pattern of changes is frequently observed.20 Related to this, Mazieres et al.23 showed that electrical stimulation of the afferent urethral branch of the pudendal nerve evoked both excitatory and inhibitory reflex effects in parasympathetic neurons to the bladder and suggested that urethral and bladder afferents converge in the spinal micturition reflex pathway. Groenendijk et al.30 treated 19 female patients with refractory voiding disorders, 18 with UPV, with sacral neuromodulation (SNM) therapy. Treatment was successful in 13 patients (68%), 12 of whom had UPV at baseline. UPV resolved in seven of these patients, and the authors concluded that UPV appeared to be a valuable urodynamic parameter for predicting the outcome of SNM. During SNM stimulation, somatic pudendal nerve fibers are activated and resulted in enhanced urethral sphincter tone and increased pelvic floor activity. Via afferent fibers contained in the pudendal nerve, this also results in detrusor muscle inhibition. Groenendijk et al.30 speculated that SNM may influence urethral function and that UPV should be considered in the evaluation of its working mechanisms. According to Groenendijk et al.,14 patients with refractory OAB that were successfully treated with SNM had an immediate recurrence of both DO and UPV after a bilateral pudendal blockage with lignocaine. These observations have been suggestive of the potential importance of the pudendal nerve, which stimulates urethral function with a positive stabilizing effect on both UPV and DO.
Thus, urethral stimulation appears to generate a spinal/supraspinal on/off mechanism that decreases and increases urethral activity. When urine enters the urethra (with or without provoking urgency) in a normal individual, the initial pressure variations generated are limited. However, when the afferent impulses in the nervous control system are disturbed (as could be the case in OAB), an involuntary bladder contraction may be initiated by the UPV (Fig. 3).
Urethral Innervation and Areas for Future Research
A number of nerves containing a variety of transmitters that mediate contraction or relaxation have been demonstrated in the smooth and striated muscle components of the female urethra. Relaxation of the urethra may be achieved in different ways. Because noradrenaline is generally considered to be a main factor that maintains urethral smooth muscle tone, this implies that a decrease in noradrenergic activity can relax the urethra. Similarly, the release of relaxant transmitters may relax the urethra by reducing existing contraction. Nitric oxide (NO) appears to be an important mediator of urethral smooth muscle relaxation, but the role of other transmitters cannot be excluded.31, 32 Von Heyden et al.33 investigated the density of nerves containing acetylcholine, noradrenaline, neuropeptide Y (NPY), galanin, vasoactive intestinal polypeptide (VIP), and calcitonin gene‐related peptide (CGRP) within the urethral sphincter in patients without voiding disorders. They found that the striated sphincter was densely innervated by cholinergic nerves. Adrenergic nerves next to striated fibers were rare but present in all patients. NPY was rarely observed along striated fibers. In the smooth muscle sphincteric component, noradrenaline‐, acetylcholine‐, NPY‐, and galanin‐reactive nerves were frequently observed. Many of these transmitters must be contained within the same nerves. However, no co‐localization studies were reported, and because no functional studies were performed, the clinical implications of these results can only be speculated upon.
The transmitter of the striated urethral sphincter is acetylcholine, stimulating nicotinic receptors, and variation in the release of acetylcholine seems to be the main mechanism by which its tone is regulated.
The presence of TRPV1‐immunoreactive nerves in the urethra and the effects of capsaicin on both urethral and striated muscles34 have raised the question of whether this channel is involved in urethral functions that can be linked to DO/OAB. It was speculated that urothelial TRP receptors in the proximal urethra, activated by urine flow, may stimulate detrusor contraction. Whether such a relaxant effect on urethral muscle obtained by TRP receptor activation may be involved in UPV and whether this is linked to DO remains to be established.
Little is known about the possible role of transmitters that are released from the urethral epithelium/lamina propria and participate in the signaling pathways that convey afferent activity and eventually result in urgency and/or detrusor activity. However, there is some evidence that the mucosal pathway within the proximal urethra, which involves a cascade of epithelial inhibitory and stimulatory transmitters/mediators, may play a role in continence and sensation.4, 35, 36 The urethral epithelium is likely to be part of a signaling system that involves projections of the neuroendocrine cells, interstitial cells, and sensory nerve endings.37, 38 Studies suggest that these urethral‐neuroendocrine cells release mediators, which can stimulate urethral reflexes through the activation of adjacent sensory nerves.39 Such cell types are not unlike those in other types of epithelia, such as within the airways or trachea, where a cell‐type termed “brush cells,” which are chemo‐receptive and contact nearby nerve fibers has been described.40 In addition, the densest distribution of nerves occurs throughout the bladder neck and the initial part of the urethra, where the nerves form a plexus and have a sensory function.41
Pain symptoms that arise from the lower urinary tract may originate from the bladder neck and proximal urethra.42 The bladder neck and proximal urethra contain the highest density of nerves,43 and the proximity of afferent nerves to the urothelium suggests that urothelial cells could be targets for transmitters released from nerves and/or that agents released by urothelial cells influence afferent nerve excitability. However, whether urethral epithelial–neural interactions (via the release of mediators) lead to UPV that can influence storage and voiding reflexes and result in symptoms including urgency and pain can only be remains unclear and is an area for further research.
DISCUSSION
Slow‐wave, rhythmic UPV can be observed in healthy volunteers. They may be an aspect of normal urethral physiology and contribute to continence. However, the observation that UPV are significantly more prevalent in patients with clinical symptoms of OAB than in patients with symptoms of stress urinary incontinence suggests that both UPV and DO share a common pathophysiology pathway to cause OAB.
In addition, in relation to the perception of urinary urgency with or without detrusor contraction, UPV may display a dramatic change in urethral pressure. The negative DP/DT is considerably larger than the positive and negative DP/DT of physiologically normal pressure variations.
Fundamental research suggests that crosstalk between urethra and detrusor via cells, nerves and neurotransmitters adds evidence to a possible pathophysiological interaction between urethral and detrusor muscle function. Clinical research findings suggest that pudendal nerve modulation or inhibition affects urethral smooth muscle function and that UPV may be due to the lack of more central neurological control. Either peripheral (neurotransmitters) or central (sacral and pudendal nerve or in or above the sacral reflex) lack of control may cause a cascade of events that lead to DO or OAB without DO.
Future Directions
Further investigation of urethral transmitters released from nerves or via urethral epithelial–neural interactions and resulting in urgency and/or pain can hopefully enhance our knowledge of not only UPV but also the pathophysiology of OAB and urinary incontinence. Further fundamental research should be conducted with clinical protocols that use adequately standardized urodynamics with reliable measurement of urethral pressure during continuous fluid filling cystometry in carefully prospectively recruited patient groups.
These standards should fulfill the requirements described by the ICS Sub‐Committee on the “Standardization of Urethral pressure measurement”.44 To reduce moving artifacts, continuous urethral pressure measurements throughout the cystometric phase of bladder filling should be performed in a stable position allowing a simultaneous recording of both urethral pressure as well as intravesical pressure. A three‐lumen catheter (7–9 charr) should be placed with one measurement channel placed intravesicularly and the other at the point of maximum urethral closing pressure (MUCP). Zeroing of the pressure sensors with fluid‐filled catheters should be performed with external transducers placed at the height of the superior edge of the symphysis pubis. The catheters should be rather flexible to reduce artifacts of an excessively rigid catheter. If using microtips or fiber‐optic catheters as suggested by Heeakkers et al.,45 one should consider that these catheters do not measure the urethral pressure directly; they measure the normal stress component on the surface of the transducer. Because we cannot exclude that the measurement results are due to the interaction between the urethral tissue and transducer surface itself, they cannot be recommended for the evaluation of urethral pressure variations during the filling phase.
A proportion of patients who fail on current standard management of OAB will benefit from further objective detailing of the cause of the symptoms, leading to personalized management with better outcomes. Improved diagnosis of UPV may be helpful in this regard.
Potential conflicts of interest: Nothing to disclose.
Dr. Hashim Hashim led the peer‐review process as the Associate Editor responsible for the paper.
REFERENCES
- 1. Meng E, Lin WY, Lee WC, et al. Pathophysiology of overactive bladder. LUTS 2012; 4:48–55. [DOI] [PubMed] [Google Scholar]
- 2. Banakhar MA, Al‐Shaiji TF, Hassouna MM. Pathophysiology of overactive bladder. Int Urogynecol J 2012; 23:975–82. [DOI] [PubMed] [Google Scholar]
- 3. Sakakibara R, Panicker J, Fowler CJ, et al. Is overactive bladder a brain disease? The pathophysiological role of cerebral white matter in the elderly. Int J Urol 2014; 21:33–8. [DOI] [PubMed] [Google Scholar]
- 4. Birder L, Andersson KE. Urothelial signaling. Physiol Rev 2013; 93:653–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keay SK, Birder LA, Chai TC. Evidence for bladder urothelial pathophysiology in functional bladder disorders. Biomed Res Int 2014; 2014:865463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillespie JI, van Koeveringe GA, de Wachter SG, et al. On the origins of the sensory output from the bladder: The concept of afferent noise. BJU Int 2009; 103:1324–33. [DOI] [PubMed] [Google Scholar]
- 7. Andersson KE. Detrusor myocyte activity and afferent signaling. Neurourol Urodyn 2010; 29:97–106. [DOI] [PubMed] [Google Scholar]
- 8. Eastham JE, Gillespie JI. The concept of peripheral modulation of bladder sensation. Organogenesis 2013; 9:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hindmarsh JR, Gosling PT, Deane AM. Bladder instability. Is the primary defect in the urethra? Br J Urol 1983; 55:648–51. [DOI] [PubMed] [Google Scholar]
- 10. Low JA, Armstrong JB, Mauger GM. The unstable urethra in the female. Obstet Gynecol 1989; 74:69–74. [PubMed] [Google Scholar]
- 11. Farrell SA, Tynski G. The effect of urethral pressure variation on detrusor activity in women. Int Urogynecol J Pelvic Floor Dysfunct 1996; 7:87–93. [DOI] [PubMed] [Google Scholar]
- 12. McLennan MT, Melick C, Bent AE. Urethral instability: Clinical and urodynamic characteristics. Neurourol Urodyn 2001; 20:653–60. [DOI] [PubMed] [Google Scholar]
- 13. Colstrup H, Lose G, Jorgensen L. Pressure variations in the female urethra measured by infusion technique: Is it an artefact? Neurourol Urodyn 1988; 7:457–60. [Google Scholar]
- 14. Groenendijk PM, Heesakkers JP, Ouwerkerk TJ, et al. Urethral instability: Current pathophysiological concept. Urol Int 2009; 83:125–33. [DOI] [PubMed] [Google Scholar]
- 15. Sørensen S, Kirkeby HJ, Stødkilde‐Jørgensen H, et al. Continuous recording of urethral activity in healthy female volunteers. Neurourol Urodyn 1986; 5:5–16. [Google Scholar]
- 16. Brown M, Wickham JEA. The urethral pressure profile. Brits J Urol 1969; 41:211–17. [DOI] [PubMed] [Google Scholar]
- 17. Sørensen S. Urethral pressure and pressure variation in stress incontinent women and women with unstable detrusor. Neurourol Urodyn 1991; 10:483–92. [Google Scholar]
- 18. Sørensen S. Urodynamic investigations and their reproducibility in healthy postmenopausal females. Scand J Urol Nephrol Suppl 1988; 114:42–7. [PubMed] [Google Scholar]
- 19. Sørensen S, Gregersen H, Sørensen SM, et al. Rhythmic pressure variations in urethra and anal canal: Investigations in healthy fertile female volunteers. Neurourol Urodyn 1991b; 10:493–501. [Google Scholar]
- 20. Sorensen S. Urethral pressure variations in healthy and incontinent women. Neurourol Urodyn 1992; 11:549. [Google Scholar]
- 21. Nørgaard JP, Nissen T, Djurhuus JC. A device for treatment of destrusor hyperreflexia by bio‐feedback. Urol Res 1985; 13:241–2. [DOI] [PubMed] [Google Scholar]
- 22. Barrington FJF. The relation of the hind‐brain to micturition. Brain 1921; 44:23–53. [Google Scholar]
- 23. Mazieres L, Jiang C, Lindstrom S. Bladder parasympathetic response to electrical stimulation of urethral afferents in the cat. Neurol Urodyn 1997; 16:471–2. [Google Scholar]
- 24. Robain G, Combrisson H, Mazières L. Bladder response to urethral flow in the awake ewe. Neurourol Urodyn 2001; 20:641–9. [DOI] [PubMed] [Google Scholar]
- 25. Nathan PW. Micturition reflexes in man. J Neurol Surg Psychiat 1952; 15:148–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geirsson G, Fall M. Reflex interaction between the proximal urethra and the bladder. A clinical experimental study. Scand J Urol Nephrol 1999; 33:24–6. [DOI] [PubMed] [Google Scholar]
- 27. Shafik A, Shafik AA, El‐Sibai O, et al. Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: The urethrovesical reflex. World J Urol 2003; 21:167–70. [DOI] [PubMed] [Google Scholar]
- 28. Tanagho EA, Miller ER. Initiation of voiding. Br J Urol 1970; 42:175–83. [DOI] [PubMed] [Google Scholar]
- 29. Low JA. Urethral behavior during the involuntary detrusor contraction. Am J Obstet Gynecol 1977; 128:32–42. [DOI] [PubMed] [Google Scholar]
- 30. Groenendijk PM, Heesakkers JP, Lycklama A, et al. Urethral instability and sacral nerve stimulation—A better parameter to predict efficacy? J Urol 2007; 178:568–72. [DOI] [PubMed] [Google Scholar]
- 31. Bridgewater M, MacNeil HF, Brading AF. Regulation of tone in pig urethral smooth muscle. J Urol 1993; 150:223–8. [DOI] [PubMed] [Google Scholar]
- 32. Werkström V, Persson K, Ny L. Factors involved in the relaxation of female pig urethra evoked by electrical field stimulation. Br J Pharmacol 1995; 116:1599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Heyden B, Jordan U, Hertle L. Neurotransmitters in the human urethral sphincter in the absence of voiding dysfunction. Urol Res 1998; 26:299–310. [DOI] [PubMed] [Google Scholar]
- 34. Everaerts W, Gevaert T, Nilius B, et al. On the origin of bladder sensing: Tr(i) ps in urology. Neurourol Urodyn 2008; 27:264–73. [DOI] [PubMed] [Google Scholar]
- 35. Andersson KE. Bladder activation: Afferent mechanisms. Urology 2002; 59:43–50. [DOI] [PubMed] [Google Scholar]
- 36. Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 2009; 297:F1477–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hashimoto Y, Ushiki T, Uchida T, et al. Scanning electron microscopic observation of apical sites of open‐type paraneurons in the stomach, intestine and urethra. Arch Histol Cytol 1999; 62:181–9. [DOI] [PubMed] [Google Scholar]
- 38. McCloskey KD. Interstitial cells in the urinary bladder‐localization and function. Neurourol Urodyn 2010; 29:82–7. [DOI] [PubMed] [Google Scholar]
- 39. Deckmann K, Filipski K, Krasteva‐Christ, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. PNAS 2014; 111:8267–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saunders CJ, Reynolds SD, Finger TE. Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am J Respir cell Mol Biol 2013; 49:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dixon JS, Gosling JA. Histology and fine structure of the muscularis mucosae of the human urinary bladder. J Anat 1983; 136:265–71. [PMC free article] [PubMed] [Google Scholar]
- 42. Kaur H, Arunkalaivanan AS. Urethral pain syndrome and its management. Obstet Gynecol Surv 2007; 62:348–51. [DOI] [PubMed] [Google Scholar]
- 43. Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol 1998; 27:141–55. [DOI] [PubMed] [Google Scholar]
- 44. Lose G, Griffiths D, Hosker G, et al. Report from the Standardisation Sub‐Committee of the International Continence Society. Jersey NeuroUrol Urodyn 2002–21, 258–26045. [DOI] [PubMed] [Google Scholar]
- 45. Heesakkers J1, Gerretsen R, Izeta A, et al. Circumferential urinary sphincter surface electromyography: A novel diagnostic method for intrinsic sphincter deficiency. Neurourol Urodyn 2014. doi: 10.1002/nau.22711 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


