Abstract
Peptides derived from the glucagon gene Gcg, for example, glucagon and glucagon‐like peptide 1 (GLP‐1), act as physiological regulators of fuel metabolism and are thus of major interest in the pathogenesis of diseases, such as type‐2 diabetes and obesity, and their therapeutic management. Glicentin‐related pancreatic polypeptide (GRPP) is a further, 30 amino acid Gcg‐derived peptide identified in human, mouse, rat, and pig. However, the potential glucoregulatory function of this peptide is largely unknown. Here, we synthesized rat GRPP (rGRPP) and a closely related peptide, rat GRPP‐like peptide (rGRPP‐LP), and investigated their actions in the liver and pancreas of adult male rats by employing isolated‐perfused organ preparations. Rat GRPP and rGRPP‐LP did not affect glucose output from the liver, but both elicited potent inhibition of glucose‐stimulated insulin secretion (GSIS) from the rat pancreas. This action is unlikely to be mediated by glucagon or GLP‐1 receptors, as rGRPP and rGRPP‐LP did not stimulate cyclic adenosine monophosphate (cAMP) production from the glucagon or GLP‐1 receptors, nor did they antagonize glucagon‐ or GLP‐1‐stimulated cAMP‐production at either receptor. GRPP and GRPP‐LP may be novel regulators of insulin secretion, acting through an as‐yet undefined receptor.
Keywords: GLP‐1, glucagon, GRPP, GSIS, proglucagon
Introduction
Preproglucagon, derived from the Gcg gene, is the peptide precursor for several important endocrine hormones and shows a tissue‐specific processing pattern (Fig. 1). In intestinal L‐cells and neurons, proglucagon processing results in the liberation of glicentin, oxyntomodulin, GLP‐1, and glucagon‐like peptide‐2 (GLP‐2) (Holst 1997). By contrast, in the pancreatic α‐cells, proglucagon is processed to yield glucagon along with glicentin‐related peptide (GRPP) and an apparently nonbiologically active proglucagon fragment consisting of amino acids 72–158 (Patzelt and Schiltz 1984; Holst 1997). Glucagon, GLP‐1, GLP‐2, and oxyntomodulin are well studied and are known to have numerous important functions: either directly or indirectly, they each modulate glucose homeostasis (Myers et al. 1991; Magnusson et al. 1995; Stumpel et al. 1998; Jeppesen et al. 2001; Vahl et al. 2003). GLP‐1 is an incretin that has given rise to two new classes of antidiabetic agents: the GLP‐1 agonists and, indirectly, the dipeptidyl peptidase‐4 (DPP4) inhibitors. Glucagon is another potent Gcg‐derived glucoregulatory hormone whose main physiological role is to increase glucose output from the liver.
Figure 1.
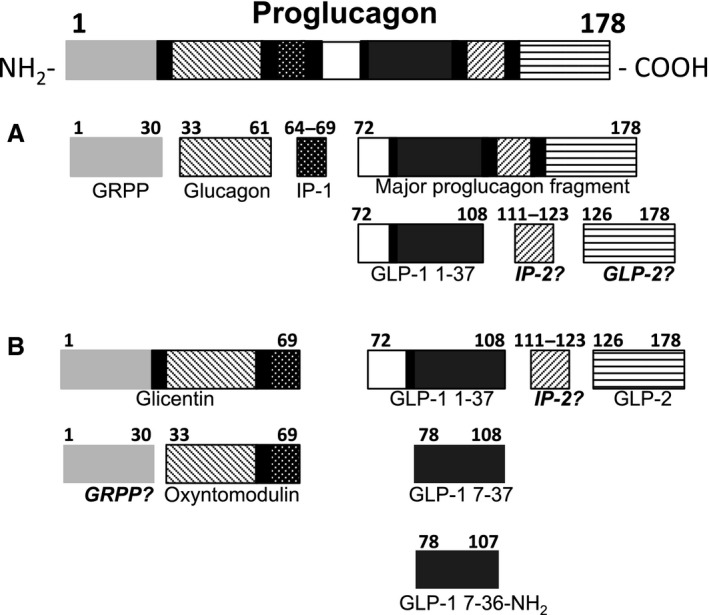
Theoretical processing of proglucagon in the pancreas and intestine. GRPP, glicentin‐related pancreatic polypeptide; IP‐1, intervening peptide; GLP‐1, glucagon‐like peptide 1; IP‐2, intervening peptide 2; GLP‐2, glucagon‐like peptide 2; modified from Holst (Holst 1997). Peptides labeled in italics have been presumed to be present but have not been identified in mammals.
Considerably less is known of the actions of GRPP, which has been little studied to date. This 30‐amino acid peptide was originally purified from porcine pancreas (Thim and Moody 1982). It has also been identified in rat, mouse, and human islets along with insulin, glucagon, and amylin (Minerva et al. 2008; Stewart et al. 2011; Taylor et al. 2013). A further peptide, closely related to GRPP, GRPP‐like peptide (GRPP‐LP) was also identified in Wistar rat islets (Stewart et al. 2011). This molecule is similar to GRPP but differs in that GRPP‐LP has the carboxyterminal sequence—PNQINE (Table 1).
Table 1.
Comparison of GRPP amino acid sequences derived from proglucagon
| Gene ID | Species | Sequence | Sequence identity |
|---|---|---|---|
| gi¦204370 | Rattus norvegicus | HAPQDTEENARSFPASQTEPLEDPDQINED | 30/30 (100%) |
| rGRPP‐LP (Patzelt and Schiltz 1984) | Rattus norvegicus | HAPQDTEENARSFPASQTEPLEDPNQINE | 28/30 (97%) |
| gi¦183270 | Homo sapiens | RSLQDTEEKSRSFSASQADPLSDPDQMNED | 20/30 (67%) |
Comparison of GRPP and GRPP‐LP from rat preproglucagon and GRPP from human preproglucagon. Residues differing from the GRPP‐like peptide are bold and underlined.
The identification of both GRPP and GRPP‐LP as potential products from the proglucagon precursor suggests that both peptides could be secreted from the α‐cell along with glucagon. As yet, there are currently no immunological tools or antagonists for GRPP or GRPP‐LP, meaning that understanding of the expression and function of this peptide is in its infancy. Glucagon fulfills a major physiological role in maintaining glucose homeostasis through its effects in the liver. The effects of GRPP and GRPP‐LP on liver have yet to be investigated.
Both GLP‐1 and glucagon are reported to act on the pancreatic β‐cell to enhance glucose‐stimulated insulin secretion (GSIS) (Metz et al. 1983; Zielmann et al. 1985; Holst et al. 1987; Shima et al. 1988; Goke et al. 1989; Ishizuka et al. 1991; Jia et al. 1995; Vahl et al. 2003). However, we know of only one study reporting the effects of GRPP on GSIS. In that study, which employed a local circulation preparation of the canine pancreas, the administration of human GRPP and human glicentin 1–16 reportedly caused slight increases in plasma insulin and decreases in plasma glucagon (Ohneda and Ohneda 1988). This tantalizing finding suggests that GRPP might also be an unrecognized regulator of GSIS. We, therefore, questioned whether rGRPP and rGRPP‐LP can act like GLP‐1 to modulate GSIS in the rat pancreas and/or, like glucagon, to modulate glucose production in the liver.
Materials and Methods
Synthesis of rGRPP and rGRPP‐LP
Peptides were synthesized by solid‐phase peptide synthesis, using the 9‐fluorenylmethoxycarbonyl/tertiary‐butyl (Fmoc/tBu) method on a 0.1‐mmol scale. Briefly, aminomethyl resin was prepared as described (Harris et al. 2011), functionalized with the appropriate linker, and the peptide elongated using a microwave peptide synthesizer (CEM, NC) as previously described (Harris et al. 2008). The peptides were cleaved from the resin with concomitant removal of side‐chain protecting groups with 94% trifluoroacetic acid, 1% triisopropylsilane, 2.5% water, and 2.5% ethane dithiol (v/v/v/v) for 2–3 h, precipitated with cold diethyl ether, isolated by centrifugation, dissolved in 50% aqueous acetonitrile containing 0.1% trifluoroacetic acid, and lyophilized. Crude peptides were purified by semipreparative reversed phase HPLC. Fractions containing the pure peptide were identified by electrospray mass spectrometry and/or HPLC, pooled, and lyophilized. All peptides were >95% purity as judged by integration of the HPLC chromatogram at 210 nm, and peptide masses were confirmed by electrospray mass spectrometry.
Animals
All studies were performed under approval of the University of Auckland Animal Ethics Committee and were consistent with the ARRIVE guidelines (Kilkenny et al. 2012). Male Sprague‐Dawley rats (220–300 g) were obtained from a breeding colony in the Integrated Physiology Unit, School of Biological Sciences at the University of Auckland. Animals were housed in standard environmental conditions under a 12‐hour light/dark cycle and a temperature of 20 ± 1°C. They were maintained on Teklad TB 2018 (Harlan, UK) rat chow and tap water ad libitum.
Isolated liver perfusion
Under general anesthesia (isoflurane 2–5%; 2 L min−1 O2 via nasal cone), nonfasted rats underwent laparotomy between 8:00 and 9:00 am on the experimental day. The liver was removed and quickly connected to a custom‐made, temperature‐controlled, organ perfusion system, whereby it was perfused (in nonrecirculating mode) in a manner similar to that reported previously (Englisch et al. 2000; Whiting et al. 2013). Livers were perfused at a rate of 2 mL g−1 min−1 with perfusion media containing final concentrations (in mmol L−1): NaCl, 128; NaHCO3, 23.9; KCl, 6.0; CaCl2, 2.4; MgSO4, 1.18; D‐glucose, 5; 3‐(‐N‐morpholino)propane sulfonate, 1.29; with fatty‐acid‐free BSA, 0.5% (w/v) at pH 7.4 gassed with O2:CO2 95:5% (v/v) at 37°C. Each liver was stabilized for 40 min: thereafter, hormone‐containing solutions or vehicle (buffer only) was delivered via a side‐arm infusion. Hormones were also investigated with regard to their ability to attenuate epinephrine‐stimulated hepatic glucose output (Epinephrine, Sigma‐Aldrich, St. Louis, MO). Epinephrine was added 30 min after the addition of hormones was commenced. All livers were viable over the period of the perfusion as indicated by their prompt responsiveness to glucagon (Glucagen; Novodisk, Novo Allé, Denmark), which was added alone to the buffer at 60 min.
Isolated pancreas perfusion
General aspects of the preparation for the isolated pancreas perfusions were the same as those for the liver perfusion studies (above). Particular aspects of the preparation for the pancreas perfusion studies were as follows. After laparotomy, the pancreas and duodenum were removed en bloc and perfused (in nonrecirculating mode) consistent with our previous report (Buchanan et al. 2001). Each pancreatic preparation was quickly connected to the custom‐made, temperature‐controlled, organ perfusion system and perfused at 3 mL min−1 with oxygenated perfusion buffer (containing at final concentrations in mmol L−1): NaCl, 112; NaHCO3, 29.3; KCl, 4.4; CaCl2, 2.4; MgSO4, 1.2; D‐glucose, 5.0; with BSA, 0.5% (w/v), Dextran 4% (w/v) at pH 7.4 gassed with O2:CO2 95:5% (v/v) at 37°C. Peristalsis of the duodenum was visible throughout the entire perfusion period as an indicator of the preparation's viability. Each pancreas was stabilized for 30 min before hormones or vehicle (buffer only) was delivered via a side‐arm infusion. The human GLP‐1 7–36 was obtained from Abcam (Cambridge, UK).
Measurement of organ perfusion parameters
Insulin was measured in perfusate from the pancreas preparation using an ELISA (Merck Millipore, Darmstadt, Germany) and glucagon using an RIA (Merck Millipore, Darmstadt, Germany): however, concentrations of the latter were uniformly below the minimum detectable concentration (data not shown). Glucose and lactate concentrations were measured in both organ preparations using a YSI2300 Stat glucose and lactate analyzer (Yellow Springs, Ohio). Portal pressure was measured in isolated‐perfused liver preparations in mmH2O via a T‐tube located 10 cm upstream from the preparation.
Transfection of Cos 7 cells
Cos 7 cells were maintained and subcultured as previously described (Bailey and Hay 2006) and plated at a density of 15,000 cells per well into 96‐well plates 1 day prior to transfection. Expression clones for the human GLP‐1 receptor (hGLP‐1R) and human glucagon receptor (hGL‐R) were kindly provided by Professor Patrick Sexton (Monash University, Melbourne, Australia) and were here transfected using polyethylenimine as previously described with 0.25 μg of plasmid DNA per well (Bailey and Hay 2006). Cyclic AMP assays were performed 48 h after transfection (Bailey and Hay 2006). The human GRPP was obtained from Bachem (Bubendorf, Switzerland).
Cyclic AMP stimulation of transfected Cos 7 cells
Transfected cells were serum‐deprived in DMEM containing 1 mmol L−1 IBMX and 0.1% (w/v) bovine serum albumin (BSA) for 30 min. GRPP, GRPP‐LP, glucagon, or GLP‐1 7–36 at different concentrations were added to wells, along with forskolin (50 μmol L−1, Tocris Bioscience, Wiltshire, UK) which was included as a positive control on each plate. Plates were incubated at 37°C for 10 min. For antagonist experiments, GRPP was preincubated with the cells for 5 min before glucagon or hGLP‐1 7–36 were added at indicated concentrations. Media were then aspirated from the wells and reactions terminated by addition of ice‐cold absolute ethanol. Ethanol was then evaporated to dryness and cell extracts re‐suspended in 45 μL cAMP assay buffer: cAMP concentrations were determined using ALPHA‐screen assay kits (Perkin Elmer, Massachusetts) as previously described (Gingell et al. 2010).
Glucose stimulation of isolated rat islets
Rat islets were isolated by the collagenase digestion method. For each experiment, 2–4 rats were used. Rats were killed under anesthetic (isoflurane 2–5%; 2 L min−1 O2 via nasal cone) and collagenase solution (collagenase type 4, 350 μ mg−1; Bioconcept, Switzerland) at 0.05 mg mL−1 infused into the common bile duct after occlusion of the distal end just proximal to the duodenum. Pancreata were then excised, and incubated in a waterbath at 37°C for 30 min. At the end of digestion, the tissue was shaken for 1 min and washed with HBSS. Tube contents were filtered through a 500 μm plastic mesh to discard any undigested tissue and washed again in HBSS. The resulting pellet was filtered onto a 70‐μmol L−1 strainer, and islets were then transferred into RPMI 1640 medium containing 10% BSA and 1% of penicillin/streptomycin and handpicked. Islets were plated at 10 islets/well and cultured in RPMI media for 48 h. Insulin secretion was measured by static incubation using KRB (in mmol L−1: NaCl, 129; NaHCO3, 5; KCl, 4.8; KH2PO4, 1.2; MgSO4, 1.2; HEPES, 10; CaCl2, 2.5; 0.1% (w/v) BSA. The incubation experiments were started with a 30 min preincubation of the islets in KRB with 2.8 mmol L−1 glucose. Islets were then incubated for 1 h each in KRB supplemented with glucose (2.8 and 16.7 mmol L−1) and different concentrations of hGRPP (1–100 nmol L−1) or 20 nmol L−1 GLP‐1 7–36. The medium was collected for each incubation period and insulin content of islets were extracted using 0.18 mol L−1 HCl‐70% ethanol. Insulin concentration was measured by ELISA technique (Mercodia, Sweden). Experiments were repeated three times with three incubations per condition in each experiment.
Data analysis and statistics
Maximum pancreatic first‐phase insulin secretion in the presence of hormone infusion was contrasted with corresponding control values by unpaired Welch's t‐tests for preplanned comparisons, to allow for unequal variance between groups. Linear mixed‐effects models were fitted by restricted maximum likelihood (LME‐REML) to data obtained from each indicated phase of isolated perfused–organs studies, using S‐PLUS version 8.2 (TIBCO, Spotfire). Calculated P‐values <0.05 are presented in the article and reflect the likelihood ratio between two models, the effect in question (treatment, time, or treatment:time) against the model without the effect in question. Insulin secreted from isolated islets was expressed as a percentage of insulin content and compared using one‐way ANOVA (GraphPad Prism version 6.0: GraphPad Software Inc., San Diego, CA). Cyclic AMP data have been normalized to the response obtained to forskolin and fitted to obtain concentration–response curves using a three‐parameter logistic equation (GraphPad Prism version 6.0: GraphPad Software Inc., San Diego, CA): pEC50 values were obtained from the concentration–response curves and compared using one‐way ANOVA. All data are presented as mean ± SEM of three to four independent experiments.
Results
Rat GRPP and rGRPP‐LP do not modify glucose production in the perfused rat liver
The effects of hormones on glucose production, lactate production, and portal pressure in perfused livers are presented in Figure 2. Glucose output decreased slightly in all 6 groups over the 30‐min basal period, but there were no significant differences between any of the 6 groups in this phase. At t = 30 min, the addition of 100 pmol g−1 min−1 epinephrine evoked an increase in glucose output in all groups that was not modified by rGRPP or GRPP‐LP. At t = 60 min, infusion of vehicle, rGRPP, rGRPP‐LP, and epinephrine were terminated, and an infusion of 2.3 pmol g−1 min−1 glucagon commenced to check viability and the potential for glucagon receptor‐responsiveness. Glucagon increased glucose output by at least 2.5‐fold. There were no significant differences in the glucose responses of livers to glucagon between those perfused with any treatment compared with the control group. During the basal period, lactate output and portal pressure were not different between all groups verifying viability of the preparations. Epinephrine induced the expected elevation of lactate release and portal pressure in all groups without differences between the groups. During glucagon infusion, lactate output decreased to below basal levels and portal pressure returned to basal levels, consistent with the stimulation of gluconeogenesis—further indications of viability (Exton and Park 1968). There were no significant differences in lactate and portal pressure between livers perfused with any treatment compared with the control group.
Figure 2.
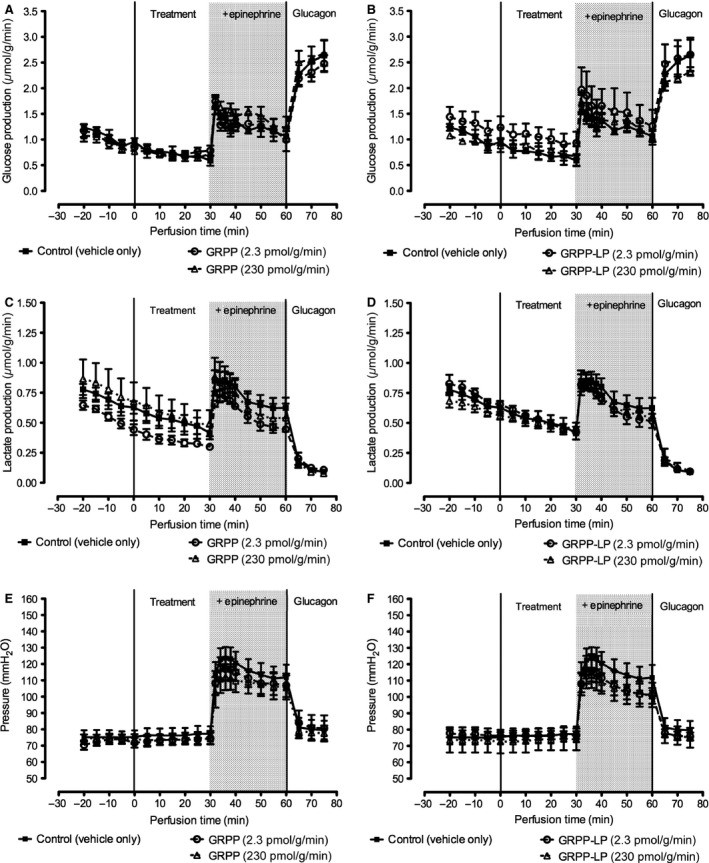
Effect of rGRPP and rGRPP‐LP on glucose output, lactate output, and portal pressure in the isolated perfused rat liver. Livers from Sprague‐Dawley rats were isolated and perfused with rGRPP (left panels: 2.3 or 230 pmol g−1 min−1, n = 5 and n = 3, respectively), rGRPP‐LP (right panels: 2.3 or 230 pmol g−1 min−1, n = 5 and n = 3), or vehicle (all panels: control, n = 5). Livers were perfused with hormones or vehicle from 0 to 60 min with 0–30 min as the basal period. At 30 min, the addition of epinephrine (100 pmol g−1 min−1, 30–60 min) increased glucose production (A–B), lactate production (C–D), and portal pressure (E–F). Neither dose of rGRPP nor rGRPP‐LP modified these parameters compared with control values. Glucagon (2.3 pmol g−1 min−1) alone was administered from 60 to 75 min to verify organ viability.
Rat GRPP and rGRPP‐LP inhibit GSIS in the isolated perfused rat pancreas
No significant difference in insulin secretion was observed during the perfusion of 5‐mmol L−1 glucose between pancreases perfused with rGRPP, rGRPP‐LP, and glucagon compared with the control group (Fig. 3). When hGLP‐1 7–36 was perfused, there was a pulsatile change in insulin secretion over the 20 min during 5‐mmol L−1 glucose perfusion. At 20 min, glucose was increased to 20 mmol L−1 in all groups. The control (vehicle) group demonstrated the characteristic biphasic increase in insulin secretion, and this response was substantially elevated by infusion of the incretin hGLP‐1 7–36. The main effect of GLP‐1 7–36 occurs during the first phase, whereas during the second phase of GSIS, the curves for this hormone and for the control infusion are essentially parallel and this is reflected in the nonsignificant P value for the time:treatment (Table 2). In contrast, rGRPP, rGRPP‐LP, and glucagon significantly suppressed GSIS during the 20‐min perfusion with 20‐mmol L−1 glucose (Fig. 3). Whereas the second phase progressively increased in the control infusion, these peptides exert an ongoing suppression of insulin secretion throughout the second phase, and these effects are reflected in the strongly significant time‐dependent P‐values for each of these infusions (Table 2). Lactate was also measured in the perfusate at 5‐min intervals, and no significant differences were found between any of the groups.
Figure 3.
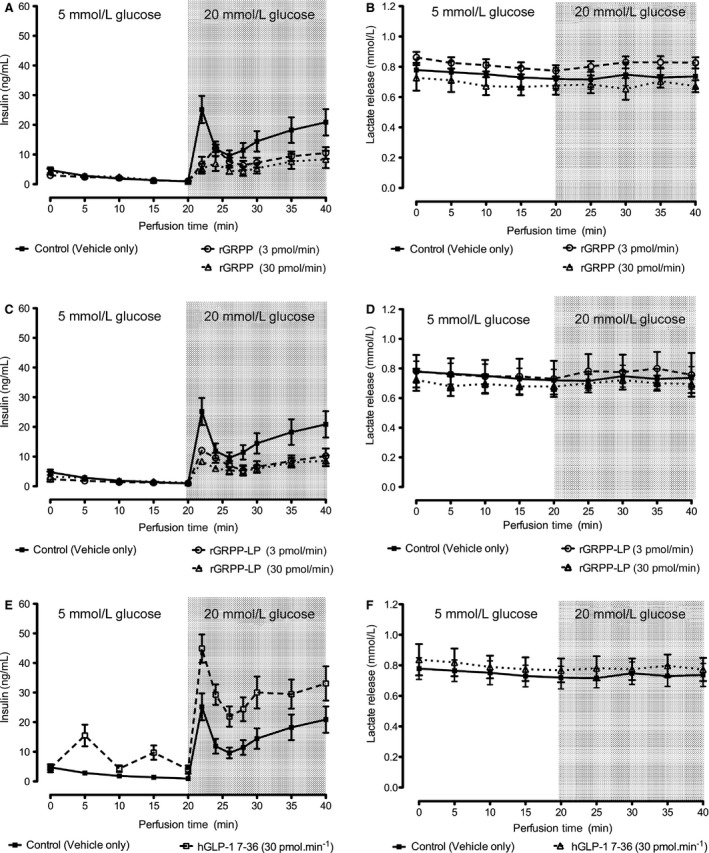
Effects of proglucagon products on insulin secretion in the isolated‐perfused rat pancreas. Pancreases isolated from Sprague‐Dawley rats were perfused with 5‐mmol L−1 glucose and either 30 pmol min−1 hGLP‐1 7–36 (A, B), 3 or 30 pmol min−1 rGRPP (C,D), 3 or 30 pmol min−1 rGRPP‐LP (E, F), or vehicle (A–F, solid lines). Glucose was increased to 20 mmol L−1 at 20 min (shaded area): n = 5/group. hGLP‐1 7–36 increased insulin secretion at 5‐mmol L−1 glucose and at 20‐mmol L−1 glucose compared with the control (A). By contrast, rGRPP and rGRPP‐LP all inhibited GSIS (C,E). Lactate output (B, D, F) remained at a constant level throughout the perfusion in all groups, and there were no significant differences between the groups.
Table 2.
Effects of infusion of Gcg‐derived peptide hormones on the first and second phases of insulin secretion from isolated perfused rat pancreas preparations
| Peptide infusion | First phase | Second phase | ||
|---|---|---|---|---|
| Time | Treatment | Time:Treatment | ||
| GLP‐1 7‐36, 30 pmol−1 min−1 | 0.015 | 0.0005 | NS | NS |
| rGRPP, 30 pmol−1 min−1 | 0.009 | <0.0001 | NS | 0.0039 |
| rGRPP, 3 pmol−1 min−1 | 0.011 | <0.0001 | NS | 0.0063 |
| rGRPP‐LP, 30 pmol−1 min−1 | 0.019 | <0.0001 | 0.047 | 0.0006 |
| rGRPP‐LP, 3 pmol−1 min−1 | 0.044 | <0.0001 | NS | 0.018 |
Calculated P‐values derived from Welch's t‐tests for first‐phase insulin secretion data, and linear mixed‐effects models (LMEM) of the form (Insulin Secretion ~ [Time + Treatment + Time:Treatment]) fitted by restricted maximum likelihood to data describing the second phase of insulin secretion following infusion of peptide solutions. Time values used for these calculations were at t = 22 min for the first phase and from t = 26 min until the end of the infusion at t = 40 for the second phase. NS, not significant (i.e., P > 0.05) comparing insulin secretion in control pancreases with that in pancreases perfused with hGLP‐1 7–36 (30 pmol min−1), rGRPP (3 or 30 pmol min−1), or rGRPP‐LP (3 or 30 pmol min−1).
Human GRPP does not affect insulin secretion in rat islets
Incubation of islets in 16.7‐mmol L−1 glucose alone increased insulin secretion compared with insulin secreted when islets were incubated in 2.8‐mmol L−1 glucose (Fig. 4). As expected, 20‐nmol L−1 hGLP‐1 increased insulin secretion in rat islets at both 2.8‐mmol L−1 and 16.7‐mmol L−1 glucose compared with the corresponding controls (Fig. 4). The addition of hGRPP at concentrations of 1, 10, and 100 nmol L−1 did not affect insulin secretion at 2.8‐mmol L−1 glucose and at 16.7‐mmol L−1 glucose compared with the corresponding controls (Fig. 4).
Figure 4.
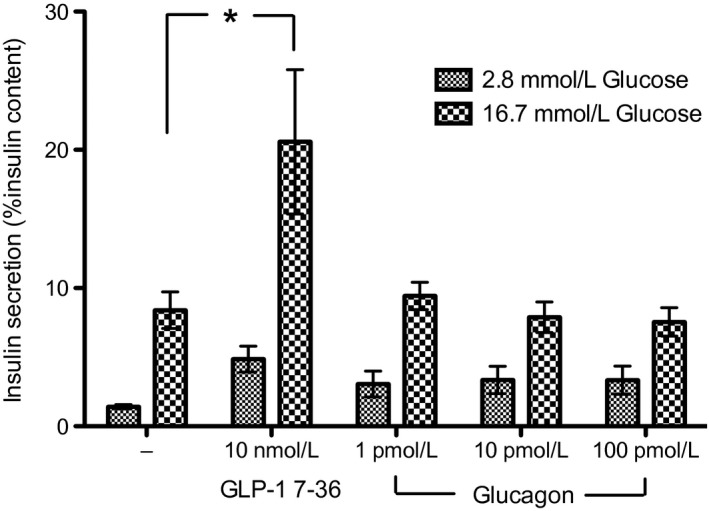
Human GRPP and glucagon do not affect GSIS in isolated rat islets. Effects of hGRPP on basal (2.8 mmol L−1 glucose) and stimulated (16.7 mmol L−1 glucose) insulin secretion. Each bar represents mean ± SEM from 8 to 9 incubations of 10 islets/well performed in three separate experiments. GLP‐1 7–36 increases GSIS compared with untreated islets (*P < 0.05 compared with control). In contrast, hGRPP at all concentrations did not affect basal or stimulated insulin compared with untreated islets.
Rat GRPP and rGRPP‐LP do not activate or antagonize cAMP production via GL‐R or GLP‐1R in transfected Cos 7 cells
The receptor for GRPP is currently unknown. We investigated the possibility that GRPP or GRPP‐LP might act via either the GLP‐1R or GL‐R in transfected Cos 7 cells. Glucagon at the hGL‐R and hGLP‐1 7–36 at the hGLP‐1R produced concentration‐dependent increases in cAMP (Fig. 5). The mean pEC50 (±SEM) for glucagon was 10.30 (0.14, n = 4) and for hGLP‐1 7–36 it was 9.59 (0.43, n = 3): by contrast, neither rGRPP nor rGRPP‐LP stimulated cAMP production at either receptor (Fig. 5). Furthermore, there were no shifts in the GLP‐1 7–36 or glucagon concentration–response curves in the presence of rGRPP (P > 0.05, Fig. 5). The hGL‐R exhibits 82% identity to the rat receptor (Lok et al. 1994; MacNeil et al. 1994). Furthermore, RAMP2 has an association with the GL‐R receptor (Christopoulos et al. 2003; Weston et al. 2015). In an effort to determine whether RAMP2 is required for hGL‐R activation with hGRPP, Cos 7 cells were transfected with hGL‐R with the addition of a vector or RAMP2. However, in this preliminary experiment, hGRPP did not stimulate cAMP production at hGL‐R alone or in the presence of RAMP2 (data not shown).
Figure 5.
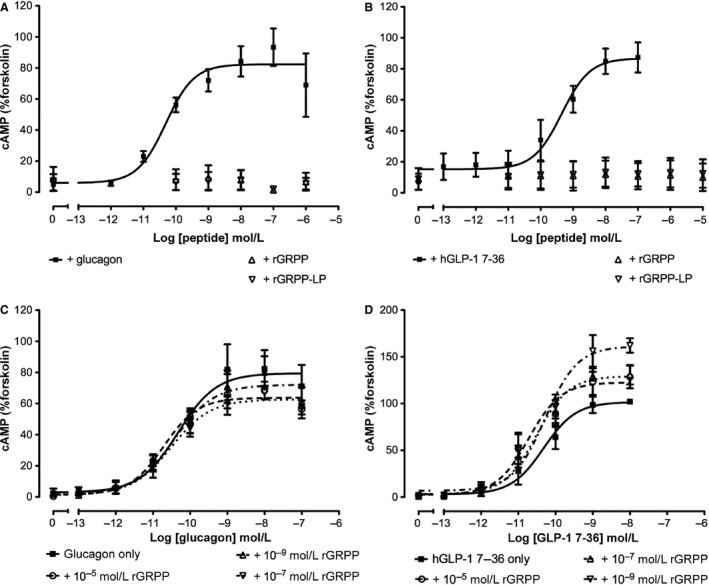
GRPP or GRPP‐LP does not stimulate or inhibit cAMP production through GL‐R and GLP‐1R. Glucagon stimulated cAMP production (A) and hGLP‐1 7–36 stimulated cAMP production (B) in Cos 7 cells transfected with their respective receptors, hGL‐R and hGLP‐1R. In contrast, rGRPP and rGRPP‐LP did not stimulate cAMP production at either receptor (A, B). Rat GRPP did not modify glucagon‐stimulated (C) or GLP‐1 7–36‐stimulated (D) cAMP production in Cos 7 cells transfected with hGL‐R or hGLP‐1R, respectively.
Discussion
GRPP and GRPP‐LP inhibited the initial first phase of GSIS in the isolated rat pancreas, and this inhibition was additionally sustained in the second phase. All pancreata were viable over the period of the perfusion as indicated by a lack of any statistically significant change in lactate output between the groups. This finding is noteworthy as it offers the possibility that GRPP and GRPP‐LP could play physiological roles in the regulation of GSIS, along with the other known proglucagon‐derived products, glucagon, and GLP‐1.
GLP‐1 7–36 evoked a significant increase in GSIS in both the rat pancreas and isolated islets. Although it is recognized that GLP‐1 7–36 amide and GLP‐1 7–37 are the more likely forms produced, it has been reported that the carboxyterminal of GLP‐1 does not have a significant effect on insulin secretion in the perfused pancreas (Suzuki et al. 1989).
Although GRPP and rat GRPP‐LP have been identified in islets, it has yet to be established whether GRPP and GRPP‐LP are secreted from α‐cells in response to physiological stimuli, such as glucose or arginine. Glucagon is secreted from the α‐cells of the pancreas in response to a decrease in blood glucose and is also stimulated by arginine (Weir et al. 1976; Dunbar and Brown 1984; Myers et al. 1991). Given that GRPP and GRPP‐LP are also processing products from the same precursor, it could be proposed that these peptides are also secreted in response to low glucose. However, this proposal does not easily fit with the present findings that the addition of GRPP and GRPP‐LP inhibits GSIS. Furthermore, neither peptide inhibited insulin secretion against a low glucose background. According to the theoretical model of proglucagon, processing for each tissue type is as put forward by Holst (Fig. 1). GRPP could also be present in the intestinal L‐cells along with oxyntomodulin; however, this has yet to be explored.
Previously, porcine GRPP was found to have a small effect on glucagon and insulin secretion in the canine pancreas, whereby insulin was possibly increased and glucagon decreased (Ohneda and Ohneda 1988). Furthermore, human glicentin 12–69 (which encompasses both GRPP and glucagon sequences) enhanced GSIS and inhibited glucagon secretion in the perfused canine pancreas (Yanaihara et al. 1985). The precise experimental conditions such as amount of glucose and experimental model used may have influences on the different outcomes; however, the reason for the opposing actions of GRPP between these studies could be due to species differences. Interestingly, in our study, GRPP did not affect GSIS in isolated rat islets. GRPP is not fully conserved between species; hGRPP has 67% of its amino acids conserved with rat (Table 1). The lack of effect of hGRPP in rat islets could relate to this. Nevertheless, ours is the first matched‐species study in the whole pancreas and shows that rGRPP has an effect on insulin secretion: this finding warrants further investigation.
GRPP and GRPP‐LP may inhibit insulin secretion indirectly, perhaps by influencing other islet‐derived hormones known to modulate hormone secretion from the β‐cell. For example, somatostatin (SST) is an influential peptide produced in pancreatic‐islet δ‐cells that inhibits both insulin and glucagon secretion. In humans, infusion of SST decreased arginine‐stimulated plasma insulin and glucagon levels in a dose‐dependent manner (Skamene and Patel 1984). In the isolated‐perfused rat pancreas, perifused rat islets, and cultured islets, SST inhibits GSIS as well as arginine‐stimulated glucagon secretion (Norfleet et al. 1975; Fujimoto 1981; Bolaffi et al. 1990). Recently, an additional peptide of the SST preprohormone, neuronostatin, has been identified and also found to inhibit GSIS in rat islets (Salvatori et al. 2014). Furthermore, the authors found that this effect was more likely a result of neuronostatin directly acting on the α‐cell as administration of neuronostatin in both islets, and the α‐cell line αTC1‐9 increases glucagon secretion, but failed to affect insulin secretion in the β‐cell line INS 832/13 where α‐cells are not present (Salvatori et al. 2014). Other islet hormones influence the secretion of SST. For example, exogenous glucagon at supraphysiological levels in the presence of glucose increases SST in isolated rat islets (Patel et al. 1979; Dolais‐Kitabgi et al. 1981). Therefore, it is possible that the administration of rGRPP or rGRPP‐LP in the perfused rat pancreas could increase SST or neuronostatin secretion, inhibiting GSIS. However, there is as yet no direct evidence for this putative mechanism.
These current findings raise the possibility that there is a more complex feedback system present in islets, whereby islet‐derived peptide hormones could influence each other's production and effects. It is important to acknowledge that although physiological events such as GSIS are similar between rodent and human, the functional organization within the islet to achieve this broad similarity is different between rodent and man (Barker et al. 2013; Caicedo 2013). Human islets contain more α‐cells than mouse islets and, unlike rodents, these cells are aligned along blood vessels with no particular order or arrangement within the islet (Brissova et al. 2005, 2015; Cabrera et al. 2006). As a result, the human islet contains a heterogeneous cell population through which blood flows. It is, therefore, unlikely that there is a hierarchy in the sequence in which the different endocrine cells are perfused in the human islet. This is thought to predispose human islets to strong paracrine interactions (Barker et al. 2013). These anatomical arrangements are likely to have consequences for the effects of GRPP in human islets. Therefore, it is also of interest to investigate the role human GRPP plays in the human pancreas. Further investigations are needed to more fully comprehend how islet hormones influence each other with the ultimate goal of understanding how these various influences might sum to modulate GSIS in health and disease.
Given that we found an inhibitory effect of rGRPP and rGRPP‐LP on GSIS in the isolated perfused rat pancreas, and glucagon and GLP‐1 increase cAMP production through binding to and activating signaling pathways via GLP‐1R and GL‐R, respectively, we investigated the activity of rGRPP and rGRPP‐LP at these receptors to search for a possible mechanism for their action. However, neither rGRPP nor rGRPP‐LP activated these receptors when added alone: nor did rGRPP block cAMP production evoked by glucagon or GLP‐1 7–36. It is possible that GRPP and GRPP‐LP could activate another pathway via these receptors. Further studies are needed with rat receptors and radioligand binding assays to confirm the lack of interaction at the GL‐R and GLP‐1R. However, the lack of effect of GRPP and GRPP‐LP in the liver supports a lack of activation of the glucagon receptor.
There is also the possibility that GRPP and GRPP‐LP may require a receptor activity‐modifying protein (RAMP). In transfected HEK‐293 cells, GL‐R was found to interact specifically with RAMP‐2, and not other RAMPs, leading to translocation of RAMP‐2 to the cell surface as well as an increase in total RAMP‐2 levels (Christopoulos et al. 2003; Weston et al. 2015). The specific pairing of GL‐R with only one of the RAMPs and the known impact for their diversity on function, particularly on the calcitonin family of receptors, suggests that this may be biologically significant (Christopoulos et al. 2003). However, a preliminary experiment where hGRPP was tested in Cos 7 cells transfected with hGL‐R and with the addition of a vector or RAMP2 indicates that the modification of this receptor by RAMP2 does not allow activation by GRPP. Further, it suggests that the lack of effect of rGRPP on cAMP production at the human receptors is not due to species differences.
Rat GRPP and rGRPP‐LP may exert their effects through an as‐yet unknown receptor such as one of the many orphan G‐protein‐coupled receptors expressed in islets but whose effects on islet hormone secretion are largely unknown due to a lack of currently available pharmacological tools (Nambi and Aiyar 2003; Amisten et al. 2013). Two possible mechanisms that have been found to enhance GSIS and both involve an increase in intracellular Ca2+ (Ahren 2009). The activation of the receptor‐coupled enzyme phospholipase C (PLC) leads to the production of diacylglycerol (DAG), which liberates Ca2+ from intracellular storage sites, thereby increasing exocytosis of insulin. Second, increased intracellular levels of cAMP activate protein kinase A (PKA), which activates kinases that are important in exocytosis (Ahren 2009). It is possible that GRPP and GRPP‐LP are capable of inhibiting either of these pathways through any of these orphan G‐protein‐coupled receptors.
GRPP and GRPP‐LP inhibited GSIS in the rat pancreas. This finding is significant and leads to the question as to whether GRPP and/or GRPP‐LP are physiological regulators of glucose homeostasis. This requires further exploration. The importance of controlling GSIS in diabetes together with the potential of rGRPP and rGRPP‐LP to regulate GSIS warrants further investigation into the putative effects and mechanisms of GRPP.
Conflict of Interest
None declared.
Acknowledgments
We thank C. A. Tse for her excellent research management and for proof‐reading the article and A. Petzer for her excellent support with the animal experiments.
Whiting L., Stewart K. W., Hay D. L., Harris P. W., Choong Y. S., Phillips A. R. J., Brimble M. A., Cooper G. J. S.. Glicentin‐related pancreatic polypeptide inhibits glucose‐stimulated insulin secretion from the isolated pancreas of adult male rats. Physiol Rep, 3 (12), 2015, e12638, doi: 10.14814/phy2.12638
Funding Information
This work was funded by grants from the Endocore Research Associates, the Maurice and Phyllis Paykel Trust, Lottery Health (New Zealand), the University of Auckland, and the Waikato Institute of Technology and by program grants from the Health Research Council of New Zealand and the Ministry for Business, Innovation, and Employment (New Zealand) and was facilitated by the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network.
References
- Ahren, B. 2009. Islet G protein‐coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discovery 8:369–385. [DOI] [PubMed] [Google Scholar]
- Amisten, S. , Salehi A., Rorsman P., Jones P. M., and Persaud S. J.. 2013. An atlas and functional analysis of G‐protein coupled receptors in human islets of Langerhans. Pharmacol. Ther. 139:359–391. [DOI] [PubMed] [Google Scholar]
- Bailey, R. J. , and Hay D. L.. 2006. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides 27:1367–1375. [DOI] [PubMed] [Google Scholar]
- Barker, C. J. , Leibiger I. B., and Berggren P. O.. 2013. The pancreatic islet as a signaling hub. Adv. Biol. Regul. 53:156–163. [DOI] [PubMed] [Google Scholar]
- Bolaffi, J. L. , Rodd G., Ma Y. H., and Grodsky G. M.. 1990. Effect of glucagon or somatostatin on desensitized insulin secretion. Endocrinology 126:1750–1755. [DOI] [PubMed] [Google Scholar]
- Brissova, M. , Fowler M. J., Nicholson W. E., Chu A., Hirshberg B., Harlan D. M., et al. 2005. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53:1087–1097. [DOI] [PubMed] [Google Scholar]
- Brissova, M. , Shostak A., Fligner C. L., Revetta F. L., Washington M. K., Powers A. C., et al. 2015. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J. Histochem. Cytochem. 63:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan, C. M. , Phillips A. R., and Cooper G. J.. 2001. Preptin derived from proinsulin‐like growth factor II (proIGF‐II) is secreted from pancreatic islet beta‐cells and enhances insulin secretion. Biochem. J. 360:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera, O. , Berman D. M., Kenyon N. S., Ricordi C., Berggren P. O., and Caicedo A.. 2006. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. U. S. A. 103:2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo, A. 2013. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin. Cell Dev. Biol. 24:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos, A. , Christopoulos G., Morfis M., Udawela M., Laburthe M., Couvineau A., et al. 2003. Novel receptor partners and function of receptor activity‐modifying proteins. J. Biol. Chem. 278:3293–3297. [DOI] [PubMed] [Google Scholar]
- Dolais‐Kitabgi, J. , Kitabgi P., and Freychet P.. 1981. Glucose and glucagon do stimulate somatostatin release from isolated pancreatic islets. Diabetologia 21:238. [DOI] [PubMed] [Google Scholar]
- Dunbar, J. C. , and Brown A.. 1984. Glucagon secretion by dispersed alpha cell enriched islets from streptozotocin treated hamsters in perifusion. Horm. Metab. Res. 16:221–225. [DOI] [PubMed] [Google Scholar]
- Englisch, R. , Wurzinger R., Furnsinn C., Schneider B., Frisch H., Waldhausl W., et al. 2000. Effects of insulin‐like growth factor I on basal and stimulated glucose fluxes in rat liver. Biochem. J. 351:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton, J. H. , and Park C. R.. 1968. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3′,5′‐monophosphate on gluconeogenesis in the perfused rat liver. J. Biol. Chem. 243:4189–4196. [PubMed] [Google Scholar]
- Fujimoto, W. Y. 1981. Effect of gut peptides on glucose‐stimulated insulin release by monolayer cultures of neonatal rat islet cells. Horm. Metab. Res. 13:135–138. [DOI] [PubMed] [Google Scholar]
- Gingell, J. J. , Qi T., Bailey R. J., and Hay D. L.. 2010. A key role for tryptophan 84 in receptor activity‐modifying protein 1 in the amylin 1 receptor. Peptides 31:1400–1404. [DOI] [PubMed] [Google Scholar]
- Goke, R. , Fehmann H. C., Richter G., Trautmann M., and Goke B.. 1989. Interaction of glucagon‐like peptide‐1(7‐36)amide and somatostatin‐14 in RINm5F cells and in the perfused rat pancreas. Pancreas 4:668–673. [DOI] [PubMed] [Google Scholar]
- Harris, P. W. R. , Williams G. M., Shepherd P., and Brimble M. A.. 2008. The synthesis of phosphopeptides using microwave‐assisted solid phase peptide synthesis. Int. J. Pept. Res. Ther. 14:387–392. [Google Scholar]
- Harris, P. W. R. , Yang S. H., and Brimble M. A.. 2011. An improved procedure for the preparation of aminomethyl polystyrene resin and its use in solid phase (peptide) synthesis. Tetrahedron Lett. 52:6024–6026. [Google Scholar]
- Holst, J. J. 1997. Enteroglucagon. Annu. Rev. Physiol. 59:257–271. [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , Orskov C., Nielsen O. V., and Schwartz T. W.. 1987. Truncated glucagon‐like peptide I, an insulin‐releasing hormone from the distal gut. FEBS Lett. 211:169–174. [DOI] [PubMed] [Google Scholar]
- Ishizuka, J. , Tatemoto K., Cohn D. V., Thompson J. C., and Greeley G. H. Jr. 1991. Effects of pancreastatin and chromogranin A on insulin release stimulated by various insulinotropic agents. Regul. Pept. 34:25–32. [DOI] [PubMed] [Google Scholar]
- Jeppesen, P. B. , Hartmann B., Thulesen J., Graff J., Lohmann J., Hansen B. S., et al. 2001. Glucagon‐like peptide 2 improves nutrient absorption and nutritional status in short‐bowel patients with no colon. Gastroenterology 120:806–815. [DOI] [PubMed] [Google Scholar]
- Jia, X. , Brown J. C., Ma P., Pederson R. A., and McIntosh C. H.. 1995. Effects of glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐I‐(7‐36) on insulin secretion. Am. J. Physiol. 268:E645–E651. [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne W. J., Cuthill I. C., Emerson M., and Altman D. G.. 2012. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage 20:256–260. [DOI] [PubMed] [Google Scholar]
- Lok, S. , Kuijper J. L., Jelinek L. J., Kramer J. M., Whitmore T. E., Sprecher C. A., et al. 1994. The human glucagon receptor encoding gene: structure, cDNA sequence and chromosomal localization. Gene 140:203–209. [DOI] [PubMed] [Google Scholar]
- MacNeil, D. J. , Occi J. L., Hey P. J., Strader C. D., and Graziano M. P.. 1994. Cloning and expression of a human glucagon receptor. Biochem. Biophys. Res. Commun. 198:328–334. [DOI] [PubMed] [Google Scholar]
- Magnusson, I. , Rothman D. L., Gerard D. P., Katz L. D., and Shulman G. I.. 1995. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes 44:185–189. [DOI] [PubMed] [Google Scholar]
- Metz, S. A. , Fujimoto W. Y., and Robertson R. P.. 1983. A role for the lipoxygenase pathway of arachidonic acid metabolism in glucose‐ and glucagon‐induced insulin secretion. Life Sci. 32:903–910. [DOI] [PubMed] [Google Scholar]
- Minerva, L. , Clerens S., Baggerman G., and Arckens L.. 2008. Direct profiling and identification of peptide expression differences in the pancreas of control and ob/ob mice by imaging mass spectrometry. Proteomics 8:3763–3774. [DOI] [PubMed] [Google Scholar]
- Myers, S. R. , Diamond M. P., Adkins‐Marshall B. A., Williams P. E., Stinsen R., and Cherrington A. D.. 1991. Effects of small changes in glucagon on glucose production during a euglycemic, hyperinsulinemic clamp. Metabolism 40:66–71. [DOI] [PubMed] [Google Scholar]
- Nambi, P. , and Aiyar N.. 2003. G protein‐coupled receptors in drug discovery. Assay Drug Dev. Technol. 1:305–310. [DOI] [PubMed] [Google Scholar]
- Norfleet, W. T. , Pagliara A. S., Haymond M. W., and Matschinsky F.. 1975. Comparison of alpha‐ and beta‐cell secretory responses in islets isolated with collagenase and in the isolated perfused pancreas of rats. Diabetes 24:961–970. [DOI] [PubMed] [Google Scholar]
- Ohneda, A. , and Ohneda M.. 1988. Effect of glicentin‐related peptides upon the secretion of insulin and glucagon in the canine pancreas. Tohoku J. Exp. Med. 155:197–204. [DOI] [PubMed] [Google Scholar]
- Patel, Y. C. , Amherdt M., and Orci L.. 1979. Somatostatin secretion from monolayer cultures of neonatal rat pancreas. Endocrinology 104:676–679. [DOI] [PubMed] [Google Scholar]
- Patzelt, C. , and Schiltz E.. 1984. Conversion of proglucagon in pancreatic alpha cells: the major endproducts are glucagon and a single peptide, the major proglucagon fragment, that contains two glucagon‐like sequences. Proc. Natl. Acad. Sci. U. S. A. 81:5007–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori, A. S. , Elrick M. M., Samson W. K., Corbett J. A., and Yosten G. L.. 2014. Neuronostatin inhibits glucose‐stimulated insulin secretion via direct action on the pancreatic alpha‐cell. Am. J. Physiol. Endocrinol. Metab. 306:E1257–E1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima, K. , Hirota M., and Ohboshi C.. 1988. Effect of glucagon‐like peptide‐1 on insulin secretion. Regul. Pept. 22:245–252. [DOI] [PubMed] [Google Scholar]
- Skamene, A. , and Patel Y. C.. 1984. Infusion of graded concentrations of somatostatin in man: pharmacokinetics and differential inhibitory effects on pituitary and islet hormones. Clin. Endocrinol. 20:555–564. [DOI] [PubMed] [Google Scholar]
- Stewart, K. W. , Phillips A. R., Whiting L., Jullig M., Middleditch M. J., and Cooper G. J.. 2011. A simple and rapid method for identifying and semiquantifying peptide hormones in isolated pancreatic islets by direct‐tissue matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry. Rapid Commun. Mass Spectrom. 25:3387–3395. [DOI] [PubMed] [Google Scholar]
- Stumpel, F. , Scholtka B., Hunger A., and Jungermann K.. 1998. Enteric glucagon 37 rather than pancreatic glucagon 29 stimulates glucose absorption in rat intestine. Gastroenterology 115:1163–1171. [DOI] [PubMed] [Google Scholar]
- Suzuki, S. , Kawai K., Ohashi S., Mukai H., and Yamashita K.. 1989. Comparison of the effects of various C‐terminal and N‐terminal fragment peptides of glucagon‐like peptide‐1 on insulin and glucagon release from the isolated perfused rat pancreas. Endocrinology 125:3109–3114. [DOI] [PubMed] [Google Scholar]
- Taylor, S. W. , Nikoulina S. E., Andon N. L., and Lowe C.. 2013. Peptidomic profiling of secreted products from pancreatic islet culture results in a higher yield of full‐length peptide hormones than found using cell lysis procedures. J. Proteome Res. 12:3610–3619. [DOI] [PubMed] [Google Scholar]
- Thim, L. , and Moody A. J.. 1982. Purification and chemical characterization of a glicentin‐related pancreatic peptide (proglucagon fragment) from porcine pancreas. Biochim. Biophys. Acta 703:134–141. [DOI] [PubMed] [Google Scholar]
- Vahl, T. P. , Paty B. W., Fuller B. D., Prigeon R. L., and D'Alessio D. A.. 2003. Effects of GLP‐1‐(7‐36)NH2, GLP‐1‐(7‐37), and GLP‐1‐ (9‐36)NH2 on intravenous glucose tolerance and glucose‐induced insulin secretion in healthy humans. J. Clin. Endocrinol. Metab. 88:1772–1779. [DOI] [PubMed] [Google Scholar]
- Weir, G. C. , Knowlton S. D., Atkins R. F., McKennan K. X., and Martin D. B.. 1976. Glucagon secretion from the perfused pancreas of streptozotocin‐treated rats. Diabetes 25:275–282. [DOI] [PubMed] [Google Scholar]
- Weston, C. , Lu J., Li N., Barkan K., Richards G. O., Roberts D. J., et al. 2015. Modulation of glucagon receptor pharmacology by RAMP2. J. Biol. Chem. doi: 10.1074/jbc.M114.624601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting, L. , Danaher R. N., Ruggiero K., Lee C. C., Chaussade C., Mulvey T., et al. 2013. D‐chiro‐inositol attenuates epinephrine‐stimulated hepatic glucose output in the isolated perfused liver independently of insulin. Horm. Metab. Res. 45:394–397. [DOI] [PubMed] [Google Scholar]
- Yanaihara, C. , Matsumoto T., Hong Y. M., and Yanaihara N.. 1985. Isolation and chemical characterization of glicentin C‐terminal hexapeptide in porcine pancreas. FEBS Lett. 189:50–56. [DOI] [PubMed] [Google Scholar]
- Zielmann, S. , Schutte G., Lenzen S., and Panten U.. 1985. Effects of isoprenaline and glucagon on insulin secretion from pancreatic islets. Naunyn‐Schmiedeberg's Arch. Pharmacol. 329:299–304. [DOI] [PubMed] [Google Scholar]


