Abstract
The prediction of the consequences of our own actions through internal models is an essential component of motor control. Previous studies showed improvement of anticipatory behaviors with age for grasping, drawing, and postural control. Since these actions require visual and proprioceptive feedback, these improvements might reflect both the development of internal models and the feedback control. In contrast, visual tracking of a temporarily invisible target gives specific markers of prediction and internal models for eye movements. Therefore, we recorded eye movements in 50 children (aged 5–19 yr) and in 10 adults, who were asked to pursue a visual target that is temporarily blanked. Results show that the youngest children (5–7 yr) have a general oculomotor behavior in this task, qualitatively similar to the one observed in adults. However, the overall performance of older subjects in terms of accuracy at target reappearance and variability in their behavior was much better than the youngest children. This late maturation of predictive mechanisms with age was reflected into the development of the accuracy of the internal models governing the synergy between the saccadic and pursuit systems with age. Altogether, we hypothesize that the maturation of the interaction between smooth pursuit and saccades that relies on internal models of the eye and target displacement is related to the continuous maturation of the cerebellum.
Keywords: cerebellum, development, occlusion, pursuit, saccades
to overcome delays, the control of our actions is based on knowledge about the dynamics of the world and about the future consequences of our actions, which is acquired during development. For instance, anticipatory behaviors are observed in the grip force during object lift (Flanagan and Wing 1997) or in the forearm in reaction to the unloading of an object [anticipatory postural adjustment (APA)] (Hugon et al. 1982). Such predictive behaviors are acquired early in life [∼2 yr for grip force (Forssberg et al. 1991) and 3–4 yr for APA (Schmitz et al. 1999)] but improve with age and only reach adult-like levels a few years later [8–11 yr for grip force (Forssberg et al. 1992) and after 7 yr for APA (Girolami et al. 2010)]. Prediction of the trajectory of a moving target, which requires an internal model of the environment, follows a similar developmental course but only reaches adult levels at ∼17 yr old (van Roon et al. 2008). In addition, the ability of children to learn such predictive behaviors is another key component to understand better the development of internal models. For instance, Vasudevan et al. (2011) showed that young children (<6 yr old) could learn the timing but not the spatial coordination during a split-belt treadmill walking task (with 1 leg going 2 times faster than the other). Finally, visual tracking of a visible target moving on a predictable trajectory also evolved with age (Accardo et al. 1995; Haishi and Kokubun 1995; Salman et al. 2006b), showing that prediction about the dynamics of the world is also acquired during childhood.
All of these actions are driven both by predictive control (based on internal model and state estimation) and by sensory feedback (visual, vestibular, tactile, and/or proprioceptive). For instance, a smooth pursuit response to a predictable moving target relies both on predictive mechanisms and internal models and on visual feedback control (Orban de Xivry et al. 2013). Therefore, a pure signature of internal models during development was not obtained by any of the above-mentioned studies.
Such a pure signature of internal models without any interference from sensory feedback can be observed during ocular tracking of temporarily invisible moving targets. Indeed, in the absence of visual feedback, eye movements are only driven by internal models, as proprioception does not play any role in the control of eye movements (Wang et al. 2007; Xu et al. 2011). Studies on infants have shown the development in the first months of life of the ability to predict the reappearance of a target that transiently disappeared. At 12 wk old, infants begin to predict the reappearance (Rosander and von Hofsten 2004; von Hofsten 2007). This ability largely increases in the first year of life (Bertenthal et al. 2007; Gredebäck and von Hofsten 2004). However, such predictive mechanisms only refer to the ability to perceive continuous motion and direct their eyes to the other side of the occluder. In contrast, during such blanking periods, adults show more advanced predictive oculomotor responses (Becker and Fuchs 1985; Bennett and Barnes 2003, 2004, 2005; Bennett et al. 2007; Coppe et al. 2010; Madelain and Krauzlis 2003; Mitrani and Dimitrov 1978). When the target disappears, the smooth pursuit eye velocity typically decreases to a plateau value (Becker and Fuchs 1985; Mitrani and Dimitrov 1978). If the duration of blanking is predictable, then the eye velocity increases again in anticipation of target reappearance (Bennett and Barnes 2003, 2004; Orban de Xivry et al. 2006). This predictive reacceleration of the eye is called predictive recovery. Moreover, the observed decrease in eye velocity during blanking is compensated by saccades, such that the total amplitude of saccades is inversely proportional to the pursuit displacement (Coppe et al. 2012; Orban de Xivry et al. 2006, 2008). Indeed, saccades compensate for the variability of the smooth eye displacement (SED) during blanking and contribute to the predictive mechanisms that improve the perception of the target at reappearance. This synergy between pursuit and saccades is regulated on a trial-by-trial basis by internal models of the eye and target motion.
In the present study, we will use these behavioral markers of predictive abilities and internal models to characterize the developmental time course of these mechanisms during childhood.
MATERIALS AND METHODS
Subjects.
Eye movements were recorded in a total of 60 subjects, categorized in 6 groups of 10 subjects, ranging from 5 yr to adults (5 groups of children: 5–7, 8–10, 11–13, 14–16, and 17–19 yr; 1 group of adults: 20–34 yr). All subjects were healthy and had normal or corrected-to-normal vision. All procedures were approved by the Université Catholique de Louvain Ethics Committee and were in accordance with the Declaration of Helsinki. Written consents were obtained from the participants or from their parents if they were under 18 yr old.
Experimental setup.
The stimulus was projected on a screen (195 × 145 cm), placed 1.5 m away from the subjects, with a Cine 8 projector (refresh rate, 100 Hz; Barco, Kortrijk, Belgium). Eye movements of the dominant eye were recorded with the EyeLink 1000 (SR Research, Ottawa, ON, Canada) at 1000 Hz. The dominant eye was determined using a classic test, where subjects have to look at a focus point through a small hole made in a sheet of paper. With the use of the hole as a viewing window, only one eye may fixate on the focus point. With the covering of one eye or the other, we determine the dominant fixating eye. Chin and forehead supports were used to stabilize the head.
Paradigm.
Subjects were asked to pursue a red dot (diameter, 0.6°), centered in a small green bird (width, 4°), moving horizontally on the screen (Fig. 1). Each trial started with an initial fixation of 1 s on one side of the screen at a position randomly selected between 16° and 25° to the left or to the right of the screen center (the head of the bird was oriented in the direction of its future motion). Then, the visual stimulus disappeared for 300 ms (gap period) before starting to move at a constant velocity of 15 or 20°/s toward the center of the screen. In control trials, the target stayed visible throughout the trial and moved at a constant velocity (15 or 20°/s) for 2 s. In the test trials, after 0.6 s of visible motion, the target was blanked for 0.8 s (blanking period) and then reappeared and continued to move for another 0.6 s (Fig. 1). Subjects were instructed to follow the target as accurately as possible, even when the target was not visible. In 10 randomly chosen test trials of each block, the bird that was green before the blanking period reappeared blue after it. In this case, the subjects were instructed to press any key of the keyboard placed in front of them to report this change of color. This color-change detection task was used to maintain attention. Each subject performed 8 blocks of 20 trials. Each block consisted of 4 control trials (trials 1; 2; 9 or 10; and 13, 14, or 15), only used to reinforce the continuous movement of the target, and 16 test trials. Target direction and velocity were kept constant within a block but randomized across blocks.
Fig. 1.
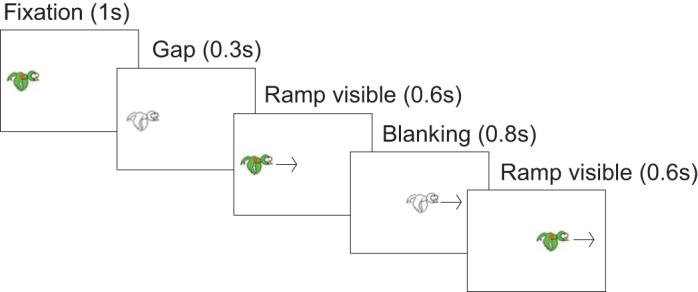
Time course of a (test) trial. After 1 s of fixation and a gap period (target blanked for 300 ms), the target started moving horizontally at a constant velocity. After 600 ms, the target was blanked for 800 ms (blanking period) and continued moving for another 600 ms. Target velocity (15 or 20°/s) and direction (to the left or to the right) were randomized across blocks but kept constant within a block. Each frame in the figure corresponds to a specific period of the trial, with duration reported in parentheses.
Data analysis.
Data analysis was similar to the one described in Coppe et al. (2012). Eye movements were low-pass filtered at 50 Hz, with a bidirectional, autoregressive zero-phase filter implemented in MATLAB (de Brouwer et al. 2001). Velocity and acceleration signals were obtained from a position with a central difference algorithm on a 20-ms window. Saccades onset and offset were detected based on an acceleration criterion of 500°/s2 and a minimum duration of 30 ms. These saccades were removed from the velocity traces to analyze the smooth pursuit performance. Saccades were replaced by a linear interpolation between the velocity before and after each saccade.
Each block was divided in four periods of five trials. Control trials were removed for the analysis. Therefore, each period contained between three and five test trials [T1: trials 3–5 (Early trials), T2: trials 6–10, T3: trials 11–15, T4: trials 16–20 (Late trials)]. All trials with blinks during the blanking period were removed from the analysis (3%).
We analyzed separately the anticipatory pursuit response (during the gap period) and the predictive pursuit response (during the blanking period).
In all trials, we quantified the anticipatory pursuit with the gain at trial onset. This gain was computed as the ratio between eye velocity at the onset of target motion and the target velocity.
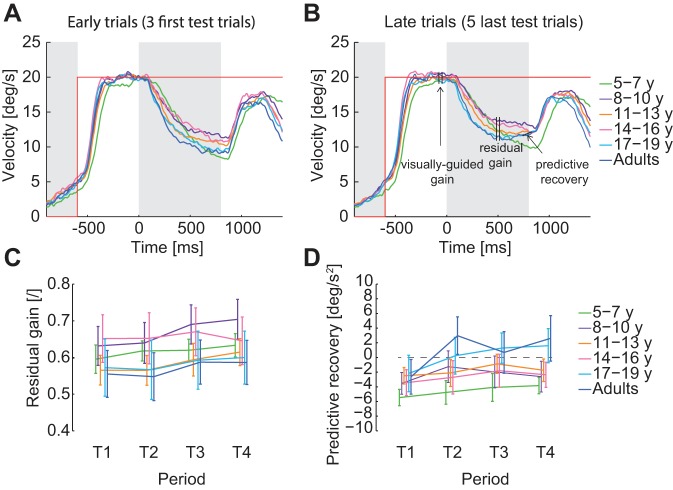
In test trials, we computed the visually guided gain, the residual gain, and the predictive reacceleration during the blanking period to quantify the predictive smooth pursuit. The visually guided gain was defined as the mean eye velocity in a 50-ms interval, centered 100 ms before target blanking, divided by target velocity (see Fig. 4B). When the target disappeared, the eye velocity exponentially decayed to a plateau level, called residual velocity (Becker and Fuchs 1985). The residual gain was defined as the ratio between the mean residual eye velocity in a 50-ms interval, centered 500 ms after blanking onset and target velocity (see Fig. 4B). This time interval was chosen to fall before any predictive increase in eye velocity observed in the last trials of the block. Predictive reacceleration was defined as the slope of the regression line fitted on the desaccaded eye velocity between 100 ms before and 50 ms after the end of target blanking (see Fig. 4B).
Fig. 4.
Predictive smooth pursuit. A: mean eye velocity per age group for the 3 first test trials of each block. B: mean eye velocity per age group for the 5 last test trials of each block. Gray areas represent the gap and blanking periods (when the target is not visible on the screen). C: evolution of the residual gain through the blocks for the different age groups. D: evolution of the predictive recovery through the blocks for the different age groups. For these 2 last panels, data points are the average per age group computed with the means by subjects. The error bars represent the SE of these means. Residual gains and predictive recovery are averaged across the 2 target velocities.
Saccades were defined as predictive when executed between 120 and 800 ms after target-blanking onset. This interval was used as we observed a clear transition in the saccade latency histogram between visually guided saccades and predictive saccades. A similar transition was observed in an earlier study (Orban de Xivry et al. 2009). Therefore, this interval excludes visually guided saccades from these analyses.
To analyze the saccades executed during the blanking period, we built heat maps of saccade end points for each age group. For heat maps of saccade end points, each ending point (in position and time) was replaced by a two-dimensional (2D) Gaussian. The x coordinate of the center of the 2D Gaussian was the time from target-blanking onset when the saccade ended, and the y coordinate of the center of the Gaussian was the horizontal position of the saccade end point. The height of each Gaussian for one participant was equal to 1/n, where n is equal to the total number of saccades elicited by all participants of this age group during the blanking periods. The SD of the Gaussian was 25 ms along the x-axis and 0.25° along the y-axis. Data from all subjects belonging to a given age group were pooled together to build the heat map of this age group.
Position error (PE) at the end of blanking is an additional indicator of how subjects predict target blanking. PE at the end of blanking was defined as the difference between target and eye position at the end of target blanking.
Finally, as saccades and pursuit alone can be predictive, the combination of both types of eye movements can also be a marker of prediction. To study saccade-pursuit interaction, we first computed the distance traveled by saccades during blanking (sum of the saccadic amplitudes during the blanking period) and normalized this distance by the target displacement (target velocity × blanking duration) to obtain the saccadic eye displacement (SAD). The SED was defined as follows
In other words, the SAD was removed from the total eye displacement during the blanking period and normalized to the target displacement to obtain the SED. For each subject separately, a regression line was fitted to quantify the relationship between SAD and SED. For this particular analysis, trials with no saccades were excluded. We used the slope of the regression as well as the root mean square error (RMSE) of the fit to quantify the quality of the relationship. Since SAD and SED are proportions, none of these parameters (SAD, SED, slope, or RMSE) has units.
Due to the presence of noise in the measure of SED, we performed a control analysis by using the maximum likelihood approach described in Haith et al. (2015). For each measure, the likelihood is given by the following
In this expression, e represents the possible values for SED, given the noisy measure SEDi. The values of σSAD and σSED, which represent the variability associated with the SAD and SED measures, were set to 0.2 and 0.1, respectively. A trapezoid integration over e was used to compute the likelihood. The sum of the likelihood over all observations of a subject was computed, and we found the values of a (slope of the regression) and b (intercept of the regression) that maximized this log likelihood.
For all analyses, data from both target directions were collapsed, because none of the studied parameters was influenced by the direction of the target motion. Furthermore, the results from both target velocities were averaged, since all of the results were the same for both target velocities. The use of two target velocities only increased the randomization and task difficulty. For the different parameters, we performed repeated-measures ANOVA with age group as between-subject factor and period (T1, T2, T3, T4) as within-subject factor. Main statistical analyses were performed using R. Regression parameters were computed using the robustfit function in MATLAB.
Finally, to control the attention of subjects, we used the detection task that involved responding to a color change of the target that may have occurred during the blanking. The percentage of correct color-change detection was used as a first way to assess the absence of difference in attention/fatigue among subjects.
In addition, we used the pursuit gain on the control trials (target continuously visible) as a second marker of attention. This gain was computed on a 50-ms interval, centered 500 ms after that the target started to move. Three different periods were defined for this analysis: the two first control trials, the third control trial, and the last one.
RESULTS
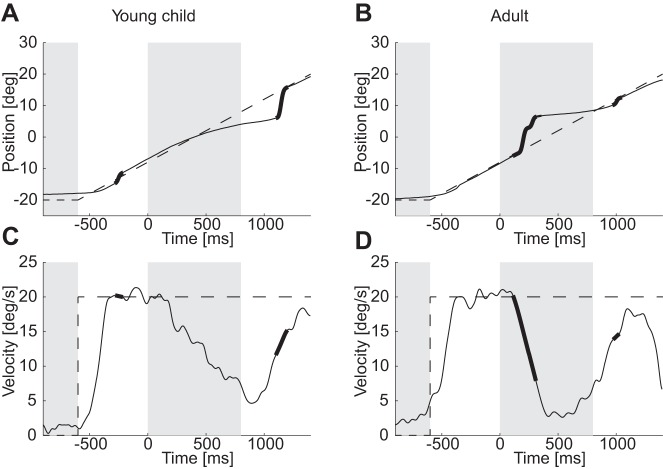
In this experiment, we investigated the ability of children to track a moving target that is transiently blanked and how this ability evolves with age. Typical oculomotor responses from one of the youngest children and one adult are displayed in Fig. 2 and will be used to describe qualitatively the main results of this study. Quantitative analyses will then be reported in details. Both subjects tracked the moving target accurately when it was visible. When the target was blanked (start of the second gray area), the eye velocity of both children and adults rapidly dropped, as reported in earlier studies (Fig. 2, C and D). During the first trials of each block, the eye velocity continued to decay until target reappearance and increased again when the visual feedback became available again (e.g., Fig. 2C). In adults, after a few trials, subjects anticipated the time of target reappearance, and the eye reaccelerated before target reappearance (Fig. 2D). During the blanking periods, both smooth pursuit and saccades were combined to pursue the invisible target (Fig. 2B). However, adults had a higher tendency to execute saccades than children (Fig. 2B compared with Fig. 2A). A better synergy between saccades and pursuit during blanking in adults led to differences in the PE at target reappearance (Fig. 2B compared with Fig. 2A).
Fig. 2.
Typical trials. A and B: position of the eye during typical test trials from a young child and an adult. C and D: the corresponding desaccaded eye velocities. Bold parts of lines represent saccades on the position graphs and the timing of saccades on desaccaded eye-velocity graphs. Dashed lines represent, respectively, the target displacement or velocity.
This difference in the error at target reappearance was confirmed at the group level. Whereas the younger children lag the target at its reappearance, adults tend to lead it [Fig. 3; main effect of age group on PE: F(5,54) = 5.611, P < 0.001]. It is worth noting that leading the target at its reappearance is intuitively more appropriate, since eye velocity is lower than target velocity. In the following sections, we analyzed specifically three aspects of the oculomotor response during the blanking period. We first analyzed the pursuit component of the response during the blanking period, we then quantified the saccadic component of the response, and finally, we analyzed the saccade pursuit interaction. We performed these analyses to identify which component of the oculomotor response had the largest impact on the PE at target reappearance.
Fig. 3.
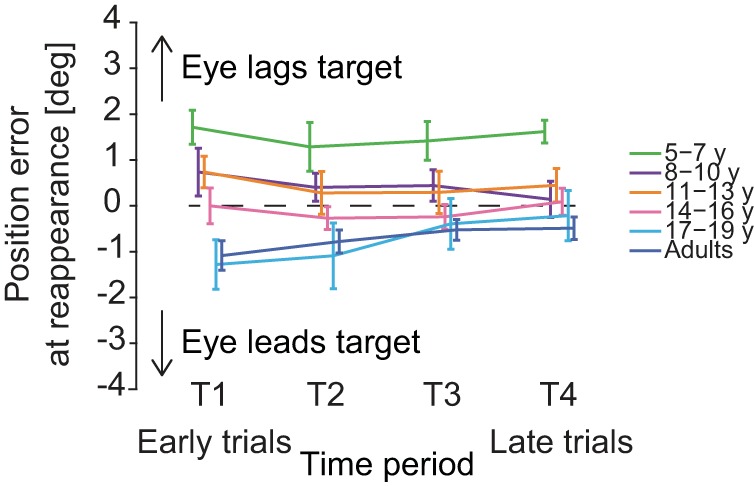
Position error (PE) between the eye and the target when the target reappears at the end of the blanking period. Younger children tend to lag the target at reappearance (positive error), whereas older children and adults tend to lead the target slightly when it reappears. Data points are the average values per age group computed with the means by subjects. Error bars are SE. T1: trials 3–5 (Early trials), T2: trials 6–10, T3: trials 11–15, T4: trials 16–20 (Late trials), respectively.
Similar pursuit behavior during blanking across age.
Overall, the smooth pursuit behavior was very similar across age groups for the range of target velocity tested. All subjects had similar visually guided pursuit [Fig. 4, A and B; no main effect of age group on visually guided pursuit gain: F(5,54) = 1.15, P = 0.34]. After target-blanking onset (100 ms), the eye velocity started decreasing exponentially until a plateau (Fig. 4, A and B). The decrease in eye velocity was observed for all age groups, and on average, the velocity reached the same plateau level for all age groups. The residual gain computed 500 ms after target disappearance did not depend on age [Fig. 4C; no main effect of age group: F(5,54) = 0.51, P = 0.77] but slightly increased with training for all age groups [main effect of period: F(3,162) = 10.41, P < 0.001, but no interaction between periods and age groups: F(15,162) = 1.35, P = 0.17].
For all age groups, the eye velocity initially decayed until ∼100 ms after target reappearance when the visual feedback became available (Fig. 4A). However, after the three first test trials, adults and adolescents (17–19 yr old) learned to increase their eye velocity before the end of the blanking period (Fig. 4, A and B). This predictive recovery of eye velocity was absent in the youngest children (Fig. 4B; 5–7 yr old). To quantify the predictive recovery, we measured the acceleration of the eye at the end of the blanking period. This predictive recovery was negative for all age groups for the first three trials (T1) and increased after [T2, T3, T4; main effect of period: F(3,162) = 8.74, P = 2.10−5]. However, this increase was similar across age groups [interaction between periods and age groups: F(15,162) = 1.28, P = 0.22].
In addition, the anticipatory pursuit observed in the gap period was similar across age [no main effect of age on the gain at trial onset: F(5,54) = 0.48, P = 0.78, and no interaction between periods and age groups: F(15,162) = 1.05, P = 0.40].
Overall, this suggests that predictive smooth pursuit did not differ largely across age groups and might not be responsible for the rather large difference observed in PE at target reappearance.
Saccades land ahead of the target for all age groups.
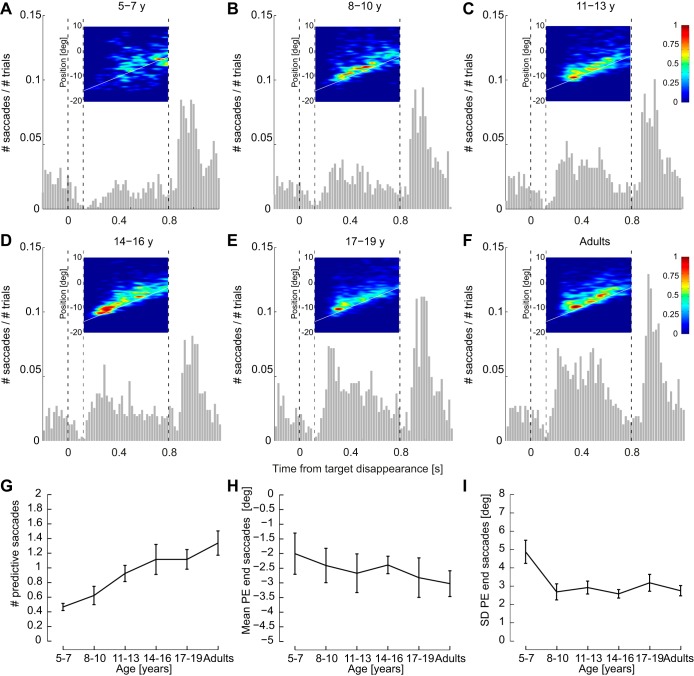
In most trials (69%), at least one saccade was executed during the blanking period to compensate for the decrease in eye velocity. However, this percentage largely varied with age (Fig. 5). The number of saccades was much lower in the youngest children (Fig. 5A) than in adults (Fig. 5F) and gradually increased with age [Fig. 5G; main effect of age on the number of predictive saccades: F(5,54) = 5.71, P < 0.001].
Fig. 5.
Development of predictive saccades with age. A–F: histograms of the number of saccades during blanking per age group. The time 0 corresponds to the onset of target blanking. Insets: heat map of predictive saccade endpoints that shows where the saccades land in space and time. The red color represents the locations in space and time where the eye lands with a high frequency and the blue color locations with nearly no saccade endpoints. The white line represents the virtual target displacement when blanked. G: evolution with age of the number of predictive saccades during blanking. H: evolution with age of the mean PE at the end of each predictive saccade. I: evolution with age of the SD of PE that represents the variability in space of the saccade endpoints. For these 3 last panels, data points are the average per age group computed with the means by subjects. Error bars represent the SE.
In addition, heat maps of saccade endpoints (Fig. 5, A–F) revealed that saccades of all age groups mainly landed ahead of the position of the invisible target. However, the variability in saccade endpoints appears larger for the younger children (Fig. 5A). These two observations were quantified by the means and SD of the PE at the end of saccades. The mean PE appeared similar across age groups [Fig. 5H; Kruskal-Wallis test: χ2(5) = 2.66, P = 0.75], whereas its SD was not [Fig. 5I; F(5,54) = 4.22, P = 0.003]. It was larger for the youngest children than for all of the other age groups (Tukey honest significant difference, P < 0.03 for all age groups, except children aged 17–19 yr, P = 0.065). Therefore, the endpoint of saccades does not seem to be responsible for the observed PE at reappearance, since the PE at the end of saccades does not change with age.
The ability to compensate for one's own variability improves with age.
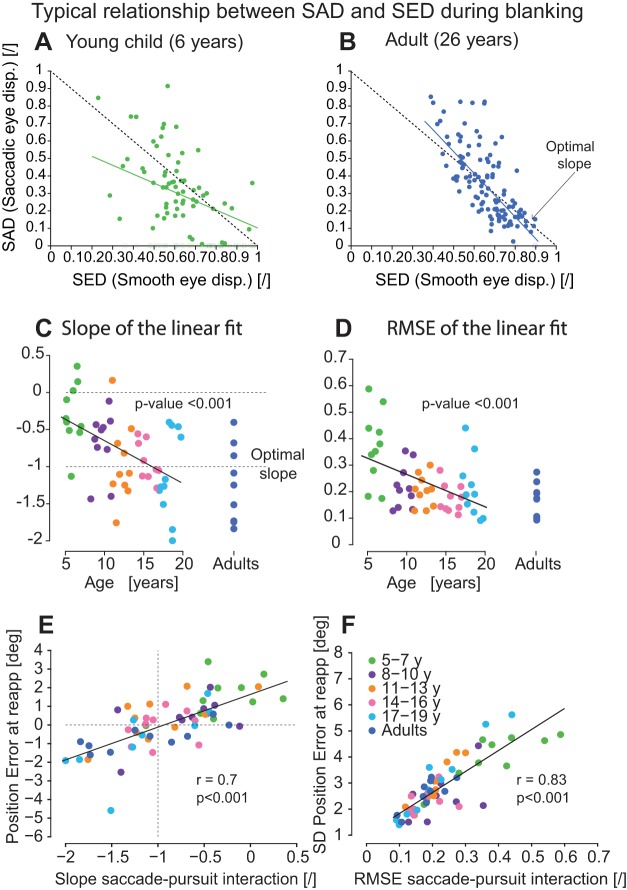
On a trial-by-trial basis, the amplitude of saccades during blanking needs to be adjusted to the decrease in eye velocity to align the eye with the target at its reappearance. That is, during the blanking period, the amplitude of the predictive saccades should be larger when the eye velocity drops more and vice versa (see Fig. 6, A and B). Because there is no visual information on the screen, the subjects need to rely on an internal model of their eye movements to estimate the decrease in eye velocity and to adjust their saccade amplitude on the flight. For each subject, we quantified the relationship between the amount of distance covered by saccades (SAD) as a function of the SED (which is the integral of the smooth eye velocity during the blanking period). The SED can be quite variable on a trial-by-trial basis (see variability of the value on the x-axis in Fig. 6, A and B). As reported before (Coppe et al. 2012; Orban de Xivry et al. 2006, 2008), adults were able to adjust the amplitude of their predictive saccades to the actual drop in eye velocity on a trial-by-trial basis (Fig. 6B). Such good compensation gave rise to a strong relationship between SAD and SED. This relationship was quantified by the slope of the regression line (Fig. 6B; slope = −1.2) and by the RMSE around the regression line (Fig. 6B; RMSE = 0.19). Perfect compensation would give rise to a slope of −1 and an RMSE of zero. In contrast, young children did not compensate for their eye-velocity variability as well as adults (Fig. 6A; slope = −0.5, RMSE = 0.33). This worse compensation gave rise to a lower slope in absolute value and a larger RMSE.
Fig. 6.
The interaction between smooth pursuit and saccades improves with age. A and B: two typical examples of the relationship between the saccadic eye displacement (SAD) and smooth eye displacement (SED) for a young child (6 yr) and an adult, respectively. The colored lines represent the regression line fitted on all of the disks (SAD different from 0). The dashed lines are the optimal slopes. C: evolution with age of the slope of the regression line. Each dot is the slope for each individual subject. D: evolution of the quality of the relationship between SAD and SED with age. Each dot represents the root mean square error (RMSE) from the regression for each individual subject. E: relationship between the PE at target reappearance and the quality of the saccade-pursuit interaction (slope in C). F: relationship between variability of the PE at target reappearance (SD) and the variability of the saccade-pursuit interaction (RMSE in D). E and F: each dot represents an individual subject.
Given that there is no visual information during the blanking period, this compensation can only take place thanks to an internal estimate of eye displacement. The developmental evolution of internal models can thus be assessed by quantifying the evolution of the strength of the relationship between SAD and SED across age. The absolute value of the slope showing the relationship between SAD and SED increases with age [Fig. 6C; significance of slope: t(48) = −3.91, P < 0.001] and became, on average, close to −1 in adulthood (Fig. 6C). Similar results are obtained if the slopes are computed using a maximum likelihood approach (see materials and methods), where the noise in the measurement of SED is specifically taken into account [correlation between the slopes and age: r = −0.48, t(48) = −3.78, P < 0.001]. In addition, the quality of the linear fits (how dots are scattered around the line) also improves with age (Fig. 6D). Indeed, the RMSE of the regression decreases with age [significance of the slope: t(48) = −3.64, P < 0.001].
Interestingly, the quality of saccade-pursuit interaction (slope in Fig. 6C) is strongly correlated with the PE at target reappearance [Fig. 3; r(58) = 0.7, P < 0.001], as shown in Fig. 6E. Furthermore, this relationship stays significant if the effect of age is taken into account [partial correlation: r(56) = 0.63, P < 0.001]. In particular, an ideal slope of −1 yields, on average, a zero PE of the eye at target reappearance [the regression line in Fig. 6E crosses the (−1,0) point].
Finally, it is worth mentioning that there is also a strong correlation between the variability of the saccade-pursuit interaction (RMSE in Fig. 6D) and the variability of the PE at target reappearance [Fig. 6F; r(58) = 0.83, P < 0.001]. Thus it can be hypothesized that both average PE at target reappearance and the variability of this parameter are explained by the quality of the saccade-pursuit interaction (slope and RMSE) for each subject, i.e., by the quality of their internal model.
Similar attention with age.
Our results cannot be explained by a change in attention, as we did not detect such a change. Indeed, age did not influence any of our two markers of attention. First, the percentage of correct color change detection did not change with age [main effect of the age group: F(5,54) = 0.71, P = 0.61]. The mean percentage was 92.9% and ranged from 85.8% for children aged 5–7 yr to 99.8% for children aged 8–10 yr (and to 89.3% for adults). Second, a differential decrease in pursuit gain on control trials could reflect a difference in attention/fatigue with age. This gain decreased across the time course of a block [main effect of period: F(2,108) = 24.9, P < 0.001] but did not change differently with age [no main effect of age group: F(5,54) = 1.04, P = 0.4, and no interaction age group × period: F(10,108) = 1.36, P = 0.21].
DISCUSSION
In the present paper, we studied the development of predictive visual tracking and internal models during childhood. Overall, all children starting at 5 yr old exhibited some predictive tracking during the blanking period, and their oculomotor behavior was similar to the adults. Besides this similarity, we found that the youngest children (aged 5–7 yr) lack from anticipation in predictive pursuit. In addition, the number of predictive saccades gradually increased with age, and the landing position of these saccades was more variable in the youngest children. Finally, our results allow us to identify precisely the development of internal models in children, as measured by the ability of the children to adjust their saccade amplitude to the variability of their smooth pursuit response on a trial-by-trial basis.
The oculomotor behavior of children is close to adult starting at 5 yr old.
Overall, 5-yr-old children presented a general oculomotor behavior during blanking that was close to the one observed previously in adults (Becker and Fuchs 1985; Bennett and Barnes 2003, 2004, 2005, 2006; Bennett et al. 2007; Coppe et al. 2012; Orban de Xivry et al. 2006, 2008). First, the predictive smooth pursuit response during the blanking period was globally similar across age groups. For instance, at the time of target disappearance, the eye velocity started decreasing to a plateau level that was not significantly different with age. However, adults, but not the youngest children, were able to reaccelerate their eyes slightly before target reappearance. This predictive recovery relies on the integrity of the frontal lobe (among others), which plays an important role in the spatial representation of an invisible moving target (Barborica and Ferrera 2003, 2004; Ferrera and Barborica 2010; Xiao et al. 2007). Predictive recovery is specifically altered in patients with frontotemporal lobar degeneration (Coppe et al. 2012). The maturation of the frontal zone of the brain during childhood comes late in adolescence (after 16 yr) (Giedd et al. 1999; Gogtay et al. 2004; Paus 2005; Sowell et al. 1999). However, we found only slight improvement with age in the predictive recovery. Despite a tendency for adults to have a higher predictive recovery than other age groups, no statistical differences could be found in this measure that is particularly sensitive to noise. Only the youngest children did not show such a predictive recovery. The absence of predictive recovery could be a sign of a late development of the representation of target displacement.
Second, both children and adults used a combination of smooth pursuit and saccades during blanking, even though the number of saccades triggered during the blanking period increased dramatically with age (see Fig. 5G). From age 5, children executed saccades that landed ahead of the invisible target. Again, this position lead of the eye with respect to the target was comparable across age groups, although the variability of this measure decreased with age.
Finally, the timing of the saccades during the blanking period was qualitatively similar across ages. For instance, we observed in all age groups a large drop in the number of saccades, ∼120 ms after disappearance, as documented previously (Orban de Xivry et al. 2009).
A lower sensitivity to error may explain the increased error at reappearance in the youngest children.
We used the PE at reappearance as a marker of the visual tracking performance of the blanked target. The increased PE of the younger children is the consequence of the low-quality saccade-pursuit interaction, as well as the higher number of trials without predictive saccades during blanking. This number of saccades gradually increases with age. This reveals that older children and adults tend to correct their movement more in the absence of visual feedback. The “accuracy” of saccade endpoints during blanking suggests that even the youngest children have some estimate of the target displacement. However, the fact that in youngest children, the variability of the saccadic response is much larger (Fig. 5I) and that they trigger much less saccades during blanking (Fig. 5G) is fully compatible with the hypothesis that their internal models are less mature than those of older subjects.
The rate of catch-up saccades was previously found to increase with age during visually guided pursuit (Ego et al. 2013). This increase was associated with an increased sensitivity to errors, a progressive maturation of internal models, and a decrease of processing delays. A study on drawing movements (Contreras-Vidal 2006) also reported a greater endpoint variability for the youngest children (between 5 and 7 yr). The study attributed this phenomenon to a better internal representation of target position with increasing age.
Precision of internal models improves with age.
We found that the variability in the smooth pursuit response during blanking is better compensated by the saccadic system with increasing age. This improved coordination between smooth pursuit and saccades, which also partially determined the average PE at the end of the blanking, relies on the ability to monitor the target correctly but also the eye position. Since there is no visual feedback during blanking and since proprioception is not available online to the oculomotor system (Wang et al. 2007), this eye position estimation (or eye state) relies on the integrity/maturity of a representation of the eye position by an internal model (Miall and Wolpert 1996; Shadmehr et al. 2010; Wolpert et al. 1998). A correct internal model of the target displacement is also essential for the interaction between saccade and pursuit. However, the interaction is independent of the reliability of timing estimation, since adults present this interaction even when the duration of the blanking period is not predictable (Orban de Xivry et al. 2008).
Our results show that children have a good estimate of the target displacement during blanking. Indeed, heat maps show that even for our youngest children, the saccades landed ahead of the target. This suggests that children have an internal model of the target displacement, which might reside either in the frontal eye field (Barborica and Ferrera 2003; Xiao et al. 2007) or in the cerebellum (Cerminara et al. 2009). However, the increased variability of saccade landing positions during blanking, together with the reduced synergy between saccades and smooth pursuit in the youngest children, indicates that these internal models might still be immature. This late immaturity is consistent with our previous work (Ego et al. 2013), where we found that reflexive oculomotor responses to visible targets followed a similar developmental time course to the time course of predictive internal model maturity reported in this study (evolving throughout adolescence).
In young children, the increased uncertainty about the estimated eye position with respect to the target during the blanking period refrains them from executing predictive saccades, as they cannot accurately localize the target position with respect to their eye. This unreliability of the internal models of young children contrasts with their rate of saccadic adaptation. Indeed, young children adapt at the same speed as adults (Doré-Mazars et al. 2011; Salman et al. 2006a). The scarcity of predictive saccades observed in the present study and the use of compensatory strategies for lifting or tracking objects reported for young children in another study (Gachoud et al. 1983) suggest that the central nervous system of young children is well aware of the unreliability of its internal models.
Finally, an interesting comparison can be made between the maturation of children in motor adaptation tasks and in our oculomotor task. Indeed, it has been reported that motor adaptation in a simple reaching task is mature as early as at 6 yr old (Takahashi et al. 2003). This contrasts with the report made by Vasudevan et al. (2011) on the development of locomotor adaptation in a split-belt paradigm, where it was shown that some aspects of adaptation (timing) are mature as early as 3 yr, whereas others (spatial) show slower adaptation rates until 12 yr. This is compatible with our results showing a dramatic effect of age on spatial accuracy (PE) compared with its effect on timing (predictive recovery). Vasudevan et al. (2011) made the interesting hypothesis that these differences might be due to the complexity of the task: the split-belt paradigm involving the adaptation of a much more complex system with multiple joints. The link could be made with the maturation of the different parts of the cerebellum, as revealed by magnetic resonance, with the midline cerebellum (involving the vermis) being mature much earlier than the hemispheres (Hashimoto et al. 1995; ten Donkelaar et al. 2003; Tiemeier et al. 2010). Thus one could speculate that simpler aspects of motor control (single-joint motor adaptation or saccades) might be mature earlier, because they rely more on the vermis. In contrast, more complex mechanisms (multiple-joint motor adaptation or saccade-pursuit interaction) might become mature later, because they rely more on the intermediate and lateral parts of the cerebellum. This possible interpretation is consistent with earlier studies showing that the lateral cerebellum is involved in the implementation of forward models (Miall et al. 2007; Pasalar et al. 2006), which are critical to control the interaction between saccades and pursuit.
GRANTS
Support for this work was provided by the Belgian Program on Interuniversity Attraction Poles, initiated by the Belgian Federal Science Policy Office, Actions de Recherche Concertées (French community, Belgium), and the European Space Agency (ESA) of the European Union. Support for J.-J. Orban de Xivry was provided by the Brains Back to Brussels program from the Brussels region (Belgium). Support for C. Ego was provided by Fondation JED Belgique.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.E., D.Y., J.-J.O., and P.L. conception and design of research; C.E. performed experiments; C.E. and J.-J.O. analyzed data; C.E., J.-J.O., and P.L. interpreted results of experiments; C.E. prepared figures; C.E., D.Y., J.-J.O., and P.L. drafted manuscript; C.E., D.Y., J.-J.O., and P.L. edited and revised manuscript; C.E., D.Y., J.-J.O., and P.L. approved final version of manuscript.
REFERENCES
- Accardo AP, Pensiero S, Da Pozzo S, Perissutti P. Characteristics of horizontal smooth pursuit eye movements to sinusoidal stimulation in children of primary school age. Vision Res 35: 539–548, 1995. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Estimating invisible target speed from neuronal activity in monkey frontal eye field. Nat Neurosci 6: 66–74, 2003. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Modification of saccades evoked by stimulation of frontal eye field during invisible target tracking. J Neurosci 24: 3260–3267, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp Brain Res 57: 562–575, 1985. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Barnes GR. Combined smooth and saccadic ocular pursuit during the transient occlusion of a moving visual object. Exp Brain Res 168: 313–321, 2006. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Barnes GR. Human ocular pursuit during the transient disappearance of a visual target. J Neurophysiol 90: 2504–2520, 2003. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Barnes GR. Predictive smooth ocular pursuit during the transient disappearance of a visual target. J Neurophysiol 92: 578–590, 2004. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Barnes GR. Timing the anticipatory recovery in smooth ocular pursuit during the transient disappearance of a visual target. Exp Brain Res 163: 198–203, 2005. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Orban de Xivry JJ, Barnes GR, Lefèvre P. Target acceleration can be extracted and represented within the predictive drive to ocular pursuit. J Neurophysiol 98: 1405–1414, 2007. [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Longo MR, Kenny S. Phenomenal permanence and the development of predictive tracking in infancy. Child Dev 78: 350–363, 2007. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J Physiol 587: 429–442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Vidal JL. Development of forward models for hand localization and movement control in 6- to 10-year-old children. Hum Mov Sci 25: 634–645, 2006. [DOI] [PubMed] [Google Scholar]
- Coppe S, Orban de Xivry JJ, Missal M, Lefèvre P. Biological motion influences the visuomotor transformation for smooth pursuit eye movements. Vision Res 50: 2721–2728, 2010. [DOI] [PubMed] [Google Scholar]
- Coppe S, Orban de Xivry JJ, Yüksel D, Ivanoiu A, Lefèvre P. Dramatic impairment of prediction due to frontal lobe degeneration. J Neurophysiol 108: 2957–2966, 2012. [DOI] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Lefèvre P. Role of retinal slip in the prediction of target motion during smooth and saccadic pursuit. J Neurophysiol 86: 550–558, 2001. [DOI] [PubMed] [Google Scholar]
- Doré-Mazars K, Vergilino-Perez D, Lemoine C, Bucci MP. Adaptation of reactive saccades in normal children. Invest Ophthalmol Vis Sci 52: 4813–4818, 2011. [DOI] [PubMed] [Google Scholar]
- Ego C, Orban de Xivry JJ, Nassogne MC, Yüksel D, Lefèvre P. The saccadic system does not compensate for the immaturity of the smooth pursuit system during visual tracking in children. J Neurophysiol 110: 358–367, 2013. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Barborica A. Internally generated error signals in monkey frontal eye field during an inferred motion task. J Neurosci 30: 11612–11623, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci 17: 1519–1528, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Eliasson AC, Kinoshita H, Johansson RS, Westling G. Development of human precision grip. I: Basic coordination of force. Exp Brain Res 85: 451–457, 1991. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Kinoshita H, Eliasson AC, Johansson RS, Westling G, Gordon AM. Development of human precision grip. II. Anticipatory control of isometric forces targeted for object's weight. Exp Brain Res 90: 393–398, 1992. [DOI] [PubMed] [Google Scholar]
- Gachoud JP, Mounoud P, Hauert CA, Viviani P. Motor strategies in lifting movements: a comparison of adult and child performance. J Mot Behav 15: 202–216, 1983. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos F, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2: 861–863, 1999. [DOI] [PubMed] [Google Scholar]
- Girolami GL, Shiratori T, Aruin AS. Anticipatory postural adjustments in children with typical motor development. Exp Brain Res 205: 153–165, 2010. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein DK, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredebäck G, von Hofsten C. Infants' evolving representations of object motion during occlusion: a longitudinal study of 6- to 12-month-old infants. Infancy 6: 165–184, 2004. [DOI] [PubMed] [Google Scholar]
- Haishi K, Kokubun M. Developmental trends in pursuit eye movements among preschool children. Percept Mot Skills 81: 1131–1137, 1995. [DOI] [PubMed] [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. Hedging your bets: intermediate movements as optimal behavior in the context of an incomplete decision. PLoS Comput Biol 30: e1004171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, Kuroda Y. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord 25: 1–18, 1995. [DOI] [PubMed] [Google Scholar]
- Hugon M, Massion J, Wiesendanger M. Anticipatory postural changes induced by active unloading and comparision with passive unloading in man. Pflugers Arch 393: 292–296, 1982. [DOI] [PubMed] [Google Scholar]
- Madelain L, Krauzlis RJ. Effects of learning on smooth pursuit during transient disappearance of a visual target. J Neurophysiol 90: 972–982, 2003. [DOI] [PubMed] [Google Scholar]
- Miall RC, Christensen LO, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5: e316, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw 9: 1265–1279, 1996. [DOI] [PubMed] [Google Scholar]
- Mitrani L, Dimitrov G. Pursuit eye movements of a disappearing moving target. Vision Res 18: 537, 1978. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Bennett SJ, Lefèvre P, Barnes GR. Evidence for synergy between saccades and smooth pursuit during transient target disappearance. J Neurophysiol 95: 418–427, 2006. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Coppe S, Blohm G, Lefèvre P. Kalman filtering naturally accounts for visually guided and predictive smooth pursuit dynamics. J Neurosci 33: 17301–17313, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Missal M, Lefèvre P. A dynamic representation of target motion drives predictive smooth pursuit during target blanking. J Vis 8: 6.1–13, 2008. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Missal M, Lefèvre P. Smooth pursuit performance during target blanking does not influence the triggering of predictive saccades. J Vis 9: 7.1–16, 2009. [DOI] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9: 1404–1411, 2006. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 9: 60–68, 2005. [DOI] [PubMed] [Google Scholar]
- Rosander K, von Hofsten C. Infants' emerging ability to represent occluded object motion. Cognition 91: 1–22, 2004. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Eizenman M, Lillakas L, To T, Westall C, Dennis M, Steinbach MJ. Saccadic adaptation in children. J Child Neurol 21: 1025–1031, 2006a. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Lillakas L, Dennis M, Steinbach MJ. Smooth pursuit eye movements in children. Exp Brain Res 169: 139–143, 2006b. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Martin N, Assaiante C. Development of anticipatory postural adjustments in a bimanual load-lifting task in children. Exp Brain Res 126: 200–204, 1999. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith AM, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Colin J, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature 2: 859–861, 1999. [DOI] [PubMed] [Google Scholar]
- Takahashi CD, Nemet D, Rose-Gottron CM, Larson JK, Cooper DM, Reinkensmeyer DJ. Neuromotor noise limits motor performance, but not motor adaptation, in children. J Neurophysiol 90: 703–711, 2003. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar HJ, Lammens M, Wesseling P, Thijssen HO, Renier WO. Development and developmental disorders of the human cerebellum. J Neurol 250: 1025–1036, 2003. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage 49: 63–70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon D, Caeyenberghs K, Swinnen SP, Smits-Engelsman BC. Development of feedforward control in a dynamic manual tracking task. Child Dev 79: 852–865, 2008. [DOI] [PubMed] [Google Scholar]
- Vasudevan EV, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci 31: 3055–3065, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten C. Action in development. Dev Sci 10: 54–60, 2007. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci 10: 640–646, 2007. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Barborica A, Ferrera VP. Modulation of visual responses in macaque frontal eye field during covert tracking of invisible targets. Cereb Cortex 17: 918–928, 2007. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang X, Peck C, Goldberg ME. The time course of the tonic oculomotor proprioceptive signal in area 3a of somatosensory cortex. J Neurophysiol 106: 71–77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]