Abstract
The neuropeptide proctolin (RYLPT) plays important roles as both a neurohormone and a cotransmitter in arthropod neuromuscular systems. We used third-instar Drosophila larvae as a model system to differentiate synaptic effects of this peptide from its direct effects on muscle contractility and to determine whether proctolin can work in a cell-selective manner on muscle fibers. Proctolin did not appear to alter the amplitude of excitatory junctional potentials but did induce sustained muscle contractions in preparations where the CNS had been removed and no stimuli were applied to the remaining nerves. Proctolin-induced contractions were dose-dependent, were reduced by knocking down expression of the Drosophila proctolin receptor in muscle tissue, and were larger in some muscle cells than others (i.e., larger in fibers 4, 12, and 13 than in 6 and 7). Proctolin also increased the amplitude of nerve-evoked contractions in a dose-dependent manner, and the magnitude of this effect was also larger in some muscle cells than others (again, larger in fibers 4, 12, and 13 than in 6 and 7). Increasing the intraburst impulse frequency and number of impulses per burst increased the magnitude of proctolin's enhancement of nerve-evoked contractions and decreased the threshold and EC50 concentrations for proctolin to enhance nerve-evoked contractions. Reducing proctolin receptor expression decreased the velocity of larval crawling at higher temperatures, and thermal preference in these larvae. Our results suggest that proctolin acts directly on body-wall muscles to elicit slow, sustained contractions and to enhance nerve-evoked contractions, and that proctolin affects muscle fibers in a cell-selective manner.
Keywords: proctolin, muscle force, cotransmitter, basal tonus, peripheral modulation, synapse
signaling molecules such as neuropeptides and biogenic amines are found extensively throughout the animal kingdom, and the genomes of most organisms encode many such molecules and their receptors. There are roughly 100 neuropeptides in the human central nervous system (CNS) and several hundred in invertebrates, and they can exert a wide range of biological actions during all stages of development (Christie 2014; Geary and Maule 2010; Hummon et al. 2006; Hurlenius and Lagercrantz 2001; Kastin et al. 2013; Ma et al. 2008; Yew et al. 1999). One hypothesis to rationalize this complexity of signaling molecules is that different modulatory substances may act on different subsets of neurons to activate specific neural circuits and/or inhibit others, to elicit specific physiological or behavioral outcomes (Dickinson et al. 2015; Harris-Warrick and Kravitz 1984; Marder and Calabrese 1996; Ormerod et al. 2015; Selverston 2010). This concept of modulators working in a “circuit-specific” manner may help to explain why so many peptides and their receptors are conserved within genomes. Neuromodulators can act in a cell-selective way to recruit specific sets of synaptically connected interneurons into activity patterns that generate rhythmic movements. These sets of interneurons comprise central pattern generators (CPGs; Harris-Warrick and Johnson 2010; Hooper and DiCaprio 2004; Marder and Bucher 2007; Marder and Calabrese 1996; Selverston 2010). The participation of a given interneuron within a specific CPG depends on the presence of specific modulator substances outside the cell and the expression of receptors to those modulators in the cell membrane. Neuromodulators can also act outside the central nervous system by altering both the release of neurotransmitters at neuromuscular synapses and the responsiveness of muscle cells to neurotransmitters (Dickinson et al. 2015; Marder and Calabrese 1996; Orchard et al. 1989). Modulatory substances also act on sensory neurons, changing their sensitivity to sensory stimuli (El Manira et al. 1991; Pasztor and Bush 1987, 1989; Pasztor and Macmillan 1990; Ramirez and Orchard 1990). Thus all the key components responsible for generating behavior (sensory neurons, interneurons, motor neurons, and muscle cells) can be modulated by neuropeptides and biogenic amines. Our current thinking is that modulatory actions at all these levels work in concert to generate physiologically and/or behaviorally appropriate responses in the context of constantly changing internal and external environments.
In addition to cell-specific effects on neurons, our work and that of others have shown that aminergic and peptidergic modulators can enhance nerve-evoked contractions and excitatory junctional potentials (EJPs) more strongly in some muscle cells than others (Gutovitz et al. 2001; Jorge-Rivera et al. 1998; Ormerod et al. 2013, 2015; Pasztor and Golas 1993). Our recent work with Drosophila third-instar larvae showed that the Drosophila peptide DPKQDFMRFamide elicits stronger contractions and enhances nerve-evoked contractions more strongly in some muscle fibers than others, and these effects corresponded to differences in expression of the FMRFamide receptor in the muscle fibers (Ormerod et al. 2015). The modulatory actions of DPKQDFMRFamide in Drosophila larvae appear to reflect neurohormonal effects, since this peptide is contained in a neurosecretory structure, the ring gland, and the motor axons innervating the larval body wall muscles do not contain FMRFamide-like immunoreactivity (White et al. 1986). Other modulators, such as proctolin (RYLTP), can be released both as hormones and as cotransmitters.
The primary purpose of this paper was to determine whether proctolin can elicit cell-selective effects on muscle fibers in Drosophila third-instar larvae. The muscle cells of the third-instar larvae are easily identifiable, their innervation is well characterized, and the larvae are considerably larger than the preceding developmental stages, which facilitates electrophysiological recording. Proctolin is known to elicit contractions and to enhance nerve-evoked contractions in muscles of many arthropod species (Baines et al. 1990; Bensen et al. 1981; Erxleben et al. 1995; Jorge-Rivera et al. 1998; Kimura et al. 1989; Lange 1990; Mercier and Lee 2002). In Drosophila larvae, proctolin is present in the ring gland, indicating a hormonal function, and it is also present in motor axons and terminals innervating muscle cells 4, 12, and 13 but not those axons innervating muscle cells 6 and 7 (Anderson et al. 1988; Taylor et al. 2004). We used cell ablation techniques to determine whether or not proctolin induces and/or enhances contractions to the same extent in all these fibers. The results indicate that proctolin can elicit modulatory effects in all these muscle cells, but the modulatory effects are more pronounced in muscle cells 4, 12, and 13. We also show that the effects of proctolin are reduced by knocking down a G protein-coupled receptor (GPCR) previously identified as a proctolin receptor in Drosophila, and that modulation of nerve-evoked contractions by proctolin depends on stimulus frequency. Drosophila has also proven to be an effective and high-throughput system in which to assess the behavioral role for modulators, or to assess the behavioral ramifications of altered modulator expression (Sokolowski 2001). As Drosophila has been used successfully to elucidate putative behavioral functions of other modulators (octopamine: Certel et al. 2010; serotonin: Alekseyenko et al. 2014; dopamine: Alekseyenko et al. 2013), we also attempted to make use of these tools to uncover potential behavioral roles of proctolin.
MATERIALS AND METHODS
Fly lines.
Drosophila melanogaster Canton S. (CS) flies, obtained from Bloomington Drosophila stock center (BDSC), were used for all control trials unless otherwise indicated. All flies were provided with commercial fly media (Formula 4–24 Instant Drosophila medium, Plain, 173200), including dry yeast (Saccharomyces cereviciae), and were reared at 21°C, constant humidity, and on a 12:12-h light-dark cycle. To examine the effects of knocking down expression of the mRNA encoding the proctolin receptor (ProcR), a transgenic line containing dsRNA for RNAi against the ProcR downstream of an upstream activating sequence (UAS) was obtained from BDSC (29414). Two tissue-specific drivers were utilized to examine reduced ProcR expression: elav-GAL4 (BDSC) and 24B-GAL4 (BDSC). elav-Gal4 was used for pan-neuronal expression of the UAS-ProcR-IR transgene (Luo et al. 1994; Sink et al. 2001), and 24B-GAL4 (Brand and Perrimon 1993; Luo et al. 1994) was used to express UAS-FR-IR in all larval somatic muscles (Schuster et al. 1996).
Dissection.
In all physiological experiments, wandering, third-instar larvae were used. Larvae were isolated from the sides of their culture vials and were placed immediately onto a dissecting dish containing a modified hemolymph-like (HL6) Drosophila saline (Macleod et al. 2002) with the following composition (in mM): 23.7 NaCl, 24.8 KCl, 0.5 CaCl2, 15.0 MgCl2, 10.0 NaHCO3, 80.0 trehalose, 20.0 isethionic acid, 5.0 BES, 5.7 l-alanine, 2.0 l-arginine, 14.5 glycine, 11.0 l-histidine, 1.7 l-methionine, 13.0 l-proline, 2.3 l-serine, 2.5 l-threonine, 1.4 l-tyrosine, 1.0 valine (pH = 7.2). Larvae were pinned dorsal-side up at the anterior and posterior ends, a small incision was made along the entire dorsal midline, and the visceral organs were removed. All nerves emerging from the central nervous system (CNS) were severed at the ventral nerve cord, and the CNS, including ventral nerve cord and the right and left lobes, was removed, leaving long nerve bundles innervating the body wall muscles. This preparation was sufficient for muscle contraction recordings. To record intracellularly from muscle cells, the body wall was pinned out, and the body-wall muscles were viewed under a dissecting microscope.
Electrophysiological recordings.
Compound EJPs were elicited by stimulating all severed abdominal nerves using a suction electrode with an inner diameter of typically 150–180 μm connected to a Grass S88 stimulator via a Grass stimulus isolation unit (Grass Technologies, Warwick, RI). Impulses were generated via the stimulator at 0.2 Hz. EJPs were recorded using sharp, glass microelectrodes containing a 2:1 mixture of 3 M potassium chloride:3 M potassium acetate, electrode resistance typically 40–80 MΩ. Signals were detected with an intracellular electrometer with a 50-Hz low-pass filter (Warner Instrument, model IE:210), viewed on a HAMEG oscilloscope, and sent to a personal computer via an analog-to-digital converter (Brock University, Electronics Division). Signals were acquired at 10 kHz and processed in digital format using custom-made software (“Evoke,” Brock University, Electronics Division). Microsoft Excel was used for further analysis. The acquisition software detected the maximum amplitude of each EJP. For each trial, EJP amplitudes were averaged over 30-s time intervals (6 responses). Proctolin was obtained from Abbiotec (350353, San Diego, CA).
Nerve-evoked contractions.
All force recordings were obtained using a Grass FT03 Force-Displacement Transducer connected to a Grass MOD CP122A amplifier. Nerve-evoked contractions were elicited using bursts of electrical stimuli from a Grass S48 stimulator. Impulse bursts 250 ms in duration were applied every 15 s. All force recordings were made using HL6 physiological saline (described above) except that the CaCl2 concentration was 1.5 mM. Larvae were dissected as outlined above. To attach the larvae to the force transducer, a hook was made from a fine minutien pin and placed onto the posterior end of the larvae. In selected trials, a fine angled tip dissecting knife was used to selectively ablate a subset of muscles in each hemisegment, as described elsewhere (Ormerod et al. 2013). Care was taken to avoid damage to any other tissue in the larvae.
Muscle cells 6 and 7 contributed ∼50% of the ventral longitudinal force generated by these semi-intact preparations, muscle cells 12 and 13 contributed ∼30%, and muscle cells 4, 12, and 13 contributed ∼70%, which was consistent with cellular volume/sarcomeric potential (Ormerod et al. 2013). To determine whether or not proctolin enhanced muscle cells in a cell-selective manner, we used cell ablation to eliminate sets of muscle cells that contribute to longitudinal force production, and then compared proctolin's effects on nerve-evoked contractions. All other muscle cells were left intact for recording contractions unless deliberately ablated, or ablated during dissection. To determine the relative contribution made by these muscle cells, contractions were compared with those of control preparations that were identical in every respect except that no proctolin was applied.
Proctolin-induced contractions.
To determine whether proctolin would induce contractions in the absence of nerve stimulation, larvae were dissected as described above, and the anterior pin was replaced with a custom minutien pin attached to a Grass FT03 tension transducer (Grass Instruments, Quincy, MA) as described previously (Clark et al. 2008; Milakovic et al. 2014; Ormerod et al. 2013, 2015). Contractions were amplified using a MOD CP 122A amplifier (Grass Telefactor, West Warwick, RI), digitized using DATAQ data-acquisition sampling at 30 Hz (model DI-145, Akron, OH), and viewed using WinDaq software (DATAQ Instruments). Data were subsequently exported to Microsoft Excel for further analysis and graphing. The recording dish had a volume of ∼0.2–0.4 ml and was perfused continuously at a rate of 0.7 ml/min. Excess fluid was removed by continuous suction.
RT-qPCR.
To quantify changes in ProcR expression in knockdown lines, total RNA was isolated from whole larvae (5 larvae per replicate) using Norgen's Total RNA Purification Kit (St. Catharines, ON, Canada), 500 ng of total RNA were reverse transcribed with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA), and PCR was performed to validate primers (see Table 1). For real-time qPCR, SYBR Green qPCR Supermix (Invitrogen) was added to the cDNA and the primers. Each sample was amplified for 40 cycles in a thermocycler (Bio-Rad) as follows: 5 min at 95°C, 15 s at 95°C, 90 s at 56°C, and 30 s at 72°C. The delta delta Ct (2−ΔΔCt) method was used for data analysis, and ribosomal protein 49, a housekeeping gene, was used for data normalization.
Table 1.
Primers used for PCR
| Primers | Sequence |
|---|---|
| rp49 forward | 5′-GATCGTGAAGAAGCGCCA-3′ |
| rp49 reverse | 5′-CGCTCGACAATCTCCTTG-3′ |
| ProcR forward | 5′-TGTGCCGCATACAACGACTA-3′ |
| ProcR reverse | 5′-GCTATCAGGCGACCCGTATT-3′ |
Larval thermal preference.
To assess thermal preference, a linear thermal gradient of 15–30°C was generated using an aluminum dish machined into a rectangular lane (48 mm × 20 mm × 9 mm), which was placed on top of two Peltier units, each positioned at either end of the lane. Each Peltier unit had an independent digitally controlled power supply box (Brock University Electronics Division), ensuring accurate and constant temperature extremes. The temperature range and linear nature of the gradient were confirmed using a thermal camera (FLIR SC660, FLIR Systems), connected to a computerized data-acquisition system (Examine-R, FLIR Systems). During experiments, the thermal gradient was monitored using two thermocouples, one at each end of the lane. Third-instar larvae were removed from stock vials without anesthesia and were washed in distilled water (to avoid contaminating the testing surface with food) and placed in the center of the lane. Testing was performed in the dark, and images were captured under infrared light using a Vario-Sonnar Super Steady Shot camcorder (Sony Carl Zeiss); the video stream was captured to still images every second using HandyAvi software (AZcendant Software, Tempe, AZ). ImageJ (NIH) software was used to assess the final positional coordinates and the corresponding temperature for each larva after 10 min, and mean positioning was calculated as a measure of thermal preference. Prior to experimentation any potential side or wall bias was assessed by examining larval distribution in the lane set ubiquitously at 22°C. We arbitrarily divided the lane into four equal quadrants and observed no statistically significant differences between them, nor were there any side or wall biases.
Velocity of locomotion.
We made use of our gradient testing apparatus from the thermotaxis experiments and knowledge that Drosophila larvae show a negative phototaxis to assess larval locomotory velocity (Xiang et al. 2010). Larvae were placed at one end of the lane, with the entire gradient chamber set to a constant temperature 21°C. Then using fiber-optic cables, light was cast directly onto the larvae, which caused them to crawl away. We recorded their behavior on a video camcorder (see above) and calculated their velocity over a distance of 4 mm using ImageJ software.
Statistics.
SigmaPlot software was used to examine statistical significances. To look for comparisons within conditions a one-way ANOVA was used if the data were normally distributed and the variance was homogenous. In circumstances where these two conditions were not met, a comparable nonparametric test was used. Specifically, in Figs. 6, 7, and 8B, a Kruskal-Wallis one-way analysis of variance by ranks was used with a Dunn's post hoc test. For comparisons both within and between conditions a two-way repeated-measures ANOVA was used. In all cases if a significant difference was obtained a Tukey (for ANOVA) or Dunn's (for ANOVA on ranks) post hoc test was performed to establish specific differences. GraphPad software was used for generating dose-response curves and for calculating EC50 concentrations. Within the figure headings both standard error of the means (SE) and standard deviation (SD) are used. SE is only reported when averages of averages were plotted, while SD is used to depict the error of a sample of data relative to their own means.
Fig. 6.
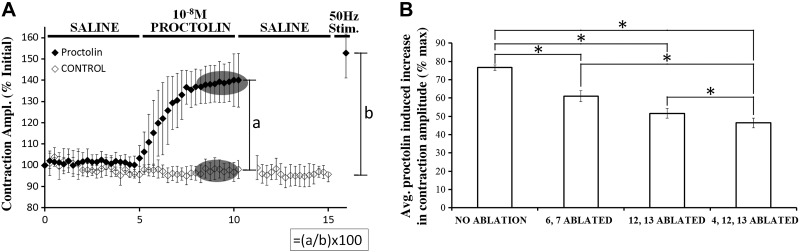
Proctolin increases the amplitude of evoked contractions to a greater extent in muscle fibers 4, 12, and 13 than muscle fibers 6 and 7. A: data from Fig. 5B are used to demonstrate how proctolin-induced increases in contraction amplitude were expressed as a function of maximal contraction. Within each trial, contraction amplitudes (expressed as % of initial contraction) were averaged during the 8- to 10-min time period for both proctolin and “no-proctolin” application trials. The averaged amplitude for the trials with no proctolin was subtracted from amplitude obtained in each of the proctolin application trials to estimate the increase in nerve-evoked contraction attributed to proctolin (a). The maximal contraction value for that preparation (b) was estimated by subtracting the averaged contraction at 15 min in control trials from the contraction elicited by a single burst of impulses at 50 Hz in the presence of the peptide. The ratio a/b was then multiplied by 100 to estimate the relative effectiveness of proctolin in each trial. This procedure estimates the modulatory effect on only the muscle fibres present and allowed a comparison between preparations with different levels of ablation by compensating for differences in total muscle mass. B: effects of cell ablation on modulation by 10−8 M proctolin were assessed by estimating the relative effectiveness as described in A. Error bars, SD. (Kruskal-Wallis, H = 123.455, P < 0.001; Dunn's post hoc, *P < 0.05).
Fig. 7.
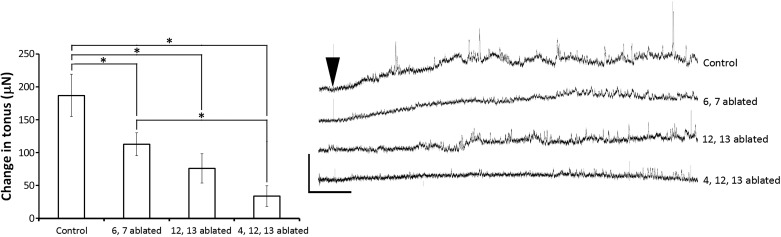
Proctolin-induced sustained contractions are larger in cells 4, 12, and 13 than in cells 6 and 7. Average change in tonus induced by 10−6 M proctolin application compared across preparations with: no ablation (Control); with cells 6 and 7 ablated; cells 12 and 13 ablated; and cells 4, 12, and 13 ablated. Significances were observed between the controls and all other groups, and between those preparations with cells 6 and 7 ablated and those preparations with cells 4, 12, and 13 ablated (*P < 0.05). Error bars, SD; N = 10–19.
Fig. 8.
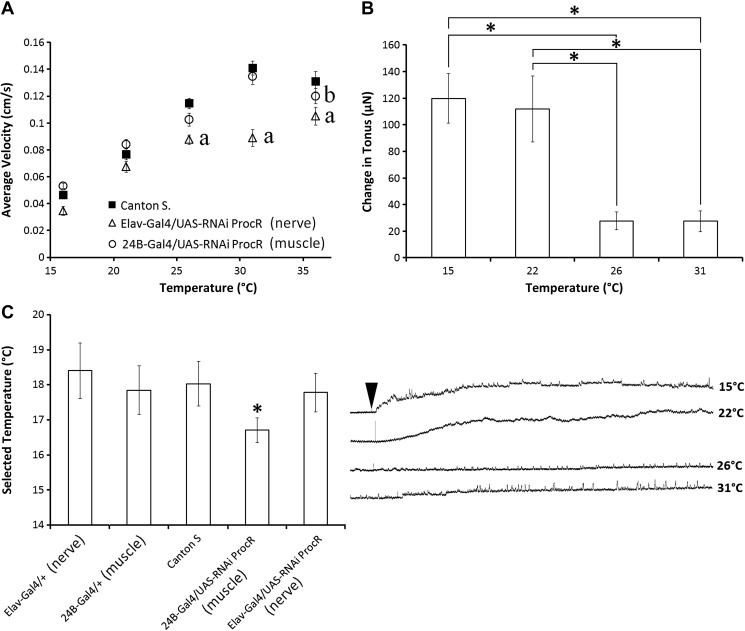
Reducing the expression of the proctolin receptor alters larval speed and thermal preference. A: using the Gal4/UAS system to knock down the expression of the proctolin receptor in muscle (24B-Gal4/UAS-RNAi ProcR) and in nervous tissue (Elav-Gal4/UAS - RNAi ProcR), to determine if reducing proctolin receptor expression alters the velocity of larval crawling at different temperatures. aSignificant differences between control and nerve (elav) trials. bSignificant differences between control and muscle (24B) trials. Error bars, SE; N = 14–19. B: ability of proctolin to increase changes in tonus is temperature-dependent. Increasing temperature decreases the force of sustained contractions elicited by 10−6 M proctolin application. Error bars, SD; N = 10. *P < 0.05. C: using the Gal4/UAS system to knock-down proctolin receptor expression revealed that altering proctolin receptor expression in muscle tissue significantly alters the preferred temperature of larvae. Error bars, SD; N = 18–20. *P < 0.05.
RESULTS
Excitatory junctional potentials.
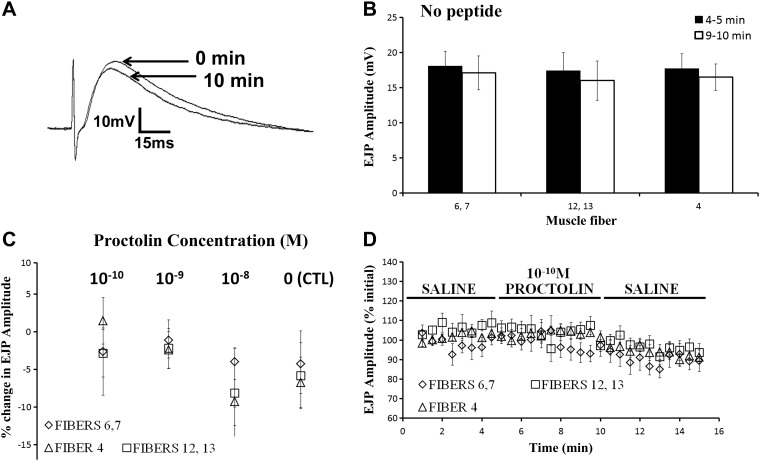
We first assessed the effect of proctolin on 5 different muscle fibers (fibers 4, 6, 7, 12 and 13) to determine if this peptide also elicits differential effects on muscle cells. Electrical stimuli were delivered to severed nerve branches continuously at 0.2 Hz, and EJPs were recorded for 15 min (5 min in saline, 5 min in proctolin, and 5 min of washout with saline). At the stimulus frequency used there was a gradual decrease in EJP amplitude over the 15-min recording period in control recordings with no peptide (Fig. 1, A–D). Such a reduction in EJP amplitude has been referred to as low-frequency depression and has been reported previously for this preparation (Dunn and Mercier 2005) and in other arthropod muscles (Bruner and Kennedy 1970; Bryan and Atwood 1981). The degree of low-frequency depression was not significantly different between the five fibers in control trials with no peptide after 10 min (2-way ANOVA, df = 58, between: F = 0.448, P = 0.642; within: F = 1.48, P = 0.237). Figure 1A depicts representative EJPs from muscle cell 6 (fiber 6) at the start of a recording (0 min) and after 5 min of saline application followed by 5 min of saline with 10−8 M proctolin (10 min). Application of 10−10 to 10−8 M proctolin did not consistently alter the amplitude of EJPs in any of the identified muscle fibers investigated (Fig. 1C). The slight reduction in amplitude in Fig. 1 (raw traces in Fig. 1A) is due to low-frequency depression and not an inhibitory effect of proctolin. Figure 1D depicts averaged EJP values (6 EJPs per 30 s time point) for the 5 muscle fibers during the 15-min recording period. Resting membrane potential was monitored continuously throughout each trial and no significant difference was observed in any of the 5 muscle fibers: (0 min: muscles 6, 7: −43.7 ± 4.9 mV; muscles 12, 13: −42.7 ± 6.5 mV; muscle 4: −42.3 ± 5.6 mV; 10 min: muscles 6, 7: −42.7 ± 7.0 mV; muscles 12, 13: −43.5 ± 5.4 mV; muscle 4: −41.5 ± 5.4 mV).
Fig. 1.
Proctolin application does not alter the amplitude of excitatory junctional potentials (EJPs). A: representative raw recordings of an EJP after 0 and 10 min of the recording protocol. B: average EJP amplitudes across the 5 muscle fibers of interest at 4–5 min and 9–10 min in physiological saline to emphasize change in EJP amplitude as a function of time is conserved across these fibers. Error bars, SE; N = 6–9. C: EJPs were recorded intracellularly in Drosophila muscle fibers prior to and during exogenous application of 10−10 to 10−8 M proctolin. Each trial consisted of 5 min of saline application followed by 5 min of proctolin application and subsequently a 5-min washout period. EJPs were elicited using continuous 0.2-Hz stimulation, and in each trial the average amplitude of 6 EJPs recorded between 9.5 and 10 min are shown (corresponding to 4.5–5 min of proctolin application). None of the muscle fibers showed a significant change in EJP amplitude at any of the proctolin concentrations shown. Error bars, SE; N = 6–9. D: average EJP values (expressed as a percentage of the initial EJP) for the 5 muscle fibers during the 15-min recording period. Each data point represents an average of 6 EJPs per replicate trial. Error bars, SE; N = 7.
Proctolin-induced changes in tonus.
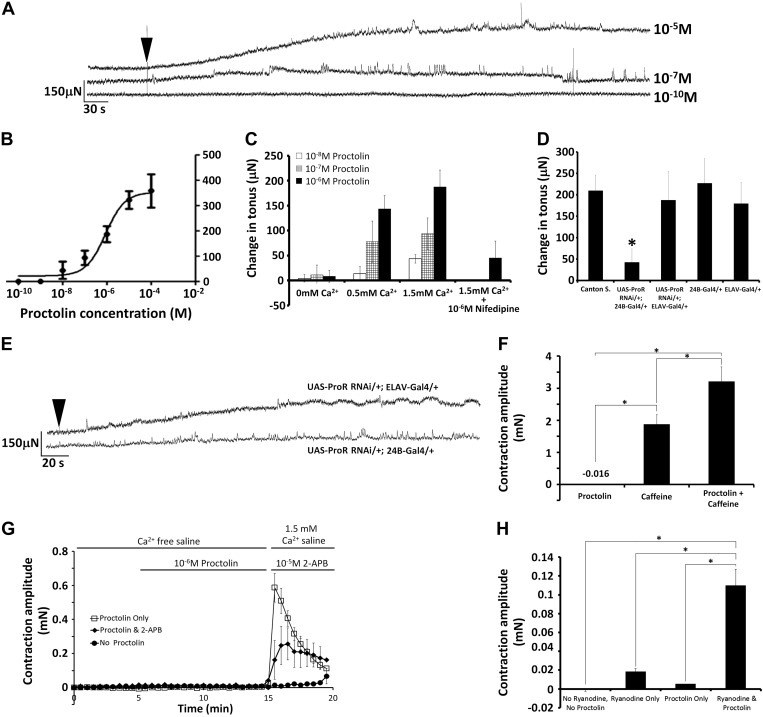
To determine whether proctolin could elicit contractions in third-instar larval body-wall muscles, changes in muscle force were recorded in dissected larvae with no CNS and no nerve stimulation. Figure 2A depicts representative recordings of muscle contractions from larvae following the application of 10−10, 10−7, and 10−5 M proctolin. Exogenous application of proctolin elicited small, sustained muscle contractions (increases in muscle tonus) that were dose-dependent (Fig. 2). The threshold for eliciting sustained contractions was between 10−9 M and 10−8 M, and the effect saturated at or above 10−5 M. The EC50 for the effect of proctolin on sustained contractions was 8.5 × 10−7 M (Fig. 2B).
Fig. 2.
Proctolin induces dose-dependent sustained contractions in third-instar larvae. A: representative force traces of proctolin-induced (10−5 M, 10−7 M, and 10−10 M) contractions from semi-intact preparations. Arrow indicates when proctolin was applied. B: dose-response curve for the effect of proctolin on sustained contractions. Independent replicates for each concentration. Error bars, SD; N = 8–18. C: effects of altering the external calcium concentration (0, 0.5, and 1.5 mM Ca2+) in saline on the ability of exogenous proctolin (at 10−8 M to 10−6 M) to evoke sustained contractions in third-instar larval body-wall muscles. Coapplication of 10−6 M nifedipine with 1.5 mM Ca2+ saline significantly reduced the amplitude of 10−6 M proctolin-induced sustained contractions. Error bars, SD; N = 5–7. D: using the Gal4/UAS system to knock-down the expression of the proctolin receptor separately in muscle (UAS-ProcR RNAi/+; 24B-Gal4/+) and in nervous tissue (UAS-ProcR RNAi/+; Elav-Gal4/+) shows that 10−6 M proctolin-induced changes in tonus require proctolin receptor expression in muscle tissue, but not in nervous tissue. Error bars, SD; N = 7–8. *P < 0.05 compared with Canton S. controls. E: representative traces from muscle and nerve knockdown lines. Arrow indicates when proctolin was applied. F: in Ca2+-free saline, coapplication of 3 mM caffeine and 1 μM proctolin induced a contraction that was significantly greater than that induced by caffeine or proctolin alone. *P < 0.05. G: sizable larval contractions were induced by a 10 min “pretreatment” of 1 μM proctolin in Ca2+-free saline, followed by a 1.5 mM Ca2+ saline containing no proctolin. These contractions were reduced significantly by including a blocker of store-operated Ca2+ channels, 2-aminoethoxydiphenyl borate (2-APB), in the 1.5 mM Ca2+. H: coapplication of ryanodine and proctolin in Ca2+-saline elicited contractions that were significantly greater than application of ryanodine or proctolin alone. *P < 0.05.
Proctolin and other neuropeptides have previously been demonstrated to require external calcium to elicit their effects on muscle fibers (Lange et al. 1987; Nwoga and Bittar 1985; Schwarz et al. 1980; Wilcox and Lange 1995). To determine if proctolin-induced contractions in Drosophila require external calcium, the concentration of calcium in the physiological saline was altered. Figure 2C demonstrates the effects of altering the external calcium concentration (0, 0.5, and 1.5 mM) on the ability of proctolin (10−8, 10−7, and 10−6 M) to induce sustained contractions in body-wall muscle. Reducing the external calcium from 1.5 to 0.5 mM significantly reduced the amplitude of contractions in 10−8 M and 10−6 M proctolin, and reducing calcium from 1.5 to 0 mM significantly reduced the amplitude of contractions at all three proctolin concentrations examined (Fig. 2C: 2-way ANOVA, F = 66.415, df = 68, P < 0.001, Tukey post hoc, P < 0.05). To examine the possibility that calcium influx through L-type channels might be necessary for the contractions, we coapplied 10−6 M proctolin with the L-type selective blocker, nifedipine, at a concentration (1 × 10−6 M) slightly lower than the IC50 (3 × 10−6 M) reported to inhibit L-type channels in Drosophila muscle cells (Morales et al. 1999). Coapplication of 10−6 M nifedipine and 10−6 M proctolin significantly reduced the amplitude of sustained contractions compared with application of 10−6 M proctolin alone (Fig. 2C: Mann-Whitney rank sum, t = 29, P < 0.01).
To determine whether proctolin-induced changes in tonus are elicited via this G protein-coupled receptor [GPCR, the proctolin receptor (ProcR)] and are dependent upon postsynaptic receptor localization, we used the Gal4/UAS system to drive the expression of RNAi against the proctolin receptor in a tissue-selective manner to knock-down receptor expression in nervous and muscle tissue. Figure 2D depicts changes in tonus elicited by 10−6 M proctolin for control lines [Canton S (wild-type) and Gal4 driver lines (24B-Gal4/+, and ELAV-Gal4/+) that were not crossed with RNAi-knockdown line] and to lines where the ProcR was knocked-down postsynaptically in muscle cells (UAS-ProcR RNAi/+; 24b-Gal4/+) and in nervous tissue (UAS-ProcR RNAi/+; ELAV-Gal4/+). Knocking down ProcR expression selectively in muscle fibers significantly reduced the proctolin-induced changes in tonus compared with control lines, but knocking down ProcR in nervous tissue did not (1-way ANOVA, F = 17.452, P < 0.001, Tukey post hoc P < 0.05). There were no statistically significant differences between the responses to proctolin between control lines (1-way ANOVA, F = 0.975, P = 0.419). Figure 2E depicts representative traces from nerve (top) and muscle (bottom) knockdown lines. qPCR was carried out to confirm knock-down of proctolin receptor expression across the various lines. Expression was reduced in muscle (24B-Gal4/UAS-ProcR) and nerve (elav-Gal4/UAS-ProcR) by 67% and 53%, respectively.
To begin to elucidate the intracellular mechanism(s) underlying proctolin's effects, we examined whether proctolin could act downstream of calcium influx if calcium is released from the sarcoplasmic reticulum. We elicited contractions in calcium-free saline by applying 3 mM caffeine, a voltage-independent activator of ryanodine receptors (Fig. 2F). These large contractions were significantly different (1-way ANOVA, P < 0.05) from the effect of 1 μM proctolin alone, which did not elicit contractions in calcium-free saline. Coapplication of 3 mM caffeine and 1 μM proctolin induced a contraction that was significantly greater than that induced by caffeine alone (Fig. 2F, P < 0.05). In a separate set of trials, larvae were perfused with 1 μM proctolin in calcium-free saline for 10 min, and then the perfusion was changed to 1.5 mM calcium saline that contained no proctolin (Fig. 2G). Sizable contractions occurred when proctolin was washed-out and 1.5 mM calcium was simultaneously “washed-in.” The amplitude of these contractions was reduced significantly by including a blocker of store-operated calcium channels, 2-aminoethoxydiphenyl borate (2-APB), in the 1.5 mM calcium saline that was used to wash-out proctolin (Fig. 2G, 1-way ANOVA, P < 0.05). In trials with no proctolin, simply washing-in 1.5 mM calcium had little or no effect on contraction. Last, perfusing larvae with calcium-free saline containing ryanodine at a concentration that activates ryanodine receptors (10 μM; Xu et al. 2000) induced small muscle contractions, and coapplication of ryanodine and proctolin in calcium-free saline elicited contractions that were significantly larger (Fig. 2H). These observations indicate that proctolin can act downstream of calcium influx if calcium is released from intracellular stores, and they suggest that proctolin can activate store-operated calcium influx in calcium-free saline.
Nerve-evoked contractions.
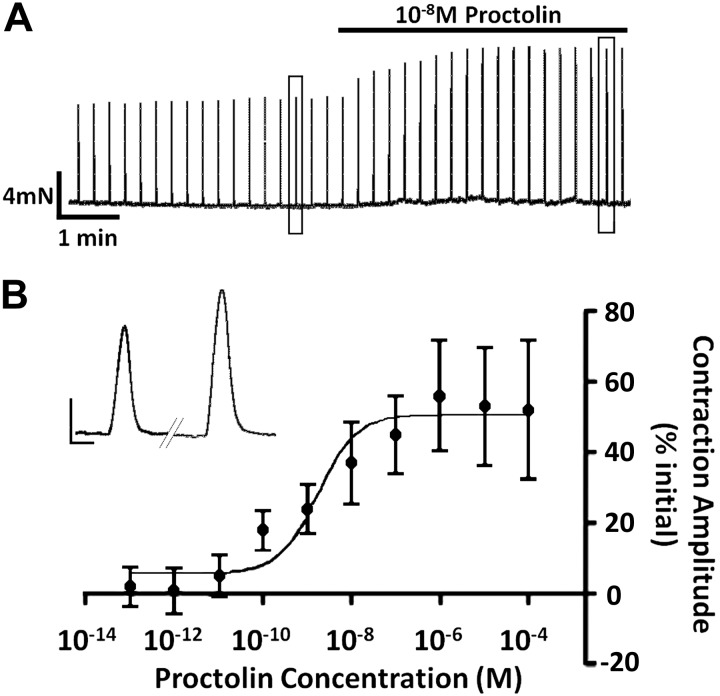
We next examined whether exogenous application of proctolin would alter nerve-evoked contractions in this larval preparation. Initially, we used a previously established stimulus protocol to evoke and record contractions (32-Hz intraburst impulse frequency, 250-ms burst duration, 1 burst every 15 s; Ormerod et al. 2013). This stimulus protocol is within the range of motor output patterns underlying contractions recorded from tethered larvae (Paterson et al. 2010). Figure 3A depicts a 10-min recording showing that 10−8 M proctolin increased nerve-evoked contractions and shows the dose-dependence for this enhancement of nerve-evoked contractions. Interestingly, the threshold for proctolin to alter nerve-evoked contractions (∼10−11 M) was 2–3 orders of magnitude lower than its threshold for eliciting contractions. At this intraburst impulse frequency (32 Hz), the dose-response relationship was quite broad, with a saturating effect at 10−6 M, and the EC50 value was 1.7 × 10−9 M (Fig. 3B).
Fig. 3.
Proctolin application increased the amplitude of evoked contractions. A: representative trace of the temporal effects of proctolin application on contractions that were evoked using a 250-ms duration burst at 32 Hz, every 15 s. The representative trace is about 9 min in duration. The black application bar depicts when 10−8 M proctolin was applied. The two boxes (one prior to proctolin application and one roughly 4.5 min after 10−8 M proctolin) indicate which individual contractions were taken to create the inset shown in the middle-left. Inset: single evoked contraction before (left) and after (right) 10−8 M proctolin. The scale bars for the inset: 0.4 mN, 0.2 s. B: dose-response curve for the effect of proctolin on nerve-evoked contractions. Independent replicates for each concentration; error bars, SD; N = 7–10.
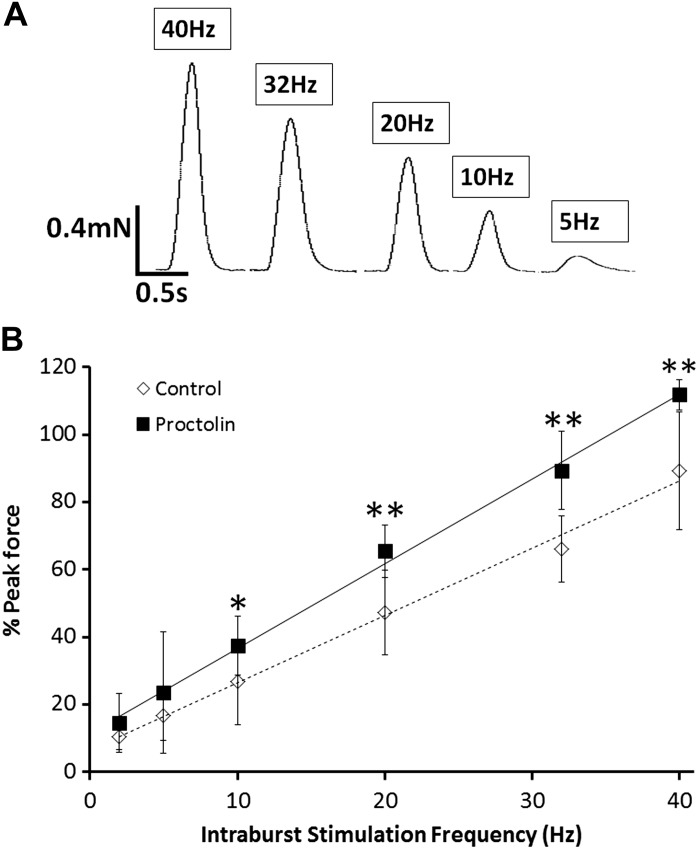
We next examined whether proctolin-induced enhancement of nerve-evoked contractions was sensitive to changes in the intra-burst impulse frequency. Figure 4 illustrates the effects of 10−9 M proctolin on nerve-evoked contractions for several intraburst frequencies. In these experiments peak force was expressed as a percentage of the maximal contraction elicited with 50-Hz stimulation, which elicits maximal contractions in these preparations (Ormerod et al. 2013). The concentration of proctolin used, which was very close to the EC50 value, caused significant increases in nerve-evoked contractions at intraburst frequencies of 10–40 Hz but not at intraburst frequencies of 2–5 Hz (Fig. 4B). Thus proctolin was more effective at increasing nerve-evoked contractions at higher intraburst frequencies.
Fig. 4.
Force-frequency curve for control and proctolin groups. A: representative traces shown above depict the effect of varying the intraburst frequency on evoked-contractions from control animals. B: effect of 10−9 M proctolin on nerve-evoked contractions was determined for various intraburst frequencies (motor neuron stimulation) in third-instar larvae. Proctolin enhanced nerve-evoked contractions at higher impulse frequencies but not at low intraburst frequencies. Maximal force was estimated using a single 50-Hz burst (250-ms duration) at the end of each trial. Statistical comparisons between contractions with and without proctolin were made using a Mann-Whitney test (*P < 0.05; **P < 0.01). Error bars, SD; n = 7–10.
To further explore the frequency-dependent effects of proctolin, dose-response curves were generated for five of the intraburst stimulus frequencies used in Fig. 3 and were used to estimate threshold and EC50 values (Table 2). The threshold for proctolin to enhance nerve-evoked contractions decreased as the intraburst stimulus frequency was raised (Table 2). Thresholds were between 10−10 and 10−9 M for 2 and 5 Hz stimulation, between 10−11 and 10−10 M for 10 and 20 Hz, and between 10−12 and 10−11 M for 32 and 40 Hz stimulation (Table 2). There was also a concomitant reduction in the EC50 concentration as the intraburst impulse frequency increased (Table 2).
Table 2.
Increasing impulse frequency within bursts increases the effectiveness of proctolin at enhancing nerve-evoked contractions
| Intraburst Impulse Rate, Hz | No. of Impulses | Threshold | EC50 |
|---|---|---|---|
| 2 | 1 | 10−10–10−9 M | 8.4 × 10−8 M |
| 5 | 2 | 10−10–10−9 M | 4.0 × 10−8 M |
| 10 | 3 | 10−11–10−10 M | 1.8 × 10−8 M |
| 20 | 6 | 10−11–10−10 M | 4.6 × 10−9 M |
| 32 | 9 | 10−12–10−11 M | 1.7 × 10−9 M |
A dose-response curve was generated for the 5 listed stimulus rates. Both the threshold for enhancement of contractions and the EC50 values for proctolin decreased with increases in intraburst stimulus rate.
Muscle-cell ablation.
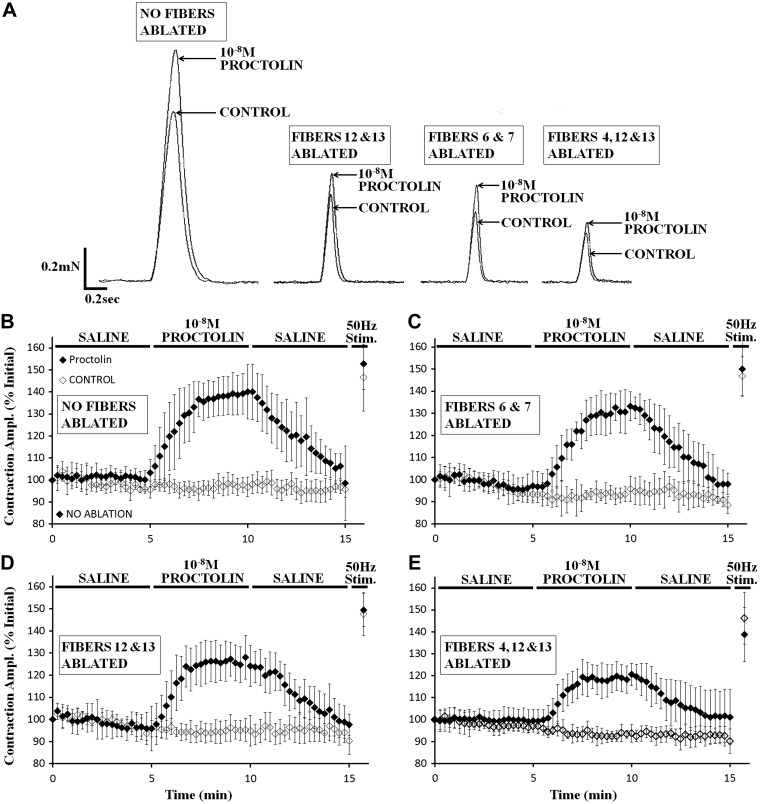
Anderson et al. (1988) demonstrated that the motor neurons innervating muscle fibers 3, 4, 12, and 13 contain the highest relative abundance of proctolin immunoreactivity (14–34%), with very little apparent expression (0–4%) in the other 26 fibers. Thus, to determine if these fibers are preferentially modulated by proctolin, we used an established cell-ablation technique, where muscle cells of interest are serially ablated (Ormerod et al. 2013; 2015), to assess the relative contribution of selected muscle fibers to proctolin-induced enhancement of nerve-evoked contractions (Fig. 5). Contractions were expressed as % of initial value and were plotted against time. At 10−8 M, proctolin enhanced nerve-evoked contractions in all cases. The effect of proctolin was reduced only slightly by ablating muscles cells 6 and 7 or by ablating muscle cells 12 and 13. Ablating cells 4, 12, and 13, however, appeared to reduce proctolin's effectiveness more. To quantify the effect of proctolin, nerve-evoked contractions at 8–10 min in each proctolin trial (corresponding to 4–5 min of proctolin exposure) were averaged, and the averaged value at 8–10 min control trials (with no proctolin) was subtracted. This difference was then expressed as a percentage of the maximal contraction elicited by a 50-Hz impulse burst in that trial. This procedure corrected for differences in force between the different preparations and provided a measure of proctolin's ability to enhance nerve-evoked contractions in the subset of fibers remaining after ablation (Fig. 6). With no cells ablated, 10−8 M proctolin enhanced contractions by up to 78% of maximal contraction. Ablating cells 6 and 7 significantly decreased the effectiveness of proctolin to 62% of maximal force (a 16% drop in effectiveness compared with no ablation). Ablating cells 12 and 13 significantly decreased proctolin's effect to 52% of maximal contraction (a 26% drop compared with no ablation), and ablating cells 4, 12, and 13 significantly decreased proctolin's ability to enhance contractions to 47% of maximal contraction (a 31% decrease in the modulatory effect of proctolin). Thus modulation of muscle cells 4, 12 and 13 appeared to contribute proportionately more to proctolin's effectiveness than did modulation of cells 6 and 7. (Fig. 6: Kruskal-Wallis, H = 123.455, P < 0.001, Dunn's post hoc, P < 0.05).
Fig. 5.
Proctolin application increased the amplitude of nerve-evoked contraction in muscle cells 4, 12, and 13 more than in muscle cells 6 and 7. A: representative traces from intact preparations and those preparations with muscle cells ablated, before and after 10−8 M proctolin application. B: nerve-evoked contractions from semi-intact preparations with no muscle cell ablation show that 10−8 M proctolin application induced a significant increase in contraction amplitude (P < 0.001). C: 10−8 M proctolin significantly increased the amplitude of nerve-evoked contractions in preparations with muscle cells 6 and 7 ablated (P < 0.001). D: 10−8 M proctolin significantly increased the amplitude of nerve-evoked contractions in preparations with muscle cells 12 and 13 ablated (P < 0.001). E: 10−8 M proctolin significantly increased the amplitude of nerve-evoked contractions in preparations with muscle cells 4, 12, and 13 ablated (P < 0.001). B–E: error bars, SD; N = 6–9.
We next sought to determine if proctolin-induced contractions in the absence of neuron stimulation (Fig. 2) might also reflect cell-selective effects. To determine this, contractions were elicited using 10−6 M proctolin, as in Fig. 2, but selective cell ablation was performed. Ablating muscle fibers 6 and 7 significantly reduced the force of proctolin-induced contractions compared with control preparations with no ablation (Fig. 7: Kruskal-Wallis ANOVA on ranks, H = 42.803, P < 0.001, Dunn's post hoc, P < 0.05). Ablating muscle fibers 12 and 13 also significantly reduced the force of sustained contractions compared with control preparations (P < 0.05), and ablating fibers 4, 12, and 13 significantly reduced the force of sustained contractions compared with controls (P < 0.05). A statistically significant difference was also observed between preparation with fibers 6 and 7 ablated and those preparations with fibers 4, 12, and 13 ablated. Thus, as with nerve-evoked contractions, fibers 4, 12, and 13 appeared to contribute more to the proctolin-induced contractions.
Behavioral experiments.
In addition to examining physiological effects of proctolin in third-instar Drosophila larvae, we examined its possible roles in behavior. Given proctolin's ability to enhance muscle contractions, we first examined whether knocking down the proctolin receptor would alter the ability of larvae to crawl. A light-avoidance assay was employed to estimate maximal crawling speed of larvae in Canton S. larvae, muscle and nerve-selective ProcR knockdown lines, as well as the corresponding genetic controls (Fig. 8A). At room temperature, Canton S. flies had an average velocity of 0.08 mm/s, which was not significantly different from either of the knockdown lines (Elav-Gal4/UAS-RNAi ProcR or 24B-Gal4/UAS-RNAi ProcR; 2-way ANOVA with Tukey for pairwise comparisons, P > 0.05). Given that larval crawling in Drosophila and neuromodulation by neuropeptides have both been demonstrated to be temperature-dependent, along with many other biological processes, we examined larval crawling velocity at five different temperatures, ranging from 16 to 36°C (Dunn and Mercier 2005; Ohyama et al. 2013). In response to stepwise increments of 5°C, Canton S. larvae crawled at a significantly faster velocity with each temperature increase up to 36°C, at which larval velocity appeared to saturate (2-way ANOVA, Tukey P < 0.05). At 16°C, there were no significant differences between crawling velocities of the Canton S. and proctolin receptor knockdown groups. Increasing temperature above 21°C increased crawling velocity in larvae with reduced proctolin receptor expression in nervous tissue and muscle tissue, but velocity was significantly lower (P < 0.05) at 26–36°C in larvae with reduced expression in nervous tissue than in Canton S. larvae. At 36°C, larvae with reduced proctolin receptor expression in muscle tissue were also significantly slower compared with Canton S. controls (P < 0.05). Nerve and muscle control lines (Elav-Gal4/+; 24B-Gal4/+) were also tested, and none showed a significant difference in larval crawling velocity compared with Canton S. larvae. Taken together, the results indicated that altering proctolin receptor expression affects the temperature dependence of crawling velocity.
To further explore the temperature dependence of proctolin's effects in third-instar larvae, we next examined proctolin-induced contractions at different temperatures. Contractions induced by 10−6 M proctolin at 16°C and 21°C were not significantly different (Fig. 8B: Kruskal-Wallis 1-way ANOVA, H = 23.674, P < 0.001, Tukey test for pairwise comparisons, P > 0.05). Increasing the temperature to 31 or 36°C, however, significantly decreased proctolin-induced contractions to ∼24% of the amplitude observed at 16 and 21°C (P < 0.05). One possible confounding effect was the role of pH during these experiments. We monitored the saline pH and found a strongly negative correlation (R2 = 0.999) with increasing temperature. At 16°C the pH was 7.30, and at 36°C the pH was 7.06. Thus, for every degree Celsius change, a 0.12 pH shift occurred. We next examined whether altering proctolin receptor expression altered the thermal preference of third-instar larvae (Fig. 8C). Canton S. flies had a preferred temperature of 18.0 ± 0.6°C, and the nerve-selective proctolin receptor knockdown line had a preferred temperature of 17.8 ± 0.5°C. The muscle-selective proctolin receptor knockdown line had a significantly decreased preferred temperature of 16.7 ± 0.4°C (1-way ANOVA, F = 3.529, P = 0.037, Tukey test for pairwise comparisons, P < 0.05). No significant differences were observed between muscle and nerve genetic control lines (P < 0.05).
DISCUSSION
To date, few studies have examined the physiological role of proctolin in Drosophila (Taylor et al. 2004). Here we characterize several roles for proctolin in body-wall muscles in third-instar Drosophila larvae. Johnson et al. (2003) identified and characterized the Drosophila proctolin receptor by expressing genes for “orphaned” Drosophila GPCRs in HEK cells and examining responses to numerous modulators. Only one receptor (the one studied here) was highly selective for proctolin compared with 14 other modulators investigated. The only other agent reported to activate this receptor was substance P, which was effective at a concentration that was 10,000 times greater than that of proctolin. Substance P has previously been identified in the Drosophila CNS and in cell lines derived from the Drosophila CNS, but we were unable to identify a receptor for substance P within the Drosophila genomic database (Nassel et al. 2002; Ui-Tei et al. 1995). Our results demonstrate that proctolin-induced contractions were significantly reduced by knock-down of this receptor in Drosophila muscle tissue and, thus, help to confirm its identity as a bona fide Drosophila proctolin receptor. The inability of receptor knockdown in neurons to reduce proctolin-induced contractions confirms that this effect of proctolin is mediated postsynaptically and not presynaptically.
The proctolin-induced contractions of Drosophila body-wall muscles required extracellular calcium and were blocked by 10−6 M nifedipine, which has been shown by others to block L-type calcium channels in these muscle fibers (Gielow et al. 1995). Observations similar to ours have been reported for cockroach hyperneural muscle (Wegener and Nassel 2000), where the actions of proctolin requires calcium entry through L-type channels in the plasma membrane to trigger release of calcium from intracellular stores. A similar dependence on extracellular calcium has been reported for proctolin-induced contractions in numerous arthropod muscles, including locust oviduct muscles (Lange et al. 1987; Wilcox and Lange 1995), lobster opener muscle (Schwarz et al. 1980), barnacle muscle (Nwoga and Bittar 1985), and Lucilia body wall muscle (Irving and Miller 1980), and proctolin is reported to increase calcium channel activity in the plasma membrane of crayfish abdominal flexor muscles (Bishop et al. 1991). The prolonged time course of proctolin-induced contractures and the requirement of a GPCR imply the involvement of second messengers. Proctolin has been shown by others to activate adenylate cyclase (Hiripi et al. 1979; Wright et al. 1986), and both cyclic adenosine monophosphate (cAMP) and inositol trisphosphate (IP3) have been implicated as mediators of proctolin-induced contractions (Erxleben et al. 1995; Lange et al. 1987). Here we provide evidence that proctolin can act downstream of calcium influx through the plasma membrane. In calcium-free saline, coapplication of caffeine and proctolin elicited a significantly greater contraction than caffeine alone, and coapplication of ryanodine and proctolin caused a greater contraction than ryanodine alone. Additionally, the store operated calcium channel blocker, 2-APB, significantly reduced the ability of proctolin to induce contractions when calcium was washed into the extracellular saline. These observations suggest the involvement of calcium release from intracellular stores. Details about the intracellular signaling pathways underlying effects of proctolin in Drosophila remain to be elucidated.
Although proctolin increased the amplitude of nerve-evoked contractions, it had no significant effect on EJP amplitude at concentrations of 1 × 10−10 to 1 × 10−8 M in any of the five Drosophila muscle fibers examined. This suggests that proctolin increases force generation by acting downstream of postsynaptic depolarization. Similar effects of proctolin have been reported in crayfish abdominal flexor muscles (Bishop et al. 1987), locust oviduct muscles (Lange and Orchard 1984; Orchard and Lange 1986) and crab ventilatory muscles (Mercier and Wilkens, 1985). Direct evidence that proctolin enhances excitation-contraction coupling has been reported for isolated muscle fibers of the isopod crustacean, Idotea baltica, where proctolin decreases membrane conductance and potassium channel activity and, in some fibers, increases excitability (Erxleben et al. 1995). In other arthropod muscles, however, proctolin does increase EJP amplitude and appears to do so by presynaptic actions (Beilin and Pasztor 1989; Belanger and Orchard 1993; Jorge-Rivera et al. 1998; Rathmayer et al. 2002).
Proctolin increased the amplitude of nerve-evoked contractions in Drosophila larvae to a greater degree when the frequency and number of impulses delivered to the motor axons was increased. Other reports also indicate that modulation of evoked contractions is sensitive to changes in nerve impulse pattern. Serotonin and dopamine increased nerve-evoked contractions in crab gastric mill muscle (gm4) more substantially at 20–40 Hz than at lower frequencies (with a constant burst duration of 0.5 s; Jorge-Rivera et al. 1998). In the same muscle, however, proctolin and the crustacean peptide TNRNFLRFamide increased nerve-evoked contractions less substantially at 30–40 Hz than at lower impulse frequencies (Jorge-Rivera et al. 1998). In Drosophila larval body wall muscles, octopamine increased nerve-evoked contractions more effectively at 5–20 Hz (with 250 ms burst duration) than at higher intraburst frequencies (30–40 Hz; Ormerod et al. 2013). Thus different modulatory substances can be either more effective or less effective at increasing nerve-evoked contractions when the frequency and number of stimuli increase. The mechanisms underlying such frequency-dependent effects of aminergic and peptidergic modulators are not known.
One possible rationale for bath application of proctolin being more effective at higher frequencies in our experiments is that these stimuli may release proctolin and other peptides that are present as cotransmitters in synaptic terminals of the motorneurons. Belanger and Orchard (1993) showed that proctolin was released from motor neurons innervating locust ovipositor opener muscles by 30-Hz stimulation but not by 10-Hz stimulation. In Drosophila larvae high-frequency stimulation of motor axons supplying the body wall muscles increases both the mobilization of dense core vesicles in the synaptic terminals and the release of neuropeptides (Shakiryanova et al. 2005). Thus increased release of proctolin or other cotransmitters at the higher stimulus frequencies in our experiments might contribute to the increased amplitude of nerve-evoked contractions. We were unable to address this question directly because the amount of proctolin released from Drosophila larvae is below the detection level of current analytical methods. Using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry, we found that extracts from ten larvae contained a peak at 649 Da that was just above detection level and was identical in mass to a proctolin standard (data not shown). Dircksen et al. (2011) also identified a proctolin-specific peak at 649 Da using MS in another arthropod, Daphnia pulex. Since electrical stimulation typically releases 6–8% of endogenous stores or proctolin in locust muscle (Belanger and Orchard 1993; Lange 2002), other approaches are needed to assess release of proctolin and other cotransmitters in Drosophila.
Our data show that increasing the frequency and number of stimuli applied to the motor axons decreases the threshold concentration and EC50 for proctolin to increase nerve-evoked contractions. To our knowledge, this is the first report of any modulatory substance having such an effect. This finding suggests that caution is needed in interpreting threshold and EC50 values for modulation of nerve-evoked processes, and this might be particularly important for modulatory substances that are released as cotransmitters. Increasing the frequency and number of nerve impulses increases synaptic release of peptide cotransmitters, including proctolin (Belanger and Orchard 1993; Shakiryanova et al. 2005). We propose that such an effect may effectively lower the concentration needed for bath application of the same substance to elicit a noticeable effect on the postsynaptic cell. We tested this hypothesis by comparing the effectiveness of proctolin to increase nerve-evoked contractions between muscle cells that receive proctolin as a cotransmitter with muscle cells that do not. We reasoned that bath-applying a submaximal concentration of proctolin (that would not “overpower” the effects of cotransmission) would increase nerve-evoked contractions to a greater degree in muscle cells innervated by motor neurons that contain proctolin (muscles 4, 12 and 13) than in muscle cells that do not (muscles 6 and 7; Anderson et al. 1988). Ablating muscle cells 4, 12, and 13 reduced the effect of proctolin to a greater extent than did ablating cells 6 and 7, supporting the hypothesis that cotransmission reduces threshold and EC50 concentrations. Since the effectiveness of proctolin was assessed on all muscle cells that remained after ablation and were quantified as a percentage of maximal contraction induced by tetanic stimulation of those fibers, the differences cannot be attributed to differences in muscle fiber size. Instead, our data indicate differential modulation of muscle fibers, with proctolin increasing nerve-evoked contractions to a greater degree in cells 4, 12, and 13 than in cells 6 and 7.
There are alternative explanations for the reduction in threshold and EC50 that occurred in response to increasing the number and frequency of nerve impulses. Such changes in the stimulus regimen could increase the release of cotransmitters other than proctolin, and these may alter intracellular signaling pathways and “prime” the postsynaptic cell to increase its responsiveness to proctolin (Meyrand and Marder 1991). On the other hand, proctolin's ability to increase evoked contractions depends on postsynaptic depolarization and concomitant opening of calcium channels in the plasma membrane (Bishop et al. 1987, 1991), both of which should occur as the number and frequency of impulses increase. Thus the differential modulation of nerve-evoked contraction could be explained if changes in the stimulus regimen generated larger depolarization in muscle cells 4, 12, and 13 than in 6 and 7, or if the proctolin receptor is more highly expressed in cells 4, 12, and 13. Since proctolin also elicited larger contractions in cells 4, 12, and 13, the latter explanation seems plausible.
To at least some extent, the cell-selective modulation of muscle contraction reported here mirrors the effects of the Drosophila peptide DPKQDFMRFamide, which increases nerve-evoked contractions to a greater degree in muscle cells 6 and 7 than in 12 and 13 (Ormerod et al. 2015). The cell-selective modulation by the latter peptide appears to result from differences in expression level of the FMRFamide receptor between these muscle fibers (Ormerod et al. 2015), but it remains to be determined whether differences in expression of the proctolin receptor also occur. The apparent complementarity of modulation of these muscle fibers by proctolin and DPKQDFMRFamide suggests that these two peptides may play some complementary role in either recruiting or maximizing the contraction of specific subsets of muscle cells during specific behaviors.
The behavioral experiments reported here indicate that a pathway involving the proctolin receptor may mediate the ability of larvae to detect and respond to an altered thermal environment. Reducing proctolin receptor expression in the nervous system reduced the ability of larvae to increase crawling velocity at 25–36°C, but reducing receptor expression in muscle only had a significant effect at 36°C. These results indicate that the ability of larvae to increase crawling speed in response to warming involves activation of proctolin receptors on neurons, with little or no contribution from proctolin release onto muscle, despite the ability of bath-applied proctolin to enhance contractions in neuromuscular preparations at room temperature. In Drosophila larvae, proctolin immunoreactivity occurs in about 50 neuronal cell bodies distributed across the brain, subesophageal, thoracic, and abdominal regions of the central nervous system (Anderson et al. 1988). Proctolin receptors have been reported on neurosecretory cells and interneurons of Drosophila (Johnson et al. 2003; Nassel and Homberg 2006), but the distribution of proctolin receptors in Drosophila nervous system is not well mapped. Thus proctolin might act on sensory neurons, interneurons, or even on the motor neurons themselves to increase impulse bursting as temperature increases. Since proctolin did not alter EJP amplitude (Fig. 1), it is unlikely that synaptic terminals of the motor axons are targets of proctolin's action.
Our contraction data (Fig. 8B) indicate that proctolin is less effective at inducing contractions at higher temperatures. However, we also noticed that pH decreased (pH = 7.06) when temperature increased to 36°C. While seemingly minor, a 0.1–0.2 shift in pH is physiologically relevant and could affect both pre- and postsynaptic cellular function (Sinning and Huber 2013).
Surprisingly, larvae with reduced proctolin receptor expression in muscle exhibited a preference for slightly lower temperatures, but reducing proctolin receptor expression in neurons had no effect on temperature preference. These observations are difficult to reconcile with the fact that proctolin receptors in muscle contribute very little to warmth-induced increases in locomotion while proctolin receptors in neurons contribute substantially more. The effect of reducing receptor expression in muscle only manifests as a 1°C change in preference at temperatures of ∼16–19°C, which is below the temperature range in which we observed any effects on crawling. It is possible that removing the proctolin receptor may alter behavior in some fundamental way that alters locomotion, but the mechanisms underlying the change in preferred temperature are not known.
GRANTS
K. G. Ormerod was supported by a Queen Elizabeth II scholarship in science and technology and National Science and Engineering Research Council of Canada (NSERC) PGS funding. O. K. LePine was supported by a NSERC undergraduate student research award. A. J. Mercier was supported by NSERC Grant 46292, and G. J. Tattersall was supported by NSERC Grant 262 087. G. J. Tattersall also acknowledges Brock University Chancellor's Chair funding.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.G.O., G.J.T., and A.J.M. conception and design of research; K.G.O., O.K.L., M.S.B., and J.J. performed experiments; K.G.O., O.K.L., M.S.B., and J.J. analyzed data; K.G.O., M.S.B., J.J., G.J.T., and A.J.M. interpreted results of experiments; K.G.O. prepared figures; K.G.O. and A.J.M. drafted manuscript; K.G.O., G.J.T., and A.J.M. edited and revised manuscript; K.G.O., O.K.L., G.J.T., and A.J.M. approved final version of manuscript.
REFERENCES
- Alekseyenko OV, Chan YB, Li R, Kravtiz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci USA 110: 6151–6156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, Chan YB, Fernandez ML, Bulow T, Pankratz MJ, Kravtiz EA. Single serotonergic neurons that modulate aggression in Drosophila. Curr Biol 17: 2700–2707, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Halpern ME, Keshishian H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: Characterization of muscle fiber-specific neuromuscular endings. J Neurosci 8: 242–255, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Lange AB, Downer RG. Proctolin in the innervation of the locust mandibular closer muscle modulates contractions through the elevation of inositol triphosphate. J Comp Neurol 297: 479–486, 1990. [DOI] [PubMed] [Google Scholar]
- Beilin SA, Pasztor VM. Modulation of a rhythmically active crayfish muscle by the neuropeptide proctolin. Can J Zool 67: 73–81, 1989. [Google Scholar]
- Belanger JH, Orchard I. The locust ovipositor opener muscle: Proctolinergic central and peripheral neuromodulation in a centrally driven motor system. J Exp Biol 174: 343–362, 1993. [Google Scholar]
- Benson JA, Sullivan RE, Watson WH, Augustine GJ. The neuropeptide proctolin acts directly on limulus cardiac muscle to increase the amplitude of contraction. Brain Res 213: 449–454, 1981. [DOI] [PubMed] [Google Scholar]
- Bishop CA, Wine JJ, Nagy F, O'Shea MR. Physiological consequences of a peptide cotransmitter in a crayfish nerve-muscle preparation. J Neurosci 7: 1769–1779, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CA, Krouse ME, Wine JJ. Peptide cotransmitter potentiates calcium channel activity in crayfish skeletal muscle. J Neurosci 11: 269–276, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993. [DOI] [PubMed] [Google Scholar]
- Bruner J, Kennedy D. Habituation: occurrence at a neuromuscular junction. Science 169: 92–94, 1970. [DOI] [PubMed] [Google Scholar]
- Bryan JS, Atwood HL. Two types of synaptic depression at synapses of a single crustacean motor axon. Marine Behav Physiol 8: 99–121, 1981. [Google Scholar]
- Certel SJ, Leung A, Lin CY, Perez P, Chiang AS, Kravitz EA. Octopamine neuromodulatory effects on social behaviour decision-making networks in Drosophila males. PLos One 5: e13248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie AE. Identification of the first neuropeptides from the Amphipoda (Arthropoda, Crustacea). Gen Comp Endocrinol 260: 96–110, 2014. [DOI] [PubMed] [Google Scholar]
- Clark J, Milakovic M, Cull A, Klose MK, Mercier AJ. Evidence for postsynaptic modulation of muscle contraction by a Drosophila neuropeptide. Peptides 29: 1140–1149, 2008. [DOI] [PubMed] [Google Scholar]
- Dickinson CP, Calkins A, Stevens JS. Related neuropeptides use different balances of unitary mechanisms to modulate the cardiac neuromuscular system in the American lobster, Homarus americanus. J Neurophysiol 113: 856–870, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dircksen H, Neupert S, Predel R, Verleyen P, Huybrechts J, Strauss J, Hauser F, Stafflinger E, Schneider M, Pauwels K, Schoofs L, Grimmelikhuijzen CJ. Genomics, transcriptomics, and peptideomics of Daphnia pulex neuropeptides and protein hormones. J Proteome Res 7: 4478–4504, 2011. [DOI] [PubMed] [Google Scholar]
- Dunn TW, Mercier AJ. Synaptic modulation by a Drosophila neuropeptide is motor neuron specific and requires CaMKII activity. Peptides 26: 269–276, 2005. [DOI] [PubMed] [Google Scholar]
- El Manira A, Rossi-Durand C, Clarac F. Serotonin and proctolin modulate the response of a stretch receptor in crayfish. Brain Res 54: 157–162, 1991. [DOI] [PubMed] [Google Scholar]
- Erxlenben CF, deSantis A, Rathmayer W. Effects of proctolin on contractions, membrane resistance, and non-voltage-dependent sarcolemmal ion channels in crustacean muscle fibers. J Neurosci 15: 4356–4369, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary TG, Maule AG. Neuropeptide systems as targets for parasite and pest control. Adv Exp Med Biol 692: v–vi, 2010. [PubMed] [Google Scholar]
- Gielow ML, Gu GG, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci 15: 6085–6093, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutovitz S, Birmingham JT, Luther JA, Simon DJ, Marder E. GABA enhances transmission at an excitatory glutamatergic synapse. J Neurosci 21: 5935–5943, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick R, Johnson BR. Checks and balances in neuromodulation. Front Behav Neurosci 4: 1–9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Kravitz EA. Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J Neurosci 4: 1976–1993, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiripi I, Rosza KS, Miller TA. Effect of proctolin on the adenylate and guanylate cyclases in the Locusta brain at various developmental stages. Experientia 35: 1287–1288, 1979. [DOI] [PubMed] [Google Scholar]
- Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals 13: 50–69, 2004. [DOI] [PubMed] [Google Scholar]
- Hummon AB, Amare A, Sweedler JV. Discovering new invertebrate neuropeptides using mass spectrometry. Mass Spectrom Rev 25: 77–98, 2006. [DOI] [PubMed] [Google Scholar]
- Hurlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev 65: 21–37, 2001. [DOI] [PubMed] [Google Scholar]
- Irving SN, Miller TA. Octopamine and proctolin mimic spontaneous membrane depolarisations in Lucilia larvae. Experientia 15: 566–568, 1980. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. l-Glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol 262: 215–236, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Garczynski SF, Dongkook P, Crim JW, Nassel DR, Taghert PH. Identification and characterization of a G protein-coupled receptor for the neuropeptide proctolin in Drosophila melanogaster. Proc Natl Acad Sci USA 100: 6198–6203, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge-Rivera JC, Sen K, Birmingham JT, Abbott LF, Marder E. Temporal dynamics of convergent modulation at a crustacean neuromuscular junction. J Neurophysiol 80: 2559–2570, 1998. [DOI] [PubMed] [Google Scholar]
- Kastin AJ. Handbook of Biologically Active Peptides (2nd ed). San Diego, CA: Academic, 2013. [Google Scholar]
- Kimura T, Yasuyama K, Yamaguchi T. Proctolinergic innervation of the accessory gland in male crickets (Gryllus bimaculatus): detection of proctolin and some pharmacological properties of myogenically and neurogenically evoked contractions. J Insect Physiol 35: 251–264, 1989. [Google Scholar]
- Lange AB. The presence of proctolin in the reproductive system of Rhodnius prolixus. J Insect Physiol 36: 345–351, 1990. [Google Scholar]
- Lange AB. A review of the involvement of proctolin as a cotransmitter and local neurohormone in the oviduct of the locust, Locusta migratoria. Peptides 23: 2063–2070, 2002. [DOI] [PubMed] [Google Scholar]
- Lange AB, Orchard I. Some pharmacological properties of neuromuscular transmission in the oviduct of the locust, Locusta migratoria. Arch Insect Biochem Physiol 13: 231–241, 1984. [Google Scholar]
- Lange AB, Orchard I, Lam W. Mode of action of proctolin on locust visceral muscle. Arch Insect Biochem Physiol 5: 285–295, 1987. [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev 8: 1787–1802, 1994. [DOI] [PubMed] [Google Scholar]
- Ma M, Chen R, Sousa GL, Bors EK, Kwiatkowski M, Goiney CC, Goy MF, Christie AE, Li L. Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen Comp Endocrinol 156: 395–409, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod GT, Hegstrom-Wojtowicz M, Charlton MP, Atwood HL. Fast calcium signals in Drosophila motor neuron terminals. J Neurophysiol 88: 2659–2663, 2002. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor patter generation. Physiol Rev 76: 687–716, 1996. [DOI] [PubMed] [Google Scholar]
- Mercier AJ, Lee J. Differential effects of neuropeptides on circular and longitudinal muscles of the crayfish hindgut. Peptides 23: 1751–1757, 2002. [DOI] [PubMed] [Google Scholar]
- Mercier AJ, Wilkens JL. Modulatory effect of proctolin on a crab ventilatory muscle. J Neurobiol 16: 401–408, 1985. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Marder E. Matching neural and muscle oscillators: control by FMRFamide-like peptides. J Neurosci 11: 1150–1161, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milakovic MM, Ormerod KG, Klose MK, Mercier AJ. Mode of action of a Drosophila FMRFamide in inducing muscle contraction. J Exp Biol 217: 1725–1736, 2014. [DOI] [PubMed] [Google Scholar]
- Morales M, Ferrus A, Martinez-Padron M. Presynaptic calcium-channel currents in normal and csp mutant Drosophila peptidergic terminals. Eur J Neurosci 11: 1818–1826, 1999. [DOI] [PubMed] [Google Scholar]
- Nassel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol 68: 1–84, 2002. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Homberg U. Neuropeptides in interneurons of the insect brain. Cell Tissue Res 326: 1–24, 2006. [DOI] [PubMed] [Google Scholar]
- Nwoga J, Bittar EE. Stimulation by proctolin of the ouabain-insensitive sodium efflux in single barnacle muscle fibres. Comp Biochem Physiol 81C: 345–350, 1985. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Jovanic T, Denisov G, Dang TC, Hoffman D, Kerr RZ, Zlatic M. High-throughput analysis of stimulus-evoked behaviors in Drosophila larva reveals multiple modality-specific escape strategies. PLos One 8: 0071706, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard I, Lange AB. Neuromuscular transmission in an insect visceral muscle. J Neurobiol 17: 359–372, 1986. [DOI] [PubMed] [Google Scholar]
- Orchard I, Belanger JH, Lange AB. Proctolin: a review with emphasis on insects. J Neurobiol 20: 470–496, 1989. [DOI] [PubMed] [Google Scholar]
- Ormerod KG, Hadden JK, Deady LD, Mercier AJ, Krans JL. Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae. J Neurophysiol 110: 1984–1996, 2013. [DOI] [PubMed] [Google Scholar]
- Ormerod KG, Krans JL, Mercier AJ. Cell-selective modulation of the Drosophila neuromuscular system by a neuropeptide. J Neurophysiol 113: 1631–1643, 2015. [DOI] [PubMed] [Google Scholar]
- Pasztor VM, Bush BM. Peripheral modulation of mechano-sensitivity in primary afferent neurons. Nature 326: 793–795, 1987. [DOI] [PubMed] [Google Scholar]
- Pasztor VM, Bush BM. Primary afferent responses of a crustacean mechanoreceptor are modulated by proctolin, octopamine, and serotonin. J Neurobiol 20: 234–254, 1989. [DOI] [PubMed] [Google Scholar]
- Pasztor VM, Golas LB. The modulatory effects of serotonin, neuropeptide F1 and proctolin on the receptor muscles of the lobster abdominal stretch receptor and their exoskeletal muscle homologues. J Exp Biol 174: 363–374, 1993. [Google Scholar]
- Pasztor VM, MacMillan DL. The actions of proctolin, octopamine and serotonin on crustacean proprioceptors show species and neurone specificity. J Exp Biol 152: 485–504, 1990. [Google Scholar]
- Paterson BA, Anikin IM, Krans JL. Hysteresis in the production of force by larval Dipteran muscle. J Exp Biol 213: 2483–2493, 2010. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Orchard I. Octopaminergic modulation of interneurones in the flight system of the locust. Brain Res 549: 332–337, 1990.1884227 [Google Scholar]
- Rathmayer W, Djokaj S, Gaydukov A, Kreissl S. The neuromuscular junctions of the slow and the fast excitatory axon in the closer of the crab Eriphia spinifrons are endowed with different Ca2+ channel types and allow neuron-specific modulation of transmitter release by two neuropeptides. J Neurosci 22: 708–717, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17: 641–654, 1996. [DOI] [PubMed] [Google Scholar]
- Schwarz TL, Harris-Warrick RM, Glusman S, Kravitz E. A peptide action in a lobster neuromuscular preparation. J Neurobiol 11: 623–628, 1980. [DOI] [PubMed] [Google Scholar]
- Selverston AI. Invertebrate central pattern generator circuits. Philos Trans R Soc Lond B Biol Sci 365: 2329–2345, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci 8: 173–178, 2005. [DOI] [PubMed] [Google Scholar]
- Sink H, Rehm EJ, Richstone L, Bulls YM, Goodman CS. sidestep encodes a target-derived attractant essential for motor axon guidance in Drosophila. Cell 105: 57–67, 2001. [DOI] [PubMed] [Google Scholar]
- Sokolowski MA. Social interactions in “simple” model systems. J Neuron 65: 780–794, 2001. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Winther AM, Siviter RJ, Shirras AD, Isaac RE, Nassel DR. Identification of a proctolin preprohormone gene (Proct) of Drosophila melanogaster: expression and predicted prohormone processing. J Neurobiol 58: 379–391, 2004. [DOI] [PubMed] [Google Scholar]
- Ui-Tei K, Sakuma M, Watanabe Y, Miyake T, Miyata Y. Chemical analysis of neurotransmitter candidates in clonal cell lines from Drosophila central nervous system. II. Neuropeptides and amino acids. Neurosci Lett 11: 187–190, 1995. [DOI] [PubMed] [Google Scholar]
- Wegener C, Nassel DR. Peptide-induced Ca2+ movements in a tonic insect muscle: effects of proctolin and periviscerokinin-2. J Neurophysiol 84: 3056–3066, 2000. [DOI] [PubMed] [Google Scholar]
- White K, Hurteau T, Punsal P. Neuropeptide-FMRFamide-like immunoreactivity in Drosophila: development and distribution. J Comp Neurol 247: 430–438, 1986. [DOI] [PubMed] [Google Scholar]
- Wilcox CL, Lange AB. Role of extracellular and intracellular calcium on proctolin-induced contractions in an insect visceral muscle. Regul Pept 56: 49–59, 1995. [DOI] [PubMed] [Google Scholar]
- Wright MS, Cook BJ, Holman GM. Activation of an insect hindgut adenylate-cyclase by the neuropeptide proctolin. J Gen Physiol 88: A63, 1986. [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 16: 921–926, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bhat MB, Nishi M, Takeshima H, Ma J. Molecular cloning of cDNA encoding a Drosophila ryanodine receptor and functional studies of the carboxyl-terminal calcium release channel. Biophys J 78: 1270–1281, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew DT, Chan WY, Luo CB, Zheng DR, Yu MC. Neurotransmitters and neuropeptides in the developing human central nervous system. A review. Biol Signals Recept 8: 149–159, 1999. [DOI] [PubMed] [Google Scholar]