Abstract
Conventional anti-Parkinsonian dopamine replacement therapy is often complicated by side effects that limit the use of these medications. There is a continuing need to develop nondopaminergic approaches to treat Parkinsonism. One such approach is to use medications that normalize dopamine depletion-related firing abnormalities in the basal ganglia-thalamocortical circuitry. In this study, we assessed the potential of a specific T-type calcium channel blocker (ML218) to eliminate pathologic burst patterns of firing in the basal ganglia-receiving territory of the motor thalamus in Parkinsonian monkeys. We also carried out an anatomical study, demonstrating that the immunoreactivity for T-type calcium channels is strongly expressed in the motor thalamus in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys. At the electron microscopic level, dendrites accounted for >90% of all tissue elements that were immunoreactive for voltage-gated calcium channel, type 3.2-containing T-type calcium channels in normal and Parkinsonian monkeys. Subsequent in vivo electrophysiologic studies in awake MPTP-treated Parkinsonian monkeys demonstrated that intrathalamic microinjections of ML218 (0.5 μl of a 2.5-mM solution, injected at 0.1–0.2 μl/min) partially normalized the thalamic activity by reducing the proportion of rebound bursts and increasing the proportion of spikes in non-rebound bursts. The drug also attenuated oscillatory activity in the 3–13-Hz frequency range and increased gamma frequency oscillations. However, ML218 did not normalize Parkinsonism-related changes in firing rates and oscillatory activity in the beta frequency range. Whereas the described changes are promising, a more complete assessment of the cellular and behavioral effects of ML218 (or similar drugs) is needed for a full appraisal of their anti-Parkinsonian potential.
Keywords: Parkinsonism, T-type calcium channel, nondopaminergic therapy, rebound burst
degeneration of the dopaminergic nigrostriatal tract in Parkinson's disease leads to abnormalities in neuronal activity throughout the basal ganglia-thalamocortical circuitry that may eventually trigger the development of Parkinsonian motor signs (Galvan et al. 2015). Conventional dopamine replacement therapy for the treatment of Parkinson's disease is highly effective but often leads to significant side effects, such as drug-induced dyskinesias or psychosis, which limit the usefulness of these agents in many patients. An alternative therapeutic strategy would be the use of nondopaminergic medications that directly reduce Parkinsonism-related changes in neuronal firing patterns throughout the basal ganglia-thalamocortical circuits.
One of the prominent Parkinsonism-related discharge abnormalities in the basal ganglia is an increase of the incidence of burst discharges (Baufreton et al. 2005; Magnin et al. 2000; Soares et al. 2004; Wichmann et al. 1999; Wichmann and Soares 2006). The same has been described to occur in the motor thalamus, in Parkinsonian patients, and in monkeys rendered Parkinsonian by treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Magnin et al. 2000; Pessiglione et al. 2005; Zirh et al. 1998). The bursting activity of thalamic neurons is at least partially due to the fact that they strongly express T-type calcium channels (Hammond et al. 2002; Jahnsen and Llinas 1984). These channels are rapidly inactivated by depolarization and then de-inactivated by a period of hyperpolarization, allowing them to drive strong bursting activity upon the subsequent onset of depolarization (Destexhe et al. 1998; Jahnsen and Llinas 1984; Sherman and Koch 1986). The three subtypes of T-type calcium channels that have been identified [voltage-gated calcium channel, types 3.1, 3.2, and 3.3 (Cav3.1, Cav3.2, and Cav3.3)] have similar functional characteristics (Catterall et al. 2005; Perez-Reyes 2003; Shin et al. 2008; Talley et al. 1999) but differ in their distribution across brain regions and in their activation and inactivation kinetics. In rodent species, Cav3.1 is the most strongly expressed subtype in thalamocortical projection neurons, whereas Cav3.3 is expressed abundantly in reticular thalamic neurons (Talley et al. 1999).
If T-type calcium channel-driven bursting in the thalamus is a feature of the pathophysiology of Parkinson's disease, then the use of T-type calcium channel blockers could be a useful, nondopaminergic anti-Parkinsonian strategy. Preliminary support for this idea comes from the fact that several antiepileptic drugs with T-type calcium channel-blocking properties have anti-Parkinsonian effects in animal models of Parkinsonism and in patients with Parkinson's disease (Bermejo 2007; Gomez-Mancilla et al. 1992; Handforth et al. 2010; Miwa et al. 2008, 2011; Murata et al. 2001, 2007; Pourcher et al. 1992). However, most of the agents used in the existing studies were not specific for T-type calcium channels (Miwa 2007). Recently, a family of highly specific T-type calcium channel blockers has become available (Shipe et al. 2008; Xiang et al. 2011; Yang et al. 2008). The primary agent in this group, ML218, which blocks the three subtypes of T-type calcium channels, robustly inhibits low-threshold spike (LTS)-driven bursting and has anticataleptic properties in haloperidol-treated rats (Xiang et al. 2011).
To characterize further the potential relevance of T-type calcium channel blockers to treat Parkinsonism in primates, the present study examined the cellular and subcellular distribution of Cav3.1 and characterized the electrophysiological effects of local microinjections of ML218 on neurons in the basal ganglia-receiving area of the ventral motor thalamus in Parkinsonian monkeys.
MATERIALS AND METHODS
Animals
Nine adult Rhesus monkeys (Macaca mulatta, 5–10 kg) were used for these studies. Six of these animals were used for anatomical experiments (3 normal and 3 MPTP-treated monkeys) and three for the electrophysiology studies (with recordings in each animal before and after MPTP treatment). The animals were pair housed and had ad libitum access to food and water. All experiments were performed in accordance with the U.S. Public Health Service Policy on the humane care and use of laboratory animals, including the provisions of the “Guide for the Care and Use of Laboratory Animals” (Garber et al. 2011). All studies were approved by the Biosafety and Animal Care and Use Committees of Emory University.
Six of the animals were treated with MPTP (0.2–0.6 mg/kg im; Sigma, St. Louis, MO; cumulative doses: 2.8–8.8 mg/kg), once weekly, until they reached comparable states of stable, moderate Parkinsonism. The degree and stability of the MPTP-induced motor disability were assessed as described previously (Devergnas et al. 2014). The animals' spontaneous behavior was evaluated weekly for 15-min periods in an observation cage that was equipped with infrared beams, allowing us to measure their body movements as infrared beam break events. In addition, we used a Parkinsonism rating scale to quantify impairments in 10 aspects of motor function (speed of movement, incidence and severity of “freezing” episodes, extremity posture, trunk posture, presence and severity of tremor, frequency of arm movements, frequency of leg movements, finger dexterity, home cage activity, and balance). Each item was scored on a 0–3 scale (maximal score, 30). The final Parkinsonism scores ranged between 13 and 19, corresponding to moderately severe Parkinsonism. The severity of the Parkinsonian motor signs had to be stable for a period of at least 6 wk after the last MPTP injection before euthanasia (in the case of anatomical studies) or before the beginning of the recording experiments (in the case of electrophysiology studies).
To terminate the experiment, the animals were first injected with an overdose of sodium pentobarbital (100 mg/kg iv) and then transcardially perfused with cold oxygenated Ringer's solution, followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in a phosphate buffer (PB) solution. After perfusion, the brains were removed from the skull, cut coronally into 10 mm-thick blocks, and postfixed overnight in 4% paraformaldehyde. The blocks were then cut into 60 μm-thick coronal sections with a vibrating microtome and stored at −20°C in an antifreeze solution containing 30% ethylene glycol and 30% glycerol in PB until the time of the immunohistochemistry studies.
Immunohistochemistry
Specificity of Cav3.1 antibodies.
For the localization of Cav3.1, we used highly specific mouse MAb (NeuroMab; UC Davis/NIH NeuroMab Facility; Catalog #73-206; used at 1:1,000 dilution). According to the supplier, this antibody reacts with the ∼250-kD molecular weight protein associated with Cav3.1, does not crossreact with other subtypes of Cav3 channels, and does not result in significant immunostaining or Western blot band labeling in tissue from Cav3.1 knockout mice (NeuroMab; Accession #Q9WUT2). To assess further the specificity of this antibody for its use in the present study, a Western blot was performed on fresh monkey brain tissue (gift from Dr. Anthony Chan, Yerkes National Primate Research Center, Emory University, Atlanta, GA).
The frozen tissue was homogenized by sonication in buffer (150 mM NaCl, 10 mM HEPES, 1 mM EGTA, and 0.1 mM MgCl2, pH 7.4, with 0.5% Triton X-100) with complete antiprotease (Cat No. 11245200; Roche, Indianapolis, IN), and the protein concentration was determined by using the Bradford reagent (Bio-Rad Laboratories, Hercules, CA). Samples were resolved by SDS-PAGE on a 4–15% gel (Invitrogen, Thermo Fisher Scientific, Waltham, MA) and the protein content analyzed by immunoblotting with the Cav3.1 antibody (NeuroMab) and antibodies against beta-actin (Sigma). Immunoreactivity was detected by enhanced chemiluminescence using Amersham Hyperfilm ECL (GE Healthcare Life Sciences, Pittsburgh, PA), as described previously (Gokhale et al. 2012). These experiments confirmed the antibody specificity, showing a high level of an ∼250-kD protein (corresponding to the molecular weight of Cav3.1) in the monkey thalamus compared with a much lower level of expression in the striatum (see Fig. 2F).
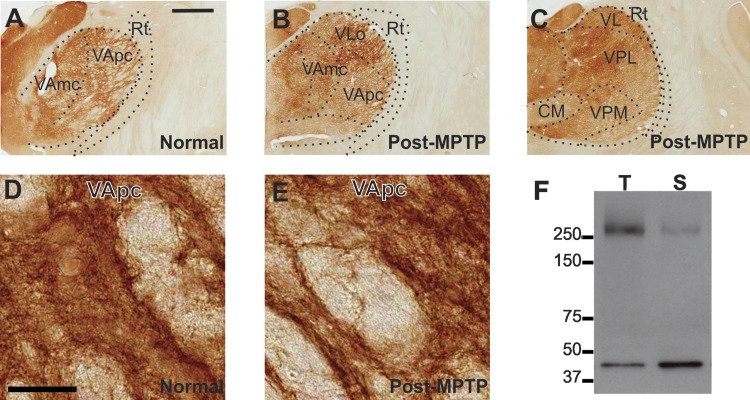
Fig. 2.
A–E: light micrographs of coronal brain sections showing immunostaining for voltage-gated calcium channel, type 3.1 (Cav3.1) in the ventrolateral and ventroposterior thalamus of a normal (A) and MPTP-treated monkey (B and C). The overall intensity of labeling is similar across the ventral motor [ventral anterior nucleus magnocellularis (VAmc), ventral anterior nucleus parvocellularis (VApc), ventral lateral nucleus oral part (VLo)] and somatosensory [ventral posterolateral (VPL), ventral posteromedial (VPM)] thalamic nuclei. D and E: high-power views of Cav3.1 neutrophil immunostaining in VApc in a normal (D) and MPTP-treated (E) monkey. Original scale bars, 2 mm (A; also applies to B and C); 50 μm (D; also applies to E). F: Western blot showing the specificity of the NeuroMab Cav3.1 antibody when applied on monkey thalamic (T) and striatal (S) tissue. A single band corresponding to the predicted molecular weight of Cav3.1 protein (∼250 kD) is shown in this immunoblot. The lower band corresponds to beta-actin probing that was used as a loading control. Note the higher amount of Cav3.1 protein in the thalamic tissue compared with the striatal tissue. Rt, reticular nucleus; VL, ventral lateral nucleus; CM, centromedian nucleus.
Light microscopic pre-embedding immunoperoxidase procedures.
Sections of tissue to be processed were removed from the antifreeze solution (see above) and then placed in PBS (0.01 M, pH 7.4). They were immersed in sodium borohydride (1% in PBS) for 20 min and incubated for 1 h in PBS containing 10% normal goat serum (NGS), 1% BSA, and 0.3% Triton X-100, followed by incubation in the primary antibody (anti-Cav3.1) solution containing 1% NGS, 1% BSA, and 0.3% Triton X-100 in PBS for 24 h at 4°C. Sections were then rinsed three times in PBS and subsequently incubated in the secondary antibody solution containing 1% NGS, 1% BSA, 0.3% Triton X-100, and biotinylated horse anti-mouse IgGs (Vector Laboratories, Burlingame, CA; used at 1:200 dilution) for 90 min at room temperature. After three rinses in PBS, the sections were incubated for 90 min in avidin-biotin peroxidase complex (ABC) solution (Vectastain standard ABC kit; Vector Laboratories; used at 1:100 dilution), including 1% BSA. To reveal the antigenic sites, the sections were first rinsed with PBS and Tris buffer (50 mM; pH 7.6) and then incubated in a solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma), 10 mM imidazole, and 0.005% hydrogen peroxide in Tris buffer for 10 min. The sections were subsequently washed several times in PBS, mounted on gelatin-coated glass slides, dehydrated, and coverslipped with Permount (Fisher Scientific, Thermo Fisher Scientific). The slides were scanned at 20× using a scanning light microscope system (ScanScope CS; Aperio Technologies, Vista, CA). Digital representations of the slides were then saved and later analyzed using the ImageScope software (Aperio Technologies). The borders between the basal ganglia- and cerebellar-receiving territories of the ventral motor nuclei were delineated using adjacent Nissl-stained sections (1 section out of 6) combined with drawings of corresponding levels from two rhesus monkey brain stereotaxic atlases (Lanciego and Vazquez 2012; Paxinos et al. 1999). In addition, one out of six sections was immunostained for the vesicular glutamate transporter 2 to confirm further the border of the cerebellar-receiving region of the ventrolateral nucleus of the thalamus (Kuramoto et al. 2011; Raju et al. 2008; Villalba et al. 2006). As controls, sections of the thalamus adjacent to those processed to localize Cav3.1 immunoreactivity were incubated in solutions, in which the primary MAb were replaced by nonimmune mouse serum. These sections were devoid of significant immunoreactivity.
Tyrosine hydroxylase immunohistochemistry.
Striatal and nigral tissue from all MPTP-treated monkeys that were used in this study was immunostained with mouse tyrosine hydroxylase (TH) MAb (catalog no. MAB 318; Millipore, Billerica, MA) to confirm the degeneration of the nigrostriatal dopamine system. The immunoperoxidase ABC method with DAB as chromogen was used to localize TH immunoreactivity, according to procedures described in previous studies (Hsu et al. 1981; Smith and Bolam 1991). After completion of the immunostaining reaction, sections were rinsed in PBS, mounted onto gelatin-coated slides, dehydrated, coverslipped with Permount (Fisher Scientific, Thermo Fisher Scientific), and examined with a light microscope. With the use of the ImageScope software (Aperio Technologies), 0.7× magnification digital images of the stained tissue slides containing the caudate nucleus, putamen, or substantia nigra (SN) were selected and imported into ImageJ (National Institutes of Health, Bethesda, MD) for additional processing. For optical density measurements, the images were converted into 16-bit grayscale format and inverted. To control for differences in background staining, the optical density measurement in the internal capsule (for caudate and putamen) was subtracted from that obtained in striatal measurements, and the intensity measurement values at the level of the cerebral peduncle were used as background measurements for the SN analysis. Measurements of the intensity of labeling were obtained in two sections at the striatal and nigral levels from control and MPTP-treated animals. In each section, the intensity of TH staining was measured in four representative areas in each structure. The resulting values were averaged in each animal. Data from the MPTP-treated animals were compared with data obtained in controls with Mann-Whitney rank tests to determine the percent of TH immunostaining loss in the striatum (caudate nucleus + putamen) and the SN.
Electron microscopic pre-embedding immunoperoxidase protocol.
Sections were immersed in sodium borohydride (1% in PBS) for 20 min and placed in a cryoprotectant solution (0.05 M PB, pH 7.4, 25% sucrose, and 10% glycerol) for 20 min before being frozen at −80°C for 20 min and thawed to permeabilize cell membranes. Then, sections were put through a graded series of cryoprotectant solution (100, 70, 50, and 30% in PBS) and finally washed in PBS. The subsequent tissue processing was identical to that used for light microscopy, up to the point of the use of DAB, although Triton X-100 was omitted from all solutions, and sections were incubated in the primary antibody solution for 48 h at 4°C. After DAB exposure, the tissue was rinsed in PB and treated with 1% osmium tetroxide for 20 min. The sections were then dehydrated through 30 min immersion in increasing concentrations of ethanol solution (50, 70, 90, and 100%). Uranyl acetate (1%) was added to the 70% ethanol solution to increase contrast under the electron microscopy (EM). The sections were then placed in propylene oxide for 30 min and subsequently embedded in epoxy resin (FLUKA Durcupan ACM; Sigma-Aldrich, St. Louis, MO) for 24 h. Lastly, the sections were mounted on microscope slides, dabbed with epoxy resin, covered with mineral oil-coated coverslips, and put in the oven at 60°C for 48 h to cure the resin. After resin polymerization, the coverslips were taken off, and small blocks of tissue from the basal ganglia-receiving area of the thalamus, as defined in two different stereotaxic atlases (Lanciego and Vazquez 2012; Paxinos et al. 1999), were cut from the slides and glued onto resin blocks with cyanoacrylate glue. The blocks were cut into serial 60 nm-thick ultrathin sections using a diamond knife ultramicrotome (Ultracut T2; Leica, Nussloch, Germany) and collected on single-slot, Pioloform-coated copper grids. The sections were then stained with lead citrate for 5 min, rinsed in distilled water, and viewed under a transmission EM (JEM-1011; JEOL USA, Peabody, MA).
EM pre-embedding immunogold protocol.
The tissue was prepared to localize Cav3.1 immunoreactivity using the pre-embedding immunogold method, according to a protocol used routinely in our laboratory (Gonzales et al. 2013; Kuwajima et al. 2007; Mitrano et al. 2010). Sections were treated with sodium borohydride and processed with the cryoprotectant protocol described above. This was followed by rinses in PBS and preincubation for 30 min in a PBS solution containing 5% dry milk. Sections were then rinsed in a Tris-buffered saline (TBS)-gelatin buffer (0.02 M, 0.1% gelatin, pH 7.6) and incubated with the Cav3.1 antibody solution prepared with 1% dry milk in TBS-gelatin buffer for 24 h at room temperature. One day later, sections were rinsed in TBS-gelatin buffer and then treated for 2 h at room temperature with the secondary antibody solution (goat anti-mouse Fab′ fragments conjugated to 1.4 nm gold particles 1:100; Nanoprobes, Yaphank, NY) prepared with TBS-gelatin buffer in 1% milk. Sections were then washed in TBS-gelatin buffer and 2% sodium acetate buffer before incubation with the HQ Silver Kit (Nanoprobes) for 4–10 min to increase gold particle sizes to 30–50 nm through silver intensification. Sections were then rinsed in PB and exposed to osmium treatment, dehydration, embedding, and tissue selection, as described above, with the exception that sections were kept in 0.5% OsO4 for 10 min, and the tissue was stained with 1% uranyl acetate in 70% ethanol for 10 min in the dark.
Analysis of EM material.
To compare the overall distribution of Cav3.1 labeling between normal and Parkinsonian animals, 50 digital micrographs of randomly encountered Cav3.1-labeled cellular elements in each animal were digitally imaged at 40,000× (DualView 300W; Gatan, Pleasanton, CA), yielding 738 μm2 of tissue analyzed per animal. Immunoperoxidase labeling could be identified as a dark, amorphous deposit within neuronal elements, whereas immunogold labeling appeared as small, dark, and round particles within neuronal elements. The immunoreactive elements were classified based on ultrastructural features (Peters et al. 1991), and the relative distribution of Cav3.1 immunoreactivity amongst neuronal elements was then statistically compared between normal and MPTP-treated animals using a Mann-Whitney test. Labeled dendrites were divided into small (<0.5 μm), medium (0.5–1 μm), or large (>1 μm) profiles, based on their cross-sectional diameter (Galvan et al. 2014; Gonzales et al. 2013; Raju et al. 2008; Villalba et al. 2006). The immunogold-stained sections were used to confirm expression of Cav3.1 labeling on the plasma membrane and at synaptic sites along immunoreactive dendrites.
Electrophysiology
Animal preparation and surgical procedures.
The three animals used for the electrophysiologic studies (monkeys A, B, and C) were first conditioned to being handled by the experimenter and to sit in a primate chair. They then underwent an aseptic surgical procedure under anesthesia with isoflurane (1–3%) for placement of stainless-steel recording chambers (Crist Instrument, Hagerstown, MD; inner chamber diameter, 19 mm). The chambers were stereotactically positioned over trephine holes in the skull and embedded into an acrylic skull “cap,” along with a stainless-steel head holder and multiple bone screws. For monkeys A and B, the chambers were positioned at a 36° angle anterior to the vertical in the sagittal plane aimed at the thalamus. For monkey C, the chamber was directed at the thalamus with a 40° angle from the vertical in the coronal plane. After surgery, the monkeys were given analgesics and prophylactic antibiotic treatment for 1 wk. Recordings started after the end of this postprocedural treatment.
Electrophysiological recordings.
The animals were seated in a primate chair with their head immobilized and allowing for movement of body and limbs. All recordings were performed while the animals were at rest and awake, as determined online by video observations (eyes open, occasional movements). For the purpose of electrophysiologic mapping, tungsten microelectrodes (FHC, Bowdoinham, ME; Z = 0.5–1.0 MΩ at 1 kHz) were lowered into the brain with a microdrive (MO-95B; Narishige, Tokyo, Japan) to record single-unit signals. The electrical signals were amplified (DAM-80 amplifier; World Precision Instruments, Sarasota, FL), filtered (400−6,000 Hz bandpass filter; Krohn-Hite, Brockton, MA), displayed on a digital oscilloscope (DL1540; Yokogawa, Tokyo, Japan), and made audible via an audio amplifier. The locations and borders of the motor thalamus were provisionally defined with these single-unit extracellular recordings. The eventual decision of whether recorded neurons were located in the basal ganglia-receiving area of the thalamus was made based on postmortem anatomical reconstructions of the electrode tracks (see below).
Microinjections and recordings.
ML218 hydrochloride, provided by the Center for Neuroscience Drug Discovery in the Department of Pharmacology, Vanderbilt University (Nashville, TN), was dissolved in artificial cerebrospinal fluid [aCSF; comprised of (in mM): 143 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, and 1 Na2HPO4, pH 7.2–7.4] to a final concentration of 2.5 mM. Before being loaded into the injection syringe, all solutions were filtered with a 0.2-μm pore-size nylon membrane (Fisher Scientific, Hampton, NH). For control injections, aCSF was used. Intracerebral microinjections were then performed with a custom-built device (“injectrode”), consisting of a tungsten microelectrode glued to a thin silica tube (Kliem and Wichmann 2004). The injection tubing was connected to a 1-ml gas-tight syringe (CMA Microdialysis AB, Solna, Sweden), driven by a computer-controlled injection pump (model 102; CMA Microdialysis AB). The injectrode was lowered into the thalamus using the microdrive and positioned to isolate single neurons for recording at locations identified to be within the ventral motor thalamus based on the mapping procedures. The recording of the neuron's spontaneous activity at baseline started only after a few minutes (when the cell was considered stable) and lasted for at least 100 s. Recording then continued throughout the drug injections (0.5 μl at 0.1–0.2 μl/min) and if possible, for 5 min thereafter. Injections along the same tract were separated by at least 0.5 mm and spaced by at least 15 min.
Histological reconstruction.
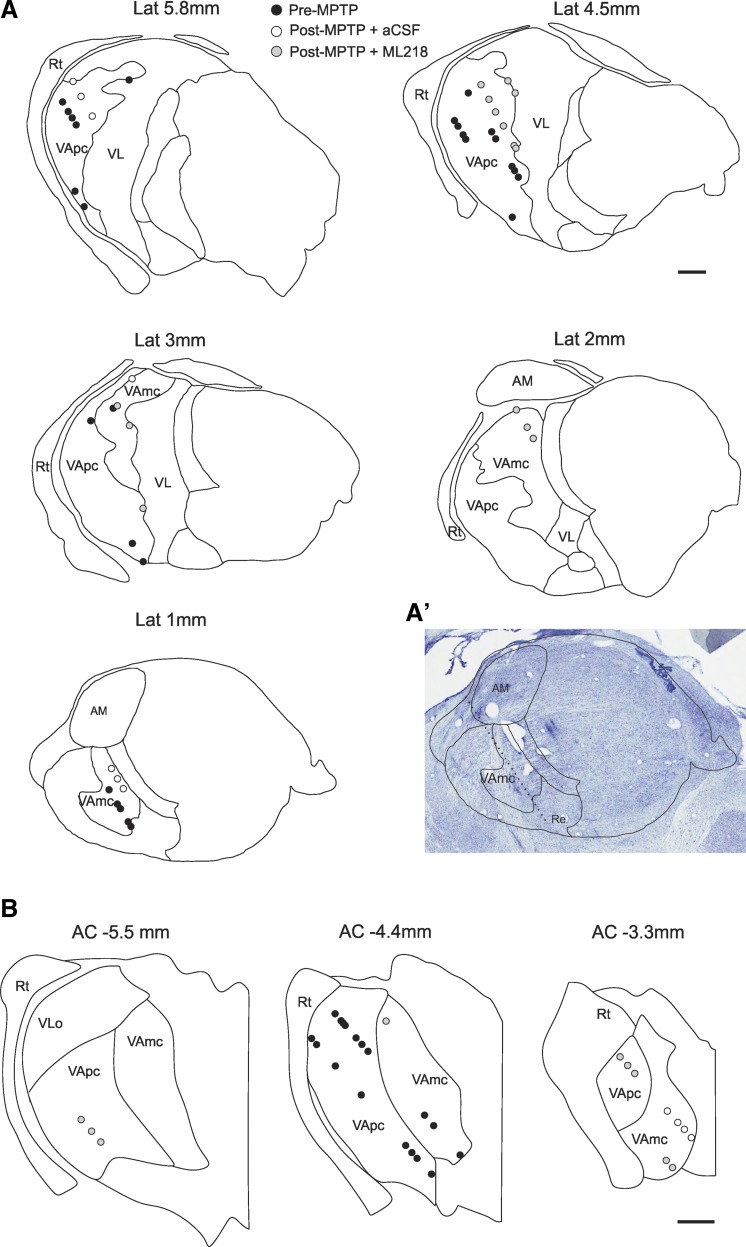
At the conclusion of the electrophysiological experiments, the animals were deeply anesthetized and perfused and the brains processed as described above, except that the brains from monkeys A and B were cut in the sagittal plane. A series of sections containing the motor thalamus was stained for Nissl substance to visualize electrode penetrations and to localize the borders of the thalamus. Based on the Nissl staining, the atlas of the macaque thalamus (Lanciego and Vazquez 2012), and depth readings obtained during the injectrode recording sessions, individual recording sites were verified to be located in the basal ganglia-receiving area of the ventral thalamus. This region is subdivided into the ventral anterior nucleus parvocellularis and magnocellularis (VApc and VAmc, respectively), corresponding, respectively, to the areas receiving information from the pallidum and the SN reticulata (SNr; see Fig. 4). However, there is a lack of consistency in the nomenclature applied to different thalamic nuclei across the different nonhuman primate atlases (Smith and Raju 2008). For example, according to Jones (2007), the pallidal-receiving area corresponds to VApc (or VLa), and the SNr-receiving area corresponds to VAmc (or VM); Paxinos et al. (2000) called the same areas VAL (for VApc) and VAM (for VAmc), and Ilinsky and Kultas-Ilinsky (1987) named them VAdc (for VApc) and VAmc. Neurons recorded outside of this region of the basal ganglia-receiving area of the ventral thalamus were not analyzed further (see Fig. 4). In addition, TH immunostaining served to evaluate the extent of nigrostriatal dopaminergic denervation (see above).
Fig. 4.
Location of thalamic neurons recorded in the normal (black circles) and post-MPTP states, after local microinjections of artificial cerebrospinal fluid (aCSF; white circles) or ML218 (gray circles). Delineation of nuclear boundaries and tracks in the thalamus are based on Nissl staining and the sagittal and coronal atlas of the macaque brain (Lanciego and Vazquez 2012). A: sagittal views of the location of neurons studied in monkeys A and B. A′: example of track reconstruction based on Nissl staining of a slice in the L1 plane (Lanciego and Vazquez 2012). B: coronal views of the location of neurons studied in monkey C. Lat, lateral; AM, anteromedian nucleus; Re, reticular nucleus. Original scale bars, 1 mm.
Analysis of electrophysiological data.
Spikes of neurons that had been recorded with an adequate signal-to-noise ratio were detected offline with a waveform-matching algorithm (see example in Fig. 5). Principal component analysis (Spike2; Cambridge Electronic Design, Cambridge, UK) and an analysis of the distribution of interspike intervals (ISIs) were used to verify the quality of the spike sorting. All subsequent data analysis steps were based on the ISI data and were done with custom-written Matlab scripts (Matlab 7.6; MathWorks, Natick, MA).
Fig. 5.
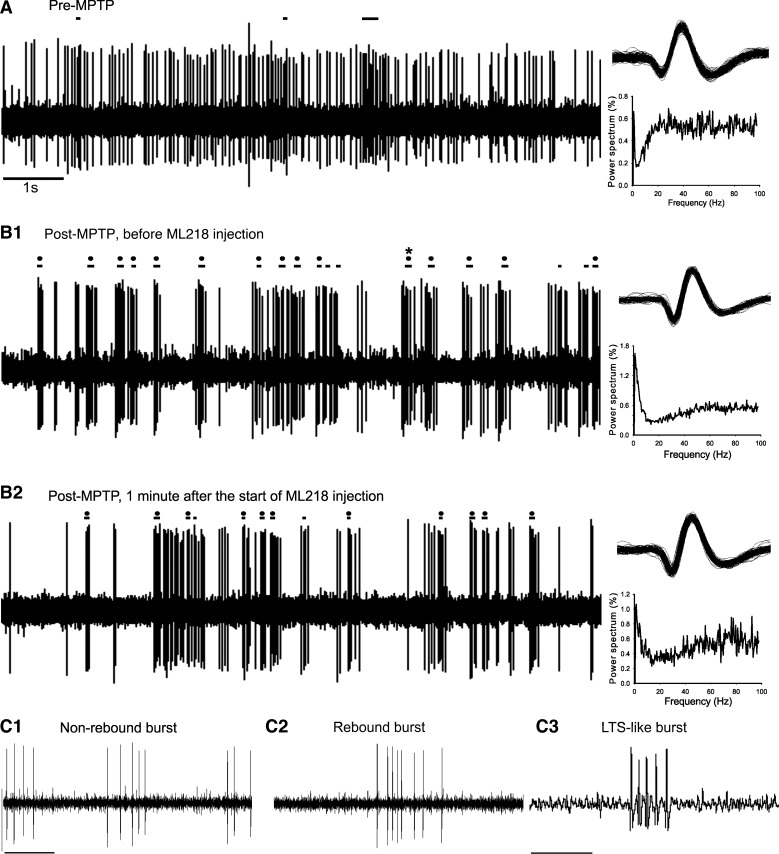
Effect of MPTP and ML218 on thalamic activity. Examples of 10 s of thalamic recordings and corresponding power spectra [normalized to the 1–100-Hz range; nonequispaced fast Fourier transform (nfft): 11; bin size: 0.49], before (A) and after (B) MPTP treatment. Same cell before (B1) and after (B2) ML218 injection. Horizontal lines above the recordings indicate events identified as burst. Horizontal lines with a black dot denote rebound bursts, and the 1 burst with an asterisk (*) was identified as a low-threshold spike (LTS)-like burst (see materials and methods). C: example of non-rebound burst (C1), rebound burst (C2), and LTS-like burst (C3). Original scale bars, 1 s (A; also applies to B); 0.1 s (C1; also applies to C2); 0.02 s (C3).
The effects of Parkinsonism were analyzed by comparing recordings in the same monkeys from the normal state with the baseline recordings from the drug-injection experiments. To analyze the effects of microinjections of aCSF or ML218 on the firing pattern of neurons, we compared in each cell a “control,” a “random effect,” and a “maximal effect” period, each 60 s long. The control epoch was the 60-s segment of data immediately preceding the drug or aCSF injection. The random-effect period was a randomly selected, 60-s segment of data within 30–420 s after the start of the injection (∼298 ± 29 s after the start of the injection). An advantage of using a randomly chosen postinjection period is that it is not potentially biased by user input. However, this approach has the disadvantage that the maximal effect of the injected drug may not be fully captured within the chosen random period. Therefore, we also analyzed the maximal injection effects. The maximal effect period was defined based on the firing-rate changes induced by the injected solutions. To determine the time point at which the maximal effect occurred, we generated second-by-second readouts of the cell's firing rate (based on a sliding, 30-s window), which were subsequently smoothed using a 20-point moving-average technique (see example in Fig. 6A). This readout was plotted and used to mark the maximal effects of the first sustained (>60 s) deviation of the cell's firing rate beyond the 90th or below the 10th percentile of the preinjection baseline data and within 30–420 s of the start of the injection. If an effect were detected, a 60-s period, centered on the maximal firing-rate deflection, was selected for further analysis. In addition, we measured the delay of the start of the effect (i.e., the amount of time elapsed from the start of the injection to the moment when the firing rate passed beyond the 90th or below the 10th percentile) and the delay for the peak effect.
Fig. 6.
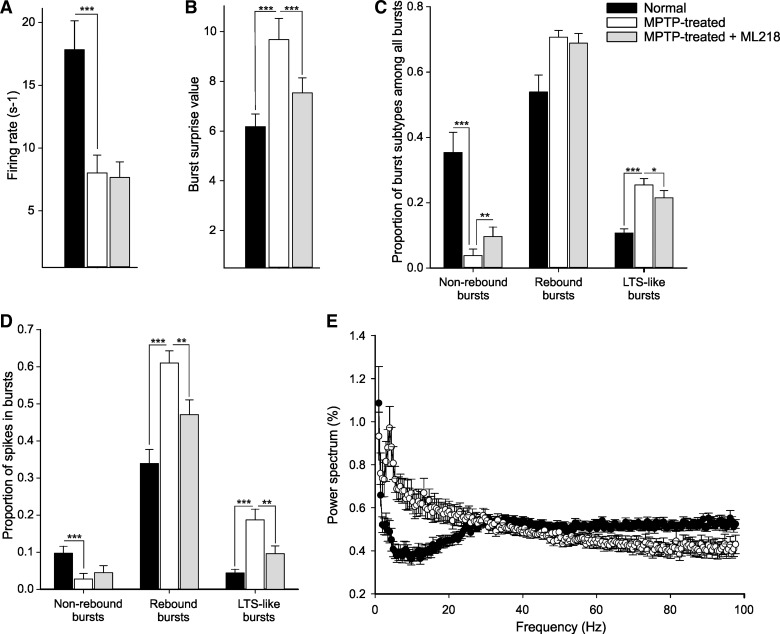
Effect of MPTP and ML218 on thalamic activity. A: firing rate in normal animals (black bars), Parkinsonian animals (white bars), and Parkinsonian animals after ML218 injection (at the random effect period; gray bars). B: averaged burst surprise value for all bursts. C: proportion of burst subtypes among all bursts. D: proportion of spikes in bursts for each burst subtype. E: average power spectra (normalized to the 1–100-Hz range; nfft: 11; bin size: 0.49) for the normal (black circles) and MPTP-treated (white circles) animals. Values are means ± SE compared with a Mann-Whitney rank sum test (*P < 0.05, **P < 0.01, and *** P < 0.001).
Several parameters of neuronal firing were considered in our analysis of ISI sequences, including the neuron's average firing rates (number of spikes per second in the analyzed segments), the coefficients of variation of the ISIs (ISI CVs), the power spectra of neuronal firing, as well as detections of bursts and pauses in discharge. The power spectral analysis of the ISI [sampling 1,000 Hz, fast Fourier transform (FFT) segment length of 2,048 samples nonequispaced FFT (11), resulting in a spectral resolution of 0.49 Hz] used the NeuroSpec 2.0 Matlab functions for frequency domain analyses of neuronal spiking data (Nielsen et al. 2005). For each neuron, raw spectra were integrated in the 1–3-, 3–8-, 8–13-, 13–30-, and 30–100-Hz ranges and the resultant values expressed as a fraction of the power in the entire 1–100-Hz range. Similar methods have been used in our previous publications (Galvan et al. 2010; Sanders et al. 2013).
To detect bursts, we used the method described by Legendy and Salcman (1985) [see also Sanders et al. (2013) and Wichmann and Soares (2006)], with a “surprise” value of three. Bursts were classified as a “rebound burst” if the ISI immediately preceding the burst was at least three times longer than the average ISI of the neuron's discharge. A subgroup of rebound bursts was classified as “LTS-like” bursts. The identification of these was based on literature indicating that the mechanisms underlying the induction of LTS bursts lead to a progressive increment of the ISIs inside of the burst (see Fig. 5C3), referred to as the descelerando signature (David et al. 2013). Bursts that failed to meet the criteria of rebound bursts were defined as non-rebound bursts. We calculated the frequency of occurrence, the mean intraburst firing rate, the proportion of spikes in bursts (compared with the total number of spikes), the average number of spikes per burst, and the burst surprise value. These values were generated separately for all bursts, non-rebound bursts, rebound bursts, and LTS-like bursts. In addition, we identified a descelerando pattern in a small proportion (0.1) of non-rebound bursts; the frequency of this particular burst was not affected by the drug (these bursts were part of the non-rebound burst).
The presence of decelerations was evaluated by using a surprise method similar to that used for burst detection. Decelerations were identified as sudden reductions in firing rate to less than one-half of the average firing rate for at least one ISI, satisfying a surprise cutoff value of three for the entire deceleration (Elias et al. 2007). Based on these parameters, we evaluated the amount of time spikes spent in decelerations.
We also calculated the proportion of spikes that were neither in bursts nor in decelerations and generated ISI sequences in which all ISIs that belonged to bursts or decelerations were removed to calculate the “background” (non-modulated) firing rate and ISI CV. The values (see Table 2) correspond to the means ± SE for each firing property of thalamic neurons in the pre-MPTP- and post-MPTP-treated states. The values are the means ± SE of the postinjection values (see Tables 3 and 4), normalized to the corresponding preinjection baseline of the same cell. In this case, values above one indicate that the postinjection power was higher than the corresponding preinjection value.
Table 2.
Firing properties of thalamic neurons in the pre-MPTP- and post-MPTP-treated states
| Pre-MPTP, n = 50 | Post-MPTP, n = 40 | P, Mann-Whitney Test | ||
|---|---|---|---|---|
| Firing rate, s−1 | 17.83 ± 2.30 | 8.01 ± 1.43 | <0.001 | |
| Firing rate of non-modulated spikes, s−1 | 17.17 ± 2.3 | 6.81 ± 1.4 | <0.001 | |
| CV of all ISIs | 1.64 ± 0.13 | 2.25 ± 0.23 | 0.013 | |
| CV of non-modulated ISIs | 0.725 ± 0.024 | 0.822 ± 0.022 | <0.001 | |
| Analysis of spectral power | ||||
| Spectral power (relative to power in the 1–100 Hz range) | 1–3 Hz | 0.020 ± 0.002 | 0.032 ± 0.002 | <0.001 |
| 3–8 Hz | 0.041 ± 0.004 | 0.077 ± 0.004 | <0.001 | |
| 8–13 Hz | 0.038 ± 0.003 | 0.065 ± 0.004 | <0.001 | |
| 13–30 Hz | 0.163 ± 0.007 | 0.203 ± 0.009 | 0.001 | |
| 30–100 Hz | 0.738 ± 0.015 | 0.622 ± 0.016 | <0.001 | |
| Burst and deceleration analysis | ||||
| Proportion of spikes in bursts | All bursts | 0.436 ± 0.033 | 0.598 ± 0.026 | <0.001 |
| Non-rebound bursts | 0.097 ± 0.019 | 0.043 ± 0.017 | <0.001 | |
| Rebound bursts | 0.339 ± 0.038 | 0.555 ± 0.036 | <0.001 | |
| LTS-like bursts | 0.044 ± 0.010 | 0.153 ± 0.023 | <0.001 | |
| Intraburst firing rate, s−1 | All bursts | 68.92 ± 8.57 | 115.67 ± 16.49 | 0.087 |
| Non-rebound bursts | 84.37 ± 7.66 | 54.39 ± 11.77 | 0.008 | |
| Rebound bursts | 60.78 ± 8.59 | 116.08 ± 18.18 | 0.013 | |
| LTS-like bursts | 73.18 ± 10.57 | 144.97 ± 22.68 | 0.025 | |
| Proportion of burst subtypes among all bursts | Non-rebound bursts | 0.354 ± 0.062 | 0.077 ± 0.035 | <0.001 |
| Rebound bursts | 0.539 ± 0.052 | 0.703 ± 0.031 | 0.440 | |
| LTS-like bursts | 0.107 ± 0.013 | 0.219 ± 0.019 | <0.001 | |
| Frequency of bursts, s−1 | All bursts | 1.202 ± 0.114 | 1.03 ± 0.106 | 0.728 |
| Non-rebound bursts | 0.628 ± 0.132 | 0.322 ± 0.125 | <0.001 | |
| Rebound bursts | 0.574 ± 0.061 | 0.709 ± 0.077 | 0.207 | |
| LTS-like bursts | 0.128 ± 0.025 | 0.262 ± 0.039 | 0.007 | |
| Burst surprise value | All bursts | 6.18 ± 0.51 | 9.12 ± 0.67 | <0.001 |
| Non-rebound bursts | 7.65 ± 2.32 | 8.61 ± 1.05 | 0.013 | |
| Rebound bursts | 4.93 ± 0.53 | 8.63 ± 0.61 | <0.001 | |
| LTS-like bursts | 3.96 ± 0.48 | 8.10 ± 0.70 | <0.001 | |
| Preburst ISI, ms | All bursts | 223.10 ± 38.04 | 508.79 ± 130.11 | <0.001 |
| Non-rebound bursts | 25.82 ± 1.58 | 15.08 ± 2.39 | 0.008 | |
| Rebound bursts | 299.78 ± 39.03 | 515.31 ± 63.13 | <0.001 | |
| LTS-like bursts | 344.63 ± 48.53 | 571.95 ± 105.07 | 0.039 | |
| Proportion of non-modulated spikes | 0.58 ± 0.03 | 0.44 ± 0.02 | <0.001 | |
| Proportion of spikes in deceleration | 0.050 ± 0.067 | 0.067 ± 0.003 | 0.003 | |
Means ± SE. CV, coefficient of variation; ISI, interspike interval; LTS, low-threshold spike.
Table 3.
Firing properties of thalamic neurons in MPTP-treated animals during the baseline and “random effect” periods after ML218 or aCSF injections
| ML218, n = 27 |
aCSF, 13 |
||||
|---|---|---|---|---|---|
| Values | P, Wilcoxon's Test | Values | P, Wilcoxon's Test | ||
| Firing rate | 1.71 ± 0.54 | 0.819 | 1.05 ± 0.17 | 0.787 | |
| Firing rate of non-modulated spikes | 1.79 ± 0.48 | 0.782 | 1.10 ± 1.16 | 0.893 | |
| CV of all ISIs | 0.94 ± 0.08* | 0.047 | 1.07 ± 0.16 | 0.376 | |
| CV of non-modulated ISIs | 1.19 ± 0.17 | 0.524 | 1.08 ± 0.05 | 0.445 | |
| Analysis of spectral power | |||||
| Spectral power | 1–3 Hz | 1.01 ± 0.08 | 0.477 | 1.13 ± 0.21 | 0.622 |
| 3–8 Hz | 0.89 ± 0.05* | 0.021 | 1.08 ± 0.17 | 0.376 | |
| 8–13 Hz | 0.90 ± 0.04* | 0.016 | 1.07 ± 0.10 | 1 | |
| 13–30 Hz | 0.94 ± 0.03 | 0.104 | 0.95 ± 0.04 | 0.146 | |
| 30–100 Hz | 1.09 ± 0.03* | 0.007 | 0.99 ± 0.06 | 1 | |
| Burst and deceleration analysis | |||||
| Proportion of spikes in bursts | All bursts | 0.83 ± 0.06† | 0.009 | 1.08 ± 0.15 | 0.97 |
| Non-rebound bursts | 2.94 ± 1.91 | 0.216 | 1.34 ± 0.26 | 0.297 | |
| Rebound bursts | 0.82 ± 0.07† | 0.006 | 2.64 ± 1.61 | 0.558 | |
| LTS-like bursts | 0.71 ± 0.14† | 0.008 | 0.75 ± 0.10 | 0.078 | |
| Intraburst firing rate | All bursts | 1.24 ± 0.37 | 0.106 | 1.03 ± 0.18 | 0.791 |
| Non-rebound bursts | 0.96 ± 0.06 | 0.685 | 1.05 ± 0.07 | 0.08 | |
| Rebound bursts | 0.94 ± 23* | 0.047 | 1.03 ± 0.17 | 0.414 | |
| LTS-like bursts | 1.22 ± 0.56 | 0.130 | 1.01 ± 0.089 | 0.051 | |
| Proportion of burst subtypes among all bursts | Non-rebound bursts | 4.46 ± 2.27† | 0.003 | 4.16 ± 2.91 | 0.05 |
| Rebound bursts | 0.99 ± 0.55 | 0.879 | 1.81 ± 0.93 | 1 | |
| LTS-like bursts | 0.88 ± 0.10* | 0.025 | 0.93 ± 0.24 | 0.206 | |
| Frequency of bursts | All bursts | 1.48 ± 0.41 | 0.589 | 1.01 ± 0.14 | 0.733 |
| Non-rebound bursts | 4.84 ± 2.10† | 0.006 | 1.33 ± 0.31 | 0.110 | |
| Rebound bursts | 1.24 ± 0.17 | 0.407 | 1.74 ± 0.84 | 0.542 | |
| LTS-like bursts | 0.98 ± 0.16* | 0.045 | 0.81 ± 0.14 | 0.094 | |
| Burst surprise value | All bursts | 0.84 ± 0.03‡ | <0.001 | 1.24 ± 0.13 | 0.376 |
| Non-rebound bursts | 1.38 ± 0.56 | 0.203 | 0.99 ± 0.16 | 0.219 | |
| Rebound bursts | 0.27 ± 0.07‡ | <0.001 | 1.19 ± 0.10 | 0.151 | |
| LTS-like bursts | 0.76 ± 0.08† | 0.004 | 1.00 ± 0.05 | 0.640 | |
| Preburst ISI | All bursts | 1.06 ± 0.17 | 0.187 | 1.64 ± 0.30 | 0.064 |
| Non-rebound bursts | 1.49 ± 0.49 | 0.946 | 3.94 ± 2.66 | 0.244 | |
| Rebound bursts | 1.06 ± 0.18 | 0.095 | 1.61 ± 0.28 | 0.191 | |
| LTS-like bursts | 0.96 ± 0.12 | 0.06 | 1.05 ± 0.16 | 0.110 | |
| Proportion of non-modulated spikes | 1.17 ± 0.12 | 0.464 | 0.87 ± 0.1 | 0.110 | |
| Proportion of spike in deceleration | 0.88 ± 0.09 | 0.132 | 1.33 ± 0.25 | 0.414 | |
Means ± SE of postinjection values normalized to the preinjection baseline of the respective cell. Boldface indicates significant results.
P < 0.05,
P < 0.01,
P < 0.001.
Table 4.
ML218 effects on firing properties, according to the “maximal effect” analysis
| Cells with Increased Firing, n = 11 |
Cells with Decreased Firing, n = 7 |
||||
|---|---|---|---|---|---|
| Values | P, Wilcoxon's Test | Values | P, Wilcoxon's Test | ||
| Firing rate | 3.50 ± 1.22‡ | <0.001 | 0.19 ± 0.06* | 0.016 | |
| Firing rate of non-modulated spikes | 3.64 ± 1.14‡ | <0.001 | 0.17 ± 0.07 | 0.125 | |
| CV of all ISIs | 1.27 ± 0.24 | 0.831 | 0.82 ± 0.11 | 0.219 | |
| CV of non-modulated ISIs | 1.09 ± 0.09 | 0.571 | 0.53 ± 0.19 | 1 | |
| Analysis of spectral power | |||||
| Spectral power | 1–3 Hz | 0.97 ± 0.11 | 0.577 | 1.01 ± 0.13 | 0.438 |
| 3–8 Hz | 0.96 ± 0.07 | 0.413 | 0.72 ± 0.09 | 0.125 | |
| 8–13 Hz | 1.02 ± 0.06 | 0.831 | 0.81 ± 0.09 | 0.188 | |
| 13–30 Hz | 1.01 ± 0.06 | 0.765 | 0.87 ± 0.08 | 0.625 | |
| 30–100 Hz | 1.03 ± 0.03 | 0.700 | 1.19 ± 0.10 | 0.125 | |
| Burst and deceleration analysis | |||||
| Proportion of spikes in bursts | All bursts | 0.89 ± 0.04* | 0.024 | 0.57 ± 0.19 | 0.156 |
| Non-rebound bursts | 11.15 ± 7.77* | 0.02 | 0.57 ± 0.20 | 0.250 | |
| Rebound bursts | 0.73 ± 0.07† | 0.003 | 0.56 ± 0.20* | 0.047 | |
| LTS-like bursts | 0.65 ± 0.15* | 0.024 | 0.56 ± 0.41* | 0.047 | |
| Intraburst firing rate | All bursts | 2.41 ± 1.00 | 0.7 | 0.94 ± 0.42 | 0.078 |
| Non-rebound bursts | 0.74 ± 1.78 | 1 | 0.57 ± 0.20 | 1 | |
| Rebound bursts | 0.17 ± 0.10 | 0.125 | 2.96 ± 1.25 | 0.278 | |
| LTS-like bursts | 1.12 ± 0.08 | 0.250 | 1.91 ± 0.703 | 0.625 | |
| Proportion of burst subtypes among all bursts | Non-rebound bursts | 17.95 ± 6.98* | 0.042 | 23.90 ± 9.93 | 0.438 |
| Rebound bursts | 0.98 ± 0.08 | 0.625 | 0.90 ± 0.18 | 0.469 | |
| LTS-like bursts | 0.84 ± 0.13 | 0.123 | 1.09 ± 0.26 | 0.813 | |
| Frequency of bursts | All bursts | 2.73 ± 0.96* | 0.042 | 0.49 ± 0.31* | 0.031 |
| Non-rebound bursts | 18.03 ± 8.76* | 0.012 | 2.29 ± 1.62 | 0.625 | |
| Rebound bursts | 2.05 ± 0.39 | 0.102 | 0.21 ± 0.12* | 0.016 | |
| LTS-like bursts | 1.61 ± 0.32 | 0.413 | 0.26 ± 0.19* | 0.031 | |
| Burst surprise value | All bursts | 0.91 ± 0.06 | 0.175 | 0.52 ± 0.20 | 0.625 |
| Non-rebound bursts | 0.79 ± 0.11 | 0.125 | 0.61 ± 0.18 | 0.250 | |
| Rebound bursts | 0.55 ± 0.12† | 0.002 | 0.05 ± 0.008* | 0.016 | |
| LTS-like bursts | 0.71 ± 0.11* | 0.019 | 0.60 ± 0.22 | 0.219 | |
| Preburst ISI | All bursts | 0.43 ± 0.07‡ | <0.001 | 2.19 ± 0.80 | 0.125 |
| Non-rebound bursts | 5.72 ± 2.07 | 0.946 | 0.57 ± 0.20 | 0.244 | |
| Rebound bursts | 0.55 ± 0.07‡ | <0.001 | 2.23 ± 0.96 | 0.125 | |
| LTS-like bursts | 0.57 ± 0.12* | 0.037 | 1.05 ± 0.53 | 0.375 | |
| Proportion of non-modulated spikes | 1.18 ± 0.10 | 0.083 | 0.53 ± 0.21 | 0.877 | |
| Proportion of spikes in deceleration | 0.76 ± 0.11* | 0.019 | 0.82 ± 0.14 | 0.877 | |
Means ± SE of postinjection values normalized to the preinjection baseline of the respective cell. Boldface indicates significant results.
P < 0.05,
P < 0.01,
P < 0.001.
Statistics
The effects of Parkinsonism were evaluated by comparing the values from normal and MPTP-treated animals with nonparametric Mann-Whitney tests (see Table 2). For analyses of the drug-injection effects, comparisons between control and effect periods were carried out by using the nonparametric (paired) Wilcoxon's signed-rank test (see Table 3) separately for ML218 and aCSF. The proportion of cells with decreased or increased firing after ML218 or aCSF was assessed using a χ2 test. For the two groups of cells (decreased firing rate vs. increased firing rate; see Table 4), comparison of the values at control and at the maximal effect was evaluated with Wilcoxon's signed-rank tests.
RESULTS
Anatomical Experiments
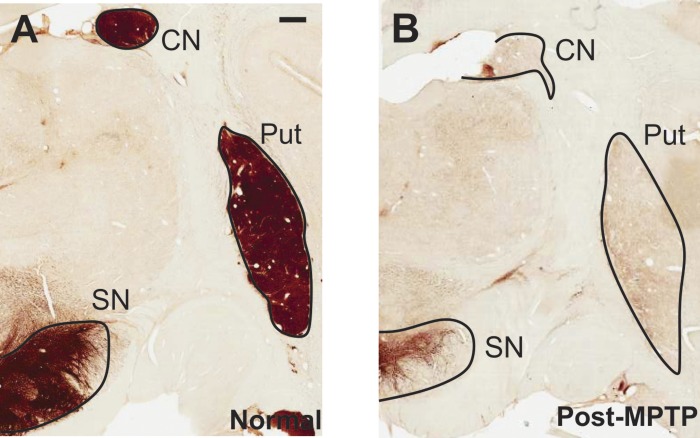
As shown in Fig. 1, MPTP-treated animals used in this study displayed a significant loss of TH immunoreactivity throughout the caudate nucleus (80 ± 1%), the putamen (88 ± 2%), and the SN (60 ± 2%).
Fig. 1.
Examples of immunolabeling to reveal tyrosine hydroxylase in the basal ganglia of a normal monkey (A) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkey (B; monkey C in this study). In both cases, a coronal section, corresponding to the anterior commissure (AC) 7.7-mm plane in the macaque atlas by Lanciego and Vazquez (2012), is shown. CN, caudate nucleus; Put, putamen; SN, substantia nigra.
All nuclei in the ventral motor thalamus displayed strong Cav3.1 immunoreactivity in normal and MPTP-treated monkeys, whereas the reticular nucleus was devoid of labeling (Fig. 2, A–C). The overall intensity of labeling in ventral motor thalamic nuclei was comparable with that seen in the somatosensory ventral posterior nuclei. At high magnification, Cav3.1 immunoreactivity in the VApc of both normal and MPTP-treated animals was found to be heavily concentrated in a dense meshwork of fine neutrophil elements, dendritic processes, and cell bodies, without any obvious difference in pattern of distribution and intensity of labeling between the two states (Fig. 2, D and E). Western blot analysis confirmed the specificity of the Cav3.1 MAb on thalamic and striatal tissue of rhesus monkeys (Fig. 2F). In both cases, a single band corresponding to the approximate molecular weight of Cav3.1 (∼250 kD) was detected.
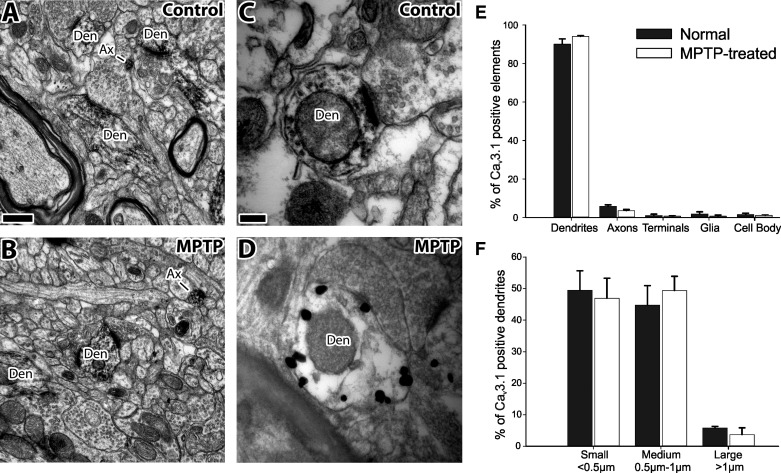
At the EM level, strong immunoreactivity was observed in various neuronal elements of the basal ganglia-receiving areas of the thalamus in normal and MPTP-treated monkeys (Fig. 3). Dendrites accounted for 90% of all immunoreactive elements in normal monkeys and 94% in MPTP-treated animals (Fig. 3). Of the labeled dendrites, 49% were categorized as small (<0.5 μm in diameter), 45% as medium (0.5–1 μm), and 6% as large (>1 μm) dendrites in normal monkeys; corresponding numbers were 47, 49, and 4%, respectively, in MPTP-treated animals (Fig. 3, A, B, and F). Because of its amorphous texture, the peroxidase deposit in labeled dendrites was often diffusely distributed across the whole element (Fig. 3, A–C). However, in sections immunostained with the pre-embedding immunogold method, most gold particles were closely apposed to the plasma membrane of dendritic shafts (Fig. 3D). In addition to dendrites, less-frequent labeling was found in unmyelinated axons (6% of all labeled elements in normal animals and 4% in the MPTP-treated monkeys), terminals (1/1%), glia (1/1%), and neuronal cell bodies (1/1%; Fig. 3E). There was no significant difference in the distribution of labeled elements between the normal and Parkinsonian states.
Fig. 3.
Electron micrographs of Cav3.1-immunoreactive neuronal elements in the basal ganglia-receiving area of the thalamus of normal and MPTP-treated monkeys, as revealed with the pre-embedding immunoperoxidase (A–C) and immunogold methods (D). Note that the labeling is mostly expressed in dendrites of various sizes. Note also that the gold particles are associated with the plasma membrane (D). E: distribution of Cav3.1-labeled elements in the VApc of normal (black bars) and MPTP-treated (white bars) animals. F: distribution of Cav3.1-labeled dendritic profiles, separated by size. Values are means ± SE. There were no differences between the normal and Parkinsonian animals (tested with Mann-Whitney tests). Ax, axon; Den, dendrite. Original scale bars, 0.5 μm (A; also applies to B); 0.1 μm (C; also applies to D).
Electrophysiology
Parkinsonism-associated changes.
We recorded thalamic neurons in primates, first in the normal state and then in a moderately Parkinsonian state. Table 1 shows the number of recorded cells in the two states. We compared electrophysiological parameters of the baseline activity of 50 neurons recorded in the normal state with 40 neurons recorded in the Parkinsonian state in the basal ganglia-receiving nuclei of the ventral motor thalamus [i.e., the VApc and VAmc (Lanciego and Vazquez 2012); Fig. 4]. Data for pre- and post-MPTP are presented as means and SE values in Table 2 and Fig. 5. Compared with the normal state, thalamic neurons fired at a significantly lower rate in the Parkinsonian state (Table 2 and Fig. 6). This effect was still present when the bursts and decelerations were removed from the data stream (creating “non-modulated” data). The variability of firing (measured with the CV of ISIs) increased in both the original data and in the non-modulated portions of the data. Significant differences were also detected in the power spectral distribution of firing-rate fluctuations. Oscillatory activity in the 1–3-, 3–8-, 8–13-, and 13–30-Hz ranges was increased, whereas oscillatory activity in the 30–100-Hz band was reduced (see Fig. 6E).
Table 1.
Number of neurons recorded for each animal in the different conditions
| State of Animals | Microinjections | Total Number of Cells | Monkey A | Monkey B | Monkey C |
|---|---|---|---|---|---|
| Pre-MPTP | (None) | 50 | 14 | 15 | 21 |
| Post-MPTP | aCSF | 13 | 4 | 4 | 5 |
| ML218 | 27 | 7 | 9 | 11 |
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; aCSF, artificial cerebrospinal fluid.
A large portion of the increased variability was likely caused by changes in bursts, as generally indicated by a lower proportion of non-modulated spikes in the Parkinsonian state. The average surprise value assigned to rebound and LTS-like bursts increased, whereas the one assigned to non-rebound bursts was decreased. The frequency of LTS-like bursts, the proportion of spikes, the intraburst frequency, and the preburst ISI of rebound and LTS-like bursts were increased, whereas the proportion of spikes, the frequency, the preburst ISI, and the intraburst frequency in non-rebound bursts were decreased in the Parkinsonian state. In addition, cells spent significantly more time in decelerations in the Parkinsonian state.
Changes induced by ML218 and aCSF injection (analysis of random effect periods).
The thalamic microinjections of ML218 or aCSF did not induce behavioral changes, as qualitatively assessed by visual examination of the animals seated in the primate chair. As shown in Table 3, local aCSF injections in Parkinsonian monkeys did not affect the activity of the thalamic neurons. ML218 injections, on the other hand, significantly increased the proportion and frequency of the non-rebound burst and reduced the proportion and frequency of LTS-like bursts (Fig. 6). The variability of the firing (measured with the CV of ISIs), the oscillatory power in the 1–13-Hz range of frequencies, the burst surprise value of the rebound and LTS-like burst, the intraburst frequency in rebound bursts, and the proportion of spikes in rebound and LTS-like bursts were reduced following ML218 injection.
Changes induced by ML218 and aCSF injection (analysis of “maximum effect” periods).
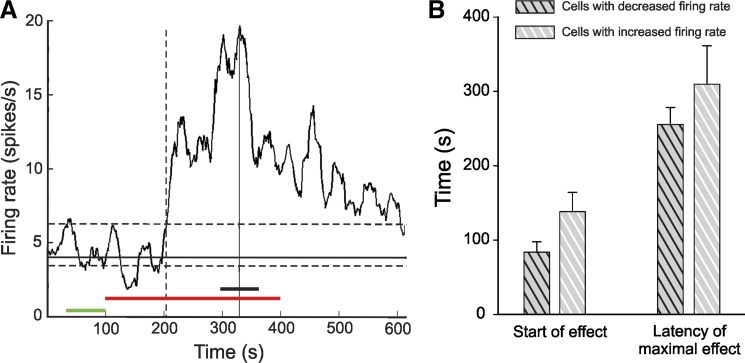
We separately analyzed data from cells that showed increases or decreases in firing (Table 4). ML218- and aCSF-induced decreases in firing were seen in 7/27 and 3/13 cells, respectively, whereas increases occurred in 11/27 and 0/13 cells (χ2 test, ML218 vs. aCSF; P = 0.02). Because of the low numbers of cells responding to aCSF injections, there is not aCSF data shown in Table 4. As shown in Fig. 7B, the average onset of effects or the timing of the maximal effects did not differ between cells with ML218 injection-induced increases or decreases in firing. In the group of cells that increased its firing rate in response to ML218, the proportion of non-rebound bursts among all bursts, the frequency of the non-rebound burst (per second), the proportion of spikes in non-rebound bursts, and the firing rate of spikes in the non-modulated sections of the record were increased (Table 4). In contrast, the duration of ISIs preceding rebound and LTS-like bursts, the proportion of the spike, and the surprise values of rebound and LTS-like bursts were decreased. In the group of cells with decreased firing in response to ML218, the proportion of spikes in rebound bursts or LTS-like bursts, the frequency of these types of bursts, and the average surprise values of the rebound burst were reduced during the maximal effect period (Table 4).
Fig. 7.
A: example trace of a thalamic neuron that increased its firing rate in response to ML218 injections. Green bar, 60-s control period; red bar, drug injection; black bar, maximal effect period; solid horizontal line, median firing rate at baseline; dashed lines, 90th and 10th percentiles; solid vertical line, maximal effect; vertical dashed line, moment at which the effect was considered to have started. B: delay of the onset of the ML218-induced effects and the timing of the maximal effect (both measured from the start of the injection), shown for cells with firing-rate decreases (black-striped bars) and cells with firing-rate increases (white-striped bars). Values are means ± SE compared with a Mann-Whitney rank sum test.
DISCUSSION
Our anatomical data demonstrate strong expression of Cav3.1 T-type calcium channel immunoreactivity along the entire somatodendritic domain of neurons in the basal ganglia-receiving area of the thalamus in control and Parkinsonian monkeys. Microinjections of the selective T-type calcium channel blocker ML218 in the basal ganglia-receiving thalamic region of MPTP-treated monkeys partially normalized the increases in burst activity by reducing the proportion of spikes in rebound and LTS-like bursts and by increasing the frequency of non-rebound bursts. MPTP treatment-related changes in spectral power were also partly reversed, with an increase in the 3–13-Hz range and a decrease of the gamma frequency range. However, ML218 did not reverse MPTP treatment-induced decreases in firing rates. It even further decreased the firing of some cells. Thus although effective in alleviating some features of abnormal thalamic activity, the lack of effects of ML218 on other parameters may limit the anti-Parkinsonian effects of T-type calcium channel blockers, such as ML218.
Subcellular Localization of Cav3.1 in the Monkey Thalamus
In line with previous rodent studies (Craig et al. 1999; McKay et al. 2006; Parajuli et al. 2010; Perez-Reyes 2003; Talley et al. 1999), our findings demonstrate that Cav3.1 immunoreactivity is strongly expressed throughout the monkey thalamus, with the exception of the reticular nucleus, which is known to be enriched in Cav3.3 (Liu et al. 2011; Talley et al. 1999). At the subcellular level, Cav3.1 immunostaining was found throughout the entire dendritic tree of thalamic projection neurons in normal and Parkinsonian monkeys. This widespread dendritic distribution in thalamocortical cells may indicate that T-type calcium channels mediate various physiological effects upon ventral motor thalamocortical neurons (Allken et al. 2014; Lai and Jan 2006; Nusser 2009; Perez-Reyes 2003), including regulatory functions at the cellular level, such as amplification of synaptic responses from distal synaptic inputs and various forms of synaptic plasticity and exocytosis, or that they may serve as a source of calcium for the activation of Ca2+-activated potassium channels (Allken et al. 2014; Perez-Reyes 2003). Although our findings do not allow us to quantify the amount of channels distributed along the plasma membrane of proximal vs. distal dendrites and between the normal and Parkinsonian state, the fact that both large and small dendrites displayed strong immunostaining in the immunoperoxidase and immunogold material suggests comparable levels of expression across the somatodendritic domain of thalamocortical neurons. The fact that the expression level and pattern of distribution of Cav3.1 immunoreactivity did not differ between normal and Parkinsonian animals suggests that altered localization of T-type calcium channels may not explain the increased bursting activity in the thalamus in the Parkinsonian state [see present results and Magnin et al. (2000), Pessiglione et al. (2005), and Zirh et al. (1998)].
MPTP-Induced Changes of Thalamic Activity
With the comparison of thalamic recordings from the normal and Parkinsonian states, we found that the overall firing rate of thalamic cells was reduced. These results are consistent with the classical model of the functional basal ganglia circuitry, in which increased inhibitory basal ganglia output in the Parkinsonian state is thought to decrease activity in the thalamus (Albin et al. 1989; DeLong 1990; Galvan et al. 2015). Similar decreases in firing rates were found in several previous studies, including Parkinsonian patients (Chen et al. 2010; Molnar et al. 2005), MPTP-treated cats (Schneider and Rothblat 1996), and 6-hydroxydopamine (6-OHDA)-lesioned rats (Ni et al. 2000). However, other studies found either no change in thalamic firing activity in MPTP-treated monkeys (Pessiglione et al. 2005) or increases in firing in 6-OHDA-treated rats (Bosch-Bouju et al. 2014). The discrepancies between these studies may be related to species and experimental differences across studies, such as the specific cell types selected for recording, the state of wakefulness of the animals, or the severity of Parkinsonism.
The oscillatory activity of the thalamic firing was also altered by MPTP treatment, with an increase of oscillatory activity in the 1–30-Hz band and a reduction in the 30–100-Hz range. An increase of low-frequency oscillatory spiking activity was described previously in the subthalamic nucleus (STN) and globus pallidus internus of MPTP-treated monkeys (Bergman et al. 1994; Galvan et al. 2014; Leblois et al. 2007; Raz et al. 2000; Soares et al. 2004). We also found that the variability of firing in thalamic cells (as measured by the CV of ISIs) was higher in the Parkinsonian than in the normal state. A significant portion of the increased variability was likely caused by changes in bursts and decelerations.
The surprise values assigned to rebound and LTS-like bursts were higher in the recordings from the Parkinsonian state than in the control recordings, indicating a more intense tendency of neurons to show this type of burst in the Parkinsonian state. In addition, we found an increase of the proportion of spikes in rebound and LTS-like bursts. Increases in bursting were described previously in the motor thalamus of MPTP-treated monkeys (Guehl et al. 2003; Pessiglione et al. 2005). A similarly high incidence of burst firing has been found in corresponding areas in Parkinson's disease patients (Magnin et al. 2000; Molnar et al. 2005; Zirh et al. 1998). Interestingly, in Parkinsonian monkeys, Pessiglione et al. (2005) found that burst activity increased in both basal ganglia and cerebellar territories but that this reached significance only in the cerebellar-receiving area of the thalamus. We also found a decrease of the proportion of spike and the ISI preceding non-rebound bursts (in addition to the decrease of the surprise value), suggesting that Parkinsonism differently affects rebound and non-rebound bursting activity. Perhaps related to the increased rebound bursting (which is associated with preburst episodes of reduced firing), decelerations were also more noticeable in the Parkinsonian state, as indicated by the larger amount of time that neurons spent in decelerations.
In addition, we found that the incidence of LTS-like bursts increased in the Parkinsonian state. Increased rebound and LTS-like bursting may be related to increased activation of T-type calcium channels in the thalamus, which, in turn, may have resulted from increased inhibitory (hyperpolarizing) input to the recorded cells (Jahnsen and Llinas 1984). A likely source of direct inhibitory inputs to the thalamus is that from the basal ganglia output nuclei, although other inhibitory afferents, such as projections from the reticular nucleus or the thalamic interneurons, may also play a role. Whereas increased LTS bursting may relate to the motor dysfunction in the Parkinsonian state, LTS-like bursts have also been associated with rest and inattention (Llinas and Steriade 2006) and thus may signify a more general state change in this condition.
ML218 Effects on Bursting and Oscillatory Activity
ML218 is a specific blocker of T-type calcium channels (Xiang et al. 2011). Not surprisingly, local ML218 injections reduced the burst surprise values, the incidence of LTS-like bursts, and the proportion of spikes in rebound and LTS-like bursts. These results confirm previous findings about the effects of this drug on bursting activity in the rat STN (Tai et al. 2011; Xiang et al. 2011). As expected, ML218 did not strongly affect non-rebound bursting, suggesting that mechanisms other than T-type calcium channel activation may drive non-rebound bursting. For example, voltage-gated potassium channels (Kv7.2 and Kv7.3) have been found abundantly in the mouse thalamus, and their activation reduces tonic firing and increases bursting activity in relay neurons (Cerina et al. 2015).
The T-type calcium blockade strongly reduced the Parkinsonism-related abnormalities in oscillatory activity by decreasing the oscillatory activity in the 3–13-Hz range and increasing oscillations in the gamma (30–100 Hz) band. Whereas not explained completely, these effects are potentially highly important, as spectral changes in these ranges of firing are considered to be core abnormalities in Parkinsonism [see review by Galvan et al. (2015)].
ML218 Effects on Firing Rate
Whereas T-type calcium channel activity is often linked to bursting [for review, see Lambert et al. (2014)], activation of these channels may have additional effects on tonic firing activity, as shown in mouse brain-slice recordings of thalamocortical neurons (Deleuze et al. 2012). In addition, administration of a T-type calcium channel antagonist to the initial axon segment of cochlear interneurons reduced not only bursting but also the probability of single action potential generation (Bender and Trussell 2009). Recently, Bosch-Bouju et al. (2014) found that the overall firing rate of motor thalamic neurons was reduced following local injection of a selective T-type calcium channel blocker in freely moving 6-OHDA-lesioned rats and suggested that a proportion of single spikes recorded in the thalamus may be due to partial disinhibition of T-type calcium channels that failed to lead to a burst. In our analysis of random-effect periods, ML218 injections did not lead to a change in the overall firing rate of the recorded basal ganglia-receiving thalamic neurons, but the analysis of maximal effect periods revealed that ML218 injections significantly affected the firing rate of most thalamic cells, leading to significant decreases or increases in firing. Both types of responses were associated with decreases in rebound and LTS-like bursting activity. The divergent effects on firing rates may be explained by the fact that the drug acted on different populations of neurons or that some of the observed changes may have been secondary effects, mediated via intrathalamic networks (see below).
Potential Study Limitations
The borders between the cerebellar- and the basal ganglia-receiving territories are interdigitating; however, the cerebellar- and basal ganglia-receiving thalamic cells are distinct in primates [for review, see Bosch-Bouju et al. (2013)]. The overlap should not be a concern for the present study, because almost all cells included in the presented analysis were at least 0.5 mm away from the border between the cerebellar and basal ganglia territories, as defined in Lanciego's atlas (Lanciego and Vazquez 2012). Furthermore, the firing pattern of the recorded cells was not noticeably different between cells close to the border and those in the core of the basal ganglia-receiving territory. Finally, as shown in Fig. 2, the overall intensity of Cav3.1 labeling was similar across the motor thalamus, suggesting that drug effects on neurons close to or away from the border would not be expected to be different.
Given their relative density and large size (Jones 2007), we expect that most of the recorded cells were thalamocortical projection neurons (Hunt et al. 1991). In some cases, however, the injected drug may not have acted directly on these neurons but on inhibitory thalamic interneurons [constituting ∼30% of all neurons in the primate ventral thalamus (Jones 2007; Smith et al. 1987)], leading to secondary effects in the recorded cells and an overall increase in variability of the recordings.
In addition, it has been suggested that the specific effects of T-type calcium channel activation may be determined, in part, by their distribution (somatic vs. dendritic) (Destexhe et al. 1998). However, this is an unlikely explanation for the observed heterogeneity, because our EM data show that the channels are expressed similarly along the entire extent of the dendritic tree of thalamic cells.
Thalamic activity is known to be sensitive to the state of vigilance and sleep activity (Llinas and Steriade 2006; Weyand et al. 2001), and T-type calcium channels have been implicated in the regulation of sleep-wake states (Crunelli et al. 2014; Hughes et al. 2002; Shin et al. 2008). The possibility that changes in the state of arousal of our animals during the recording sessions contributed to some of our findings cannot be ruled out completely. However, every effort was made to maintain a stable level of wakefulness in our animals during the recordings.
Another limitation of our study is that the drug concentration at the recording site (i.e., 100–200 μm away from the injection location) is not known. The attainment of a high drug concentration at the recording site is very important, because experiments in rodent-slice preparations and computer-modeling studies have suggested that robust LTS bursting can still be present when a large proportion (up to 70%) of T-type calcium channels is blocked (Broicher et al. 2007; Dreyfus et al. 2010). However, the concentration of ML218 used in our study (2.5 mM) was high compared with its effective concentrations at Cav3.3 and Cav3.2 channels found in in vitro electrophysiology [IC50 of 270 and 310 nM, respectively (Xiang et al. 2011)], making us confident that a very large proportion of the T-type calcium channels were, in fact, blocked in our experiments. Further confirmation comes from the fact that we saw, as expected, a reversal of many Parkinsonism-associated changes in parameters describing burst behaviors.
Finally, we cannot rule out that some of the effects described in our study were mediated by diffusion of the drug to the reticular thalamic nucleus, which likely expresses Cav3.2 and Cav3.3 (Talley et al. 1999). ML218 is not selective among the subtypes of channels that comprise the Cav3 family (Xiang et al. 2011) and may therefore have acted on reticular nucleus neurons, with secondary effects on neurons in the ventral motor thalamus. However, because ML218 effects were found, regardless of the distance of the microinjections to the reticular nucleus (see Fig. 4), we believe that most of the changes described in our study were due to local drug effects in the basal ganglia-receiving territory of the thalamus (David et al. 2013; Li et al. 2005).
Possible Use of T-Type Calcium Channel Blockers As Treatment of Parkinsonism
Given the increase in burst discharges in the basal ganglia and thalamus in the Parkinsonian state, T-type calcium channel blockade has been discussed as a possible strategy to treat Parkinsonism. Drugs that block T-type calcium channels were shown to have anti-tremor effect in rats (Miwa et al. 2008, 2011), mice (Handforth et al. 2010), and perhaps monkeys (Gomez-Mancilla et al. 1992), as well as anti-Parkinsonian effects in patients with Parkinson's disease (Bermejo 2007; Murata et al. 2001, 2007). However, the previously tested agents have multiple effects other than T-type calcium channel blockade [see, for instance, data on zonisamide (Murata 2004)] so that the anti-Parkinsonian potential of selective T-type calcium channel blockade remains essentially untested. The only previous study of the anti-Parkinsonian properties of the specific agent ML218 occurred in haloperidol-treated rats, in which the drug was shown to have anticataleptic properties (Xiang et al. 2011). The current study shows that the basal ganglia-receiving thalamic nuclei display abnormal bursting activity in the Parkinsonian state and that the amount and location of T-type calcium channels in these thalamic nuclei do not differ significantly between normal and MPTP-treated monkeys, thus confirming that the manipulation of T-type calcium channel activity to alter thalamic burst discharges and oscillatory firing patterns could be a viable strategy to treat Parkinsonism. The global effect of ML218 on burst surprise values, rebound bursting, the incidence of LTS-like bursts, and oscillatory activity is, therefore, potentially relevant. However, because other parameters of thalamic neuronal activity that were strongly affected by MPTP did not respond to the drug injections (such as the firing rate or the oscillation in the beta frequency range), the nature and origin of these effects need to be understood more fully before advancing the use of specific T-type calcium channel blockers as potential therapeutics in Parkinsonism.
GRANTS
Support for this project was provided through grants from the National Institute of Neurological Disorders and Stroke [R01-NS054976 and P50-NS071669 (Udall Center Grant) to T. Wichmann and R25-NS080688 to Y. Smith]; National Institute of General Medical Science (R01-GM077569 to V. Faundez); and U.S. National Institutes of Health, Office of Research Infrastructure Programs (P51 OD011132 to the Yerkes National Primate Research Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.D., C.W.L., C.J., Y.S., and T.W. conception and design of research; A.D., E.C., Y.M., I.H., D.P., S.K., A.P.M., C.W.L., and C.J. performed experiments; A.D., E.C., Y.M., V.F., Y.S., and T.W. analyzed data; A.D., E.C., Y.S., and T.W. interpreted results of experiments; A.D., E.C., Y.S., and T.W. prepared figures; A.D., E.C., Y.S., and T.W. drafted manuscript; A.D., Y.S., and T.W. edited and revised manuscript; A.D., E.C., Y.M., I.H., D.P., S.K., A.P.M., C.W.L., C.J., Y.S., and T.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jean-Francois Pare and Susan Jenkins for their technical support for the anatomical studies.
REFERENCES
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. [DOI] [PubMed] [Google Scholar]
- Allken V, Chepkoech JL, Einevoll GT, Halnes G. The subcellular distribution of T-type Ca2+ channels in interneurons of the lateral geniculate nucleus. PLoS One 9: e107780, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci 25: 8505–8517, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron 61: 259–271, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of Parkinsonism. J Neurophysiol 72: 507–520, 1994. [DOI] [PubMed] [Google Scholar]
- Bermejo PE. Zonisamide in patients with essential tremor and Parkinson's disease. Mov Disord 22: 2137–2138, 2007. [DOI] [PubMed] [Google Scholar]
- Bosch-Bouju C, Hyland BI, Parr-Brownlie LC. Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and Parkinsonian conditions. Front Comput Neurosci 7: 163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Bouju C, Smither RA, Hyland BI, Parr-Brownlie LC. Reduced reach-related modulation of motor thalamus neural activity in a rat model of Parkinson's disease. J Neurosci 34: 15836–15850, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broicher T, Seidenbecher T, Meuth P, Munsch T, Meuth SG, Kanyshkova T, Pape HC, Budde T. T-Current related effects of antiepileptic drugs and a Ca2+ channel antagonist on thalamic relay and local circuit interneurons in a rat model of absence epilepsy. Neuropharmacology 53: 431–446, 2007. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005. [DOI] [PubMed] [Google Scholar]
- Cerina M, Szkudlarek HJ, Coulon P, Meuth P, Kanyshkova T, Nguyen XV, Gobel K, Seidenbecher T, Meuth SG, Pape HC, Budde T. Thalamic Kv 7 channels: pharmacological properties and activity control during noxious signal processing. Br J Pharmacol 172: 3126–3140, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhuang P, Miao SH, Yuan G, Zhang YQ, Li JY, Li YJ. Neuronal firing in the ventrolateral thalamus of patients with Parkinson's disease differs from that with essential tremor. Chin Med J (Engl) 123: 695–701, 2010. [PubMed] [Google Scholar]
- Craig PJ, Beattie RE, Folly EA, Banerjee MD, Reeves MB, Priestley JV, Carney SL, Sher E, Perez-Reyes E, Volsen SG. Distribution of the voltage-dependent calcium channel alpha1G subunit mRNA and protein throughout the mature rat brain. Eur J Neurosci 11: 2949–2964, 1999. [DOI] [PubMed] [Google Scholar]
- Crunelli V, David F, Leresche N, Lambert RC. Role for T-type Ca2+ channels in sleep waves. Pflugers Arch 466: 735–745, 2014. [DOI] [PubMed] [Google Scholar]
- David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, Renger JJ, Lambert RC, Leresche N, Crunelli V. Essential thalamic contribution to slow waves of natural sleep. J Neurosci 33: 19599–19610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C, David F, Behuret S, Sadoc G, Shin HS, Uebele VN, Renger JJ, Lambert RC, Leresche N, Bal T. T-Type calcium channels consolidate tonic action potential output of thalamic neurons to neocortex. J Neurosci 32: 12228–12236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. J Neurosci 18: 3574–3588, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergnas A, Pittard D, Bliwise D, Wichmann T. Relationship between oscillatory activity in the cortico-basal ganglia network and Parkinsonism in MPTP-treated monkeys. Neurobiol Dis 68: 156–166, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci 30: 99–109, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. J Neurosci 27: 2525–2538, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Devergnas A, Wichman T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the Parkinsonian state. Front Neuroanat 9: 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Rommelfanger KS, Pare JF, Khan ZU, Smith Y, Wichmann T. Localization and function of dopamine receptors in the subthalamic nucleus of normal and Parkinsonian monkeys. J Neurophysiol 112: 467–479, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and function of GABA transporters in the globus pallidus of Parkinsonian monkeys. Exp Neurol 223: 505–515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Barbee R, Bielitzki J, Clayton L, Donovan J, Hendriksen C, Kohn D, Lipman N, Locke P, Melcher J, Quimby F, Turner P, Wood G, Wurbel H. Guide for the Care and Use of Laboratory Animals (8th ed). Washington, DC: National Academies Press, 2011. [Google Scholar]
- Gokhale A, Larimore J, Werner E, So L, Moreno-De-Luca A, Lese-Martin C, Lupashin VV, Smith Y, Faundez V. Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1. J Neurosci 32: 3697–3711, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mancilla B, Latulippe JF, Boucher R, Bedard PJ. Effect of ethosuximide on rest tremor in the MPTP monkey model. Mov Disord 7: 137–141, 1992. [DOI] [PubMed] [Google Scholar]
- Gonzales KK, Pare JF, Wichmann T, Smith Y. GABAergic inputs from direct and indirect striatal projection neurons onto cholinergic interneurons in the primate putamen. J Comp Neurol 521: 2502–2522, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehl D, Pessiglione M, Francois C, Yelnik J, Hirsch EC, Feger J, Tremblay L. Tremor-related activity of neurons in the ‘motor’ thalamus: changes in firing rate and pattern in the MPTP vervet model of Parkinsonism. Eur J Neurosci 17: 2388–2400, 2003. [DOI] [PubMed] [Google Scholar]
- Hammond C, Beurrier C, Garcia L, Bioulac B. Physiological and pathological patterns in subthalamic neurons related to Parkinson's disease. Neurophysiology 34: 96, 2002. [Google Scholar]
- Handforth A, Homanics GE, Covey DF, Krishnan K, Lee JY, Sakimura K, Martin FC, Quesada A. T-Type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology 59: 380–387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577–580, 1981. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron 33: 947–958, 2002. [DOI] [PubMed] [Google Scholar]
- Hunt CA, Pang DZ, Jones EG. Distribution and density of GABA cells in intralaminar and adjacent nuclei of monkey thalamus. Neuroscience 43: 185–196, 1991. [DOI] [PubMed] [Google Scholar]
- Ilinsky IA, Kultas-Ilinsky K. Sagittal cytoarchitectonic maps of the Macaca mulatta thalamus with a revised nomenclature of the motor-related nuclei validated by observations on their connectivity. J Comp Neurol 262: 331–364, 1987. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol 349: 205–226, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus (2nd ed), Cambridge, UK: Cambridge University Press, 2007. [Google Scholar]
- Kliem MA, Wichmann T. A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods 138: 45–49, 2004. [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Fujiyama F, Nakamura KC, Tanaka Y, Hioki H, Kaneko T. Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur J Neurosci 33: 95–109, 2011. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, Smith Y. Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J Neurosci 27: 6249–6260, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 7: 548–562, 2006. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Bessaih T, Crunelli V, Leresche N. The many faces of T-type calcium channels. Pflugers Arch 466: 415–423, 2014. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Vazquez A. The basal ganglia and thalamus of the long-tailed macaque in stereotaxic coordinates. A template atlas based on coronal, sagittal and horizontal brain sections. Brain Struct Funct 217: 613–666, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci 26: 1701–1713, 2007. [DOI] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985. [DOI] [PubMed] [Google Scholar]
- Li M, Hansen JB, Huang L, Keyser BM, Taylor JT. Towards selective antagonists of T-type calcium channels: design, characterization and potential applications of NNC 55-0396. Cardiovasc Drug Rev 23: 173–196, 2005. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Low-threshold calcium channel subunit Ca(v) 3.3 is specifically localized in GABAergic neurons of rodent thalamus and cerebral cortex. J Comp Neurol 519: 1181–1195, 2011. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol 95: 3297–3308, 2006. [DOI] [PubMed] [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in Parkinsonian patients. Neuroscience 96: 549–564, 2000. [DOI] [PubMed] [Google Scholar]
- McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci 24: 2581–2594, 2006. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Pare JF, Smith Y. Ultrastructural relationships between cortical, thalamic, and amygdala glutamatergic inputs and group I metabotropic glutamate receptors in the rat accumbens. J Comp Neurol 518: 1315–1329, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H. Zonisamide for the treatment of Parkinson's disease. Expert Rev Neurother 7: 1077–1083, 2007. [DOI] [PubMed] [Google Scholar]
- Miwa H, Hama K, Kajimoto Y, Kondo T. Effects of zonisamide on experimental tremors in rats. Parkinsonism Relat Disord 14: 33–36, 2008. [DOI] [PubMed] [Google Scholar]
- Miwa H, Koh J, Kajimoto Y, Kondo T. Effects of T-type calcium channel blockers on a Parkinsonian tremor model in rats. Pharmacol Biochem Behav 97: 656–659, 2011. [DOI] [PubMed] [Google Scholar]
- Molnar GF, Pilliar A, Lozano AM, Dostrovsky JO. Differences in neuronal firing rates in pallidal and cerebellar receiving areas of thalamus in patients with Parkinson's disease, essential tremor, and pain. J Neurophysiol 93: 3094–3101, 2005. [DOI] [PubMed] [Google Scholar]
- Murata M. Novel therapeutic effects of the anti-convulsant, zonisamide, on Parkinson's disease. Curr Pharm Des 10: 687–693, 2004. [DOI] [PubMed] [Google Scholar]
- Murata M, Hasegawa K, Kanazawa I. Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology 68: 45–50, 2007. [DOI] [PubMed] [Google Scholar]
- Murata M, Horiuchi E, Kanazawa I. Zonisamide has beneficial effects on Parkinson's disease patients. Neurosci Res 41: 397–399, 2001. [DOI] [PubMed] [Google Scholar]
- Ni ZG, Gao DM, Benabid AL, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces a transient decrease of firing rate with no change in the firing pattern of neurons of the parafascicular nucleus in the rat. Neuroscience 101: 993–999, 2000. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Conway BA, Halliday DM, Perreault MC, Hultborn H. Organization of common synaptic drive to motoneurones during fictive locomotion in the spinal cat. J Physiol 569: 291–304, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci 32: 267–274, 2009. [DOI] [PubMed] [Google Scholar]
- Parajuli LK, Fukazawa Y, Watanabe M, Shigemoto R. Subcellular distribution of alpha1G subunit of T-type calcium channel in the mouse dorsal lateral geniculate nucleus. J Comp Neurol 518: 4362–4374, 2010. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Tonga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego: Academic, 1999. [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego: Academic, 2000. [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83: 117–161, 2003. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci 25: 1523–1531, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. (editors). The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. New York: Oxford University Press, 1991. [Google Scholar]
- Pourcher E, Gomez-Mancilla B, Bedard PJ. Ethosuximide and tremor in Parkinson's disease: a pilot study. Mov Disord 7: 132–136, 1992. [DOI] [PubMed] [Google Scholar]
- Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of Parkinsonism. Eur J Neurosci 27: 1647–1658, 2008. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of Parkinsonism. J Neurosci 20: 8559–8571, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TH, Clements MA, Wichmann T. Parkinsonism-related features of neuronal discharge in primates. J Neurophysiol 110: 720–731, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Rothblat DS. Alterations in intralaminar and motor thalamic physiology following nigrostriatal dopamine depletion. Brain Res 742: 25–33, 1996. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res 63: 1–20, 1986. [DOI] [PubMed] [Google Scholar]
- Shin HS, Cheong EJ, Choi S, Lee J, Na HS. T-Type Ca2+ channels as therapeutic targets in the nervous system. Curr Opin Pharmacol 8: 33–41, 2008. [DOI] [PubMed] [Google Scholar]
- Shipe WD, Barrow JC, Yang ZQ, Lindsley CW, Yang FV, Schlegel KA, Shu Y, Rittle KE, Bock MG, Hartman GD, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Uebele VN, Nuss CE, Connolly TM, Doran SM, Fox SV, Kraus RL, Marino MJ, Graufelds VK, Vargas HM, Bunting PB, Hasbun-Manning M, Evans RM, Koblan KS, Renger JJ. Design, synthesis, and evaluation of a novel 4-aminomethyl-4-fluoropiperidine as a T-type Ca2+ channel antagonist. J Med Chem 51: 3692–3695, 2008. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience 44: 45–73, 1991. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV. The thalamus. In: Neuroscience in Medicine, edited by Conn PM. Totowa, NJ: Humana, 2008. [Google Scholar]
- Smith Y, Seguela P, Parent A. Distribution of GABA-immunoreactive neurons in the thalamus of the squirrel monkey (Saimiri sciureus). Neuroscience 22: 579–591, 1987. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate Parkinsonism: comparison of the effects of MPTP-induced Parkinsonism and lesions of the external pallidal segment. J Neurosci 24: 6417–6426, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CH, Yang YC, Pan MK, Huang CS, Kuo CC. Modulation of subthalamic T-type Ca(2+) channels remedies locomotor deficits in a rat model of Parkinson disease. J Clin Invest 121: 3289–3305, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19: 1895–1911, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Raju DV, Hall RA, Smith Y. GABA(B) receptors in the centromedian/parafascicular thalamic nuclear complex: an ultrastructural analysis of GABA(B)R1 and GABA(B)R2 in the monkey thalamus. J Comp Neurol 496: 269–287, 2006. [DOI] [PubMed] [Google Scholar]
- Weyand TG, Boudreaux M, Guido W. Burst and tonic response modes in thalamic neurons during sleep and wakefulness. J Neurophysiol 85: 1107–1118, 2001. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res 125: 397–409, 1999. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in Parkinsonism. J Neurophysiol 95: 2120–2133, 2006. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Thompson AD, Brogan JT, Schulte ML, Melancon BJ, Mi D, Lewis LM, Zou B, Yang L, Morrison R, Santomango T, Byers F, Brewer K, Aldrich JS, Yu H, Dawson ES, Li M, McManus O, Jones CK, Daniels JS, Hopkins CR, Xie XS, Conn PJ, Weaver CD, Lindsley CW. The discovery and characterization of ML218: a novel, centrally active T-type calcium channel inhibitor with robust effects in STN neurons and in a rodent model of Parkinson's disease. ACS Chem Neurosci 2: 730–742, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZQ, Barrow JC, Shipe WD, Schlegel KA, Shu Y, Yang FV, Lindsley CW, Rittle KE, Bock MG, Hartman GD, Uebele VN, Nuss CE, Fox SV, Kraus RL, Doran SM, Connolly TM, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Marino MJ, Graufelds VK, DiLella AG, Reynolds IJ, Vargas HM, Bunting PB, Woltmann RF, Magee MM, Koblan KS, Renger JJ. Discovery of 1,4-substituted piperidines as potent and selective inhibitors of T-type calcium channels. J Med Chem 51: 6471–6477, 2008. [DOI] [PubMed] [Google Scholar]
- Zirh TA, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during Parkinsonian tremor. Neuroscience 83: 107–121, 1998. [DOI] [PubMed] [Google Scholar]