Abstract
Counterstimuli such as scratching, pinching, noxious heat and cold, and innocuous cooling and warming have been shown to inhibit itch in humans. In the present study, the effects of each of these counterstimuli were determined on baseline firing rates and on sustained pruriceptive responses of rat trigeminothalamic tract neurons. We found that scratching had little, if any, effect on baseline firing levels but greatly reduced mean pruriceptive firing following scratching for nearly 1 min. None of the other noxious or innocuous counterstimuli significantly inhibited pruriceptive responses. Our results indicate that scratching, but not other counterstimuli, significantly reduces itch-induced responses of trigeminothalamic tract neurons.
Keywords: itch, pain, pruriception, scratching
counterstimuli are strong, frequently noxious stimuli that reduce itch (Ward et al. 1996). Scratching the skin is the most commonly employed counterstimulus that reduces or eliminates itch (Greaves and Wall 1996; Vierow et al. 2009). Other forms of noxious mechanical stimuli, including pricking or pinching skin, also can reduce or eliminate itch in human subjects (Chapman et al. 1960; Graham et al. 1951). In addition, application of either noxious cold (Fruhstorfer et al. 1986; Melton and Shelly 1950; Mochizuki et al. 2003; Murray and Weaver 1975; Simone et al. 1991; Yosipovitch et al. 2005) or noxious heat (Ward et al. 1996; Yosipovitch et al. 2005, 2007) has been shown to reduce itch. Mixed effects of noxious heat on itch also have been reported; immersion of itchy skin in 45°C water reduced itch in two-thirds of subjects, whereas itch was increased in the remaining subjects (Fruhstofer et al. 1986). Electrical stimulation at levels sufficient to induce pain also can reduce itch (Bickford 1938; Nilsson et al. 1997; Ward et al. 1996). Finally, application of the pain-inducing chemical capsaicin reduces histamine-induced itch in humans (Brull et al. 1999). These findings suggest that many forms of noxious stimulation can reduce itch.
The effects of innocuous thermal stimuli on itch also have been examined in human subjects. Cormia and Kuykendall (1953) examined the effects of altered skin temperatures on thresholds for itch induced with various concentrations of histamine. They found that lower concentrations of histamine caused itch in skin warmed to 39°C, whereas cooling the skin to 25°C increased itch thresholds.
Seemingly conflicting evidence exists related to the effects of cooling on itch. Bromm et al. (1995) found that gently cooling the skin to a mean of 29.7°C reduced itch induced with histamine, as did topical application of menthol, which induced the sensation of cooling without lowering skin temperature. These results suggest that activation of circuitry underlying cooling sensation, through lowering of skin temperatures or application of menthol, reduces itch in humans. On the other hand, cooling skin to 25°C has been shown to increase itch induced with histamine (Pfab et al. 2006; van de Sand et al. 2015).
Several electrophysiological studies have examined the effects of counterstimulation on responses of spinal cord neurons to pruriceptive stimuli. In monkeys, pruriceptive responses of spinothalamic tract neurons induced with intradermal injections of histamine were strongly inhibited by scratching of cutaneous receptive fields (Davidson et al. 2009). The inhibition of firing was found to outlast the scratching for nearly 1 min. In mice, scratching the injection site and areas 5–17 mm away from injection sites potently reduced the pruritic responses of dorsal horn neurons (Akiyama et al. 2012). In rats, scratching of the receptive fields of neurons in the lumbar superficial dorsal horn significantly reduced responses to the pruritogen serotonin (Nishida et al. 2013). Thermal counterstimulation also has been shown to reduce pruriceptive responses of neurons. For example, pruriceptive responses of wide dynamic range neurons in the dorsal horn of rats were reduced by noxious cold stimuli (3°C) applied to the receptive field (Jinks and Carstens 1998). In a chronic dry skin model of itch in mice, ongoing activity in superficial dorsal horn neurons was reduced by scratching, pinching, and application of noxious heat (48–56°C) to receptive fields (Akiyama et al. 2011). The results from electrophysiological studies of the effects of counterstimuli to date indicate that various forms of counterstimuli can reduce pruriceptive responses of spinal cord neurons. In the studies described above that were performed in rats and mice, the axonal projections of the recorded neurons were not determined, making the role of the examined neurons in sensation uncertain.
In this study, the effects of a variety of mechanical and thermal counterstimuli were examined on pruriceptive responses of trigeminothalamic tract (VcTT) neurons with receptive fields on the cheeks of rats. Sensory processing related to the cheek of rodents is of particular interest because, unlike much of the rest of the body of rodents, injections of algogens and pruritogens into the cheek produce easily distinguished behavioral responses (Klein et al. 2011; Moser and Giesler 2014a; Shimada and LaMotte 2008). In rats, a large percentage of VcTT neurons are capable of transmitting pruriceptive information from the cheek (Moser and Giesler 2013, 2014b). The goal of this study was to examine the effects of counterstimuli on pruriceptive responses of VcTT neurons, cells likely to contribute ascending information that underlies the sensation of itch.
EXPERIMENTAL PROCEDURES
Animals.
Adult male Sprague-Dawley rats (300–450 g) were used according to protocols approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Animals were anesthetized with urethane (1.5 mg/kg ip; Sigma) and received supplemental injections as needed. A tracheotomy was performed to ease breathing. A laminectomy was performed over C1 and C2 vertebrae to allow recording of single units within the lower medulla and upper cervical spinal cord (Moser and Giesler 2014b). A craniotomy was performed over the thalamus, and the dura was removed from both the exposed brain and spinal cord.
Identification of VcTT neurons.
A low-impedance stainless steel stimulation electrode was positioned at stereotaxic coordinates for the ventroposterior medial (VPM) nucleus of the thalamus as determined by using a rat brain atlas (Paxinos and Watson 1982). Cathodal pulses (100–300 μA, 200 μs, 3 Hz) were delivered through the electrode as a search stimulus. A stainless steel recording electrode (∼10 MΩ; FHC) was lowered into the dorsal horn of the contralateral caudal medulla and C1 segment to record single-unit responses. All examined neurons met the following criteria for antidromic activation: 1) stable antidromic response latency (<0.05-ms variation), 2) ability to follow high-frequency (>300 Hz) antidromic stimulus trains, and 3) collision of orthodromic spikes with putative antidromic responses. Single-unit responses that met these criteria and had receptive fields on the cheek were used for further study. Action potentials were amplified and filtered (model DAM80; WPI) and then digitized and waveform discriminated using DAPSYS data acquisition software (http://www.dapsys.net).
Characterization of VcTT neurons.
Mechanical receptive fields of VcTT neurons were delineated using innocuous brushing and mild pinching. Neurons were functionally characterized using brushing with a soft-bristled brush, pressure with a weak arterial clip (7.4 bar), and pinch with a stronger arterial clip (15.4 bar) that produced pain when delivered to human skin. Each stimulus was applied for 10 s. Neurons characterized as low threshold (LT) responded maximally to brushing. High-threshold (HT) neurons were activated by pinching and by brushing at <1.5 spikes/s (Dado et al. 1994b). Wide-dynamic range (WDR) cells were activated by brushing (≥1.5 spikes/s) and at higher levels of firing by pinching (Dado et al. 1994b). Our previous studies have shown that only VcTT neurons classified as WDR or HT respond to pruritogens (Moser and Giesler 2014b). As a result, LT neurons were not further examined.
Pruritogens.
An intradermal injection of the vehicle solution (10 μl, 0.9% NaCl) was made within the receptive field. Its effects on neuronal firing were recorded for ≥5 min. Our previous studies have shown that intradermal injections of 5-HT activate a higher percentage of VcTT neurons than does any of several other pruritogens (Moser and Giesler 2014b). As a result, in the present studies, 5-HT (10 μl, 47 mM; Sigma) was the first pruritogen intradermally injected. Injections of this dose of 5-HT produce robust scratching and little wiping in awake rats (Klein et al. 2011; Moser and Giesler 2014a). Criteria for a response to injection of a pruritogen included 1) firing rates following injection of a pruritogen ≥150% of those seen during the 1 min preceding the injections, 2) firing rates following injection of a pruritogen ≥150% of those induced by injection of vehicle, and 3) firing rate increases endured for at least 1 min. The termination of a pruriceptive response was defined as the time at which firing returned to the mean baseline firing level. In one case, 5-HT failed to induce a response; for this neuron, the partial pruritogen histamine (10 μl, 900 mM; Sigma) was intradermally injected (Klein et al. 2011). All injections were separated by ≥5 mm within the receptive field. Each pruritogen was injected only once during the study of an individual neuron.
Counterstimuli.
Counterstimuli were applied to the receptive fields of pruriceptive VcTT neurons under baseline conditions both before and during responses to injection of pruritogens. Scratching stimuli were produced using a 7-mm length of a coping saw blade attached perpendicularly to a metal rod. This was lightly dragged by hand over the injection site at a rate ∼2.5 sweeps/s in a dorsal-to-ventral path for 10 s. Scratching was delivered at a force sufficient to reduce itch induced by application of cowhage in the experimenters and produce superficial abrasions on human skin (which disappeared within a few minutes). To determine the effects of noxious pinching on pruriceptive responses, an arterial clip (15.4 bar) was applied to the skin for 10 s within 5 mm of the injection site. A feedback-controlled Peltier device (3 × 3-mm contact surface; Yale Instruments) was used to apply thermal stimuli within 5 mm of the injection site. Five-second-duration thermal stimuli of 40°, 45°, and 50°C followed by 25°, 20°, 15°, and 10°C were separated by at least 1 min. Between stimuli, skin temperature was maintained at 30°C. The order of application of counterstimuli was scratching, pinching, and thermal stimuli of 40°, 45°, 50°, 25°, 20°, 15°, and 10°C.
Mapping of VcTT axonal projections.
The stimulating electrode was moved in a series of dorsal to ventral tracks within the thalamus contralateral to the recording point. Tracks were separated mediolaterally by 300 μm. The electrode was also moved rostrocaudally in 500-μm intervals and the process of tracking repeated to determine the most rostral region in the thalamus to which the axon projected. Lowest threshold points (≤30 μA) of each neuron were determined at all levels. Antidromic stimulation points with thresholds ≤30 μA have been shown to reflect the location of examined axons within 200 μm (Burstein et al. 1991; Dado et al. 1994a). The location of the most rostral projection of an axon was defined as the location of the most rostral LT points.
Histology.
Lesions (continuous anodal current, 10–15 s, 25 μA) were made at the LT and recording points at the conclusion of each experiment. Rats were perfused with 0.9% normal saline, followed by 10% formalin and 1% ferrocyanide, the latter to mark lesion sites with a Prussian blue reaction product. The borders of C1 and C2 segments were identified, and then the brain and the rostral spinal cord were dissected. A freezing microtome was used to section the brain at 75 μm and the spinal cord at 50 μm. Sections were mounted on glass slides, Nissl stained using neutral red, and coverslipped. A rat brain atlas (Paxinos and Watson 1982) was used to help identify thalamic nuclei.
Data analysis.
Spike times were collected and then binned in histograms (1-s bins) for display and analysis. Mean firing levels were determined during the 10-s period preceding application of the various counterstimuli, during the 5- or 10-s period in which counterstimuli were applied, and during the 10 s following the termination of counterstimuli. These were statistically compared. Standard errors of the mean are presented. The Wilcoxon signed rank test was used for all statistical calculations. Data found to differ at a level of P < 0.05 were considered to be significant.
RESULTS
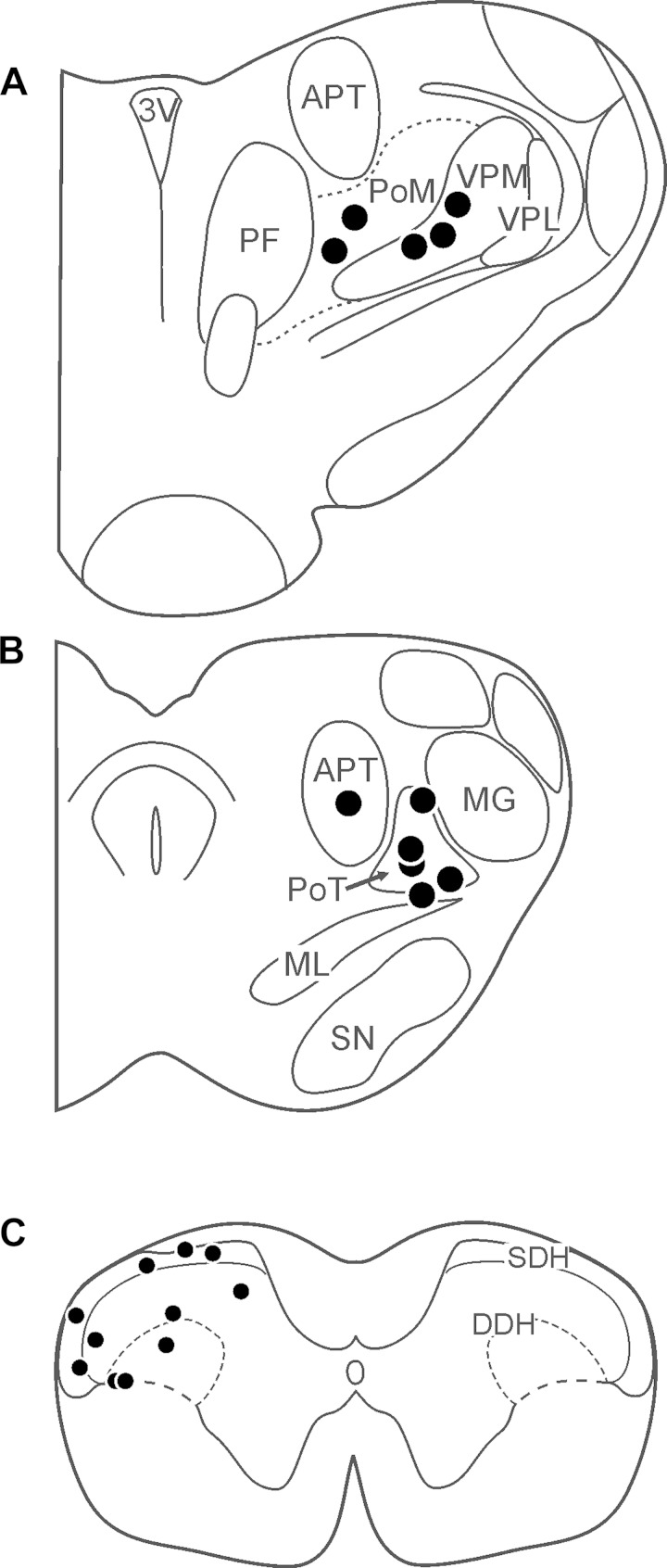
The effects of various counterstimuli on responses to pruritic stimuli were examined on 14 identified VcTT neurons. Ten were classified as WDR and four as HT. Lesions were recovered at low threshold points for antidromic activation of 11 neurons (Fig. 1, A and B); 5 were located within or on the border of the posterior triangular nucleus (PoT), 3 in the ventral posterior medial nucleus (VPM), 2 in the posterior medial nucleus (PoM), and 1 in the anterior pretectal nucleus. All 11 recovered recording points were located in the C1 segment; 5 were located in the superficial dorsal horn (SDH) and 6 in the deep dorsal horn (DDH; Fig. 1C). Seven neurons were sensitive to noxious cooling (5 WDR, 2 HT), and 8 neurons were sensitive to noxious heating (6 WDR, 2 HT) under baseline conditions.
Fig. 1.
A and B: locations of points in the thalamus at which antidromic activation was achieved using low-threshold (≤30 μA) current pulses. C: locations of points in C1 dorsal horn where examined trigeminothalamic tract (VcTT) neurons were recorded. 3V, third ventricle; APT, anterior pretectal nucleus; DDH, deep dorsal horn; MG, medial geniculate nucleus; ML, medial lemniscus; PF, parafascicular thalamic nucleus; PoM, posterior medial nucleus; PoT, posterior triangular nucleus; SDH, superficial dorsal horn; SN, substantia nigra; VPL, ventral posterior lateral nucleus; VPM, ventral posterior medical nucleus.
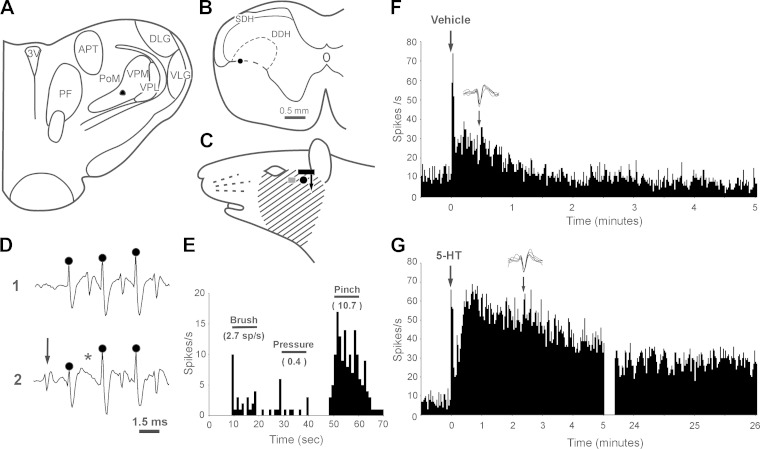
Figure 2 depicts the characteristics of a VcTT neuron that was activated by injection of the pruritogen 5-HT. The neuron was recorded in the DDH (Fig. 2B). Its axon was surrounded with antidromic stimulation tracks and appeared to terminate in the PoM nucleus (Fig. 2A). Its receptive field covered the cheek and lower face (Fig. 2C). The cell was activated by high-frequency antidromic stimulation (Fig. 2D1), and orthodromic action potentials (arrow in Fig. 2D2) collided with antidromic responses (asterisk in Fig. 2D2). This WDR neuron was activated by innocuous brush and pressure stimuli and more strongly by noxious pinch (Fig. 2E). An intradermal injection of vehicle (10 μl) caused a mild burst of firing that endured for less than 2 min (Fig. 2F). In contrast, an intradermal injection of 5-HT (10 μl) induced higher frequency firing that persisted for more than 26 min (Fig. 2G).
Fig. 2.
Identification of a pruriceptive VcTT neuron. A: lesion at low-threshold point in PoM. B: lesion at recording point in DDH of C1 segment. C: receptive field (cross hatched). Gray square indicates site of vehicle injection. Black circle indicates site of 5-HT injection. Black bar shows approximate size of scratching device; and arrow indicates direction of scratching. D: unit responses to high-frequency antidromic spike train (trace 1). Circles represent stimulus artifacts. Note collision of antidromic spike (asterisk) with orthodromic spike (arrow) in trace 2. E: responses to brush, pressure, and pinch. The neuron was classified as a wide-dynamic range (WDR) neuron. F: response to intradermal injection of vehicle. Five consecutive action potentials are depicted above histogram; arrow indicates period from which action potentials were sampled. G: sustained response to intradermal 5-HT injection. The roughly 22-min period in which counterstimuli were applied has been deleted from this record. Note that action potential size and shape are similar in F and G.
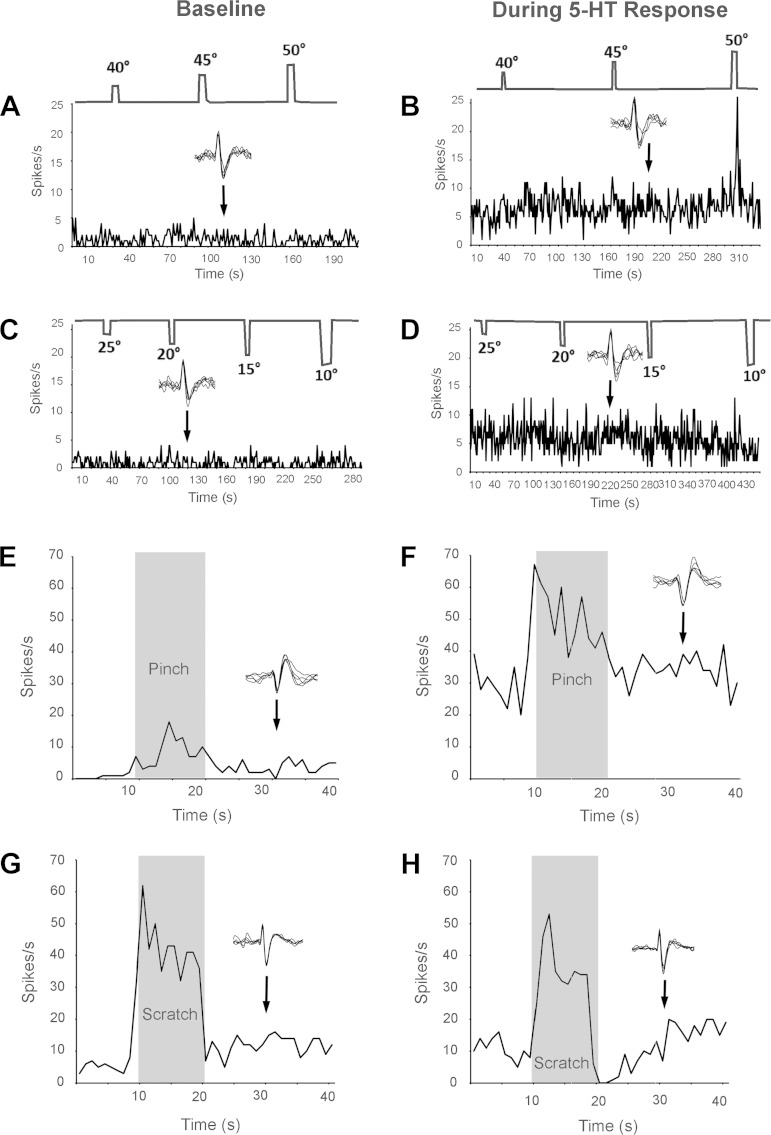
Figure 3 displays examples of the effects of various thermal and mechanical counterstimuli on the responses of pruriceptive VcTT neurons. Under baseline conditions (before injection of pruritogen), stimulating the receptive field with innocuous (40°C) and noxious (45°, 50°C) heat had no clear effect on activity of the neuron (Fig. 3A). In addition, the firing rates following the termination of these stimuli did not vary greatly from those seen immediately before thermal stimulation (Fig. 3A). During the pruriceptive response of the neuron, 40° or 45°C stimuli also failed to activate the neuron. Stimulation with 50°C activated the neuron during the pruriceptive response. However, firing levels following cessation of each of the heat stimuli did not vary greatly from those seen during the period preceding thermal stimulation (Fig. 3B).
Fig. 3.
Effects of various counterstimuli on activity of 3 pruriceptive VcTT neurons. A and C: stimulus temperature (top) and frequency histograms of neuronal activity (bottom) during baseline conditions. B and D: same histograms showing neuronal activity during response to pruritogen 5-HT. E and F: firing rate of a second neuron before, during (shaded rectangle), and after the termination of pinch during baseline condition (E) and during response to pruritogen 5-HT (F). Firing rate did not decrease after pinch during baseline or during pruriceptive response. G and H: firing rates of a third VcTT pruriceptive neuron before, during, and after scratching during baseline condition (G) and during pruriceptive response (H). During scratching, the neuron was strongly activated in baseline condition and during the response to 5HT. During the period after scratching, activity rates were increased in baseline condition compared with rates before scratching. However, during the pruriceptive response (H), firing rates were sharply reduced for about 10 s after scratching. Five consecutive action potentials are depicted above each histogram, indicating that the same neurons were examined during both conditions.
Under both baseline conditions and during the response to the pruritogen, innocuous (25°C) and noxious (20°, 15°C; Chery-Croze 1983; Fig. 3, C and D) cold stimuli failed to alter firing rates either during the stimuli or during the 10-s period that followed stimulation.
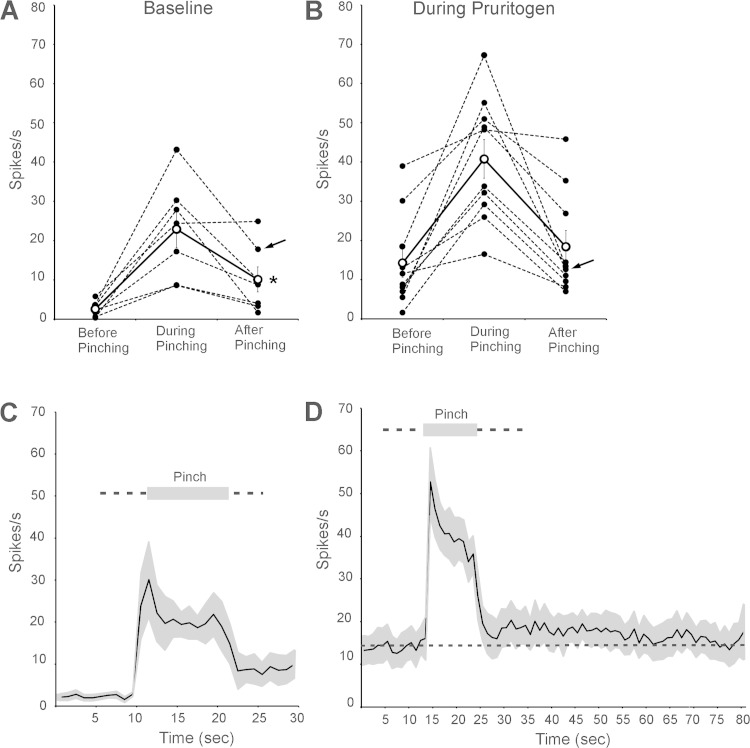
The effects of the counterstimulus of noxious pinching in the receptive field on firing levels of a second pruritogen-responsive VcTT neuron (same cell depicted in Fig. 2) are illustrated in Fig. 3, E and F. During baseline conditions and during the response to serotonin, pinch activated the neuron during the stimulus (Fig. 3, E and F; see Table 2) and also caused a small increase in firing following the pinch; that is, afterdischarge was induced (Fig. 3, E and F). No inhibition of firing was caused by pinch during baseline conditions or during the pruriceptive response of this neuron.
Table 2.
Effects of pinching and scratching counterstimuli on mean firing frequencies of VcTT neurons during baseline conditions and during responses to pruritogens
| Counterstimulus | Before (10 s) | During (10 s) | After (10 s) |
|---|---|---|---|
| Pinch (baseline) | 2.5 ± 0.7 | 22.9 ± 4.7 | 10.1 ± 3.2* |
| Pinch (during pruriceptive responses) | 14.3 ± 3.7 | 40.8 ± 4.9 | 18.4 ± 4.1 |
| Scratching (baseline) | 5.1 ± 2.4 | 24.8 ± 5.1 | 4.7 ± 1.7 |
| Scratching (during pruriceptive responses) | 14.6 ± 3.3 | 29.8 ± 4.1 | 8.3 ± 2.0* |
Values are mean firing levels (in spikes/s) during the 10-s periods before, during, and after mechanical counterstimuli. Pinch during baseline significantly increased firing. Scratching during pruriceptive responses significantly reduced the firing rate.
P < 0.05.
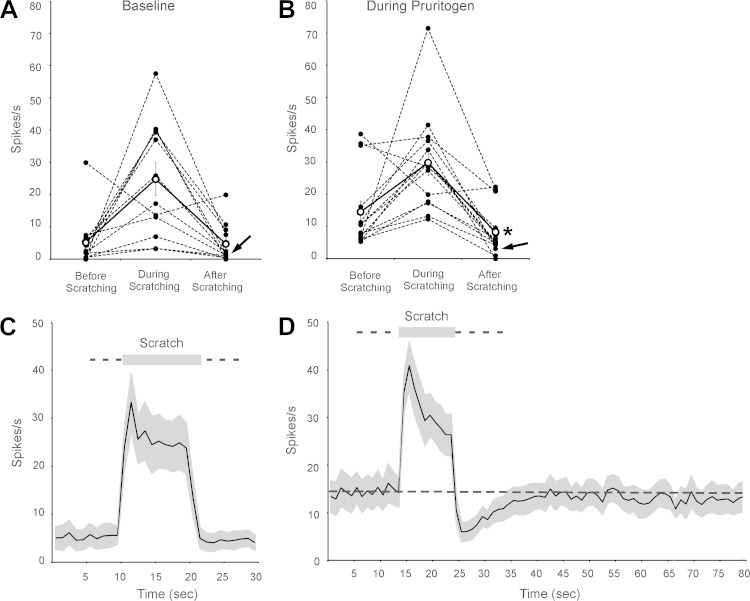
The effects of scratching clearly differed from those produced by the other tested counterstimuli. The effects of scratching the receptive field of another pruriceptive VcTT cell are shown in Fig. 3, G and H. During baseline conditions, a large increase in firing (Fig. 3G) was seen during scratching of the receptive field and a small increase in firing appeared to occur during the period following scratching. During the pruriceptive response, activity levels were again increased during the period of scratching. However, in contrast to the effects of scratching under baseline conditions, scratching greatly reduced firing levels following the end of scratching (Fig. 3H). Firing rate was reduced immediately following the termination of scratching to zero and returned to the level seen before scratching about 10 s following the end of scratching.
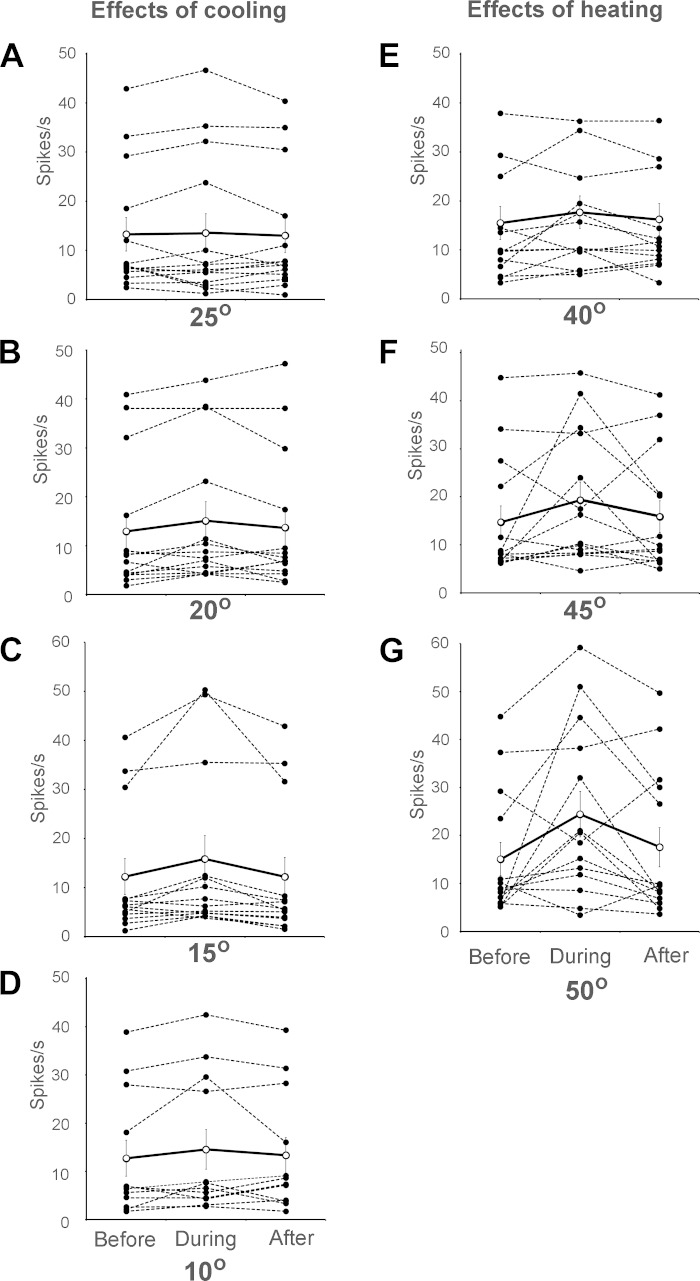
The effects of innocuous cooling, noxious cold, innocuous warming, and noxious heat stimuli on firing during pruriceptive responses of all examined VcTT neurons are illustrated in Fig. 4. None of the examined thermal counterstimuli significantly reduced mean firing levels during stimulus presentation under baseline conditions (Table 1) or during pruriceptive responses (Fig. 4, A–G). Mean firing levels during the 10-s period following each of the thermal stimuli did not significantly differ from firing levels seen during the 10-s period preceding stimulus presentation (Fig. 4, A–G).
Fig. 4.
Effects of cooling (A and B), noxious cold (C and D), warming (E), and noxious heat (F and G) within receptive fields on activity levels of VcTT neurons during responses to pruritogen. Mean firing rates for individual VCTT neurons are indicated before, during, and after the 10 s following changes in temperature (dashed lines, filled circles). Mean responses for each temperature are indicated (solid line, open circles). None of the examined temperatures significantly reduced firing levels of the neurons during or after application of thermal stimuli (n = 13 for 15°C, 12 for 10°C, and 14 for all other temperatures).
Table 1.
Effects of warm, noxious heat, cool, and cold counterstimuli on mean firing of VcTT neurons during baseline conditions
| Temperature, °C |
|||||||
|---|---|---|---|---|---|---|---|
| 40° | 45° | 50° | 25° | 20° | 15° | 10° | |
| Before | 5.6 ± 1.1 | 5.5 ± 1.4 | 5.3 ± 1.2 | 5.1 ± 1.3 | 4.2 ± 1.4 | 4.8 ± 1.4 | 6.7 ± 2.0 |
| During | 6.5 ± 1.0 | 7.6 ± 1.4 | 10.2 ± 2.5 | 6.1 ± 1.4 | 7.5 ± 1.8 | 8.8 ± 2.3 | 11.7 ± 2.9 |
| After | 6.3 ± 1.3 | 6.2 ± 1.3 | 7.0 ± 1.9 | 5.1 ± 1.2 | 4.9 ± 1.3 | 5.0 ± 1.7 | 6.6 ± 2.1 |
Values are mean firing levels (in spikes/s) of trigeminothalamic tract (VcTT) neurons before, during, and after thermal counterstimuli. Note that none of the counterstimuli reduced firing levels following stimulation below those seen before application of counterstimuli.
Figure 5 illustrates the effects of the noxious mechanical counterstimulus pinching within receptive fields on the firing levels of the examined VcTT neurons under baseline conditions and during responses to pruritogens. Mean firing levels during the 10-s periods before, during, and after the termination of pinch are presented in Table 2. Firing during the period after pinch was terminated was significantly greater than that observed before pinch (Fig. 5A), likely reflecting afterdischarge produced by pinching (n = 7). During responses to pruritogens, the mean firing level seen after the cessation of pinching did not significantly differ from the mean firing level during the period that preceded pinching (Table 2; Fig. 5B, n = 10). Therefore, the counterstimulus pinching did not reduce mean firing levels of VcTT neurons during baseline conditions or during pruriceptive responses. Figure 5C illustrates the time course of mean firing rates of the examined neurons before and after pinching under baseline conditions. Under these conditions, the examined neurons were activated during pinching, and firing during the period after pinching was increased to levels roughly three times those seen before pinching. Figure 5D depicts the time course of the effects of pinching on firing rate during pruriceptive responses. Firing levels after pinching remained greater than firing levels observed before pinching (indicated by dashed line) for more than 50 s.
Fig. 5.
A: effects of pinching on activity of VcTT neurons under baseline conditions [5-HT, n = 6; histamine (HA), n = 1]. B: effects of pinching during responses to pruritogens (5-HT, n = 9: HA, n = 1). Arrows indicate firing levels of the neuron responsive to HA; all other neurons were activated by 5-HT. Firing levels significantly increased (asterisk) following the end of pinch in baseline condition. Mean firing levels were not reduced, even during pruriceptive responses. C: time course of effects of pinching on mean firing levels of VcTT neurons during baseline conditions. SE are indicated in gray. Mean firing levels were ∼3-fold greater after pinching than before pinching. D: time course of effects of pinching on mean firing levels during responses to pruritogens. Mean firing level before pinching is indicated with dashed line. Mean firing levels after pinching remained elevated (above the dashed line) for most of the 55 s recorded.
Figure 6 illustrates the effects of scratching within receptive fields on the firing levels of the VcTT neurons under baseline conditions and during responses to pruritogens. Mean firing levels during the 10-s periods before, during, and after the termination of scratching are presented in Table 2. During baseline conditions, firing levels were increased in 11 of the 12 neurons examined. One, an HT neuron, was inhibited during scratching but not during the 10 s after scratching. The mean firing rates after scratching were virtually identical to those seen before scratching (Table 2; Fig. 6A); three neurons appeared to increase firing during the 10-s period that followed the termination of scratching, firing of four neurons appeared to decrease slightly, and five neurons did not change firing greatly (i.e., change <2.0 spikes/s, Fig. 6A). In contrast, during responses to pruritogens, the mean firing rate of 12 of 14 examined neurons decreased after the cessation of scratching, firing of one increased, and firing of one did not change greatly (Fig. 6B). During responses to pruritogens, the mean firing rate after scratching was significantly lower than that seen before scratching (Table 2; Fig. 6B). Figure 6C illustrates the time course of mean firing rates of all examined neurons before and after scratching under baseline conditions. Under these conditions, the examined neurons were activated during scratching, but firing during the period that followed scratching was virtually identical to the firing levels observed during the period that preceded scratching. Figure 6D depicts the time course of the effects of scratching on firing rate during pruriceptive responses. Note that the firing levels before scratching (Fig. 6D) were roughly three times that seen before scratching in baseline conditions (Fig. 6C), reflecting the robust excitatory response to pruritogens. During responses to pruritogens, scratching activated the neurons but sharply inhibited firing after scratching ended, initially nearly to levels observed before injections of pruritogens (Fig. 6C). Firing levels did not return fully to those seen before scratching (indicated by dashed line) for more than 50 s.
Fig. 6.
Effects of scratching the receptive field on activity of VcTT neurons during baseline conditions and during responses to a pruritogen. A: effects of scratching on firing rates of VcTT neurons during baseline conditions (5-HT, n = 11; HA, n = 1). B: effects of scratching during responses to pruritogens (5-HT, n = 13; HA, n = 1). Arrows indicate firing levels of the neuron responsive to HA. Note the clear reduction of firing levels after the end of scratching compared with firing during the period that preceded scratching in 12 of 14 examined neurons. Mean firing after scratching was significantly reduced (asterisk). C: time course of effects of scratching on mean firing levels of VcTT neurons during baseline conditions. SE are indicated in gray. Mean firing levels before and after scratching were virtually identical. D: time course of effects of scratching on mean firing levels during responses to pruritogens. Mean firing levels before scratching is indicated with dashed line. Note that mean firing levels during response to pruritogen (before scratching) were ∼3-fold greater than those seen in the same cells before injection of pruritogen (C), reflecting the response to pruritogens. Mean firing rates were sharply reduced by scratching, to levels near those seen before injection of pruritogens (C). Full recovery from the inhibitory effects of scratching did not occur during the 55 s examined after scratching.
The mean rate of firing during scratching appeared to decline more rapidly during responses to a pruritogen (Fig. 6D) than it did during baseline conditions (Fig. 6C), possibly suggesting that the inhibition began during the 10-s period of scratching. However, slopes fitting the mean levels of firing during the two conditions did not differ significantly (t-test). Additional study is needed to determine precisely the latency of the inhibition produced by scratching.
DISCUSSION
A large number of psychophysical studies in humans have shown that scratching the skin or application of other noxious mechanical or thermal counterstimuli reduce or eliminate itch. Our intent was to determine the effects of these counterstimuli on pruriceptive responses of identified VcTT neurons in rats, a projection that is capable of contributing to itch generated from the skin of the cheek (Moser and Giesler 2013, 2014b).
Effects of scratching.
We found that firing frequencies of pruriceptive responses in rat VcTT neurons were significantly reduced during the period that followed scratching, often to levels equal to those seen during the period that preceded injections of pruritogens (i.e., baseline firing). This indicates that scratching completely eliminated pruriceptive responses in these cases, a finding that is consistent with the observation that scratching can block itch entirely. Scratching produced inhibition of pruriceptive responses that outlasted the period of scratching. We found that pruriceptive responses did not return to firing levels seen before scratching for the nearly 1-min period that was examined after the termination of 10 s of scratching. Vierow et al. (2009) showed that scratching strongly reduced itch induced in human subjects for roughly 10 s and that itch ratings continued to recover for the following 30 s, a time course similar to that of the inhibition of pruriceptive responses seen in this study. Similar effects of scratching, in both intensity and duration, were seen in our previous study of the effect of scratching on pruriceptive responses of primate spinothalamic tract neurons (Davidson et al. 2009). The similarity in the effects of scratching in this and in our previous study is another indication (Moser and Giesler 2013, 2014b) that the VcTT in rats shares a number of important functional properties related to coding of information for both pain and itch with those of primate spinothalamic tract neurons.
Scratching did not reduce mean levels of firing of VcTT neurons seen during baseline conditions (before injection of pruritogens). Previously, we found that scratching significantly increased responses of primate spinothalamic tract neurons to the algogen capsaicin (Davidson et al. 2009), a finding that suggested that the inhibition of pruriceptive responses by scratching was a state-dependent phenomenon. The failure of scratching to inhibit baseline firing in the current study is consistent with the idea that the inhibitory mechanisms underlying the effects of scratching are only engaged during pruriceptive responses.
Nishida et al. (2013) found that scratching on the site in which serotonin had been injected activated lumbar spinal cord neurons during scratching and inhibited the pruriceptive firing for at least 40 s following the scratching, a result that is very similar to those found in the present study. In contrast, Akiyama et al. (2011) found that scratching reduced pruriceptive responses of spinal cord neurons in mice only during the period of scratching.
Effect of other counterstimuli.
We also examined the effects of pinching, a second noxious mechanical counterstimulus, on pruriceptive responses of VcTT neurons. Pinch has been reported to reduce itch (Chapman et al. 1960; Graham et al. 1951) in human psychophysical studies. Akiyama et al. (2011) found that pinching strongly reduced pruriceptive responses of mouse spinal cord neurons. However, the observed inhibition stopped immediately following cessation of the pinch. Surprisingly, we found that noxious pinching did not inhibit mean pruriceptive responses of rat VcTT neurons either during the pinching or during the period that followed it.
An additional surprising result in the present study was that noxious heat (45° and 50°C) and cold (20°, 15°, and 10°C), stimuli that are also known to reduce itch in humans (Fruhstofer et al. 1986; Murray and Weaver 1975; Ward et al. 1996; Yosipovitch et al. 2005, 2007), failed to reduce mean pruriceptive firing levels during or after application of the thermal stimuli.
The effects of innocuous cooling and warming of the receptive fields on baseline firing and on pruriceptive responses of rat VcTT neurons were also examined. Cormia and Kuykendall (1953) reported that warming the skin to 39°C reduced the concentration of histamine necessary to produce itch, indicating that warming potentiated itch. In contrast, Yosipovitch et al. (2005) found that warming the skin (41°C) did not affect the intensity of histamine-induced itch. We found that warming the skin of the receptive fields of VcTT neurons to 40°C failed to significantly affect pruriceptive discharges. Cormia and Kuykendall (1953) found that cooling the skin to 25°C increased the concentration of histamine necessary to produce itch, indicating that cooling inhibited itch. Bromm et al. (1995) found that a small decrease of skin temperature (29.7°C) was sufficient to reduce experimentally induced itch. These same authors showed that application of menthol to the skin, which induces the sensation of cooling without changing skin temperature, also reduces itch. These results suggest that activation of cooling afferent fibers either via reduced skin temperature or chemical activation can reduce itch in humans. Again, in contrast to previous psychophysical studies in humans, we found that cooling receptive fields of VcTT neurons to 25°C did not reduce pruriceptive discharges.
Methodological concerns.
The failure of counterstimuli other than scratching to inhibit pruriceptive responses raises concerns that the methods employed in this study were not ideal for revealing inhibitory effects of these counterstimuli. For example, it is possible that longer duration or more intense noxious stimuli would have produced inhibition. It should be noted, however, that the durations and intensities used presently were similar to those used in previous psychophysical studies in humans and electrophysiological studies in animals. It is also possible that the use of the anesthetic urethane in this study reduced the inhibitory effects of these counterstimuli, although scratching was found to produce potent inhibition of the same examined neurons. Another concern could be the site at which counterstimuli were applied in the current study. Our studies were focused on application of counterstimuli to the area in which pruritogens were injected. This was based on the observation that humans often appear to direct scratching and other counterstimuli to the area of the itch. Also, in our previous study in monkeys, scratching an area that included the receptive field produced strong inhibition of pruriceptive responses of lumbar spinothalamic tract neurons (Davidson et al. 2009). However, it has been shown that heating the skin 3 cm from the area of application of a pruritogen reduces itch in humans (Yosipovitch et al. 2007). In addition, it has been shown that scratching and pinching outside of the receptive fields of spinal cord neurons reduce pruriceptive responses in rats (Akiyama 2012). Additional studies are needed to address these concerns.
Effects of nociceptive counterstimuli on itch in animals.
Many species of mammals, including primates, rodents, and carnivores, can be observed scratching the skin, indicating that scratching relieves itch in these species. However, we are unaware of studies in any animals in which the effects of thermal or mechanical stimuli including warming, cooling, noxious heat, cold, or pinch have been shown to reduce behavioral responses to itch. To our knowledge, the only counterstimulus that has been shown to reduce itch in behavioral studies in animals is menthol. Kardon et al. (2014) found that topical application of menthol reduced scratching in mice. We did not examine the effects of menthol, or algogens such as capsaicin or mustard oil, because of the likelihood that they would produce long-duration changes in neuronal responses, making it difficult to examine their effects under baseline conditions and during responses to pruritogens. Behavioral studies of the effects of counterstimuli on pruriceptive responses in awake animals are needed.
Implications.
It is possible that future behavioral studies will establish that application of noxious pinching, noxious heat and cold, and innocuous warming and cooling reduce itch in experimental animals. Our results suggest that any relief from itch that might be observed in such studies focused on the rat cheek likely would not result from inhibition of VcTT neurons. A possible mechanism underlying relief from itch might be that counterstimuli activate inhibitory circuits that block pruriceptive firing at higher levels of the nervous system, for example, in the thalamus or cortex. There have been no studies examining the effects of noxious counterstimuli on single neurons in these regions of the brain. In fact, there have been no single-unit studies of pruriceptive processing by thalamic and cortical neurons. It is also possible that itch is induced by pruriceptive responses of several types of ascending tract neurons. We (Jansen and Giesler 2015) recently examined the responses of trigeminoparabrachial tract (VPbT) neurons to intradermal injections of pruritogens, including serotonin, within receptive fields on the cheek. We found that the pruriceptive responses of VPbT neurons more closely resemble, in duration and latency, behavioral responses of rats than do the responses of VcTT neurons. This result suggests that VPbT neurons contribute prominently to pruriception. It is possible that the pruriceptive responses of VPbT or other types of projection neurons contribute to itch sensation and that such responses are inhibited by cooling, warming, noxious pinching, heat, and cold counterstimuli.
Activation of inhibitory circuits.
Graham et al. (1951) concluded that relief produced by counterstimuli is produced by mechanisms within the central nervous system (CNS). Understanding of the areas of the CNS involved in producing the relief from itch caused by counterstimuli remains incomplete. It is likely that mechanisms in both the brain and spinal cord contribute. Akiyama et al. (2011) showed that removal of descending brain influences using cold block of the spinal cord reduced by about one-third the inhibitory effects of scratching on pruriceptive responses of spinal cord neurons, a finding that indicates strongly that the brain contributes to scratch-induced inhibition. Papoiu et al. (2013) found in studies using functional MRI that a number of brain areas in humans, including the anterior cingulate cortex and the midbrain central gray, are deactivated during scratching of the skin. We (Davidson and Giesler 2010) and others (Akiyama et al. 2011; Ross 2011) have speculated that inhibition of itch by scratching depends in part on activation of inhibitory interneurons within the spinal cord, interneurons that require simultaneous input from pruriceptors and mechanical nociceptors for activation. The current results showing that scratching generated inhibition only during pruriceptive responses supports the idea that activation of both pruriceptors and nociceptors is required to produce inhibition. However, the present results also suggest, at least for inhibition of VcTT neurons, that activation of nociceptors via noxious cold, heat, and pinching is insufficient for activation of the proposed inhibitory circuit. It is conceivable that primary afferent fibers exist that are activated by scratching and not by the other noxious counterstimuli employed in this study. If such fibers exist, and if it is proven that they feed into the proposed inhibitory circuit, it would help account for the present results. Alternatively, it is possible that scratching and the other counterstimuli activate overlapping populations of nociceptors but that scratching activates these fibers in patterns or frequencies that are required for activation of the inhibitory circuit. These possibilities need attention.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants P01 NS-047399 and NS-062158 (core funding).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.L. and G.J.G. conception and design of research; B.L. performed experiments; B.L. analyzed data; B.L. and G.J.G. interpreted results of experiments; B.L. and G.J.G. prepared figures; B.L. and G.J.G. drafted manuscript; B.L. and G.J.G. edited and revised manuscript; B.L. and G.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mr. H. Truong for valuable technical assistance and Drs. C. Honda and D. Simone for critically reading an early version of the manuscript.
REFERENCES
- Akiyama T, Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One 6: e22665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Misutoshi T, Carstens M, Carstens E. Site-dependent and state-dependent inhibition of pruritogen-responsive spinal neurons by scratching. Eur J Neurosci 36: 2311–2316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford R. Experiments relating to the itch sensation, its peripheral mechanism, and central pathways. Clin Sci 3: 377–386, 1938. [Google Scholar]
- Bromm B, Scharein E, Darsow U, Ring J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett 187: 157–160, 1995. [DOI] [PubMed] [Google Scholar]
- Brull S, Atanassoff P, Silverman D, Zhang J, LaMotte R. Attenuation of experimental pruritus and mechanically evoked dysesthesiae in an area of cutaneous allodynia. Somatosens Mot Res 16: 299–303, 1999. [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Cliffer KD, Giesler GJ Jr. Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. J Neurophysiol 66: 261–284, 1991. [DOI] [PubMed] [Google Scholar]
- Chapmann L, Goodell H, Wolff HG. Structures and processes involved in the sensation of itch. In: Advances in Biology of Skin, edited by Montagna W. NY: Pergamon, 1960, vol. 1, p. 161–188. [Google Scholar]
- Chery-Croze S. Relationship between noxious cold stimuli and the magnitude of pain sensation in man. Pain 15: 265–269, 1983. [DOI] [PubMed] [Google Scholar]
- Cormia F, Kuykendall V. Experimental histamine pruritus. II. Nature; physical and environmental factors influencing development and severity. J Invest Dermatol 20: 429–446, 1953. [PubMed] [Google Scholar]
- Dado RJ, Katter JT, Giesler GJ. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. I. Locations of axons antidromically activated from the thalamus and hypothalamus. J Neurophysiol 71: 959–980, 1994a. [DOI] [PubMed] [Google Scholar]
- Dado RJ, Katter JT, Giesler GJ. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. J Neurophysiol 71: 981–1002, 1994b. [DOI] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ Jr. The multiple pathways for itch and their interactions with pain. Trends Neurosci 33: 550–558, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov S, Simone D, Giesler GJ Jr. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci 12: 544–546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstofer H, Hermanns M, Latze L. The effects of thermal stimulation on clinical and experimental itch. Pain 24: 259–269, 1986. [DOI] [PubMed] [Google Scholar]
- Graham D, Goodell H, Wolff H. Neural mechanisms involved in itch, “itchy skin,” and tickle sensations. J Clin Invest 30: 37–49, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves MW, Wall PD. Pathophysiology of itching. Lancet 348: 938–940, 1996. [DOI] [PubMed] [Google Scholar]
- Jansen NA, Giesler GJ Jr. Response characteristics of pruriceptive and nociceptive trigeminoparabrachial tract neurons in the rat. J Neurophysiol 113: 58–70, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Skin cooling attenuates rat dorsal horn neuronal responses to intracutaneous histamine. Neuroreport 9: 4145–4149, 1998. [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber R, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82: 1–14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol 106: 1078–1088, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton F, Shelley W. The effect of topical antipruritic therapy on experimentally induced pruritus in man. J Invest Dermatol 15: 325–332, 1950. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain 105: 339–346, 2003. [DOI] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ Jr. Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci 33: 6093–6101, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ Jr. Itch elicited by intradermal injection of serotonin, intracisternal injection of morphine, and their synergistic interactions in rats. Neuroscience 274: 119–127, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ Jr. Characterization of pruriceptive trigeminothalamic tract neurons in rats. J Neurophysiol 111: 1574–1589, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray F, Weaver M. Effects of ipsilateral and contralateral counterirritation on experimentally produced itch in human beings. J Comp Physiol Psychol 89: 819–826, 1975. [DOI] [PubMed] [Google Scholar]
- Nilsson HJ, Levinsson A, Schouenborg J. Cutaneous field stimulation (CFS): a new powerful method to combat itch. Pain 71: 49–55, 1997. [DOI] [PubMed] [Google Scholar]
- Nishida K, Takechi Akiyama TK, Carstens MI, Carstens E. Scratching inhibits serotonin-evoked responses of rat dorsal horn neurons in a site- and state-dependent manner. Neuroscience 250: 275–281, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu A, Nattkemper LA, Sanders KM, Kraft RA, Yiong-Huak C, Coghill RC, Yosipovitch G. Brain's reward circuits mediate itch relief. A functional MRI study of active scratching. PLoS One 8: e82389, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1982. [Google Scholar]
- Pfab F, Valet M, Toelle TR, Athanasiadis GI, Behrendt H, Ring J, Darsow U. Short-term alternating temperature enhances histamine-induced itch: a biphasic stimulus model. J Invest Dermatol 126: 2673–2678, 2006. [DOI] [PubMed] [Google Scholar]
- Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol 21: 880–887, 2011. [DOI] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res 8: 271–279, 1991. [DOI] [PubMed] [Google Scholar]
- Van de Sand M, Sprenger C, Buchel C. BOLD responses to itch in the human spinal cord. Neuroimage 108: 138–143, 2015. [DOI] [PubMed] [Google Scholar]
- Vierow V, Fukuoka M, Ikoma A, Dorlfer A, Handwerker HO, Forster C. Cerebral representation of the relief of itch by scratching. J Neurophysiol 102: 3216–3224, 2009. [DOI] [PubMed] [Google Scholar]
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious, and innocuous counterstimuli on experimentally induced itch and pain. Pain 64: 129–138, 1996. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Duque M, Fast K, Dawn A, Coghill R. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol 156: 629–634, 2007. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Fast K, Bernhard J. Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J Invest Dermatol 125: 1268–1272, 2005. [DOI] [PubMed] [Google Scholar]