Abstract
Deep brain stimulation (DBS) is widely used for the treatment of movement disorders including Parkinson's disease, essential tremor, and dystonia and, to a lesser extent, certain treatment-resistant neuropsychiatric disorders including obsessive-compulsive disorder. Rather than a single unifying mechanism, DBS likely acts via several, nonexclusive mechanisms including local and network-wide electrical and neurochemical effects of stimulation, modulation of oscillatory activity, synaptic plasticity, and, potentially, neuroprotection and neurogenesis. These different mechanisms vary in importance depending on the condition being treated and the target being stimulated. Here we review each of these in turn and illustrate how an understanding of these mechanisms is inspiring next-generation approaches to DBS.
Keywords: deep brain stimulation, basic science of clinical practice, Parkinson's disease, tremor, dystonia, obsessive-compulsive disorder
deep brain stimulation (DBS) is the therapeutic use of chronic electrical stimulation of the brain via an implanted electrode. It is most commonly used to treat the motor symptoms of Parkinson's disease (PD), essential tremor, and dystonia, and it is in more limited use or under active investigation to treat a wide variety of neurological and psychiatric conditions including epilepsy, obsessive-compulsive disorder (OCD), and major depression (Table 1).
Table 1.
Deep brain stimulation indications and targets
Partial list of indications and targets for deep brain stimulation therapy, divided by approved and experimental indications. Within the approved indications, less well-validated targets are included in parentheses. Many of the experimental indications have been explored only in small series without randomization. Seminal clinical trials are referenced, and for investigational targets relevant reviews that summarize the clinical motivation and pilot clinical series are cited. ALIC, anterior limb of the internal capsule; ATN, anterior thalamic nucleus; CC, corpus callosum; Cg25, cingulate area 25 or subgenual cingulate; CM, centromedian nucleus of the thalamus; CN, caudate nucleus; CT, central thalamus; GPi, globus pallidus internus; ITP, inferior thalamic peduncle; LC, locus of caudate; LH, lateral hypothalamus; LoC, locus coeruleus; MB, mammillary bodies; NAc, nucleus accumbens; NBM, nucleus basalis of Meynert; PAG, periaqueductal gray; PH, posterior hypothalamus; PPN, pedunculopontine nucleus; STN, subthalamic nucleus; VC/VS, ventral capsule/ventral striatum; Vim, ventral intermediate nucleus of the thalamus; VMH, ventromedial hypothalamus; VPL, ventral posterolateral thalamus; VPM, ventral posteromedial thalamus; VTA, ventral tegmental area.
Neuropace RNS detects and stimulates at the seizure focus, customized to each patient.
The most commonly used DBS system uses a four-contact stimulating electrode stereotactically implanted in the target and connected via a subcutaneous wire to a pacemaker-like unit called an implantable pulse generator (IPG) that is placed on the chest wall underneath the collarbone. Electrodes are typically placed bilaterally, although clinical needs sometimes dictate unilateral stimulation. Most targets are deep brain structures (including deep white matter tracts) rather than cortical areas (Table 1). A clinician uses a handheld device to wirelessly communicate with the IPG to adjust the parameters of stimulation, tuning stimulation to maximize symptom relief and minimize side effects.
Here we review what is known about the effects of DBS on local and network neural activity and plasticity, how these effects are thought to result in clinical benefits and side effects, and how our understanding of these mechanisms is driving next-generation approaches to neuromodulation.
Time Course of DBS Clinical Effects
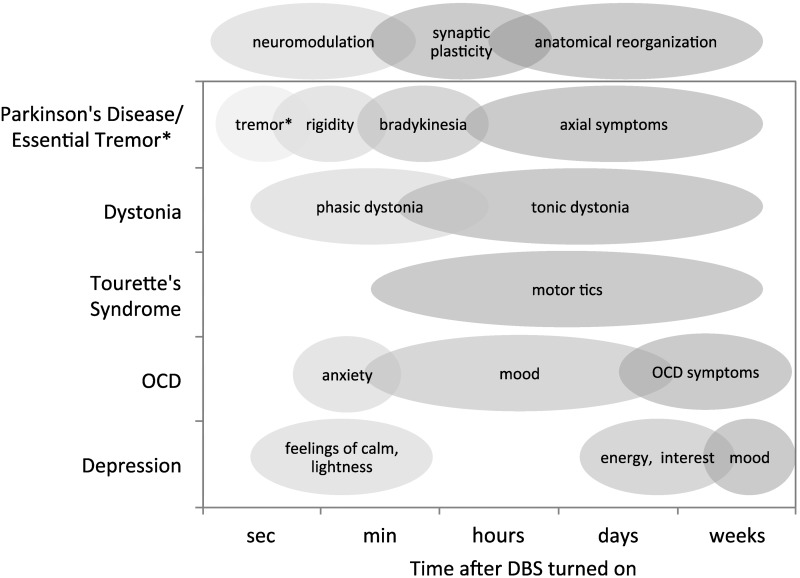
In the treatment of movement disorders and psychiatric disease, different symptoms characteristically respond to DBS with different time courses (Fig. 1) (Agnesi et al. 2013b). Ventral intermediate (Vim) thalamus DBS for essential tremor provides relief of tremor over seconds (Flora et al. 2010). Subthalamic nucleus (STN) DBS for PD provides relief of tremor over seconds (Blahak et al. 2009), relief of rigidity and bradykinesia over minutes to hours (Temperli et al. 2003), and relief of axial symptoms that is less profound and often delayed hours or days (Fasano et al. 2015). Similarly, the time course with which symptoms return when STN DBS is stopped mirrors the time course of symptom relief when stimulation is initiated (Temperli et al. 2003). In dystonia, globus pallidus internus (GPi) DBS can induce an early improvement in phasic dystonic movements, while tonic symptoms require months of DBS treatment to become fully realized (Krauss et al. 2004; Yianni et al. 2003). In subgenual cingulate gyrus DBS for the treatment of depression, investigators found that patients experienced immediate intraoperative effects of stimulation such as feelings of sudden calm or lightness, heightened awareness, and changes in positive and negative affect (Mayberg et al. 2005). These immediate effects were followed by subacute effects over days including improvements in interest and activity level and finally remission of disease with chronic stimulation in some patients (Mayberg et al. 2005). However, despite the success in some patients, two larger, randomized clinical trials of DBS targeting the anterior limb of the internal capsule (ALIC) and cingulate area 25 failed to show a lasting clinical benefit (Dougherty et al. 2015). In the case of DBS of the ALIC for OCD, investigators reported some immediate effects including improvement in mood and anxiety, although ultimately reduction in OCD symptoms evolved gradually over months (Greenberg et al. 2010; Tierney et al. 2014). In some cases, short-term responses to stimulation may not be predictive of eventual disease remission as has been described in thalamic and GPi DBS for Tourette's syndrome (Motlagh et al. 2013). Also, symptom onset and return do not always follow the same time course. For example, in Tourette's syndrome, DBS of the anterior-medial GPi or thalamic centromedian-parafascicular complex-ventral oral complex (CM-Pfc-Voa) results in improvement in tics over months (Sachdev et al. 2014; Servello et al. 2008). However, when thalamic DBS is turned off, tics may reappear rapidly (Servello et al. 2008).
Fig. 1.
Various disease symptoms exhibit different latencies in response to deep brain stimulation (DBS) treatment, supporting the theory that different mechanisms of DBS are responsible, including immediate neuromodulation effects, synaptic plasticity, and long-term effects that may involve anatomical reorganization. *DBS exerts a therapeutic effect on tremor within seconds, in both Parkinson's disease (PD) and essential tremor. In the case of depression, it has been reported that stimulation induced immediate positive subjective experiences that varied in individual patients, including feelings of calm, lightness, heightened awareness, etc. (Mayberg et al. 2005). Patients had improved interest, energy, and psychomotor speed within days of stimulation, but maximal improvements in mood, anxiety, sleep, and somatic symptoms were achieved after months of stimulation (Lozano et al. 2008; Mayberg et al. 2005). OCD, obsessive-compulsive disorder.
That symptoms respond to treatment on dramatically different timescales suggests that DBS is acting via several different mechanisms that have their own intrinsic time courses (Fig. 1). Symptoms that respond rapidly are mediated by rapidly reversible DBS mechanisms such as the immediate neuromodulation of pathological network activity. Symptoms that respond more slowly are at least in part mediated by longer-term mechanisms such as synaptic plasticity and ultimately anatomical remodeling (Agnesi et al. 2013b; Temperli et al. 2003).
Perielectrode Targets of DBS
Figure 2 illustrates the typical placement of a DBS electrode in the STN, the most commonly used DBS target for PD. The most effective site of STN stimulation appears to be in the dorsolateral STN or just dorsal to the STN in the zona incerta (ZI) and fields of Forel (Butson et al. 2011; but see Richardson et al. 2011). This white matter tract includes efferents from the STN/ZI (Parent and Hazrati 1995a), afferents from the pallidum (Groenewegen and Berendse 1990), hyperdirect projections from the cortex (Haynes and Haber 2013; Nambu et al. 1996, 1997), and fibers of passage including efferents from the pallidum to the thalamus (Severin et al. 1976) and the pedunculopontine nucleus (PPN) (Lee et al. 2000).
Fig. 2.

A: typical placement of a DBS electrode (Medtronic model 3387) in the subthalamic nucleus (STN) and zona incerta (ZI; green) near the thalamus (blue), substantia nigra (orange and yellow), and striatum/pallidum (red). Adapted with permission from Mai et al. (2008) (copyright Elsevier 2008). B: the electric field produced when in a “monopolar” configuration, in which a single electrode contact is the cathode and the implantable pulse generator (IPG) case, located distantly in the chest, is the anode. The field is roughly spherical in shape. C: electric field in a “bipolar” configuration in which the anode and cathode are both on electrode contacts. The bipolar configuration generates a more focused electric field concentrated between the anode and cathode. Using varied combinations of anodes and cathodes, the field of stimulation can be molded. Amg, amygdala; APr, anteroprincipal nucleus; Cl, claustrum; ec, external capsule; ex, extreme capsule; fx, fornix; GPe, globus pallidus externus; GPi, globus pallidus internus; H1, H2, fields of Forel; LV, lateral ventricle; MD, medial dorsal nucleus; opt, optic tract; PuV, ventral putamen; Rt, reticular nucleus; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; st, stria terminalis; TCd, tail of the caudate nucleus; TLV, tail of the lateral ventricle; VA, ventral anterior; VLA/VLP, ventrolateral anterior/posterior nucleus; VP, ventral pallidum; VTA, ventral tegmental area; VM, ventral medial nucleus; iml, internal medullary lamina.
The clinician has a limited ability to adjust the shape of the electrical stimulation field by adjusting the number and configuration of anodal (positive) or cathodal (negative) electrode contacts and the voltage or current of the stimulation (Fig. 2). In addition, the clinician can alter the duration of each charge-balanced pulse (called the pulse width) as well as the frequency of the pulses. Stimulator settings are chosen empirically to maximize benefit and minimize side effects. For example, whereas stimulation in the region of the dorsal STN and ZI is associated with relief of parkinsonian rigidity, bradykinesia, and tremor, stimulation of surrounding structures is thought to give rise to side effects including tonic muscular contractions and slurred speech (internal capsule), declines in executive function (ventral STN and its cortical connections), and mood disorders including mania, anxiety, and depression [ventral STN and substantia nigra pars reticulata (SNr)] (Kumar and Johnson 2011). Although these structure-effect relationships are used as rules of thumb to guide clinical programming, the precise neuroanatomical substrate for the clinical benefits and side effects of DBS remains an area of active investigation.
The subset of neural elements stimulated is more complicated than the electric field diagram suggests. Many factors influence which neural elements are stimulated (Montgomery 2010; for review see Brocker and Grill 2013). Stimulation acts predominantly on axons and dendrites near the electrode, rather than on the soma, which have substantially higher stimulation thresholds. The result is that a neuron whose soma is distant from the electrode may be more readily stimulated than one adjacent to the electrode if the former happens to have dendritic or axonal processes in close proximity to the electrode (Histed et al. 2009). Action potentials in axons propagate both orthodromically (away from the soma) and antidromically (toward the soma). Larger axons and those oriented perpendicularly to the electric field are activated more readily (i.e., at lower voltages and pulse widths) than smaller axons oriented parallel to the electric field (Montgomery 2010; Rattay 1999). In theory, nonsquare stimulation waveforms, conditioning pulses, and other modifications to stimulation parameters could offer greater efficiency and selectivity for specific neural elements (Foutz and McIntyre 2010; Hofmann et al. 2011; Wongsarnpigoon and Grill 2010). However, these remain largely untested in human subjects, as clinically available IPGs deliver only cathodal-leading, charge-balanced, square-wave pulses. Additionally, novel electrodes that allow stimulation fields that are asymmetric around the electrode, sometimes called current steering, may offer further control over the specific neural elements stimulated (Contarino et al. 2014; Martens et al. 2011).
DBS Acts as a Reversible Lesion
Initial hypotheses about the mechanism of DBS were based on the observed similarity between the effects of high-frequency stimulation and the effects of lesions in the same regions, i.e., pallidotomy for the treatment of PD (Guridi and Lozano 1997) or capsulotomy for the treatment of OCD (Jenike 1998). Because high-frequency stimulation had a therapeutic effect similar to that of ablative surgery, DBS was thought to function as a reversible lesion by inhibiting neurons near the stimulating electrode. Consistent with this idea, chemical inhibition of the STN or GPi reduced parkinsonian motor symptoms in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) primate model (Baron et al. 2002; Wichmann et al. 1994).
The reversible lesion hypothesis also fit well with the prevailing model of basal ganglia function. The cortical-basal ganglia-thalamo-cortical loop had been divided into a direct pathway (cortex-striatum-GPi/SNr-thalamus), which functioned to initiate and facilitate voluntary movement, and an indirect pathway [cortex-striatum-globus pallidus externus (GPe)-STN-GPi/SNr-thalamus] that inhibits movement (Parent and Hazrati 1995a, 1995b; Penney and Young 1983) (Fig. 3). D1-receptor-expressing striatal medium spiny neurons (MSNs) project primarily to the direct pathway, and D2-receptor-expressing MSNs project primarily to the indirect pathway (Alexander and Crutcher 1990). Dopaminergic input from the substantia nigra pars compacta (SNc) to the striatum increases activity in the direct pathway via D1 receptors and decreases activity in the indirect pathway via D2 receptors, facilitating movement (Gerfen et al. 1990). In addition to the motor system, multiple parallel circuits exist that are thought to subserve oculomotor, limbic, and associative functions but maintain this same fundamental organization (Alexander et al. 1986, 1990; Hoshi et al. 2005; Jung et al. 2014; Kelly and Strick 2004; Middleton and Strick 2000; Postuma and Dagher 2006).
Fig. 3.
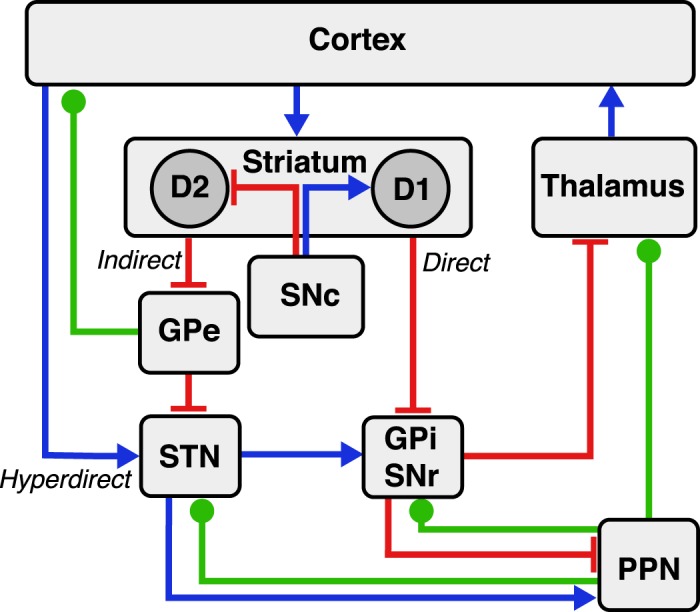
Cortico-basal-ganglia-thalamo-cortical circuitry. The direct, indirect, and hyperdirect pathways are indicated. Red lines denote inhibitory connections, blue lines denote excitatory connections, and green lines denote mixed cholinergic, GABAergic, and glutamatergic connections. Of note, the pedunculopontine nucleus (PPN) also exhibits anatomic projections to striatum and cortex (omitted for clarity).
Over the years, additional, functionally important pathways were recognized. The hyperdirect pathway consists of a direct excitatory input from the cortex to the STN (Nambu et al. 2000; Tokuno and Nambu 2000) and is thought to function in conflict-related response inhibition (Frank et al. 2007). The PPN is a part of the mesencephalic locomotor region that has reciprocal connections with the STN, GPe, GPi, and thalamus (Mena-Segovia et al. 2004) and is an experimental target of DBS therapy for PD with a potential role in the treatment of axial symptoms (Moro et al. 2010; Pereira et al. 2008; Stefani et al. 2007). Other projections, for example, the mixed GABAergic and cholinergic inhibitory projection from GPe to frontal cortex (Bolam et al. 1986; Furuta et al. 2004; Ingham et al. 1988; Sarter and Bruno 2002; Saunders et al. 2015), are also likely functionally important, although they have received less attention in pathophysiological models of movement and psychiatric disease.
In the classical model, movement disorders were conceptualized as hyperkinetic (e.g., hemiballism, dyskinesia, chorea) due to relative direct pathway overactivation or hypokinetic (e.g., PD) due to relative indirect pathway overactivation (DeLong 1990; Penney and Young 1983; Young and Penney 1989). The core validity of this model was confirmed in recent optogenetic experiments in a rodent model in which striatal direct or indirect pathway MSNs were selectively activated with resulting alleviation or exacerbation of parkinsonism, respectively (Kravitz et al. 2010). Similarly, a functional lesion induced by high-frequency DBS in either the STN or GPi would be expected to decrease GPi output to thalamus, promoting movement and alleviating parkinsonism.
Early experimental evidence supported the hypothesis that DBS inhibits neuronal activity at the site of stimulation. Intraoperative recordings in the GPi (Dostrovsky et al. 2000) and STN (Filali et al. 2004; Welter et al. 2004) of human subjects showed decreased neuronal spike rates during stimulation. This somatic inhibition may develop via several mechanisms. In vitro, high-frequency stimulation can cause sustained depolarization of neural membranes, inactivating sodium channels (Benazzouz et al. 1995; Beurrier et al. 2001; Magariños-Ascone et al. 2002) and increasing potassium currents (Shin et al. 2007) preventing the initiation or propagation of action potentials (i.e., depolarization block). Additionally, DBS may act through a synaptic mechanism, by activating inhibitory presynaptic terminals on afferents to the cell body. The time course of neuronal inactivation (100 ms after stimulation onset) during GPi stimulation supports the hypothesis that inhibition occurs through the release of the inhibitory neurotransmitter GABA from striatal and GPe afferents to the GPi (Dostrovsky et al. 2000). This would also explain the observation that DBS has been reported to cause local activation rather than inhibition in regions that have primarily excitatory input such as Vim (Dostrovsky and Lozano 2002) as well as for some STN neurons (Tai et al. 2003).
In contrast to the observation that soma near the stimulating electrodes is inhibited, evidence accumulated that axons and dendrites in the area of stimulation were activated, leading to an increase in the frequency of action potential output from the region of interest and representing a dissociation between neuronal somatic and axonal activity (Anderson et al. 2004; Dostrovsky et al. 2000; McIntyre et al. 2004b; Nowak and Bullier 1998; Vitek 2002). Computational models suggest that axons and dendrites have lower stimulation thresholds than soma, and so most somatic effects are probably due to propagation of stimulation effects from the membranes of its local arborization rather than on the soma itself (McIntyre et al. 2004a). In the MPTP parkinsonian primate model, neuronal activity in the GPi increased during clinically effective STN DBS, consistent with an increase in excitatory output from the STN (Elder et al. 2003). In humans undergoing STN DBS implantation, microdialysis during clinically effective DBS resulted in increased extracellular cGMP concentration in the putamen, GPi (Stefani et al. 2005, 2006, 2011), and SNr (Galati et al. 2006). Extracellular cGMP is an indirect marker of local glutamatergic synaptic input, consistent with stimulation increasing STN output (Fedele and Raiteri 1999). In a human subject with dystonia, stimulation in the GPi resulted in net inhibition of Voa thalamus (Montgomery 2006). There is also evidence that DBS induces action potentials in the passing afferent fibers that are in the region of stimulation (Anderson et al. 2004; Johnson et al. 2012; Sato et al. 2000), and in some cases these tracts are emerging as a principal DBS target. For example, the anterior and ventral internal capsule adjacent to the striatum is one target used for the treatment of OCD (Greenberg et al. 2010; Machado et al. 2009). Stimulation of the fields of Forel and the ZI dorsal to the STN appears to mediate at least some of the effects of DBS (Blomstedt et al. 2012; Butson et al. 2011; Plaha et al. 2006). DBS also elicits antidromic action potentials to cortex that have been observed with intracellular cortical recordings in rodents (Li et al. 2007) and by short-latency (∼1 ms) evoked potentials in human subjects stimulated in the STN or Vim thalamus (Baker et al. 2002; Walker et al. 2012a, 2012b), potentially altering local activity within those regions (Ashby et al. 2001; Baker et al. 2002; Li et al. 2007; MacKinnon et al. 2005). Whereas optogenetic inhibition of excitatory STN neurons in a parkinsonian [6-hydroxydopamine (6-OHDA) lesion] rat model did not have a therapeutic effect, high-frequency selective activation of afferent fibers terminating in the STN resulted in a robust therapeutic effect and a decrease in STN neuronal activity (Gradinaru et al. 2009).
An increase in the action potential output of a target region is not necessarily incompatible with the hypothesis that high-frequency stimulation is acting similarly to a lesion. A change in the mean action potential output of a brain region is only a very coarse representation of an area's function. Grill and others advanced the concept of an “informational lesion” whereby regularization of neural output from an area by high-frequency stimulation is equivalent to a lesion in that information normally contained in the time-varying neuronal activity cannot pass through the stimulated nucleus (Dorval et al. 2008; Grill et al. 2004). This hypothesis was recently explored in two studies in nonhuman primates in whom high-frequency stimulation of the GPi partially reduced encoding of joint kinematics in the GP and ventralis lateralis pars oralis (VLo) thalamus (Agnesi et al. 2013a) and completely inhibited GPi responses to electrical stimulation of the motor cortex (Nambu 2013).
However, regularization of neuronal activity does not obligatorily decrease information content in the network. For example, in the 6-OHDA rat model of parkinsonism, the induction of parkinsonism increased neuronal entropy while clinically effective DBS decreased entropy (Dorval and Grill 2014). A measure of directed entropy derived from multiunit recordings suggested that information transfer was decreased in the parkinsonian state (despite increased entropy) both between and within the GPi and SNr and that information transfer was partially recovered in the DBS ON state (despite decreased entropy). This fits within a computational framework whereby low-entropy (i.e., correlated or regular) activity is important for information transfer (Buehlmann and Deco 2010).
Neurochemical Effects of Stimulation
In addition to the local electrical effects of DBS, there are a myriad of other neurochemical changes including DBS-induced release of neurotransmitters locally and throughout the stimulated network. DBS of the anterior thalamus for the management of seizures may depend in part on stimulation-induced release of adenosine. In a rodent seizure model, stimulation increased adenosine release in the hippocampus and adenosine antagonists blocked the antiepileptic effect of DBS (Bekar et al. 2008; Miranda et al. 2014). Local, nonsynaptic generation of adenosine in the thalamus may also account for some of the antitremor effects of DBS (Bekar et al. 2008).
DBS in the caudate nucleus results in increased extracellular dopamine as measured in vivo via fixed potential amperometry, and stimulation of the dorsal STN or ZI also results in dopamine release in the caudate, presumably by stimulation of the nearby median forebrain bundle (Gale et al. 2013). The relevance of this finding to PD is uncertain given the relative paucity of intact dopamine fibers, although high-frequency stimulation of the STN or GPi can induce dopamine release detected by microdialysis in human subjects (Martinez et al. 2013; Zsigmond et al. 2012). Clinically, the effect of DBS on PD symptoms appears to be additive with the effect of levodopa, suggesting that DBS acts via a dopamine-independent mechanism (Piboolnurak et al. 2007). Additionally, symptoms of PD that worsen or are unresponsive to dopamine replacement in some subjects, such as dyskinesias or tremor, nevertheless can respond to DBS. In contrast, dopaminergic mechanisms may be relevant to the use of STN DBS for the treatment of cervical dystonia (Ostrem et al. 2011) or OCD (Mallet et al. 2008a). In a rodent obesity model, DBS of the nucleus accumbens (NAc) shell increased extracellular dopamine levels as well as D2 receptor gene expression (Zhang et al. 2015). In a rodent addiction model, NAc DBS led to decreased glutamate and increased GABA concentrations in the ventral tegmental area, NAc, and ventral pallidum in rats that had been exposed to morphine (Yan et al. 2013) and increased dopamine, serotonin, and norepinephrine concentrations in the prefrontal cortex (van Dijk et al. 2012), with less consistent effects on monoamines near the site of stimulation (van Dijk et al. 2011). DBS-mediated change in prefrontal monoamine signaling is a potential mechanism by which NAc DBS might alter symptoms of OCD, depression, addiction, and other neuropsychiatric disorders (Hirschfeld 2000).
Most preclinical and clinical studies of the neurochemical effects of DBS report only short-term effects of DBS over seconds or minutes. In contrast, neurochemical changes relevant to the chronic effects of DBS must last years. Developing technology to chronically assess CNS neurochemical state, and perhaps using this state as a signal for closed-loop control of DBS, is an area of active investigation (Grahn et al. 2014).
Role of Pathological Oscillations in Parkinson's Disease
Oscillations are a ubiquitous finding in normally functioning neural networks, are remarkably conserved across mammalian evolution (Buzsáki et al. 2013), and are thought to facilitate dynamic communication and plasticity between spatially disparate populations of neurons by temporally aligning the collective synaptic activity related to a particular neural process (Fries 2009). Rather than being composed of a single circuit with a characteristic oscillatory frequency, the brain is a complex combination of countless nested oscillators functioning in parallel and in series, including intrinsic cellular membrane oscillations, oscillations arising in local microcircuits, and long-range networks (Montgomery 2010).
Pathological oscillatory activity in sensorimotor loops between the cortex, basal ganglia, thalamus, and cerebellum is thought to contribute to the motor symptoms of PD, specifically tremor, bradykinesia, and rigidity. Oscillations in the beta frequency band (12–30 Hz) are of particular interest in PD. In healthy subjects, beta-band oscillations are observed throughout the brain but are most prominent in the sensorimotor cortex and associated regions of the thalamus, basal ganglia, and cerebellum (Courtemanche and Lamarre 2005). Beta-band oscillations are observed in the local field potential (LFP), in the coherence of the LFP across brain regions, in the synchronization of single neurons with the LFP, and in the synchronization between single neurons (Hammond et al. 2007). In the motor network, beta-band oscillations are greatest in the resting state or during tonic contraction and decrease during movement, where they are replaced by higher-frequency oscillations in the gamma (30–100 Hz) and higher (100–500 Hz) bands called high-frequency oscillations (HFOs) (Amirnovin et al. 2004; Brown 2007; Cassidy et al. 2002; Courtemanche et al. 2003; Doyle et al. 2005b; Jenkinson and Brown 2011; Labyt et al. 2005; Özkurt et al. 2011; Pogosyan et al. 2010). Computational models suggest that beta oscillations may transiently decrease the computational flexibility of the neural network, promoting maintenance of the status quo over new patterns of activity (Brittain et al. 2014).
In the healthy brain, beta oscillations occur in bursts lasting 200–600 ms (Courtemanche et al. 2003; Murthy and Fetz 1996; Spinks et al. 2008). In PD there is an increase in the coherence and spread of beta oscillations compared with healthy control subjects and patients with dystonia (but see Moshel et al. 2013; Pollok et al. 2012; Starr et al. 2005; Weinberger et al. 2006, 2012), and these beta oscillations persist during attempted movement (Doyle et al. 2005a; Little et al. 2012; Oswal et al. 2012). The increase in beta power is most robust in the sensorimotor basal ganglia (STN and GPi) but is also evident in the motor cortex (Crowell et al. 2012; de Hemptinne et al. 2013). Similar changes are seen in dopamine-depleted rodent and nonhuman primate models of PD (Bergman et al. 1994; Magill et al. 2001; Mallet et al. 2008b; McCairn and Turner 2009).
In addition to the increased prominence of beta oscillations, PD is associated with an increase in the entrainment of HFOs (phase-amplitude coupling) and single-neuron action potentials (spike-field coupling) to the local beta rhythm in the cortex, STN, and GPi (de Hemptinne et al. 2013, 2015; Schrock et al. 2009; Shimamoto et al. 2013) (Fig. 4). Although dynamic phase-amplitude coupling in motor cortex is a normal feature of motor cortex (Miller et al. 2012), it is exaggerated in PD compared with subjects with dystonia or epilepsy (de Hemptinne et al. 2013). Broadband gamma and high-frequency oscillations are thought to reflect the organization of local population spiking activity (Manning et al. 2009; Miller et al. 2009) and to have a central role in cortical computation (Fries 2009), although recent recordings in human STN showed that spiking activity was not correlated with changes in local HFO power in that nucleus (Yang et al. 2014). Taken together, these results suggest that elevated phase-amplitude coupling reflects the enslavement of local cortical computation to the pathological, sensorimotor circuit-wide beta oscillations, locking the network into a computationally ineffective state.
Fig. 4.
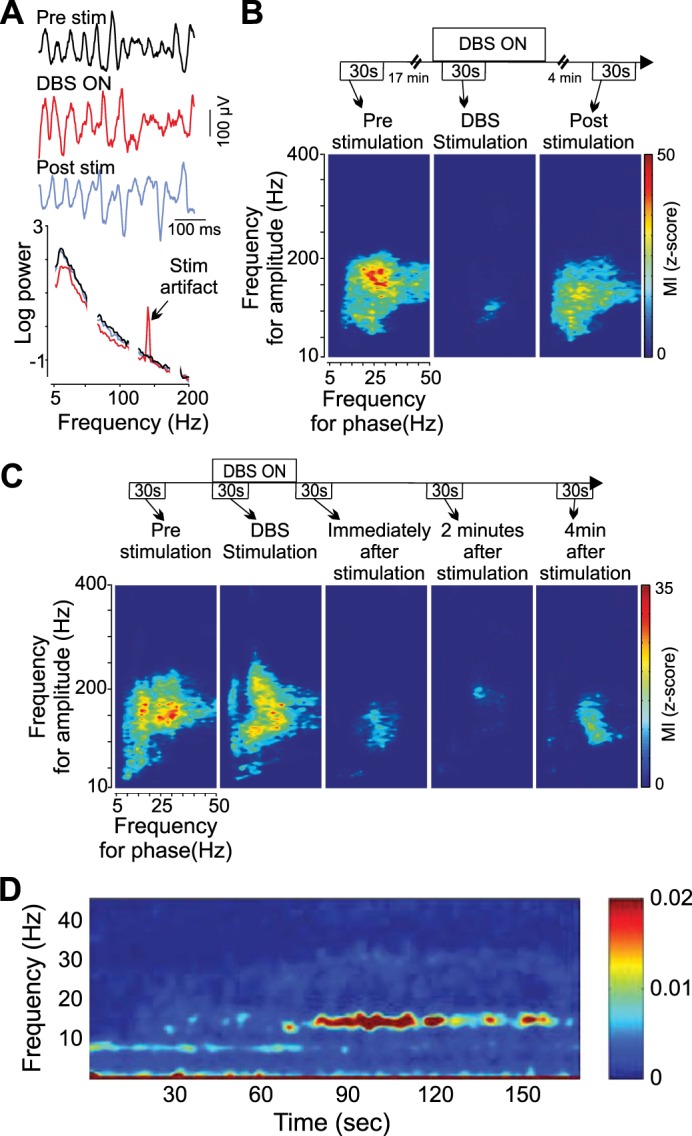
High-frequency STN DBS reduces phase-amplitude coupling in the cortex and beta power in the STN in humans with PD. A: motor cortex local field potentials (LFPs) before, during, and after STN DBS and log power spectral densities. DBS reduces the peak beta power along with a broadband decrease in low-frequency power. B: single-subject reduction in phase-amplitude coupling in motor cortex during DBS, which partially recovers within 4 min of DBS cessation. Phase-amplitude plots show how fluctuations in power as a function of frequency (y-axis) are entrained to background LFP phase and demonstrate that gamma-band power (100–200 Hz) is entrained to beta (15–30 Hz) phase. C: single-subject example showing prolonged (>4 min) suppression of phase-amplitude coupling after cessation of DBS. D: time-frequency plot of STN LFP beginning at cessation of high-frequency DBS. Beta oscillations are suppressed during DBS and reemerge over ∼1 min after DBS is stopped. Reproduced with permission from de Hemptinne et al. (2013) (A–C) and Kühn et al. (2008) (D).
Beta-band oscillations appear to emerge and are amplified within the parkinsonian cortico-basal ganglia circuit. Based on resonant amplification of evoked potentials, this circuit has a resonance frequency of ∼20 Hz in parkinsonian patients (Eusebio et al. 2009). Pharmacological lesion studies in parkinsonian nonhuman primates suggests that beta-band oscillations depend critically on both cortico-STN hyperdirect projections and reciprocal connections between the STN and GPe, as pharmacological blockade of any of these pathways attenuates beta-band oscillations (Nambu and Tachibana 2014; Tachibana et al. 2011). In contrast, blockade of striatal projections to GPe and GPi did not attenuate beta-band oscillations (Tachibana et al. 2011), although beta-band oscillations can be induced in a non-dopamine-depleted mouse by intrastriatal cholinergic agonists (McCarthy et al. 2011). M1 gamma activity precedes STN spiking, consistent with a role of M1 HFOs in driving basal ganglia spiking (Shimamoto et al. 2013). The beta-entrainment of this M1-STN coupling appears relatively specific for PD; while M1 gamma activity also precedes spiking in the STN of patients with dystonia, the gamma band activity and STN spiking were not phase locked to the beta rhythm (Shimamoto et al. 2013).
These oscillations, and the entrainment of high-frequency activity, correlate with the presence of motor symptoms between subjects and within subjects as a function of their medication state. Beta-band power in the STN and GPi decreases with levodopa medication, and this decrease correlates with the magnitude of clinical improvement in bradykinesia and rigidity (but not tremor) (Brown et al. 2001; Cassidy et al. 2002; Kühn et al. 2008, 2009; Ray et al. 2008; Weinberger et al. 2006; Williams et al. 2002). Phase-amplitude coupling in the STN is 100 times greater in amplitude in the OFF medication vs. the ON medication state, and HFOs exhibit greater perimovement phasic modulation in the ON medication state (López-Azcárate et al. 2010). Although changes in cortical beta power after levodopa administration have been somewhat inconsistent (Litvak et al. 2011; Melgari et al. 2014; Silberstein et al. 2005; Whitmer et al. 2012), levodopa robustly decreases phase-amplitude coupling in motor cortex (de Hemptinne et al. 2013; Shimamoto et al. 2013). Finally, STN HFO power inversely correlates with clinical severity in PD (Wang et al. 2014). In contrast, in a nonhuman primate progressive dopamine depletion model of PD, excessive synchronous oscillations in the pallidum emerged only after severe bradykinesia was observed, arguing that these pathological oscillations are not required for the expression of parkinsonism after dopamine depletion (Leblois et al. 2007).
Role of Pathological Oscillations in Tremor
Unlike for bradykinesia and rigidity in PD, beta-band oscillations and phase-amplitude coupling are not robustly linked to tremor in PD (Amirnovin et al. 2004; Kühn et al. 2008, 2009; Ray et al. 2008; Silberstein et al. 2003; Weinberger et al. 2006). However, unlike bradykinesia and rigidity, which are not inherently oscillatory phenomenon, the existence of tremor demands a neural oscillator. In both Parkinson's and essential tremor, even tremor in the ipsilateral arm and leg are not coherent (Raethjen et al. 2000), suggesting that multiple, parallel oscillators can occur within the motor circuit in the same hemisphere. Multiple neural structures have been found to oscillate at tremor frequency or its first harmonic (4–8 or 8–16 Hz) including motor cortex (Shimamoto et al. 2013; Timmermann et al. 2003), STN (Hirschmann et al. 2013; Reck et al. 2009; Rodriguez-Oroz et al. 2001, 2011; Shimamoto et al. 2013; Weinberger et al. 2009), GPi (Bergman et al. 1998; Hurtado et al. 1999; Magnin et al. 2000), and the cerebellar-receiving thalamus (Vim) (Hua and Lenz 2005; Lenz et al. 1994; Magnin et al. 2000). In essential tremor, similar oscillations are seen in Vim (Hua and Lenz 2005). However, for each of these observations the possibility remains that the tremor-synchronized activity is secondary to sensory feedback from the tremulous limb rather than the tremor generator per se.
The origin of the oscillatory activity remains uncertain, although the most prominent hypotheses center on cerebellothalamic bursting inputs that drive the thalamic tremor-synchronized oscillations, analogous to those recorded in the cat harmaline model of essential tremor (de Montigny and Lamarre 1973). In an fMRI study of Parkinson's tremor, activity in the cerebellar-thalamic circuit correlated closely with tremor amplitude (Helmich et al. 2011). In contrast, the presence of tremor in PD was most closely associated with pallidal (but not striatal) dopamine depletion, suggesting a model whereby pallidal dopamine depletion allows for tremor-driving oscillations to arise in the cerebellothalamic circuit.
DBS Disrupts Pathological Oscillations to Treat Tremor, Rigidity, and Bradykinesia
Several lines of evidence suggest that disruption of beta-band oscillations underlies some of the DBS effect on bradykinesia and rigidity. Placement of the DBS electrode in an area of coherent beta oscillations recorded during DBS electrode implantation is predictive of subsequent clinical response to DBS (Zaidel et al. 2010). High-frequency STN DBS suppresses local beta oscillations in a manner that lingers after DBS cessation, analogous to DBS clinical effects (Bronte-Stewart et al. 2009; Eusebio et al. 2011; Giannicola et al. 2010; Kühn et al. 2008; Wingeier et al. 2006) (Fig. 4). High-frequency DBS of the STN also decreases beta-band oscillations and phase-amplitude coupling in the GPi (Brown et al. 2004), motor cortex (de Hemptinne et al. 2013, 2015; Devos et al. 2004; Silberstein et al. 2005), and STN-cortical beta coherence (Kühn et al. 2008) (Fig. 4). GPi DBS reduced beta oscillations in the GPi (Bar-Gad et al. 2004; McCairn and Turner 2009) and motor cortex (McCairn and Turner 2015). In contrast to high-frequency stimulation, STN stimulation at beta frequencies, which increases beta-band oscillations (Brown et al. 2004), worsens bradykinesia (Chen et al. 2007; Eusebio et al. 2008).
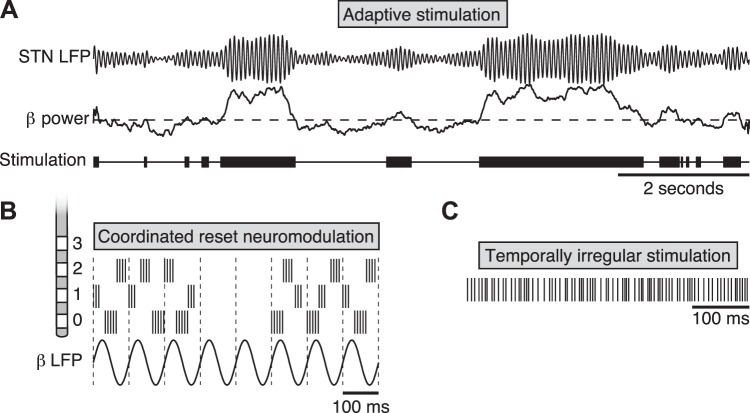
If abnormal oscillatory activity is at the root of Parkinsonian motor symptoms, then stimulation patterns designed specifically to disrupt this oscillatory activity should be at least as good as, if not superior to, continuous, high-frequency DBS. Little et al. tested this directly in eight PD patients who had been acutely implanted with externalized DBS electrodes, which allowed for application of stimulation in response to real-time measurements of beta-oscillation power. Delivery of high-frequency STN DBS triggered on an STN beta power threshold set to achieve 50% stimulation on time resulted in a 50% improvement in blinded motor ratings compared with traditional, continuous high-frequency stimulation and was superior to an randomly delivered stimulation with a similar on time (Little et al. 2013) (Fig. 5). It remains to be seen whether similar closed-loop strategies will perform in ambulatory subjects, given the natural beta fluctuations associated with movement (Quinn et al. 2015).
Fig. 5.
Novel approaches to DBS. A: adaptive or closed-loop stimulation. Top: simulated STN LFP data showing beta-band power fluctuating over time. In adaptive or closed-loop stimulation, electrical stimulation (bottom) is delivered only when a control signal, in this case beta-band power (middle), is elevated above a threshold (Little et al. 2013). Other potential control signals in PD include phase-amplitude coupling in motor cortex or the basal ganglia or M1 spiking (Little and Brown 2012). B: coordinated reset neuromodulation (Adamchic et al. 2014). Short bursts of 3–5 stimulation pulses at 130 Hz are delivered in a staggered fashion to 3 adjacent contacts of the STN DBS electrode (labeled 0, 1, and 2), with stimulation times staggered to 3 different phases of the dominant low-frequency rhythm (in this case, 10 Hz). Contacts are stimulated in a random order such that each contact is stimulated in an aperiodic fashion. Cycles of stimulation on and off are cycled in a 3-to-2 ratio that computational studies suggest is optimal for facilitating desynchronization of the population, which evolves during the off periods (Lysyansky et al. 2011). C: temporally irregular stimulation (here, a log-uniform distribution of instantaneous spike rates between 90 and 380 Hz) is superior to temporally regular stimulation in one study of bradykinesia in PD (Brocker et al. 2013) but inferior for the suppression of tremor (see text for discussion) (Birdno et al. 2012).
In an MPTP primate model of PD, short (∼50 ms) bursts of 130-Hz stimulation were delivered to the GPi triggered on single-neuron action potentials in motor cortex (Rosin et al. 2011). By measures of movement speed and tremor, this cortex-triggered DBS was superior to continuous, high-frequency stimulation of the GPi. Importantly, this effect critically depended on the latency between the cortical action potential and the GPi stimulation. Latencies of 10, 20, or 40 ms were ineffective, whereas a latency of 80 ms was highly effective. Stimulation at 80-ms latency uniquely reduced oscillatory power in the 9–15 Hz frequency range in both cortex and GPi and reduced firing rates in the GPi. This result offers compelling evidence that the precise timing of stimulation relative to the underlying oscillations was critical to the clinical effect and indirectly supports the importance of cortical input in driving pathological low-frequency oscillations. However, as triggering on other nodes in the cortico-thalamic-basal ganglia loop was not studied, it is not known whether this efficacy was unique to triggering on motor cortex.
An alternative approach is the asynchronous delivery of stimulation at spatially separated electrode contacts, termed coordinated reset neuromodulation, that is designed to desynchronize local neuronal populations with respect to the dominant slow (theta or beta) oscillation (Fig. 5). The concept, backed by computational modeling, is that by forcing local populations out of pathological synchrony, the network will settle back into a desynchronized state and that over time the network can in effect be trained out of its abnormal synchrony (Popovych and Tass 2012). A test of this approach in an MPTP primate showed sustained relief of bradykinesia lasting several days after cessation of coordinated reset stimulation, as opposed to effects lasting <30 min after standard high-frequency DBS (Tass et al. 2012). A preliminary test of this approach in six patients with PD and STN DBS leads was recently conducted with further promising results (Adamchic et al. 2014). Coordinated reset neuromodulation was applied intermittently in two sessions per day, each <2 h long, over a period of 3 days. The authors observed a cumulative decrease in beta-band power and a correlated improvement of motor performance. These effects were also long-lasting, persisting overnight despite the lack of overnight stimulation.
Despite the converging lines of modeling and experimental evidence that the clinical effects of DBS are in part mediated by disruption of pathological oscillations, the evidence linking the two remains correlational. One could imagine an experimental intervention that alters neuronal synchrony but leaves other properties of the network activity, such as average firing rate, patterns of time-varying firing rates, entropy, etc., unchanged. Notwithstanding the inherent challenge of experimentally separating these fundamentally interrelated features, this highlights the need for improved tools to record and manipulate neural activity at fine temporal and spatial scales.
Why High Frequency?
For most applications, DBS has been found empirically to be most effective at high frequency (>130 Hz). In essential tremor, stimulation between 5 and 50 Hz worsened tremor or was ineffective, while stimulation > 100 Hz is effective (Grill et al. 2004; Kuncel et al. 2006; Pedrosa et al. 2013; Ushe et al. 2006). In PD, DBS at 5–10 Hz worsens bradykinesia, stimulation at 30–100 Hz is generally ineffective, and stimulation at 130–200 Hz is effective (Moro et al. 2002; Timmermann et al. 2004). In contrast, there is more limited evidence that 60- to 70-Hz stimulation can be effective in focal and generalized dystonia (Alterman et al. 2007; Kim et al. 2012; Velez-Lago et al. 2012) and PD (Khoo et al. 2014). In MPTP primate models, burst stimulation of the GPi at 80 Hz has been reported to be effective (Baker and Vitek 2011). In particular, dystonia and dyskinesia may respond more effectively to lower frequencies than rigidity and tremor (Merola et al. 2013). In PD there may be specific low frequencies that are effective for individual patients that can only be discovered through extensive empirical testing that is not routinely performed in clinical practice (Huang et al. 2014). Other targets, like the PPN for PD, are typically stimulated at lower frequencies (∼25 Hz) (Hamani et al. 2011).
There is presently no unifying hypothesis as to why different individuals and targets require different frequencies (for review see Birdno and Grill 2008). Based on the hypothesis that DBS in PD is effective by increasing the gamma/beta power ratio that is decreased in PD, Tsang et al. customized the stimulation frequency to each patient's intrinsic gamma frequency and found it was no more effective than standard high-frequency stimulation at 130 Hz (Tsang et al. 2012). Computational modeling of coordinated reset stimulation suggested that optimal desynchronization of the theta and beta oscillations occurs when coordinated reset cycles are aligned with the dominant LFP rhythm so as to maximally distribute the phases of the spiking neuronal subpopulations relative to that dominant rhythm. Whereas high-frequency stimulation may attenuate a range of low-frequency rhythms, lower-frequency stimulation may allow pathological oscillations to pass in the interpulse intervals unless stimulation is precisely aligned with the pathological oscillation.
Alternatively, high-frequency stimulation may shift the intrinsic resonant frequency of the circuit. Garcia et al. proposed that antidromic action potentials in cortico-subthalamic projections induced by high-frequency STN DBS would preferentially disrupt slow cortico-subthalamic projections over fast ones, because slow fibers are more susceptible to antidromic action potential collisions because of the slower velocity and thus longer duration of the antidromic action potential (Garcia et al. 2013). By functionally removing the slowest cortical projections, the latency of the cortico-basal-ganglia-thalamo-cortical loop shortens and the resonant frequency elevates out of the range that supports tremor. This model awaits empiric testing.
How critical is the regularity of high-frequency stimulation? Although current DBS IPGs can only deliver regular pulse trains, investigators have used custom pulse generators connected to externalized DBS leads to test temporally irregular pulse sequences. For a given mean stimulation frequency, irregular stimulation trains were shown to be less effective than regular stimulation trains to treat tremor in essential tremor (Birdno and Grill 2008) and bradykinesia in Parkinson's (Dorval et al. 2010). In subsequent experiments the decreased efficacy of irregular trains was shown to be related to the long pauses in the irregular trains used rather than the irregularity per se. Irregular stimulus trains that avoided long pauses were in fact superior to regular high-frequency stimulation for the treatment of finger-tapping bradykinesia in PD (Brocker et al. 2013) (Fig. 5C). The degree to which these spike trains disrupted beta oscillations in a simple computational model of the basal ganglia correlated with their effect on finger-tapping bradykinesia in PD patients. To our knowledge, the effect of these irregular trains on beta oscillations in a parkinsonian patient or PD model system has not yet been tested.
In essential tremor, temporally irregular trains were still not as effective as regular trains, but the efficacy inversely correlated with the degree of pausing, further suggesting that the presence of pauses was the primary factor in reducing DBS efficacy (Birdno et al. 2012). A computational model suggested that the efficacy of DBS was in its suppressing burst-driver input to the thalamus from the cerebellum, and that pauses in DBS trains allowed for burst-driver inputs to pass. This is consistent with the observation that the most effective DBS location for essential tremor is near the cerebello-thalamic afferents (Coenen et al. 2011; Hamel et al. 2007; Herzog et al. 2007; Jiménez et al. 2000; Kitagawa et al. 2005; Struppler et al. 1978).
Pathological Synchrony in Other Disorders as Targets for DBS
Although abnormal neuronal oscillations have been most rigorously implicated in the pathology of PD and essential tremor, many neurological and psychiatric disorders exhibit abnormal neuronal oscillations. If disruption of pathological oscillations is a general principle by which DBS operates, pathological oscillations themselves may represent new pathophysiological targets for DBS. In tic disorders, for example, suppression of tics was associated with increased, frontal cortical alpha-band coherence (Serrien et al. 2005) and broad premotor cortical spectral changes were reported during the premonitory phase before a tic (Almeida et al. 2015). These signals are being actively pursued as potential closed-loop stimulation control signals (Almeida et al. 2015).
In dystonia, simultaneous pallidal LFP and magnetoencephalography has identified dissociable cortical-pallidal networks coherent in distinct frequency bands: pallido-temporal (theta, 4–8 Hz), pallido-cerebellar (alpha, 7–13 Hz), and sensorimotor-cortex-pallidal (beta, 13–30 Hz) (Neumann et al. 2015). In that study, pallido-cerebellar alpha-band power was negatively correlated with dystonia severity. In patients with generalized dystonia, there is a reduced beta desynchronization during movement similar to PD (Crowell et al. 2012). In the GPe and GPi of patients with cervical dystonia, there are enhanced theta-band oscillations in the LFP and neural spiking and coupling of gamma power to theta phase (Moll et al. 2014; Silberstein et al. 2005). Dystonic spasms are associated with increases in broad low-frequency (3–18 Hz) power in the GPi (Liu et al. 2008).
In OCD, intraoperative LFP recordings from two patients undergoing STN DBS showed a significant increase in low-frequency (1–12 Hz) anterior STN oscillatory activity during acute OCD symptoms, although the specificity of this finding is uncertain (Bastin et al. 2014). Compared with PD patients, STN neurons in OCD patients displayed more theta (4–11 Hz) activity (Welter et al. 2011). OCD symptom severity also correlated with higher intraburst frequency and increased oscillations in low-frequency bands in STN neurons (Welter et al. 2011). Finally, DBS of the NAc reduced excessive prefrontal-striatal resting-state functional connectivity and reduced prefrontal low-frequency (2–5 Hz) oscillations associated with symptom-provoking stimuli (Figee et al. 2013).
Pursuing novel neurophysiological signatures as targets for DBS will require continued, basic research to better understand the role of neuronal synchrony both in normal brain function and in illness, as well as a refined ability to use stimulation to target pathological synchrony while preserving normal neural dynamics.
Synaptic Plasticity and Network Reorganization
DBS effects that emerge over minutes to days likely result at least in part from synaptic plasticity-related changes in the stimulated neural network; such network changes occur over similar timescales in natural behaviors such as learning (Caroni et al. 2014). High-frequency stimulation of STN in rat brain slices induced varied forms of synaptic plasticity in different subpopulations of STN neurons including short-term potentiation (STP), long-term potentiation (LTP), and long-term depression (LTD) (Shen et al. 2003). In dopamine-depleted rats, high-frequency stimulation induced short-term depression (STD) and LTD, an effect that was abolished by treatment of the slice with a dopamine agonist (apomorphine), suggesting that stimulation-related synaptic plasticity is sensitive to dopaminergic state (Yamawaki et al. 2012). In contrast, in human subjects with PD, a study of STN stimulation-evoked potentials in SNr showed that LTP-like potentiation of evoked potentials was obtained when patients were treated with levodopa but not when their levodopa was withheld (Prescott et al. 2009). Although these results highlight the potential for DBS-like stimulation to induce synaptic plasticity, to date there is scant direct evidence that any of these synaptic changes underlie the clinical effects of DBS. However, a recent study in a rodent addiction model used low-frequency stimulation of the NAc paired with a dopamine receptor D1 antagonist to selectively depotentiate excitatory inputs on D1-expressing MSNs, with a resulting reversal of cocaine-evoked plasticity (Creed et al. 2015). This approach represents a novel use of combined pharmacology and DBS to specifically shape neural plasticity, and a potential model for plasticity-targeted DBS for other disorders.
Imaging studies using functional MRI (fMRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) have also provided a window into the global and long-term changes in network activity due to DBS (Tang and Eidelberg 2013) (Fig. 6). In nonhuman primates, STN DBS increases fMRI BOLD activation in a broad sensorimotor network including sensorimotor, supplementary motor and cingulate cortex, insula, caudate nucleus, PPN, and cerebellum (Min et al. 2014). Patients with PD exhibit a particular spatial covariance pattern of glucose metabolism on fluoro-d-glucose (FDG)-PET imaging, called the PD-related metabolic pattern (PDRP), which includes hypermetabolism in the pons, globus pallidus, and thalamus and hypometabolism in the premotor cortex, supplementary motor area, and parietal association areas (Ma et al. 2007; Wu et al. 2013). The expression of PDRP can be quantified and has been shown to correlate with clinical disease severity (Eidelberg 2009). Both GPi (Fukuda et al. 2001) and STN (Asanuma et al. 2006; Trost et al. 2006) DBS have been reported to decrease PDRP expression, suggesting that both treatments may normalize network activity in a similar way. Tremor-predominant PD patients with Vim thalamic DBS exhibit a different change in the spatial covariance pattern between DBS on and off, termed PD tremor-related metabolic pattern (PDTP), that correlates with tremor severity and is distinct from PDRP (Mure et al. 2011). PDTP is characterized by increased activity in the cerebellar dentate nucleus, primary motor cortex, and, to a smaller degree, striatum (Mure et al. 2011). Whereas Vim DBS modulated PDTP expression but not PDRP expression, STN DBS reduced the activity of both PDTP and PDRP (Mure et al. 2011), consistent with the observation that Vim DBS treats tremor only whereas STN DBS treats tremor, bradykinesia, and rigidity. Similarly, multiple SPECT studies have provided evidence that STN and GPI DBS both lead to the normalization of abnormal regional cerebral blood flow (rCBF) patterns associated with PD (Antonini et al. 2003; Cilia et al. 2009; Tang and Eidelberg 2013). Others have argued that these patterns of metabolic changes in PD are nonspecific and an artifact of global mean normalization, in which regional tracer uptake is normalized to the global mean of all gray matter voxels (Borghammer et al. 2009; but see Dhawan et al. 2012). By using alternative methods of normalization (including normalization to the mean of all white matter voxels), they instead report widespread cortical hypometabolism in untreated, newly diagnosed PD patients (Berti et al. 2012), early-stage PD patients (Borghammer et al. 2010), and later-stage PD patients (Moeller et al. 1999). This remains an area of unsettled debate.
Fig. 6.
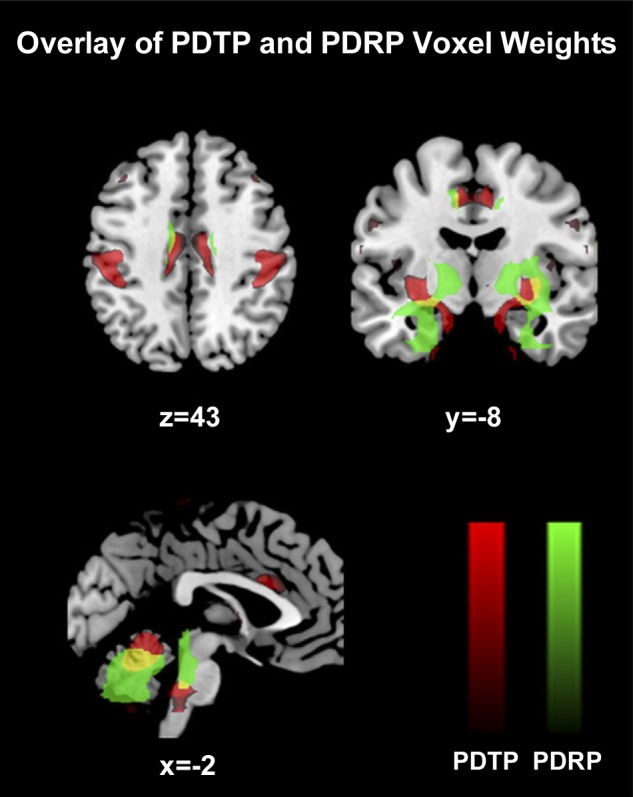
Fluoro-d-glucose (FDG)-PET identifies candidate neural networks underlying bradykinesia, rigidity, and tremor in PD. Comparison between PD-related metabolic pattern (PDRP, in green) and PD tremor-related pattern (PDTP, in red), which are spatial covariance patterns derived from FDG-PET imaging studies of PD patients and tremor-predominant PD patients, respectively (Mure et al. 2011). Areas of overlap are denoted in yellow. PDTP is characterized by increased metabolic activity in the anterior cerebellum, dentate nucleus, primary motor cortex, and, to a lesser extent, caudate and putamen. PDRP is characterized by relative hypermetabolism in the globus pallidus, putamen, thalamus, pons, cerebellum, and sensorimotor cortex, with metabolic decreases in the lateral premotor cortex and parieto-occipital association regions. Reproduced with permission from Mure et al. (2011) (copyright Elsevier 2011).
The above results demonstrate that, perhaps unsurprisingly, continuous DBS results in network-level changes in activity. However, these could reflect acute stimulation-related effects alone rather than network plasticity. In a recent single-subject study, a patient underwent diffusion tensor imaging and resting-state fMRI both before DBS and after 5 mo of DBS, at which time the system was explanted because of emotional lability and motor side effects (van Hartevelt et al. 2014). Using a graph theoretic measure of nodal efficiency, the authors argue that the structural connectivity of the subject's brain after DBS had shifted toward values more typical of healthy control subjects. Future studies will be required to assess whether these changes are reproducible, or whether they emerge from appropriately therapeutic DBS lead placement. Numerous PET imaging studies have also demonstrated long-term changes in metabolic activity after DBS, including DBS of the ALIC for OCD (Rauch et al. 2006; Suetens et al. 2014), GPi DBS for dystonia (Kumar et al. 1999; Yianni et al. 2005), PPN DBS for PD (Strafella et al. 2008), and subgenual cingulate DBS for depression (Lozano et al. 2008; Mayberg et al. 2005). Successful subgenual cingulate DBS was shown to reverse some abnormalities seen in depressed patients at baseline (elevated subgenual cingulate blood flow and decreased prefrontal blood flow) (Mayberg et al. 2005), similar to the changes seen in patients responsive to antidepressant medication (Mayberg et al. 2000). Subgenual cingulate DBS-induced changes in metabolic activity include decreases in orbital, medial frontal, and insular cortex and increases in lateral prefrontal, parietal, anterior midcingulate, and posterior cingulate areas (Lozano et al. 2008).
Neuroprotection and Neurogenesis
It has been hypothesized that DBS could be neuroprotective in PD by slowing the degeneration of dopaminergic neurons in the substantia nigra (Charles et al. 2008). However, several observations suggest that DBS does not arrest or reverse PD. Clinically, PD symptoms continue to progress despite effective DBS therapy (Fasano et al. 2010; Hilker et al. 2005; Krack et al. 2003; Lilleeng et al. 2014; Merola et al. 2011), and dopamine terminal loss continues to accrue at a rate similar to non-DBS-treated PD patients as assessed by 18F-fluorodopa (F-dopa)-PET (Hilker et al. 2005). One study compared 106 PD patients who underwent DBS implantation to 41 PD patients who chose to have medical management only (Ngoga et al. 2014) and showed that DBS-treated patients had a significantly longer survival. However, the nonrandomized design was sensitive to selection bias. Furthermore, DBS may improve survival by minimizing PD motor disability, allowing for maintenance of a healthier lifestyle. There is evidence from a postmortem study of PD patients that DBS leads to increased neuronal precursor cell proliferation in the subventricular zone of the lateral ventricles, the third ventricle lining, and the tissue surrounding the DBS leads in these patients compared with age-matched normal control subjects and PD patients who did not undergo DBS implantation (Vedam-Mai et al. 2014). The clinical significance of these neural precursors is uncertain.
Stronger evidence for a neuroprotective effect of DBS comes from animal models. Intermittent STN DBS (1 h of high-frequency stimulation daily for 3 mo) improves the survival of SNc neurons in a parkinsonian rat model (6-OHDA lesion) (Temel et al. 2006). In these experiments, bilateral STN DBS electrodes were implanted in rats during the same surgical session as bilateral striatal 6-OHDA injections (Temel et al. 2006). Similar results have been described in another STN DBS study and for STN lesions (Benazzouz and Benabid 1996; Maesawa et al. 2004). This protective effect was also seen when the STN DBS was initiated 2 wk after ipsilateral striatal 6-OHDA injection, a period of time in which neuronal degeneration is expected to continue in this parkinsonian model. Although SNc neurons were greater in number with DBS, dopaminergic neurite density in the striatum was unchanged (Spieles-Engemann et al. 2010). In an MPTP primate parkinsonian model, investigators demonstrated that both STN lesioning (kainic acid lesion) and high-frequency STN DBS result in increased survival of dopaminergic cells in the SNc compared with controls, whether the animals were treated before or after MPTP lesioning (Wallace et al. 2007). They postulated that this protective effect was due to a reduction in glutamate excitotoxicity from STN hyperactivity in the dopamine-deficient state (Benazzouz et al. 2000; Rodriguez et al. 1998; Wallace et al. 2007). Alternatively, STN DBS has been shown to induce the neuroprotective growth factor brain-derived neurotrophic factor (BDNF) in the substantia nigra, GPi, and M1 cortex (Spieles-Engemann et al. 2011) and GPi DBS has been reported to alter splice isoforms of glial-derived growth factor (GDNF) expression in the basal ganglia in a nonparkinsonian rat model (Ho et al. 2014). The potential neuroprotective effects of DBS remain an area of active investigation; a pilot clinical trial of STN DBS for early PD has been conducted (Kahn et al. 2012), and a larger definitive trial is planned.
In an analogous fashion, stimulation of the anterior nucleus of the thalamus induces hippocampal neurogenesis in rodent models (Toda et al. 2008), and a phase I trial suggested that chronic, high-frequency stimulation of the fornix can reverse some of the temporoparietal hypometabolism seen in Alzheimer's and might improve cognitive function (Laxton et al. 2010). A larger follow-up trial of forniceal DBS for Alzheimer's dementia is underway.
The Future of Neuromodulation
The fundamental approach to DBS—continuous, temporally regular stimulation to a single (often bilateral) target—has remained largely unchanged since the late 1980s and was derived primarily from empiric observation rather than mechanistic understanding. This is poised to change. The clinical benefits of DBS emerge via multiple, nonexclusive mechanisms of action including the shaping of perielectrode electrical activity via electrical and neurochemical mechanisms, modulation of neural network activity and plasticity, and possibly by influencing neurogenesis and neurodegeneration directly. In recent years our understanding has advanced in all of these areas. Translating this knowledge into improved therapeutics will require investment in well-controlled, translational and interdisciplinary preclinical and clinical studies.
The next generation of DBS systems promises to be more flexible in stimulation parameters and patterns, to allow greater steering of stimulation current and to be able to respond to ongoing neural activity. In addition, other techniques such as optogenetic neuromodulation and DREADDS (Designer Receptors Exclusively Activated by Designer Drugs) hold promise for manipulating neural network activity in ways that are distinct from electrical stimulation but will ultimately target the same pathological neural circuits. Together these innovations hold enormous promise to improve the efficacy and side effect profile of neuromodulation for the treatment of neurological and psychiatric disease, and to open up a range of new neuropsychiatric conditions to neuromodulation therapy.
GRANTS
T. M. Herrington has received support from the Anne Young Fellowship in Movement Disorders, the Bachman-Strauss Dystonia & Parkinson Foundation Fellowship, and the Defense Advanced Research Projects Agency (DARPA). E. N. Eskandar has received support from National Institute of Neurological Disorders and Stroke and DARPA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.M.H. and J.J.C. prepared figures; T.M.H. and J.J.C. drafted manuscript; T.M.H., J.J.C., and E.N.E. edited and revised manuscript; T.M.H., J.J.C., and E.N.E. approved final version of manuscript.
REFERENCES
- Adamchic I, Hauptmann C, Barnikol UB, Pawelczyk N, Popovych O, Barnikol TT, Silchenko A, Volkmann J, Deuschl G, Maarouf M, Sturm V, Freund HJ, Tass PA. Coordinated reset neuromodulation for Parkinson's disease: proof-of-concept study. Mov Disord 29: 1679–1684, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnesi F, Connolly AT, Baker KB, Vitek JL, Johnson MD. Deep brain stimulation imposes complex informational lesions. PLoS One 8: e74462, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnesi F, Johnson MD, Vitek JL. Deep brain stimulation: how does it work? Handb Clin Neurol 116: 39–54, 2013b. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271, 1990. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85: 119–146, 1990. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381, 1986. [DOI] [PubMed] [Google Scholar]
- Almeida L, Martinez-Ramirez D, Rossi PJ, Peng Z, Gunduz A, Okun MS. Chasing tics in the human brain: development of open, scheduled and closed loop responsive approaches to deep brain stimulation for Tourette syndrome. J Clin Neurol 11: 122–131, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman RL, Miravite J, Weisz D, Shils JL, Bressman SB, Tagliati M. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology 69: 681–688, 2007. [DOI] [PubMed] [Google Scholar]
- Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN. Visually guided movements suppress subthalamic oscillations in Parkinson's disease patients. J Neurosci 24: 11302–11306, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T, Hu B, Pittman Q, Kiss ZH. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol 559: 301–313, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Marotta G, Benti R, Landi A, De Notaris R, Mariani C, Gerundini P, Pezzoli G, Gaini SM. Brain flow changes before and after deep brain stimulation of the subthalamic nucleus in Parkinson's disease. Neurol Sci 24: 151–152, 2003. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson's disease. Brain 129: 2667–2678, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P, Paradiso G, Saint-Cyr JA, Chen R, Lang AE, Lozano AM. Potentials recorded at the scalp by stimulation near the human subthalamic nucleus. Clin Neurophysiol 112: 431–437, 2001. [DOI] [PubMed] [Google Scholar]
- Baker KB, Montgomery EB, Rezai AR, Burgess R, Lüders HO. Subthalamic nucleus deep brain stimulus evoked potentials: physiological and therapeutic implications. Mov Disord 17: 969–983, 2002. [DOI] [PubMed] [Google Scholar]
- Baker KB, Vitek JL. Pallidal stimulation: effect of pattern and rate on bradykinesia in the non-human primate model of Parkinson's disease. Exp Neurol 231: 309–313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci 24: 7410–7419, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron MS, Wichmann T, Ma D, DeLong MR. Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci 22: 592–599, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin J, Polosan M, Piallat B, Krack P, Bougerol T, Chabardès S, David O. Changes of oscillatory activity in the subthalamic nucleus during obsessive-compulsive disorder symptoms: two case reports. Cortex 60: 145–150, 2014. [DOI] [PubMed] [Google Scholar]
- Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, Bakos R, Nedergaard M. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med 14: 75–80, 2008. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 84: 203–214, 1996. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Benabid AL. Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci 8: 1408–1414, 1996. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Ni ZG, Koudsie A, Pollak P, Benabid AL. Implication of the subthalamic nucleus in the pathophysiology and pathogenesis of Parkinson's disease. Cell Transplant 9: 215–221, 2000. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett 189: 77–80, 1995. [DOI] [PubMed] [Google Scholar]
- Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O'Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, Seale CG. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84: 810–817, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72: 507–520, 1994. [DOI] [PubMed] [Google Scholar]
- Bergman H, Raz A, Feingold A, Nini A, Nelken I, Hansel D, Ben-Pazi H, Reches A. Physiology of MPTP tremor. Mov Disord 13, Suppl 3: 29–34, 1998. [DOI] [PubMed] [Google Scholar]
- Berti V, Polito C, Borghammer P, Ramat S, Mosconi L, Vanzi E, De Cristofaro MT, De Leon M, Sorbi S, Pupi A. Alternative normalization methods demonstrate widespread cortical hypometabolism in untreated de novo Parkinson's disease. Q J Nucl Med Mol Imaging 56: 299–308, 2012. [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85: 1351–1356, 2001. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology 37: 1975–1985, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdno MJ, Grill WM. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics 5: 14–25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Gross RE, Grill WM. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. J Neurophysiol 107: 364–383, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blahak C, Bäzner H, Capelle HH, Wöhrle JC, Weigel R, Hennerici MG, Krauss JK. Rapid response of parkinsonian tremor to STN-DBS changes: direct modulation of oscillatory basal ganglia activity? Mov Disord 24: 1221–1225, 2009. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Fytagoridis A, Åström M, Linder J, Forsgren L, Hariz MI. Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat Disord 18: 1062–1066, 2012. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord 25: 1350–1356, 2010. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Ingham CA, Izzo PN, Levey AI, Rye DB, Smith AD, Wainer BH. Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Res 397: 279–289, 1986. [DOI] [PubMed] [Google Scholar]
- Borghammer P, Chakravarty M, Jonsdottir KY, Sato N, Matsuda H, Ito K, Arahata Y, Kato T, Gjedde A. Cortical hypometabolism and hypoperfusion in Parkinson's disease is extensive: probably even at early disease stages. Brain Struct Funct 214: 303–317, 2010. [DOI] [PubMed] [Google Scholar]
- Borghammer P, Cumming P, Aanerud J, Förster S, Gjedde A. Subcortical elevation of metabolism in Parkinson's disease—a critical reappraisal in the context of global mean normalization. Neuroimage 47: 1514–1521, 2009. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Sharott A, Brown P. The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci 39: 1951–1959, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker DT, Grill WM. Principles of electrical stimulation of neural tissue. Handb Clin Neurol 116: 3–18, 2013. [DOI] [PubMed] [Google Scholar]
- Brocker DT, Swan BD, Turner DA, Gross RE, Tatter SB, Koop MM, Bronte-Stewart H, Grill WM. Improved efficacy of temporally non-regular deep brain stimulation in Parkinson's disease. Exp Neurol 239: 60–67, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol 215: 20–28, 2009. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol 17: 656–664, 2007. [DOI] [PubMed] [Google Scholar]
- Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease. Exp Neurol 188: 480–490, 2004. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci 21: 1033–1038, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehlmann A, Deco G. Optimal information transfer in the cortex through synchronization. PLoS Comp Biol 6: e1000934, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, Wolgamuth B, McIntyre CC. Probabilistic analysis of activation volumes generated during deep brain stimulation. Neuroimage 54: 2096–2104, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80: 751–764, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Chowdhury A, Lahr M. Synapse rearrangements upon learning: from divergent-sparse connectivity to dedicated sub-circuits. Trends Neurosci 37: 604–614, 2014. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain 125: 1235–1246, 2002. [DOI] [PubMed] [Google Scholar]
- Charles PD, Gill CE, Davis TL, Konrad PE, Benabid AL. Is deep brain stimulation neuroprotective if applied early in the course of PD? Nat Clin Pract Neurol 4: 424–426, 2008. [DOI] [PubMed] [Google Scholar]
- Chen CC, Litvak V, Gilbertson T, Kühn A, Lu CS, Lee ST, Tsai CH, Tisch S, Limousin P, Hariz M, Brown P. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson's disease. Exp Neurol 205: 214–221, 2007. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Larson PS. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience 169: 1768–1778, 2010. [DOI] [PubMed] [Google Scholar]
- Cilia R, Marotta G, Landi A, Isaias IU, Mariani CB, Vergani F, Benti R, Sganzerla E, Pezzoli G, Antonini A. Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson's disease: a prospective case-control study. Clin Neurol Neurosurg 111: 140–146, 2009. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Allert N, Mädler B. A role of diffusion tensor imaging fiber tracking in deep brain stimulation surgery: DBS of the dentato-rubro-thalamic tract (drt) for the treatment of therapy-refractory tremor. Acta Neurochir (Wien) 153: 1579–1585, 2011. [DOI] [PubMed] [Google Scholar]
- Contarino MF, Bour LJ, Verhagen R, Lourens MA, de Bie RM, van den Munckhof P, Schuurman PR. Directional steering: a novel approach to deep brain stimulation. Neurology 83: 1163–1169, 2014. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci 23: 11741–11752, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche R, Lamarre Y. Local field potential oscillations in primate cerebellar cortex: synchronization with cerebral cortex during active and passive expectancy. J Neurophysiol 93: 2039–2052, 2005. [DOI] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Lüscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347: 659–664, 2015. [DOI] [PubMed] [Google Scholar]
- Crowell AL, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Shimamoto S, Lim DA, Starr PA. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain 135: 615–630, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci USA 110: 4780–4785, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci 18: 779–786, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res 53: 81–95, 1973. [DOI] [PubMed] [Google Scholar]
- Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med 345: 956–963, 2001. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Bosch A, Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 67: 1061–1068, 2010. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Agid Y. Subthalamic neurostimulation for Parkinson's disease with early fluctuations: balancing the risks and benefits. Lancet Neurol 12: 1025–1034, 2013. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, German Parkinson Study Group, Neurostimulation Section. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 355: 896–908, 2006. [DOI] [PubMed] [Google Scholar]
- Devos D, Labyt E, Derambure P, Bourriez JL, Cassim F, Reyns N, Blond S, Guieu JD, Destée A, Defebvre L. Subthalamic nucleus stimulation modulates motor cortex oscillatory activity in Parkinson's disease. Brain 127: 408–419, 2004. [DOI] [PubMed] [Google Scholar]
- Dhawan V, Tang CC, Ma Y, Spetsieris P, Eidelberg D. Abnormal network topographies and changes in global activity: absence of a causal relationship. Neuroimage 63: 1827–1832, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Grill WM. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J Neurophysiol 111: 1949–1959, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol 104: 911–921, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Russo GS, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson's disease. J Neurophysiol 100: 2807–2818, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol 84: 570–574, 2000. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Lozano AM. Mechanisms of deep brain stimulation. Mov Disord 17: S63–S68, 2002. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O'Reardon JP, Eskandar EN, Baltuch GH, Machado AD, Kondziolka D, Cusin C, Evans KC, Price LH, Jacobs K, Pandya M, Denko T, Tyrka AR, Brelje T, Kubu C, Malone DA. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry 78: 240–248, 2015. [DOI] [PubMed] [Google Scholar]
- Doyle LM, Kühn AA, Hariz M, Kupsch A, Schneider GH, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson's disease. Eur J Neurosci 21: 1403–1412, 2005a. [DOI] [PubMed] [Google Scholar]
- Doyle LM, Yarrow K, Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin Neurophysiol 116: 1879–1888, 2005b. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci 32: 548–557, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: 1916–1923, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Chen CC, Lu CS, Lee ST, Tsai CH, Limousin P, Hariz M, Brown P. Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson's disease. Exp Neurol 209: 125–130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Pogosyan A, Wang S, Averbeck B, Gaynor LD, Cantiniaux S, Witjas T, Limousin P, Azulay JP, Brown P. Resonance in subthalamo-cortical circuits in Parkinson's disease. Brain 132: 2139–2150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Thevathasan W, Doyle Gaynor L, Pogosyan A, Bye E, Foltynie T, Zrinzo L, Ashkan K, Aziz T, Brown P. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry 82: 569–573, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol 11: 98–110, 2015. [DOI] [PubMed] [Google Scholar]
- Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Albanese A. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain 133: 2664–2676, 2010. [DOI] [PubMed] [Google Scholar]
- Fedele E, Raiteri M. In vivo studies of the cerebral glutamate receptor/NO/cGMP pathway. Prog Neurobiol 58: 89–120, 1999. [DOI] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, de Koning P, Levar N, Droge L, van den Munckhof P, Schuurman PR, Nederveen A, van den Brink W, Mazaheri A, Denys D. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 16: 386–387, 2013. [DOI] [PubMed] [Google Scholar]
- Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res 156: 274–281, 2004. [DOI] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, SANTE Study Group . Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51: 899–908, 2010. [DOI] [PubMed] [Google Scholar]
- Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: a systematic review. Mov Disord 25: 1550–1559, 2010. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, CSP 468 Study Group. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 362: 2077–2091, 2010. [DOI] [PubMed] [Google Scholar]
- Foutz TJ, McIntyre CC. Evaluation of novel stimulus waveforms for deep brain stimulation. J Neural Eng 7: 066008, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318: 1309–1312, 2007. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224, 2009. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Mentis MJ, Ma Y, Dhawan V, Antonini A, Lang AE, Lozano AM, Hammerstad J, Lyons K, Koller WC, Moeller JR, Eidelberg D. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson's disease: a PET study of resting-state glucose metabolism. Brain 124: 1601–1609, 2001. [DOI] [PubMed] [Google Scholar]
- Furuta T, Koyano K, Tomioka R, Yanagawa Y, Kaneko T. GABAergic basal forebrain neurons that express receptor for neurokinin B and send axons to the cerebral cortex. J Comp Neurol 473: 43–58, 2004. [DOI] [PubMed] [Google Scholar]
- Galati S, Mazzone P, Fedele E, Pisani A, Peppe A, Pierantozzi M, Brusa L, Tropepi D, Moschella V, Raiteri M, Stanzione P, Bernardi G, Stefani A. Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson's disease. Eur J Neurosci 23: 2923–2928, 2006. [DOI] [PubMed] [Google Scholar]
- Gale JT, Amirnovin R, Roberts DW, Williams ZM, Blaha CD, Eskandar EN. Electrical stimulation-evoked dopamine release in the primate striatum. Stereotact Funct Neurosurg 91: 355–363, 2013. [DOI] [PubMed] [Google Scholar]
- Garcia MR, Pearlmutter BA, Wellstead PE, Middleton RH. A slow axon antidromic blockade hypothesis for tremor reduction via deep brain stimulation. PLoS One 8: e73456, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: 1429–1432, 1990. [DOI] [PubMed] [Google Scholar]
- Giannicola G, Marceglia S, Rossi L, Mrakic-Sposta S, Rampini P, Tamma F, Cogiamanian F, Barbieri S, Priori A. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson's disease. Exp Neurol 226: 120–127, 2010. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn PJ, Mallory GW, Khurram OU, Berry BM, Hachmann JT, Bieber AJ, Bennet KE, Min HK, Chang SY, Lujan JL. A neurochemical closed-loop controller for deep brain stimulation: toward individualized smart neuromodulation therapies. Front Neurosci 8: 169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 15: 64–79, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 15: 1137–1140, 2004. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol 294: 607–622, 1990. [DOI] [PubMed] [Google Scholar]
- Guridi J, Lozano AM. A brief history of pallidotomy. Neurosurgery 41: 1169–1180, 1997. [DOI] [PubMed] [Google Scholar]
- Halpern CH, Wolf JA, Bale TL, Stunkard AJ, Danish SF, Grossman M, Jaggi JL, Grady MS, Baltuch GH. Deep brain stimulation in the treatment of obesity. J Neurosurg 109: 625–634, 2008. [DOI] [PubMed] [Google Scholar]
- Hamani C, Moro E, Lozano AM. The pedunculopontine nucleus as a target for deep brain stimulation. J Neural Transm 118: 1461–1468, 2011. [DOI] [PubMed] [Google Scholar]
- Hamel W, Herzog J, Kopper F, Pinsker M, Weinert D, Müller D, Krack P, Deuschl G, Mehdorn HM. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir (Wien) 149: 749–758, 2007. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007. [DOI] [PubMed] [Google Scholar]
- Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J Neurosci 33: 4804–4814, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69: 269–281, 2011. [DOI] [PubMed] [Google Scholar]
- Herzog J, Hamel W, Wenzelburger R, Pötter M, Pinsker MO, Bartussek J, Morsnowski A, Steigerwald F, Deuschl G, Volkmann J. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain 130: 1608–1625, 2007. [DOI] [PubMed] [Google Scholar]
- Hilker R, Portman AT, Voges J, Staal MJ, Burghaus L, van Laar T, Koulousakis A, Maguire RP, Pruim J, de Jong BM, Herholz K, Sturm V, Heiss WD, Leenders KL. Disease progression continues in patients with advanced Parkinson's disease and effective subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry 76: 1217–1221, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 61, Suppl 6: 4–6, 2000. [PubMed] [Google Scholar]
- Hirschmann J, Hartmann CJ, Butz M, Hoogenboom N, Ozkurt TE, Elben S, Vesper J, Wojtecki L, Schnitzler A. A direct relationship between oscillatory subthalamic nucleus-cortex coupling and rest tremor in Parkinson's disease. Brain 136: 3659–3670, 2013. [DOI] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63: 508–522, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DX, Tan YC, Tan J, Too HP, Ng WH. High-frequency stimulation of the globus pallidus interna nucleus modulates GFRα1 gene expression in the basal ganglia. J Clin Neurosci 21: 657–660, 2014. [DOI] [PubMed] [Google Scholar]
- Hofmann L, Ebert M, Tass PA, Hauptmann C. Modified pulse shapes for effective neural stimulation. Front Neuroeng 4: 9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci 8: 1491–1493, 2005. [DOI] [PubMed] [Google Scholar]
- Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol 93: 117–127, 2005. [DOI] [PubMed] [Google Scholar]
- Huang H, Watts RL, Montgomery EB. Effects of deep brain stimulation frequency on bradykinesia of Parkinson's disease. Mov Disord 29: 203–206, 2014. [DOI] [PubMed] [Google Scholar]
- Hurtado JM, Gray CM, Tamas LB, Sigvardt KA. Dynamics of tremor-related oscillations in the human globus pallidus: a single case study. Proc Natl Acad Sci USA 96: 1674–1679, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Bolam JP, Smith AD. GABA-immunoreactive synaptic boutons in the rat basal forebrain: comparison of neurons that project to the neocortex with pallidosubthalamic neurons. J Comp Neurol 273: 263–282, 1988. [DOI] [PubMed] [Google Scholar]
- Jenike MA. Neurosurgical treatment of obsessive-compulsive disorder. Br J Psychiatry Suppl 1998: 79–90, 1998. [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci 34: 611–618, 2011. [DOI] [PubMed] [Google Scholar]
- Jiménez F, Velasco F, Velasco M, Brito F, Morel C, Márquez I, Pérez ML. Subthalamic prelemniscal radiation stimulation for the treatment of Parkinson's disease: electrophysiological characterization of the area. Arch Med Res 31: 270–281, 2000. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Ghosh D, McIntyre CC, Vitek JL. Neural targets for relieving parkinsonian rigidity and bradykinesia with pallidal deep brain stimulation. J Neurophysiol 108: 567–577, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Jang JH, Park JW, Kim E, Goo EH, Im OS, Kwon JS. Unravelling the intrinsic functional organization of the human striatum: a parcellation and connectivity study based on resting-state fMRI. PLoS One 9: e106768, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn E, D'Haese PF, Dawant B, Allen L, Kao C, Charles PD, Konrad P. Deep brain stimulation in early stage Parkinson's disease: operative experience from a prospective randomised clinical trial. J Neurol Neurosurg Psychiatry 83: 164–170, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res 143: 449–459, 2004. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry 168: 502–510, 2011. [DOI] [PubMed] [Google Scholar]
- Khoo HM, Kishima H, Hosomi K, Maruo T, Tani N, Oshino S, Shimokawa T, Yokoe M, Mochizuki H, Saitoh Y, Yoshimine T. Low-frequency subthalamic nucleus stimulation in Parkinson's disease: a randomized clinical trial. Mov Disord 29: 270–274, 2014. [DOI] [PubMed] [Google Scholar]
- Kim JP, Chang WS, Park YS, Chang JW. Effects of relative low-frequency bilateral globus pallidus internus stimulation for treatment of cervical dystonia. Stereotact Funct Neurosurg 90: 30–36, 2012. [DOI] [PubMed] [Google Scholar]
- Kiss ZH, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O, Functional and Stereotactic Section of the Canadian Neurosurgical Society, Canadian Movement Disorders Group. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain 130: 2879–2886, 2007. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Murata JI, Uesugi H, Kikuchi S, Saito H, Tashiro K, Sawamura Y. Two-year follow-up of chronic stimulation of the posterior subthalamic white matter for tremor-dominant Parkinson's disease. Neurosurgery 56: 281–289, 2005. [DOI] [PubMed] [Google Scholar]
- Koller W, Pahwa R, Busenbark K, Hubble J, Wilkinson S, Lang A, Tuite P, Sime E, Lazano A, Hauser R, Malapira T, Smith D, Tarsy D, Miyawaki E, Norregaard T, Kormos T, Olanow CW. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol 42: 292–299, 1997. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardès S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 349: 1925–1934, 2003. [DOI] [PubMed] [Google Scholar]
- Krauss JK, Yianni J, Loher TJ, Aziz TZ. Deep brain stimulation for dystonia. J Clin Neurophysiol 21: 18–30, 2004. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Kempf F, Brücke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci 28: 6165–6173, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol 215: 380–387, 2009. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Bührle CP, Lenartz D, Sturm V. Deep brain stimulation in addiction due to psychoactive substance use. Handb Clin Neurol 116: 259–269, 2013. [DOI] [PubMed] [Google Scholar]
- Kumar R, Dagher A, Hutchison WD, Lang AE, Lozano AM. Globus pallidus deep brain stimulation for generalized dystonia: clinical and PET investigation. Neurology 53: 871–874, 1999. [DOI] [PubMed] [Google Scholar]
- Kumar R, Johnson L. Managing Parkinson's disease patients treated with deep brain stimulation. In: Deep Brain Stimulation Management, edited by Marks WJ. New York: Cambridge Univ. Press, 2011. [Google Scholar]
- Kuncel AM, Cooper SE, Wolgamuth BR, Clyde MA, Snyder SA, Montgomery EB, Rezai AR, Grill WM. Clinical response to varying the stimulus parameters in deep brain stimulation for essential tremor. Mov Disord 21: 1920–1928, 2006. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Müller JU, Deuschl G. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355: 1978–1990, 2006. [DOI] [PubMed] [Google Scholar]
- Labyt E, Cassim F, Devos D, Bourriez JL, Destée A, Guieu JD, Defebvre L, Derambure P. Abnormal cortical mechanisms in voluntary muscle relaxation in de novo parkinsonian patients. J Clin Neurophysiol 22: 192–203, 2005. [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol 68: 521–534, 2010. [DOI] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci 26: 1701–1713, 2007. [DOI] [PubMed] [Google Scholar]
- Lee MS, Rinne JO, Marsden CD. The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J 41: 167–184, 2000. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin RL, Tasker RR, Dostrovsky JO, Lenz YE. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain 117: 531–543, 1994. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol 98: 3525–3537, 2007. [DOI] [PubMed] [Google Scholar]
- Lilleeng B, Brønnick K, Toft M, Dietrichs E, Larsen JP. Progression and survival in Parkinson's disease with subthalamic nucleus stimulation. Acta Neurol Scand 130: 292–298, 2014. [DOI] [PubMed] [Google Scholar]
- Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry 66: 289–296, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, Woodside B, Lozano AM. Evaluating the potential of deep brain stimulation for treatment-resistant anorexia nervosa. Handb Clin Neurol 116: 271–276, 2013. [DOI] [PubMed] [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson's disease? Ann NY Acad Sci 1265: 9–24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Kühn AA, Brown P. β band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol 236: 383–388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, Fitzgerald J, Green AL, Aziz TZ, Brown P. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74: 449–457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain 134: 359–374, 2011. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang S, Yianni J, Nandi D, Bain PG, Gregory R, Stein JF, Aziz TZ. The sensory and motor representation of synchronized oscillations in the globus pallidus in patients with primary dystonia. Brain 131: 1562–1573, 2008. [DOI] [PubMed] [Google Scholar]
- López-Azcárate J, Tainta M, Rodriguez-Oroz MC, Valencia M, González R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson's disease. J Neurosci 30: 6667–6677, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, Debonnel G, Sadikot AF, Lam RW, Howard AK, Ilcewicz-Klimek M, Honey CR, Mayberg HS. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg 116: 315–322, 2012. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 64: 461–467, 2008. [DOI] [PubMed] [Google Scholar]
- Lysyansky B, Popovych OV, Tass PA. Desynchronizing anti-resonance effect of m: n ON-OFF coordinated reset stimulation. J Neural Eng 8: 036019, 2011. [DOI] [PubMed] [Google Scholar]
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow Metab 27: 597–605, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Haber S, Sears N, Greenberg B, Rezai A. Functional topography of the ventral striatum and anterior limb of the internal capsule determined by electrical stimulation of awake patients. Clin Neurophysiol 120: 1941–1948, 2009. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Webb RM, Silberstein P, Tisch S, Asselman P, Limousin P, Rothwell JC. Stimulation through electrodes implanted near the subthalamic nucleus activates projections to motor areas of cerebral cortex in patients with Parkinson's disease. Eur J Neurosci 21: 1394–1402, 2005. [DOI] [PubMed] [Google Scholar]
- Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, Yoshida J. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg 100: 679–687, 2004. [DOI] [PubMed] [Google Scholar]
- Magariños-Ascone C, Pazo JH, Macadar O, Buño W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: a possible cellular mechanism in Parkinson's disease. Neuroscience 115: 1109–1117, 2002. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience 106: 313–330, 2001. [DOI] [PubMed] [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience 96: 549–564, 2000. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain (3rd ed). San Diego, CA: Elsevier Academic, 2008. [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardès S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Vérin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B, Pelissolo A, STOC Study Group . Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 359: 2121–2134, 2008a. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Márton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci 28: 14245–14258, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 65: 267–275, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci 29: 13613–13620, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens HC, Toader E, Decré MM, Anderson DJ, Vetter R, Kipke DR, Baker KB, Johnson MD, Vitek JL. Spatial steering of deep brain stimulation volumes using a novel lead design. Clin Neurophysiol 122: 558–566, 2011. [DOI] [PubMed] [Google Scholar]
- Martinez RC, Hamani C, de Carvalho MC, de Oliveira AR, Alho E, Navarro J, Dos Santos Ghilardi MG, Bor-Seng-Shu E, Heinsen H, Otoch JP, Brandão ML, Barbosa ER, Teixeira MJ, Fonoff ET. Intraoperative dopamine release during globus pallidus internus stimulation in Parkinson's disease. Mov Disord 28: 2027–2032, 2013. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843, 2000. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660, 2005. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol 101: 1941–1960, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Pallidal stimulation suppresses pathological dysrhythmia in the parkinsonian motor cortex. J Neurophysiol 113: 2537–2548, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Moore-Kochlacs C, Gu X, Boyden ES, Han X, Kopell N. Striatal origin of the pathologic beta oscillations in Parkinson's disease. Proc Natl Acad Sci USA 108: 11620–11625, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 91: 1457–1469, 2004a. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115: 1239–1248, 2004b. [DOI] [PubMed] [Google Scholar]
- Melgari JM, Curcio G, Mastrolilli F, Salomone G, Trotta L, Tombini M, di Biase L, Scrascia F, Fini R, Fabrizio E, Rossini PM, Vernieri F. Alpha and beta EEG power reflects L-dopa acute administration in parkinsonian patients. Front Aging Neurosci 6: 302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci 27: 585–588, 2004. [DOI] [PubMed] [Google Scholar]
- Merola A, Zibetti M, Angrisano S, Rizzi L, Ricchi V, Artusi CA, Lanotte M, Rizzone MG, Lopiano L. Parkinson's disease progression at 30 years: a study of subthalamic deep brain-stimulated patients. Brain 134: 2074–2084, 2011. [DOI] [PubMed] [Google Scholar]
- Merola A, Zibetti M, Artusi CA, Rizzi L, Angrisano S, Lanotte M, Lopiano L, Rizzone MG. 80 Hz versus 130 Hz subthalamic nucleus deep brain stimulation: effects on involuntary movements. Parkinsonism Relat Disord 19: 453–456, 2013. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31: 236–250, 2000. [DOI] [PubMed] [Google Scholar]
- Mikell CB, McKhann GM, Segal S, McGovern RA, Wallenstein MB, Moore H. The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereotact Funct Neurosurg 87: 256–265, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Hebb AO, Ramsey NF, Knight RT, Ojemann JG, Fetz EE. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comp Biol 8: e1002655, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comp Biol 5: e1000609, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HK, Ross EK, Lee KH, Dennis K, Han SR, Jeong JH, Marsh MP, Striemer B, Felmlee JP, Lujan JL, Goerss S, Duffy PS, Blaha CD, Chang SY, Bennet KE. Subthalamic nucleus deep brain stimulation induces motor network BOLD activation: use of a high precision MRI guided stereotactic system for nonhuman primates. Brain Stimul 7: 603–607, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MF, Hamani C, de Almeida AC, Amorim BO, Macedo CE, Fernandes MJ, Nobrega JN, Aarão MC, Madureira AP, Rodrigues AM, Andersen ML, Tufik S, Mello LE, Covolan L. Role of adenosine in the antiepileptic effects of deep brain stimulation. Front Cell Neurosci 8: 312, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieres P, Antonini A, Missimer J, Leenders KL, Eidelberg D. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med 40: 1264–1269, 1999. [PubMed] [Google Scholar]
- Moll CK, Galindo-Leon E, Sharott A, Gulberti A, Buhmann C, Koeppen JA, Biermann M, Bäumer T, Zittel S, Westphal M, Gerloff C, Hamel W, Münchau A, Engel AK. Asymmetric pallidal neuronal activity in patients with cervical dystonia. Front Syst Neurosci 8: 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery EB. Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol 117: 2691–2702, 2006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB. Deep Brain Stimulation Programming: Principles and Practice. Oxford, UK: Oxford Univ. Press, 2010. [Google Scholar]
- Moro E, Esselink RJ, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson's disease of electrical parameter settings in STN stimulation. Neurology 59: 706–713, 2002. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain 133: 215–224, 2010. [DOI] [PubMed] [Google Scholar]
- Moshel S, Shamir RR, Raz A, de Noriega FR, Eitan R, Bergman H, Israel Z. Subthalamic nucleus long-range synchronization—an independent hallmark of human Parkinson's disease. Front Syst Neurosci 7: 79, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlagh MG, Smith ME, Landeros-Weisenberger A, Kobets AJ, King RA, Miravite J, de Lotbinière AC, Alterman RL, Mogilner AY, Pourfar MH, Okun MS, Leckman JF. Lessons learned from open-label deep brain stimulation for Tourette syndrome: eight cases over 7 years. Tremor Other Hyperkinet Mov (NY) 3: tre-03-170-4428-1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure H, Hirano S, Tang CC, Isaias IU, Antonini A, Ma Y, Dhawan V, Eidelberg D. Parkinson's disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 54: 1244–1253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol 76: 3949–3967, 1996. [DOI] [PubMed] [Google Scholar]
- Nambu A. High-frequency pallidal stimulation disrupts information flow through the pallidum by GABAergic inhibition. J Neurosci 33: 2268–2280, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tachibana Y. Mechanism of parkinsonian neuronal oscillations in the primate basal ganglia: some considerations based on our recent work. Front Syst Neurosci 8: 74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci 16: 2671–2683, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol 84: 289–300, 2000. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Inase M, Takada M. Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neurosci Lett 239: 13–16, 1997. [DOI] [PubMed] [Google Scholar]
- Neumann WJ, Jha A, Bock A, Huebl J, Horn A, Schneider GH, Sander TH, Litvak V, Kühn AA. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain 138: 1894–1906, 2015. [DOI] [PubMed] [Google Scholar]
- Ngoga D, Mitchell R, Kausar J, Hodson J, Harries A, Pall H. Deep brain stimulation improves survival in severe Parkinson's disease. J Neurol Neurosurg Psychiatry 85: 17–22, 2014. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res 118: 489–500, 1998. [DOI] [PubMed] [Google Scholar]
- Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RM. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12: 37–44, 2013. [DOI] [PubMed] [Google Scholar]
- Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, Alterman R, Jankovic J, Simpson R, Junn F, Verhagen L, Arle JE, Ford B, Goodman RR, Stewart RM, Horn S, Baltuch GH, Kopell BH, Marshall F, Peichel D, Pahwa R, Lyons KE, Tröster AI, Vitek JL, Tagliati M. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurol 11: 140–149, 2012. [DOI] [PubMed] [Google Scholar]
- Ostrem JL, Racine CA, Glass GA, Grace JK, Volz MM, Heath SL, Starr PA. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology 76: 870–878, 2011. [DOI] [PubMed] [Google Scholar]
- Oswal A, Litvak V, Sauleau P, Brown P. Beta reactivity, prospective facilitation of executive processing, and its dependence on dopaminergic therapy in Parkinson's disease. J Neurosci 32: 9909–9916, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkurt TE, Butz M, Homburger M, Elben S, Vesper J, Wojtecki L, Schnitzler A. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson's disease. Exp Neurol 229: 324–331, 2011. [DOI] [PubMed] [Google Scholar]
- Pahwa R, Lyons KE, Wilkinson SB, Simpson RK, Ondo WG, Tarsy D, Norregaard T, Hubble JP, Smith DA, Hauser RA, Jankovic J. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg 104: 506–512, 2006. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev 20: 128–154, 1995a. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 20: 91–127, 1995b. [DOI] [PubMed] [Google Scholar]
- Pedersen JL, Barloese M, Jensen RH. Neurostimulation in cluster headache: a review of current progress. Cephalalgia 33: 1179–1193, 2013. [DOI] [PubMed] [Google Scholar]
- Pedrosa DJ, Auth M, Eggers C, Timmermann L. Effects of low-frequency thalamic deep brain stimulation in essential tremor patients. Exp Neurol 248: 205–212, 2013. [DOI] [PubMed] [Google Scholar]
- Penney JB, Young AB. Speculations on the functional anatomy of basal ganglia disorders. Annu Rev Neurosci 6: 73–94, 1983. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Green AL, Aziz TZ. Deep brain stimulation for pain. Handb Clin Neurol 116: 277–294, 2013. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Muthusamy KA, De Pennington N, Joint CA, Aziz TZ. Deep brain stimulation of the pedunculopontine nucleus in Parkinson's disease. Preliminary experience at Oxford. Br J Neurosurg 22, Suppl 1: S41–S44, 2008. [DOI] [PubMed] [Google Scholar]
- Piboolnurak P, Lang AE, Lozano AM, Miyasaki JM, Saint-Cyr JA, Poon YY, Hutchison WD, Dostrovsky JO, Moro E. Levodopa response in long-term bilateral subthalamic stimulation for Parkinson's disease. Mov Disord 22: 990–997, 2007. [DOI] [PubMed] [Google Scholar]
- Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain 129: 1732–1747, 2006. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Yoshida F, Chen CC, Martinez-Torres I, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Brown P. Parkinsonian impairment correlates with spatially extensive subthalamic oscillatory synchronization. Neuroscience 171: 245–257, 2010. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Südmeyer M. Motor-cortical oscillations in early stages of Parkinson's disease. J Physiol 590: 3203–3212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovych OV, Tass PA. Desynchronizing electrical and sensory coordinated reset neuromodulation. Front Hum Neurosci 6: 58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 16: 1508–1521, 2006. [DOI] [PubMed] [Google Scholar]
- Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson's disease patients. Brain 132: 309–318, 2009. [DOI] [PubMed] [Google Scholar]
- Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, Hill BC, Kilbane C, Henderson JM, Bronte-Stewart H. Beta oscillations in freely moving Parkinson's subjects are attenuated during deep brain stimulation. Mov Disord (September 11, 2015). doi: 10.1002/mds.26376. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Lindemann M, Schmaljohann H, Wenzelburger R, Pfister G, Deuschl G. Multiple oscillators are causing parkinsonian and essential tremor. Mov Disord 15: 84–94, 2000. [DOI] [PubMed] [Google Scholar]
- Rattay F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 89: 335–346, 1999. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Rezai A, Friehs G, Fischman AJ, Alpert NM, Haber SN, Stypulkowski PH, Rise MT, Rasmussen SA, Greenberg BD. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg 104: 558–565, 2006. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol 213: 108–113, 2008. [DOI] [PubMed] [Google Scholar]
- Reck C, Florin E, Wojtecki L, Krause H, Groiss S, Voges J, Maarouf M, Sturm V, Schnitzler A, Timmermann L. Characterisation of tremor-associated local field potentials in the subthalamic nucleus in Parkinson's disease. Eur J Neurosci 29: 599–612, 2009. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Freed CR, Shimamoto SA, Starr PA. Pallidal neuronal discharge in Parkinson's disease following intraputamenal fetal mesencephalic allograft. J Neurol Neurosurg Psychiatry 82: 266–271, 2011. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson's disease: a target for neuroprotection. Ann Neurol 44: S175–S188, 1998. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, López-Azcárate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, Guridi J, Artieda J, Obeso JA. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson's disease. Brain 134: 36–49, 2011. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA. The subthalamic nucleus in Parkinson's disease: somatotopic organization and physiological characteristics. Brain 124: 1777–1790, 2001. [DOI] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72: 370–384, 2011. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Mohan A, Cannon E, Crawford JD, Silberstein P, Cook R, Coyne T, Silburn PA. Deep brain stimulation of the antero-medial globus pallidus interna for Tourette syndrome. PLoS One 9: e104926, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. The neglected constituent of the basal forebrain corticopetal projection system: GABAergic projections. Eur J Neurosci 15: 1867–1873, 2002. [DOI] [PubMed] [Google Scholar]
- Sato F, Lavallée P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J Comp Neurol 417: 17–31, 2000. [PubMed] [Google Scholar]
- Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL, Xie T, Gerfen CR, Sabatini BL. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521: 85–89, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Biondi T, O'Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 448: 600–603, 2007. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33: 368–377, 2008. [DOI] [PubMed] [Google Scholar]
- Schrock LE, Ostrem JL, Turner RS, Shimamoto SA, Starr PA. The subthalamic nucleus in primary dystonia: single-unit discharge characteristics. J Neurophysiol 102: 3740–3752, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Hälbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltête D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Krüger R, Pinsker MO, Amtage F, Régis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, EARLYSTIM Study Group . Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 368: 610–622, 2013. [DOI] [PubMed] [Google Scholar]
- Schüpbach WM, Maltête D, Houeto JL, du Montcel ST, Mallet L, Welter ML, Gargiulo M, Béhar C, Bonnet AM, Czernecki V, Pidoux B, Navarro S, Dormont D, Cornu P, Agid Y. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology 68: 267–271, 2007. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Orth M, Evans AH, Lees AJ, Brown P. Motor inhibition in patients with Gilles de la Tourette syndrome: functional activation patterns as revealed by EEG coherence. Brain 128: 116–125, 2005. [DOI] [PubMed] [Google Scholar]
- Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry 79: 136–142, 2008. [DOI] [PubMed] [Google Scholar]
- Severin CM, Young PA, Massapust LC. Pallidothalamic projections in the rat. J Comp Neurol 166: 491–502, 1976. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Zhu ZT, Munhall A, Johnson SW. Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse 50: 314–319, 2003. [DOI] [PubMed] [Google Scholar]
- Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson's disease. J Neurosci 33: 7220–7233, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DS, Samoilova M, Cotic M, Zhang L, Brotchie JM, Carlen PL. High frequency stimulation or elevated K+ depresses neuronal activity in the rat entopeduncular nucleus. Neuroscience 149: 68–86, 2007. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Kühn AA, Kupsch A, Trottenberg T, Krauss JK, Wöhrle JC, Mazzone P, Insola A, Di Lazzaro V, Oliviero A, Aziz T, Brown P. Patterning of globus pallidus local field potentials differs between Parkinson's disease and dystonia. Brain 126: 2597–2608, 2003. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Pogosyan A, Kühn AA, Hotton G, Tisch S, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P. Cortico-cortical coupling in Parkinson's disease and its modulation by therapy. Brain 128: 1277–1291, 2005. [DOI] [PubMed] [Google Scholar]
- Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, Paumier K, Daley BF, Gombash S, Madhavan L, Mandybur GT, Lipton JW, Terpstra BT, Sortwell CE. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis 39: 105–115, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieles-Engemann AL, Steece-Collier K, Behbehani MM, Collier TJ, Wohlgenant SL, Kemp CJ, Cole-Strauss A, Levine ND, Gombash SE, Thompson VB, Lipton JW, Sortwell CE. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis 1: 123–136, 2011. [PMC free article] [PubMed] [Google Scholar]
- Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J Neurosci 28: 10961–10971, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA. Deep brain stimulation for other tremors, myoclonus, and chorea. Handb Clin Neurol 116: 209–215, 2013. [DOI] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. J Neurophysiol 93: 3165–3176, 2005. [DOI] [PubMed] [Google Scholar]
- Stefani A, Fedele E, Galati S, Pepicelli O, Frasca S, Pierantozzi M, Peppe A, Brusa L, Orlacchio A, Hainsworth AH, Gattoni G, Stanzione P, Bernardi G, Raiteri M, Mazzone P. Subthalamic stimulation activates internal pallidus: evidence from cGMP microdialysis in PD patients. Ann Neurol 57: 448–452, 2005. [DOI] [PubMed] [Google Scholar]
- Stefani A, Fedele E, Galati S, Raiteri M, Pepicelli O, Brusa L, Pierantozzi M, Peppe A, Pisani A, Gattoni G, Hainsworth AH, Bernardi G, Stanzione P, Mazzone P. Deep brain stimulation in Parkinson's disease patients: biochemical evidence. J Neural Transm Suppl 2006: 401–408, 2006. [DOI] [PubMed] [Google Scholar]
- Stefani A, Fedele E, Pierantozzi M, Galati S, Marzetti F, Peppe A, Pastore FS, Bernardi G, Stanzione P. Reduced GABA content in the motor thalamus during effective deep brain stimulation of the subthalamic nucleus. Front Syst Neurosci 5: 17, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain 130: 1596–1607, 2007. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Lozano AM, Ballanger B, Poon YY, Lang AE, Moro E. rCBF changes associated with PPN stimulation in a patient with Parkinson's disease: a PET study. Mov Disord 23: 1051–1054, 2008. [DOI] [PubMed] [Google Scholar]
- Struppler A, Erbel F, Velho F. An overview on the pathophysiology of parkinsonian and other pathological tremors. In: Physiological Tremor, Pathological Tremors and Clonus, edited by Desmedt JE. Basel: Karger, 1978. [Google Scholar]
- Suetens K, Nuttin B, Gabriëls L, Van Laere K. Differences in metabolic network modulation between capsulotomy and deep-brain stimulation for refractory obsessive-compulsive disorder. J Nucl Med 55: 951–959, 2014. [DOI] [PubMed] [Google Scholar]
- Sydow O, Thobois S, Alesch F, Speelman JD. Multicentre European study of thalamic stimulation in essential tremor: a six year follow up. J Neurol Neurosurg Psychiatry 74: 1387–1391, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y, Iwamuro H, Kita H, Takada M, Nambu A. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur J Neurosci 34: 1470–1484, 2011. [DOI] [PubMed] [Google Scholar]
- Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB J 17: 1820–1830, 2003. [DOI] [PubMed] [Google Scholar]
- Tang CC, Eidelberg D. Brain stimulation and functional imaging with fMRI and PET. Handb Clin Neurol 116: 77–95, 2013. [DOI] [PubMed] [Google Scholar]
- Tass PA, Qin L, Hauptmann C, Dovero S, Bezard E, Boraud T. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol 72: 816–820, 2012. [DOI] [PubMed] [Google Scholar]
- Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, Blokland A, Schmitz C, Steinbusch HW. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res 1120: 100–105, 2006. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60: 78–81, 2003. [DOI] [PubMed] [Google Scholar]
- Tierney TS, Abd-El-Barr MM, Stanford AD, Foote KD, Okun MS. Deep brain stimulation and ablation for obsessive compulsive disorder: evolution of contemporary indications, targets and techniques. Int J Neurosci 124: 394–402, 2014. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain 126: 199–212, 2003. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, Treuer H, Sturm V, Schnitzler A. Ten-Hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson's disease. Mov Disord 19: 1328–1333, 2004. [DOI] [PubMed] [Google Scholar]
- Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg 108: 132–138, 2008. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: an electrophysiological study in the macaque monkey. Cereb Cortex 10: 58–68, 2000. [DOI] [PubMed] [Google Scholar]
- Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, Ma Y, Eidelberg D. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. Neuroimage 31: 301–307, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang EW, Hamani C, Moro E, Mazzella F, Saha U, Lozano AM, Hodaie M, Chuang R, Steeves T, Lim SY, Neagu B, Chen R. Subthalamic deep brain stimulation at individualized frequencies for Parkinson disease. Neurology 78: 1930–1938, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushe M, Mink JW, Tabbal SD, Hong M, Schneider Gibson P, Rich KM, Lyons KE, Pahwa R, Perlmutter JS. Postural tremor suppression is dependent on thalamic stimulation frequency. Mov Disord 21: 1290–1292, 2006. [DOI] [PubMed] [Google Scholar]
- van Dijk A, Klompmakers AA, Feenstra MG, Denys D. Deep brain stimulation of the accumbens increases dopamine, serotonin, and noradrenaline in the prefrontal cortex. J Neurochem 123: 897–903, 2012. [DOI] [PubMed] [Google Scholar]
- van Dijk A, Mason O, Klompmakers AA, Feenstra MG, Denys D. Unilateral deep brain stimulation in the nucleus accumbens core does not affect local monoamine release. J Neurosci Methods 202: 113–118, 2011. [DOI] [PubMed] [Google Scholar]
- van Hartevelt TJ, Cabral J, Deco G, Møller A, Green AL, Aziz TZ, Kringelbach ML. Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson's disease. PLoS One 9: e86496, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedam-Mai V, Gardner B, Okun MS, Siebzehnrubl FA, Kam M, Aponso P, Steindler DA, Yachnis AT, Neal D, Oliver BU, Rath SJ, Faull RL, Reynolds BA, Curtis MA. Increased precursor cell proliferation after deep brain stimulation for Parkinson's disease: a human study. PLoS One 9: e88770, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez-Lago FM, Oyama G, Foote KD, Hwynn N, Zeilman P, Jacobson C, Wu S, Okun MS. Low-frequency deep brain stimulation for dystonia: lower is not always better. Tremor Other Hyperkinet Mov (NY) 2: tre-02-55-272-1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet M, Yelnik J, Lagrange C, Fraix V, Grabli D, Thobois S, Burbaud P, Welter ML, Xie-Brustolin J, Braga MC, Ardouin C, Czernecki V, Klinger H, Chabardès S, Seigneuret E, Mertens P, Cuny E, Navarro S, Cornu P, Benabid AL, Le Bas JF, Dormont D, Hermier M, Dujardin K, Blond S, Krystkowiak P, Destée A, Bardinet E, Agid Y, Krack P, Broussolle E, Pollak P, French SPIDY2 Study Group. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol 8: 709–717, 2009. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo JF, Jankovic J. Deep brain stimulation for Tourette syndrome: target selection. Stereotact Funct Neurosurg 90: 213–224, 2012. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 17: S69–S72, 2002. [DOI] [PubMed] [Google Scholar]
- Walker HC, Huang H, Gonzalez CL, Bryant JE, Killen J, Cutter GR, Knowlton RC, Montgomery EB, Guthrie BL, Watts RL. Short latency activation of cortex during clinically effective subthalamic deep brain stimulation for Parkinson's disease. Mov Disord 27: 864–873, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker HC, Huang H, Gonzalez CL, Bryant JE, Killen J, Knowlton RC, Montgomery EB, Cutter GC, Yildirim A, Guthrie BL, Watts RL. Short latency activation of cortex by clinically effective thalamic brain stimulation for tremor. Mov Disord 27: 1404–1412, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, Benabid AL. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain 130: 2129–2145, 2007. [DOI] [PubMed] [Google Scholar]
- Wang J, Hirschmann J, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. High-frequency oscillations in Parkinson's disease: spatial distribution and clinical relevance. Mov Disord 29: 1265–1272, 2014. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, CSP 468 Study Group. Bilateral deep brain stimulation vs. best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301: 63–73, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Alavi M, Hodaie M, Lozano AM, Moro E, Dostrovsky JO. Oscillatory activity in the globus pallidus internus: comparison between Parkinson's disease and dystonia. Clin Neurophysiol 123: 358–368, 2012. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson's disease patients. J Neurophysiol 101: 789–802, 2009. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol 96: 3248–3256, 2006. [DOI] [PubMed] [Google Scholar]
- Welter ML, Burbaud P, Fernandez-Vidal S, Bardinet E, Coste J, Borg M, Besnard S, Sauleau P, Devaux B, Pidoux B, Chaynes P, Tézenas du Montcel S, Bastian A, Langbour N, Teillant A, Haynes W, Yelnik J, Karachi C, Mallet L, French Stimulation dans Trouble Obsessionnel Compulsif (STOC) Study Group. Basal ganglia dysfunction in OCD: subthalamic neuronal activity correlates with symptoms severity and predicts high-frequency stimulation efficacy. Transl Psychiatry 1: e5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter ML, Houeto JL, Bonnet AM, Bejjani PB, Mesnage V, Dormont D, Navarro S, Cornu P, Agid Y, Pidoux B. Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch Neurol 61: 89–96, 2004. [DOI] [PubMed] [Google Scholar]
- Whiting DM, Tomycz ND, Bailes J, de Jonge L, Lecoultre V, Wilent B, Alcindor D, Prostko ER, Cheng BC, Angle C, Cantella D, Whiting BB, Mizes JS, Finnis KW, Ravussin E, Oh MY. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg 119: 56–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease. Front Hum Neurosci 6: 155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 72: 521–530, 1994. [DOI] [PubMed] [Google Scholar]
- Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K, PD SURG Collaborative Group . Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 9: 581–591, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Tijssen M, van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain 125: 1558–1569, 2002. [DOI] [PubMed] [Google Scholar]
- Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM. Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson's disease. Exp Neurol 197: 244–251, 2006. [DOI] [PubMed] [Google Scholar]
- Wongsarnpigoon A, Grill WM. Energy-efficient waveform shapes for neural stimulation revealed with a genetic algorithm. J Neural Eng 7: 046009, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wang J, Peng S, Ma Y, Zhang H, Guan Y, Zuo C. Metabolic brain network in the Chinese patients with Parkinson's disease based on 18F-FDG PET imaging. Parkinsonism Relat Disord 19: 622–627, 2013. [DOI] [PubMed] [Google Scholar]
- Yamawaki N, Magill PJ, Woodhall GL, Hall SD, Stanford IM. Frequency selectivity and dopamine-dependence of plasticity at glutamatergic synapses in the subthalamic nucleus. Neuroscience 203: 1–11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Chen N, Zhu H, Zhang J, Sim M, Ma Y, Wang W. High-frequency stimulation of nucleus accumbens changes in dopaminergic reward circuit. PLoS One 8: e79318, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AI, Vanegas N, Lungu C, Zaghloul KA. Beta-coupled high-frequency activity and beta-locked neuronal spiking in the subthalamic nucleus of Parkinson's disease. J Neurosci 34: 12816–12827, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yianni J, Bain PG, Gregory RP, Nandi D, Joint C, Scott RB, Stein JF, Aziz TZ. Post-operative progress of dystonia patients following globus pallidus internus deep brain stimulation. Eur J Neurol 10: 239–247, 2003. [DOI] [PubMed] [Google Scholar]
- Yianni J, Bradley K, Soper N, O'Sullivan V, Nandi D, Gregory R, Stein J, Aziz T. Effect of GPi DBS on functional imaging of the brain in dystonia. J Clin Neurosci 12: 137–141, 2005. [DOI] [PubMed] [Google Scholar]
- Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. [DOI] [PubMed] [Google Scholar]
- Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson's disease. Brain 133: 2007–2021, 2010. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wei NL, Wang Y, Wang X, Zhang JG, Zhang K. Deep brain stimulation of the nucleus accumbens shell induces anti-obesity effects in obese rats with alteration of dopamine neurotransmission. Neurosci Lett 589C: 1–6, 2015. [DOI] [PubMed] [Google Scholar]
- Zhang K, Bhatia S, Oh MY, Cohen D, Angle C, Whiting D. Long-term results of thalamic deep brain stimulation for essential tremor. J Neurosurg 112: 1271–1276, 2010. [DOI] [PubMed] [Google Scholar]
- Zsigmond P, Dernroth N, Kullman A, Augustinsson LE, Dizdar N. Stereotactic microdialysis of the basal ganglia in Parkinson's disease. J Neurosci Methods 207: 17–22, 2012. [DOI] [PubMed] [Google Scholar]