Abstract
In nonhuman primates, tactile representation at the cortical level has mostly been studied using single-cell recordings targeted to specific cortical areas. In this study, we explored the representation of tactile information delivered to the face or the shoulders at the whole brain level, using functional magnetic resonance imaging (fMRI) in the nonhuman primate. We used air puffs delivered to the center of the face, the periphery of the face, or the shoulders. These stimulations elicited activations in numerous cortical areas, encompassing the primary and secondary somatosensory areas, prefrontal and premotor areas, and parietal, temporal, and cingulate areas as well as low-level visual cortex. Importantly, a specific parieto-temporo-prefrontal network responded to the three stimulations but presented a marked preference for air puffs directed to the center of the face. This network corresponds to areas that are also involved in near-space representation, as well as in the multisensory integration of information at the interface between this near space and the skin of the face, and is probably involved in the construction of a peripersonal space representation around the head.
Keywords: tactile, face, fMRI, head, nonhuman primate
the representation of tactile information at the cortical level has been studied for a long time, first by virtue of single-cell recordings in animal models like the macaque monkey, and more recently using imaging techniques in humans. Most of the studies have focused on describing how the tactile information is spatially represented in the primary and secondary somatosensory areas. In the nonhuman primate, the primary somatosensory area (SI) encompasses areas 1, 2, 3a, and 3b in the central sulcus and on its posterior convexity and contains several somatotopic representations of the body (e.g., Kaas et al. 1979; Nelson et al. 1980; Pons et al. 1985, 1987; Sur et al. 1982). The secondary somatosensory area (SII) is located within the lateral sulcus and, with its neighboring areas such as the parietal ventral area (PV), also contains several somatotopic representations (e.g., Coq et al. 2004; Fitzgerald et al. 2004, 2006; Krubitzer et al. 1995). In humans, most of the functional imaging studies have aimed at identifying the homolog of the primary and secondary somatosensory areas and their somatotopic organization, using mainly tactile stimulations of the hand and digits (e.g., Ann Stringer et al. 2014; Besle et al. 2013; Burton et al. 2008; Eickhoff et al. 2007, 2008; Martuzzi et al. 2014; McGlone et al. 2002; Polonara et al. 1999; Sanchez-Panchuelo et al. 2010, 2012; Schweisfurth et al. 2014) and/or on the face (e.g., Eickhoff et al. 2007, 2008; Kopietz et al. 2009; Lin et al. 2010; Nguyen et al. 2004, 2005).
However, tactile responses have been observed outside of SI and SII in the nonhuman primate. Neuronal responses to somatosensory stimulations have notably been described in the parietal cortex [area 5, area 7b, ventral intraparietal area (VIP); e.g., Avillac et al. 2005, 2007; Dong et al. 1994; Duhamel et al. 1998; Murray and Mishkin 1984] and in the premotor cortex (area 6; e.g., Rizzolatti et al. 1981). In contrast, few human studies describe tactile-related neuronal activations outside of areas SI or SII, and when they do, these studies often focus on very specific, theory-driven regions of interest (e.g., Bremmer et al. 2001 for the identification of human ventral intraparietal area). For example, the team of Sereno and colleagues has very nicely described the somatotopic representation of the face and body in the parietal cortex (Huang and Sereno 2007; Huang et al. 2012; Sereno and Huang 2006) but has not described how their stimulations evoked activations in other cortical areas.
In this study, we addressed the question of the representation of the tactile information at the whole brain level by using functional magnetic resonance imaging (fMRI) in the nonhuman primate. This approach allows us to bridge the gap between single-cell recordings and anatomical studies performed in this animal model, and the whole brain imaging methodology used in humans. We used three locations of tactile stimulations, on the center of the face, the periphery of the face, and the shoulders, to assess possible topographic organizations or reveal tactile coding preferences without restricting our observations to the primary or secondary somatosensory areas (e.g., Chen et al. 2011). During the experiment, the acquisition of tactile data was interleaved with visual and auditory conditions. The multisensory convergence results have been reported previously within the intraparietal sulcus (Guipponi et al. 2013) and at the whole brain level (Guipponi et al. 2015). In this article, we focus on the tactile representation.
MATERIAL AND METHODS
Subjects and Materials
Two rhesus monkeys (monkey M1, female; monkey M2, male; 5–7 yr old, 5–7 kg) participated in the study. The animals were implanted with a plastic MRI-compatible headset covered by dental acrylic. The anesthesia during surgery was induced by tiletamine-zolazepam (Zoletil, 15 mg/kg; Virbac, NSW, Australia) and followed by isoflurane (Belamont, 1–2%). Postsurgery analgesia was ensured with buprenorphine (Temgesic, 0.3 mg/ml, 0.01 mg/kg). During recovery, proper analgesic and antibiotic coverage were provided. All procedures were in compliance with the guidelines of the European Community on animal care (European Community Council, Directive No. 86-609, November 24, 1986). All the protocols used in this experiment were approved by the animal care committee (Department of Veterinary Services, Health & Protection of Animals, permit no. 69 029 0401) and the biology department of the University Claude Bernard Lyon 1. The animals' welfare and the steps taken to ameliorate suffering were in accordance with the recommendations of the Weatherall report, The use of nonhuman primates in research (https://royalsociety.org/topics-policy/publications/2006/weatherall-report/).
During the scanning sessions, monkeys sat in a sphinx position in a plastic monkey chair positioned within a horizontal magnet (1.5T MR scanner Sonata; Siemens, Erlangen, Germany) facing a translucent screen placed 90 cm from the eyes. The head was restrained and equipped with MRI-compatible headphones customized for monkeys (MR Confon, Magdeburg, Germany). A radial receive-only surface coil (10-cm diameter) was positioned above the head. Eye position was monitored at 120 Hz during scanning using a pupil-corneal reflection tracking system (ISCAN, Cambridge, MA). Monkeys were rewarded with liquid dispensed by a computer-controlled reward delivery system (Crist Instruments). The task and all the behavioral parameters, as well as the sensory stimulations, were controlled by two computers running MATLAB and Presentation software. The fixation point the monkeys were instructed to fixate was projected onto a screen with a Canon XEED SX60 projector. Tactile stimulations were delivered through Teflon tubing and six articulated plastic arms connected to distant air pressure electrovalves.
Task and Stimuli
The animals were trained to maintain fixation on a red central spot (0.24° × 0.24°) while tactile stimulations were delivered. The monkeys were rewarded for staying within a 2° × 2° tolerance window centered on the fixation spot. The reward delivery was scheduled to encourage long fixation without breaks (i.e., the interval between successive deliveries was decreased and their amount was increased, up to a fixed limit, as long as the eyes did not leave the window).
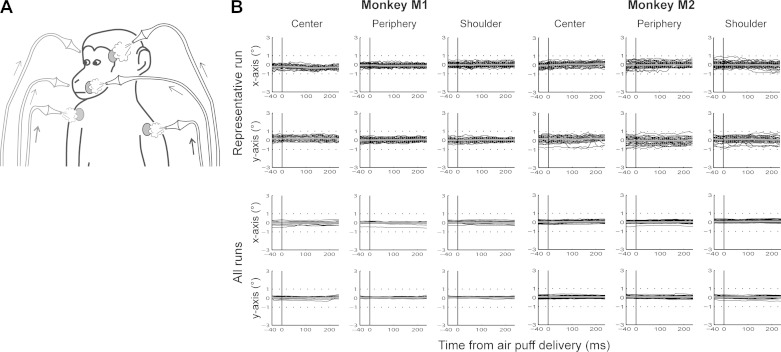
The tactile stimulations consisted in air puffs delivered to three different locations bilaterally on the animals' body (Fig. 1A): 1) center of the face, close to the nose and the mouth; 2) periphery of the face, above the eyebrows; and 3) shoulders. The intensity of the stimulations ranged from 0.5 bar (center/periphery) to 1 bar (shoulders), to adjust for the larger distance between the extremity of the stimulation tubes and the skin, as well as for the difference in hair density. Within a given block, left and right stimulations were pseudorandomly interleaved, with each stimulation lasting between 400 and 500 ms and the interstimulation interval between 500 and 1,000 ms (Guipponi et al. 2013).
Fig. 1.
A: tactile stimulations. Air puffs were delivered to the center of the face, the periphery of the face, or the shoulders, pseudorandomly on the left and right sides of the monkeys. B: eye movements (x- and y-axes, in degrees) following air-puff delivery (0 ms) for each monkey for a representative run (chosen randomly: monkey M1, session 12 run 03; monkey M2, session 06 run 03) and for all runs. For the individual runs, the black lines correspond to individual eye traces following air-puff delivery, and the gray lines to the average over all these eye traces. For all runs, the black lines correspond to average eye traces following air-puff delivery for individual runs, and the gray lines to the grand average over all runs. For each monkey, panels at left correspond to stimulations delivered to the center of the face, at middle to stimulations to the periphery of the face, and at right to stimulations to the shoulders.
Functional time series (runs) were organized as follows: a 10-volume block of pure fixation (baseline) was followed by a 10-volume block of each of the three tactile stimulation types; this sequence was played 4 times, resulting in a 160-volume run. The three tactile blocks were presented in six counterbalanced possible orders.
Scanning
Before each scanning session, a contrast agent, monocrystalline iron oxide nanoparticle [Sinerem (Guerbet) or Feraheme (AMAG Pharmaceuticals); Vanduffel et al. 2001], was injected into the animal's femoral/saphenous vein (4–10 mg/kg). For the sake of clarity, the polarity of the contrast agent MR signal changes, which are negative for increased blood volumes, was inverted. We acquired gradient-echo echoplanar images covering the whole brain [1.5T; repetition time, 2.08 s; echo time, 27 ms; 32 sagittal slices; 2 × 2 × 2-mm voxels]. During each scanning session, the tactile runs were interleaved with visual and auditory runs, not exploited in this report (Guipponi et al. 2013).
Analysis
On the 36 and 40 runs acquired in monkeys M1 and M2, respectively, the 21 best runs were selected in each monkey, based on the quality of the monkeys' fixation throughout each run (>85% within the tolerance window). Time series were analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). For spatial preprocessing, functional volumes were first realigned and rigidly coregistered with the anatomy of each individual monkey (T1-weighted MPRAGE 3D 0.6 × 0.6 × 0.6-mm or 0.5 × 0.5 × 0.5-mm voxel acquired at 1.5T) in stereotactic space. The JIP program (Mandeville et al. 2011) was used to perform a nonrigid coregistration (warping) of a mean functional image onto the individual anatomies. The same procedure was used to coregister the functional images of monkey M1 onto the anatomy of monkey M2 (i.e., realignment followed by a rigid and then a nonrigid coregistration).
Fixed-effect individual analyses were performed in each monkey, with a level of significance set at P < 0.001 uncorrected and P < 0.05 corrected for multiple comparisons [familywise error (FEW), t > 4.89]. We also performed individual conjunction analyses, i.e., the conjunction of several individual contrasts, aiming at revealing the regions commonly activated in all the individual contrasts of choice. For these conjunction analyses, the statistical levels were set at P < 0.001 at uncorrected level. In all analyses, realignment parameters, as well as eye movement traces, were included as covariates of no interest to remove eye movement and brain motion artifacts. When coordinates are provided, they are expressed with respect to the anterior commissure. Results are displayed on flattened maps obtained with Caret (Van Essen et al. 2001; http://www.nitrc.org/projects/caret/).
Potential Covariates
In all analyses, as stated above, realignment parameters, as well as eye movement traces, were included as covariates of no interest to remove eye movement and brain motion artifacts. However, some of the stimulations might have induced a specific behavioral pattern biasing our analysis, not fully accounted for by the above regressors. Although we cannot completely rule out this possibility, our experimental setup allows us to minimize its impact. Monkeys worked head-restrained (to maintain the brain at the optimal position within the scanner, to minimize movement artifacts on the fMRI signal, and to allow for a precise monitoring of their eye movements).
Uncertainty about tactile stimulation location.
As a result of the head restraint, the tactile stimulations to the center were stable in a given session. When monkeys were drinking the liquid reward, small lip movements occurred. These movements thus correlated with reward timing and were on average equally distributed over the different sensory runs and the different conditions within each run (we checked that the monkeys had equal performance among the different conditions within a given run). The center of the face air puffs was placed on the cheeks on each side of the monkey's nose, at a location that was not affected by the lip movements. Peripheral body stimulation air puffs were directed to the shoulders, at a location that was not affected by possible arms movements by the monkey. This was possible because the monkey chair tightly fit the monkey's width.
Perceived intensity of the tactile stimulations.
Air-puff intensity was adjusted to be fully perceived by the monkeys. Center and periphery of the face stimulations were set at the same air-puff intensity. As a result, they thus stimulated an equivalent skin surface (same physical properties), although periphery of the face stimuli most probably were perceived as less intense due to a thicker skin (different perceived intensities). This allows for the direct comparison of the resultant activations performed in the present study. Shoulder air-puff stimulations had to go through the monkeys' fur, reaching the skin with an equal or higher intensity than at the face or periphery of the face stimulations (although possibly a lower perceived intensity). The overall lower shoulder tactile activations thus reflect a genuine difference between face and shoulder somatosensory representations, when these activations are referenced to the objective stimulus strength.
Air puffs.
Air puffs are often considered as aversive stimuli evoking a wide variety of emotional overt behavioral reactions such as eye movements, blinks, or facial mimics. In our study, the intensity of the air puffs was chosen so that the tactile stimulations were well above detection threshold, yet did not evoke any overt behavior in the monkey following the habituation period. However, at the beginning of the training period, monkeys were curious about these novel stimuli and did have typical blink and eye exploration patterns for the center and periphery of the face stimuli and hand exploration patterns for the shoulder tactile stimulations, indirectly indicating that these stimuli were indeed perceived by the monkeys. Such advert movements interrupted the reward schedule because the fixation criterion was violated. The monkeys quickly habituated to the stimuli and reverted to the expected fixation behavior. As a result, the air puffs did not evoke eye movements during scanning acquisitions (Fig. 1B). Confirming this, the monkeys' fixation performance did not vary between the tactile and visual runs (not used in this report but interleaved with the tactile runs; 1-way ANOVA: monkey M1, P = 0.98; monkey M2, P = 0.23) or between the different conditions of the tactile runs (repeated-measures 1-way ANOVA: monkey M1, P = 0.596; monkey M2, P > 0.2). The potential eye movement or blink confounds are further explored below. Air puffs are also often suspected to activate the auditory system. In the present study, the air-puff delivery system was placed outside the MRI scanner room, and the monkeys were wearing headphones to protect their hearing from the high-intensity sound produced by the scanner. By placing a microphone inside the headphones, we confirmed that no air puff-triggered sound could be recorded, whether in the absence or presence of a weak MRI scanner noise (see additional information of Guipponi et al. 2015). We also showed that none of the tactile contrasts of interest produced an activation similar to that obtained for pure auditory stimulations (see additional information of Guipponi et al. 2015) in the core auditory region on the inferior lateral bank of the lateral sulcus.
Eye movements.
Monkeys were required to maintain their gaze on a small fixation point within a tolerance window of 2° × 2°. This was controlled online and was used to motivate the animal to maximize fixation rates (because fixation disruptions, such as saccades or drifts, affected the reward schedule). Eye traces were also analyzed offline for the selection of the runs to include in the analysis (good fixation for 85% of the run duration, with no major fixation interruptions). A statistical analysis (already mentioned above) indicates that the monkeys' performance was not significantly different across the visual or tactile (1-way ANOVA: monkey M1, P = 0.75; monkey M2, P = 0.65). This suggests that the overall oculomotor behavior was constant across types of runs. Importantly, the frontal eye fields (Bruce and Goldberg 1985) and the lateral intraparietal area (Barash et al. 1991; Ben Hamed et al. 2001, 2002), two key cortical oculomotor regions that are activated by eye movements including microsaccades, are not identified in our successive analyses, although their activity can be shown to correlate with the eye movements regressor of our fMRI design (data not shown).
Eye blinks.
Last, air puffs to the face might have evoked eye blinks, inducing some degree of variability in the point of impact of the air puffs, but most importantly might have led to a confound between eye blink activations and face tactile activations. In a recent study (Guipponi et al. 2014), we identified the cortical network activated by eye blinks. Several regions highlighted by this analysis, such as the lateral most portion of precentral area ProM, are not reported in the present study. This greatly minimizes blinks as a potential confound.
Reward schedule.
The reward schedule was the same between the different conditions of the tactile runs and was directly related to the fixation performance of the animals. Since performance was similar in the different conditions (see above), reward schedule was also similar.
Nomenclature
Several competing nomenclatures coexist in the literature to describe specific cortical sectors on the basis of their distinctive cytological and architectonic patterns, connectivity patterns, and/or functional single-cell responses. For the description of premotor and prefrontal activations, we have mostly relied on the “F” notation proposed by Matelli and Luppino (2001) and Rizzolatti and Luppino (2001). To allow for a direct comparison with human fMRI studies, we also use a more standard reference to the rostrocaudal medial, dorsal, and ventral premotor areas (Dum and Strick 2002; Mendoza and Merchant 2014).
RESULTS
A total of 21 runs were used for the analysis in each monkey, selected on the basis of quality of the fixation. Behavioral parameters such as fixation quality and number of blinks were quantified and did not differ as a function of the location of tactile stimulation (see material and methods). Only individual analyses were performed, and except for the initial description of the activations, only the cortical regions activated in at least three of four hemispheres were considered as robust results. Subcortical activations are not described in the present work.
Description of the Regions Activated for Each Tactile Stimulation
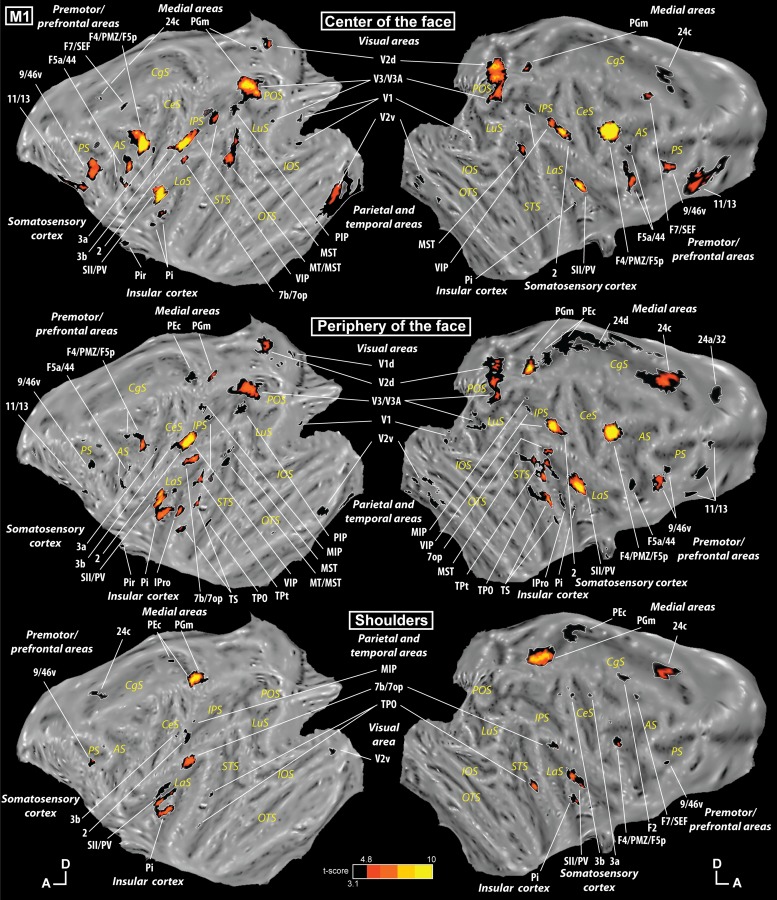
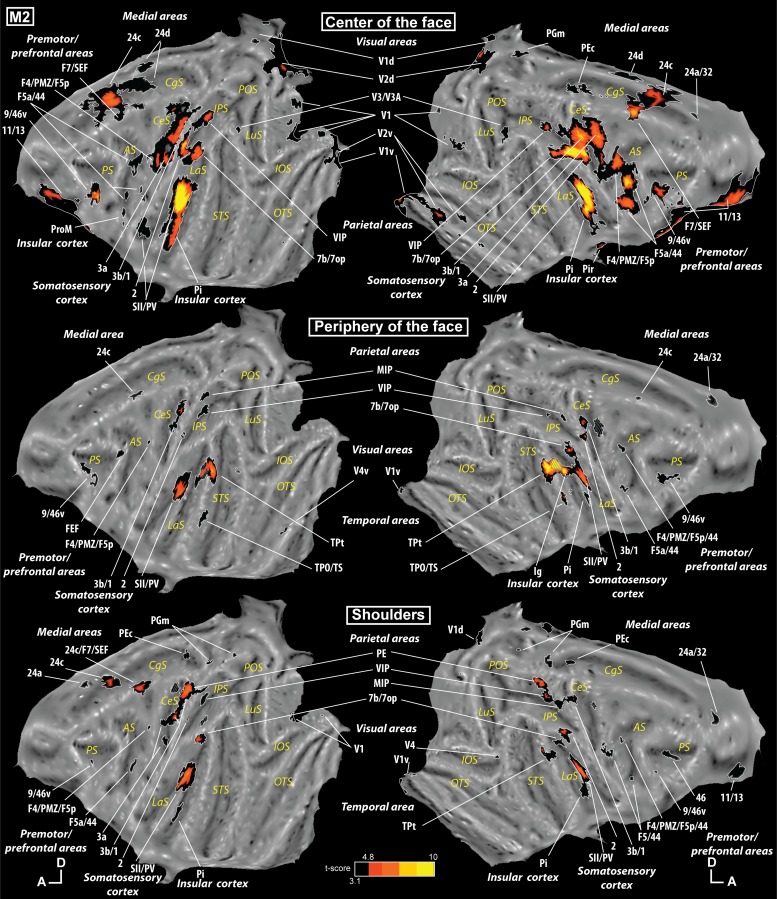
Our tactile stimulations elicited responses in numerous cortical areas. The tactile stimulation at the center of the face was the one activating the largest number of regions (contrast [center of the face vs. fixation]). As can be seen in Fig. 2, top (monkey M1), and Fig. 3, top (monkey M2), this stimulation elicited a response in somatosensory regions such as areas 1, 2, 3a, and 3b in the central sulcus and areas SII and PV in the lateral sulcus. However, it also produced an activation in several frontal regions (orbitofrontal cortex, ventrolateral prefrontal cortex, premotor regions), in the parietal cortex (in the intraparietal sulcus, in the inferior parietal convexity, and on the medial wall), in the temporal cortex (but only in M1), in the cingulate cortex, in the insular cortex, and in the occipital cortex. All the individual activations are listed in Supplemental Tables S1, S2, and S3 for the center of the face, periphery of the face, and shoulders, respectively. (Supplemental material for this article is available online at the Journal of Neurophysiology website.)
Fig. 2.
Individual activations in monkey M1. Statistical parametric maps (SPMs) are presented on the flattened representation of the cortex of monkey M1 obtained with Caret. Top shows the activations corresponding to stimulation to the center of the face (contrast [center of the face vs. fixation]), middle shows the activations corresponding to stimulation to the periphery of the face (contrast [periphery of the face vs. fixation]), and bottom shows the activations corresponding to stimulation to the shoulders (contrast [shoulders vs. fixation]). The results are presented at corrected and uncorrected levels [black regions correspond to t-scores ≥ 3.1, P < 0.001 uncorrected level, and hot-colored regions correspond to t-scores > 4.8, P < 0.05, familywise error (FEW)-corrected level]. Gray hyphenated areas represent activations spilling over the other bank of a sulcus or a gyrus during the flattening. Scale: A, anterior; D, dorsal. Cortical sulci: AS, arcuate sulcus; CgS, cingulate sulcus; CeS, central sulcus; IOS, inferior occipital sulcus; IPS, intraparietal sulcus; LaS, lateral sulcus; LuS, lunate sulcus; PS, principal sulcus; STS, superior temporal sulcus.
Fig. 3.
Individual activations in monkey M2. Same conventions as in Fig. 2.
The tactile stimulation at the periphery of the face (contrast [periphery of the face vs. fixation]) also activated several cortical regions: somatosensory regions in the central and lateral sulci, frontal activations (ventrolateral prefrontal and premotor regions), parietal cortex (in the intraparietal sulcus, in the inferior parietal convexity, and on the medial wall in M1), cingulate cortex, and insular cortex, but also large temporal activations in the superior bank of the superior temporal sulcus and the adjacent convexity (Figs. 2 and 3, middle).
The tactile stimulation on the shoulders (contrast [shoulders vs. fixation]) elicited the least activations but with a similar pattern to that for stimulation at the periphery of the face: somatosensory regions in the central and lateral sulci, frontal activations (ventrolateral prefrontal and premotor regions), parietal cortex (in the intraparietal sulcus, in the inferior parietal convexity, and on the medial wall), cingulate cortex, insular cortex, and temporal activations (superior bank of the superior temporal sulcus) as shown in Figs. 2 and 3, bottom.
Regions Activated by Both Face and Shoulder Stimulations
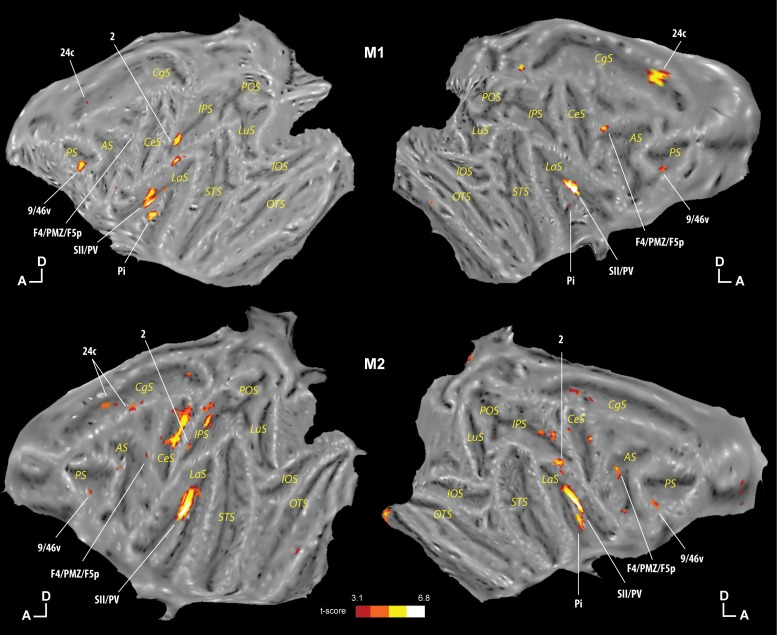
To identify the cortical regions that respond to tactile stimulations irrespectively of whether these were directed to the face or to the shoulders, i.e., the regions with no apparent topographical organization, we performed a conjunction analysis of the three contrasts previously described ([center of the face vs. fixation] ∩ [periphery of the face vs. fixation] ∩ [shoulders vs. fixation]). We describe only the regions present in the two monkeys (at least 3 of 4 hemispheres). As can be seen in Fig. 4, these regions are somatosensory regions area 2 and SII/PV, insular region Pi, premotor region F4/PMZ/F5p (PMv), ventrolateral prefrontal area 9/46v, and cingulate area 24c.
Fig. 4.
Regions commonly activated for the 3 tactile stimulations. The results of the conjunction analysis ([center of the face vs. fixation] ∩ [periphery of the face vs. fixation] ∩ [shoulders vs. fixation]) are presented individually for monkey M1 (top) and monkey M2 (bottom). The results are presented at uncorrected levels (hot-colored regions correspond to t-scores ≥ 3.1, P < 0.001 uncorrected level). Other conventions are the same as in Fig. 2.
Regions Preferentially or Exclusively Representing Stimulation of One Body Part
The previous analysis describes cortical regions that are activated by both shoulder and face tactile stimulations. Here, we are interested in identifying either cortical regions that are significantly more activated by tactile stimulations to one body part than the others, or cortical regions that are activated exclusively by only one type of tactile stimulation. Specifically, to test for a preference for either the center of the face, the periphery of the face, or the shoulders, we performed conjunction analyses of pairs of contrasts. For example, the conjunction analysis revealing the preference for stimulation at the center of the face was [center of the face vs. periphery of the face] ∩ [center of the face vs. shoulders] (masked inclusively by [center of the face vs. fixation] to show only positive activations). The results of this analysis are shown in Fig. 5 in purple for the preference for stimulation at the center of the face, in green for the preference for stimulation at the periphery of the face, and in blue for the preference for stimulation on the shoulders. This set of analyses reveals regions that prefer one site of stimulation over the two other sites, but not necessarily that represent exclusively that location of stimulation. To assess exclusivity, we further performed the simple contrast between one site of stimulation and fixation, masked exclusively by the conjunction between the two other contrasts and fixation, so as to subtract any regions responding to the two other sites of stimulation (for example, for stimulation at the center of the face: [center of the face vs. fixation] masked exclusively by [periphery of the face vs. fixation] + [shoulders vs. fixation]). These analyses allowed us to draw the black outlines on the purple, green, and blue regions in Fig. 5, showing the regions representing exclusively the center of the face (purple), the periphery of the face (green), or the shoulders (blue). As previously, only the regions present in the two monkeys are described (at least 3 of 4 hemispheres).
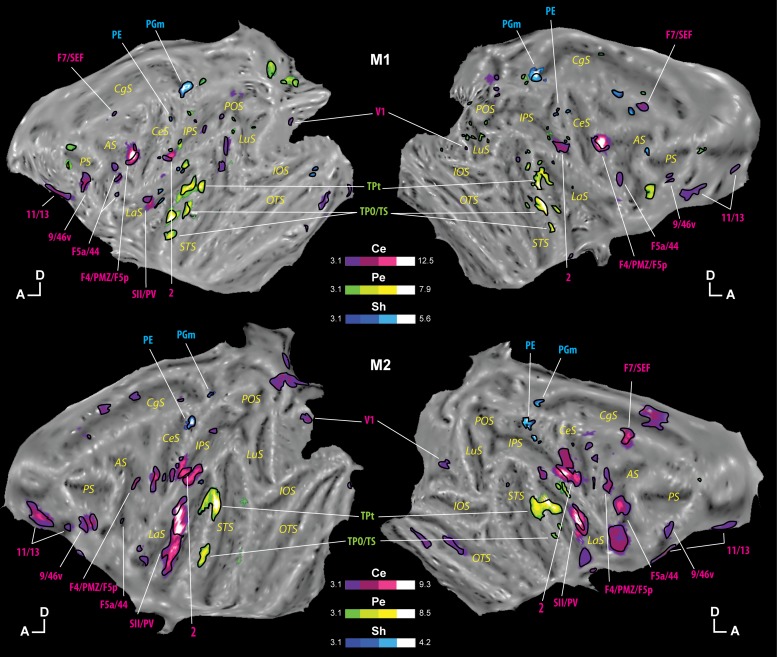
Fig. 5.
Preferential representations of each of the 3 tactile stimulations. Regions that statistically represent preferentially the center of the face over the 2 other stimulations are presented in purple (Ce), those preferring the periphery of the face in green (Pe), and those preferring the shoulders in blue (Sh; t-scores ≥ 3.1, P < 0.001 uncorrected level). Within these regions, those representing exclusively the center of the face, periphery of the face, or shoulders are outlined in black (see results for the exact statistical analyses). The results are presented individually for monkey M1 (top) and monkey M2 (bottom). Other conventions are the same as in Fig. 2.
Representation of the center of the face.
Very interestingly, within the regions preferring the tactile stimulation to the center of the face, we observed four regions already described as responding to the three sites of stimulation: area 2, area SII/PV, area F4/PMZ/F5p (PMv), and area 9/46v. In these four regions, several parts can be described: a part representing the three stimulations but preferring the site at the center of the face, and a part (or multiple subparts) representing exclusively the site at the center of the face. It is to be noted that two regions representing the three sites of stimulation, areas Pi and 24c, do not exhibit any preferential topographical representation.
The other regions preferring the location at the center of the face appear to represent it exclusively: orbitofrontal areas 11/13, premotor areas F5a/44 (PMvr), and F7/SEF, as well as parts of area V1. With the use of an independent retinotopic localizer, these V1 activations could be localized in both the central and the peripheral visual field representations, although activations were more pronounced in the peripheral visual field representation, confirming what we have already described in Guipponi et al. (2015; see specifically Fig. 4).
Representation of the periphery of the face.
Very few regions prefer stimulation at the periphery of the face. The regions common to both animals are the temporal areas TPt and TPO/TS. Importantly, these areas show a strong exclusive representation of the periphery of the face.
Representation of the shoulders.
Even fewer regions show a preference for stimulation of the shoulders. These are medial parietal areas PE and PGm, which appear to represent the shoulders exclusively.
DISCUSSION
We describe a large bilateral cortical network activated by tactile stimulations of the center of the face, periphery of the face, and shoulders. Cortical activations are observed in expected somatosensory areas SI and SII but also in parietal, cingulate, premotor, prefrontal, and visual cortices. Distinguishing between unilateral contralateral, ipsilateral, or bilateral activations within these regions was not a goal of our experiment, so we do not discuss this aspect (e.g., in humans, Hansson and Brismar 1999). It is important to note that fMRI activations reflect both the spikes produced by the neurons and the modulation of local field potentials that do not necessarily result in the generation of spikes (Goense and Logothetis 2008; Logothetis et al. 2001; Logothetis and Pfeuffer 2004; Magri et al. 2012). As a result, our study identifies regions whose neurons are responsive to the three types of tactile stimulations as well as regions receiving long-range (from other cortical areas) or short-range (from lateral nearby functional subdivisions) modulatory influences, which can be either excitatory or inhibitory. This has to be kept in mind all throughout the discussion.
No Somatotopic Organization but Regions with Specific Representations
We did not observe the expected somatotopic organization in the central and lateral sulci, as was described with the use of single-cell recordings (Coq et al. 2004; Fitzgerald et al. 2004, 2006; Kaas et al. 1979; Krubitzer et al. 1995; Nelson et al. 1980; Pons et al. 1985, 1987; Sur et al. 1982). This is possibly a limitation of our study, which was performed on a 1.5T scanner, with a voxel size of 2 × 2 × 2 mm. At this magnetic field and at this voxel size, it is most likely that the operated spatial filtering does not allow for the fine-grained resolution needed to reveal a body map. Indeed, most monkey imaging studies revealing a somatotopic organization were carried out using scanners with a higher field in the monkey (9.4 T; Chen et al. 2011) or with imaging (Friedman et al. 2004). Nonetheless, our study revealed very interesting, and mostly unsuspected, specific representations.
First, most of the regions preferring one of the three tactile stimulations represented almost exclusively that location. We thus revealed regions specific to the center of the face, the periphery of the face, and the shoulders. For the center of the face, if we exclude the parieto-temporo-frontal network comprising areas 9/46v, F4/PMZ/F5p (PMv), 2, and SII/PV that is discussed in the next section, we observed specific activations in orbitofrontal areas 11/13, premotor areas F5a/44 (PMvr) and F7/SEF and parts of area V1. Tactile responses in the orbitofrontal cortex have been reported in monkeys and in humans, but to our knowledge, no specificity to the location of the tactile stimulation has ever been proposed (e.g., Frey et al. 2009). This region has been associated with nociceptive stimulations or pleasant touch on hairy skin (McGlone et al. 2012, zu Eulenburg et al. 2013), and it has been proposed to be part of an orbitofrontal-insular network. Corroborating this, our study also identifies systematic activations within the insular cortex. Tactile responses in the ventral premotor cortex (PMv; areas F4 and F5) have already been described, with receptive fields preferentially located on the face, near the mouth, and on the hand (Fogassi et al. 1996; Gentilucci et al. 1988; Rizzolatti et al. 1981). In the dorsal premotor cortex, an activation in the supplementary motor area (SMA) could have been expected, because it is known to contain tactile neurons (e.g., Romo et al. 1993) and a face representation (e.g., Luppino et al. 1991). An activation in this region was in fact observed in one monkey (M2; Fig. 3) but not in the other one. Since the SMA is involved in the production of movement, it may not be optimally activated in our passive paradigm. In contrast, and to our knowledge, no tactile responses have been reported in area F7/SEF. The connectivity pattern of this region possibly suggests that it could be part of a tactile network, due to its reciprocal connections with PGm (whose activation we also observe in our study; Matelli et al. 1998). Most interestingly, this region was also activated by visual stimulations close to the face, possibly indicating a role in a higher order cognitive processing of peripersonal space, yet to be determined (unpublished observations). Finally, tactile responses to center of the face stimulation were also observed in visual cortex V1. In fact, tactile activations were observed in visual cortex (mostly V1 and V2) for the three locations of stimulations in both monkeys (but not the same cortical location for each stimulation), but only for the center of the face did it reach the statistical level for the spatial specificity analysis, probably because this stimulation elicited the strongest activations overall and because tactile activations in the occipital cortex were overall, and expectedly, rather weak. The fact that the visual cortex is not unimodal has now been described both in monkeys and humans with several approaches (connectivity in the monkey: Cappe and Barone 2005; Clavagnier et al. 2004; Falchier et al. 2002; single-cell recording in the monkey: Iurilli et al. 2012; Vasconcelos et al. 2011; fMRI in humans: Amedi et al. 2001; Macaluso et al. 2000; Sathian et al. 1997), including our own work, which describes that multisensory convergence is widely observed within the cortex, in high-level cortical areas as well as in low-level sensory areas (Guipponi et al. 2015). Specifically, in a previous study using the same data set, on a group analysis, we have described visuotactile convergence to occur mainly in the peripheral visual representation (see Fig. 4 in Guipponi et al. 2015). In the present study, we replicate these observations at the single-monkey level (Figs. 2 and 3), although we also identify small activations within the central visual field representation. Importantly, these occipital visual activations do not depend on the type of tactile stimulation used and appear to often co-occur in V1. More finely grained analyses are required to better understand the topographical organization of these tactile V1 activations. Importantly, different V1 activations might be unveiled using a more diverse range of tactile stimulations. As described at the beginning of the discussion, these low-level activations most probably do not reflect spiking activity but rather long-range modulations from somatosensory primary or associative areas (Amedi et al. 2001; Macaluso et al. 2000; Sathian et al. 1997).
Regions specifically activated by the periphery of the face or the shoulders stimulations are also observed. Only temporal regions are specifically activated for the periphery of the face. The regions observed, TPt and TPO/TS, are connected to the auditory cortex (Smiley et al. 2007), suggesting that they are multisensory. They could thus correspond, at least partly, to the polymodal temporal region, STP. This region contains tactile neurons, but no spatial selectivity has been previously reported (Bruce et al. 1981; Hikosaka et al. 1988). The neurons of this cortical region have been described to be sensitive to the expectation of the tactile stimulus (Mistlin and Perrett 1990), a variable that could partially account for the observed activations given the block structure of our experimental design. The multisensory neurons of this area have additionally been described to respond to the observation of faces (Perrett et al. 1982), possibly pointing toward an overlap between regions encoding the face and regions encoding others' faces, as described in the parietal cortex (Ishida et al. 2010). It is, however, to be noted that such a specificity for the periphery of the face is not observed in humans (Beauchamp et al. 2008). The regions specific to the shoulders correspond to the medial parietal regions PE and PGm. Supporting our observations, neurons with a tactile receptive field mostly on the arm have been described in area PEc (Breveglieri et al. 2006, 2008).
Finally, we observed two regions, cingulate area 24c and insular area Pi, that presented tactile activations without any spatial specificity, suggesting that the tactile information is integrated, in these areas, at a more global or cognitive level.
Given that our tactile mapping procedure only investigated tactile responsiveness around the head and shoulder area, we obviously do not report activations in areas selectively tuned to arm or hand stimulations (e.g., visual area V6A, Breveglieri et al. 2002). A different tactile network would have been revealed if different locations had been stimulated or tactile stimulations of a different nature had been used (e.g., pressure, heat, pain). In addition, there is growing evidence that tactile activations are dependent on task requirements (e.g., visuotactile representations in the human parietal cortex for orientation identification, Kitada et al. 2006; or motion discrimination, Nakashita et al. 2008).
Specificity of the Parieto-Temporo-Frontal Network
Our results reveal the existence of a functional network with outstanding functional characteristics. Areas 9/46v, F4/PMZ/F5p (PMv), 2, and SII/PV are activated for the three tactile stimulations we used, with a preference for the center of the face and with a subpart of each region responding exclusively to the center of the face. This suggests that, at the neuronal level, their tactile representation is centered on the center of the face, possibly the mouth. Such neuronal responses biased for the center of the face representation have been described for premotor areas F4 and F5 (Fogassi et al. 1996; Gentilucci et al. 1988; Rizzolatti et al. 1981). Likewise, somatotopic representations have also been well described in area 2 and SII/PV, and each contains a representation of the face (Coq et al. 2004; Fitzgerald et al. 2004, 2006; Kaas et al. 1979; Krubitzer et al. 1995, Nelson et al. 1980; Pons et al. 1985, 1987; Sur et al. 1982). Although it is possible that our area 2 activations are restricted to this face-selective subsector, our SII/PV activations are, on the other hand, too large to be restricted to what has been described as face related, suggesting that, in these non-face SII/PV-activated voxels, even if the neurons respond to other tactile stimulations, they are modulated by the position of the tactile stimulus relatively to the center of the face, due, for example, to short-range lateral connections within area SII/PV. Finally, ventral prefrontal area 9/46v receives connections from the somatosensory area SII (Preuss and Goldman-Rakic 1989) and presents visual responses and auditory responses (face and vocalizations; e.g., Cohen et al. 2004; O Scalaidhe et al. 1997; Romanski 2005) consistent with a representation of face or identity, in association with higher cognitive functions such as working memory. Indeed, the neurons of this region respond during a delayed somatosensory discrimination (Romo et al. 1999).
Area VIP has often been associated with the F4/PMZ/F5p (PMv) complex, based on common functional responses and strong connectivity patterns (Cléry et al. 2015; Matelli and Luppino 2001; Rizzolatti and Luppino 2001). This region represents visual information in a head-centered frame of reference (Duhamel et al. 1997), as well as the multisensory space around the head (Avillac et al. 2005; Duhamel et al. 1998; Guipponi et al. 2013, 2015). This region additionally integrates tactile and visual information (Avillac et al. 2004, 2007) and represents observed touch on others' faces (Ishida et al. 2010). Whereas in monkey M1, fundal intraparietal sulcus tactile activations are composed of two subparts (Guipponi et al. 2013), with a marked preference for the center of the face stimulations, in monkey M2, fundal intraparietal sulcus tactile activations are constituted of only one region that is activated by the three stimulations (Fig. 4, bottom; Duhamel et al. 1998), possibly suggesting that the parietal functional definition of head-centered processes changes from one individual to another.
Very interestingly, this temporal(SII/PV)-parieto(VIP)-prefrontal(F4/PMZ/F5a) network also appears to specifically encode visual near space as compared to visual far space (Bremmer et al. 2013, Cléry et al. 2015), as well as the prediction of the impact to the face of a looming visual stimulus (Cléry et al. 2015; see also poster cited in Cléry et al. 2015). These convergent lines of evidence strongly suggest that this tactile network is involved in the multisensory representation of the peripersonal space around the head (Cléry et al. 2015), possibly in relation with self-defense (Graziano et al. 2002; Graziano and Cooke 2006), feeding behavior, and mouth-directed actions (Bruni et al. 2015). It is extremely tempting to propose that the enhanced tactile representation for the center of the face perioral region in this parieto-temporo-prefrontal network results from a life-long experience of objects touching the mouth during feeding behavior, at a higher rate than any other body part, rather than on a hardwired neuronal organization.
Conclusion
Overall, we show that air-puff tactile stimulations effectively elicit fMRI activations not only in the macaque monkey primary and secondary somatosensory areas but also in a larger cortical network involving prefrontal, premotor, parietal, temporal, cingulate, and striate and extrastriate visual areas. Within this large cortical network, a parieto-temporo-prefrontal subnetwork appears to be also involved in representing the visual space around the head. Interestingly, numerous cortical areas represent exclusively touch to the center of the face, to the periphery of the face, or to the shoulders in the absence of any evident topographical organization, indicating a functional segregation in the processing of these incoming tactile sources of information, possibly due to their distinct ecological relevance. A similar systematic whole brain fMRI mapping of tactile organization in the human brain would allow us to directly probe the homology between the human and nonhuman primate regarding higher order somatosensory processing, i.e., beyond the primary and secondary processing areas.
GRANTS
This work was supported by Agence Nationale de la Recherche Award ANR-05-JCJC-0230-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.W., O.G., and S.B.H. conception and design of research; C.W., O.G., S.P., and S.B.H. performed experiments; C.W., O.G., and S.B.H. analyzed data; C.W. and S.B.H. interpreted results of experiments; C.W. prepared figures; C.W. drafted manuscript; C.W. and S.B.H. edited and revised manuscript; S.B.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Maurin for technical support and J. L. Charieau and F. Hérant for animal care.
REFERENCES
- Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nat Neurosci 4: 324–330, 2001. [DOI] [PubMed] [Google Scholar]
- Ann Stringer E, Qiao PG, Friedman RM, Holroyd L, Newton AT, Gore JC, Min Chen L. Distinct fine-scale fMRI activation patterns of contra- and ipsilateral somatosensory areas 3b and 1 in humans. Hum Brain Mapp 35: 4841–4857, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avillac M, Olivier E, Denève S, Ben Hamed S, Duhamel JR. Multisensory integration in multiple reference frames in the posterior parietal cortex. Cogn Process 5: 159–166, 2004. [Google Scholar]
- Avillac M, Denève S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci 8: 941–949, 2005. [DOI] [PubMed] [Google Scholar]
- Avillac M, Ben Hamed S, Duhamel JR. Multisensory integration in the ventral intraparietal area of the macaque monkey. J Neurosci 27: 1922–1932, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol 66: 1109–1124, 1991. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. Neuroimage 41: 1011–1020, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp Brain Res 140: 127–144, 2001. [DOI] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Visual receptive field modulation in the lateral intraparietal area during attentive fixation and free gaze. Cereb Cortex 12: 234–245, 2002. [DOI] [PubMed] [Google Scholar]
- Besle J, Sánchez-Panchuelo RM, Bowtell R, Francis S, Schluppeck D. Single-subject fMRI mapping at 7 T of the representation of fingertips in S1: a comparison of event-related and phase-encoding designs. J Neurophysiol 109: 2293–2305, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Kaminiarz A, Hoffmann KP. Encoding of movement in near extrapersonal space in primate area VIP. Front Behav Neurosci 7: 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29: 287–296, 2001. [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Kutz DF, Fattori P, Gamberini M, Galletti C. Somatosensory cells in the parieto-occipital area V6A of the macaque. Neuroreport 13: 2113–2116, 2002. [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Gamberini M, Passarelli L, Fattori P. Somatosensory cells in area PEc of macaque posterior parietal cortex. J Neurosci 26: 3679–3684, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Monaco S, Fattori P. Visual, somatosensory, and bimodal activities in the macaque parietal area PEc. Cereb Cortex 18: 806–816, 2008. [DOI] [PubMed] [Google Scholar]
- Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol 46: 369–384, 1981. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53: 603–635, 1985. [DOI] [PubMed] [Google Scholar]
- Bruni S, Giorgetti V, Bonini L, Fogassi L. Processing and integration of contextual information in monkey ventrolateral prefrontal neurons during selection and execution of goal-directed manipulative actions. J Neurosci 35: 11877–11890, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, Wingert JR, Dierker D. Multiple parietal operculum subdivisions in humans: tactile activation maps. Somatosens Mot Res 25: 149–162, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci 22: 2886–2902, 2005. [DOI] [PubMed] [Google Scholar]
- Chen LM, Dillenburger BC, Wang F, Friedman RM, Avison MJ. High-resolution functional magnetic resonance imaging mapping of noxious heat and tactile activations along the central sulcus in New World monkeys. Pain 152: 522–532, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavagnier S, Falchier A, Kennedy H. Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cogn Affect Behav Neurosci 4: 117–126, 2004. [DOI] [PubMed] [Google Scholar]
- Cléry J, Guipponi O, Wardak C, Ben Hamed S. Neuronal bases of peripersonal and extrapersonal spaces, their plasticity and their dynamics: knowns and unknowns. Neuropsychologia 70: 313–326, 2015. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Russ BE, Gifford IIIGW, Kiringoda R, MacLean KA. Selectivity for the spatial and nonspatial attributes of auditory stimuli in the ventrolateral prefrontal cortex. J Neurosci 24: 11307–11316, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coq JO, Qi H, Collins CE, Kaas JH. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World titi monkey (Callicebus moloch). J Comp Neurol 476: 363–387, 2004. [DOI] [PubMed] [Google Scholar]
- Dong WK, Chudler EH, Sugiyama K, Roberts VJ, Hayashi T. Somatosensory, multisensory, and task-related neurons in cortical area 7b (PF) of unanesthetized monkeys. J Neurophysiol 72: 542–564, 1994. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Bremmer F, Ben Hamed S, Graf W. Spatial invariance of visual receptive fields in parietal cortex neurons. Nature 389: 845–848. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol 79: 126–136, 1998. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682, 2002. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex 17: 1800–1811, 2007. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR. Functional lateralization of face, hand, and trunk representation in anatomically defined human somatosensory areas. Cereb Cortex 18: 2820–2830, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci 22: 5749–5759, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Pramodsingh HT, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: evidence for multiple functional representations. J Neurosci 24: 11193–11204, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Pramodsingh HT, Hsiao SS. Receptive field (RF) properties of the macaque second somatosensory cortex: RF size, shape, and somatotopic organization. J Neurosci 26: 6485–6495, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4). J Neurophysiol 76: 141–157, 1996. [DOI] [PubMed] [Google Scholar]
- Frey S, Zlatkina V, Petrides M. Encoding touch and the orbitofrontal cortex. Hum Brain Mapp 30: 650–659, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RM, Chen LM, Roe AW. Modality maps within primate somatosensory cortex. Proc Natl Acad Sci USA 101: 12724612729, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res 71: 475–490, 1988. [DOI] [PubMed] [Google Scholar]
- Goense JBM, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18: 631–640, 2008. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851, 2002. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 44: 2621–2635, 2006. [DOI] [PubMed] [Google Scholar]
- Guipponi O, Wardak C, Ibarrola D, Comte JC, Sappey-Marinier D, Pinède S, Ben Hamed S. Multimodal convergence within the intraparietal sulcus of the macaque monkey. J Neurosci 33: 4128–4139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guipponi O, Odouard S, Pinède S, Wardak C, Ben Hamed S. fMRI cortical correlates of spontaneous eye blinks in the nonhuman primate. Cereb Cortex 25: 2333–2345, 2014. [DOI] [PubMed] [Google Scholar]
- Guipponi O, Cléry J, Odouard S, Wardak C, Ben Hamed S. Whole brain mapping of visual and tactile convergence in the macaque monkey. Neuroimage 117: 93–102, 2015. [DOI] [PubMed] [Google Scholar]
- Hansson T, Brismar T. Tactile stimulation of the hand causes bilateral cortical activation: a functional magnetic resonance study in humans. Neurosci Lett 271: 29–32, 1999. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Iwai E, Saito H, Tanaka K. Polysensory properties of neurons in the anterior bank of the caudal superior temporal sulcus of the macaque monkey. J Neurophysiol 60: 1615–1637, 1988. [DOI] [PubMed] [Google Scholar]
- Huang RS, Sereno MI. Dodecapus: an MR-compatible system for somatosensory stimulation. Neuroimage 34: 1060–1073, 2007. [DOI] [PubMed] [Google Scholar]
- Huang RS, Chen CF, Tran AT, Holstein KL, Sereno MI. Mapping multisensory parietal face and body areas in humans. Proc Natl Acad Sci USA 109: 18114–18119, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Nakajima K, Inase M, Murata A. Shared mapping of own and others' bodies in visuotactile bimodal area of monkey parietal cortex. J Cogn Neurosci 22: 83–96. [DOI] [PubMed] [Google Scholar]
- Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F, Medini P. Sound-driven synaptic inhibition in primary visual cortex. Neuron 73: 814–828, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Lin CS, Merzenich MM. Multiple representations of the body within the primary somatosensory cortex of primates. Science 204: 521–523, 1979. [DOI] [PubMed] [Google Scholar]
- Kitada R, Kito T, Saito DN, Kochiyama T, Matsumura M, Sadato N, Lederman SJ. Multisensory activation of the intraparietal area when classifying grating orientation: a functional magnetic resonance imaging study. J Neurosci 26: 7491–7501, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopietz R, Sakar V, Albrecht J, Kleemann AM, Schöpf V, Yousry I, Linn J, Fesl G, Wiesmann M. Activation of primary and secondary somatosensory regions following tactile stimulation of the face. Klin Neuroradiol 19: 135–144, 2009. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci 15: 3821–3839, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CCK, Sun YN, Huang CI, Yu CY, Ju MS. Cortical activation by tactile stimulation to face and anterior neck areas: an fMRI study with three analytic methods. Hum Brain Mapp 31: 1876–1885, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging 22: 1517–1531, 2004. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol 311: 463–482, 1991. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science 289: 1206–1208, 2000. [DOI] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci 32: 1395–1407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Choi JK, Jarraya B, Rosen BR, Jenkins BG, Vanduffel W. FMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia. Neuropsychopharmacology 36: 1187–1198, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuzzi R, van der Zwaag W, Farthouat J, Gruetter R, Blanke O. Human finger somatotopy in areas 3b, 1, and 2: a 7T fMRI study using a natural stimulus. Hum Brain Mapp 35: 213–226, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neurol 402: 327–352, 1998. [PubMed] [Google Scholar]
- Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage 14: S27–32, 2001. [DOI] [PubMed] [Google Scholar]
- McGlone F, Kelly EF, Trulsson M, Francis ST, Westling G, Bowtell R. Functional neuroimaging studies of human somatosensory cortex. Behav Brain Res 135: 147–158, 2002. [DOI] [PubMed] [Google Scholar]
- McGlone F, Olausson H, Boyle JA, Jones-Gotman M, Dancer C, Guest S, Essick G. Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. Eur J Neurosci 35: 1782–1788, 2012. [DOI] [PubMed] [Google Scholar]
- Mendoza G, Merchant H. Motor system evolution and the emergence of high cognitive functions. Prog Neurobiol 122: 73–93, 2014. [DOI] [PubMed] [Google Scholar]
- Mistlin AJ, Perrett DI. Visual and somatosensory processing in the macaque temporal cortex: the role of ‘expectation’. Exp Brain Res 82: 437–450, 1990. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res 11: 67–83, 1984. [DOI] [PubMed] [Google Scholar]
- Nakashita S, Saito D, Kochiyama T, Honda M, Tanabe HC, Sadato N. Tactile-visual integration in the posterior parietal cortex: a functional magnetic resonance imaging study. Brain Res Bull 75: 513–525, 2008. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Sur M, Felleman DJ, Kaas JH. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol 192: 611–643, 1980. [DOI] [PubMed] [Google Scholar]
- Nguyen BT, Tran TD, Hoshiyama M, Inui K, Kakigi R. Face representation in the human primary somatosensory cortex. Neurosci Res 50: 227–232, 2004. [DOI] [PubMed] [Google Scholar]
- Nguyen BT, Inui K, Hoshiyama M, Nakata H, Kakigi R. Face representation in the human secondary somatosensory cortex. Clin Neurophysiol 116: 1247–1253, 2005. [DOI] [PubMed] [Google Scholar]
- O Scalaidhe SP, Wilson FA, Goldman-Rakic PS. Areal segregation of face-processing neurons in prefrontal cortex. Science 278: 1135–1138, 1997. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Exp Brain Res 47: 329–342, 1982. [DOI] [PubMed] [Google Scholar]
- Polonara G, Fabri M, Manzoni T, Salvolini U. Localization of the first and second somatosensory areas in the human cerebral cortex with functional MR imaging. Am J Neuroradiol 20: 199–205, 1999. [PMC free article] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Cusick CG, Kaas JH. A sequential representation of the occiput, arm, forearm and hand across the rostrocaudal dimension of areas 1, 2 and 5 in macaque monkeys. Brain Res 335: 350–353, 1985. [DOI] [PubMed] [Google Scholar]
- Pons TP, Wall JT, Garraghty PE, Cusick CG, Kaas JH. Consistent features of the representation of the hand in area 3b of macaque monkeys. Somatosens Res 4: 309–331, 1987. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Connections of the ventral granular frontal cortex of macaques with perisylvian premotor and somatosensory areas: anatomical evidence for somatic representation in primate frontal association cortex. J Comp Neurol 282: 293–316, 1989. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. I. Somatosensory responses. Behav Brain Res 2: 125–146, 1981. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron 31: 889–901, 2001. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol 93: 734–747, 2005. [DOI] [PubMed] [Google Scholar]
- Romo R, Ruiz S, Crespo P, Zainos A, Merchant H. Representation of tactile signals in primate supplementary motor area. J Neurophysiol 70: 2690–2694, 1993. [DOI] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernández A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399: 470–473, 1999. [DOI] [PubMed] [Google Scholar]
- Sanchez-Panchuelo RM, Francis S, Bowtell R, Schluppeck D. Mapping human somatosensory cortex in individual subjects with 7T functional MRI. J Neurophysiol 103: 2544–2556, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Panchuelo RM, Besle J, Beckett A, Bowtell R, Schluppeck D, Francis S. Within-digit functional parcellation of Brodmann areas of the human primary somatosensory cortex using functional magnetic resonance imaging at 7 tesla. J Neurosci 32: 15815–15822, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST. Feeling with the mind's eye. Neuroreport 8: 3877–3881, 1997. [DOI] [PubMed] [Google Scholar]
- Schweisfurth MA, Frahm J, Schweizer R. Individual fMRI maps of phalanges and digit bases of all fingers in human primary somatosensory cortex. Front Hum Neurosci 8: 658, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci 9: 1337–1343, 2006. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE. Multisensory convergence in auditory cortex. I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol 502: 894–923, 2007. [DOI] [PubMed] [Google Scholar]
- Sur M, Nelson RJ, Kaas JH. Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J Comp Neurol 211: 177–192, 1982. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron 32: 565–577, 2001. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 8: 443–459, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos N, Pantoja J, Belchior H, Caixeta FV, Faber J, Freire MAM, Cota VR, Anibal de Macedo E, Laplagne DA, Gomes HM, Ribeiro S. Cross-modal responses in the primary visual cortex encode complex objects and correlate with tactile discrimination. Proc Natl Acad Sci USA 108: 15408–15413, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zu Eulenburg P, Baumgärtner U, Treede RD, Dieterich M. Interoceptive and multimodal functions of the operculo-insular cortex: tactile, nociceptive and vestibular representations. Neuroimage 83: 75–86, 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.