Abstract
Normal aging is associated with a decrease in motor function, a concomitant increase in muscle stiffness and tone, and a decrease in dopamine (DA) levels in the spinal cord. The striatum plays a critical role in the control of motor function, and it receives strong DA innervation from the substantia nigra. However, locomotor activity also requires the activation of motoneurons in the lumbar spinal cord, which in the mouse express all five DA receptor subtypes (D1–D5). Of these, the D3 receptor (D3R) expresses the highest affinity to DA and mediates inhibitory actions, while activation of the lower-affinity D1 receptor (D1R) system promotes excitatory effects. To test whether the aging-related decrease in DA levels is associated with corresponding changes in DA receptor protein expression levels, we probed with Western blot and immunohistochemical techniques for D1R and D3R protein expression levels over the normal life span of the mouse. We found that with age D1R expression levels increased in both striatum and spinal cord, while D3R expression levels remained stable in the striatum or slightly decreased in the spinal cord. The resulting D1-to-D3 ratio indicates a strong upregulation of D1R-mediated pathways in old animals, which is particularly pronounced in the lumbar spinal cord. These data suggest that aging may be associated with a shift in DA-mediated pathways in striatum and spinal cord, which in turn could be an underlying factor in the emergence of aging- and DA-related motor dysfunctions such as Parkinson's disease or Restless Legs Syndrome (RLS).
Keywords: normal aging, dopamine receptor expression, striatum, spinal cord
aging is associated with a gradual decline in motor function (Allen and Cavanaugh 2014) and a concomitant increase in muscle stiffness and tone (Cham et al. 2007). The striatum is a key player in the control of locomotion, and it is well established that the striatal dopamine (DA) system plays an important role in the control of movement, via direct D1 receptor (D1R)- and indirect D2 receptor (D2R)-mediated pathways (Gerfen and Surmeier 2011), and in the decline of motor performance with normal aging (Bjorklund and Dunnett 2007; Darbin 2012; Reeves et al. 2002; Stark and Pakkenberg 2004). However, the coordination and execution of movement also require the motor systems in the spinal cord. All DA receptor subtypes are expressed in the spinal cord (Zhu et al. 2007), where their actions are mediated by excitatory (D1-like) and inhibitory (D2-like, including D3) receptor subtypes. D1R activation can induce or strengthen fictive locomotion in the isolated spinal cord (Clemens et al. 2012; Gordon and Whelan 2006; Han et al. 2007; Lapointe et al. 2009). In contrast, activation of the D2-like system in the spinal cord can reduce the number of spontaneously occurring motor episodes (Clemens et al. 2012), and activation of the D3 receptor (D3R) system decreases the amplitudes of somatosensory reflexes (Clemens and Hochman 2004).
As with the striatum, the spinal DA system undergoes aging-related changes that lead to a decrease of DA and in particular D2R function in the cord (Ko et al. 1997), but details on D1R and D3R changes are missing. We recently reported evidence of an interplay between D1R and D3R pathways in the rodent spinal cord (Brewer et al. 2014), and as both striatum and spinal cord are directly involved in locomotor output, we here examined the protein expression levels of D1R and D3R in striatum and lumbar spinal cord over the life span of mice. We found that the ratio of excitatory (D1) to inhibitory (D3) receptor protein expression increased with age in both striatum and spinal cord, with a higher increase in the spinal cord than in the striatum. We suggest that this altered D1-to-D3 ratio might play a role in the increase of overall excitatory drive in these tissues with senescence.
METHODS
Animals and tissue collection.
All experimental procedures complied with the National Institutes of Health guidelines for animal care and were reviewed and approved by the East Carolina University Institutional Animal Care and Use Committee. For the Western blot receptor protein expression studies, we used male C57BL/6 mice aged 2 mo, 1 yr, and 2 yr (n = 4 per group). Mice were deeply anesthetized with isoflurane and decapitated, and brains and spinal cords were dissected out. Striatal tissue was isolated from whole brains as described previously (Mhyre et al. 2005) and placed in RNAlater (Life Technologies), while spinal cords were divided into lumbar and thoracic segments and preserved in RNAlater. Tissues were kept at −20°C until use. Only lumbar parts of the spinal cord were used in this study. For immunohistochemistry (IHC) we used animals aged 2 mo and 2 yr only, and their spinal cords and brain tissue were fixed in 10% zinc formalin (Fisher Scientific) for processing and paraffin embedding. For additional control experiments aimed at validating the D1R antibody used, brain striatal tissues from D1R-knockout mice (D1KO; donated by Dr. Rosario Moratalla, Cajal Institute, Madrid, Spain; Darmopil et al. 2009; Granado et al. 2014) were dissected out, frozen and kept at −20°C, and processed in parallel with C57BL/6 tissue from our animals (data not shown).
Protein isolation and Western blot.
Striatal and lumbar tissues (∼30–60 mg) were homogenized in 1 ml of RIPA buffer with protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO) and centrifuged at >16,000 g, and the protein concentration of the supernatant was determined with the EZQ Protein Quantification Kit (Life Technologies). Forty micrograms of each sample were denatured at 95–100°C for 10 min and loaded onto a Criterion precast gel (Any KD, TGX; Bio-Rad, Hercules, CA). Proteins were transferred to an Immobilon-P PVDF transfer membrane (Millipore, Billerica, MA), and membranes were blocked overnight at 4°C in 5% milk in Tris-buffered saline plus Tween 20 (Sigma-Aldrich). Primary antibodies were diluted in fresh blocking solution, and membranes were incubated overnight at 4°C and then in secondary antibody at room temperature for 2 h. Amersham ECL Plus Western Blotting Detecting Agents and Amersham Hyperfilm Blue (GE Healthcare) were used to visualize the proteins.
Between primary antibody exposures, membranes were stripped, washed, and exposed to other antibodies as described above. Each experiment was performed twice, with a different sample layout and order of antibody application, to verify with these technical replicates the accuracy of the results.
Antibodies.
We used the following primary DA receptor antibodies and concentrations: D1 (ab78021, 1:500) and D3 (ab42114, 1:2,500). β-Actin (ab8227, 1:2,000; Abcam) served as an internal loading control. All primary antibodies were purchased from Abcam (Cambridge, MA). HRP-tagged secondary antibodies HAF007 and HAF008 (both R&D Systems, Minneapolis, MN) were used at 1:2,000.
We also performed control Western blot experiments, to verify the specificity of the D1R antibody we used, in striatal D1KO tissue donated by Dr. Moratalla (D1KO: B6;129P2-Drd1tm1Stl/Mmjax). We compared D1KO tissues with our own wild-type (WT) controls. As D1KO express D1R, albeit at significantly lower quantities (Xu et al. 1994), we expected to observe a strong reduction in D1R signals in D1KO. Indeed, we detected a matching band in both WT and D1KO, with a substantially lower intensity in D1KO than in WT tissues thus confirming our choice for the D1R antibody we used (data not shown).
Immunohistochemistry.
We also performed quantitative IHC for D1R expression on lumbar spinal cord slices to verify the data obtained with the Western blot approaches. Paraffin-embedded tissues were sectioned at 5-μm thickness, and two sections were laid on a Superfrost slide (Fisher Scientific). From each slide, one section was used for D1R expression analysis and the second section was used as negative control. Slides were deparaffinized in xylene and sequentially dehydrated in 100%, 95%, and 70% alcohol. After rinsing in distilled water, antigen retrieval was accomplished at ∼98°C for 15 min (BD Retrievagen Antigen Retrieval Systems; Fisher Scientific). Sections were allowed to cool down for 20 min. Endogenous peroxidase activity was quenched for 30 min at room temperature (hydrogen peroxide; Sigma-Aldrich), followed by nonspecific blocking for 1 h [blocking solution: 10% normal goat serum in phosphate-buffered saline (PBS)]. Sections were incubated overnight at 4°C with either D1R antibody (1:100 dilution, Abcam ab78021) in blocking solution or just blocking solution (negative controls). Next day sections were thoroughly rinsed in PBS and the secondary antibody was applied for 1 h at room temperature (1:3,000 dilution, biotinylated goat anti-mouse Ig; Sigma-Aldrich) and detected with the ABC staining system (Thermo Scientific) and DAB substrate (Vector Laboratories) according to the manufacturers' instructions. Nuclei counterstain was obtained with methyl green for 10 min, excess stain was removed by washing in isopropanol, and slides were allowed to air dry and mounted with DPX aqueous medium.
Analysis.
For Western blots, exposed films were scanned at 1,200 dpi, and densitometry measurements were made with ImageJ (W. S. Rasband, National Institutes of Health, Bethesda, MD, http://imagej.nih.gov/ij/, 1997–2011). We used the ratio of expression intensities for the proteins of interest over β-actin for statistical analysis. Values are presented as means ± SE, and ratios were analyzed, displayed, and compared in multiple groups with either one-way ANOVA or ANOVA on rank tests (SigmaPlot 11; Systat Software, San Jose, CA), with α set <0.05, and appropriate post hoc comparisons (Holm-Sidak or Dunn's).
For IHC experiments, sectioned slices were photographed at identical settings on an Olympus BX 51 with a cellSens platform (cellSens Entry, version 1.13; Olympus). For quantification, we acquired a minimum of five images randomly per section at ×40 magnification by a group-blinded researcher, using the same intensity settings across all photos. We then quantified the positive D1R staining (% of positive area per total tissue area of field of view) with Image Pro Premier (MediaCybernetics). T-tests (P = 0.5) were performed to compare datasets between young (2 mo) and old (2 yr) animals.
RESULTS
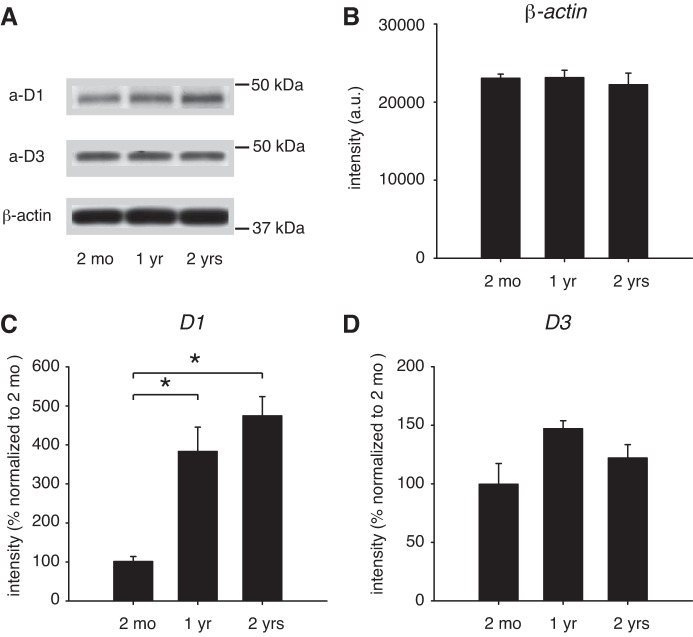
Using Western blots, we first analyzed the aging-related changes of D1R and D3R protein expression in the striatum (Fig. 1). Measurements for each age group were obtained from n = 4 animals with two independent technical replicates. Figure 1A shows representative examples of one of the replicates of D1R (Fig. 1A, top), D3R (Fig. 1A, middle), and β-actin (Fig. 1A, bottom) expression with age. We did not compare D1R and D3R expression levels to each other.
Fig. 1.
Aging-related changes in dopamine receptor protein expression in the striatum. A: representative Western blots of D1 receptor (D1R), D3 receptor (D3R), and β-actin expression. a-D1, D1R antibody; a-D3, D3R antibody; β-actin, β-actin antibody. B: β-actin expression. C: D1R protein expression. D: D3R protein expression. a.u., Arbitrary units. *Significant differences.
Protein expression of the internal control, β-actin, did not change with age (ANOVA, P = 0.8; Fig. 1B); thus we normalized both D1R (Fig. 1C) and D3R (Fig. 1D) expression to β-actin protein expression of 2-mo-old animals and set the values at this age at 100% for each receptor subtype. This normalization allowed us to compare 1- and 2-yr-old animals to this standard. We found that D1R protein expression increased in 1-yr-old animals to 383.2 ± 62.4% over the 2-mo-old control and to 474.4 ± 49.5% in the 2-yr-old animals (ANOVA, P < 0.001; Fig. 1C). The subsequent post hoc comparisons revealed significant differences between 2 mo and 2 yr (P < 0.001) and between 2 mo and 1 yr (P = 0.002). In contrast, we found no significant aging-related change in striatal D3R protein expression between 2-mo-old and 2-yr-old animals (ANOVA, P = 0.556; Fig. 1D), despite a transient increase in D3R expression in 1-yr-old animals to 147.1 ± 6.8%.
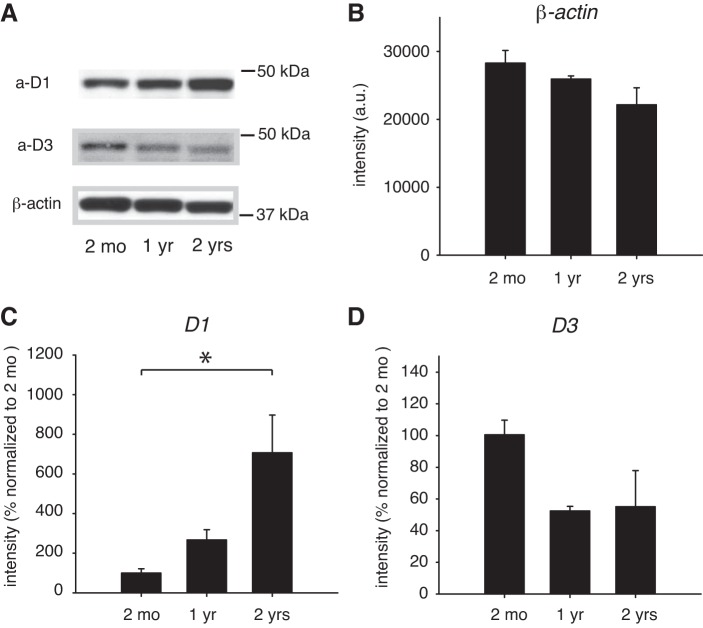
We next assessed DA receptor expression levels in the lumbar spinal cord (Fig. 2). As in the striatum, β-actin expression did not significantly change over time (ANOVA, P = 0.11; Fig. 2B), and we therefore again normalized respective D1R and D3R protein expressions to β-actin and this ratio in 1- and 2-yr-old animals to that in the 2-mo-old animals.
Fig. 2.
Aging-related changes in dopamine receptor protein expression in the lumbar spinal cord. A: representative Western blots of D1R, D3R, and β-actin expression. B: β-actin expression. C: D1R protein expression. D: D3R protein expression. Abbreviations as in Fig. 1. *Significant differences.
We found that D1R protein expression increased significantly with age, similar to the striatum, to 267 ± 51.9% in 1-yr-old animals and to 706.9 ± 190.3% in 2-yr-old animals (ANOVA, P = 0.004; Fig. 2C). The subsequent post hoc comparisons revealed significant differences between 2-mo-old and 2-yr-old animals (P < 0.05). In contrast, and similar to the striatum, D3R expression values in the lumbar spinal cord were not significantly different over the life span of the animals (55.1 ± 22.8% at 2 yr of age, ANOVA, P = 0.086; Fig. 2D).
Next, we calculated the relative fold change of D1-to-D3 ratios in striatum and lumbar spinal cord with age, with the ratios at 2 mo set to 1 (Fig. 3). We found that, in striatum, D1-to-D3 ratio increased slightly to 2.6 ± 0.5-fold at 1 yr and significantly to 3.9 ± 0.1-fold at 2 yr of age (ANOVA, P = 0.002). In the lumbar spinal cord, D1-to-D3 ratio increased significantly at 1 yr of age (5.1 ± 1-fold, ANOVA, P < 0.001) and even further at 2 yr of age (12.9 ± 1.9-fold, ANOVA, P < 0.001; Fig. 3).
Fig. 3.
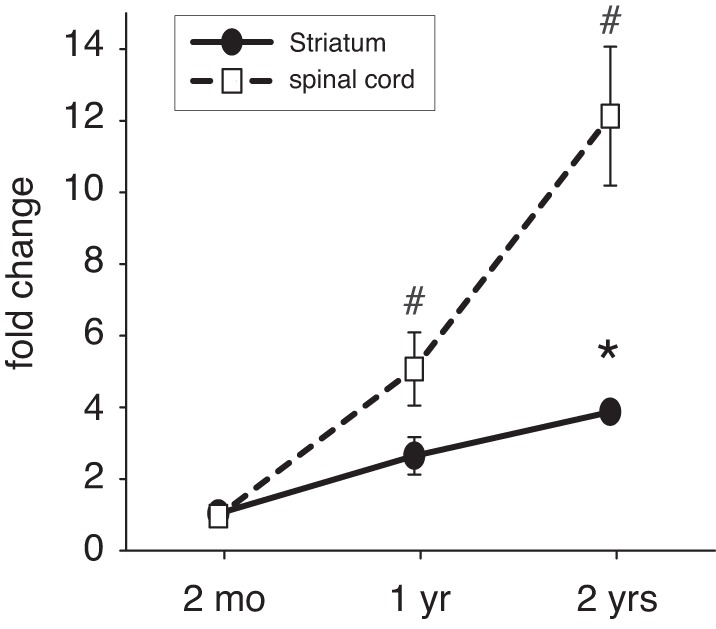
Ratio of D1 to D3 receptor expression with age in striatum and lumbar spinal cord. In the striatum, D1-to-D3 ratio increases gradually with age (solid line) but is only significantly increased at 2 yr of age over 2-mo-old controls (asterisk). In contrast, in the lumbar spinal cord, D1-to-D3 ratios grow faster (dashed line) and are significantly increased over 2-mo-old animals at both 1 yr and 2 yr of age (octothorpes).
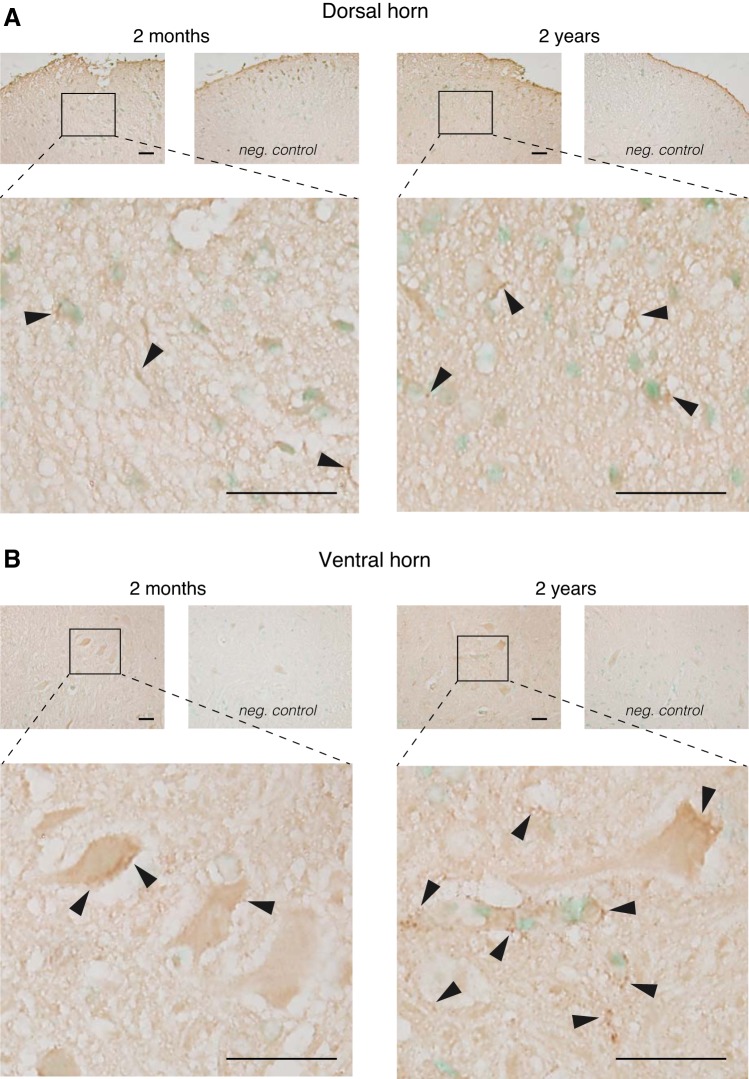
To verify the strong aging-related increase in D1R protein expression detected with the Western blots, we next performed a series of quantitative IHC experiments on lumbar spinal cord tissue (Fig. 4) and striatum (Fig. 5). Additionally, we divided the IHC analysis of D1R expression in the lumbar spinal cord with age into dorsal (sensory) horn and ventral (motor) horn areas. In the dorsal horn (Fig. 4A), we observed only sparse labeling of D1R expression in both young and old animals, but labeling appeared to be slightly more widespread in the 2-yr-old animals. In contrast, in the ventral horn the effect of aging on D1R expression was much more prominent than in the dorsal horn (Fig. 4B). In young animals, we observed that D1R labeling was mostly associated with large cell bodies (likely motoneurons), while D1R-positive labeling was greatly enhanced in the 2-yr-old animals and no longer only restricted to the large cell bodies.
Fig. 4.
Immunohistochemical (IHC) analysis of D1R expression with age in the lumbar spinal cord. A: dorsal horn. Top left: representative low-resolution micrograph of D1R expression in the dorsal horn of a 2-mo-old animal and its negative control. Bottom left: high-resolution micrograph of the area outlined in the low-resolution picture. Top right: representative low-resolution micrograph of D1R expression in the dorsal horn of a 2-yr-old animal and its negative control. Bottom right: high-resolution micrograph of the area outlined in the low-resolution picture. B: ventral horn. Top left: representative low-resolution micrograph of D1R expression in the ventral horn of a 2-mo-old animal and its negative control. Bottom left: high-resolution micrograph of the area outlined in the low-resolution picture. Top right: representative low-resolution micrograph of D1R expression in the ventral horn of a 2-yr-old animal and its negative control. Bottom right: high-resolution micrograph of the area outlined in the low-resolution picture. D1R labeling is ubiquitous and can be detected throughout the ventral horn (arrowheads). Scale bars, 20 μm.
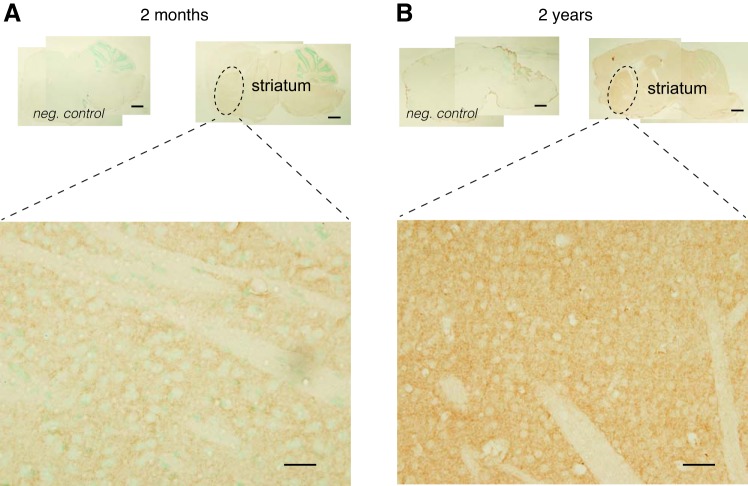
Fig. 5.
IHC analysis of D1R expression with age in the striatum. A, top: representative low-resolution micrograph of D1R expression in a sagittal brain section of a 2-mo-old animal (right) and its negative control (left). Bottom: high-resolution micrograph of a typical area within the dorsal striatum. Note that D1R-positive staining is present throughout the striatum. B: typical sagittal brain section from a 2-yr-old animal. Top: representative low-resolution micrograph of D1R expression in a sagittal brain section of a 2-yr-old animal (right) and its negative control (left). Bottom: high-resolution micrograph of a typical area within the dorsal striatum. Note that D1R-positive staining is much more intense throughout the striatum. Scale bars, 500 μm (top panels), 20 μm (bottom panels).
In the striatum (Fig. 5) we found that in young animals (Fig. 5A) D1R expression was present throughout, with pockets of stronger labeling. Furthermore, and in support of the evidence provided by the Western blot data (cf. Fig. 1C), D1R protein staining in the striatum of the 2-yr-old animals was strongly increased over the young cohort (Fig. 5B), and we did not detect any notable differences in the intensity of D1R staining.
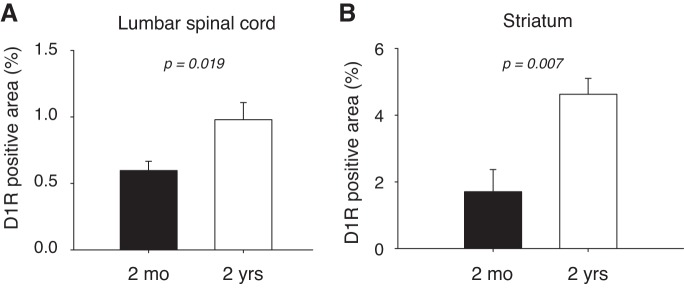
Quantification of the IHC data in Figs. 4 and 5 is presented in Fig. 6. Data for the lumbar spinal cord (Fig. 6A) were obtained from randomly chosen areas throughout the spinal gray matter, irrespective of dorsal or ventral areas. We found that D1R-positive areas increased significantly from 0.6 ± 0.07% in 2 mo-old animals to 0.98 ± 0.13% (an increase of 63%, t-test, P = 0.019). In the striatum (Fig. 6B), D1R-positive areas increased significantly from 1.7 ± 0.7% in 2-mo-old animals to 4.6 ± 0.5% in 2-yr-old animals (an increase of 270%, t-test, P = 0.007). Note that in both young and old animals D1R positive staining in the striatum occupies a larger percent area than in the spinal cord.
Fig. 6.
Quantification of IHC experiments. A: lumbar spinal cord (combined quantification of dorsal and ventral horns). With age, D1R-positive staining increases significantly (P = 0.019, t-test). B: striatum. With age, D1R-positive staining increases significantly (P = 0.007, t-test).
Thus these findings mimic the results observed with the Western blot experiments, albeit at a different scale, which is likely due to the inherent difficulties in quantifying IHC staining. Taken together, our data provide evidence 1) that aging is associated with a shift in the ratio of excitatory D1R to inhibitory D3R expression and 2) that this reorganization is present in both the spinal cord and the striatum.
DISCUSSION
Normal aging is associated with neuronal loss in the striatum (Han et al. 1989), a decrease in DA levels (Haycock et al. 2003), and a decrease in D2-like receptor expression (D2, D3, and D4) (Mesco et al. 1991; Valerio et al. 1994; Wong et al. 1997). Together with the high affinity of the D3R to DA and the association of the D3R system with overall inhibitory modulatory effects, these data alone point to a gradual disinhibition of the DA system in the basal ganglia. In contrast, the role of the D1R system with age remains less well understood, and the aging-related changes of DA receptors (D1 and D3) are virtually unexplored in the spinal cord.
The D1R system has a prominent neuromodulatory role in activating spinal cord locomotor circuits (Han et al. 2007; Lapointe et al. 2009; Tran et al. 2005). Conversely, a dysfunctional D3R system is associated with increased locomotor activity (Accili et al. 1996; Dowling et al. 2011), increased motor outputs upon stimulation of the monosynaptic stretch reflex (Clemens and Hochman 2004), and increased excitability of spinal pain reflexes (Keeler et al. 2012).
D1Rs have a substantially lower affinity to DA than D3Rs (Richtand et al. 2001); hence the aging-related change of the D1-to-D3 ratio suggests a strong upregulation of Gs-coupled DA-mediated actions on D1R pathways with age in both striatum and spinal cord, possibly leading to a reduction in the relative expression of D1R-D3R heterodimers within the frame of a D1R-D3R heterotetramer (Guitart et al. 2014).
An arguable shortcoming of our study is that we only assessed overall D1R and D3R protein levels in the striatum. We thus may have failed to detect potential pathway-specific changes in direct vs. indirect pathways in the basal ganglia that have been the focus of understanding D1R- vs. D2R-mediated responses in the basal ganglia (Kravitz et al. 2010). Their findings suggested that activation of the indirect and D2R-mediated pathway could elicit a Parkinson's disease (PD) phenotype, while activation of the D1R-mediated direct pathway reduced freezing and increased locomotion. Our findings of a substantial increase in overall D1R protein levels with age in both striatum and lumbar spinal cord might be part of a compensatory mechanism by which the system tries to counterbalance for the reduction in DA levels that occur with normal aging.
According to this model, the high-affinity D3R system would be virtually unaffected by a decrease of DA levels and still be able to exert its modulatory actions via the Gi pathway, while such a decrease in DA levels would more heavily impact low-affinity D1R-mediated Gs pathways. A parallel upregulation of D1R protein levels might be able to maintain D1R responses in the face of reduced DA presence to maintain or reestablish an environment that can respond to descending DA modulation in a controlled fashion. Eventually, however, the D1R-driven modulatory balance will shift toward excitation, paving the way for aging-related changes in motor control and the spinal sensorimotor system.
Interestingly, DA-related dysfunctions [e.g., PD, Restless Legs Syndrome (RLS)] worsen with age, but their symptoms can be, at least initially, improved by treatment with DA receptor agonists that target the inhibitory D3R system (Castro-Hernandez et al. 2015; Das et al. 2015; Jenner 2005; Manconi et al. 2010, 2011; Reichmann et al. 2006). Moreover, motor dysfunctions such as Tourette's syndrome can be improved by blocking the excitatory D1R system (Gilbert et al. 2014). A caveat of the D3R treatment options in RLS is the gradual emergence of tolerance or augmentation, the switch of initially beneficial therapeutic actions into adverse effects with prolonged therapy (Garcia-Borreguero et al. 2007; Garcia-Borreguero and Williams 2010). The origins of this D3R-driven augmentation in RLS are not fully understood, but since the D3R can develop tolerance (Cote and Kuzhikandathil 2014; Gil-Mast et al. 2013; Kuzhikandathil et al. 2004; Westrich et al. 2010) and we here show that D3R levels do not change with age, a testable hypothesis arising from this study is that the D3R may be less effective in aged animals compared with young ones. Such a study could then also help resolve the question of whether augmentation is truly only a drug-induced consequence or perhaps, at least in part, also a repercussion of altered DA receptor ratios with age.
Moreover, as DA deficit or inappropriate DA/other neurotransmitter imbalances are also playing an important role in PD (e.g., Beitz 2014), it is tempting to speculate that the gradual reduction in treatment efficiency of D3R therapeutics with age not only in RLS but also in PD may be in part due to the aging-associated change in excitatory and inhibitory DA receptor expression we report here. We therefore suggest that continuous monitoring of RLS and PD patients may be needed to titrate their treatments against these dynamic changes and to maintain the effectiveness of the DA-based therapies.
Conclusions.
Our data suggest that normal aging is associated with a strong increase in excitatory D1R but not inhibitory D3R protein expression levels in striatum and spinal cord. We speculate that this divergence may play a role in the gradual emergence of aging-related motor dysfunctions.
GRANTS
This study was supported in part by a Start-up Award of the Brody School of Medicine (S. Clemens).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.E.K. and S.C. conception and design of research; B.E.K., P.L., M.M.P., and L.E.d.C.B. performed experiments; B.E.K., P.L., M.M.P., L.E.d.C.B., and S.C. analyzed data; B.E.K., L.E.d.C.B., and S.C. prepared figures; B.E.K. drafted manuscript; B.E.K., P.L., M.M.P., L.E.d.C.B., and S.C. approved final version of manuscript; P.L. and S.C. edited and revised manuscript; L.E.d.C.B. and S.C. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Dr. Rosario Moratalla (Cajal Institute, Madrid, Spain) for donating brain tissue of D1R-knockout mice and Madeleine Neuhaus for optimizing the protocol for the IHC experiments.
REFERENCES
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA 93: 1945–1949, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EN, Cavanaugh JE. Loss of motor coordination in an aging mouse model. Behav Brain Res 267: 119–125, 2014. [DOI] [PubMed] [Google Scholar]
- Beitz JM. Parkinson's disease: a review. Front Biosci (Schol Ed) 6: 65–74, 2014. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci 30: 194–202, 2007. [DOI] [PubMed] [Google Scholar]
- Brewer KL, Baran CA, Whitfield BR, Jensen AM, Clemens S. Dopamine D3 receptor dysfunction prevents anti-nociceptive effects of morphine in the spinal cord. Front Neural Circuits 8: 62, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hernandez J, Afonso-Oramas D, Cruz-Muros I, Salas-Hernandez J, Barroso-Chinea P, Moratalla R, Millan MJ, Gonzalez-Hernandez T. Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiol Dis 74: 325–335, 2015. [DOI] [PubMed] [Google Scholar]
- Cham R, Perera S, Studenski SA, Bohnen NI. Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults. Gait Posture 26: 516–525, 2007. [DOI] [PubMed] [Google Scholar]
- Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol 107: 2250–2259, 2012. [DOI] [PubMed] [Google Scholar]
- Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci 24: 11337–11345, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote SR, Kuzhikandathil EV. In vitro and in vivo characterization of the agonist-dependent D3 dopamine receptor tolerance property. Neuropharmacology 79: 359–367, 2014. [DOI] [PubMed] [Google Scholar]
- Darbin O. The aging striatal dopamine function. Parkinsonism Relat Disord 18: 426–432, 2012. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry 66: 603–613, 2009. [DOI] [PubMed] [Google Scholar]
- Das B, Modi G, Dutta A. Dopamine D3 agonists in the treatment of Parkinson's disease. Curr Top Med Chem 15: 908–926, 2015. [DOI] [PubMed] [Google Scholar]
- Dowling P, Klinker F, Stadelmann C, Hasan K, Paulus W, Liebetanz D. Dopamine D3 receptor specifically modulates motor and sensory symptoms in iron-deficient mice. J Neurosci 31: 70–77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Allen RP, Kohnen R, Hogl B, Trenkwalder C, Oertel W, Hening WA, Paulus W, Rye D, Walters A, Winkelmann J, Earley CJ. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine-International Restless Legs Syndrome Study Group consensus conference at the Max Planck Institute. Sleep Med 8: 520–530, 2007. [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Williams AM. Dopaminergic augmentation of restless legs syndrome. Sleep Med Rev 14: 339–346, 2010. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34: 441–466, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mast S, Kortagere S, Kota K, Kuzhikandathil EV. An amino acid residue in the second extracellular loop determines the agonist-dependent tolerance property of the human D3 dopamine receptor. ACS Chem Neurosci 4: 940–951, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Budman CL, Singer HS, Kurlan R, Chipkin RE. A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome. Clin Neuropharmacol 37: 26–30, 2014. [DOI] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Monoaminergic control of cauda-equina-evoked locomotion in the neonatal mouse spinal cord. J Neurophysiol 96: 3122–3129, 2006. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Moratalla R. D1 but not D4 dopamine receptors are critical for MDMA-induced neurotoxicity in mice. Neurotox Res 25: 100–109, 2014. [DOI] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sanchez-Soto M, Kumar-Barodia S, Naidu YT, Mallol J, Cortes A, Lluis C, Canela EI, Casado V, McCormick PJ, Ferre S. Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1–D3 receptor heterotetramer. Mol Pharmacol 86: 417–429, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci 27: 13192–13204, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Kuyatt BL, Kochman KA, DeSouza EB, Roth GS. Effect of aging on concentrations of D2-receptor-containing neurons in the rat striatum. Brain Res 498: 299–307, 1989. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem 87: 574–585, 2003. [DOI] [PubMed] [Google Scholar]
- Jenner P. A novel dopamine agonist for the transdermal treatment of Parkinson's disease. Neurology 65, Suppl 1: S3–S5, 2005. [DOI] [PubMed] [Google Scholar]
- Keeler BE, Baran CA, Brewer KL, Clemens S. Increased excitability of spinal pain reflexes and altered frequency-dependent modulation in the dopamine D3-receptor knockout mouse. Exp Neurol 238: 273–283, 2012. [DOI] [PubMed] [Google Scholar]
- Ko ML, King MA, Gordon TL, Crisp T. The effects of aging on spinal neurochemistry in the rat. Brain Res Bull 42: 95–98, 1997. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Westrich L, Bakhos S, Pasuit J. Identification and characterization of novel properties of the human D3 dopamine receptor. Mol Cell Neurosci 26: 144–155, 2004. [DOI] [PubMed] [Google Scholar]
- Lapointe NP, Rouleau P, Ung RV, Guertin PA. Specific role of dopamine D1 receptors in spinal network activation and rhythmic movement induction in vertebrates. J Physiol 587: 1499–1511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manconi M, Ferri R, Zucconi M, Clemens S, Giarolli L, Bottasini V, Ferini-Strambi L. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology 77: 110–117, 2011. [DOI] [PubMed] [Google Scholar]
- Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A, Ferini-Strambi L. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med 12: 47–55, 2010. [DOI] [PubMed] [Google Scholar]
- Mesco ER, Joseph JA, Blake MJ, Roth GS. Loss of D2 receptors during aging is partially due to decreased levels of mRNA. Brain Res 545: 355–357, 1991. [DOI] [PubMed] [Google Scholar]
- Mhyre TR, Chesler EJ, Thiruchelvam M, Lungu C, Cory-Slechta DA, Fry JD, Richfield EK. Heritability, correlations and in silico mapping of locomotor behavior and neurochemistry in inbred strains of mice. Genes Brain Behav 4: 209–228, 2005. [DOI] [PubMed] [Google Scholar]
- Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int J Geriatr Psychiatry 17: 359–370, 2002. [DOI] [PubMed] [Google Scholar]
- Reichmann H, Bilsing A, Ehret R, Greulich W, Schulz JB, Schwartz A, Rascol O. Ergoline and non-ergoline derivatives in the treatment of Parkinson's disease. J Neurol 253, Suppl 4: IV36–IV38, 2006. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Woods SC, Berger SP, Strakowski SM. D3 dopamine receptor, behavioral sensitization, and psychosis. Neurosci Biobehav Rev 25: 427–443, 2001. [DOI] [PubMed] [Google Scholar]
- Stark AK, Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res 318: 81–92, 2004. [DOI] [PubMed] [Google Scholar]
- Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci USA 102: 2117–2122, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Belloni M, Gorno ML, Tinti C, Memo M, Spano P. Dopamine D2, D3, and D4 receptor mRNA levels in rat brain and pituitary during aging. Neurobiol Aging 15: 713–719, 1994. [DOI] [PubMed] [Google Scholar]
- Westrich L, Gil-Mast S, Kortagere S, Kuzhikandathil EV. Development of tolerance in D3 dopamine receptor signaling is accompanied by distinct changes in receptor conformation. Biochem Pharmacol 79: 897–907, 2010. [DOI] [PubMed] [Google Scholar]
- Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab 17: 316–330, 1997. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79: 729–742, 1994. [DOI] [PubMed] [Google Scholar]
- Zhu H, Clemens S, Sawchuk M, Hochman S. Expression and distribution of all dopamine receptor subtypes (D1–D5) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience 149: 885–897, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]