Abstract
Adenomyosis is a common debilitating gynaecological disease. Transvaginal sonography (TVS) has been shown to be capable of diagnosing adenomyosis with an acceptable degree of accuracy. However, the reported appearances of adenomyosis on TVS are numerous and there is no consensus in the literature as to which image characteristics are unequivocally diagnostic; clarification would assist the sonographer in confidently providing a diagnosis. Following a thorough search of the electronic databases Embase and Medline, nine articles assessing the diagnostic accuracy of the sonographic features of adenomyosis on TVS against a gold standard reference test (histology post hysterectomy), using sensitivity and specificity, were selected for inclusion. The methodological quality of each of the nine included articles was assessed using a valid and reliable checklist tool. Four articles were considered suitable for inclusion in meta-analysis, which was facilitated by Meta-DiSc (Clinical Biostatistics Unit, Ramón y Cajal Hospital, Madrid, Spain). Meta-analysis showed that the heterogeneity between the studies was too great to allow statistical pooling of data. There was a wide between-study variation in the proficiency of six well-documented ultrasound characteristics of adenomyosis to correctly identify the disease, despite an apparent similarity in the studies’ populations, interventions and outcomes. This systematic review has been unable to draw a concise conclusion about which ultrasound image characteristics are most reliable in the correct diagnosis of adenomyosis. Further research is required into the sonographic features of adenomyosis with much larger study groups to attempt to establish those features that could enhance the reliability of ultrasound image interpretation.
Keywords: Adenomyosis, adenomyoma, transvaginal sonography, systematic review
Introduction
Adenomyosis is a common debilitating yet poorly understood gynaecological disease, which until recently could only be diagnosed histologically post hysterectomy.1 It is not possible to obtain an accurate assessment of the epidemiology of the disease from the literature, because there is such wide variation in the diagnostic criteria used. However, it affects 20–30% of women undergoing hysterectomy, independent of the indication for surgery,2 causes substantial morbidity3,4 and may be present for many years without correct diagnosis.5
Adenomyosis occurs when the normal interface between the endometrial basal layer and the myometrium is disrupted, causing the invasion of ectopic endometrial glands into the myometrium.2 This invasion can be either diffuse (adenomyosis) or focal (adenomyoma) and can affect any part of the uterus, although the posterior uterine wall is most frequently affected.2 It is considered to be a variant of endometriosis and the two conditions coexist in approximately 20% of affected patients.6 The symptoms of the disease are non-specific and varied, and include uterine tenderness and enlargement, dysmenorrhoea, menorrhagia and dyspareunia.4,5 Such symptoms can occur with many other gynaecologic disorders, such as fibroids, endometriosis and dysfunctional uterine bleeding, making clinical diagnosis difficult. Risk factors include multiparity, spontaneous and induced abortions and endometrial hyperplasia.2
Traditionally, the diagnosis of adenomyosis is confirmed histologically post hysterectomy and this is the accepted gold standard. However, there is no consensus definition for the pathological diagnosis of adenomyosis3,7 and consequently the reported prevalence of adenomyosis in the general population varies widely depending on the definition used.8,9 It is accepted that the incidence of the disease is higher in multiparous women.7 However, it is claimed that the disease may be grossly under-diagnosed among symptomatic women of reproductive age since the desire among these patients to preserve their fertility makes a confirmed diagnosis of adenomyosis by histopathology impossible.4,10
The limited image resolution of transabdominal ultrasound is frequently insufficient to detect the subtle sonographic features of adenomyosis.4 However, advances in imaging techniques, including magnetic resonance imaging (MRI) and transvaginal ultrasound (TVS) have provided a minimally invasive means of reliably diagnosing the disease. Studies have shown that there is no significant difference in accuracy between the two modalities; both are highly specific at detecting adenomyosis, meaning that false positive rates are low.3
Ultrasound examination is frequently the first-line diagnostic test in the investigation of gynaecological symptoms, informing clinicians in the primary care setting whether referral to a gynaecological specialist is necessary, assisting gynaecologists in deciding whether further diagnostic tests are required and in selecting appropriate patient management pathways and treatment options. TVS is accessible, minimally invasive, well tolerated by most patients and relatively inexpensive. Several studies have concluded TVS to be at least moderately accurate in diagnosing adenomyosis3–5,9,11,12 providing an acceptable, minimally invasive test that facilitates the early detection of the disease, thus assisting the clinician and patient in selecting the most appropriate treatment option.4
The reported characteristics of adenomyosis on TVS are numerous and varied and include myometrial heterogeneity, myometrial cysts, linear striations or focal areas with ill-defined borders within the myometrium, indistinct endomyometrial junction and asymmetric thickening of the myometrium.3,4,10,9,13 Power Doppler TVS has also been shown to be useful in differentiating adenomyosis from leiomyomas.9,13,14 However, there is a lack of general agreement as to which ultrasound characteristics of adenomyosis have the highest diagnostic accuracy in defining the presence of the disease and in determining the depth of penetration and the degree of spread.4,2
Over the last two decades, the sonographer’s role has been extended and has progressed from the expectation to merely produce images of adequate quality for interpretation by others to formulating an opinion in the form of a clear and concise report that can be relied upon by clinicians, thereby assisting them in arriving at an accurate diagnosis to ensure appropriate subsequent patient management.
Anecdotal evidence suggests that ultrasound practitioners are often reluctant to definitively diagnose adenomyosis. This is likely to be because of the wide variation of ultrasound appearances and lack of pathognomonic features, leading to a lack of confidence in the diagnosis, and concern that they may be unnecessarily committing patients to more invasive testing or procedures.
The aim of this systematic review was to identify and evaluate primary studies which empirically test the effectiveness of the measureable ultrasound features of adenomyosis in order to determine the reported features that are most reliable in the correct diagnosis of adenomyosis on TVS.
Methods
Inclusion and exclusion criteria
The study selection criteria determine which studies will be selected for inclusion in the review, based on their populations, interventions and outcomes. The inclusion and exclusion criteria were defined a priori in order to avoid bias being introduced by the knowledge of individual study results.15,16 Table 1 summarises the inclusion and exclusion criteria utilised.
Table 1.
Summary of inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population | Pre- and postmenopausal adult females scheduled for hysteroscopy | Pregnant patients Patients unable to tolerate TVS |
| Intervention | Transvaginal ultrasound (TVS) | Transabdominal ultrasound |
| Outcome | All relevant clinical outcomes Description of ultrasound features Diagnosis confirmed histopathologically Sensitivity Specificity | Diagnosis not confirmed No histopathological correlation |
| Study design | Observational studies Prospective Retrospective | Clinical trials Case reports |
| Other | From peer-reviewed journals | Non-English papers Journals more than 15 years old |
Search strategy
An initial list of potentially relevant articles was compiled by searching electronic databases. The general medical databases EMBASE and MEDLINE were searched using a combination of search terms (and appropriate MeSH terms where appropriate) relevant to the population, intervention and outcome.15 Boolean logic was employed to ensure that all relevant articles were retrieved and extraneous and duplicate material was excluded. The reference lists of all articles retrieved in full were interrogated for any articles that had been omitted by the electronic search. In addition, the reference lists of all review articles relevant to the subject were scrutinised for additional studies not already identified.
The included studies are Kepkep et al.10, Sun et al.11 and Bazot and Cortez12, and Exacoustos et al.17, Hak18, ElKattan et al.,19 Bazot et al.,20 Atri et al.,21 and Botsis D et al.22
Quality assessment
Observational studies are often more susceptible to bias.23 Quality assessment of the articles and assessment for risk of bias was achieved using a version of the QUADAS checklist, a tool specifically designed to assess the quality of diagnostic accuracy studies.24 An adapted checklist recommended by Reitsma et al.25 was utilised. Table 2 reports the compliance of each study with each checklist item using the judgements ‘YES’, ‘NO’ or ‘UNCLEAR’.
Table 2.
Compliance checklist
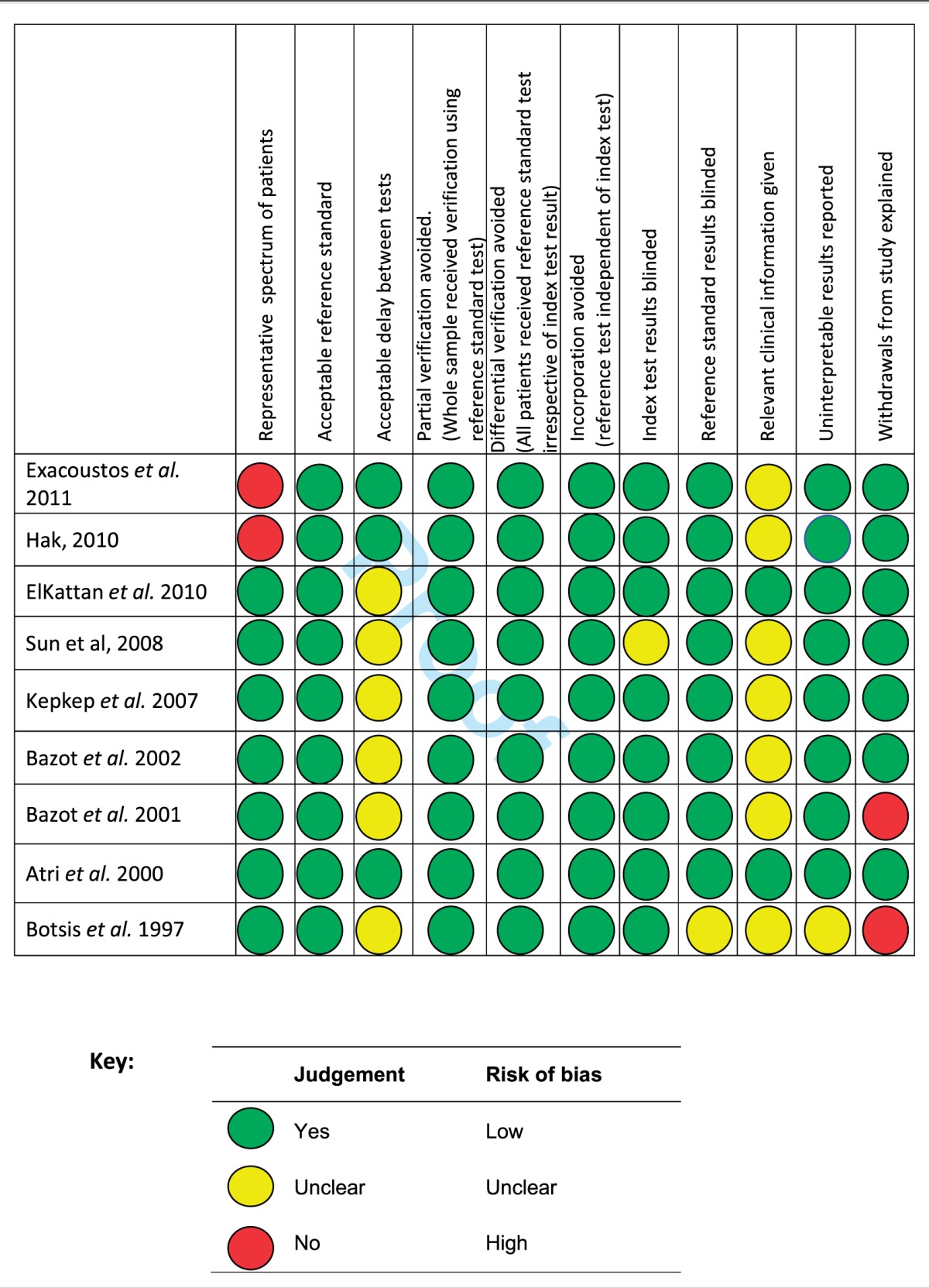
The detailed systematic analysis of the nine selected studies demonstrated elements of heterogeneity such that some were considered unsuitable for inclusion in the meta-analysis. The patient spectrum included in the studies by Exacoustos et al.17 Hak18 and Atri et al.21 were not fully representative of the population of patients on which the test will be used in clinical practice (see Table 3). In addition, the decision by these authors to only include selected individuals with specific clinical symptoms may well affect both the sensitivity of the test (due to possible greater disease severity) and the specificity of the test (due to the possible presence of other diseases).25 The study by Botsis et al.22 was also excluded from meta-analysis due to insufficient data to allow construction of 2 × 2 tables. On close inspection, it is clear that the study by Bazot et al.20 is drawn from the same study population as Bazot et al.12 It was decided to include the later study as the earlier study’s data set is incomplete. Bazot et al.20 divided the study population into two groups, according to the presence or absence of ‘recurrent menometrorrhagia, but no evidence of leiomyoma and endometrial diseases on TAS’. It was decided for the purposes of meta-analysis to treat the study population as a whole, thus producing a full spectrum of patients. Hence 2 × 2 tables for this study were constructed using combined results. The studies included in the meta-analysis were therefore reduced to four: ElKattan et al.,19 Sun et al.,11 Kepkep et al.10 and Bazot et al.20
Table 3.
Study characteristics
| Patient group | Main results | ||||||
|---|---|---|---|---|---|---|---|
| Study | No. of patients | Study design | Inclusions | Exclusions | Indications for hysterectomy | Sensitive | Specific |
| Exacoustos et al.17 | 72 | Prospective | Consecutive premenopausal scheduled for hysterectomy for benign pelvic pathology | Pregnant & postmenopausal patients, those on hormonal therapy, fibroids >8 cm, 3× fibroids >5 cm | Menorrhagia/abnormal uterine bleeding 76%, uterine prolapsed 10%, ovarian pathology 10% | Heterogenous myometrium 88% | Myometrial cysts 98%, linear striations 90%, asymmetrical myometrium 80% |
| Hak18 | 50 | Prospective | Perimenopausal planned for hysterectomy for menorrhagia | Patients with chronic pain | Menorrhagia | Hypoechoic areas 83%, heterogenous areas 75% | Myometrial cysts, linear striations, globular uterine configuration all 100%, hypoechoic areas 95%, heterogenous areas 87% |
| ElKattan et al.19 | 352 | Prospective | All patients scheduled for hysterectomy.? consecutive | Patients who refused hysterectomy or were unfit for surgery. | Leiomyoma 42%, abnormal uterine bleeding 31%, endometrial hyperplasia 9%, prolapse 8%, adnexal masses 8%, cervical masses 1%. | Heterogenous myometrial echo texture 95%, poor endomyometrial delineation 76%, myometrial cysts 70% | Linear myometrial striations 91% |
| Sun et al.11 | 213 | Retro-spective | Consecutive patients scheduled for hysterectomy | None | Dysmenorrhoea, menometrorrhagia, cervical masses, adnexal masses, prolapsed, endometrial hyperplasia or carcinoma | Linear myometrial striations 92%, heterogenous myometrium 87%, myometrial cysts 82%. | Globular uterine configuration 78%, myometrial A-P asymmetry 75% |
| Kepkep et al.10 | 70 | Prospective | Consecutive patients scheduled for hysterectomy | None | Leiomyoma 40%, endometrial hyperplasia 25%, adnexal tumours 11%, abnormal uterine bleeding 11%, prolapsed 6%, cervical dysplasia 3%, postmenopausal bleeding 3% | Heterogenous myometrium 81% | Linear myometrial striations 96%, globular configuration 86%, poor delineation of endomyometrial junction 82%, myometrial cysts 82% |
| Bazot et al.20 | 129 Group 1 23 Group 2 106 | Prospective | ? consecutive patients scheduled for hysterectomy Group 1 – with menometrorrhagia but free of myoma & endometrial disorders on TAS Group 2 – all other | None | Menorrhagia and/or metrorrhagia 71%, endometrial carcinoma 10%, cervical masses 6%, adnexal masses 8%, prolapse 10% | Group 1: heterogenous myometrial echo texture 100%, myometrial cysts 81% Group 2: Globular uterine configuration 92%, heterogenous myometrial echo texture 88% | Group 1: Myometrial cysts, linear myometrial striations, asymmetric myometrium, globular configuration all 100% Group 2: Linear myometrial striations 99%, myometrial cysts 98%, globular uterine configuration 96%, asymmetric myometrium 83%. |
| Bazot et al.12 | 120 | Prospective | ? consecutive patients referred for hysterectomy | 47 excluded – lack of US/MRI findings 55% cancelled surgery, conservative surgery, endometrial resection | Menorrhagia and/or metrorrhagia 45%, postmenopausal bleeding 13%, adnexal masses 11%, cervical masses 9%, pelvic pain 12%, prolapsed 8%, miscellaneous 2% | Myometrial cyst 60% | Myometrial cyst 99%, globular uterine configuration 96%, heterogenous myometrial echo texture 90% |
| Atri et al.21 | 102 | Prospective | Intact hysterectomy specimens (pre- and postmenopausal) | Substantial distortion due to leiomyomas | Uterine prolapse 14%, incontinence 7%, premenopausal uterine bleeding & dysmenorrhoea 31%, pelvic pain 5%, postmenopausal bleeding 3%, suspicious endometrial biopsy 16%, adnexal mass 19%, transexuality 2%, familial ovarian cancer 3%. | Hypoechoic myometrium (OR) 24.5, asymmetric myometrium (OR) 10.7 | Heterogenous myometrium (OR) 1.8 |
| Botsis et al.22 | 194 | Prospective | Enlarged uterus on TVS | Uterine nodules <2 cm | Menorrhagia and/or dysmenorrhoea 83%, pressure/pain consistent with a mass lesion 2%, dyspareunia 10%, urinary incontinence 3%, rapid tumour growth 1% | Irregular enlarged uterus 100% | |
 Studies included in meta-analysis
Studies included in meta-analysis
Meta-analysis
Meta-DiSc is a software package designed specifically for the purpose of implementing meta-analysis of test accuracy studies.26 True-positive, false-positive, false-negative and true-negative figures derived from 2 × 2 tables produced using the results from the four studies were entered into a spreadsheet within the software. Using this information, tabular results, Forest plots of sensitivity, specificity, LRs, DORs and ROC curves were produced. In addition, Chi square and Cochran’s Q were implemented in order to evaluate if the differences across the studies are greater than chance alone, indicated by a low p value.26 Cochran’s Q is inherently dependent on the number of studies included in the meta-analysis and has a low power when the number of studies is small.27 The inconsistency (I2) index is, however, independent of the number of chosen studies and is particularly useful because it describes the percentage variation between studies, which can be used to quantify the clinical heterogeneity that has not occurred due to chance. However, caution should be applied when comparing studies of different sizes as the I2 index is dependent on study precision, or sample size. Values range from 0% to 100%. A value close to 0% indicates little observed heterogeneity; where there is evidence of heterogeneity, the use of summary statistics to pool results should be considered carefully. In each case, the meta-analysis was carried out using a random-effects model, which assumes no single underlying value of the effect but a distribution of effects depending on the studies’ characteristics.15
Results and discussion
Introduction
The aim of this systematic review was to determine the sonographic features that are most reliable in the diagnosis of adenomyosis in order to assist the sonographer in producing clear and concise reports that direct the clinician in the appropriate management of the patient. This is the only systematic review to focus specifically on the sonographic features of adenomyosis. Other systematic reviews3,4,9 have been carried out to assess the accuracy of TVS in the diagnosis of adenomyosis.
The nine studies included in this review were prospective or retrospective observational cohort studies. Of these, only four studies were considered of high enough quality to be included in the meta-analysis. Although every effort has been made to ensure that these four studies are of high quality and are as similar as possible in terms of their populations, interventions and outcomes, the heterogeneity between the studies is too great to allow statistical pooling of data, the simplest method of summarising results. It is of note that even between these four apparently similar studies, the reported sensitivity and specificity of TVS for the diagnosis of adenomyosis ranges from 87.1% to 57.4% and 97.5% to 60.1%, respectively. The results of the meta-analysis show that there is a wide between-study variation in the proficiency of six well-documented ultrasound characteristics of adenomyosis to correctly identify the disease, making it very difficult to draw meaningful conclusions.
The main results from each of these studies is summarised in Table 3. There is some agreement between the studies with regard to the following:
The presence of myometrial cysts (Figure 1) on TVS has a moderate sensitivity in correctly identifying patients with adenomyosis; the presence of this ultrasound feature raises the probability that the patient has the disease. The absence of myometrial cysts lowers the probability of the presence of disease.
The results from all but the oldest study to be included in the meta-analysis suggest that a heterogenous myometrium raises the probability of the presence of adenomyosis.20
The presence of linear myometrial striations (Figure 2) raises the probability of the presence of disease; however, the absence of this ultrasound characteristic does not lower the probability of the presence of adenomyosis.
Poor delineation of the endomyometrial junction (Figure 3) raises the probability that the patient has adenomyosis and a negative result for this ultrasound characteristic reduces the probability that the disease is present. However, the SROC curve suggests that the cut-off point for a positive result varies between studies.
The presence of myometrial anteroposterior asymmetry neither raises nor lowers the probability of the disease: this is not a useful ultrasound feature in the assessment of the uterus for the presence of adenomyosis.
There was no agreement between the studies as to the usefulness of the ultrasound feature of globular uterine configuration.
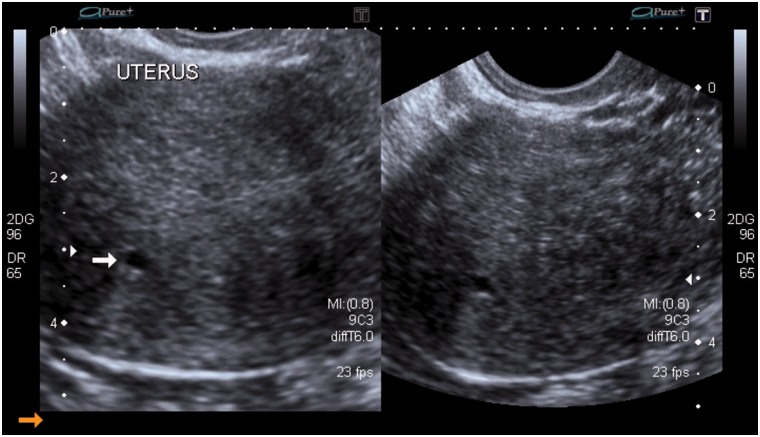
Figure 1.
Longitudinal and transverse sections of the uterus on transvaginal sonography (TVS) showing a well-defined anechoic myometrial cyst (arrow) within the posterior uterine wall
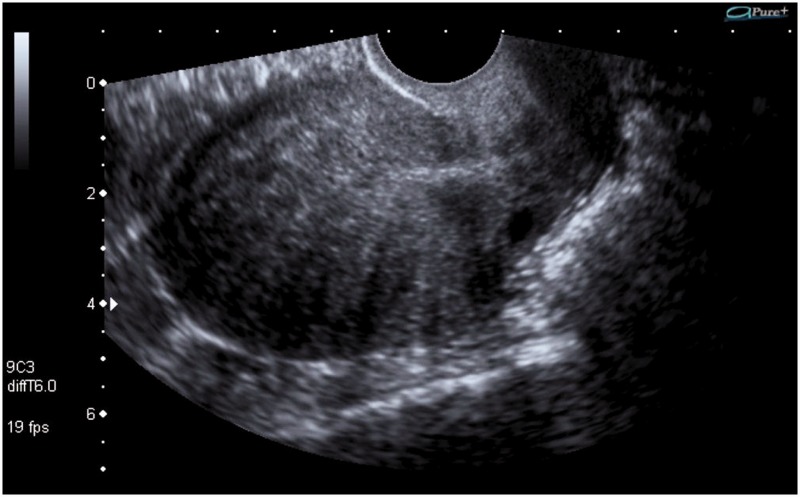
Figure 2.
Longitudinal section of the uterus on transvaginal sonography (TVS) showing hypoechoic linear myometrial striations into the myometrium
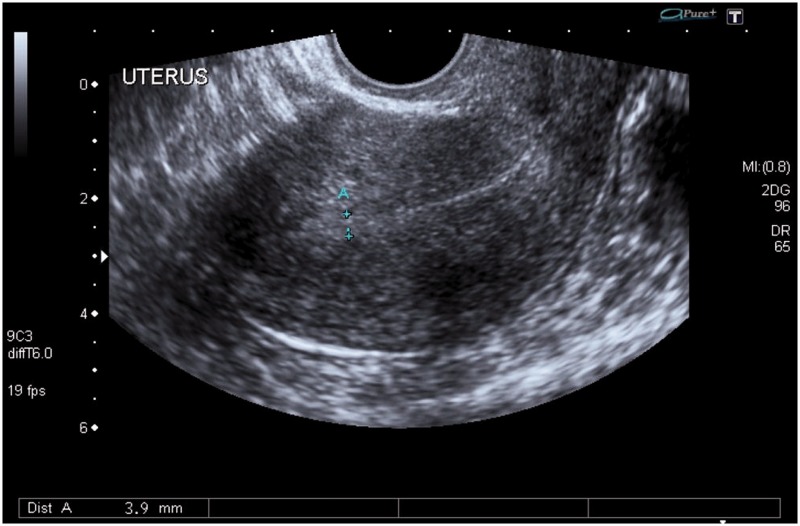
Figure 3.
Longitudinal section of the uterus on transvaginal sonography (TVS) showing a poorly defined endomyometrial junction
Potential sources of heterogeneity
Metaregression analysis could have been utilised to identify possible sources of heterogeneity between the studies, but this is outside the bounds of this review and the statistical capabilities of the review author. However, several potential sources of heterogeneity have been explored in the analysis that follows.
Sample population
The major limiting factor in the studies excluded from the meta-analysis was bias in the patient spectra included in the studies. The authors of three of the studies excluded from meta-analysis each chose not to include certain patients, the exclusion of which may have affected estimates of sensitivity and specificity of the index test.25 However, it is entirely possible that, although not explicit within the research methodologies, important differences may have existed between the patient spectra included in the included studies. When the indications for hysterectomy are interrogated (see Table 3), it is of note that ElKattan et al.19 and Kepkep et al.10 specifically list leiomyoma as the primary indication. However, in the studies carried out by Sun et al.11 and Bazot et al.,20 leiomyoma is not even listed as an indication for hysterectomy. It is unclear whether such patients were deliberately excluded or whether the indications have been described instead in terms of the symptoms of menorrhagia, dysmenorrhoea or metrorrhagia.
Consecutive enrolment
Consecutive enrolment of participants into each of the individual studies included in the meta-analysis is important because it emulates, as closely as possible, the range of patients that a clinician utilising such a test will see in practice. ElKattan et al.19 and Bazot et al.20 fail to state explicitly in their methodology whether the cohort of included patients was in fact consecutive (see Table 3). This may have affected the prevalence of the target disease in the study population.
Disease prevalence
Disease prevalence differs between the four included studies and is notably lower in the study by ElKattan et al.19 Disease prevalence can affect a test’s sensitivity and specificity and it is not necessarily possible to predict in which direction.28 Where disease prevalence is low, sensitivity may be lower, because there may be more patients in whom the disease is barely present (and therefore more difficult to detect) and fewer patients in whom the disease is clearly present.28 The opposite may also be true: the sensitivity of a test may be higher in a population where disease prevalence is higher, because the characteristics of severe disease are often easier to identify. It has been suggested that many of the ultrasound features of adenomyosis such as myometrial cysts and asymmetric myometrium may only be present in patients with advanced disease.17
Disease prevalence may be affected by the clinical pathway by which patients are referred to the setting from which they were enrolled into a study, including work-up diagnostic testing.28 The resulting differences in patient spectrum will not necessarily be evident from a study authors’ description of the patient inclusion/exclusion criteria.
Imperfect reference standards bias the reported prevalence of disease28 as well as affect the index test’s sensitivity and specificity. ElKattan et al.,19 Sun et al.11 and Kepkep et al.10 all refer to the reference standard as a possible study limitation. This may be due to differences in the histological criteria for the diagnosis of adenomyosis, inter-observer differences when different pathologists examine the specimens, the level of care undertaken during sampling or the number of sampling blocks taken during the pathological examination.
Ultrasound criteria for diagnosis
The description of the ultrasound criteria used to reach a positive diagnosis of adenomyosis in each included study is very similar and is supported by early research into the appearance of adenomyosis on ultrasound. Each study deems the presence of at least one ultrasound characteristic as definitive for a diagnosis of adenomyosis. Thus, the reported diagnostic threshold for each included study is the presence of at least one of the ultrasound characteristics of adenomyosis. However, ultrasound is highly subjective and operator-dependent and true thresholds will differ due to individual variance in test interpretation, even when the reported thresholds are constant between studies.29 This may be affected by operator experience: more experienced ultrasound operators will be better able to recognise the often subtle changes in echo texture that may denote the presence of disease. All but one of the four included studies reported the use of experienced operators, however, the study by ElKattan et al.19 described the three ultrasound operators as ‘trained’ but did not comment on the level of experience. Another difference between the studies is that the study by Sun et al.11 utilised an experienced investigator who interpreted still images. This may have affected test accuracy as ultrasound is considered a real-time examination, whereby interpretation is most accurately carried out by the operator at the time of image acquisition. In addition, base-line calibration of the ultrasound machine and the operator’s ability to manipulate machine settings in order to optimise the image for detection of specific disease characteristics will affect the reported presence of disease.
Adenomyosis is rarely an isolated condition;10 where there is co-existing disease, the appearances may mimic the target condition and vice versa. This will inevitably affect sensitivity and specificity. In addition, it is well documented that the sensitivity of TVS is limited in large uteri or in uteri with large or multiple fibroids, where it is not always possible to fully examine the myometrium.
Study limitations
The only recognised reference test for the diagnosis of adenomyosis is histopathology following hysterectomy, a highly invasive test which can only be carried out on patients referred for hysterectomy, who are likely to be older and more symptomatic than many of the affected population. In such patients, the severity of disease may be more advanced and easier to detect. The reported sensitivity and specificity of TVS for adenomyosis should only be applied to this group of patients.
This review and meta-analysis was carried out by a single reviewer as part of a taught Higher Degree (MSc) within a limited time frame. In order to reduce bias, electronic searching of databases for the retrieval of potentially relevant articles and the subsequent selection of articles for inclusion in the review should be carried out by at least two reviewers. However, with the limited financial resources and time available this was not possible and inevitably introduced an element of bias to the review process.
Foreign language studies were not considered for inclusion in this review due to time and financial implications of translation to an academic standard. This may represent a source of bias as it is likely that studies with relevant and important results were not considered for inclusion.
New technology
Ultrasound technology is continually improving with more efficient transducers producing images with ever higher resolution. In addition, new techniques are emerging and show promising results for use in diagnosing gynaecological disorders. Three-dimensional TVS (3D-TVS) is one such technique and its use in interrogating the endomyometrial junction is being explored. In a recent study, Exacoustos et al.17 reported that reconstructed 3D-TVS images provide superior visualisation of the junctional zone on the coronal section, facilitating unrivalled views of the endomyometrial junction. Elastography is another emerging ultrasound technique which is already being used to assess diseases of the liver and breast among others. Real-time transvaginal elastography is a technique that uses slight external tissue compression to quantify the strain produced in the structures examined.30 Two small studies30,31 found that there are definite differences in strain distribution between adenomyosis, which demonstrated softer tissue regions, and ‘stiffer’ leiomyomas suggesting that this technique can be used to either confirm or raise suspicion of the presence of adenomyosis.
Recommendations and future research
The characteristics of adenomyosis are often subtle but are identifiable on TVS, however, the operator’s ability to recognise and confidently report these features will depend on their experience and technique. Specialist training in pattern recognition of the subtle features of adenomyosis will improve the diagnostic accuracy of TVS. There is a need for more studies into the sonographic features of adenomyosis with larger sample volumes. Studies are also required to assess test-retest and inter-observer reliability in the diagnosis of adenomyosis at TVS.
Conclusion
Due to extensive heterogeneity between the included studies, this systematic review has been unable to draw concise conclusions about which ultrasound characteristics are most reliable in the correct diagnosis of adenomyosis. However, the presence of myometrial cysts, linear myometrial striations, poor delineation of the endomyometrial junction and a heterogenous myometrium all raise the probability of the presence of disease. Myometrial anteroposterior asymmetry is not a useful ultrasound feature in the assessment of the uterus for the presence of adenomyosis, as the presence of this feature neither raises nor lowers the probability of disease.
Acknowledgements
I acknowledge Ms Rosie Simpson and Mr Paul Seed, King’s College, London, for their advice, The Ultrasound Department at Portsmouth Hospitals NHS Trust, my partner Stephen Barton and my three children, John, Carys and Peter for their support and encouragement.
Declarations
Funding: This work was carried out as part of an MSc in Medical Ultrasound funded by Portsmouth Hospitals NHS Trust
Competing interests: None
References
- 1.Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Prac Res Clin Obstet Gynaecol 2006; 20: 603–16. [DOI] [PubMed] [Google Scholar]
- 2.Vercellini P, Viganò P, Somigliana E, et al. Adenomyosis: epidemiological factors. Best Prac & Res Clin Obstet Gynaecol 2006; 20: 465–77. [DOI] [PubMed] [Google Scholar]
- 3.Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstericia et Gynecologica 2010; 89: 1374–84. [DOI] [PubMed] [Google Scholar]
- 4.Meredith S, Sanchez-Ramos L, Kaunitz A. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and metaanalysis. Am J Obstet Gynecol 2009; 201: 107.e1–6. [DOI] [PubMed] [Google Scholar]
- 5.Andreotti F, Fleischer A. The Sonographic diagnosis of adenomyosis. Ultrasound Quarterly 2005; 21: 167–70. [DOI] [PubMed] [Google Scholar]
- 6.Bates J. Practical Gynaecological Ultrasound, 2nd edn Cambridge: Cambridge University Press, 2006. [Google Scholar]
- 7.Peric H, Fraser I. The symptomatology of adenomyosis. Best Prac & Res Clin Obstet Gynaecol 2006; 20: 547–55. [DOI] [PubMed] [Google Scholar]
- 8.Dueholm M, Lundorf E. Transvaginal ultrasound or MRI for diagnosis of adenomyosis. Current Opin Obstet Gynecol 2007; 19: 505–12. [DOI] [PubMed] [Google Scholar]
- 9.Dueholm M. Transvaginal ultrasound for diagnosis of adenomyosis: a review. Best Prac Research Clin Obstet Gynaecol 2006; 20: 569–82. [DOI] [PubMed] [Google Scholar]
- 10.Kepkep K, Tuncay Y, Göynümer G, et al. Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound Obstet Gynecol 2007; 30: 341–5. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y-L, Wang C-B, Lee C-Y, et al. Transvaginal sonographic criteria for the diagnosis of adenomyosis based on histopathologic correlation. Taiwanese J Obstet Gynecol 2008; 49: 40–4. [DOI] [PubMed] [Google Scholar]
- 12.Bazot M, Cortez A. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Human Reprod 2001; 16: 2427–33. [DOI] [PubMed] [Google Scholar]
- 13.Tonni G, Ventura A. Adenomyosis: a novel approach using transvaginal power Doppler sonography. J Diag Med Sonography 2005; 21: 267–72. [Google Scholar]
- 14.Perrot N, Frey I, Mergui J-L, et al. Adenomyosis: power Doppler findings. Ultrasound Obstet Gynaecol 2001; 17: 177–8. [DOI] [PubMed] [Google Scholar]
- 15.Khan K, Kunz R, Kleijnen J, et al. Systematic Reviews to Support Evidence-Based Medicine, 2nd edn London: Hodder Arnold, 2011. [Google Scholar]
- 16.Egger M, Davey Smith G, Altman D. Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd edn London: John Wiley & Sons, 2008. [Google Scholar]
- 17.Exacoustos C, Brienza L, Di Giovanni A, et al. Adenomyosis: three-dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet Gynecol 2011; 37: 471–9. [DOI] [PubMed] [Google Scholar]
- 18.Hak A. Accuracy of sonographic criteria for diagnosis of adenomyosis in perimenopausal women with menorrhagia. Mid East Fert Soc J 2010; 15: 35–8. [Google Scholar]
- 19.ElKattan E, Omran E, Al Inany H. The accuracy of transvaginal ultrasound and uterine artery Doppler in the prediction of adenomyosis. Mid East Fert Soc J 2010; 15: 73–8. [Google Scholar]
- 20.Bazot M, Daraï E, Rouger J, et al. Limitations of transvaginal sonography for the diagnosis of adenomyosis, with histopathological correlation. Ultrasound Obst Gynecol 2002; 20: 605–11. [DOI] [PubMed] [Google Scholar]
- 21.Atri M, Reinhold C, Mehio A, et al. Adenomyosis: US features with histologic correlation in an in vitro study. Radiology 2000; 215: 783–90. [DOI] [PubMed] [Google Scholar]
- 22.Botsis D, Kassanos D, Antoniou G, et al. Adenomyoma and leiomyoma: Differential diagnosis with transvaginal sonography. J Clin Ultrasound 1998; 26: 21–5. [DOI] [PubMed] [Google Scholar]
- 23.Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare, York: University of York, 2009, pp. 1–281. [Google Scholar]
- 24.Whiting P, Rutjes A, Reitsma J, et al. The Development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reitsma J, Rutjes A, Whiting P, et al. Assessing methodological quality. In: Deeks J, Bossuyt P, Gatsonis C, eds. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 1.0.0. The Cochrane Collaboration. 2009. See http://srda.cochrane.org/ (last checked 15 February 2012).
- 26.Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6: 31.–31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavaghan D, Moore A, McQay H. An evaluation of homogeneity tests in meta-analysis in pain using simulations of patient data. Pain 2000; 85: 415–24.. [DOI] [PubMed] [Google Scholar]
- 28.Leeflang M, Bossuyt P, Irwig L. Diagnostic test accuracy may vary with prevalence: implications for evidence-based diagnosis. J Clin Epid 2009; 62: 5–12. [DOI] [PubMed] [Google Scholar]
- 29.Jones C, Ashrafian H, Skapinakis P, et al. Diagnostic accuracy meta-analysis: A review of the basic principles of interpretation and application. Int J Cardiol 2010; 140: 138–44. [DOI] [PubMed] [Google Scholar]
- 30.Tessarolo M, Bunino L, Camanni M, et al. Elastography: a possible new tool for diagnosis of adenomyosis. Eur Rad 2011; 21: 1546–52. [DOI] [PubMed] [Google Scholar]
- 31.Veldman J, Von Hols eke C, Werbrouck E, et al. Differentiation of uterine pathology by transvaginal elastography: preliminary results. Ultrasound Obstet Gynecol 2010; 36(Suppl. 1): 14–5. [Google Scholar]