Abstract
A 14-year-old girl was admitted to hospital with fever, headache, sore throat and abdominal pain. Her blood lymphocyte count and inflammatory markers were raised. Acute Epstein–Barr virus (EBV) infection was suspected and confirmed serologically and by measuring the viral load. On day 7, she developed jaundice with abnormal liver function tests. An abdominal ultrasound scan revealed thickening of the gallbladder and bile duct walls without calculi suggesting acute acalculous cholecystitis. The patient improved slowly with symptomatic treatment, and a repeat ultrasound scan six months later was normal. Acalculous cholecystitis is a rare complication of EBV infection and usually has a good prognosis.
Keywords: Epstein–Barr virus infection, acalculous cholecystitis, children, adolescents
Case report
A 14-year-old girl was admitted with a two-day history of sore throat, cough and headache. She did not complain of nausea, vomiting or photophobia. In the past, she had suffered from migraine. On examination, she was pyrexial at 38.4℃, alert, pink, well perfused and not dehydrated. She had slightly puffy and red eyes. There was no periorbital involvement and no discharge. Chest was clear with normal heart sounds, and abdomen was soft without organomegaly. She did not have neck stiffness and both fundi had clear margins. Neurological examination was normal. A differential diagnosis of probable allergic rhinitis and migraine was made, and after a normal urinalysis, she was reassured and discharged home.
She returned 36 hours later to our hospital because of ongoing pyrexia, worsening eyelid swelling, vomiting and anorexia. A thorough examination only revealed tenderness in the right hypochondrium. A primary panel of routine blood tests was ordered, which showed raised white cell count with lymphocytosis, mild thrombocytopenia, deranged liver function tests but normal coagulation screen and kidney function (Table 1). A blood smear showed reactive/atypical mononuclear cells, and Monospot was positive. Therefore, a revised diagnosis of infectious mononucleosis with associated hepatitis was made. She was admitted to the inpatient department for further observation and management due to poor oral intake, vomiting and pyrexia.
Table 1.
Laboratory investigations of patient
| Tests (normal ranges) | Results |
|---|---|
| White cell count (4.0–11.0 × 109/L) | 15.5 × 109/L (max. rise to 39.2 × 109/L) |
| Lymphocytes (1.5–4.0 × 109/L) | 11.3 × 109/ L (max. rise to 31.5 × 109/L) |
| Platelet count (140–400 × 109/L) | 119 × 109/L (max. drop to 106 × 109/L) |
| C-reactive protein (<5 mg/L) | 12 mg/L |
| Bilirubin (<17 µmol/L) | 40 µmol/L (max. rise to 100 µmol/L) |
| Alkaline phosphatase (80–280 IU/L) | 178 IU/L (max. rise to 341 IU/L) |
| Alanine transaminase (<40 IU/L) | 207 IU/L (max. rise to 272 IU/L) |
| γ-Glutamyl transferase (<50 IU/L) | 111 IU/L (max. rise to 153 IU/L) |
| Nuclear antibody | All negative |
| Gastric parietal cell antibody | |
| Mitochondrial antibody | |
| Liver kidney microsomal antibody | |
| Smooth muscle antibody | 1:80 Positive (can be raised in viral infections) |
| Hepatitis A, B, C serology | Negative |
| Mycoplasma IgM antibody | Negative |
| Caeruloplasmin (0.15–0.45 g/L) | 0.38 g/L |
Over the next 48 hours, she continued to be febrile and suffered prostration due to vomiting and severe right upper quadrant abdominal pain. Repeat blood chemistry was ordered the next day, and her case was discussed with a paediatric hepatologist, who advised a battery of tests to look for other causes of hepatitis. These tests included EBV serology, EBV PCR, autoantibody screen, hepatitis A, B, and C serology, immunoglobulins and caeruloplasmin levels. Her liver transaminases, gamma-glutamyl transferase and bilirubin continued to rise over the next few days. She started developing jaundice on day 7 of her illness, and an ultrasound of her abdomen was requested. On the ultrasound scan, the bile ducts were hyperechoic and thickened but not dilated. The gallbladder wall was markedly thickened and oedematous, and there were no calculi. The spleen was moderately enlarged. The findings were consistent with acalculous cholecystitis (Figure 1). This was thought to be due to Epstein–Bar virus infection as EBV IgM and IgG capsid antigen were positive, and EBV viral load was detected at a concentration of 7.2 × 103 copies/ml. Secondary panel blood tests were negative and ruled out other potential causes of hepatitis.
Figure 1.
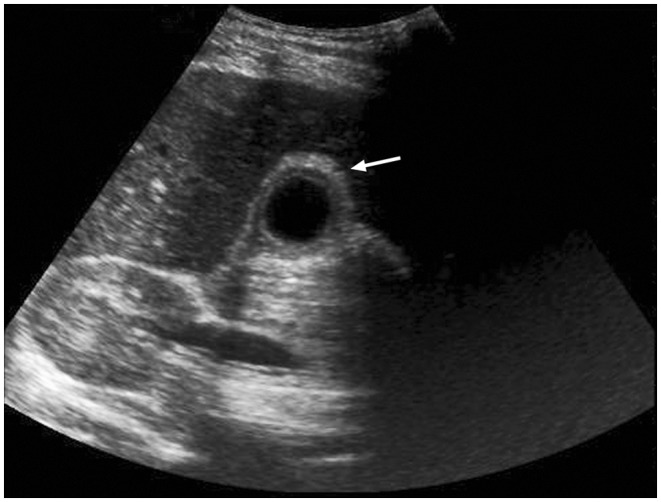
Ultrasound scan of patient showing normal liver tissue with thickened and oedematous gallbladder wall (arrow)
The girl was managed conservatively without surgical intervention and parenteral antibiotics. She became afebrile from day 8 of illness, and her appetite improved. She was discharged home a few days later. In the following weeks, her abdominal pain and jaundice steadily improved and her liver function returned to normal. She continued to suffer from post-viral fatigue for several months. A repeat ultrasound six months later showed complete resolution of the oedematous gall bladder wall.
Discussion
Epstein–Barr virus (EBV type 1 and 2) commonly manifests as acute infectious mononucleosis (glandular fever), a self-limiting illness that affects predominantly adolescents and young adults. The clinical picture includes tiredness, pyrexia, myalgia, headaches, pharyngitis, tonsillitis and lymphadenopathy. While hepatosplenomegaly is frequently found, jaundice and cholecystitis have been rarely reported as part of this condition.1
Acute acalculous cholecystitis (AAC) accounts for 2–15% of all cases of acute cholecystitis.2 It presents with fever, jaundice, right upper quadrant pain and leukocytosis. Imaging studies reveal oedematous gall bladder and ducts but no stones. Specific ultrasound criteria (six major and two minor) exist to make a diagnosis of AAC.3 They include gallbladder wall thickening and oedema, intramural gas, mucosal sloughing, pericholecystic fluid and positive Murphy’s sign. At least two major criteria are required. An oedematous gallbladder wall can be observed in primary disease of the gallbladder such as acute and chronic cholecystitis, porcelain gallbladder and gallbladder carcinoma. It can also be seen as a secondary phenomenon in hepatitis, pancreatitis, liver cirrhosis and congestive heart failure.4 Typically, AAC occurs in critically ill patients (septicaemia, trauma, burns or post-surgery) and is associated with a significant mortality. However, milder forms of the disease have been observed in non-hospitalised patients. There appears to be some debate in the literature over the correct terminology. Debnath and Mathur5 comment on a case of AAC in a teenager with hepatitis A described by Souza et al.6 Based on the ultrasound images, they question the diagnosis of AAC and instead suggest pericholecystitis in acute hepatitis as an alternative diagnosis.
Several risk factors for AAC have been identified, such as child birth, cardiovascular and renal disease, diabetes mellitus, immunosuppression, total parenteral nutrition and use of opiates.7 Infections are the commonest cause of AAC in paediatrics. The pathogenesis of acalculous cholecystitis is not fully understood despite numerous experimental studies. It is hypothesised that bile stasis, primary and secondary infection, and ischaemia lead to gallbladder distension, inflammation and, in some cases, necrosis.8
Due to its rare occurrence in previously healthy young adults, AAC can be a diagnostic challenge for the clinician unfamiliar with its association with EBV. However, since 2007, there have been several case reports on this topic.9 Gora-Gebka et al.10 reported two paediatric cases of ACC secondary to EBV and cytomegalovirus infection, respectively. Iaria et al. described a case of ACC occurring in the course of primary EBV infection and compared it with three similar cases. All four females had signs and symptoms of cholestatic hepatitis and showed gallbladder inflammation on imaging but no gallstones. They recovered spontaneously without the need for surgical intervention.11 A literature review of EBV-associated acalculous cholecystitis by Arya et al. identified 10 cases, of which all but one patient were female. They shared similar clinical and laboratory findings, such as presence of lymphocytosis, atypical lymphocytes, and direct hyperbilirubinaemia. The patients also had moderately elevated liver enzymes with transaminase levels of less than 1000 IU/L. There were no markers of severe liver involvement, such as hyper-ammonaemia or prolonged prothrombin time. Common ultrasound findings included gallbladder wall thickening and collapse. There was no evidence of biliary obstruction. All patients in their series made an excellent recovery without cholecystectomy.12
Conclusion
Cholecystitis in adults is mainly caused by gallstones, whereas infections are the main cause in children. Acalculous cholecystitis is a rare condition usually observed in seriously ill patients. However, milder forms of AAC have been described in the literature. The preferred diagnostic imaging modality is ultrasound scanning.13
Acknowledgements
We are grateful to the patient and her parents for permitting publication.
Declarations
Competing interests: None.
Funding: None.
Ethical approval: Written consent was obtained from the patient’s mother for publication of the details and image of the case.
Guarantor: Dr Strehle acts as guarantor for this manuscript.
Contributorship: Dr Strehle initiated the case report and wrote the manuscript jointly with Dr De Alwis and Dr Saleem.
References
- 1.Jenson HB. Epstein-Barr virus. In: Kliegman RM, Behrman RE, Jenson HB, et al. (eds). Nelson Textbook of Pediatrics, 18th edn Philadelphia, PA: Saunders Elsevier, 2007, pp. 1372–7. [Google Scholar]
- 2.Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol 2010; 8: 15–22. [DOI] [PubMed] [Google Scholar]
- 3.Harris EF, Younger E, Llewelyn MB. Acalculous cholecystitis occurring in the context of Plasmodium malariae infection: a case report. J Med Case Rep 2013; 7: 197–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Breda Vriesman AC, Smithuis R, Van Engelen D, et al. Gallbladder-wall thickening, http://radiologyassistant.nl (2006, accessed 15 October 2013)..
- 5.Debnath J, Mathur A. Is it acalculous cholecystitis or reactive/viral pericholecystitis in acute hepatitis? Braz J Infect Dis 2010; 14: 647–8. [PubMed] [Google Scholar]
- 6.Souza LJ, Braga LC, Rocha Nde S, et al. Acute acalculous cholecystitis in a teenager with hepatitis a infection: a case report. Braz J Infect Dis 2009; 13: 74–6. [DOI] [PubMed] [Google Scholar]
- 7.Afdhal NH. Acalculous cholecystitis, http://www.uptodate.com (2013, accessed 15 October 2013).
- 8.Zakko SF and Afdhal NH. Pathogenesis, clinical features, and diagnosis of acute cholecystitis, http://www.uptodate.com (2013, accessed 15 August 2013).
- 9.Koch AD, van den Bosch HC, Bravenboer B. Epstein-Barr virus-associated cholecystitis. Ann Intern Med 2007; 146: 826–7. [DOI] [PubMed] [Google Scholar]
- 10.Gora-Gebka M, Liberek A, Bako W, et al. Acute acalculous cholecystitis of viral etiology – a rare condition in children? J Pediatr Surg 2008; 43: e25–27. [DOI] [PubMed] [Google Scholar]
- 11.Iaria C, Arena L, Di Maio G, et al. Acute acalculous cholecystitis during the course of primary Epstein-Barr virus infection: a new case and a review of the literature. Int J Infect Dis 2008; 12: 391–5. [DOI] [PubMed] [Google Scholar]
- 12.Arya SO, Saini A, El-Baba M, et al. Epstein Barr virus-associated acute acalculous cholecystitis: a rare occurrence but favorable outcome. Clin Pediatr (Phila) 2010; 49: 799–804. [DOI] [PubMed] [Google Scholar]
- 13.Nagdev A, Md JW. Bedside ultrasound diagnosis of acalculous cholecystitis from Epstein-Barr virus. West J Emerg Med 2011; 12: 481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


