Abstract
Proliferation of imaging studies for different clinical purposes and continuous improvement of imaging technology have led to an increasing number of incidental findings of renal masses. It is estimated that over 50% of patients older than 50 years have at least one renal mass.
The majority of incidental renal masses are simple cysts that can be easily diagnosed by conventional ultrasonography. However, some incidental renal masses are not simple cysts, and differentiation between benign and malignant entities requires further imaging modalities. In the past, multiphase contrast-enhanced computed tomography and magnetic resonance imaging were considered the primary imaging modalities used to characterize and stage complex cystic and solid renal lesions. Currently, contrast-enhanced ultrasonography represents a novel alternative to contrast-enhanced computed tomography and magnetic resonance imaging.
Contrast-enhanced ultrasonography employs microbubble contrast agents that allow the study of different enhancement phases of the kidney without risk of nephrotoxicity and radiation exposure. The diagnostic accuracy of contrast-enhanced ultrasonography in the characterization of complex renal cysts is comparable to that of computed tomography and magnetic resonance imaging, and several studies have demonstrated its reliability also in identifying solid lesions such as pseudotumors, typical angiomyolipomas, and clear cell renal carcinomas. Considering the high incidence of incidental renal masses and the need for rapid and reliable diagnosis, contrast-enhanced ultrasonography could be proposed as the first step in the diagnostic work-up of renal masses because of its safety and cost effectiveness. In this paper, we propose a diagnostic algorithm for the characterization of cystic and solid renal masses.
Keywords: Kidney, cyst, tumor, microbubble contrast agents, contrast-enhanced ultrasound
BMUS members can earn 1 CPD point by reading this approved CPD article and successfully completing the online test. Please go to the members' area of the BMUS website (www.bmus.org) and log into the BMUS CPD system.
Introduction
The incidence and detection of asymptomatic renal masses have increased over the past 25 years.1 Renal lesions are detected in approximately 13–27% of adults undergoing abdominal imaging,2 and it is estimated that more than half of patients older than 50 years have at least one renal mass.3 Most renal masses (61%) are found incidentally4 during examinations performed for other reasons and unrelated to the patient’s complaint. The proliferation of abdominal imaging studies and continuous improvement of imaging technology may account for the detection of these incidental masses.
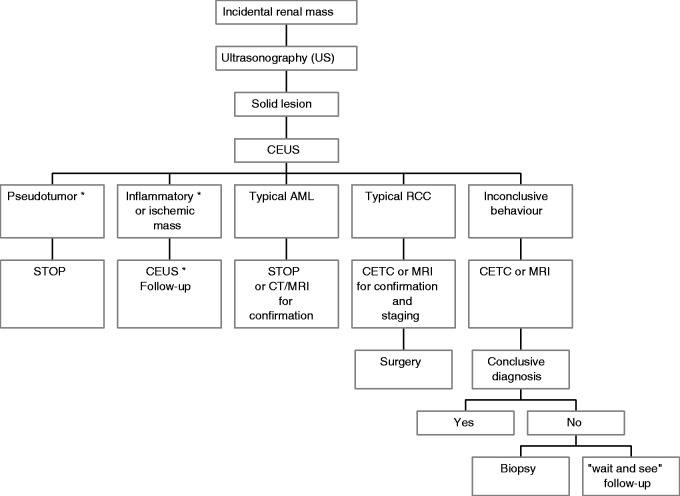
The majority of incidental renal masses are simple cysts that are easily diagnosed by ultrasonography (US) and do not need any further investigation or follow-up. Conversely, when the renal mass is not identified as a simple cyst, the next step is to assess if it is benign or malignant. To this aim, US, contrast-enhanced ultrasonography (CEUS), computed tomography (CT), and magnetic resonance imaging (MRI) are complementary imaging techniques, and the decision about which tool should be used as a first examination depends on local resources and expertise, cost efficacy, and patient characteristics. In this paper, we propose two algorithms for the diagnostic work-up of incidental solid and cystic renal masses (Figures 1 and 2).5
Figure 1.
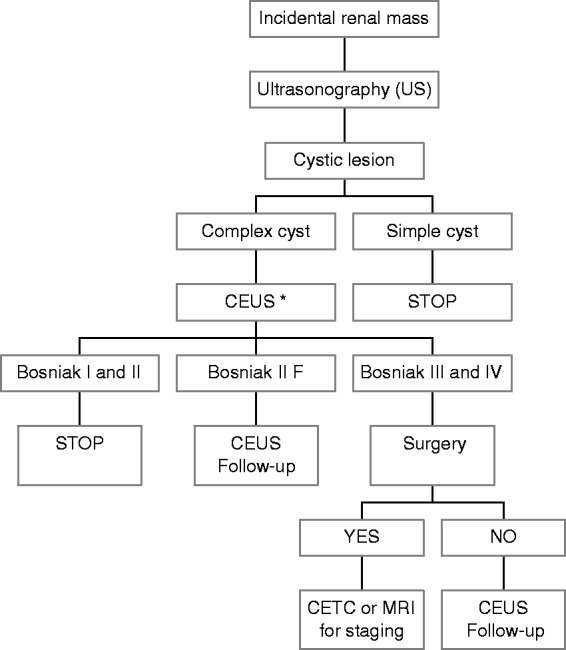
Diagnostic algorithm proposed for the work-up of incidental renal cystic lesions. *Established recommendation by EFSUMB.5
Figure 2.
Diagnostic algorithm proposed for the work-up of incidental renal solid lesions. *Established recommendation by EFSUMB.5
MRI is considered the most accurate diagnostic tool, with sensitivity and specificity in characterizing renal masses ranging from 88% to 100% and from 83% to 93%, respectively.6 However, it is also the most expensive imaging technique6 and cannot be used in patients with a pacemaker, uncooperative patients, and patients with severe renal failure. There are occasionally issues related to patient tolerance and safety, in particular the risk of nephrogenic systemic fibrosis in patients with estimated glomerular filtration rates under 30 ml/min, when exposed to MRI contrast agents.7–9 Moreover, the MRI characterization of small lesions may be difficult, as image subtraction cannot be effectively performed.10
CT exposes the subject to a relatively high dose of radiation, and the iodinated contrast agents have a potential risk of nephrotoxicity in patients with renal impairment. Furthermore, on CT examination, enhancement is defined when an increase in attenuation >15 Hounsfield Units (HU) is observed between the unenhanced and enhanced phases.10–12 However, several authors10–12 consider 20 HU as the correct value, and this criterion has some limitations in detecting hypovascular lesions13 and small cystic lesions that can show pseudo-enhancement.14
CEUS with second-generation US contrast agents is a more recent diagnostic tool, which employs microbubble contrast agents and complementary pulse sequences to demonstrate parenchymal vascularization in real time. Microbubble contrast agents consist of gas spheres, stabilized by a biodegradable surrounding shell of protein, lipid, or polymer. The small size of microbubble contrast agents marketed in western countries together with the characteristics of their shell allow for unfiltered passage through the lungs but prevent their entrance into the interstitium, making them an exclusively blood pool contrast agent. SonoVue® (Bracco, Milan, Italy) is the most widely used and studied contrast agent for abdominal applications and consists of a solution of sulfur hexafluoride microbubbles stabilized by a phospholipid shell. Unlike iodinated and paramagnetic contrast agents, SonoVue® has no renal excretion, so there is no excretory phase. Following a bolus injection of contrast agent, there is an early arterial phase with corticomedullary differentiation lasting 20–40 seconds, followed by a late phase when this differentiation is lost and renal parenchyma is homogenously enhanced for about 120 seconds.5 Some authors distinguish the late phase in two phases: a nephrographic phase that lasts approximately 40–70 seconds and a delayed phase, 70 seconds post injection. After circulating in the blood stream for several minutes, the gas components are eliminated by respiration, while the coat is metabolized by the liver. CEUS advantages include safety,15 patient tolerance, real-time imaging capability, and costs,16 but it is essential that the target of the examination is clearly visible on US. All the conditions that limit the US examination, such as obesity or meteorism, represent limits also for CEUS. CEUS has been widely studied and employed in the characterization of focal liver lesions, showing a diagnostic accuracy comparable to CECT and MRI.17,18 With respect to renal masses, CEUS has demonstrated high diagnostic accuracy for cystic lesions, while its usefulness in characterizing solid lesions is still debated.5
An early diagnosis is always desirable for both treatment efficacy and the patient’s prognosis, but the safety and costs of the examination are also to be taken into account.
Cystic lesions
The majority of renal lesions incidentally discovered on imaging studies in adults are cysts. Approximately 50% of the population aged >50 years develops simple or non-simple renal cysts.19
Simple cysts
Conventional US has a high accuracy in differentiating cysts from solid lesions, as well as simple cysts from minimally complex cysts. On baseline US, a simple cyst is defined by the presence of a linear thin wall and anechoic content without septa, calcifications, or solid components. When a simple renal cyst is detected, no further imaging examinations are necessary. Any cyst that does not conform to the well-recognized US features of a simple cyst is to be considered a complex cyst and requires further assessment.
Non-simple cysts
Up to 8% of renal cysts can have a complex appearance, such as increased intracystic echogenicity, calcifications, solid nodules, at least one intracystic septum, or wall thickening. Approximately 10% of renal cell carcinomas (RCC) may appear as complex cystic lesions.20 All imaging modalities require the administration of a contrast agent to differentiate complex benign cysts from cystic tumors. Contrast enhancement of irregular walls, septa, or nodular lesions is the most specific sign of malignancy in a cystic lesion.10 The detection of these features influences the therapeutic approach, independently of histological or cytological diagnosis.
In the past, multiphase contrast-enhanced computed tomography (CECT) and MRI have been considered the primary imaging modalities to characterize and stage complex cysts and solid renal lesions.21 More recently, CEUS has been proposed as a valid alternative to CECT and MRI. In the characterization of complex renal cysts, CEUS is more sensitive than CECT in detecting the microvascularization, enabling the depiction of even the tiny capillaries feeding thin septa. CEUS distinguishes the same Bosniak categories22 initially described on CT findings and has shown a diagnostic confidence similar to that of CECT.23 The Bosniak classification is the most widely accepted classification of complex cysts20 and has an important therapeutic impact. The Bosniak system classifies complex cysts into five groups (I, II, IIf, III, IV) with increasing probability of malignancy (Table 1).20 The most important features that guide the Bosniak classification are the number and thickness of septa, the presence of thickened wall or mural solid nodules, and the enhancement of septa and nodules. Bosniak categories I and II are benign and characterized by no enhancement or some transient enhancement of hairline thin septa and do not require further examination or intervention. Conversely, surgery is recommended for categories III and IV, because they have a medium to high risk of malignancy. Category IIf is characterized by thin walls or septa with continuous or prolonged enhancement and need a follow-up of 3 to 5 years.19
Table 1.
Bosniak classification.20
| Category | Probability of malignancy (%) | Features |
|---|---|---|
| I | 0 | No echoes within the mass, sharply marginated smooth walls No septa, calcifications, or solid components No enhancement after intravenous contrast agent injection |
| II | 0 | It may contain a few thin septa, fine calcifications, or slightly thickened calcification. It may show minimal enhancement of the septa without soft-tissue nodular enhancement |
| IIf | 5 | It may contain multiple hair-line thin septa, smooth minimal thickening of the wall, or septa and thick or nodular calcifications. It may show minimal enhancement of the septa without soft-tissue nodular enhancement |
| III | 50–70 | It may contain thickened irregular or smooth wall or septa with measurable enhancement. No solid enhancing lesions are present |
| IV | 95–100 | It may contain soft-tissue enhancing mass independent of the wall or septa |
Several studies have suggested that CEUS outperforms CT in detecting cyst vascularity,24,25 resulting in the upgrading of some lesions, similar to what has been described for MRI.10 Larger and long-term studies are needed to determine if such an increased sensitivity of CEUS will result in an undesirable number of false-positive cystic carcinoma diagnoses. However, on the basis of this evidence, the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines recommend CEUS in the characterization of complex cystic masses.5 Compared with CECT and MRI, CEUS can be performed rapidly after US examination, reducing the time to diagnosis and patient distress. CEUS is safe, well tolerated, does not expose the patient to radiation, is not associated with nephrotoxicity, and can be used in patients with iodine allergies and impaired renal function. Moreover, US contrast agents have a short half-life (approximately 5 minutes) which allows for multiple injections, with no established limit for the number of the injections and no risk for the patient. However, CEUS has the same limitations as conventional US: obesity, poorly visualized kidney, and shadowing from bowel gas, ribs or large wall calcification. Likewise, the diagnostic performance of CEUS is dependent on the operator’s skill. Given the safety profile and good accuracy of CEUS in characterizing complex cysts detected by US and indeterminate cysts identified on CECT, or MRI,19 CEUS could be considered the first tool in the diagnostic work-up of renal cystic lesions. In the case of Bosniak III or IV cysts needing a surgical approach, CECT or MRI can be considered for staging purposes, whereas they should be avoided in patients not eligible for surgery because of age or the presence of comorbidities. In these cases, a follow-up strategy with CEUS is advisable.1 The safety profile and easy availability of CEUS make it the favorite tool for the follow-up of cystic and solid lesions managed conservatively. The diagnostic algorithm proposed for the work-up of incidental renal cystic lesions is illustrated in Figure 1.
Solid lesions
Solid renal masses incidentally discovered on imaging studies are less frequent than cystic lesions, but more diagnostic possibilities must be taken into account: besides benign or malignant neoplasms, other non-neoplastic conditions such as pseudotumors, inflammatory, and ischemic lesions can mimic a neoplastic mass.
Non-neoplastic solid lesions
CEUS has good accuracy and high concordance with CECT and MRI26 in the characterization of lesions suspected for pseudotumor at color and power Doppler US. Median cortical location, echotexture, and vascular pattern equal to the normal surrounding parenchyma can suggest the diagnosis of pseudotumor without further investigations.27 However, differences in echogenicity or difficult evaluation of the color Doppler pattern are not infrequent. Moreover, infiltrative lesions that do not produce any deformity of the renal surface, such as metastases or lymphomas, can mimic a pseudotumor.28 In these cases, CEUS, when available, allows for a rapid and simple solution to the US diagnostic challenge of renal pseudotumor, avoiding more expensive and invasive exams such as CECT or MRI.22,29,30 The usefulness of CEUS in this field is recognized and its use recommended in the EFSUMB guidelines.5
CEUS can also be used to better define renal infarction and infections31 that can appear as masses hard to characterize without the use of a contrast agent. However, despite a lot of studies investigating the role of CEUS, its use in this field remains controversial and it is not recommended by the EFSUMB guidelines.
Neoplastic solid lesions
Approximately 90% of renal tumors are malignant; the remainder are benign, mostly represented by oncocytomas and angiomyolipomas (AML).32 The CEUS features of hyper- or iso-enhancement during the cortical phase, early wash-out, heterogeneous enhancement, and enhanced peritumoral rim are highly suggestive of clear cell carcinoma, the most frequent subtype of renal cell carcinoma (RCC).33 However, CEUS must be followed by another imaging test such as CECT or MRI, that can confirm the diagnosis and give important information about the stage of the disease. In up to 20% of RCC >3 cm, synchronous RCC is found in the same kidney and contralateral RCC may occur in about 5% of patients.1 Likewise, CEUS depiction of a hypovascular lesion needs further imaging tools for diagnostic and staging purposes. Renal metastases usually occur in advanced tumor stages; they can originate from bronchogenic carcinoma, breast cancer, gastroenteric cancer, and thyroid carcinoma, which are mostly hypovascular on CEUS examination, as well as primary and secondary lymphoma. Moreover, papillary RCC, which represents 10% of RCC, usually shows homogeneous hypoenhancement in the cortical phase with slow wash-out. Papillary RCC may mimic AML with low fat content, making the differential diagnosis quite hard with all imaging modalities.13
Hyperechoic lesions at baseline US, which show homogeneous, progressive, and prolonged enhancement at CEUS are considered typical renal AML,34,35 the most frequent benign solid lesion of the kidney. In these cases, the need for further examinations, like CT or MRI, is questionable, even though unenhanced CT or MRI can demonstrate the fat content of typical AML.
Similarly to CECT and MRI, CEUS has low accuracy in the characterization of RCC different from the clear cell subtype (papillary RCC, chromophobic RCC, collecting duct RCC), oncocytoma, adenoma and atypical AML with hemorrhage, calcifications, arteriovenous shunts, necrosis, and low-fat content. For these reasons, at present, CEUS is not recommended by the EFSUMB guidelines for the characterization of neoplastic solid lesions. However, the limits of all the available diagnostic tools are highlighted by the high number of misdiagnosed AML at CECT evaluation (up to 14%), which undergo unnecessary surgery.1 Pathological confirmation of incidental renal masses is increasing and is becoming a larger clinical problem, with substantial cost. Furthermore, the diagnosis of entities such as oncocytoma represents a diagnostic challenge not only for imaging method but also for pathological examination.
The diagnostic algorithm proposed for the work-up of incidental renal solid lesions is illustrated in Figure 2.
Discussion
CEUS has been shown to have high sensitivity and specificity in the characterization of incidental and indeterminate renal masses. It is more sensitive that CECT in depicting blood flow in hypovascular lesions, as well as in septa or solid components of a complex cyst. Moreover, CEUS can accurately distinguish hypovascular lesions from atypical cystic masses2 and has high positive and negative predictive values in the characterization of indeterminate renal masses, reducing the need for biopsy or other imaging modalities.2 It has good accuracy in the identification of benign lesions such as pseudotumors, cysts (even when they are not correctly identified by other imaging modalities), and typical AML. However, the correct diagnosis of AML is often a difficult and insidious challenge for all the imaging techniques because of the lack of large studies, the not infrequent possibility of atypical forms, and the low prevalence of this lesion that ranges from 0.03% to 0.07%.36 Many studies have reported an overlap between lipid-poor AML and hypovascular malignant lesions such as papillary RCC with all imaging modalities. Conversely, CEUS has been reported to have good diagnostic accuracy in identifying typical AML.29 Moreover, a retrospective study reported high negative predictive value for CEUS of malignant lesions,37 demonstrating its efficacy in diagnosing benign renal lesions.
Although the accuracy of CEUS in identifying the clear cell variant of RCC is quite good,38 other imaging modalities such as CECT or MRI are necessary for staging purposes and for pre-surgical evaluation. When CEUS is not conclusive, several strategies with different advantages and disadvantages can be adopted: to perform other non-invasive imaging modalities such as CECT or MRI, keeping well in mind that both techniques have a limited usefulness in the characterization of atypical AML and oncocytoma; to perform a biopsy with all the well known related risks and limits; or the “wait and see” strategy, with a careful follow-up of the patient. Patient characteristics (such as age and comorbidities) and lesion characteristics (such as size and location) will guide the diagnostic work-up.
Based on the safety profile and good accuracy in the identification of benign features of both cystic and solid lesions, in our opinion the departments that routinely perform CEUS should consider it as the first imaging method when an incidental renal mass is identified, according to our proposed algorithm (Figures 1 and 2). CEUS is relatively harmless, with a low incidence of side effects and it can be used when CECT and MRI are contraindicated. In the case of pseudotumor or Bosniak II cystic lesions, CEUS (performed immediately after US) can reach the final diagnosis very quickly, reducing the use of more invasive and expensive methods. In case of Bosniak IIf cystic lesions or solid lesions with benign features, CEUS can be used for the follow-up. When CEUS findings do not clearly support the benign nature of a lesion or when they are highly suspicious for malignancy, a diagnostic work-up including other imaging modalities must be planned.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Not applicable
Guarantor
SS
Contributorship
FDV and PT researched the literature and conceived the paper. FDV and FE wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved its final version.
References
- 1.Ignee A, Straub B, Schuessler G, et al. Contrast enhanced ultrasound of renal masses. World J Radiol 2010; 2: 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med 2010; 362: 624–634. [DOI] [PubMed] [Google Scholar]
- 3.Barr RG, Peterson C, Hindi A. Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study. Radiology 2014; 271: 133–142. [DOI] [PubMed] [Google Scholar]
- 4.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998; 51: 203–205. [DOI] [PubMed] [Google Scholar]
- 5.Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med 2011; 32: 1–27. [DOI] [PubMed] [Google Scholar]
- 6.Kang SK, Huang WC, Pandharipande PV, et al. Solid renal masses: what the numbers tell us. AJR Am J Roentgenol 2014; 202: 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eshed I, Althoff CE, Hamm B, et al. Claustrophobia and premature termination of magnetic resonance imaging examinations. J Magn Reson Imaging 2007; 26: 401–404. [DOI] [PubMed] [Google Scholar]
- 8.Shellock FG, Spiazzi A. MRI safety update 2008: part 1, MRI contrast agents and nephrogenic systemic fibrosis. AJR Am J Roentgenol 2008; 191: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 9.Shellock FG, Spiazzi A. MRI safety update 2008: part 2, screening patients for MRI. AJR Am J Roentgenol 2008; 191: 1140–1149. [DOI] [PubMed] [Google Scholar]
- 10.Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: comparison of CT and MR imaging by using the Bosniak classification system. Radiology 2004; 231: 365–371. [DOI] [PubMed] [Google Scholar]
- 11.Silverman SG, Israel GM, Herts BR, et al. Management of the incidental renal mass. Radiology 2008; 249: 16–31. [DOI] [PubMed] [Google Scholar]
- 12.Patel NS, Poder L, Wang ZJ, et al. The characterization of small hypoattenuating renal masses on contrast enhanced CT. Clin Imaging 2009; 33: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egbert ND, Canili EM, Cohan RH, et al. Differentiation of papillary renal cell carcinoma subtypes on CT and MRI. AJR Am J Roentgenol 2013; 201: 347–355. [DOI] [PubMed] [Google Scholar]
- 14.Tappouni R, Kissane J, Sarwani N, et al. Pseudoenhancement of renal cysts: influence of lesion size, lesion location, slice thickness, and number of MDCT detectors. AJR Am J Roentgenol 2012; 198: 133–137. [DOI] [PubMed] [Google Scholar]
- 15.Piscaglia F. Bolondi L and Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006; 32: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 16.Romanini L, Passamonti M, Aiani L, et al. Economic assessment of contrast enhanced ultrasonography for evaluation of liver lesions: a multicentre Italian experience. Eur Radiol 2007; 17: F99–F106. [DOI] [PubMed] [Google Scholar]
- 17.Seitz K, Strobel D, Bernatik T, et al. Contrast enhanced ultrasound (CEUS) for the characterization of focal liver lesions: prospective comparison in clinical practice; CEUS vs CT (DEGUM multicenter trial). Ultraschall Med 2009; 30: 383–389. [DOI] [PubMed] [Google Scholar]
- 18.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice. Recommendations for Contrast enhanced ultrasound (CEUS) in the liver- Update 2012: a WFUMB-EFSUMB Initiative in cooperation with representatives of AFSUMB, AIUM, ASUM; FLAUS and ICUS. Ultrasound Med Biol 2013; 39: 187–210. [DOI] [PubMed] [Google Scholar]
- 19.Mc Guire BB, Fitzpatrick JM. The diagnosis and management of complex renal cysts. Curr Opin Urol 2010; 20: 349–354. [DOI] [PubMed] [Google Scholar]
- 20.Nicolau C, Bunesch L, Sebastia C. Renal complex cysts in adults: contrast-enhanced ultrasound. Abdom Imaging 2011; 36: 742–752. [DOI] [PubMed] [Google Scholar]
- 21.Kang SK, Chandarana H. Contemporary imaging of the renal mass. Urol Clin North Am 2012; 39: 161–170. [DOI] [PubMed] [Google Scholar]
- 22.Robbin ML, Lockhart ME, Barr RG. Renal imaging with ultrasound contrast: current status. Radiol Clin North Am 2003; 41: 963–978. [DOI] [PubMed] [Google Scholar]
- 23.Quaia E, Bertolotto M, Cioffi V, et al. Comparison of contrast-enhanced sonography with unenhanced sonography and contrast enhanced CT in the diagnosis of malignancy in complex cystic renal masses. Am J Roentgenol 2008; 191: 1239–1249. [DOI] [PubMed] [Google Scholar]
- 24.Ascenti G, Mazziotti S, Zimbaro G, et al. Complex cystic renal masses: characterization with contrast enhanced US. Radiology 2007; 243: 158–165. [DOI] [PubMed] [Google Scholar]
- 25.Park BK, Kim B, Kim SH, et al. Assessment of cystic renal masses based on Bosniak classification: comparison of CT and contrast enhanced US. Eur J Radiol 2007; 61: 310–314. [DOI] [PubMed] [Google Scholar]
- 26.Mazziotti F, Zimbaro F, Pandolfo A, et al. Usefulness of contrast enhanced ultrasonography in the diagnosis of paseudotumors. Abdom Imaging 2010; 35: 241–245. [DOI] [PubMed] [Google Scholar]
- 27.Paspulati RM, Bhatt S. Sonography in benign and malignant renal masses. Ultrasound Clin 2006; 1: 25–41. [DOI] [PubMed] [Google Scholar]
- 28.Ascenti G, Zimbaro G, Mazziotti S, et al. Contrast enhanced power Doppler US in the diagnosis of renal pseudotumors. Eur Radiol 2001; 11: 2496–2499. [DOI] [PubMed] [Google Scholar]
- 29.Tranquart F, Correas JM, Martegani A, et al. Feasibility of real time contrast enhanced ultrasound in renal disease. J Radiol 2004; 85: 31–36. [DOI] [PubMed] [Google Scholar]
- 30.Barr RG, Robbin ML, Peterson C. Definity-enhanced ultrasound imaging of the kidney in patients with indeterminate masses: value of contrast harmonic imaging with bolus and infusion administration. Radiology 2001; 221: 316–316. [Google Scholar]
- 31.Setola SV, Catalano O, Sandomenico F, et al. Contrast enhanced sonography of the kidney. Abdom Imaging 2007; 32: 21–28. [DOI] [PubMed] [Google Scholar]
- 32.McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol 2012; 67: 909–922. [DOI] [PubMed] [Google Scholar]
- 33.Xu ZF, Xu HX, Xie XY, et al. Renal cell carcinoma: real time contrast enhanced ultrasound findings. Abdom Imaging 2009; 35: 750–756. [DOI] [PubMed] [Google Scholar]
- 34.Xu ZF, Xu HX, Xie XY, et al. Renal cell carcinoma and renal angiomyolipoma: differential diagnosis with real-time contrast enhanced ultrasonography. J Ultrasound Med 2010; 29: 709–717. [DOI] [PubMed] [Google Scholar]
- 35.Quaia E, Siracusano S, Bertolotto M, et al. Characterization of renal tumours with pulse inversion harmonic imaging by intermittent high mechanical index technique: initial results. Eur Radiol 2003; 13: 1402–1412. [DOI] [PubMed] [Google Scholar]
- 36.Frohlich T, Brands A, Thon WF, et al. Angiomyolipoma of the kydney and lymph nodes. World J Urol 1999; 17: 123–125. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Liang P, Guo M, et al. Real-time contrast-enhanced ultrasound in diagnosis of solid renal lesions. Discov Med 2013; 16: 15–25. [PubMed] [Google Scholar]
- 38.Wang C, Yu C, Yang F, et al. Diagnostic accuracy of contrast enhanced ultrasound for renal cell carcinoma: a meta-analysis. Tumor Biol 2014; 35: 6343–6350. [DOI] [PubMed] [Google Scholar]