Abstract
Externalizing psychopathology has been linked to prefrontal abnormalities. While clinically diagnosed subjects show altered frontal gray matter, it is unknown if similar deficits relate to externalizing traits in non-clinical populations. We used voxel-based morphometry (VBM) to retrospectively analyze the cerebral gray matter volume of 176 young adult social to heavy drinkers (mean age= 24.0 ± 2.9, male= 83.5%) from studies of alcoholism risk. We hypothesized that prefrontal gray matter volume and externalizing traits would be correlated. Externalizing personality trait components— Boredom Susceptibility-Impulsivity (BS/IMP) and Empathy/Low Antisocial Behaviors (EMP/LASB)— were tested for correlations with gray matter partial volume estimates (gmPVE). Significantly large clusters (pFWE < 0.05, family-wise whole-brain corrected) of gmPVE correlated with EMP/LASB in dorsolateral and medial prefrontal regions, and in occipital cortex. BS/IMP did not correlate with gmPVE, but one scale of impulsivity (Eysenck I7) correlated positively with bilateral inferior frontal/orbitofrontal, and anterior insula gmPVE. In this large sample of community-dwelling young adults, antisocial behavior/low empathy corresponded with reduced prefrontal and occipital gray matter, while impulsivity correlated with increased inferior frontal and anterior insula cortical volume. These findings add to a literature indicating that externalizing personality features involve altered frontal architecture.
Keywords: VBM, MRI, alcohol, frontal lobes, personality, impulsivity
1. Introduction
Externalizing psychopathology is marked by the outward expression of maladjustment, causing harm to others. Latent dimensional analysis of common psychiatric diagnoses shows that antisocial personality disorder (ASPD), conduct disorder (CD) and substance use disorders fall into a clear externalizing dimension (Kotov et al., 2011; Krueger, 1999; Krueger and Markon, 2006). The behavioral disinhibition and impulsivity that pervade these disorders (e.g., see Krueger and South, 2009) suggest deficits in prefrontal brain— the region most implicated in behavioral control (Knutson et al., 2015).
For example, magnetic resonance imaging (MRI) suggests that antisocial personality disorder is marked by an 11% reduction in prefrontal gray matter volume (Raine et al., 2000). Adolescent conduct disorder is characterized by functional and structural changes in brain areas related to emotion (amygdala, insula, striatum, orbital cortex, Fairchild et al., 2011; Fairchild et al., 2013; Sarkar et al., 2013; Sebastian et al., 2012). Changes in prefrontal volume have been associated with aggressive behavior and callousness in CD, with aggressive behaviors being negatively correlated with right dorsolateral prefrontal cortex volume, and emotional callousness being positively correlated with bilateral orbitofrontal cortex volumes (Fairchild et al. 2013). In a relatively small sample of adult psychiatric patients with mixed externalizing disorders, motor impulsiveness was also related to lesser orbitofrontal gray matter (Lee et al., 2011).
Studying healthy community-dwelling subjects without clinical diagnoses, Cho et al., (2013) reported an association between gray matter volume and impulsivity in 34 young adults, with greater impulsivity linked to reduced medial prefrontal, dorsolateral prefrontal and ventral striatal gray matter volumes. An inverse relationship between trait impulsiveness and orbitofrontal gray matter volume in middle age adults has also been reported (Matsuo et al., 2009). Walhovd et al. (2012) similarly showed a negative correlation between conduct problems and left orbitofrontal and supramarginal gyri thickness in children and adolescents. In the large European IMAGEN trial of community dwelling adolescents, externalizing behaviors were related to lower gray matter in left inferior and middle frontal gyri (Montigny et al., 2013), and impulsivity traits related to reductions in orbitofrontal gray matter volume (Schilling et al., 2013b) and superior frontal gyrus cortical thickness (Schilling et al., 2013a). As opposed to self-reported impulsivity, experimentally measured reward impatience (delay discounting) is associated with reduced lateral prefrontal volume in healthy adults (Bjork et al., 2009). Thus, even in healthy individuals who are not the focus of clinical attention, there seem to be relationships between regional brain volume and externalizing personality traits. However, the largest samples are limited to children and adolescents, in whom neocortical development continues in to late adolescence and early adulthood (particularly in the frontal lobes, Groeschel et al., 2010; Lenroot and Giedd, 2006). That is, these relationships between personality traits and brain volume might not persist into adulthood, or perhaps assume a different nature. Moreover, the personality assessments have usually been limited to one particular scale, which limits the dimensionality with which facets of impulsivity can be assessed.
In this study, we examined a large sample of 176 physically healthy young adults with structural MRI and several personality scales of trait impulsiveness and antisocial behaviors, which were analyzed with principal component analysis. Our primary goal was to employ voxel based morphometry (VBM) and analyze relationships between personality components and gray matter partial volume estimates (gmPVE). However, rather than studying clinically diagnosed patients or controls without any indication of pathology, our sample is instead comprised of subjects from neuroimaging studies of alcoholism risk and heavy drinking— a population in which impulsivity and antisocial behaviors are more prevalent (Finn, 2002; Haber et al., 2005; Kendler et al., 2003; Krueger and South, 2009; Moss et al., 2007; Young et al., 2000). We hypothesized that trait impulsivity and antisocial behavior would negatively correlate with prefrontal gmPVE. Secondarily, and given the nature of the sample, we also investigated the effects of alcohol use and familial alcoholism on these externalizing personality components, and on gmPVE.
2. Methods
The research procedures in this work were conducted after obtaining from all subjects written informed consent; both the consent form and procedures were approved by the Institutional Review Board (IRB) of Indiana University. All procedures were conducted according to the principles expressed in the Declaration of Helsinki.
2.1 Subjects
This study includes the anatomic brain imaging data acquired from 176 subjects who had previously participated in five different functional MRI or PET neuroimaging studies of alcoholism risk, as conducted at the Indiana University School of Medicine (including those subjects published in Kareken et al., 2012; Kareken et al., 2010; Kareken et al., 2013b; Oberlin et al., 2013; Oberlin et al., 2014). One hundred and seventeen of these subjects had participated in studies that involved pulsed arterial spin labeling MRI of regional cerebral blood flow; this subsample was recently reported in (Weafer et al., 2015) to examine relationships between resting brain physiology and impulsive personality traits. The aggregate sample (Table 1) is predominantly male (147 men, 29 women). Exclusion criteria comprised self-reported evidence of neurological disorders of central origin, self-report of symptoms consistent with mood or anxiety disorders, psychoses, bipolar disease, or current psychiatric treatment, including any psychoactive medication. At the time of the study, none of the participants were seeking treatment for alcoholism or had developed drug dependence through their lifetime. All subjects had data from the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA, Bucholz et al., 1994, see below), and 16 (9.1%) subjects satisfied criteria for a lifetime diagnosis of DSM-IV alcohol dependence. Although subjects with histories of illicit drug use were enrolled, 171 (97.2%) tested negatively on urine screens for psychoactive drug use, with 4 (2.3%) testing positive for marijuana, and 1 (0.6%) for opiates. Based on information from the SSAGA, 34 subjects (19.3%) described using marijuana or hashish at least 21 times. The majority (n= 157; 89.2%) were non-smokers.
Table 1.
Subject characteristics.
| Total (n=176) | Men (n=147) | Women (n=29) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n (%) | Mean | SD | n (%) | Mean | SD | n (%) | |
| Age (years) | 24.0 | 2.9 | 24.0 | 3.1 | 23.9 | 2.3 | |||
| Education (years)* | 15.2 | 1.6 | 15.0 | 1.6 | 15.8 | 1.4 | |||
| AUDIT* | 10.9 | 5.4 | 11.4 | 5.6 | 8.0 | 2.3 | |||
| Drinks/week1, 3* | 17.2 | 11.5 | 18.5 | 12.0 | 10.6 | 5.2 | |||
| Drinks/drinking day1* | 5.8 | 3.4 | 6.2 | 3.3 | 3.9 | 1.7 | |||
| Subjects as Classified by NIAAA-Recommended Drinking Limits2 | |||||||||
| Exceed neither weekly nor daily limits | 46 (26.1) | 38 (25.8) | 8 (27.6) | ||||||
| Exceed only weekly limit | 32 (18.2) | 22 (15.0) | 10 (34.5) | ||||||
| Exceed only daily limit | 23 (13.1) | 21 (14.3) | 2 ( 6.9) | ||||||
| Exceed both weekly & daily limits | 75 (42.6) | 66 (44.9) | 9 (31.0) | ||||||
Notes: AUDIT= Alcohol Use Disorders Identification Test (n= 169);
Drinking patterns derived from Timeline Followback Interview (Sobell et al. 1986) and compared to
recommended limits from the National Institute on Alcohol Abuse and Alcoholism for daily and weekly alcohol consumption (daily limit ≥ 5/4 drinks per day for men/women, respectively; weekly limit ≥ 14/7 drinks per day for men/women, respectively).
Drinks/week was analyzed using a square root transform; for comprehensibility, data in the table are untransformed;
Men different than women (p< 0.05) via t-test.
2.2 Measures
2.2.1 Subject assessment
All subjects were characterized with the SSAGA, using trained raters who underwent observation before examining subjects. The SSAGA’s Family History Assessment Module (FHAM) was used to assess each subject’s family history of alcoholism (only the subject was available to assess family history), and the Timeline Followback interview was used to measure subjects’ recent drinking (TLFB, Sobell et al., 1986) over 90 days prior to study participation. Alcohol Use Disorders Identification Test (AUDIT, Saunders et al., 1993) data were available for 169 subjects. Family history of alcoholism was categorized as: 1) Family history positive (FHP) having at least one first degree affected relative; 2) A more ambiguous family history (FHAmbig) consisting of one or more second degree affected relatives; and 3) A negative family history of alcoholism (FHN), as defined by no affected first or second degree relatives. Subjects with mothers who were affected with probable dependence were excluded to rule out fetal alcohol effects.
2.2.2 Personality trait measurement
Externalizing traits and self-reported behaviors were assessed with: (1) the Zuckerman Sensation Seeking Scale (Zuckerman, 1994) whose subscales reflect enjoyment of socially conventional adventure and thrill seeking (Thrill/Adventure Seeking), a liking of less conventional and more unusual sensory experiences (Experience Seeking), a preference for occasions that afford the opportunity to “let loose” (Disinhibition), and a tendency to bore easily with repetitive or sedate activity (Boredom Susceptibility), (2) the Eysenck I7 (Eysenck et al., 1985) Impulsivity scale, whose subscales reflect a tendency to act without thinking (Impulsiveness), adventure seeking (Venturesomeness), and emotional concern for others (Empathy), and (3) an in-house, computerized modification of Section M of the SSAGA semi-structured interview on which subjects endorsed or denied a variety of antisocial acts occurring after the age of 15 (total ranging from 0 – 44, see Oberlin et al., 2012). These instruments were adapted to a computer self-administration format using E-Prime software (Psychology Software Tools, Inc, Sharpsburg, PA). For all scales, higher scores represent a greater magnitude of the measured trait.
2.2.3 MRI acquisition
Imaging was performed in a Siemens (Siemens Healthcare, Erlangen, Germany) 3T Magnetom Trio-Tim scanner with a 12-channel head coil array at the Center for Neuroimaging of the Indiana University School of Medicine. A whole-brain high resolution anatomical MRI was collected using a 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MP-RAGE) with imaging parameters optimized according to the ADNI (Alzheimer’s Disease Neuroimaging Initiative) protocol, and as implemented in a large cohort study of Alzheimer’s disease, mild cognitive impairment and normal subjects (160 sagittal slices in 9.14 minutes, 1.0 × 1.0 × 1.2 mm3 voxels).
2.3 Analyses
2.3.1 Personality trait data
As a data reduction strategy, principal component analysis with varimax rotation (SPSS, v. 20) was conducted on the antisocial behavior inventory and the subscales of the Zuckerman Sensation Seeking and Eysenck I7 inventories. As some variables were abnormally skewed, they were transformed prior to inclusion in the PCA using the most appropriate transformation to reduce skewness (square root transformation for antisocial behavior count and Venturesomeness from the Eysenck I7; Log10 transformation for Thrill and Adventure Seeking from the Sensation Seeking Scale). Personality data were derived from an original superset of 198 subjects who had participated in these studies (165 men; mean age 24.3, SD=3.2). However, 22 of these subjects were eventually excluded from the VBM analyses given either incomplete data about their marijuana use (n=14) or imaging data that were acquired using a different head coil (n=8).
2.3.2 Image processing
The optimized VBM method (Good et al., 2001) was implemented within SPM8 (Wellcome Trust Centre for Neuroimaging) by first segmenting brain tissues into gray matter, white matter and cerebrospinal fluid. Subsequently, each subject’s gray matter images were normalized to the MNI gray matter template using a modulation step to adjust for spatial distortions introduced by non-linear image deformations. The gray matter images were then smoothed using a 12mm full-width at half-maximum (FWHM) isotropic Gaussian kernel, which allows the application of cluster-level statistics (Silver et al., 2011). Estimated total intracranial volume (eTIV) was derived from FreeSurfer version 5.1 (https://surfer.nmr.mgh.harvard.edu/fswiki/FreeSurferMethodsCitation) and used as a covariate to adjust for inter-individual differences in head size in the VBM analysis (see below). Freesurfer eTIV was employed, as it has a higher correlation with the gold standard of manual measurements than does SPM8 eTIV (Malone et al., 2015).
2.3.3 Image analyses
A multiple regression model in SPM8 was implemented to perform voxel-wise correlations between personality traits components derived from the PCA (independent variables of interest) and gmPVE from the modulated images. Given the centrality of impulsivity (acting without adequate thought/planning) and antisocial (socially deviant) behaviors in alcohol use disorders (rather than social use, per se, Finn, 2002; Gunn et al., 2013), we focused the analyses on the two components that predominantly loaded on trait impulsivity and the endorsement of antisocial acts. The regression model also included covariates to control for any effects from age (inverse transformed to minimize skewness), gender, drinking (drinks/week, square root transformed to minimize skewness), eTIV (as reported by FreeSurfer), smoking status, and lifetime marijuana/hashish use of greater than 21 times, as derived from the SSAGA. Smoking and marijuana use were both dummy-coded as binary variables.
Statistical inferences for VBM were made using cluster statistics (pFWE < 0.05, correcting for whole brain gray matter family-wise error [FWE]) with the cluster-forming (height) threshold p < 0.001 (uncorrected, Silver et al. 2011). The mean cluster values of each significant region were extracted for all subjects using MarsBar (Brett et al., 2002) at the cluster forming threshold to illustrate the nature of significant relationships between average gmPVE and personality components, and to assure that they were not driven by outliers.
3. Results
3.1 Personality Traits
After performing principal components analysis (PCA) on the antisocial behavior count, and the subscales of the Eysenck I7 and Sensation Seeking Scale (Table 2), three principal components emerged with eigenvalues greater than 1.0 that explained 64.9% of the total variance after varimax rotation (Table 3). The components and their pattern of loadings can be characterized as dimensions of: 1) Boredom Susceptibility and Impulsivity (BS/IMP), 2) Thrill Seeking (TS), and 3) High Empathy and Low Antisocial Behaviors (EMP/LASB).
Table 2.
Personality trait raw scores.
| Total (n=176) | Men (n=147) | Women (n=29) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Antisocial Behavior Count* | 5.6 | 4.9 | 6.0 | 5.0 | 3.5 | 3.3 |
| Eysenck I7 Inventory | ||||||
| Impulsivity | 7.5 | 4.1 | 7.6 | 4.0 | 7.0 | 4.4 |
| Venturesomeness | 11.9 | 3.0 | 12.1 | 2.8 | 10.9 | 3.5 |
| Empathy | 12.4 | 3.3 | 12.3 | 3.4 | 12.9 | 2.8 |
| Sensation Seeking Scale | ||||||
| Thrill/Adventure Seeking | 7.9 | 2.2 | 8.1 | 2.1 | 7.6 | 2.6 |
| Experience Seeking | 6.0 | 2.0 | 6.0 | 1.9 | 5.9 | 2.5 |
| Disinhibition | 6.3 | 2.2 | 6.4 | 2.2 | 6.0 | 2.5 |
| Boredom Susceptibility | 3.4 | 2.0 | 3.5 | 1.9 | 2.8 | 2.3 |
Note:
Men greater than women via t-test, p< 0.05.
Table 3.
Principal Component Loadings
| 1.
Boredom Susceptibility/Impulsivity |
2. Thrill Seeking |
3. Empathy/Low Antisocial Behavior |
|
|---|---|---|---|
| (BS/IMP; 26.2%) | (TS; 25.1%) | (EMP/LASB; 13.7%) | |
| Antisocial Behavior Count | 0.660 | 0.017 | −0.364 |
| Eysenck I7 Inventory | |||
| Impulsivity | 0.695 | −0.199 | 0.221 |
| Venturesomeness | −0.123 | 0.920 | 0.155 |
| Empathy | −0.052 | 0.125 | 0.917 |
| Sensation Seeking Scale | |||
| Thrill/Adventure Seeking | −0.028 | 0.925 | −0.012 |
| Experience Seeking | 0.416 | −0.479 | −0.046 |
| Disinhibition | 0.669 | −0.060 | −0.210 |
| Boredom Susceptibility | 0.732 | −0.125 | 0.049 |
Notes: Percentage of variance accounted for by the rotated components appears in parentheses beneath the component labels. Component loadings larger than | 0.3 | appear in boldface type.
3.2 Relationships between gmPVE and Personality Traits
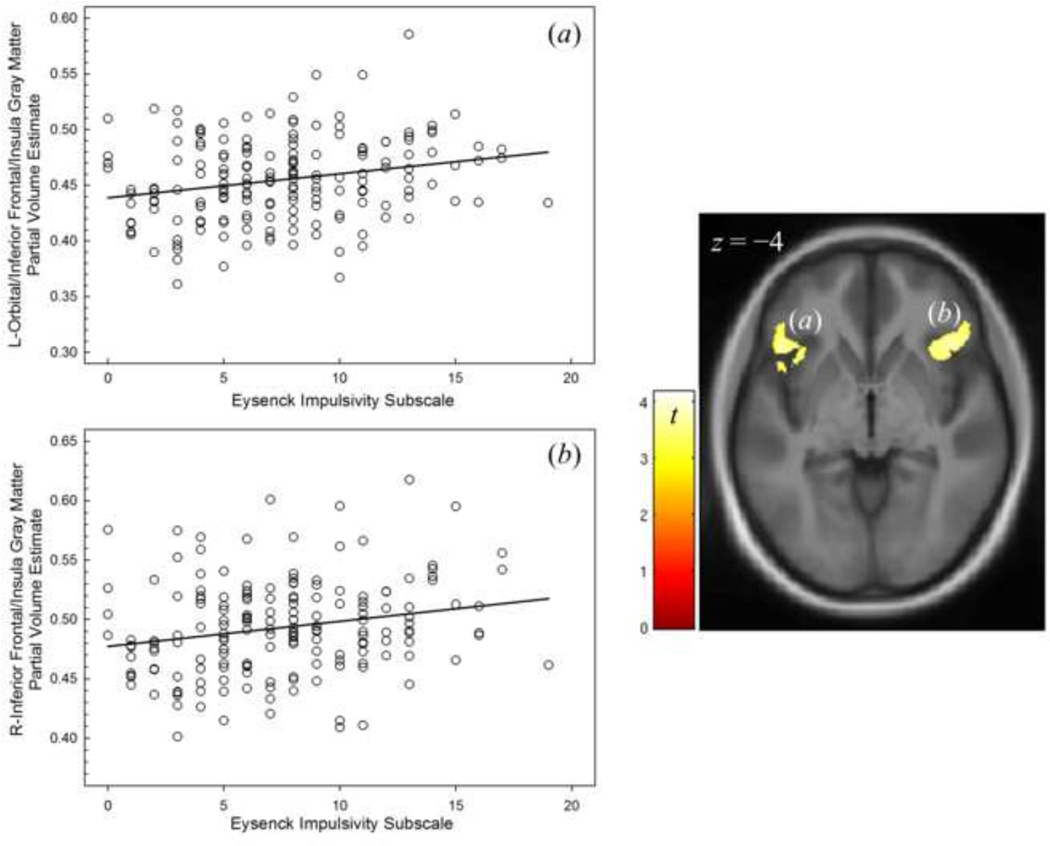
BS/IMP was neither positively nor negatively correlated with gray matter volume at FWE-corrected cluster level significance. Given our recent finding of an inverse relationship between right prefrontal (premotor) regional cerebral blood flow and Eysenck I7 impulsivity subscale (Weafer et al. 2015), we secondarily examined this scale individually, and also found no significant inverse relationship between gmPVE and impulsivity. There was, however, a left frontal cluster where the I7 impulsivity subscale was positively related to gmPVE in the inferior frontal/posterior-lateral orbitofrontal and insula areas; a similar cluster was present on the right, albeit of trend level significance (pFWE = 0.065; Figure 1, Table 4).
Figure 1.
Regions of positive correlation between the Impulsivity scale of Eysenck’s I7 inventory and gray matter partial volume estimates (gmPVE) in (a) left orbital/inferior frontal/insula and (b) right inferior frontal/insula regions. Display p < 0.001 uncorrected voxel height, showing (a) significant (pFWE< 0.05) and (b) trend-level significant (pFWE=0.065) clusters after correcting for whole brain family-wise error.
Table 4.
Positive correlations between I7 impulsivity and gray matter partial volume estimates (gmPVE).
| Voxel Statistics | Cluster Statistics | |||||
|---|---|---|---|---|---|---|
| MNI Coordinate (mm) | ||||||
| x | y | z | Peak Z | Size (k) | p(FWE) | |
| Left Inferior Frontal/Orbital/Insula | −38 | 29 | −17 | 4.05 | 8,595 | 0.001 |
| Right Inferior Frontal/Insula | 47 | 29 | −8 | 3.97 | 3,002 | 0.065 |
Notes: MNI= Montreal Neurological Institute. Voxel statistics refer to peak effects within a cluster (all p-values < 0.001 uncorrected). Cluster statistic reflects a greater number of contiguous voxels exceeding height threshold (p< 0.001, uncorrected) than expected by chance, controlling for whole brain family-wise error (FWE).
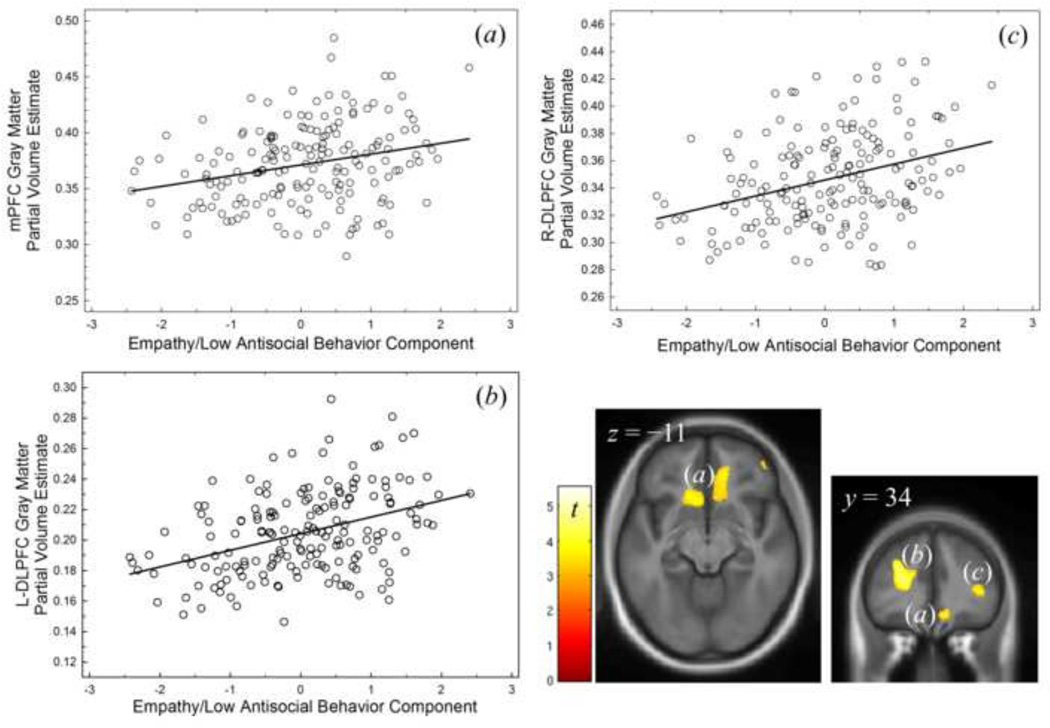
Four clusters (three frontal) of gmPVE correlated positively with EMP/LASB: a left dorsolateral prefrontal region, a right dorsolateral prefrontal cluster, and a left ventromedial prefrontal region (Figure 2; Table 5). The remaining cluster was in occipital cortex (Table 5). There were no clusters of negative correlation between gmPVE and EMP/LASB.
Figure 2.
Regions of positive correlation between the EMP/LASB component and gmPVE in (a) Left/right medial prefrontal and (b) left and (c) right dorsolateral prefrontal cortex. Display p < 0.001 uncorrected voxel height, showing only clusters of significance after correcting for whole brain family-wise error (pFWE< 0.05).
Table 5.
Positive correlations between EMP/LASB and gmPVE.
| Voxel Statistics | Cluster Statistics | |||||
|---|---|---|---|---|---|---|
| MNI
Coordinate (mm) |
Peak Z | Size (k) | p(FWE) | |||
| x | y | z | ||||
| Left DLPFC | −23 | 29 | 17 | 5.3 | 4,974 | 0.012 |
| Right DLPFC | 37 | 24 | 15 | 4.5 | 7,043 | 0.002 |
| Ventromedial Prefrontal/Ventral Anterior Cingulate | −10 | 20 | −8 | 4.0 | 4,559 | 0.016 |
| Occipital | −9 | −83 | 17 | 3.7 | 3,785 | 0.032 |
Notes: MNI= Montreal Neurological Institute. DLPFC= dorsolateral prefrontal cortex. Voxel statistics refer to peak effects within a cluster (all p-values < 0.001 uncorrected). Cluster statistic reflects a greater number of contiguous voxels exceeding p< 0.001 height threshold (uncorrected) than expected by chance, controlling for whole brain family-wise error (FWE).
3.3 Relationships between alcohol use, familial alcoholism, personality traits, and gmPVE
Average number of drinks/week from the Timeline Followback interview was related positively with BS/IMP (Spearman’s rho, ρ= 0.37, p< 0.001), negatively with EMP/LASB (ρ= -0.16, p= 0.03), but unrelated to TS (ρ= 0.13, p= 0.09). Average drinks/drinking day showed similar relationship with BS/IMP (ρ= 0.20, p= 0.008), EMP/LASB (ρ= -0.21, p= 0.006); and TS (ρ= 0.06, p= 0.42). The AUDIT was related with BS/IMP (ρ= 0.43, p< 0.001), but not with TS or EMP/LASB (ps > 0.11).
One-way analyses of variance (ANOVA) showed small, but significant mean differences between the three family history groups (FHP, n= 62; FHAmb, n= 45; and FHN, n= 69) in age (F2,173 = 6.3, p= 0.002; FHP=23.4, SD= 2.5; FHAmb= 24.9, SD=3.6; Tukey’s lease significant difference [LSD], p= 0.019), but not education, or any of the personality components (Fs < 1.9, ps > 0.16); the FHA groups also did not differ in gender (χ2= 1.3, p > 0.5). The family history groups were, however, different in drinks/week (F2,173 = 3.9, p= 0.022) and AUDIT scores (F2,173 = 5.2, p = 0.006), although not in drinks/drinking day. Tukey’s LSD showed that for drinks/week, FHN subjects (14.7, SD= 10.8) were lower than FHAmb (19.1, SD= 11.0, p = 0.038). For AUDIT, FHN subjects (9.3, SD=4.3) were lower than both FHAmb (12.1, SD=5.4) or FHP (11.9, SD=6.0; ps < 0.02). As analyzed in separate SPM ANOVA models (co-varying for age, gender, drinks/week, smoking, cannabis use, and eTIV), there were no differences between the three family history groups in gmPVE.
There were no significant clusters of correlation between the SPM model covariates of drinks/week, smoking, or cannabis use and gmPVE.
4. Discussion
In this retrospective sample of undiagnosed community-dwelling subjects recruited for studies of risky drinking, we tested for significant relationships between frontal gmPVE and impulsive/externalizing personality traits. We reasoned that such a sample should increase the variance of these traits (Finn, 2002; Haber et al. 2005; Kendler et al. 2003; Krueger and South, 2009; Moss et al. 2007; Young et al. 2000)— a rationale supported by the statistically significant relationships in this sample between drinking and the derived externalizing personality components comprising elements of impulsivity (BS/IMP) and antisocial behavior (EMP/LASB). We hypothesized that elevated trait impulsivity and antisocial behaviors would negatively correlate with prefrontal gray matter volume as observed in externalizing disorders.
4.1 Relationship between BS/IMP and gmPVE
Unlike prior studies (Boes et al., 2009; Cho et al., 2013; Lee et al. 2011; Matsuo et al. 2009; Schilling et al. 2013a; Schilling et al. 2013b; Walhovd et al., 2012), including a smaller study of patients diagnosed with DSM-IV alcohol abuse and matched controls (Asensio et al., 2015), we did not find that gmPVE correlated negatively with self-reported impulsive behavior, either as a function of our BS/IMP component (consisting of significant loadings from scales reflecting boredom susceptibility, impulsive acts, antisocial behaviors, social disinhibition, and a desire to seek out unusual experiences), or when looking specifically at the Impulsivity subscale of the Eysenck I7 inventory. The Impulsivity subscale of the Eysenck I7 inventory did, however, correlate positively with bilateral gmPVE in lateral/posterior orbital cortex and insula. While not a typical finding, some studies have reported positive correlations between impulsiveness and either insula or medial frontal gray matter (e.g., Cho et al. 2013; Kaag et al., 2014; Lee et al. 2011). Differences in sample characteristics, age or approach to measuring impulsivity could account for the contradictory results found between the literature and our study (e.g., many studies use the Barratt Impulsivity Scale, Barratt, 1959).
4.2 Relationship between EMP/LASB and gmPVE
We found significantly large clusters where frontal gmPVE (dorsolateral prefrontal extending into the ventromedial and insula areas) correlated with EMP/LASB. Of note, neither of the independent scales of Eysenck I7 empathy or antisocial acts (the primary constituents of the EMP/LASB component) was significantly associated with gray matter volume when examined independently. This suggests that statistically aggregating these anti-correlated elements allowed detecting a behavioral phenotype that is more reflective of gray matter structure.
Empathy (which by I7 inventory items includes elements of “emotional contagion,” perspective taking, and sympathy) dominates the EMP/LASB component. In the clinical neurological literature, the loss of the ability to empathize can result from acquired lesions of ventromedial prefrontal cortex (Hillis, 2014; Shamay-Tsoory et al., 2003). In 118 healthy young adults, Banissy et al. (2012) found that empathic concern was associated with reduced gray matter volume in the left precuneus, left inferior frontal gyrus/insula, and left anterior cingulate. However, a large study of 261 children (ages 5 – 15 years) found that parent-rated empathy derived from the Children's Empathy Quotient and Systemizing Quotients scale (Auyeung et al., 2009) correlated positively with gray matter volume in the left fronto-opercular and superior temporal cortex (Sassa et al., 2012; also see Sterzer et al., 2007). Most recently, higher “affective empathy” (experiencing others’ emotions) in 176 undergraduate psychology majors was associated with increased gray matter density in the insula, while “cognitive empathy” (understanding others’ motivations) was related to higher gray matter density in the middle cingulate and dorsomedial prefrontal cortex (Eres et al., 2015).
Antisocial acts loaded negatively on the EMP/LASB component, and the absence of empathy is a component of antisocial personality disorder and sociopathy. That is, the EMP/LASB component can be interpreted as the inverse of a sociopathic personality constellation. In this context, a number of studies have shown that, compared to controls, there is reduced frontal gray matter and amygdala volume in subjects with ASPD, violent offenders, and criminal psychopaths (de Oliveira-Souza et al., 2008; Gregory et al., 2012; Laakso et al., 2002; Müller et al., 2008; Pardini et al., 2014; Raine et al. 2000; Tiihonen et al., 2008). Similar anatomic locations have been implicated in 23 boys (ages 10 – 13), in whom callous-unemotional traits were related to increased gray matter concentration in medial orbital and anterior cingulate cortex, and increased gray matter volume and concentration in the bilateral temporal lobes (De Brito et al., 2009). In this case, the relative increase in gray matter in children might reflect competitive pruning during cortical maturation (Lenroot and Giedd, 2006). In older adolescent boys with conduct disorder (ages 16 – 21), Fairchild et al. (2011) found gray matter volume reductions in the amygdala and insula. The same group also noted gray matter reductions in adolescent girls with conduct disorder in the bilateral insula and right striatum, with aggressive CD symptoms negatively correlating with right dorsolateral prefrontal volume, but callous traits correlating positively with orbital volume (Fairchild et al. 2013).
A connection between antisocial behavior and dorsolateral prefrontal neocortex is certainly intuitive, as this region is well known to regulate the ability to plan, regulate, shift strategy, and exert cognitive inhibitory control (Fuster, 1997). However, antisocial activity often comprises a lack of empathy and regard for others’ well-being. In that vein, our findings extended into ventral regions of prefrontal cortex, where acquired brain lesions are most likely to result in violence (Grafman et al., 1996). Ventromedial frontal cortex also activates during moral reasoning (Shenhav and Greene, 2014), and lesions to this region most alter decisions in ethical dilemmas where subjects must decide upon actions that result in harm to others (Koenigs et al., 2007; Thomas et al., 2011). A key aspect to the ventromedial prefrontal cortex here is its role in coding the visceral sensations experienced when contemplating both the loss of assets when gambling (Bechara et al., 1999), and harm caused to others by one’s behaviors (Moretto et al., 2010). Given our results and others’ findings, it is then possible that reduced ventromedial frontal gray matter interfere with the visceral sensations that occur when contemplating how one’s actions affect others’ well-being.
In addition to the frontal findings, the whole brain VBM identified an occipital cluster that was highly correlated with the EMP/LASB component. Although not obviously implicated in disorders of behavioral control, alterations in these regions have been identified in CD and ASPD populations. Bertsch et al. (2013) showed that across different types of ASPD subjects there were common clusters of reduced gray matter volumes within the frontal pole and the occipital cortex. Sundram, et al. (2012) observed reduced white matter integrity in the inferior fronto-occipital fasciculus (the white matter tract connecting the frontal lobe and the posterior portion of the inferior occipital gyrus) in the right frontal lobe of ASPD subjects. Moreover, this altered white matter integrity was negatively correlated with measures of psychopathy. Dalwani et al.’s (2011) work also showed that boys with serious CD and substance use problems have lower gray matter volume in left DLPFC and right lingual gyrus (but higher gray matter volume in the right precuneus). A first step in empathy is to perceive another’s emotional state, and such visual processing centers play an important role in the visual identification of facial emotion (Kitada et al., 2010). One could then speculate that visual centers might, in this way, relate to empathy.
4.3 Personality traits, alcohol use, familial alcoholism, and gmPVE
Finally, although the externalizing personality traits did relate to drinking, neither alcohol consumption itself nor familial alcoholism was significantly related to gmPVE. Many studies have shown significant gray matter loss in the prefrontal cortex of alcohol-dependent individuals (up to 20% in the dorsolateral frontal cortex, e.g., Chanraud et al., 2006; Fein et al., 2002; Pfefferbaum et al., 1997). Given the nature of our sample, however, the lack of our own findings might well be accounted for by the limited range of drinking and the lack of heavy, chronic exposure. Some studies have also shown that alcohol-naïve subjects from high-risk families have alterations in frontal gray and white matter volumes (Cservenka, 2015). For example, Benegal et al. (2007) found that the alcohol naïve offspring of alcoholic parents, when compared to controls, had significantly smaller volumes of superior frontal and cingulate areas, with the left superior frontal gyrus volume correlating with externalizing symptoms. Hill’s group has found that the offspring of alcoholic relatives had lower [right > left] asymmetry ratios of combined gray and white orbital volume, and that orbital white matter asymmetry in these offspring correlated with greater impulsivity (Hill et al., 2009). The same group (Hill et al., 2001, 2013) and Dager et al (2015) also detected loss of right amygdala volume in the adolescent offspring of families with alcoholism. Most recently, Cservenka et al (2015) reported that family history density was related to left nucleus accumbens volume in adolescents, although only in girls. Our large smoothing kernel may have precluded us from detecting effects in smaller subcortical regions. Having said that, we did observe a small subthreshold effect (299 voxels at p< 0.005) in the right amygdala, where FHP subjects had reduced gmPVE when compared to FHN subjects (similar to the Hill and Dager studies above). A second consideration in our lack of findings related to this genetic risk is familial density of affected relatives, as subjects in our study could have only one first-degree affected relative, whereas the density of affected relatives in other studies was higher (e.g., Hill et al. 2001; Hill et al. 2009). Also, only the subject was available to interview with regard to family history, without the added reliability from interviewing collateral family. This could affect the nature of the family history outcome.
4.4 Limitations
Although our study is comprised of a large sample of well characterized subjects, it has limitations. First, the predominantly male sample complicates generalizing the findings to women. Second, given that the subjects were selected for their elevated drinking, it may not be possible to generalize these results to samples characterized by more social levels of alcohol consumption. Some subjects with lower empathy and higher antisocial behavior were in the age range of 27 – 35, making it difficult to tease out interactions between age and these other factors.
4.5 Conclusions
This study of 176 young healthy drinkers found significant relationships between dorsolateral/ventromedial prefrontal and occipital gray matter volume and a personality trait component reflecting a combination of high empathy and low sociopathic behavior. Trait impulsivity was unexpectedly associated with increased lateral inferior frontal/insular gray matter volume. Thus, aspects of externalizing personality traits in community-dwelling non-patients are, as in clinical samples, related to regional differences in frontal and limbic gray matter volume.
Highlights.
-
➢
We tested for relationships between externalizing behaviors and gray matter volume.
-
➢
High empathy/low sociopathic behavior correlated with frontal gray matter.
-
➢
Impulsivity positivity correlated with frontal/insula gray matter volume.
-
➢
Frontal gray matter is related to externalizing personality traits.
Acknowledgments
We gratefully acknowledge the support and assistance of Kristen Rose Case, Christina Soeurt, Stella Tran, Michele Beal, Courtney Robbins, and Dr. Jessica Weafer for assistance with data collection and entry. Supported by the National Institutes of Health (P60 AA007611, R01 AA014605, R01 AA017661, and R21 AA018020). Judith Charpentier was supported by the FidEx Inter- national program from the Initiative of Excellence programs at University of Bordeaux, and by funding from Aquimob.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asensio S, Morales JL, Senabre I, Romero MJ, Beltran MA, Flores-Bellver M, Barcia JM, Romero FJ. Magnetic resonance imaging structural alterations in brain of alcohol abusers and its association with impulsivity. Addiction Biology n/a. 2015 doi: 10.1111/adb.12257. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Wheelwright S, Allison C, Atkinson M, Samarawickrema N, Baron-Cohen S. The children's empathy quotient and systemizing quotient: sex differences in typical development and in autism spectrum conditions. J Autism Dev Disord. 2009;39:1509–1521. doi: 10.1007/s10803-009-0772-x. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Kanai R, Walsh V, Rees G. Inter-individual differences in empathy are reflected in human brain structure. NeuroImage. 2012;62:2034–2039. doi: 10.1016/j.neuroimage.2012.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES. Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills. 1959;9:191–198. [Google Scholar]
- Bechara A, Damasio H, Antonio R, Gregory P. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. The Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Grothe M, Prehn K, Vohs K, Berger C, Hauenstein K, Keiper P, Domes G, Teipel S, Herpertz S. Brain volumes differ between diagnostic groups of violent criminal offenders. Eur Arch Psychiatry Clin Neurosci. 2013;263:593–606. doi: 10.1007/s00406-013-0391-6. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biological Psychiatry. 2009;65:710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Social Cognitive and Affective Neuroscience. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. 16. 2002. p. 2. [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2006;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Cho S, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I, Strafella A. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography. 2013;26:479–487. doi: 10.1007/s10548-012-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug and Alcohol Dependence. 2015 doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Gillespie AJ, Michael PG, Nagel BJ. Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. Journal of Studies on Alcohol and Drugs. 2015;76:47–56. [PMC free article] [PubMed] [Google Scholar]
- Dager AD, McKay DR, Kent JW, Curran JE, Knowles E, Sprooten E, Goring HH, Dyer TD, Pearlson GD, Olvera RL, Fox PT, Lovallo WR, Duggirala R, Almasy L, Blangero J, Glahn DC. Shared Genetic Factors Influence Amygdala Volumes and Risk for Alcoholism. Neuropsychopharm. 2015;40:412–420. doi: 10.1038/npp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwani M, Sakai JT, Mikulich-Gilbertson SK, Tanabe J, Raymond K, McWilliams SK, Thompson LL, Banich MT, Crowley TJ. Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug and Alcohol Dependence. 2011;118:295–305. doi: 10.1016/j.drugalcdep.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S, Viding E. Size matters: Increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Azevedo Ignáício F, Tovar-Moll F, Moll J. Psychopathy as a disorder of the moral brain: Fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. NeuroImage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Eres R, Decety J, Louis WR, Molenberghs P. Individual differences in local gray matter density are associated with differences in affective and cognitive empathy. NeuroImage. 2015;117:305–310. doi: 10.1016/j.neuroimage.2015.05.038. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easton G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and Individual Differences. 1985;6:613–619. [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EAH, van Goozen SHM, Goodyer IM, Calder AJ. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 2011;168:624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical Gray Matter Loss in Treatment-Naïve Alcohol Dependent Individuals. Alcoholism: Clinical and Experimental Research. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Finn PR. Motivation, Working Memory, and Decision Making: A Cognitive-Motivational Theory of Personality Vulnerability to Alcoholism. Behavioral and Cognitive Neuroscience Reviews. 2002;1:181–203. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. 3rd. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury Study. Neurology. 1996;45:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Gregory S, ffytche D, Simmons A. The antisocial brain: Psychopathy matters. Archives of General Psychiatry. 2012;69:962–972. doi: 10.1001/archgenpsychiatry.2012.222. [DOI] [PubMed] [Google Scholar]
- Groeschel S, Vollmer B, King MD, Connelly A. Developmental changes in cerebral grey and white matter volume from infancy to adulthood. International Journal of Developmental Neuroscience. 2010;28:481–489. doi: 10.1016/j.ijdevneu.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Gunn RL, Finn PR, Endres MJ, Gerst KR, Spinola S. Dimensions of disinhibited personality and their relation with alcohol use and problems. Addictive Behaviors. 2013;38:2352–2360. doi: 10.1016/j.addbeh.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JR, Jacob T, Heath AC. Paternal alcoholism and offspring conduct disorder: Evidence for the 'common genes' hypothesis. Twin Research and Human Genetics. 2005;8:120–131. doi: 10.1375/1832427053738782. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, McDermott MD, Zezza N, Stiffler S. Amygdala Volume in Offspring from Multiplex for Alcohol Dependence Families: The Moderating Influence of Childhood Environment and 5-HTTLPR Variation. J. Alcohol. and Drug Depend. 2013;(Suppl 1):001. doi: 10.4172/2329-6488.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2009;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another's emotions. Brain. 2014;137:981–997. doi: 10.1093/brain/awt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaag AM, Crunelle CL, van Wingen G, Homberg J, van den Brink E, Reneman L. Relationship between trait impulsivity and cortical volume, thickness and surface area in male cocaine users and non-drug using controls. Drug and Alcohol Dependence. 2014;144:210–217. doi: 10.1016/j.drugalcdep.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Grahame N, Dzemidzic M, Walker MJ, Lehigh CA, O'Connor SJ. fMRI of the Brain's Response to Stimuli Experimentally Paired with Alcohol Intoxication. Psychopharmacology. 2012;220:787–797. doi: 10.1007/s00213-011-2526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill L, Dzemidzic M, Bragulat V, Cox C, Talavage T, O'Connor SJ, Foroud T. A polymorphism in GABRA2 Is associated with the medial frontal response to alcohol cues in an fMRI study. Alcoholism: Clinical and Experimental Research. 2010;34:2169–2178. doi: 10.1111/j.1530-0277.2010.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken D, Dzemidzic M, Wetherill L, Eiler W, II, Oberlin B, Harezlak J, Wang Y, O'Connor S. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology. 2013b;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. THe structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kitada R, Johnsrude IS, Kochiyama T, Lederman SJ. Brain networks involved in haptic and visual identification of facial expressions of emotion: An fMRI study. NeuroImage. 2010;49:1677–1689. doi: 10.1016/j.neuroimage.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Dal Monte O, Schintu S, Wassermann EM, Raymont V, Grafman J, Krueger F. Areas of brain damage underlying increased reports of behavioral disinhibition. The Journal of Neuropsychiatry and Clinical Neurosciences. 2015;27:193–198. doi: 10.1176/appi.neuropsych.14060126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Ruggero CJ, Krueger RF, Watson D, Yuan Q, Zimmerman M. New dimensions in the quantitative classification of mental illness. Archives of General Psychiatry. 2011;68:1003–1011. doi: 10.1001/archgenpsychiatry.2011.107. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, South SC. Externalizing disorders: Cluster 5 of the proposed meta-structure for DSM-V and ICD-11. Psychological Medicine. 2009;39:2061–2070. doi: 10.1017/S0033291709990328. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Research: Neuroimaging. 2002;114:95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Lee AKW, Jerram M, Fulwiler C, Gansler D. Neural correlates of impulsivity factors in psychiatric patients and healthy volunteers: a voxel-based morphometry study. Brain Imaging and Behavior. 2011;5:52–64. doi: 10.1007/s11682-010-9112-1. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Malone IB, Leung KK, Clegg S, Barnes J, Whitwell JL, Ashburner J, Fox NC, Ridgway GR. Accurate automatic estimation of total intracranial volume: A nuisance variable with less nuisance. NeuroImage. 2015;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MAM, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapping. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montigny C, Castellanos-Ryan N, Whelan R, Banaschewski T, Barker GJ, Büchel C, Gallinat J, Flor H, Mann K, Paillère-Martinot ML, Nees F, Lathrop M, Loth E, Paus T, Pausova Z, Rietschel M, Schumann G, Smolka MN, Struve M, Robbins TW, Garavan H, Conrod PJ and the IMAGEN Consortium. A phenotypic structure and neural correlates of compulsive behaviors in adolescents. PLoS ONE. 2013;8:e80151. doi: 10.1371/journal.pone.0080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto G, Ladavas E, Mattioli F, Di Pellegrino G. A psychophysiological investigation of moral judgment after ventromedial prefrontal damage. Journal of Cognitive Neuroscience. 2010;22:1888–1899. doi: 10.1162/jocn.2009.21367. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi Hy. Subtypes of alcohol dependence in a nationally representative sample. Drug and Alcohol Dependence. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JL, Gãnβbauer S, Sommer M, Döhnel K, Weber T, Schmidt-Wilcke T, Hajak G. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Research: Neuroimaging. 2008;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Bragulat V, Lehigh CA, Talavage T, O'Connor SJ, Kareken DA. Limbic responses to reward cues correlate with antisocial trait density in heavy drinkers. NeuroImage. 2012;60:644–652. doi: 10.1016/j.neuroimage.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: Mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Dzemidzic M, Tran S, Soeurt C, O'Connor S, Yoder K, Kareken D. Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology. 2014:1–10. doi: 10.1007/s00213-014-3720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Raine A, Erickson K, Loeber R. Lower Amygdala Volume in Men is Associated with Childhood Aggression, Early Psychopathic Traits, and Future Violence. Biol. Psychiatry. 2014;75:73–80. doi: 10.1016/j.biopsych.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell'Acqua F, Fahy T, Deeley Q, Murphy DGM. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychological Medicine. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Sassa Y, Taki Y, Takeuchi H, Hashizume H, Asano M, Asano K, Wakabayashi A, Kawashima R. The correlation between brain gray matter volume and empathizing and systemizing quotients in healthy children. NeuroImage. 2012;60:2035–2041. doi: 10.1016/j.neuroimage.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kuhn S, Paus T, Romanowski A, Banaschewski T, Barbot A, Barker GJ, Bruhl R, Buchel C, Conrod PJ, Dalley JW, Flor H, Ittermann B, Ivanov N, Mann K, Martinot JL, Nees F, Rietschel M, Robbins TW, Smolka MN, Strohle A, Kathmann N, Garavan H, Heinz A, Schumann G, Gallinat J. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol Psychiatry. 2013a;18:624–630. doi: 10.1038/mp.2012.56. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kühn S, Romanowski A, Banaschewski T, Barbot A, Barker GJ, Brühl R, Büchel C, Charlet K, Conrod PJ, Czech K, Dalley JW, Flor H, Häke I, Ittermann B, Ivanov N, Mann K, Lüdemann K, Martinot JL, Palafox C, Paus T, Poline JB, Reuter J, Rietschel M, Robbins TW, Smolka MN, Ströhle A, Walaszek B, Kathmann N, Schumann G, Heinz A, Garavan H, Gallinat J the IMAGEN consortium. Common structural correlates of trait impulsiveness and perceptual reasoning in adolescence. Human Brain Mapping. 2013b;34:374–383. doi: 10.1002/hbm.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EP, Cecil CM. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15:324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Greene JD. Integrative Moral Judgment: Dissociating the Roles of the Amygdala and Ventromedial Prefrontal Cortex. The Journal of Neuroscience. 2014;34:4741–4749. doi: 10.1523/JNEUROSCI.3390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M, Montana G, Nichols TE. False positives in neuroimaging genetics using voxel-based morphometry data. NeuroImage. 2011;54:992–1000. doi: 10.1016/j.neuroimage.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addictive Behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. NeuroImage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Sundram F, Deeley Q, Sarkar S, Daly E, Latham R, Craig M, Raczek M, Fahy T, Picchioni M, Barker GJ, Murphy DGM. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex. 2012;48:216–229. doi: 10.1016/j.cortex.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Thomas BC, Croft KE, Tranel D. Harming kin to save strangers: Further evidence for abnormally utilitarian moral judgments after ventromedial prefrontal damage. Journal of Cognitive Neuroscience. 2011;23:2186–2196. doi: 10.1162/jocn.2010.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, Repo-Tiihonen E, Vaurio O, Soininen H, Aronen HJ, Könönen M, Thompson PM, Frisoni GB. Brain anatomy of persistent violent offenders: More rather than less. Psychiatry Research: Neuroimaging. 2008;163:201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Tamnes CK, Østby Y, Due-Tønnessen P, Fjell AM. Normal variation in behavioral adjustment relates to regional differences in cortical thickness in children. Eur Child Adolesc Psychiatry. 2012;21:133–140. doi: 10.1007/s00787-012-0241-5. [DOI] [PubMed] [Google Scholar]
- Weafer J, Dzemidzic M, Eiler W, II, Oberlin BG, Wang Y, Kareken DA. Associations between regional brain physiology and trait impulsivity, motor inhibition, and impaired control over drinking. 2015 doi: 10.1016/j.pscychresns.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]
- Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. Cambridge: Cambridge Unviversity Press; 1994. [Google Scholar]