Abstract
Respiratory tract infections are an important cause of morbidity and mortality worldwide. Chief among these are infections involving the lower airways. The opportunistic bacterial pathogens responsible for most cases of pneumonia can cause a range of local and invasive infections. However, bacterial colonization (or carriage) in the upper airway is the prerequisite of all these infections. Successful colonizers must attach to the epithelial lining, grow on the nutrient-limited mucosal surface, evade the host immune response, and transmit to a susceptible host. Here, we review the molecular mechanisms underlying these conserved stages of carriage. We also examine how the demands of colonization influence progression to disease. A range of bacteria can colonize the upper airway; nevertheless, we focus on strategies shared by many respiratory tract opportunistic pathogens. Understanding colonization opens a window to the evolutionary pressures these pathogens face within their animal hosts and that have selected for attributes that contribute to virulence and pathogenesis.
Keywords: nasopharynx, commensal, opportunistic pathogen, Streptococcus pneumoniae, inflammation
INTRODUCTION
Acute respiratory tract infections are a major source of disease worldwide, with pneumonia alone responsible for more than 1.3 million child deaths annually (84). Among the most frequent bacterial causes of pneumonia are Streptococcus pneumoniae (the pneumococcus), Haemophilus influenzae, and Staphylococcus aureus. Common to all these pathogens, as well as other opportunistic invaders of the respiratory tract, is the requirement for asymptomatic bacterial colonization, or carriage, to precede local and systemic disease (9, 31, 120).
Upper respiratory tract colonization not only causes disease but also drives the evolution of these opportunistic pathogens. Transmission to new susceptible hosts occurs from the reservoir of bacteria colonizing the oro- or nasopharynx, rather than from invasive infections. As a result, the demands of colonization form the selective pressures that promote pathogen adaptation and virulence (129). Colonizing bacteria must avoid a range of host challenges, from penetrating the mucous barrier to evading professional phagocytes and obtaining a source of nutrients on the harsh mucosal surface (Table 1). These essential activities are summarized in the stages of colonization: bacterial acquisition and attachment to the epithelial lining, obtaining nutrients, replication, persistence in the face of the host immune response, and transmission to a new host (Figure 1). Here, we review the mechanisms underlying each of these steps. We focus on colonization by opportunistic pathogens that have the potential to cause disease, rather than bacteria that exclusively share a commensal relationship with their host. Most notable, and most studied, among these is the pneumococcus. We also focus on evidence from in vivo experimental models—primarily rodents, with some work in humans and nonhuman primates, as these manipulable models provide mechanistic insight. This review highlights broad themes and commonalities shared by many of these opportunistic pathogens that colonize the nasopharynx as the first step in pathogenesis.
Table 1.
Host pressures and bacterial factors to counteract them
| Host pressure | Bacterial countering factor |
|---|---|
| Anionic mucus prevents bacterial binding to epithelial surface | Polysaccharide capsule repels mucus; neuraminidases degrade mucus |
| Transporters limit free carbon on the mucosal surface | Glycosidases liberate carbon sources for uptake by carbon transporters; bacteria induce inflammation to promote secretion of substrates such as sialylated mucins |
| Lipocalin-2 binds siderophores | Nonsiderophore mechanisms obtain iron; nonenterobactin siderophores resist lipocalin-2 binding |
| Actively increases toxic zinc while limiting manganese | Zinc/manganese transporters |
| Mucociliary flow clears bacteria mechanically | Attach to host cells and tissues |
| Opsonophagocytosis | Antiphagocytic polysaccharide capsule |
| Mucosal secreted IgA1 | IgA1 protease |
| Humoral immunity | Evade antibodies through antigenic variation |
| Recognizes bacterial LPS | Mask LPS with the host mimic ChoP |
| Neutrophil influx | Polysaccharide capsule, toxins |
| Inflammatory response to colonization | Exploit host responses to drive transmission |
| Hosts a diverse beneficial commensal flora | Displace or compete with host microbiota |
| TLR-mediated epithelial opening allows immune cell infiltration | Exploit epithelial opening to promote invasion and persistence |
| Specialized M cells sample lumen for antigens | Enter through M cells as portal for invasion |
| Creates microniches with opposing selective pressures | Phenotypic or phase variation generates diversity within a bacterial population |
Abbreviations: ChoP, phosphorylcholine; LPS, lipopolysaccharide; TLR, Toll-like receptor.
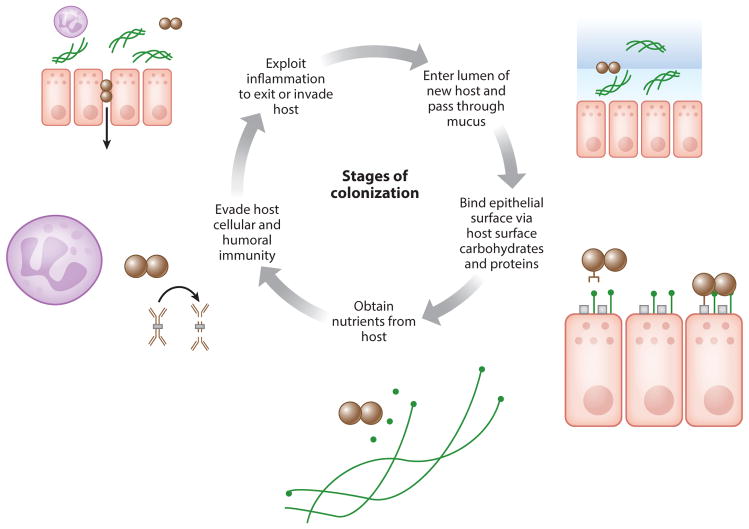
Figure 1.
The essential stages of colonization of the upper respiratory tract are presented here as a cycle. Colonizing bacteria (brown) enter the nasopharyngeal lumen and pass through the mucous layer (blue), in part facilitated by their capsule. Next, bacteria reach the epithelial surface and bind loosely and tightly to host surface carbohydrates and proteins, respectively. Bacteria obtain nutrients by exploiting host inflammation (through, for example, digestion of sialylated mucins) and evading nutritional immunity. These nutrients allow for microbial replication; persistence also involves evasion of host immune responses, both humoral (through IgA1 protease) and cellular. Opportunistic pathogens exploit these responses to drive transmission by exiting the host, and the same factors that allow for increased colonization and transmission predispose to invasion of host barriers, potentially a strategy for persistence.
ACQUISITION AND ATTACHMENT
Stable colonization of the nasopharynx requires adherence to the mucosal surface, lest colonizing bacteria be rapidly swept away by mucociliary clearance (29). So important is remaining on the epithelial surface, at least until the time of transmission, that opportunistic pathogens of the airway have developed multiple strategies for attachment, which span three broad categories: transient association with mucus, weak association with host carbohydrates, and strong association with host surface proteins.
The importance of mucus in protecting against bacterial acquisition was demonstrated by experimental colonization of ferrets with Staphylococcus aureus. Early after inoculation, nearly all staphylococci were in the mucous gel layer, rather than being bound to epithelial cells (101). Whereas some bacteria may bind to glycoconjugates in the mucous layer, others exit it to reach the epithelial surface by repelling anionic mucus with a negatively charged polysaccharide capsule (81). Using capsule to pass through mucus, however, complicates attachment to the epithelium, as the thick polysaccharide capsule also repels host cells. Airway colonizers have evolved multiple mechanisms of phenotypic variation to adjust quickly to different microniches during carriage. Pneumococci, for example, undergo phase variation, or rapid on-off switching, apparent as differences between opaque and transparent colony morphologies. Opacity is a reflection of bacteria-bacteria interaction, and differences stem from cell surface changes that affect these associations. Opacity phase variation has recently been linked to epigenetic changes in DNA methylation (62). Opaque variants display more capsule but less teichoic acid and less of other adhesins such as PspC (CbpA) (48). Only transparent variants could effectively colonize infant rats (48). Opacity variation, while leading to the emergence of more virulent, highly encapsulated organisms during sepsis, could in the nasopharynx serve to promote exit from mucus (highly encapsulated opaque variants) and then binding to the epithelium (transparent variants with less capsule to block adhesin interactions). Lipopolysaccharide (LPS) modifications causing colony opacity variation also contributed to colonization of infant rats by Haemophilus influenzae (128). Not all bacteria need to downregulate capsule to bind the epithelial surface stably, however. For example, the hyaluronic acid capsule of Streptococcus pyogenes is bound to CD44 on host cells (21).
The second stage of attachment is thought to involve weak attachment by bacterial lectin-like proteins to host surface carbohydrates (121). In studies of the chinchilla airway, pneumococcal colonization led to desialylation that was dependent on expression of the bacterial neuraminidase NanA. A nanA mutant was unable to colonize chinchillas, indicating that sialic acid cleavage was important either to exit the heavily sialylated mucus or to reveal underlying host glycoconjugate receptors (112, 113). The effect of NanA may depend on the animal model, however, as other work found no effect during colonization of infant rats (49). In vitro, the pneumococcal exoglycosidase BgaA binds specific host surface carbohydrate residues revealed by the removal of sialic acid (108).
More stable adherence comes through interaction with host cell surface proteins (121). Pili are important for adhesion and colonization for pneumococci (6), H. influenzae (42), and Moraxella catarrhalis (56); but they are not the only adhesive factor. In pneumococci, RrgA is a pilus-associated adhesin required for adhesion in both the presence and absence of pili (80). Pili may aid in extending adhesins, including beyond the capsule, to allow for binding to the host (80). Another pneumococcal adhesin is PspC (CbpA), which binds secretory component on the polymeric immunoglobulin receptor (85). Fibronectin-binding proteins PavA and PavB were also important during pneumococcal colonization (41, 43). The filamentous hemagglutinin (FHA) of Bordetella species has been implicated in adherence in vivo. In Bordetella bronchiseptica, FHA was necessary for colonization of mice, and the gene encoding FHA is one of the most expressed in Bvg+ Bordetella, the phase of growth when virulence genes are expressed under the control of the BvgAS two-component system (20). However, ectopic expression of FHA during the Bvg− phase was not sufficient to promote adherence in vivo (20), underscoring the importance of tight control over adhesin expression for effective colonization. Similarly, pilus expression in pneumococci appears to be regulated, as it peaks during the early phases of murine colonization (86).
Adhesins in S. aureus follow a similar pattern of modulated expression. Wall teichoic acids on the S. aureus cell surface were required for colonization of cotton rats (126), binding to the nasal cavity surface receptor SREC-I (7). Clumping factor B (ClfB) was necessary for experimental human colonization and bound cytokeratin 10 in vitro (130). More recent work using knockout mice and bacterial mutants demonstrated that the receptor for ClfB was not cytokeratin 10 but rather loricrin, a protein found on the cornified envelope of the squamous epithelium in the anterior nares, the site of S. aureus colonization (76). When exogenously expressed in Lactococcus lactis, ClfB was sufficient for adherence and colonization of wild-type, but not loricrin-deficient, mice (76). These results emphasize the importance of in vivo studies; in vitro studies did not identify the relevant ligand-receptor interaction necessary for colonization. Using cotton rats, Burian et al. found that wall teichoic acid adhesins were expressed in vivo early during colonization and replaced over time as ClfB and the adhesin IsdA were expressed (14).
GROWTH AND NUTRIENT SOURCES
After establishing a stable presence on the mucosal surface, colonizing organisms must find a source of nutrients to promote replication. Growth during colonization amplifies bacterial density, potentially increasing the likelihood of transmission to the next host. However, proliferation is particularly challenging in the nasopharynx. Free carbohydrates are not abundant in the normal, uninflamed upper respiratory tract (90), and the host actively depletes glucose from the airway surface fluid as a form of nutritional immunity (89).
van Opijnen & Camilli demonstrated the difficulties of replication in the nasopharynx using transposon mutagenesis of pneumococci and the method Tn-seq to catalog the genes necessary in different host and in vitro environments (115). The nasopharynx was the harsher microniche: When genes that were required in both the upper and lower respiratory tracts were absent in the nasopharynx, larger average fitness defects were observed, suggesting that these genes were more important for establishing colonization than pneumonia (115). Using dilution of a temperature-sensitive, antibiotic-resistance-expressing plasmid, they calculated pneumococcal doubling times for replication in the mouse nasopharynx (161 min) and lungs (108 min), which suggested that nutrients are less readily available at the site of initial colonization (115). This doubling time was consistent with results obtained using a flow cytometric method to quantify the dilution of CFSE, a fluorescent dye commonly used to track eukaryotic cell division, from pneumococci colonizing the mouse upper respiratory tract. Influenza coinfection accelerated CFSE dilution, and therefore replication, by providing a carbon source via mucin secretion (107).
Pneumococci depend on carbohydrates as a source of carbon for growth and are particularly adept at accessing them on the airway surface. Among commensal and pathogenic bacteria that colonize the upper respiratory tract, pneumococci express the most diverse array of sugar transporters and utilization genes (13). More than 30% of transporters in the pneumococcal genome were predicted to import carbohydrates as substrates, a higher proportion than any other bacterium sequenced (111). By contrast, H. influenzae and Neisseria meningitidis had few carbohydrate transporters (111). Pneumococcal colonization independently requires catabolism of sialic acid (65, 114), hyaluronic acid (66), and sucrose or a related disaccharide (39). It also requires an ATPase to energize multiple ABC transporters used for sialic acid, raffinose, and maltotetraose (64). This wide range of transporters and bioavailable sugars does not reflect redundant pathways, however, as suggested by the decreased colonization seen with any individual mutation (13). Rather, each transporter may be important in specific microenvironments encountered during colonization or in certain hosts, such as the particular importance of sialic acid during colonization following influenza (107).
Other opportunistic pathogens of the airway use additional sugars during colonization, though in vivo evidence is more lacking. N. meningitidis required lactate permease to utilize this carbon source in vitro and to grow in nasopharyngeal explants (28). Sialic acid catabolism was not required for H. influenzae to colonize the infant rat, though sialylation of the bacterial surface contributed to immune evasion (118). During experimental S. pyogenes pharyngitis in macaques, microarray analysis revealed high levels of maltodextrin and mannose catabolic gene expression in bacteria early during colonization (119). A maltodextrin-binding protein was required for S. pyogenes to colonize the mouse oropharynx (103). A transcriptional repressor in the same operon was also necessary for colonization, implying that tight regulation of carbohydrate utilization genes is important during carriage (102). Similarly, the nutritional repressor CodY was required during pneumococcal colonization, and its transcript was expressed in vivo in the nasopharynx (35). These modes of regulation imply there may be a temporal switch during colonization from rapid replication to a later, more silent phase of quiescent persistence.
Nearly all bacteria, including all opportunistic pathogens of the airway, require an iron source to survive (127). Mammalian hosts have developed mechanisms to combat this essential microbial activity by sequestering ferric iron or targeting bacterial uptake mechanisms (127). These mechanisms are active in the nasopharynx, as evidenced by the high levels of isdA expression in S. aureus colonizing the human airway (15). Moreover, that expression of this tightly-iron-regulated gene implies S. aureus is growing under iron-limited conditions in vivo (15). One bacterial strategy to acquire iron is secretion of siderophores, low molecular weight proteins that bind iron with high affinity (127). In contrast to inhabitants of the gut, respiratory colonizers, including pneumococci, H. influenzae, and N. meningitidis, tend not to secrete siderophores and instead obtain iron by directly retrieving iron-containing molecules such as lactoferrin, transferrin, hemin, and hemoglobin (79, 110, 127). This strategy may have evolved to avoid host antisiderophore defenses, including secretion of lipocalin-2 (siderocalin), which binds enterobactin-type siderophores to prevent their uptake by bacteria (79, 127). Lipocalin-2 was induced in the murine nasopharynx during colonization with pneumococci and H. influenzae, as well as in the human nasal mucosa, raising the possibility that these bacteria were actively inducing a host response that could eliminate competitors without being susceptible themselves (79). S. aureus and bordetellae produce siderophores that are structurally distinct from enterobactin and, as a result, can evade lipocalin-2 (79). The precise in vivo importance of many of these iron-acquisition mechanisms has not been tested in nasopharyngeal colonization, though S. aureus colonization of the infant rat airway was enhanced by the presence of hemoglobin (96).
Nutritional immunity extends to other transition metals, particularly zinc and manganese, additional critical cofactors for many bacterial functions (46). Pneumococci obtain both zinc and manganese via the PsaA protein, but excess zinc can be toxic to colonizing bacteria and can compete with manganese for uptake by pneumococci (71). The ratio of Zn2+ to Mn2+ becomes elevated in the nasopharynx during pneumococcal colonization, suggesting the host may be actively increasing zinc or limiting manganese to combat incoming bacteria (71).
IMMUNE EVASION AND PERSISTENCE
Scarcity of resources is not the only reason the mucosal surface is harsh. From the time of acquisition, colonizing bacteria interact with and must evade the host mucosal immune system long enough to spread to susceptible hosts. Bacteria have evolved a range of strategies to evade the different branches of host immunity. Innate responses can be actively thwarted by specific effectors that target host cells. Bacteria can evade detection by shielding themselves with host-mimicking or antigenically varying molecules. Rapid transmission can also be thought of as an evasion strategy, as exit to a new host can occur before the onset of adaptive immunity.
Bacterial contact with the immune system begins on entry into the nasopharynx, where colonizers are met by the layer of mucus that coats the upper respiratory tract. Mucus itself provides a physical barrier bacteria must traverse to avoid entrapment and removal by mucociliary clearance. Additionally, mucins serve as a scaffold for antimicrobial proteins (29). So important is the mucous barrier to infection that the primary virulence factor common to many opportunistic pathogens of the upper respiratory tract, capsular polysaccharide, may have evolved to combat it. While capsule is well known for its role in limiting opsonization by complement and antibody, it also contributes to evading mucus-mediated clearance. Unencapsulated pneumococci were inhibited in early colonization of mice compared with isogenic bacteria expressing a capsule (81). All but a few of the >90 pneumococcal capsule types are negatively charged, allowing for repulsion from the anionic mucous barrier. The lack of colonization without capsule was not due to increased susceptibility to neutrophils, complement, or antibody, and unencapsulated mutants were observed stuck in mucus rather than reaching the nasopharyngeal epithelium (81).
Even without capsule, successful respiratory tract colonizers have alternative methods to resist antibody-mediated clearance. Pneumococci, pathogenic Neisseria species, and H. influenzae all encode a protease specific to human immunoglobulin A1 (IgA1), the dominant IgA present in the human respiratory tract. In vitro, these proteases cleave IgA1, retaining Fab fragment binding to the bacterial surface but preventing antibody effector functions by removing the Fc fragment (93). Mice passively immunized with human protease-sensitive IgA1, but not protease-insensitive IgA2, were protected from intranasal infection with IgA1-protease-producing, but not IgA1-protease-deficient, pneumococci (40). Other bacteria do not effectively evade humoral immunity. Clearance of Bordetella pertussis, and the related species Bordetella parapertussis and B. bronchiseptica, was delayed in mice lacking mature B cells (50).
Colonizing bacteria modify their cell surface to resist antibodies and other mechanisms of targeting microbes for clearance. Phosphorylcholine (ChoP), a constituent of the surface of multiple respiratory tract bacteria, is poorly immunogenic, as it mimics host structures including phosphatidylcholine found in membrane lipids. For example, ChoP modifies the LPS of nontypeable H. influenzae (NTHi), preventing binding of bactericidal antibody by altering accessibility to underlying bacterial antigens (17). During colonization, adaptive immunity was required to select for ChoP-expressing NTHi, a process that occurs through phase variation, the rapid switching of translation of a choline kinase gene on or off through slipped-strand mispairing in a repetitive DNA motif (17). LPS biosynthetic gene phase variation and selection for the ChoP-on phase has also been observed during experimental human colonization (95). In a different form of genetic regulation, the LPS of B. bronchiseptica is modified by pagP, a palmitoyl transferase that is under the control of the BvgAS two-component system that regulates virulence gene activity (92). These LPS modifications increase evasion of antibody-dependent complement-mediated lysis (92).
After transiting the mucous layer, colonizing bacteria reach the epithelial surface, where they are rapidly sensed by multiple innate pattern recognition receptors that contribute to clearance. Lipoteichoic acids of pneumococci (116) and S. aureus (30) are sensed by Toll-like receptor (TLR) 2, whereas lipoproteins and LPS from H. influenzae can be recognized by TLRs 2 and 4, respectively (133). TLR4 has also been reported to recognize the pneumococcal pore-forming toxin pneumolysin (60). In addition to being sensed by surface receptors, these predominantly extra-cellular pathogens are recognized by host cytosolic detectors that contribute to clearance, such as nucleotide-binding oligomerization domain (Nod) receptors that detect peptidoglycan from bacterial cell walls. Signals from Nod1, which recognizes gram-negative peptidoglycan, are important in controlling H. influenzae carriage (133), whereas Nod2, which senses peptidoglycan fragments from both gram-positive and gram-negative bacteria, contributes to pneumococcal clearance (24). Further in vivo evidence of intracellular sensing of extracellular pathogens stems from findings that type I interferons are produced during pneumococcal colonization and required for clearance of the organism (88). Other work has shown, however, that synergistic, far larger increases in type I interferon during influenza-pneumococcal coinfection impairs pneumococcal clearance (78).
The initial response to colonization with a range of opportunistic pathogens is generally a mild suppurative rhinitis that can often be subclinical and inapparent but reflects infiltration of neutrophils into the nasopharynx (1, 30, 116, 133). For many respiratory tract colonizers, this initial influx is insufficient to clear colonization, either because the phagocytes are ineffective at clearing these encapsulated microbes prior to development of specific antibody or because the bacteria actively evade neutrophil-mediated killing (68). The adenylate cyclase toxin of B. bronchiseptica, for example, inhibits neutrophil effector functions by inducing cAMP overproduction from ATP, allowing the bacteria to avoid mucosal clearance (34, 72).
Neutrophils are recruited in response to pneumococcal acquisition but are insufficient for clearance from the upper respiratory tract in the absence of cellular and adaptive immunity (116). CD4 T cells are necessary and sufficient to provide acquired immunity to secondary pneumococ-cal challenge, and mice deficient in the IL-17A receptor also cannot control primary colonization (55). Sensing of pneumococci by TLR2 is required to induce Th17 responses, which lead to macrophage recruitment into the nasopharynx. These macrophages are the effector cells of pneumococcal clearance, as demonstrated by depletion experiments with clodronate liposomes (132). Th17 and macrophage responses were also dependent on intracellular signaling through Nod2, which additionally led to production of the macrophage/monocyte chemoattractant CCL2 (24). CCL2 sensing by its receptor CCR2 led to increased clearance of carriage. Pneumolysin pore formation is also required in vivo for Ccl2 expression, suggesting a potential mechanism for access of pneumococcal products to cytosolic sensors such as Nod2. Lysozyme, which digests peptidoglycan to generate fragments for Nod2-dependent signaling, was also required for these effects on clearance (24). Pneumococci have enzymatic mechanisms to limit lysozyme’s effects by modifying its peptidoglycan structure to avoid lysis (23).
Consistent with their resistance to opsonophagocytic killing in the airway, pneumococci are cleared following recognition by nonopsonic receptors. Expression of MARCO (macrophage receptor with collagenous structure), though not directly responsible for pneumococcal uptake by upper respiratory tract macrophages, contributes to monocyte and macrophage recruitment, Ccl2 upregulation, and clearance of pneumococcal carriage (27). Macrophage migration inhibitory factor is also required during pneumococcal colonization to sustain the presence of macrophages, Ccl2 upregulation, and clearance (22). Expression of the microRNA miR-155 also contributes to Th17 induction and macrophage recruitment during pneumococcal carriage (117). B. pertussis protection from whole cell vaccination is also mediated by IL-17 (36), and colonization of baboons, a model that more naturally resembles human infection than do rodents, leads to Th17 induction and downstream effector production, such as neutrophil chemoattractants (124). Acellular pertussis vaccination inducing Th1 instead of Th17 immunity did not protect against carriage in baboons (125), confirming the importance of Th17 responses in clearing B. pertussis.
Although Th17 responses seem to be broadly conserved in clearance of airway colonizers, the effector mechanisms by which IL-17 acts can vary by pathogen. For S. aureus, clearance of carriage required T cells and IL-17A, which promoted recruitment of neutrophils, not macrophages, into the nasal lumen. Neutrophils were required for clearance, though IL-23 was not needed to amplify IL-17A responses, which may have been driven by IL-1β secretion instead (4). Other work demonstrated that unencapsulated S. aureus is cleared faster from the nasopharynx, but only after the first week postinoculation (51). This time point coincides with the influx of neutrophils into the nasopharynx (4), suggesting that the S. aureus capsule resists neutrophil-mediated killing.
Paradoxically, macrophage recruitment, and eventual clearance, requires pneumococcal expression of its toxin, pneumolysin (22). It may seem counterintuitive for a colonizing organism to induce the specific host response that clears it, but it is possible that persistence in the respiratory tract is sacrificed at the expense of inducing a more robust inflammatory response, which could promote nutrient acquisition and transmission to a new host (129). Early during colonization, pneumolysin promotes higher bacterial density (44) and increased neutrophil influx (68) in the nasopharynx, supporting these possibilities.
MODIFIERS: HOST, COMPETITORS, COINFECTIONS
The balance between host and colonizer can be tipped in either direction by a range of factors that modify each of the previously described stages of attachment, growth, and immune evasion. Both congenital and acquired immunodeficiencies can predispose to disease caused by the encapsulated pathogens that colonize the respiratory tract. These conditions, such as deficiencies in signaling downstream of TLRs and IL-1 receptor (IRAK-4 deficiency) or lack of a functional spleen, lead to higher rates of invasive disease, particularly with pneumococci (16). For many of these diseases, the time of greatest susceptibility to invasive pneumococcal disease is early childhood, a period during which >50% of even healthy children are colonized with pneumococci (91). It is unclear, therefore, to what extent the pathways affected by immunodeficiencies that cause excess pneumococcal disease are also responsible for promoting bacterial colonization. In mice, deficiencies in MyD88, a signaling adapter upstream of IRAK-4, caused increased susceptibility to both invasive disease and higher density of pneumococcal carriage in the nasopharynx (2). Less dramatic but still an important determinant of disease is the age of the host. Both colonization and invasive disease occur largely in young children, and mice colonized with pneumococci as infants were impaired in clearing the bacteria (10, 106). In aged mice, pneumococcal clearance was slower than in young adult mice and was associated with decreased influx of macrophages, the effector cells of clearance (53).
Opportunistic pathogens colonizing the respiratory tract compete with a range of other bacteria, starting with the preexisting commensal flora. Although the mechanisms remain unknown, bacteria adapted to particular hosts seem to be more capable of displacing that host’s microbiota. For example, B. bronchiseptica, which can naturally colonize mice, could replace the culturable mouse nasopharyngeal flora after inoculation of <100 colony-forming units. In contrast, stable colonization of mice with B. pertussis, a human-adapted pathogen, required a more than 100-fold higher inoculum and still could not displace the indigenous flora. However, local antibiotic treatment to deplete commensal bacteria lowered the required infectious dose of B. pertussis to <100 colony-forming units; still, the presence of one species of mouse-commensal bacteria could inhibit B. pertussis colonization even in antibiotic-treated animals (131). At least one commensal bacterium has the ability to fight back against opportunistic colonizers. Staphylococcus epidermidis can secrete a serine protease, Esp, and doing so allows S. epidermidis to inhibit S. aureus carriage (38), possibly by inhibiting biofilm formation or cleaving adhesins. In experimental colonization of humans, preexisting S. aureus carriage was cleared by inoculating participants with Esp-expressing S. epidermidis or purified Esp protease (38).
In some instances, opportunistic colonizers pose a competitive threat to other colonizers. Pneumococcal carriage is associated with a lower risk of S. aureus colonization in children (98). These two species occupy different niches within the airway (the posterior nasopharynx and anterior nares, respectively), suggesting that these bacteria may not directly compete. In vivo evidence suggests that the host immune system mediates competition (54). Prior pneumococcal colonization of mice protected against secondary challenge with S. aureus, in an antibody-dependent manner. Pneumococcal colonization elicited antibodies that cross-react with S. aureus, and antibodies to a specific pneumococcal protein that could also target a homologous S. aureus protein were necessary and sufficient to protect against S. aureus carriage (54).
Another immunologic mechanism has been described as mediating the negative interaction between colonizing pathogens. Cocolonization of mice with H. influenzae and pneumococci led to synergistic increases in neutrophil chemoattractant production and neutrophil influxes, in a manner dependent on production of the pneumococcal pore-forming toxin pneumolysin (97). This increased recruitment of neutrophils was required, along with complement, for opsonophagocytic clearance of pneumococci during cocolonization, while H. influenzae persisted (58). Neutrophils had to be activated by Nod1-mediated recognition of H. influenzae peptidoglycan to promote pneumococcal clearance (57). One colonizing organism (H. influenzae) was therefore able to effectively outcompete another by inducing an immune response that it could evade—using the host to target the other organism rather than doing so directly. This process has been referred to as within-host competition (12).
Direct interactions can occur within one species as well. For instance, pneumococci express bacteriocins in vivo and can use these antimicrobial peptides to outcompete pneumococcal strains that lack a cognate immunity protein (25). Pneumococci can also benefit from other cocolonizing strains, however, due to their natural transformability. Rates of transformation were ~107-fold higher during murine colonization than during planktonic growth in sepsis (67). Transfer of antibiotic resistance occurred by homologous gene transformation even without selective pressure from antibiotic exposure, suggesting that colonization provides pneumococci with ample opportunity to obtain new genetic material and benefit from cocolonization with the same or closely related species (67).
Coinfection with viral pathogens also influences bacterial colonization of the airway. The best understood of these interactions is influenza infection predisposing to pneumococcal disease (69). Multiple mechanisms have been proposed for how influenza increases susceptibility to pneumococcal pneumonia, with more limited work addressing how influenza might alter colonization, the prerequisite to pneumonia. Early work focused on influenza leading to a denuded airway epithelium, promoting increased bacterial adherence (37, 94), but increased adherence is not required for influenza to promote secondary bacterial disease (73). Nakamura et al. (78) provided an immunologic explanation for this interaction, finding that coinfection leads to synergistic increases in type I interferons that decrease CCL2 induction and prevent macrophage recruitment to the upper respiratory tract, limiting pneumococcal clearance. Influenza can also provide a nutrient source to colonizing pneumococci (107). Host recognition of influenza leads to increased secretion of Muc5ac, an airway mucin that is heavily sialylated. Pneumococci exploit this influenza-provided sialic acid from mucins to accelerate growth in vivo (107). Influenza also predisposes to murine colonization with H. influenzae (37) and S. aureus (74), the two most common pathogens of postinfluenza disease, after pneumococci (69).
PROGRESSION TO DISEASE
Potentially pathogenic bacteria that first colonize the airway can cause a range of local and systemic disease, although this is not required to establish carriage. Local and systemic disease do not promote spread to new susceptible hosts and therefore do not exert selective pressure on these organisms (45, 129). In contrast, the demands of colonization lead to the development of virulence factors that contribute to disease (129).
Local spread within the respiratory tract can extend into normally sterile sites, including the middle ear spaces, sinuses, and lungs. Bacteria colonizing the nasopharynx can be aspirated into the lower respiratory tract, and higher density of pneumococcal carriage has been associated with increased risk of pneumonia (3). It was not clear in these human studies, however, whether the increased bacterial load in the upper airway preceded or followed pneumonia. Experimental colonization of mice followed by bronchoalveolar lavage revealed correlation between higher nasopharyngeal density and increased numbers of bacteria in the lungs (107). Influenza infection also predisposes to pneumococcal pneumonia by increasing bacterial adherence and inhibiting antibacterial innate immune defenses in the lung (69, 73).
Invasion directly from the colonizing reservoir in the nasopharynx occurs via common pathways. TLR-mediated recognition of pneumococci and H. influenzae leads to p38 MAPK and TGF-β activity, which decreases claudin expression and promotes epithelial opening, promoting bacterial translocation (18). The transcriptional repressor SNAIL1 is induced by TLR2 and TLR4 stimulation and downregulates claudin expression (8). LPS signaling through TLR4, even without bacterial colonization, is sufficient to promote serum leakage onto the mucosal surface (8). Host sensing of bacterial colonization promotes an inflammatory response that requires epithelial opening to allow professional phagocytes to enter the nasopharyngeal lumen. Encapsulated pathogens that survive once accessing the systemic circulation can exploit this patterned host response (18). Some pathogens have more specific invasion strategies, such as S. pyogenes, which transits the epithelium through M cells—specialized cells that sample the lumen for antigens (87). Invasion is not limited to pathogens, however, as viable Lactobacillus murinus can be found in the nasal-associated lymphoid tissue (NALT). This commensal is found at lower rates than S. pyogenes, however, implying either that pathogens translocate more efficiently or persist in sterile sites more readily, or both (19). In infant rats subjected to intranasal challenge, a single surviving organism of H. influenzae type b was sufficient to cause bacteremia (63). Accessing the intracellular space can also promote persistent colonization because the organism avoids immune-mediated clearance (109). Viable NTHi can be found in human adenoid tissue and bronchial epithelium samples (109).
TRANSMISSION
Comparatively little is known about the mechanisms underlying transmission of bacteria in the upper respiratory tract. Spread from the reservoir of bacteria in the nasopharynx to a susceptible host is a necessary aspect of the biology of those microbes that can only grow within a host. For common respiratory tract colonizers, survival on environmental surfaces can be as brief as 1 day, such as with pneumococci, or as long as 7 months, as with S. aureus (52). The evolution of bacteria that depend on animal hosts, therefore, is likely driven in large part by the demands and constraints of transmission. Only recently have animal models been developed to address this hypothesis.
Although rodent models have been informative about mechanisms of bacterial colonization and disease in the upper and lower airway, they have been less informative in studies of transmission. This failure in part stems from the larger bacterial inocula needed to establish colonization in some rodent models of human pathogens compared with natural infection. Mouse models of transmission of respiratory pathogens are particularly limited, however, by a lack of effective cough reflex, hampering airborne transmission that largely occurs by contact with bacteria-containing droplets (75, 77). Recent work has relied on influenza coinfection to potentiate pneumococcal transmission within litters of infant mice (26). In this model, influenza infection leads to spread of established pneumococcal colonization to new naive infant hosts, a process that occurs over a few days (26). In one set of studies, influenza increased pneumococcal titers in the directly inoculated donor, or index, mice (26), and increasing these bacterial numbers by neutrophil depletion was sufficient to promote transmission to influenza-infected recipient, or contact, mice (105). Earlier, more observational transmission models in infant rodents for pneumococci (61) and H. influenzae (32) suggested that influenza was not absolutely required for transmission. More recent studies have suggested that influenza coinfection leads to increased transmission not because of higher bacterial titers in the donor mice but rather because of increased shedding from donor mice (99).
Studies in rodents have focused on transmission among litters of suckling infants, a scenario less relevant to human disease. H. influenzae, however, was also transmitted among weaned rats, demonstrating that the presence of the dam is not required for at least some transmission models (32). Other studies have physically separated donor and recipient animals, demonstrating that airborne transmission can occur without direct contact (11, 70). However, even for respiratory pathogens, direct contact with bacteria-containing droplets may not always be required for transmission, as desiccated pneumococci were recovered and successfully used to colonize mice up to 4 weeks after drying on an environmental surface (122).
Unlike mice, ferrets do sneeze, allowing for airborne transmission. In a ferret model of coinfection influenza promoted increased pneumococcal colonization and transmission and potentiated transmission over longer distances (70). Higher bacterial density in the donor ferrets was not sufficient for increased transmission, as influenza infection also increased the colonization density of a different pneumococcal strain that was not transmitted to recipient ferrets (70).
Host pattern recognition receptors have also been implicated in transmission, as Tlr2−/− mice had increased pneumococcal transmission during influenza coinfection and Tlr4−/− mice demonstrated increased B. bronchiseptica transmission (99, 100). TLR expression in donor animals limited influenza titers and bacterial shedding, and its absence was required for high levels of transmission (99, 100).
The inflammatory response to colonization and coinfection stimulates transmission, though precisely how is not yet understood. Inflammation from LPS inoculation could substitute for influenza infection in recipient, but not donor, mice to promote transmission (105). In another model, TLR2 deficiency that led to increased transmission was associated with increased bacterial shedding, a more robust neutrophil influx, and higher induction of the secreted airway mucin Muc5ac (99). Increased mucin expression suggested a potential mechanism underlying inflammation-induced transmission. In ferrets, influenza also promoted increased mucus secretions (70). Neutrophil influx to the nasopharyngeal lumen resulted in increased mucus secretion in vivo (33), which could facilitate bacterial shedding and spreading to new hosts. In B. bronchiseptica transmission in pigs, however, expression of the type III secretion system, which induces inflammation, was not required for transmission, suggesting that perhaps not all bacteria take advantage of inflammation for transmission (83).
Bacterial factors underlying transmission are largely unknown. Using pigs as a natural model, one study found that, surprisingly, transition between Bvg+ and Bvg− phases was not required for B. bronchiseptica transmission, as Bvg+ phase–locked strains transmitted as well as wild-type bacteria (82). It is possible that even this natural model of transmission did not accurately reflect the time Bordetella must survive in the environment between hosts, a niche that would require switching to the Bvg− phase (82). In S. pyogenes, inactivation of the covR/S two-component regulatory system resulted in decreased transmission, likely at the level of acquisition by the recipient mice (1).
A new model of Bordetella transmission by droplet employs baboons and B. pertussis and recapitulates the essential clinical features of human pertussis infection (123). This model was used to demonstrate that acellular pertussis immunizations protect from infection, but not colonization or transmission, a finding with important implications for vaccine design and public health (125).
CONCLUSIONS
After being shed from a prior host, colonizing opportunistic pathogens enter the nasopharynx, where they escape from mucus and attach to the epithelial lining. These bacteria must obtain a source of nutrition on the spare mucosal surface in order to replicate, and they use multiple strategies to evade host clearance. The bacterial factors elaborated during colonization can also predispose the host to invasive disease. Ultimately, colonizing organisms that can adhere, grow, and evade clearance will transmit to a new susceptible host.
Many, and sometimes all, of these respiratory colonizers share similar strategies for each stage of colonization. Phase variation of capsule influences acquisition, immune evasion, invasion, and potentially survival outside the host during transmission (59, 81, 122). Host mimicry with phosphorylcholine modifies the cell surface of many airway pathogens and is crucial for attachment and immune evasion (17, 109). The mucosal immune responses to these pathogens have commonalities, reflected by the presence of an IgA1 protease in multiple microbes, as well as the Th17 response many elicit (4, 40, 132). Bacteria often produce toxins to target the host and typically benefit from coinfection with respiratory viruses, particularly influenza (5, 45, 69). Both reflect the effects of inducing inflammation at the expense of the commensal flora. If colonizing bacteria are resistant to its effects, inflammation promotes every stage of colonization, from upregulating receptors for adherence to providing a source of nutrition, misdirecting the immune response, increasing the rate of invasion, and facilitating transmission (9, 99, 104, 129).
The relevance of inflammation to each stage of carriage suggests that potentially pathogenic colonizers may have evolved to induce inflammation to drive colonization and transmission (12). Colonization, rather than invasive disease, is the source of selective pressure on these organisms and guides development of what should be termed colonization factors rather than virulence factors. These factors, including capsule, IgA1 protease, and pore-forming toxins, can be expressed by commensal bacteria that are rarely pathogenic, such as Streptococcus mitis (47). Further study is required to understand which combination of virulence activities separates the potentially pathogenic from the nonpathogenic colonizers. Future work should also focus on understanding the relationship between virulence or colonization factors and transmission, the end point of carriage. The key to understanding invasive disease due to these opportunistic pathogens lies in colonization.
SUMMARY POINTS.
Colonization of the upper airway is the prerequisite to invasive disease by many respiratory tract pathogens.
Colonization drives evolution of virulence factors that cause disease.
Colonizing bacteria must adhere to the mucosal surface, obtain nutrients for growth, evade host immunity, and transmit to a new host.
The stages of adherence are associating with mucus, forming weak interactions with host carbohydrates, and strong binding to host surface proteins.
Phase variation allows for rapid diversity generation within a bacterial population and promotes adherence and immune evasion.
Bacterial polysaccharide capsules have multiple roles in adherence and evasion of host immune responses.
Host inflammation can mediate bacterial competition.
Opportunistic pathogens exploit and induce host inflammation for nutrient sources, to evade sterilizing immunity and for transmission.
FUTURE ISSUES.
What separates potentially virulent from nonvirulent colonizers of the respiratory tract?
How does the host distinguish between commensals and pathogens that colonize the respiratory tract?
What determines the host range for respiratory tract colonizers?
Why does invasive disease develop in only a small proportion of colonized individuals?
Are virulence factors required for transmission?
What selective pressure does transmission exert on respiratory tract colonizers?
Can inflammation benefit commensal organisms, or only potentially pathogenic ones?
Do all respiratory tract opportunistic colonizers take advantage of inflammation to promote colonization and/or transmission?
Acknowledgments
J.N.W. received NIH grants AI038446 and AI105168. S.J.S. received NIH grant HL119030.
Glossary
- Pneumonia
inflammation of the lung alveoli and interstitial spaces caused by viral or bacterial infection
- Opportunistic pathogen
organism that does not productively infect all hosts but can take advantage of immunocompromised states to cause disease
- Colonization
the presence of a microbe on a host mucosal surface that does not cause disease in the carrier host
- Upper respiratory tract
the conducting airways of the respiratory tract from the nasal cavity, through the nasopharynx, to the larynx
- Commensal
an organism that takes advantage of a host without adverse effects
- Mucus
protective barrier produced by mucosal surfaces and composed of hydrated glycoproteins, other proteins, and small molecules
- Capsule
large polysaccharide structure that is the outermost layer of some bacteria
- Phase variation
rapid on-off switching of expression of different proteins to generate diversity in a population without mutation
- Adhesin
bacterial protein or carbohydrate that binds host structures to promote bacterial adherence
- Sialic acid
a family of nine-carbon sugars that commonly occupy the terminal ends of host glycoconjugates on cell surfaces
- Nutritional immunity
preventing pathogen access to essential nutrients; first used to describe iron-limiting mechanisms
- NTHi (nontypeable Haemophilus influenzae)
unencapsulated strains and foremost causes of H. influenzae disease after vaccination against encapsulated type b strains
- Th17 immunity
immunity directed by the cytokine IL-17 that promotes mucosal barrier function, including phagocyte recruitment
- Within-host competition
competition between bacteria that requires the presence of the host to mediate the negative interaction
- Bacteriocins
bacterial antimicrobial peptides that target the same or similar species
- Inflammation
patterned host responses to a range of potentially harmful stimuli; the body’s attempts to return to a baseline state
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Steven J. Siegel, Email: siegelst@mail.med.upenn.edu.
Jeffrey N. Weiser, Email: jeffrey.weiser@nyumc.org.
LITERATURE CITED
- 1.Alam FM, Turner CE, Smith K, Wiles S, Sriskandan S. Inactivation of the CovR/S virulence regulator impairs infection in an improved murine model of Streptococcus pyogenes naso-pharyngeal infection. PLOS ONE. 2013;8(4):e61655. doi: 10.1371/journal.pone.0061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albiger B, Sandgren A, Katsuragi H, Meyer-Hoffert U, Beiter K, et al. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol. 2005;7(11):1603–15. doi: 10.1111/j.1462-5822.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 3.Albrich WC, Madhi SA, Adrian PV, van Niekerk N, Mareletsi T, et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis. 2012;54(5):601–9. doi: 10.1093/cid/cir859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer NK, Harro JM, Shirtliff ME. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect Immun. 2013;81(6):2070–75. doi: 10.1128/IAI.00084-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol. 2010;87(2):213–22. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barocchi MA, Ries J, Zogaj X, Hemsley C, Albiger B, et al. A pneumococcal pilus influences virulence and host inflammatory responses. PNAS. 2006;103(8):2857–62. doi: 10.1073/pnas.0511017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baur S, Rautenberg M, Faulstich M, Grau T, Severin Y, et al. A nasal epithelial receptor for Staphylococcus aureus WTA governs adhesion to epithelial cells and modulates nasal colonization. PLOS Pathog. 2014;10(5):e1004089. doi: 10.1371/journal.ppat.1004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beisswenger C, Lysenko ES, Weiser JN. Early bacterial colonization induces Toll-like receptor-dependent transforming growth factor βsignaling in the epithelium. Infect Immun. 2009;77(5):2212–20. doi: 10.1128/IAI.01224-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogaert D, De Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54. doi: 10.1016/S1473-3099(04)00938-7. Reviews the importance of pneumococcal colonization in promoting invasive infection. [DOI] [PubMed] [Google Scholar]
- 10.Bogaert D, Weinberger D, Thompson C, Lipsitch M, Malley R. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect Immun. 2009;77(4):1613–22. doi: 10.1128/IAI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockmeier SL, Lager KM. Experimental airborne transmission of porcine reproductive and respiratory syndrome virus and Bordetella bronchiseptica. Vet Microbiol. 2002;89(4):267–75. doi: 10.1016/s0378-1135(02)00204-3. [DOI] [PubMed] [Google Scholar]
- 12.Brown SP, Le Chat L, Taddei F. Evolution of virulence: Triggering host inflammation allows invading pathogens to exclude competitors. Ecol Lett. 2008;11(1):44–51. doi: 10.1111/j.1461-0248.2007.01125.x. Uses ecological theory to explain how host inflammation can mediate within-host bacterial competition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckwalter CM, King SJ. Pneumococcal carbohydrate transport: food for thought. Trends Microbiol. 2012;20(11):517–22. doi: 10.1016/j.tim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burian M, Rautenberg M, Kohler T, Fritz M, Krismer B, et al. Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis. 2010;201(9):1414–21. doi: 10.1086/651619. [DOI] [PubMed] [Google Scholar]
- 15.Burian M, Wolz C, Goerke C. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLOS ONE. 2010;5(4):e10040. doi: 10.1371/journal.pone.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bustamante J, Zhang S-Y, von Bernuth H, Abel L, Casanova J-L. From infectious diseases to primary immunodeficiencies. Immunol Allergy Clin North Am. 2008;28(2):235–58. doi: 10.1016/j.iac.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Clark SE, Snow J, Li J, Zola TA, Weiser JN. Phosphorylcholine allows for evasion of bactericidal antibody by Haemophilus influenzae. PLOS Pathog. 2012;8(3):e1002521. doi: 10.1371/journal.ppat.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke TB, Francella N, Huegel A, Weiser JN. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe. 2011;9(5):404–14. doi: 10.1016/j.chom.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costalonga M, Cleary PP, Fischer LA, Zhao Z. Intranasal bacteria induce Th1 but not Treg or Th2. Mucosal Immunol. 2009;2(1):85–95. doi: 10.1038/mi.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, et al. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66(12):5921–29. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cywes C, Stamenkovic I, Wessels MR. CD44 as a receptor for colonization of the pharynx by group A Streptococcus. J Clin Investig. 2000;106(8):995–1002. doi: 10.1172/JCI10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das R, LaRose MI, Hergott CB, Leng L, Bucala R, Weiser JN. Macrophage migration inhibitory factor promotes clearance of pneumococcal colonization. J Immunol. 2014;193(2):764–72. doi: 10.4049/jimmunol.1400133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis KM, Akinbi HT, Standish AJ, Weiser JN. Resistance to mucosal lysozyme compensates for the fitness deficit of peptidoglycan modifications by Streptococcus pneumoniae. PLOS Pathog. 2008;4(12):e1000241. doi: 10.1371/journal.ppat.1000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Investig. 2011;121(9):3666–76. doi: 10.1172/JCI57761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawid S, Roche AM, Weiser JN. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun. 2007;75(1):443–51. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diavatopoulos DA, Short KR, Price JT, Wilksch JJ, Brown LE, et al. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J. 2010;24(6):1789–98. doi: 10.1096/fj.09-146779. Reports an infant mouse model of pneumococcal transmission during influenza coinfection that can be manipulated. [DOI] [PubMed] [Google Scholar]
- 27.Dorrington MG, Roche AM, Chauvin SE, Tu Z, Mossman KL, et al. MARCO is required for TLR2- and Nod2-mediated responses to Streptococcus pneumoniae and clearance of pneumococcal colonization in the murine nasopharynx. J Immunol. 2013;190(1):250–58. doi: 10.4049/jimmunol.1202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Exley RM, Goodwin L, Mowe E, Shaw J, Smith H, et al. Neisseria meningitidis lactate permease is required for nasopharyngeal colonization. Infect Immun. 2005;73(9):5762–66. doi: 10.1128/IAI.73.9.5762-5766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–47. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Zorn B, Senna JPM, Fiette L, Shorte S, Testard A, et al. Bacterial and host factors implicated in nasal carriage of methicillin-resistant Staphylococcus aureus in mice. Infect Immun. 2005;73(3):1847–51. doi: 10.1128/IAI.73.3.1847-1851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray BM, Converse GM, Dillon HC. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142(6):923–33. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 32.Halsey NA, Korock C, Johansen TL, Glode MP. Intralitter transmission of Haemophilus influenzae type B in infant rats and rifampin eradication of nasopharyngeal colonization. J Infect Dis. 1980;142(5):739–43. doi: 10.1093/infdis/142.5.739. [DOI] [PubMed] [Google Scholar]
- 33.Harkema JR, Hotchkiss JA, Harmsen AG, Henderson RF. In vivo effects of transient neutrophil influx on nasal respiratory epithelial mucosubstances: quantitative histochemistry. Am J Pathol. 1988;130(3):605–15. [PMC free article] [PubMed] [Google Scholar]
- 34.Harvill ET, Cotter PA, Yuk MH, Miller JF. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect Immun. 1999;67(3):1493–500. doi: 10.1128/iai.67.3.1493-1500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendriksen WT, Bootsma HJ, Estevão S, Hoogenboezem T, de Jong A, et al. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J Bacteriol. 2008;190(2):590–601. doi: 10.1128/JB.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins SC, Jarnicki AG, Lavelle EC, Mills KHG. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177(11):7980–89. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 37.Hirano T, Kurono Y, Ichimiya I, Suzuki M, Mogi G. Effects of influenza A virus on lectin-binding patterns in murine nasopharyngeal mucosa and on bacterial colonization. Otolaryngol Head Neck Surg. 1999;121(5):616–21. doi: 10.1016/S0194-5998(99)70068-9. [DOI] [PubMed] [Google Scholar]
- 38.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465(7296):346–49. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 39.Iyer R, Camilli A. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol Microbiol. 2007;66(1):1–13. doi: 10.1111/j.1365-2958.2007.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janoff EN, Rubins JB, Fasching C, Charboneau D, Rahkola JT, et al. Pneumococcal IgA1 protease subverts specific protection by human IgA1. Mucosal Immunol. 2014;7(2):249–56. doi: 10.1038/mi.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensch I, Gámez G, Rothe M, Ebert S, Fulde M, et al. PavB is a surface-exposed adhesin of Strep-tococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol Microbiol. 2010;77(1):22–43. doi: 10.1111/j.1365-2958.2010.07189.x. [DOI] [PubMed] [Google Scholar]
- 42.Jurcisek JA, Bookwalter JE, Baker BD, Fernandez S, Novotny LA, et al. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol. 2007;65(5):1288–99. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 43.Kadioglu A, Brewin H, Härtel T, Brittan JL, Klein M, et al. Pneumococcal protein PavA is important for nasopharyngeal carriage and development of sepsis. Mol Oral Microbiol. 2010;25(1):50–60. doi: 10.1111/j.2041-1014.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 44.Kadioglu A, Taylor S, Iannelli F, Pozzi G, Mitchell TJ, Andrew PW. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect Immun. 2002;70(6):2886–90. doi: 10.1128/IAI.70.6.2886-2890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6(4):288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 46.Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14(2):218–24. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilian M, Poulsen K, Blomqvist T, Håvarstein LS, Bek-Thomsen M, et al. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLOS ONE. 2008;3(7):e2683. doi: 10.1371/journal.pone.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177(2):368–77. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 49.King SJ, Hippe KR, Gould JM, Bae D, Peterson S, et al. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol Microbiol. 2004;54(1):159–71. doi: 10.1111/j.1365-2958.2004.04252.x. [DOI] [PubMed] [Google Scholar]
- 50.Kirimanjeswara GS, Mann PB, Harvill ET. Role of antibodies in immunity to Bordetella infections. Infect Immun. 2003;71(4):1719–24. doi: 10.1128/IAI.71.4.1719-1724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiser KB, Cantey-Kiser JM, Lee JC. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun. 1999;67(10):5001–6. doi: 10.1128/iai.67.10.5001-5006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krone CL, Trzciński K, Zborowski T, Sanders EAM, Bogaert D. Impaired innate mucosal immunity in aged mice permits prolonged Streptococcus pneumoniae colonization. Infect Immun. 2013;81(12):4615–25. doi: 10.1128/IAI.00618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lijek RS, Luque SL, Liu Q, Parker D, Bae T, Weiser JN. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. PNAS. 2012;109(34):13823–28. doi: 10.1073/pnas.1208075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y-J, Gross J, Bogaert D, Finn A, Bagrade L, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLOS Pathog. 2008;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luke NR, Jurcisek JA, Bakaletz LO, Campagnari AA. Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect Immun. 2007;75(12):5559–64. doi: 10.1128/IAI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lysenko ES, Clarke TB, Shchepetov M, Ratner AJ, Roper DI, et al. Nod1 signaling overcomes resistance of S. pneumoniae to opsonophagocytic killing. PLOS Pathog. 2007;3(8):e118. doi: 10.1371/journal.ppat.0030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLOS Pathog. 2005;1(1):e1. doi: 10.1371/journal.ppat.0010001. Shows how host inflammation can control immune-mediated competition between bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun. 2001;69(6):3755–61. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. PNAS. 2003;100(4):1966–71. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malley R, Stack AM, Ferretti ML, Thompson CM, Saladino RA. Anticapsular polysaccharide antibodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. J Infect Dis. 1998;178(3):878–82. doi: 10.1086/597600. [DOI] [PubMed] [Google Scholar]
- 62.Manso AS, Chai MH, Atack JM, Furi L, De Ste Croix M, et al. A random six-phase switch regulates pneumococcal virulence via global epigenetic changes. Nat Commun. 2014;5:5055. doi: 10.1038/ncomms6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margolis E, Levin BR. Within-host evolution for the invasiveness of commensal bacteria: an experimental study of bacteremias resulting from Haemophilus influenzae nasal carriage. J Infect Dis. 2007;196(7):1068–75. doi: 10.1086/520934. [DOI] [PubMed] [Google Scholar]
- 64.Marion C, Aten AE, Woodiga SA, King SJ. Identification of an ATPase, MsmK, which energizes multiple carbohydrate ABC transporters in Streptococcus pneumoniae. Infect Immun. 2011;79(10):4193–200. doi: 10.1128/IAI.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marion C, Burnaugh AM, Woodiga SA, King SJ. Sialic acid transport contributes to pneumococcal colonization. Infect Immun. 2011;79(3):1262–69. doi: 10.1128/IAI.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marion C, Stewart JM, Tazi MF, Burnaugh AM, Linke CM, et al. Streptococcus pneumoniae can utilize multiple sources of hyaluronic acid for growth. Infect Immun. 2012;80(4):1390–98. doi: 10.1128/IAI.05756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marks LR, Reddinger RM, Hakansson AP. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. mBio. 2012;3(5):e00200–12. doi: 10.1128/mBio.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J Immunol. 2008;180(9):6246–54. doi: 10.4049/jimmunol.180.9.6246. [DOI] [PubMed] [Google Scholar]
- 69.McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12(4):252–62. doi: 10.1038/nrmicro3231. A comprehensive review of the synergy between influenza and respiratory pathogens. [DOI] [PubMed] [Google Scholar]
- 70.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques-Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis. 2010;202(8):1287–95. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, et al. A molecular mechanism for bacterial susceptibility to zinc. PLOS Pathog. 2011;7(11):e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melvin JA, Scheller EV, Miller JF, Cotter PA. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol. 2014;12(4):274–88. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Metzger DW, Sun K. Immune dysfunction and bacterial coinfections following influenza. J Immunol. 2013;191(5):2047–52. doi: 10.4049/jimmunol.1301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mina MJ, McCullers JA, Klugman KP. Live attenuated influenza vaccine enhances colonization of Streptococcus pneumoniae and Staphylococcus aureus in mice. mBio. 2014;5(1):e01040–13. doi: 10.1128/mBio.01040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L387–98. doi: 10.1152/ajplung.00330.2007. [DOI] [PubMed] [Google Scholar]
- 76.Mulcahy ME, Geoghegan JA, Monk IR, O’Keeffe KM, Walsh EJ, et al. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLOS Pathog. 2012;8(12):e1003092. doi: 10.1371/journal.ppat.1003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musher DM. How contagious are common respiratory tract infections? N Engl J Med. 2003;348(13):1256–66. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Investig. 2011;121(9):3657–65. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelson AL, Barasch JM, Bunte RM, Weiser JN. Bacterial colonization of nasal mucosa induces expression of siderocalin, an iron-sequestering component of innate immunity. Cell Microbiol. 2005;7(10):1404–17. doi: 10.1111/j.1462-5822.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- 80.Nelson AL, Ries J, Bagnoli F, Dahlberg S, Fälker S, et al. RrgA is a pilus-associated adhesin in Streptococcus pneumoniae. Mol Microbiol. 2007;66(2):329–40. doi: 10.1111/j.1365-2958.2007.05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007;75(1):83–90. doi: 10.1128/IAI.01475-06. Showed that the importance of pneumococcal capsule during colonization is to evade mucus, not opsonophagocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicholson TL, Brockmeier SL, Loving CL, Register KB, Kehrli ME, et al. Phenotypic modulation of the virulent Bvg phase is not required for pathogenesis and transmission of Bordetella bronchiseptica in swine. Infect Immun. 2012;80(3):1025–36. doi: 10.1128/IAI.06016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nicholson TL, Brockmeier SL, Loving CL, Register KB, Kehrli ME, Shore SM. The Bordetella bronchiseptica type III secretion system is required for persistence and disease severity but not transmission in swine. Infect Immun. 2014;82(3):1092–103. doi: 10.1128/IAI.01115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 85.Ogunniyi AD, LeMessurier KS, Graham RMA, Watt JM, Briles DE, et al. Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun. 2007;75(4):1843–51. doi: 10.1128/IAI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pancotto L, De Angelis G, Bizzarri E, Barocchi MA, Del Giudice G, et al. Expression of the Streptococcus pneumoniae pilus-1 undergoes on and off switching during colonization in mice. Sci Rep. 2013;3:2040. doi: 10.1038/srep02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park H-S, Francis KP, Yu J, Cleary PP. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J Immunol. 2003;171(5):2532–37. doi: 10.4049/jimmunol.171.5.2532. [DOI] [PubMed] [Google Scholar]
- 88.Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, et al. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio. 2011;2(3):e00016–11. doi: 10.1128/mBio.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pezzulo AA, Gutiérrez J, Duschner KS, McConnell KS, Taft PJ, et al. Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLOS ONE. 2011;6(1):e16166. doi: 10.1371/journal.pone.0016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Philips BJ, Meguer J-X, Redman J, Baker EH. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29(12):2204–10. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 91.Picard C, Puel A, Bustamante J, Ku C-L, Casanova J-L. Primary immunodeficiencies associated with pneumococcal disease. Curr Opin Allergy Clin Immunol. 2003;3(6):451–59. doi: 10.1097/00130832-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Pilione MR, Pishko EJ, Preston A, Maskell DJ, Harvill ET. PagP is required for resistance to antibody-mediated complement lysis during Bordetella bronchiseptica respiratory infection. Infect Immun. 2004;72(5):2837–42. doi: 10.1128/IAI.72.5.2837-2842.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plaut AG. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–22. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- 94.Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986;134(5):1040–44. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 95.Poole J, Foster E, Chaloner K, Hunt J, Jennings MP, et al. Analysis of nontypeable Haemophilus influenzae phase-variable genes during experimental human nasopharyngeal colonization. J Infect Dis. 2013;208(5):720–27. doi: 10.1093/infdis/jit240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pynnonen M, Stephenson RE, Schwartz K, Hernandez M, Boles BR. Hemoglobin promotes Staphylococcus aureus nasal colonization. PLOS Pathog. 2011;7(7):e1002104. doi: 10.1371/journal.ppat.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ratner AJ, Lysenko ES, Paul MN, Weiser JN. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. PNAS. 2005;102(9):3429–34. doi: 10.1073/pnas.0500599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, et al. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA. 2004;292(6):716–20. doi: 10.1001/jama.292.6.716. [DOI] [PubMed] [Google Scholar]
- 99.Richard AL, Siegel SJ, Erikson J, Weiser JN. TLR2 signaling decreases transmission of Streptococcus pneumoniae by limiting bacterial shedding in an infant mouse influenza A co-infection model. PLOS Pathog. 2014;10(8):e1004339. doi: 10.1371/journal.ppat.1004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rolin O, Smallridge W, Henry M, Goodfield L, Place D, Harvill ET. Toll-like receptor 4 limits transmission of Bordetella bronchiseptica. PLOS ONE. 2014;9(1):e85229. doi: 10.1371/journal.pone.0085229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanford BA, Thomas VL, Ramsay MA. Binding of staphylococci to mucus in vivo and in vitro. Infect Immun. 1989;57(12):3735–42. doi: 10.1128/iai.57.12.3735-3742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shelburne SA, Okorafor N, Sitkiewicz I, Sumby P, Keith D, et al. Regulation of polysaccharide utilization contributes to the persistence of group A streptococcus in the oropharynx. Infect Immun. 2007;75(6):2981–90. doi: 10.1128/IAI.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shelburne SA, Sumby P, Sitkiewicz I, Okorafor N, Granville C, et al. Maltodextrin utilization plays a key role in the ability of group A Streptococcus to colonize the oropharynx. Infect Immun. 2006;74(8):4605–14. doi: 10.1128/IAI.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Short KR, Reading PC, Brown LE, Pedersen J, Gilbertson B, et al. Influenza-induced inflammation drives pneumococcal otitis media. Infect Immun. 2013;81(3):645–52. doi: 10.1128/IAI.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Short KR, Reading PC, Wang N, Diavatopoulos DA, Wijburg OL. Increased nasopharyngeal bacterial titers and local inflammation facilitate transmission of Streptococcus pneumoniae. mBio. 2012;3(5):e00255–12. doi: 10.1128/mBio.00255-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siegel SJ, Tamashiro E, Weiser JN. Clearance of pneumococcal colonization in infants is delayed through altered macrophage trafficking. PLoS Pathog. 2015;11(6):e1005004. doi: 10.1371/journal.ppat.1005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siegel SJ, Roche AM, Weiser JN. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16(1):55–67. doi: 10.1016/j.chom.2014.06.005. Revealed how bacteria can exploit host inflammation to promote replication by measuring growth directly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh AK, Pluvinage B, Higgins MA, Dalia AB, Woodiga SA, et al. Unravelling the multiple functions of the architecturally intricate Streptococcus pneumoniae β-galactosidase, BgaA. PLOS Pathog. 2014;10(9):e1004364. doi: 10.1371/journal.ppat.1004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.St Geme JW. Molecular and cellular determinants of non-typeable Haemophilus influenzae adherence and invasion. Cell Microbiol. 2002;4(4):191–200. doi: 10.1046/j.1462-5822.2002.00180.x. [DOI] [PubMed] [Google Scholar]
- 110.Tai SS, Lee CJ, Winter RE. Hemin utilization is related to virulence of Streptococcus pneumoniae. Infect Immun. 1993;61(12):5401–5. doi: 10.1128/iai.61.12.5401-5405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293(5529):498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 112.Tong HH, Blue LE, James MA, Demaria TF. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect Immun. 2000;68(2):921–24. doi: 10.1128/iai.68.2.921-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tong HH, Liu X, Chen Y, James M, Demaria T. Effect of neuraminidase on receptor-mediated adherence of Streptococcus pneumoniae to chinchilla tracheal epithelium. Acta Otolaryngol. 2002;122(4):413–19. doi: 10.1080/00016480260000111. [DOI] [PubMed] [Google Scholar]
- 114.Trappetti C, Kadioglu A, Carter M, Hayre J, Iannelli F, et al. Sialic acid: a preventable signal for pneuzmococcal biofilm formation, colonization, and invasion of the host. J Infect Dis. 2009;199(10):1497–505. doi: 10.1086/598483. [DOI] [PubMed] [Google Scholar]
- 115.van Opijnen T, Camilli A. A fine scale phenotype-genotype virulence map of a bacterial pathogen. Genome Res. 2012;22(12):2541–51. doi: 10.1101/gr.137430.112. Tn-seq study to determine the genes necessary for colonization in different host niches. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Rossum AMC, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73(11):7718–26. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verschoor CP, Dorrington MG, Novakowski KE, Kaiser J, Radford K, et al. MicroRNA-155 is required for the clearance of Streptococcus pneumoniae from the nasopharynx. Infect Immun. 2014;82:4824–33. doi: 10.1128/IAI.02251-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vimr E, Lichtensteiger C, Steenbergen S. Sialic acid metabolism’s dual function in Haemophilus influenzae. Mol Microbiol. 2000;36(5):1113–23. doi: 10.1046/j.1365-2958.2000.01925.x. [DOI] [PubMed] [Google Scholar]
- 119.Virtaneva K, Porcella SF, Graham MR, Ireland RM, Johnson CA, et al. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. PNAS. 2005;102(25):9014–19. doi: 10.1073/pnas.0503671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344(1):11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 121.Voss S, Gámez G, Hammerschmidt S. Impact of pneumococcal microbial surface components recognizing adhesive matrix molecules on colonization. Mol Oral Microbiol. 2012;27(4):246–56. doi: 10.1111/j.2041-1014.2012.00654.x. [DOI] [PubMed] [Google Scholar]
- 122.Walsh RL, Camilli A. Streptococcus pneumoniae is desiccation tolerant and infectious upon rehydration. mBio. 2011;2(3):e00092–11. doi: 10.1128/mBio.00092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infect Dis. 2012;206(6):902–6. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol. 2013;6(4):787–96. doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- 125.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. PNAS. 2014;111(2):787–92. doi: 10.1073/pnas.1314688110. Using a novel baboon transmission model, explained the lack of efficacy for acellular pertussis vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10(3):243–45. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 127.Weinberg ED. Iron availability and infection. Biochim Biophys Acta. 2009;1790(7):600–5. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 128.Weiser JN. Relationship between colony morphology and the life cycle of Haemophilus influenzae: the contribution of lipopolysaccharide phase variation to pathogenesis. J Infect Dis. 1993;168(3):672–80. doi: 10.1093/infdis/168.3.672. [DOI] [PubMed] [Google Scholar]
- 129.Weiser JN. The pneumococcus: why a commensal misbehaves. J Mol Med. 2009;88(2):97–102. doi: 10.1007/s00109-009-0557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wertheim HFL, Walsh E, Choudhurry R, Melles DC, Boelens HAM, et al. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLOS Med. 2008;5(1):e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weyrich LS, Feaga HA, Park J, Muse SJ, Safi CY, et al. Resident microbiota affect Bordetella pertussis infectious dose and host specificity. J Infect Dis. 2014;209(6):913–21. doi: 10.1093/infdis/jit597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Investig. 2009;119(7):1899–909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zola TA, Lysenko ES, Weiser JN. Mucosal clearance of capsule-expressing bacteria requires both TLR and nucleotide-binding oligomerization domain 1 signaling. J Immunol. 2008;181(11):7909–16. doi: 10.4049/jimmunol.181.11.7909. Established that macrophages and the Th17 pathway are critical for clearing pneumococcal colonization in mice. [DOI] [PubMed] [Google Scholar]