Abstract
Background
Several recent studies have investigated the relationship between telomere length and depression with inconsistent results. This meta-analysis examined whether telomere length and depression are associated and explored factors that might affect this association.
Methods
Studies measuring telomere length in subjects with clinically significant unipolar depression were included. A comprehensive search strategy identified studies in PubMed, MEDLINE, PsycINFO, Global Health, The Cochrane Library, and Web of Science. A structured data abstraction form was used and studies were appraised for inclusion or exclusion using a priori conditions. Analyses were conducted using standardized mean differences in a continuous random effects model.
Results
Thirty-eight studies (N = 34,347) met the inclusion criteria. The association between depression and telomere length was significant, with a Cohen's d effect size of -0.205 (p < 0.0001, I2 = 42%). Depression severity significantly associated with telomere length (p = 0.03). Trim and fill analysis indicated the presence of publication bias (p = 0.003), but that the association remained highly significant after accounting for the bias. Subgroup analysis revealed depression assessment tools, telomere measurement techniques, source tissue and comorbid medical conditions significantly affected the relationship.
Limitations
Other potentially important sub-groups, including antidepressant use, have not been investigated in sufficient detail or number yet and thus were not addressed in this meta-analysis.
Conclusions
There is a negative association between depression and telomere length. Further studies are needed to clarify potential causality underlying this association and to elucidate the biology linking depression and this cellular marker of stress exposure and aging.
Keywords: Telomere, meta-analysis, depression, major depressive disorder
Introduction
Individuals with major depressive disorder (MDD) have excess morbidity (Young et al., 2014) and mortality (Lou et al., 2014; Young et al., 2014; Zivin et al., 2012) as compared to the general population (Lou et al., 2014). One hypothesis regarding the cause of this excess morbidity and mortality that has gained much attention involves telomere biology. Telomeres are nucleotide sequences consisting of tandem TTAGGG repeats ranging from a few to 15 kilobases in length that provide genomic stability and shorten with each cellular division (Blackburn, 2005). Telomere shortening is strongly associated with age in most somatic tissues (Aubert and Lansdorp, 2008) and is influenced by genetic and epigenetic regulation, as well as by cellular stress and inflammation (Ridout et al., 2015). Conceptualizing chronic disease as a prolonged stress exposure, several studies have reported an association between telomere length and various somatic diseases, such as heart disease (Haycock et al., 2014; Hoen et al., 2011) and diabetes (Zhao et al., 2013). It has been proposed that telomere shortening resulting from chronic stress exposure may be a mechanism of excess morbidity or mortality (Deelen et al., 2014) or a useful indicator of progression of a process of senescence that raises mortality rates by other mechanisms (Ridout et al., 2015).
Simon et al (Simon et al., 2006) examined the relationship between mood disorders and telomere length and found that telomeres were significantly shorter in patients with mood disorders overall (n = 44) and also in the group of subjects with MDD (n = 15). Since this initial study, there have been numerous efforts to replicate these findings, which have variously reported that depression has no effect or is associated with a reduction in telomere length (see Supplementary Table 1 for references). Several factors might influence these divergent findings, including differences in telomere measurement technique, depression assessment method, population of interest, co-existing somatic illness, gender, and age. Additionally, a majority of these studies have had small sample sizes, limiting the power to draw definitive conclusions. One meta-analysis has pointed to an association between depression and telomere length (Schutte and Malouff, 2015). However, on review of the literature 39% more subjects could be included in the present meta-analysis. Additionally, that meta-analysis did not examine how depression severity, duration, tissue source, smoking, or comorbid chronic medical conditions may moderate the association between depression and telomere length. In the present study, we aimed to expand the subjects included by doubling the databases searched and expanding the search terms to capture all relevant articles. Furthermore, we included studies examining telomere length from all tissue sources, including leukocytes, brain tissue, and saliva, and studies of subjects with comorbid medical factors. The objective of this meta-analysis was to clarify the relationship between depression and telomere length by means of a systematic examination of the literature, comparing subjects with MDD to those without, and to identify moderators of this association.
Methods and Materials
Protocol and Registration
A review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42015016812) and conceptualized in October 2013. This study was designed, executed, and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (Liberati et al., 2009).
Study Eligibility Criteria
Human studies of unipolar depression meeting either clinical or rating scale thresholds for MDD and controls not meeting these thresholds were included. Prospective observational and retrospective studies were considered for inclusion. Only studies utilizing validated methods of measuring clinically significant depression and defined techniques to measure and analyze telomeres were included (these are further clarified in the moderator analysis sub-section of the Methods section below); all included studies used appropriate tools and thresholds for measurement of MDD. Studies of bipolar depression were excluded. In the case of reports that contained data from non-independent overlapping data sets, the report with the larger number of subjects was included.
Information Sources and Search Strategy
A comprehensive electronic search strategy in August 2015 identified studies indexed in PubMed, PsycINFO, Global Health, The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register), and Web of Science; no limitations on publication dates were set. The search was performed by two of the investigators with clinical and research experience in the topic of interest (K.K.R and S.J.R.) in consultation with a librarian trained in systematic reviews; both investigators reviewed titles, abstracts, and articles, and disagreements were settled by consensus. The search strategy included terms for MDD (depression, major depressive disorder, depressive episode, mood disorder, and depress*) and telomeres (telomeres, telomerase, and telo*). The search terms were adapted for use with other bibliographic databases in combination with database-specific filters limiting the search to studies published in the English language, where these are available (Supplemental Table 2 provides the full search strings). Additionally, reference lists of primary studies included in this review and the reference lists of relevant, previously published reviews were searched. Studies were appraised for inclusion or exclusion using the a priori criteria described above.
Data Extraction
Data were extracted independently (K.K.R. and S.J.R.) using the predetermined structured form. The extractors were not blinded to the study results, authors, or institutions; inter-rater reliability was high (>95%). Conflicts regarding data extraction were resolved by consensus with a third reviewer (L.H.P and A.R.T.). Data extraction variables included study design, participant clinical descriptions (age, percent subjects of male gender in the study, comorbid chronic medical condition), telomere measurement method and tissue source, telomere length for depressed and comparison subjects, depression measurement method, and measures of depression severity and treatment, in addition to bibliographic information. When possible, telomere length data that were adjusted at least for age and gender were abstracted from the studies rather than the unadjusted values. At the protocol level, study risk of bias was assessed using the guidelines suggested by Cochrane Reviews (Higgins, updated March 2011) and the Agency for Healthcare Research and Quality (Quality, 2015), through the incorporation into the study selection criteria of standard objective markers such as study design and population characteristics, as described above. The Newcastle-Ottawa Scale (NOS) for cross-sectional, case-control, or cohort designs (Stang, 2010) were used to assess risk of bias within studies. All studies were reviewed by one author (K.K.R); blinded replications of these assessments were completed with good reproducibility (94%; S.J.R.). When data were unavailable in the original manuscripts, authors of individual studies were contacted for additional information. Simon et al 2006 (Simon et al., 2006) reported telomere length for controls, subjects with mood disorders, and for subjects meeting criteria for MDD; the data regarding telomere length in MDD subjects (n = 15) and controls (n = 44) were used to calculate the effect size for this meta-analysis. Karabatsiakis et al 2014 (Karabatsiakis et al., 2014) divided telomere length results from the same subjects into groups based on tissue or cell subpopulations; the results for individual groups were converted to standardized mean differences and then pooled to a common telomere length to allow comparison to other studies (Bornstein, 2009). A similar approach was taken to group white matter oligodendrocytes in the study by Szebeni et al 2014 (Szebeni et al., 2014). Liu et al 2014 (Liu et al., 2014) presented depressed and control group data for subjects with and without diabetes separately; these were treated as separate datasets in the meta-analysis, represented as Liu et al 2014a and b. A similar approach was taken for the paper by Ruis-Ottenheim et al 2012 (Rius-Ottenheim et al., 2012), which presented data for two different regional populations.
Statistical Analysis
Data were converted into standardized mean differences (SMDs) using the effect size calculator (Wilson, 2010) and reported as Cohen's d (Cohen, 1988). The SMD is the mean difference in telomere length between the depressed and non-depressed groups divided by the pooled standard deviation of the distribution of the score used in the study. This results in a unitless effect size measure that is comparable to other studies using similar measures of outcome. By convention, effect sizes of 0.2, 0.4, and 0.8 are considered small, medium and large, respectively (Cohen, 1988). If only correlations (r) or odds ratios (OR) were reported, they were converted to Cohen's d using the formulas using the equation d = 2r/(1-r2)1/2 or d = OR(31/2/π), respectively (Bornstein, 2009).
All analyses were performed using Comprehensive Meta-Analysis Software (Version 2.2.064 Biostat, Englewood, New Jersey) utilizing the standard meta-analysis function with a random effects model (DerSimonian and Laird, 1986). Heterogeneity of effect sizes was calculated using the I2 statistic, which gives a measure of the percentage of variation between the studies attributable to between-study differences rather than to sampling error (Thorlund et al., 2012) (0% no observed heterogeneity, 25% low, 50% moderate and 75% high heterogeneity). A random effects model was utilized because initial analysis using a fixed effects model revealed significant heterogeneity between studies (I2 = 85%). Confidence intervals (CI; 95%) around the effect size and p-values for the meta-analysis and sub-group analyses were calculated. To ascertain if the results were strongly influenced by any single study, sensitivity analyses were performed utilizing the “leave-one-out” strategy (Patsopoulos et al., 2008). Publication bias was assessed by inspecting the funnel plot on primary outcome measures and quantified using Egger's regression intercept (Egger et al., 1997). Duval and Tweedie's trim and fill analysis (Duval and Tweedie, 2000), using a random effects model and looking for missing studies to the right of the mean, was used to estimate the effect size after accounting for publication bias. Random effects meta-regression models were used to examine the association between depression severity and years since MDD onset with telomere length.
Moderator analysis
To explore the possible reasons for heterogeneity, moderator analyses were performed. Meta-regression was used for analyzing the continuous moderators of mean age, smoking status, percent male gender in a study and to examine a combined model of significant moderators using the method of moments random-effects meta-regression model (Bornstein, 2009). For categorical moderators, subgroup analyses were conducted using a continuous random effects model (DerSimonian and Laird, 1986). Subgroups were assembled using the following criteria: Comorbid chronic medical condition - Studies of patients with a chronic medical condition in addition to depression were placed in the Condition group. Telomere measurement technique – The majority of studies utilized the ratio of telomere repeat copy numbers to single-copy gene numbers (T/S ratio) using quantitative real-time polymerase chain reaction (qPCR) to measure telomere length (k = 30); four studies utilized Southern blot and one study each utilized quantitative fluorescent in situ hybridization (qFISH), telomere content (TC), low-coverage whole-genome sequencing, and terminal restriction fragment lengths. Thus, we compared qPCR vs. all other techniques and also qPCR vs. Southern blot. Source tissue - The source tissue from which DNA was extracted for telomere measurement was noted in the methods section of the studies and grouped according to the categories of (1) leukocyte and (2) other (other included k = 5 studies, with brain k = 3 studies and saliva k = 2 study). Depression assessment - Measures of depression were grouped based on whether they utilized a clinical interview or self-report method. Measures determining clinically significant depression in the interview category included the Structured Clinical Interview for DSM-IV (SCID), Computerized National Institute of Mental Health Diagnostic Interview Schedule (CDIS-IV), Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria, and Composite International Diagnostic Interview (CIDI). Measures with thresholds to identify clinically significant depression in the self-report category included the Geriatric Depression Scale (GDS), Children's Depression Inventory, short version (CDI-s), Beck Depression Inventory (BDI), Hospital Anxiety and Depression Scale (HADS), Center for Epidemiologic Studies Depression Scale (CES-D), Patient Health Questionnaire (PHQ-9) and the Taiwanese depression questionnaire (TDQ). Although not a questionnaire measure, we also included here a study that reported on diagnosis of depression from a clinical sample bank that was not further defined in this category because a clinical interview was not specified. A multivariate meta-regression was performed using variables found to be statistically significant moderators.
Results
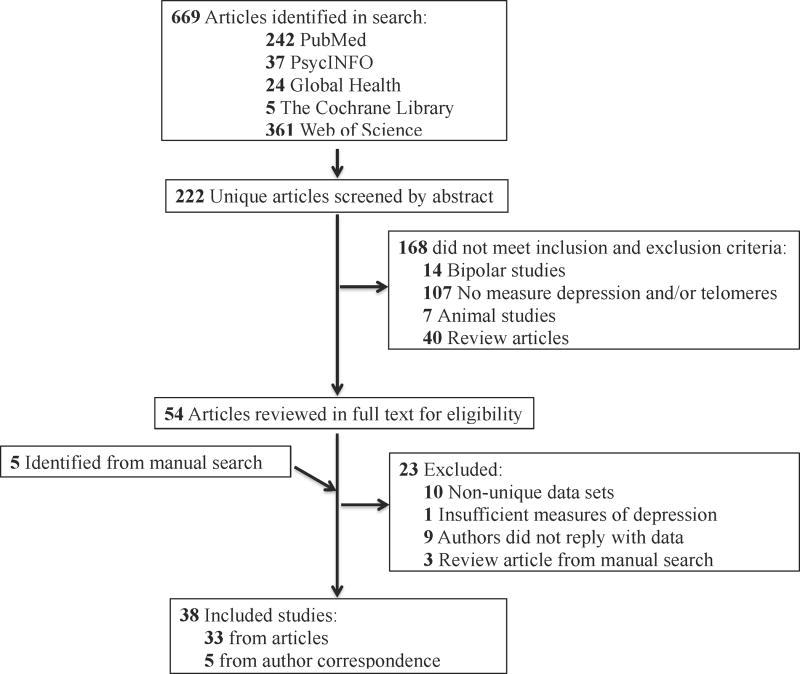
We identified 669 studies through the literature search (Figure 1). After duplicates were removed, abstracts of 222 articles were screened for eligibility and 168 were excluded. Five additional articles were identified after searching the study and review article references. Of the 54 full-text articles that were assessed for eligibility, 23 studies were excluded (9 authors did not reply to inquiries for non-published data, 10 contained overlapping data with other studies, 3 review articles resulting from the manual reference search, 1 study examined internalizing disorders rather than depression). Two papers contained two independent data sets as described in the methods section, leading to 38 data sets. These studies are summarized in Table 1; for 5 studies additional data were provided in correspondence with the authors (Garland et al., 2014; Ladwig et al., 2013; Phillips et al., 2013; Shalev et al., 2014; Tyrka et al.).
Figure 1.
PRISMA flow diagram for identification and inclusion of studies in the meta-analysis.
Table 1. Characteristics of included studies.
| Author, Year |
Setting | Study Design |
Study N (depressed) |
Male % |
Mean age ±SD |
Medical Condition |
Depression Measurement Technique |
Telomere Measurement Technique |
Tissue source |
Telomere length (p value) |
Level adjustment |
NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cai et al, 2015 | CONVERGE Study | Cross-sectional | 11,670 (5,338) | 0 | - | - | DSM-IV | Low-coverage whole-genome sequencing | Saliva | OR= 0.85 (2.84×10-14) | 3 principle components | 7/10 |
| Epel et al, 2013 | Community recruitment | Cross-sectional | 239 (-) | 0 | 57±4.4 | - | PHQ | qPCR | Leukocyte | r=-0.09 (>0.05) | None | 7/10 |
| Falci et al, 2013 | Cases: Medical oncology inpatient; Controls: Community-dwelling | Cross-sectional | 91 (-) | 28.6 | 80.7±2.3 | 26 Breast, 26 colorectal cancer | GDS | qPCR | Leukocyte | ρ=-0.10 (0.34) | None | 6/10 |
| Garcia-Rizo et al, 2013 | Cases: Psychiatric inpatient; Controls: Community-dwelling | Nested case-control | 57 (9) | 40 | 28.3±7.4 | - | SCID | Telomere content | Leukocyte | Depressed: 87.9±7.6 Control: 101.2±14.3 (0.009) | None | 5/9 |
| Garland et al, 2014 | Rowan Breast Cancer Center of the Abramson Cancer Center of the University of Pennsylvania | Nested case-control | 140 (5) | 0 | 54.6±4.4 | History of breast cancer | HADS | Terminal restriction fragment lengths | Leukocyte | Depressed: 5.6±0.2 Control: 6.2±0.7 (0.059) | None | 7/9 |
| Georgin-Lavialle et al, 2014 | French “AFIRMM protocol” Study | Cross-sectional | 19 (15) | 21 | 44.5±16.8 | Mastocytsis | BDI | qPCR | Leukocyte | r=-0.407 (0.105) | Age, gender | 8/10 |
| Gotlib et al, 2014 | Department of Psychiatry and Behavioral Sciences at Stanford University | Cross-sectional | 97 (-) | 0 | 12.0±1.5 | - | CDI | qPCR | Saliva | r=-0.039 (>0.05) | None | 8/10 |
| Hartmann et al, 2014 | Cases: Psychiatric inpatients; Controls: Community-dwelling | Case-control | 74 (54) | 43 | 49.1±14.3 | - | DSM-IV | Southern blot | Leukocyte | Depressed: 7.2±0.61 Control: 7.55±0.54 (0.007) | None | 5/9 |
| Hassett et al, 2012 | Fibromyalgia Registry at the Chronic Pain & Fatigue Research Center; University of Michigan | Cross-sectional | 66 (-) | 0 | 44.6±12.1 | Fibromyalgia | CES-D | qPCR | Leukocyte | rpartial=-0.247, (0.048) | Age | 7/10 |
| Hoen et al, 2011 | Heart and Soul Study | Nested case-control | 952 (206) | 81 | 66.7±11.0 | Coronary heart disease | CDIS-IV | qPCR | Leukocyte | Depressed: 0.86±0.02 Control: 0.90±0.01 (0.02) | Age, sex | 7/9 |
| Hoen et al, 2013 | PREVEND Study | Prospective cohort 2.2-year follow-up | 1,077 (97) | 46 | 53.5±11.3 | - | CIDI | qPCR | Leukocyte | B=0.012 (0.753) | Age, sex | 7/9 |
| Karabatsiakis et al, 2014 | recruited by newspaper announcement and public advertisements | Case-control | 94 (20) | 0 | 52.2±7.7 | - | Clinical evaluation | qFISH | Leukocyte | Depressed: 50.8±15.1 Control: 68.4±15.5 (<0.0001) | None | 7/9 |
| Ladwig et al, 2013 | Cooperative Health Research in the Region of Augsburg (KORA) F4 Study | Cross-sectional | 2,549 (126) | 47 | 52.5±1 1.0 | - | PHQ-9 | qPCR | Leukocyte | Depressed: 1.89±0.32 Control: 1.88±0.33 (0.74) | None | 7/10 |
| Lee et al, 2014 | HIV-related clinical and community sites in San Francisco, CA | Cross-sectional | 283 (137) | 74 | 44.9±8.4 | HIV | CES-D | qPCR | Leukocyte | Depressed: 0.97±0.32 Control: 0.98±0.31 (>0.05) | None | 6/10 |
| Lin et al, 2014 | The University of Texas MD Anderson Cancer Center and Baylor College of Medicine | Prospective cohort 3.6 year follow-up | 464 (-) | 79.7 | 64.9±11 | Bladder cancer | CES-D for current depression, SCID-IV for lifetime MDD | qPCR | Leukocyte | OR; 95% CI: 0.86; 0.48-1.53 (0.609) | Age, gender, ethnicity, smoking status, cancer grade, treatments by stage and vital status | 8/9 |
| Liu et al, 2014a | Division of Endocrinology, Tongji Hospital | Nested case-control | 71 (17) | 40 | 54.6±8.4 | Diabetes | HADS-D | qPCR | Leukocyte | Depressed: 1.70±0.52 Control: 2.11±0.44 (<0.05) | None | 5/9 |
| Liu et al, 2014b | Division of Endocrinology, Tongji Hospital | Nested case-control | 52 (5) | 30 | 51.3±7.7 | - | HADS-D | qPCR | Leukocyte | Depressed: 2.01±0.16 Control: 2.32±0.24 (<0.05) | None | 5/9 |
| Lung et al, 2007 | Cases: Psychiatric hospital; Controls: Community-dwelling | Case-control | 664 (253) | Not reported | 50.0±14.4 | - | SCID | Southern blot | Leukocyte | Depressed: 8.17±0.61 Control: 9.13±1.49 (<0.01) | None | 6/9 |
| Needham et al, 2014 | The National Health and Nutrition Examination Survey | Cross-sectional | 1164 (75) | 43.6 | 29.4±5.9 | - | CIDI | qPCR | Leukocyte | β=−0.03 (>0.05) | Age, gender and race/ethnicity | 8/10 |
| Phillips et al, 2013 | West of Scotland Twenty-07 Study | Prospective population-based cohort 15-year follow-up | 1063 (81) | 45 | 55.7±15.1 | - | HADS | qPCR | Leukocyte | Depressed: 0.77±0.21 Control: 0.79±0.20 (0.39) | None | 6/9 |
| Rius-Ottenheim et al, 2011 | Zutphen Elderly Study (the Netherlands) | Prospective population-based cohort 7-year follow-up | 122 (-) | 100 | 86.5±2.2 | - | GDS-15 | qPCR | Leukocyte | β=-0.51 (0.59) | Age | 8/9 |
| Rius-Ottenheim et al, 2011 | Cretan Elderly Study (Greece) | Cross-sectional | 102 (-) | 100 | 85.2±1.9 | - | GDS-15 | qPCR | Leukocyte | β=-0.044 (0.66) | Age | 8/10 |
| Savolainen et al 2012 | Helsinki Birth Cohort Study | Cross-sectional | 1950 (-) | 46.4 | 61.5±2.9 | - | BDI | qPCR | Leukocytes | β=0.058; 95% CI - 0.052, 0.168 (0.303) | Age, sex and stock DNA concentration | 8/10 |
| Schaakxs et al, 2014 | Netherlands Study of Depression in Older Persons | Case-control | 483 (355) | 35 | 70.5±7.3 | - | CIDI | qPCR | Leukocyte | Depressed: 5,035±431 Control: 5,057±729 (0.59) | Age, sex, education | 8/9 |
| Shaffer et al, 2012 | Nova Scotia Health Survey 1995 | Cross-sectional | 2225 (269) | 50 | 48.2±18.9 | - | CES-D | qPCR | Leukocyte | B=50.2; 95% CI -22.8-123.1 (0.18) | Age, sex | 10/10 |
| Shalev et al, 2014 | Dunedin Multidisciplinary Health and Development Study | Prospective, population-based cohort 35-year follow-up | 829 (177) | 51 | 38±- | - | DSM-IV | qPCR | Leukocyte | Depressed: 1.00±0.31 Control: 1.06±0.32 (0.026) | None | 7/9 |
| Simon et al, 2006 | Cases: MGH Mood Disorder Genetics Study; Controls: Harvard Health Volunteer Specimen Bank | Case-control | 59 (15) | 51 | 50.4±8.1 | - | SCID | Southern blot | Leukocyte | Depressed: 6.87±0.89 Control: 7.64±1.10 (0.018) | None | 6/9 |
| Simon et al, 2015 | Research Programs at MGH | Case-control | 332 (166) | 46 | 41.3±13.7 | - | SCID | Southern blot | Leukocyte | Depressed: 9.1±2.5 Control: 8.9±3 (0.65) | Education, exercise, smoking, htn, CIRS score, alcohol/drug disorder, ICG, TEQ, ETISR, PSS | 8/9 |
| Surtees et al, 2011 | EPIC-Norfolk | Cross-sectional | 4,012 (267) | 0 | 62(40-81)* | - | HLEQ | qPCR | Leukocyte | β=-0.004; 95% CI - 0.052, 0.044 | Age, SF-36, social class, obesity, cigarette smoking, preexisting disease, self-reported health | 7/10 |
| Szebeni et al, 2014 | Brainsobtained from Medical Examiner's Office of Cuyahoga Country | Case-control | 28 (14) | 93 | 50.9±17.3 | - | DSM-IV | End-point PCR | Astrocytes, oligodendro-cytes from Frontal white matter (Brodmann area 10) and Temporal (uncinate fasciculus). | UFastrocyte F=0.578, (0.46) BA10astrocyte F=0.354; (0.56); UFoligodendrocyte F=25.6, (<0.0005); BA10oligodendrocyte F=9.02, (0.007) | None | 6/9 |
| Teyssier et al, 2011 | Stanley Medical Research Institute | Case-control | 24 (13) | Not reported | Not reported | - | MDD diagnosis in databank | qPCR | Occipital cortex | 0.79±0.001 for depressed and controls (>0.05) | None | 4/9 |
| Teyssier et al, 2012 | Cases: Psychiatric inpatients; Controls: Hospital staff | Case-control | 33 (17) | 0 | 38.6±5.2 | - | SCID and MINI | qPCR | Leukocyte | Depressed: 13.42±0.32 Control: 13.6±0.30 (>0.05) | None | 6/9 |
| Tyrka et al, 2014 | Community-recruitment of depressed and control subjects | Cross-sectional | 290 (13) | 39 | 31.0 ± 10.7 | - | SCID | qPCR | Leukocyte | Depressed: 3398.9±4108.5 Control: 6593.6±4059.3 (<0.01) | Age, sex, SES, education, BMI | 9/10 |
| Verhoeven et al, 2014 | Netherlands Study of Depression and Anxiety | Cross-sectional | 1605 (1095) | 35 | 40.6±13.1 | - | CIDI | qPCR | Leukocyte | Depressed: 1.11±0.30 Control: 1.15±0.31 (0.003) | Age, sex, education | 9/10 |
| Wikgren et al, 2012 | Cases: Psychiatric inpatients; Controls: Betula Study | Case-control | 542 (91) | 48 | 59.1±11.9 | - | DSM-IV | qPCR | Leukocyte | Depressed: 5261±334 Control: 5538±743 (0.001) | Age, gender | 8/9 |
| Wolkowitz et al, 2011 | Case: Psychiatric outpatients; Controls: Community dwelling | Case-control | 35 (18) | 66 | 36.7±11.2 | - | SCID | qPCR | Leukocyte | Depressed: 5101±425 Control: 5141±282 (0.66) | Age, gender | 8/9 |
| Yen et al, 2012 | Household neighborhood sample | Cross-sectional | 298 (-) | 59.4 | 69.2±2.7 | - | TDQ | qPCR | Leukocyte | β=0.140; 95% CI −0.004 to 0.015 (0.235) | Sociodemo-graphics plus mental state factors | 7/10 |
| Zhang et al, 2010 | Stanley Medical Research Institute | Case-control | 63 (15) | 67 | 45.4±9.1 | - | MDD diagnosis in databank | qPCR | Gray matter cerebellum | Depressed: 0.97±0.25, Control: 1.077±0.251 (>0.1) | Disease status, age, gender | 6/9 |
(-) indicates value in study not specified.
= median (range). NOS = Newcastle-Ottawa Scale; qPCR = quantitative real-time polymerase chain reaction; SCID = Structured Clinical Interview for DSM-IV; CDIS-IV = Computerized National Institute of Mental Health Diagnostic Interview Schedule; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders IV; CIDI = Composite International Diagnostic Interview; GDS = Geriatric Depression Scale; CDI-s = Children's Depression Inventory, short version; BDI = Beck Depression Inventory; HADS = Hospital Anxiety and Depression Scale; HLEQ = Health and Life Experiences Questionnaire; CES-D = Center for Epidemiologic Studies Depression Scale; PHQ-9 = Patient Health Questionnaire; TDQ = Taiwanese depression questionnaire; MDD = major depressive disorder; BA10 = Brodmann area 10; UF = uncinate fasciculus; Htn = hypertension, CIRS = Cumulative Illness Rating Scale; ICG = Inventory of Complicated Grief; TEQ = Traumatic Events Questionnaire; ETISR = Early Trauma Inventory Self-Report; PSS = perceived stress; SF-36 = Short form-36 physical component summary; SES = socio-economic status; BMI = body mass index; OR = odds ratio; B = unstandardized regression coefficient; β = standardized regression coefficient; r = correlation coefficient; ρ = Spearman's rank correlation coefficient; F = F statistic; CI = 95% confidence interval; OR = odds ratio.
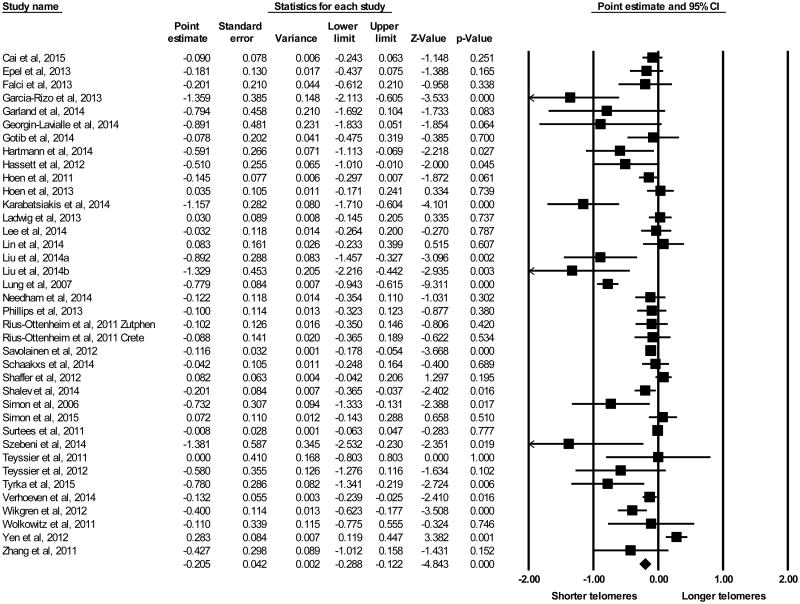
The random effects model revealed an association between depression and telomere length, with an overall effect size, reported as Cohen's d, of -0.205 (Figure 2; 95% CI −0.288 to −0.122, p < 0.0001). The association between depressive symptom severity and effect size was significant (B = -1.00, 95% CI -1.90 to -0.0985, p = 0.030, I2 = 68%). Years since onset of first episode of MDD was not significantly associated with effect size (B = -0.020, 95% CI -0.045 to 0.004, p = 0.11 I2 = 68%).
Figure 2.
Results of meta-analysis using all studies, Forest plot of point estimate effect sizes reported as Cohen's d (x-axis) evaluating depression and telomere length using the random effects model. Points represent weighted effect size, lines represent 95% confidence intervals (CI). Triangle indicates overall effect size and 95% CI.
Sensitivity analyses revealed that no one study affected the overall significance of the results. Visual inspection of the Funnel Plot (Supplemental Figure 1, white fill) showed asymmetry about the combined effect size. Egger's regression intercept was -1.63 (95% CI -2.65 to -0.606, t = 3.23, two-tailed p = 0.003), suggesting the presence of publication bias. We performed Duval and Tweedie's trim and fill analysis (Duval and Tweedie, 2000) to determine how the effect size would be changed were there no publication bias. Under the random effects model, the effect size was calculated as -0.145 (95% CI -0.231 to -0.059 Supplemental Figure 1, black fill), indicating that the effect remained highly significant after this adjustment. Heterogeneity was detected in the primary meta-analysis (I2 = 42%). The following analyses aimed to examine possible sources of heterogeneity through moderator analysis (Table 2).
Table 2. Moderator analyses.
| Parameter | k (n) | d | 95% CI | I2 (%) | p-value |
|---|---|---|---|---|---|
| Depression measurement technique | < 0.0001 | ||||
| Interview-based | 18 (19,988) | -0.337 | -0.485 to -0.188 | 34 | < 0.0001 |
| Self-report | 20 (14,359) | -0.078 | -0.217 to 0.008 | 40 | 0.076 |
| Telomere measurement technique | < 0.0001 | ||||
| Other method than qPCR | 7 (1,420) | -0.717 | -1.14 to -0.294 | 0 | 0.001 |
| qPCR | 31 (32,927) | -0.111 | -0.175 to -0.046 | 35 | 0.001 |
| Source tissue | <0.0001 | ||||
| Leukocytes | 33 (22,465) | -0.210 | -0.297 to -0.120 | 44 | < 0.0001 |
| Other | 5 (11,882) | -0.181 | -0.428 to 0.065 | 20 | 0.15 |
| Chronic comorbid medical condition | <0.0001 | ||||
| Condition | 8 (2,086) | -0.252 | -0.455 to -0.050 | 27 | 0.015 |
| Other | 30 (32,261) | -0.197 | -0.290 to -0.123 | 45 | < 0.0001 |
| Age | 37 (22,677) | B = 0.005 | -0.0002 to 0.011 | 80 | 0.06 |
| Gender | 37 (34,323) | B = 0.176 | -0.141 to 0.493 | 82 | 0.28 |
| Smokers in study (%) | 19 (12,838) | B = -0.073 | -0.969 to 0.823 | 69 | 0.87 |
The effects of all categorical variables were determined using the continuous random effects model in subgroup analyses. The effects of age and percent male gender, which were imputed as a continuous variable, were analyzed using the method of moments random-effects meta-regression model, resulting in a meta-regression coefficient (B). Highlighted p-values represent significant moderator effects on the relationship between depression and telomere length. MDD = major depressive disorder; k = number of studies within subgroup; n = number of subjects represented from all studies k; d = effect size reported as Cohen's d for subgroup; CI = confidence interval; I2 = test of heterogeneity within the subgroup, with 0% no observed heterogeneity, 25% low, 50% moderate and 75% high heterogeneity.
Study techniques as moderators of telomere length and depression
As seen in Table 2, the set of studies that utilized interview-based methods showed a larger effect of depression on telomere length (d = -0.337, 95% CI -0.485 to -0.188, p < 0.0001, I2 = 34%) than studies measuring depression using self-report instruments (d = -0.078, 95% CI -0.217 to 0.008, p = 0.076, I2 = 40%); the difference between these groups was significant (p < 0.0001).
Studies measuring telomeres using either qPCR or other measurement techniques showed a significant effect of depression on telomere length (Table 2). Studies utilizing telomere measurement techniques other than qPCR showed a larger effect and no heterogeneity between studies (d = -0.717, 95% CI -1.14 to -0.294, p = 0.001, I2 = 0%) than studies utilizing qPCR to measure telomeres (d = -0.111, 95% CI -0.175 to -0.046, p = 0.001, I2 = 35%); the difference between these groups was significant (p < 0.0001). When comparing the two most frequently used techniques, Southern blot vs. qPCR, the results were still significantly different (p < 0.0001; d = -0.500 vs. -0.113 respectively).
The majority of studies (k = 33) measured telomere length in leukocytes, and these showed a highly significant association between depression on telomere length (d = -0.210, 95% CI -0.297 to -0.120, p < 0.0001, I2 = 44% Table 2). When comparing differences between the leukocyte source tissue group and all other source tissues combined, there was a significant effect of source tissue between groups (p < 0.0001), with the Other tissue group having a smaller, non-significant, effect size (d = -0.181, 95% CI -0.428 to 0.065, p = 0.15, I2 = 20% Table 2).
Chronic medical condition, age and gender as moderators of telomere length and depression
In the analysis of comorbid medical conditions, the comparison between groups was significant (p < 0.0001, Table 3), with the Condition group showing a slightly larger effect size (d = -0.252, 95% CI -0.455 to -0.050, p = 0.015, I2 = 27%) compared to the Other group (d = -0.197, 95% CI -0.290 to -0.123, p < 0.0001, I2 = 45%). The linear relationship between study participant mean age and effect size was not significant (B = 0.005, 95% CI -0.0002 to 0.011, p = 0.06, I2 = 80% Table 2), nor was the relationship between gender and effect size (B = 0.176, 95% CI -0.141 to 0.493, p = 0.28, I2 = 82% Table 2) or percent of subjects that were smokers and effect size (B = -0.073, 95% CI -0.969 to 0.823, p = 0.87, I2 = 69% Table 2).
Table 3. Multivariate Meta-Regression.
| Covariate | B | Standard error | 95% Confidence Interval | Z-value | 2-sided p-value |
|---|---|---|---|---|---|
| Depression measurement technique | -0.098 | 0.082 | -0.260 to 0.063 | -1.19 | 0.23 |
| Telomere measurement technique | -0.438 | 0.122 | -0.677 to -0.199 | -3.59 | <0.001 |
| Source tissue | 0.075 | 0.137 | -0.194 to 0.343 | 0.54 | 0.59 |
| Chronic comorbid medical condition | -0.135 | 0.105 | -0.341 to 0.072 | -1.28 | 0.20 |
Test of model: Q = 20.3, df = 4, p = 0.0004; R2 analog for model = 0.29 (proportion of total between-study variance explained by model).
Multivariate meta-regression of significant moderators
The significant variables gleaned from the moderator analysis above were used as predictors in a multivariate meta-regression analysis. As demonstrated in Table 3, telomere measurement technique remained a statistically significant moderator (p <0.001), but source tissue, depression measurement technique and comorbid medical condition were no longer significant. A test of the model suggests that the overall effect size is related to the included variables (Q = 20.3, p = 0.0004) and that the proportion of between-study variance explained by the model was moderate (R2 = 0.29).
Discussion
The findings presented in this meta-analysis support an association between reduced telomere length and clinically significant depression. Importantly, the severity of the depressive episode correlated significantly with telomere shortening. Using Cohen's categorization of effect sizes (Cohen, 1988), the effect on telomere length is small- to medium-sized; the effect magnitude was influenced by the variables examined in the moderator analyses. We found a significant effect of small-sized studies, suggesting publication bias towards reduced telomere length with depression. However, after trim and fill analysis, the effect size was in the same direction and the 95% CI still did not cross zero (Supplemental Figure 1), indicating that the association of telomere length and depression remained significant after correcting for possible publication bias. These results are consistent with the previously published meta-analysis (Schutte and Malouff, 2015), which reported a significant correlation between depression and telomere length. Our results serve to further clarify the existing literature by including a comprehensive list of studies, such that the population presented here is approximately 40% larger than a recently published meta analysis, and by examining the effects of depression severity, years since MDD onset, chronic conditions, smoking, and tissue source on the relationship between depression and telomere length, which had previously not been examined.
Clinically significant depression has been linked to an increased risk of developing serious medical conditions including diabetes mellitus (Musselman et al., 2003) and cardiovascular disease (Musselman et al., 1998), along with earlier mortality (Evans et al., 2005), independent of socio-demographic risk factors (Gump et al., 2005) or comorbidities (Evans et al., 2005). Telomere length has been equated to a cellular clock; affecting how quickly cells reach senescence (Ridout SJ, 2015), and telomere shortening has been associated with a number of serious medical conditions (Ridout SJ, 2015). It may be that telomere shortening is a potential mechanism by which MDD may contribute to an increased risk of morbidity and mortality (Wolkowitz et al., 2010). That the effect of depression on telomere length was not solely accounted for by moderators known to significantly affect telomere length (age, gender, smoking and comorbid chronic medical conditions) indicates that other influences might be responsible for this association.
The causal nature of this association is not known; future studies will be needed to further investigate the mechanism by which depression is associated with shortened telomeres (Price et al., 2013; Ridout SJ, 2015). Telomere shortening is known to result from repeated mitotic divisions and exposure to a variety of cellular stress mechanisms (Blackburn et al., 2006). It has been speculated that MDD directly activates or is associated with increased cellular stress and replication, resulting in accelerated telomere shortening (Wolkowitz et al., 2010). Recent evidence also highlights the importance of genetic variation in telomerase, a regulator of telomere length and has been associated with depression (Wei et al., 2015). The results of this study suggest that there is a relationship between depression and biological measures of stress and aging, underscoring the importance of future studies exploring the mechanisms relating depression and telomere length.
We observed a negative association between telomere length and depression severity, but we did not find a significant association with years since first depressive episode. We were not able to examine the cumulative effect of depression on telomere length in terms of total number of episodes and length, severity, treatment, or treatment response of each episode because too few studies reported on these characteristics. It is possible that a severe current episode may be reflective of a more severe course of depressive illness. Future studies examining the total number and severity of prior depressive episodes would help elucidate this relationship.
To identify possible sources of heterogeneity between studies, the effects of study measurement techniques of depression and telomere length, source tissue, age, gender, and comorbid chronic medical conditions were examined as potential moderators. Both depression and telomere measurement techniques were significant moderators of the relationship between depression and telomere length, with studies utilizing interview-based and telomere measurement techniques other than qPCR demonstrating a larger association of depression with telomere length as shown by the effect size. A previous meta-analysis did not detect an effect of depression assessment method on the relationship between depression and telomere length (Schutte and Malouff, 2015). Compared to this previous meta-analysis, this analysis has a larger sample size, which might have facilitated our ability to detect the effect of depression measurement technique on telomere length. There is evidence that interview-based measures of depression are better able to detect outcomes in depression treatment (Cuijpers et al., 2010) and that clinicians may have higher severity thresholds than the self-report scales in these studies (Carter et al., 2010). Whether they used interview or self-report methods to detect clinically significant depression, most studies in this meta-analysis provided information regarding depression severity in their populations as well. There was no difference in severity between the studies using interview based or self-report methods in this meta-analysis (p = 0.10876). Both interview-based and telomere measurement techniques other than qPCR are more technically cumbersome and time consuming than other approaches. It is important to note that while techniques other than qPCR yielded a larger effect size, qPCR was associated with significant effects, indicating that it is an acceptable alterative. However, this was not the case for depression measurement technique: only studies utilizing interview assessments of depression had a significant association, suggesting that this may be an important consideration for future studies.
The effect of depression on telomere length was robust in the group of studies that examined DNA from leukocytes. We did find a difference between subgroups related to source tissue, although only a few studies examined brain tissue or saliva and there was variability in telomere length in these other tissues. It is possible that the finding that only leukocyte-derived data exhibit a significant association with telomere length reflects differences in preponderance of post-mitotic mass of the source tissue. We were unable to examine subgroups of leukocyte types in this meta-analysis due to a limited number of studies reporting these data, although subtypes of leukocytes have been shown to significantly differ in subjects with depression compared to controls (Karabatsiakis et al., 2014).
Decreased telomere length has been associated with a number of chronic medical conditions (Price et al., 2013; Ridout SJ, 2015); we found a significant difference between studies examining populations with and without a specific chronic medical condition, with studies examining telomere length in subjects with both depression and a comorbid chronic medical condition showing a larger effect size. However, as shown in Table 3, the effect sizes of each group would still place them in the same small to medium effect size category; any statistically significant differences noted should be evaluated cautiously, as their clinical and biological importance is unclear. While the existence of a negative association between telomere length and increasing age (Muezzinler et al., 2013), smoking (Verde et al., 2015) and effects of gender (Gardner et al., 2014) are well-established, these results show that these characteristics alone do not explain the relationship between depression and telomere length.
Limitations of this meta-analysis follow from study design differences as well as differences in baseline characteristics of study participants. The inclusion of a number of studies with small sample sizes likely contributed to the observed variation in effect size, and reduced the ability to detect differences between subgroups. The relatively small number of studies in some of the moderator categories reduced our ability to detect differences based on these categories. Many publications are based on secondary analyses from studies originally designed for other purposes, and used banked blood specimens to determine telomere length, which could affect their findings due to storage or extraction technique, inadequate power or study design (Nussey et al., 2014), and consequently the results of this meta-analysis.
Conclusions
This analysis shows that the association of depression with telomere shortening is robust and identifies factors that affect this association. Reduced telomere length in depression could be a mechanism by which depression contributes to increased morbidity and mortality risk; suggesting the need for future studies examining the effects of treating depression on telomere length. It is unclear at what point in the course of depression telomere length is affected, how the course of depression affects telomere length, and whether some individuals are more susceptible to telomere shortening with depression than others. Future research should help to elucidate these issues. Our results can help guide investigators by providing evidence regarding the optimal design and measurement of future studies in this area, which would include interview-based measures of depression. Newer prospective studies will determine whether telomere length and dynamics will serve merely as an additional biomarker of general health or as a primary target to evaluate severity of depression and treatment efficacy.
Supplementary Material
Highlights.
This is a meta-analysis of 38 studies (N = 34,347)
Depression and telomere length are significantly associated (Cohen's d of -0.205)
Depression severity is associated with telomere length
Depression and telomere measurement techniques affect this relationship
Source tissue and medical conditions affect this relationship
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature medicine. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- Bornstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons, Ltd; Hoboken, New Jersey: 2009. [Google Scholar]
- Carter JD, Frampton CM, Mulder RT, Luty SE, Joyce PR. The relationship of demographic, clinical, cognitive and personality variables to the discrepancy between self and clinician rated depression. J Affect Disord. 2010;124:202–206. doi: 10.1016/j.jad.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. second. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cuijpers P, Li J, Hofmann SG, Andersson G. Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clin Psychol Rev. 2010;30:768–778. doi: 10.1016/j.cpr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Codd V, Trompet S, Broer L, Hagg S, Fischer K, Thijssen PE, Suchiman HE, Postmus I, Uitterlinden AG, Hofman A, de Craen AJ, Metspalu A, Pedersen NL, van Duijn CM, Jukema JW, Houwing-Duistermaat JJ, Samani NJ, Slagboom PE. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. International journal of epidemiology. 2014;43:878–886. doi: 10.1093/ije/dyt267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, Shiels P, Sayer AA, Barbieri M, Bekaert S, Bischoff C, Brooks-Wilson A, Chen W, Cooper C, Christensen K, De Meyer T, Deary I, Der G, Diez Roux A, Fitzpatrick A, Hajat A, Halaschek-Wiener J, Harris S, Hunt SC, Jagger C, Jeon HS, Kaplan R, Kimura M, Lansdorp P, Li C, Maeda T, Mangino M, Nawrot TS, Nilsson P, Nordfjall K, Paolisso G, Ren F, Riabowol K, Robertson T, Roos G, Staessen JA, Spector T, Tang N, Unryn B, van der Harst P, Woo J, Xing C, Yadegarfar ME, Park JY, Young N, Kuh D, von Zglinicki T, Ben-Shlomo Y Halcyon study t. Gender and telomere length: systematic review and meta-analysis. Experimental gerontology. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SN, Palmer C, Donelson M, Gehrman P, Johnson FB, Mao JJ. A nested case-controlled comparison of telomere length and psychological functioning in breast cancer survivors with and without insomnia symptoms. Rejuvenation research. 2014;17:453–457. doi: 10.1089/rej.2014.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Eberly LE, Chang YF, Group MR. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. Bmj. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, G S. Chapter 8: Assessing risk of bias in included studies, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. updated March 2011. http://www.cochrane-handbook.org.
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, Blackburn E, Whooley MA. Depression and leukocyte telomere length in patients with coronary heart disease: data from the heart and soul study. Psychosom Med. 2011;73:541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC psychiatry. 2014;14:192. doi: 10.1186/1471-244X-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladwig KH, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, Codd V, Hafner S, Albrecht E, Illig T, Samani NJ, Wichmann HE, Gieger C, Peters A. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PloS one. 2013;8:e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Yan J, Wang Y, Li Y. Leucocyte telomere shortening in relation to newly diagnosed type 2 diabetic patients with depression. Oxidative medicine and cellular longevity. 2014;2014:673959. doi: 10.1155/2014/673959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou P, Zhu Y, Chen P, Zhang P, Yu J, Wang Y, Chen N, Zhang L, Wu H, Zhao J. Interaction of depressive and anxiety symptoms on the mortality of patients with COPD: a preliminary study. Copd. 2014;11:444–450. doi: 10.3109/15412555.2013.822856. [DOI] [PubMed] [Google Scholar]
- Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing research reviews. 2013;12:509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54:317–329. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Archives of general psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nussey DH, Baird D, Barrett E, Boner W, Fairlie J, Gemmell N, Hartmann N, Horn T, Haussmann M, Olsson M, Turbill C, Verhulst S, Zahn S, Monaghan P. Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol Evol. 2014;5:299–310. doi: 10.1111/2041-210X.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International journal of epidemiology. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Robertson T, Carroll D, Der G, Shiels PG, McGlynn L, Benzeval M. Do symptoms of depression predict telomere length? Evidence from the west of Scotland twenty-07 study. Psychosom Med. 2013;75:288–296. doi: 10.1097/PSY.0b013e318289e6b5. [DOI] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quality, A.f.H.R.a. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2015 http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=318. [PubMed]
- Ridout SJ, Ridout KK, Kao HT, Carpenter LL, Philip NS, Tyrka AR, Price LH. Telomeres, early-life stress and mental illness. Advances in psychosomatic medicine. 2015;34:92–108. doi: 10.1159/000369088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout SJ, R K, Kao HT, Carpenter LL, Philip NS, Tyrka AR, Price LH. Telomeres, early-life stress, and mental illness. In: Balon R, W T, editors. Clinical Challenges in the Biopsychosocial Interface: Update on Psychosomatics for the 21st Century Advances in Psychosomatic Medicine. Karger AG; Basel, Switzerland: 2015. pp. 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius-Ottenheim N, Houben JM, Kromhout D, Kafatos A, van der Mast RC, Zitman FG, Geleijnse JM, Hageman GJ, Giltay EJ. Telomere length and mental well-being in elderly men from the Netherlands and Greece. Behav Genet. 2012;42:278–286. doi: 10.1007/s10519-011-9498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depression and anxiety. 2015;32:229–238. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington HL, Houts RM, Israel S, Poulton R, Robertson SP, Sugden K, Williams B, Caspi A. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014 doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Szebeni A, Szebeni K, DiPeri T, Chandley MJ, Crawford JD, Stockmeier CA, Ordway GA. Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014:1–11. doi: 10.1017/S1461145714000698. [DOI] [PubMed] [Google Scholar]
- Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, Gluud C, Devereaux PJ, Wetterslev J. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PloS one. 2012;7:e39471. doi: 10.1371/journal.pone.0039471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, Welch ES, Carpenter LL. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.025. Epub ahead of print: January 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde Z, Reinoso-Barbero L, Chicharro L, Garatachea N, Resano P, Sanchez-Hernandez I, Rodriguez Gonzalez-Moro JM, Bandres F, Santiago C, Gomez-Gallego F. Effects of cigarette smoking and nicotine metabolite ratio on leukocyte telomere length. Environmental research. 2015;140:488–494. doi: 10.1016/j.envres.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Wei YB, Martinsson L, Liu JJ, Forsell Y, Schalling M, Backlund L, Lavebratt C. hTERT genetic variation in depression. J Affect Disord. 2015;189:62–69. doi: 10.1016/j.jad.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Wilson DB. Collaboration, C, editor. Effect Size Calculator. 2010 http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-Home.php.
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depression and anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Young JQ, Kline-Simon AH, Mordecai DJ, Weisner C. Prevalence of behavioral health disorders and associated chronic disease burden in a commercially insured health system: findings of a case-control study. General hospital psychiatry. 2014 doi: 10.1016/j.genhosppsych.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Zhao J, Miao K, Wang H, Ding H, Wang DW. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PloS one. 2013;8:e79993. doi: 10.1371/journal.pone.0079993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin K, Ilgen MA, Pfeiffer PN, Welsh DE, McCarthy J, Valenstein M, Miller EM, Islam K, Kales HC. Early mortality and years of potential life lost among Veterans Affairs patients with depression. Psychiatric services. 2012;63:823–826. doi: 10.1176/appi.ps.201100317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.