Abstract
Poor healing of epithelial wounds in cornea is a major clinical problem, leading to persistent epithelial defects and ulceration. The primary cause is poor cell migration over the wound. Carbohydrate-binding protein galectin-3 binds to extracellular matrixes (ECMs) and promotes lamellipodia formation by cross-linking to α3 integrin. Recombinant galectin-3 also facilitates wound healing in the rodent cornea. The purposes of the present experiments were to: (1) establish epithelial wound healing models in monkey corneal explant culture, the models more relevant to human, (2) evaluate the healing effect of galectin-3 in our models, and (3) determine if galectin-3 enhances cell adhesion by interacting with ECMs on corneal surface and their ligand integrins. Monkey corneas with central wounds produced by sodium hydroxide (NaOH) or n-heptanol were incubated with or without recombinant galectin-3. The defected area was stained with sodium fluorescein. Primary isolated corneal epithelial cells from monkey were cultured with or without galectin-3 on plates coated with ECMs or integrins, and the number of adhering cells was counted. Galectin-3 expression in various eye tissues was visualized by immunoblotting. NaOH caused loss of epithelial cells and basement membrane. n-Heptanol removed epithelial cells, but the basement membrane was retained. These corneal defects spontaneously became smaller in a time-dependent manner. Exogenous galectin-3 enhanced wound healing in both NaOH and n-heptanol models. Galectin-3 also enhanced cell adhesion onto the major ECMs found in the basement and Bowman’s membranes and onto integrins. Relatively high levels of galectin-3 were detected in corneal and conjunctival epithelium, but tear fluid contained negligible galactin-3. These results suggested that the enhanced binding of epithelial cells to ECMs and integrins caused by galectin-3 might promote cell migration over wounded corneal surfaces. Since tear fluid contained relatively low levels of galectin-3, exogenous galectin-3 may be a beneficial drug to enhance re-epithelialization in human corneal diseases.
Keywords: galectin-3, monkey corneal epithelium, wound healing, cell adhesion, extracellular matrices
1 Introduction
Following the loss of the corneal epithelium, the remaining epithelial cells attempt to cover the denuded wound. Poor healing of corneal wounds is a major clinical problem, leading to persistent epithelial defects and ulceration. Corneal epithelial defects may be caused by various situations such as refractive surgery, contact lens wear, dry eye, diabetes, and burns from chemical and thermal agents (Ambrósio et al., 2008; Galentine et al., 1984; Schultz et al., 1981; Chahud et al., 2009). Corneal epithelial wound healing is characterized by three phases; cell migration, cell proliferation, and cell differentiation (Suzuki et al., 2003). Inadequate migration of epithelial cells is the primary cause of poor healing. Although the growth factors, neurotrophins, fibronectin and cytokines accelerate corneal re-epithelialization, they have not been developed to clinical drugs. For example, epidermal growth factor accelerates corneal epithelial healing in a clinical trial (Pastor and Calonge, 1992), but corneal neovascularization was a risk factor (Yang et al., 2013; Burling et al., 2000).
Galectins are β-galactoside-binding animal lectins, and are expressed in human cornea (Hrdlicková-Cela et al., 2001). The gene homology for galectin-3 between human and mouse/rat is 87% and 83%, respectively. This is nearly the same as the 95% homology between man and monkey (Macaca mulatta). Re-epithelialization of corneal wounds is significantly slower in galectin-3 knock-out mice, and exogenous galectin-3 accelerates epithelial wound healing in rodent cornea (Cao et al., 2002; Yabuta et al., 2014). Potentially, galectin-3 ligand binding may modulate cell-to-cell and cell-to-extracellular matrix (ECM) interactions (Pricci et al., 2000). Galectin-3 cross-links N-glycans of integrin α3β1 (a ligand to laminin-5), induces lamellipodia formation, and promotes migration of corneal epithelial cells (Saravanan et al., 2009). In tumor cells, interaction of galectin-3 with integrin α5β1 modulates cell adhesion and motility through fibronectin remodeling (Lagana et al., 2006).
The corneal epithelial basement membrane plays important roles in maintaining a healthy epithelium and healing wounds (Torricelli et al., 2013). Galectin-3 enhances corneal wound healing by promoting adherence of epithelial cells onto collagen IV in the basement membrane (Yabuta et al., 2014). Other components of the basement membrane, such as fibronectin and laminin, are also involved in process of wound healing. Fibronectin increases adhesion and motility of corneal epithelial cells through activating Rac1-p21-activating kinase pathway (Kimura et al., 2006). Laminin-5 binding to α3β1 integrin accelerates keratinocytes migration by activation of focal adhesion kinase (FAK)-Rac1 pathway and formation of lamellipodia (Choma et al., 2007; Choma et al., 2004). These previous reports indicated that exogenous galectin-3 might be a good candidate drug to promote corneal wound healing. However, most studies used rodents, where Bowman’s membrane is thinner and forms a fuzzy border with the stroma, compared to the abundant Bowman’s membrane in human (Merindano et al., 2002; Hayashi et al., 2002). Thus, the purposes of the present study were to (1) establish a more relevant epithelial wound healing models using cultured explanted monkey corneas, (2) evaluate the efficacy of galectin-3 on corneal wound healing in our monkey model, and (3) measure the influence of galectin-3 on the adherence of monkey epithelial cells onto ECMs and integrins.
2 Materials and methods
2.1 Experimental animals
Sixty eyes, two tear samples, and a blood sample from 36 rhesus monkeys (Macaca mulatta) ranging in age from 1 to 12 years were obtained at necropsy from the Oregon National Primate Research Center (Beaverton, OR). Acceptable variability (see below) in tissue sampling was unavoidable, because, for ethical reasons, monkey tissues could only be obtained when they became available from experiments unrelated to the present studies. The excised eyes were soaked in ice-cold Hank’s balanced salt solution (HBSS) (Life Technologies, Carlsbad, CA), and the average time between death and use of eyes was less than 2 hrs. Experimental animals were handled in accordance with the ARVO Statement for the use of Animals in Ophthalmic and Vision Research and the Guiding Principles in the Care and Use of Animals (DHEW Publication, NIH 80-23).
2.2 Culture of corneal explants
Corneal wounds were produced in the central cornea of dissected globes by applying a 7.5 mm diameter filter paper disk soaked in 1N sodium hydroxide (NaOH) (Sigma-Aldrich, St. Louis, MO) for 60 sec or n-heptanol (Alfa Aesar, Ward Hill, MA) for 90 sec. The cornea was then rinsed with HBSS, and damaged epithelium was gently removed with a surgical blade. The cornea was then excised along with a narrow rim of sclera, and the conjunctiva was trimmed off as much as possible. The corneal sizes were similar between young and adult monkeys and were nearly equal between right and left eyes (e.g., 3 yrs = 13 mm diameter; 11 yrs = 14 mm diameter). The corneal explants were then incubated in Minimum Essential Medium (MEM) (Life Technologies) containing 1% (vol/vol) MEM non-essential amino acids (Life Technologies), 2 mM L-glutamine (Life Technologies) and 100 unit penicillin/mL and 100 μg streptomycin/mL (Life Technologies) at 37°C. Human recombinant galectin-3 (BioVision, Inc., Milpitas, CA) was added at the level of 20 μg/mL because galectin-3 was previously shown to enhance corneal wound healing in a mouse organ culture in a dose dependent manner to at least 20 μg/mL (Cao et al., 2002).
The corneal wounds were stained with 1% (wt/vol) fluorescein sodium (Sigma-Aldrich), and excess fluorescein was gently wiped from the area. Images were captured with a FluorChem FC2 imager (Alpha Innotech Corp., San Leandro, CA) and complied in image analysis software (ImageJ Launcher 1.4.3.67). Percent healing was calculated as: 100 - (stained area at each point/stained area at 0 hr) × 100. To avoid artifacts due to repeated fluorescein staining and wiping, all corneas were measured at one time point, so that 21 eyes were used in an experiment for spontaneous wound healing. Thus, twenty eyes from 10 monkeys were used to assess the effect of galectin-3: 5 eyes each for NaOH or n-heptanol treatment alone for controls, and the contralateral corneas were treated 5 eyes each with galectin-3 plus NaOH or galectin-3 plus n-heptanol.
One cornea from each time point in each group (9 eyes) was subjected to histological observations. The corneal explants were fixed in buffered formalin more than 1 day and were processed for paraffin-embedded sections. Four-micrometer of sections were stained with hematoxylin and eosin (H & E).
One cornea from each group (3 total eyes) was subjected to transmission electron microscopy. Monkey corneas were immediately fixed after wounding in 0.1 M sodium cacodylate buffer (pH7.4) containing 1.5% (wt/vol) glutaraldehyde and 1.5% (wt/vol) paraformaldehyde, then postfixed in 1% (wt/vol) osmium tetroxide, and embedded in Epon epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and observed with an electron microscope FEI Tecnai 12 TEM (FEI, Hillsboro, OR).
2.3 Isolation of monkey corneal epithelial cells
Monkey corneal epithelial cells were isolated following a protocol previously reported, with some modifications (Kawakita et al., 2004). Enucleated corneas were incubated overnight at 4°C in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) (Life Technologies) with Corneal Epithelial Cell Growth Kit (American Type Culture Collection, Manassas, VA) containing 15 mg dispase II/mL (Roche Diagnostics Corp., Indianapolis, IN). Corneal epithelial sheets were peeled off in HBSS and were then digested with 0.05% trypsin-EDTA solution (Life Technologies) at 37°C. After 3 min incubation, the reaction was stopped by addition of 1.3% (wt/vol) soybean trypsin inhibitor (Sigma-Aldrich). Corneal epithelial cells were collected after dissociation by pipetting, filtration through a 100 μm cell strainer (BD Biosciences, Franklin Lakes, NJ), and washing with DMEM/F12.
2.4 Adhesion of monkey corneal epithelial cells to ECMs and integrins
Ninety six well polystyrene culture plates (Greiner Bio-One International GmbH, Kremsmünster, Austria) were coated for 1 hr at 37°C with 50 μL/well of 0.001% (wt/vol) human collagen type I, IV or V (BD Biosciences), human fibronectin (BD Biosciences), human laminin-5 (Abcam, Cambridge, UK), or human recombinant integrins α1β1, α3β1, or α5β1 (R&D Systems, Minneapolis, MN); washed with Dulbecco’s phosphate buffer saline (DPBS); then blocked for 1 hr at 37°C with 50 μL/well of 0.1% (wt/vol) bovine serum albumin (BSA). The wells were washed with DPBS, and then soaked for 1 hr at 37°C in 50 μL/well of serum- and supplement-free DMEM/F12 with or without 20 μg human recombinant galectin-3/mL. Adsorption of galectin-3 onto the wells was confirmed qualitatively. The corneal epithelial cells were prepared from unilateral or bilateral eyes from one monkey, which were seeded into 2–3 coated wells per each group at 2 × 104 cells/100 μL/well and cultured at 37°C. At 19 hrs, non-adheren t cells were gently removed, and the number of remaining adherent cells was measured by the MTT assay (Promega Corporation, Madison, WI). The number of cells in 2–3 wells was averaged.
2.5 Immunoblotting for galectin-3 in monkey eye tissues
In anesthetized monkeys, 100 μL of Dulbecco’s phosphate-buffered saline (Life Technologies) were instilled and manually blinked into each eye, and diluted tear fluids were collected. To collect enough protein, this was repeated 2 times, and the samples were combined. Whole blood was also collected from an anesthetized monkey, and serum was obtained by centrifugation. All other eye tissues and fluids were dissected or removed from the enucleated eye globes. Aqueous humor was obtained through the cornea with a needle and syringe. Then, the eye globes were sectioned into anterior and posterior regions. After collecting the vitreous humor from the posterior cups with a syringe; cornea, conjunctiva, iris, ciliary body, lens and retina were isolated by dissection with a scissor and tweezers. Corneal and conjunctival epithelia were obtained from their stroma by scraping with a surgical knife. Each isolated tissue was diluted or lysed in buffer containing 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1% (vol/vol) Triton X-100, protease inhibitors (complete Mini-EDTA free; Roche Diagnostics Corp.), and phosphatase inhibitor cocktail I and II (EMD Millipore, Billerica, MA). The supernatants were obtained by centrifugation for 10 min at 16,000 × g, and protein concentrations were measured with the BCA protein assay (Thermo Fisher Scientific Inc., Rockford, IL) using BSA as a standard.
From each sample, three μg of denatured proteins were separated in 4–12% Bis-Tris gels (NuPAGE, Life Technologies) using MES buffer (Life Technologies). The proteins were then electro-transferred from the gels to polyvinylidene fluoride membranes (Millipore, Bedford, MA). The membranes were blocked with 1% (wt/vol) skim milk in Tris-buffered saline with 0.05% (vol/vol) Tween 20 (TTBS) for 30 min at room temperature, and incubated overnight at 4°C with primary antibodies for galectin-3 (dilution: 1:100; Santa Cruz Biotechnology Inc. Santa Cruz, CA) in 1% (wt/vol) BSA in TTBS. The membranes were then rinsed in TTBS and incubated for 1 hr at room temperature with goat anti-mouse secondary antibody conjugated to HRP (1:4000, Santa Cruz Biotechnology). Immunopositive proteins were detected with chemiluminescence (ECL plus; GE Healthcare Life Sciences, Buckinghamshire, United Kingdom) and images were captured with a FluorChem FC2 imager.
2.6 Statistical analysis
Data were analyzed by analysis of covariance or Student’s t-test with statistical software (JMP 8.0.1, 10.0.0 or 10.0.2; SAS Institute Japan Ltd., Tokyo, Japan). P<0.05 was considered statistically significant.
3 Results
3.1 Monkey corneal explant models with n-heptanol and NaOH
In normal monkey cornea, a prominent basement membrane was observed between epithelium and Bowman’s membrane (Fig.1 A). Treatment of corneas with NaOH caused loss of most of the basement membrane and epithelium (Fig.1 B). In n-heptanol treated corneas, the basement membrane was intact, but the epithelial cells were lost (Fig.1 C).
Figure 1.
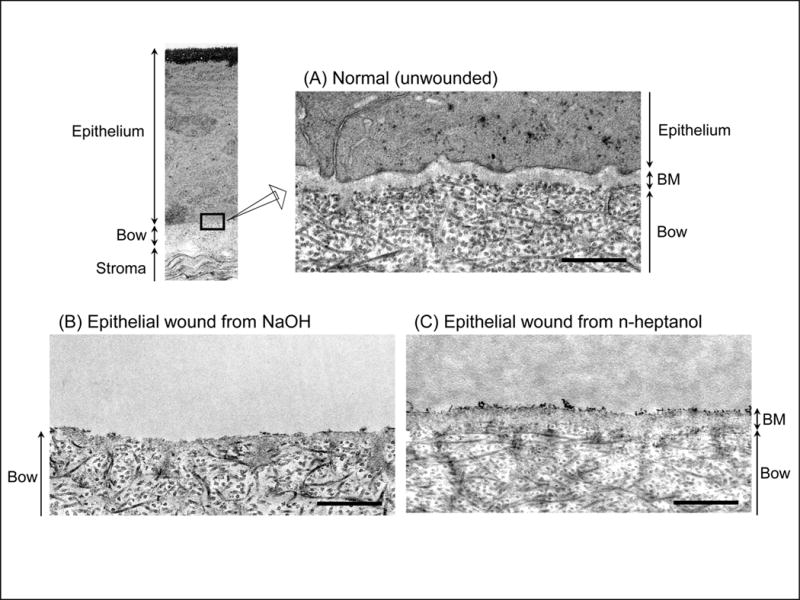
Histological differences in corneal wound models. Transmission electron microscopy of non-treated normal monkey cornea (A) and in corneas wounded with NaOH (B) or n-heptanol (C). Note loss of both basement membrane (BM) and epithelium with NaOH, but n-heptanol removed only the epithelium. Scale bars = 500 nm.
In normal cornea, 5 to 6 layers of cuboidal cells were observed in the central and peripheral epithelium (Fig.2 A, i and ii), along with 8 to 9 layers epithelial cells in the thicker limbal region (Fig.2 A, iii). Immediately following NaOH treatment, the entire epithelium in central and peripheral regions was lost, but the limbal epithelium was unaffected (Fig.2 B, 0 hr, iii). Spontaneous wound healing occurred by 12 hrs. Mono- or bi-layered epithelium covered the peripheral region (Fig.2 B, 12 hrs, ii) along with decreased cell layers in the limbal epithelium (Fig.2 B, 12 hrs, iii). The central region was covered by 36 hrs (Fig.2 B, 36 hrs, i). The limbal epithelium remained thinner at least until 36 hrs (Fig.2 B, 24 & 36 hrs, iii). These observations suggested that epithelial cells in the limbal region migrated centrally to cover the defected area.
Figure 2.
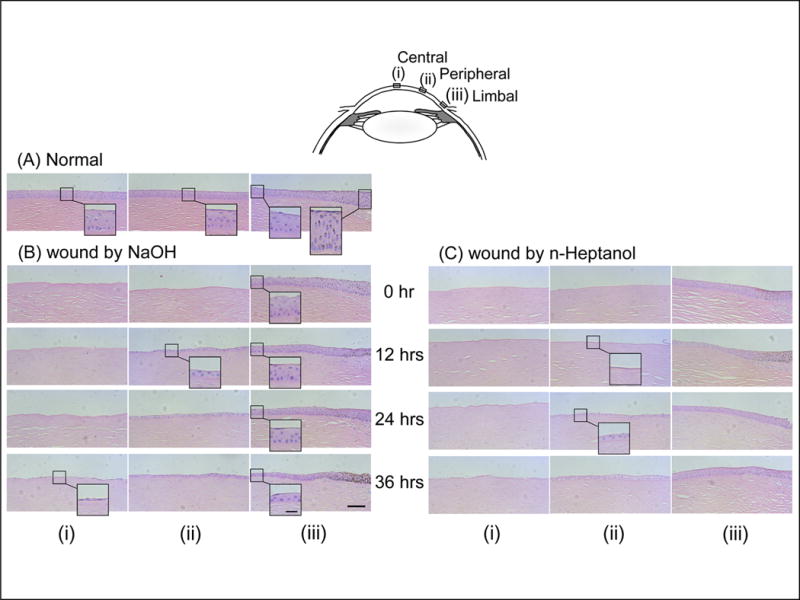
Spontaneous epithelial wound healing in explanted corneas. Central (i), peripheral (ii), and limbal (iii) regions in H & E stained sections of non-treated normal corneas (A) and in corneas at time points after treatment with NaOH (B) or n-heptanol (C). Scale bars = 100 μm and 25 μm in the inserted photos.
Similar spontaneous wound healing was observed in monkey corneas after treatment with n-heptanol. The only minor difference was that most of the peripheral region was not yet covered at 12 hrs but became covered at 24 hrs (Fig.2 C, 12 & 24 hrs, ii), suggesting that basement membrane inhibited wound healing.
After treatment with NaOH, the healed-over area was 10% of the total denuded area at 6 hrs, 60% at 18 hrs, and 80% at 30 hrs (Fig.3 A). The healing rate during the first 12 hrs was significantly higher than during second 18 hrs (Table 1). In the n-heptanol-treated corneas, the healed areas were 4% at 6 hrs, 40% at 18 hrs, and 80% at 30 hrs (Fig.3 B), and the healing rate was the same between the first 12 hrs and second 18 hrs (Table 1). Thus, the final amount of healing was similar in both models, but the late rate of healing was significantly higher in the n-heptanol model. As in the corneal wound healing results, obvious differences were not observed between young and adult monkeys or between genders (data not shown).
Figure 3.
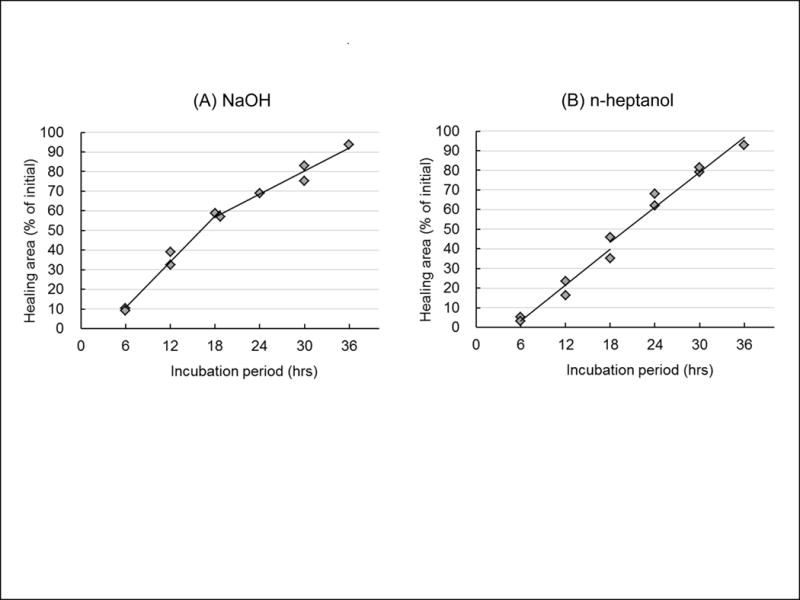
Differences in spontaneous wound healing rate in two models. Quantitative analysis of the rate of spontaneous wound healing in corneas after treatment with NaOH (A) or n-heptanol (B). Squares show individual data.
Table 1.
The calculated healing rate (slope of the regression lines from Fig.3)
P<0.05 relative to 18–36 hrs,
P<0.05 relative to NaOH (analysis of covariance).
3.2 Galectin-3 enhanced wound healing and adhesion of corneal epithelial cells
Twenty μg galectin-3/mL significantly enhanced re-epithelialization of NaOH or n-heptanol wounds (Fig.4). Exogenous galectin-3 was evaluated at 24 hrs because wound healing at 24 hrs was similar with both chemicals.
Figure 4.
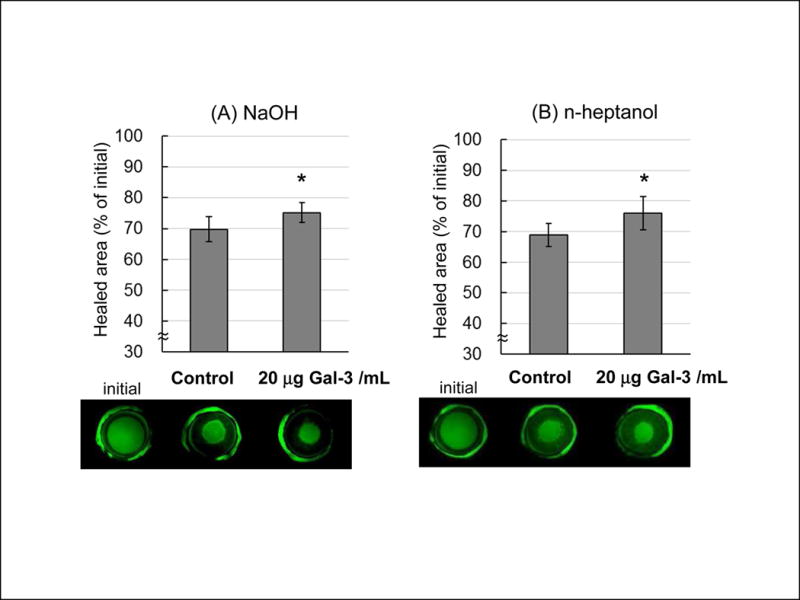
Galectin-3 accelerates corneal wound closure. Representative photographs of epithelial wounds stained with fluorescence (lower) and quantitative measurement of the healed area 24 hrs after treatment with or without galectin-3 in NaOH (A) or n-heptanol (B) treated corneas. Data are means ± S.D. (n=5). *P<0.05 relative to control (Student’s t-test).
Twenty μg galectin-3/mL significantly increased adherence of corneal epithelial cells onto the major ECM components in Bowman’s membrane (collagen types I, V) and in basement membrane (collagen type IV, fibronectin, laminin-5) (Fig.5, Upper). Galectin-3 also significantly increased adherence onto the cellular ligands of ECMs (integrins α1β1, α5β1, α3β1) (Fig.5, Lower).
Figure 5.
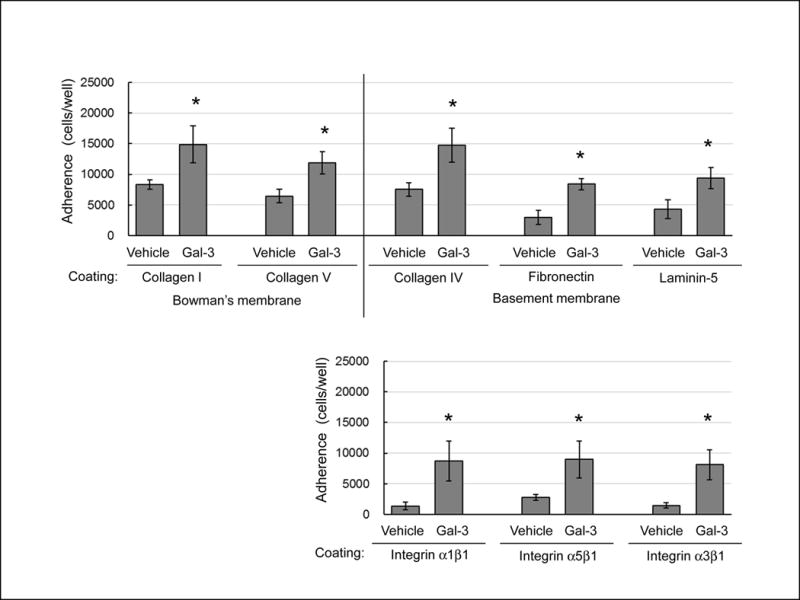
Galectin-3 enhances adhesion of monkey corneal epithelial cells. Adhesion of corneal epithelial cells onto various ECMs (Upper) and integrins (Lower) incubated with 20 μg galectin-3 (Gal-3)/mL or vehicle. Data are mean ± S.D. (n=4 monkeys). *P<0.05 relative to vehicle (Student’s t-test).
3.3 Localization of galectin-3 in monkey ocular surfaces
Relatively high levels of galectin-3 were observed in monkey corneal and conjunctival epithelia (Fig.6). Very faint bands for galectin-3 were observed in tear fluid, ciliary body and iris, and none were detected in other eye tissues or fluids.
Figure 6.
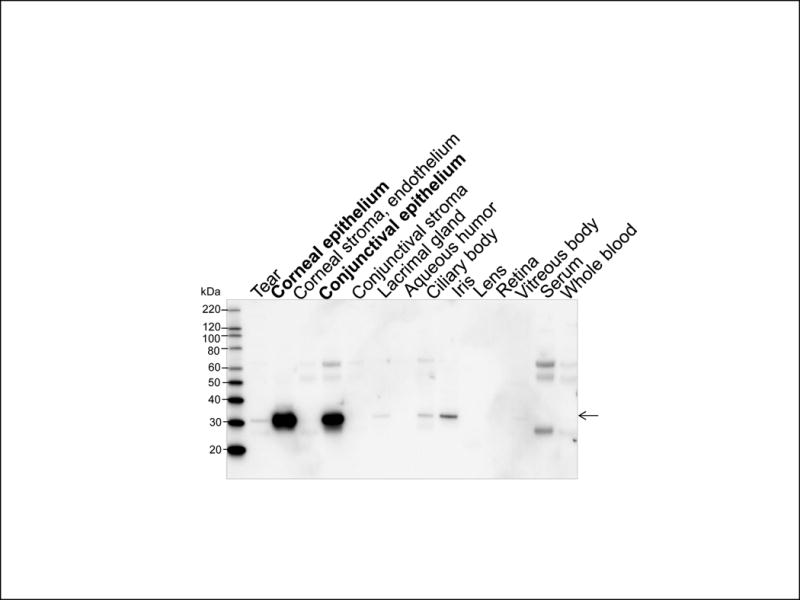
Immunoblot of galectin-3 (arrow) in the monkey ocular tissues.
4 Discussion
The current study showed that the source of cells for spontaneous re-epithelization of central wounds in monkey corneas is probably cell migration from the limbal epithelium. Exogenous galectin-3 further enhanced this wound healing. Galectin-3 promoted epithelial cell adherence onto the major ECMs found in the basement and Bowman’s membranes and onto integrins. The enhanced adherence caused by galectin-3 may be a fundamental mechanism for corneal wound healing, especially since corneal and conjunctival epithelia were found to have the highest levels of endogenous galectin-3.
4.1 Spontaneous wound healing in two chemical models
In our monkey corneal explants, the final amount of spontaneous healing was similar, but the rate of healing was different (Fig. 3). The healing rate was constant with time after n-heptanol. But in the NaOH model, the rate during the first 6 to 18 hrs was faster than during the last 18 to 36 hrs. Previous reports also showed differences in corneal healing rates depending on the agent or depth of wound. For example, an in vivo rabbit study showed that spontaneous healing rate between 6 and 30 hrs after alkali was faster than that in corneas wounded with n-heptanol (Chung et al., 1987). After trans-corneal freezing, the healing rate in diabetic rabbits (no basement membrane) during first 27 hrs was faster than that in non-diabetic rabbits (with basement membrane). After that, the healing rate slowed in diabetic rabbits(Hatchell et al., 1983).
Adherence of cells onto ECMs is important for cell migration and healing (Berrier and Yamada., 2007; Lock et al., 2008). The difference in wound healing rates noted above may have been influenced by the specific proteins on which corneal epithelial cells migrate. In our NaOH model, cells migrated on Bowman’s membrane containing collagen types I & V. In the n-heptanol model, cells migrated onto a basement membrane consisting of proteins such as collagen type IV, fibronectin and laminin-5. Our in vitro data showed that adherence of epithelial cells onto collagens was higher than onto fibronectin and laminin-5 (Fig.5). After alkali burns in rat corneas, fibronectin and laminin-5 were synthesized by the epithelial cells and keratocytes released onto the denuded cornea (Zhao et al., 2003). Synthesis of these substrates may slow the healing rate during the later period following NaOH wounds.
4.2 Galectin-3 enhanced epithelial cell adhesion and wound healing
Galectin-3 forms pentamers in the presence of multivalent ligands. These pentamers facilitate ECM interactions, receptor clustering, and formation of lattices with glycoproteins (Argüeso and Panjwani, 2011). In the present study, exogenous galectin-3 enhanced re-epithelialization onto Bowman’s membrane (NaOH model) and basement membrane (n-heptanol model) (Fig. 4). These results were consistent with our observation that galectin-3 increased the in vitro adherence of epithelial cells onto the major ECMs found in Bowman’s and basement membranes (Fig. 5). In rodent models, exogenous galectin-3 accelerated re-epithelialization after alkali burn and mechanical abrasion (Cao et al., 2002; Yabuta et al., 2014). Similar examples include experiments showing that β-glucan, ROCK inhibitor, and heparin-binding EGF-like growth factor promote cell adhesion and wound healing in corneal epithelial cells, corneal endothelial cells, and intestinal epithelial cells, respectively (Choi and Joo, 2013; Pipparelli et al., 2013; Su and Besner, 2014). This indicates that ECMs are essential for cell migration in corneal wound healing.
Interestingly, galectin-3 also increased cell adhesion onto α1β1, α5β1, and α3β1 integrins (Fig.5), suggesting that galectin-3 mediated association of integrins. Integrin clustering activates signaling pathways that regulate cell migration (Lock et al., 2008; Kariya et al., 2010; Welf et al., 2012). Saravanan et al. reported that galectin-3 induces clustering of α3β1 integrin on the cell surface (Saravanan et al., 2009). The clusters of α3β1 integrin activate FAK and Rac1, leading to lamellipodia formation, cell migration and re-epithelialization of wounds. Thus, we hypothesize that galectin-3 enhances cell migration and wound healing by increasing cell adherence onto the ECMs in basement and Bowman’s membranes and integrin clustering (Fig.7). Galectin-3 also induces clustering of CD147 and subsequent production of matrix metalloproteinase (MMP) in keratinocytes (Mauris et al., 2014). The production and activation of MMPs is required for cell motility at the leading edge of migrating epithelia during re-epithelialization, and improper regulation of MMPs contributes to the pathogenesis of wounds.
Figure 7.
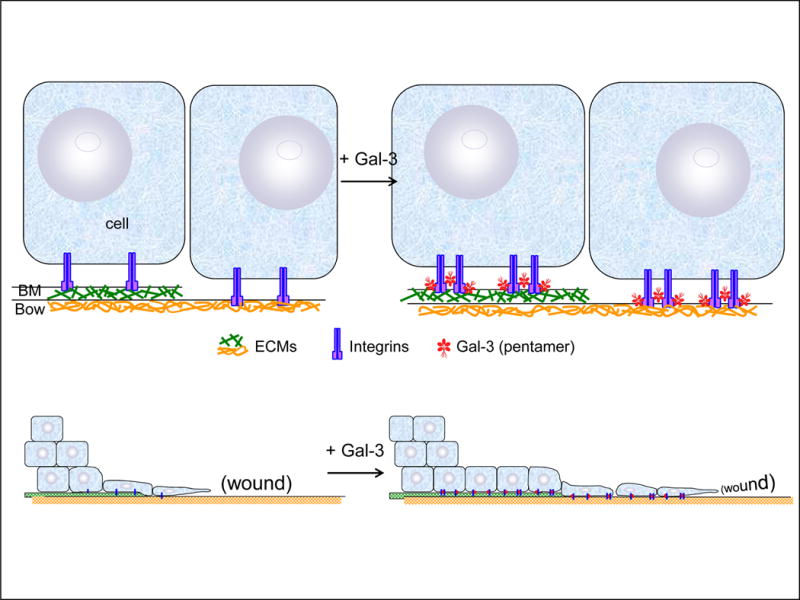
Hypothesis for galectin-3-mediated association of epithelial cells with ECMs and integrins, resulting in enhancement of cell migration. Exogenous gelectin-3 (Gal-3) binds epithelial cells to ECMs in the basement membrane (BM) and Bowman’s membrane (Bow), and enhances integrin-integrin cluster formation. Clustering and integrin-ECM associations may further activate intracellular signaling pathways to stimulate cell migration.
Since our primary epithelial cells required longer culture times to adhere onto ECMs, the effect of galectin-3 was also assessed at longer culture times than previous studies (Kuwabara and Liu, 1996; Ochieng et al., 1999; Sato et al., 2002; Yu et al., 2007). Despite the different culture times, galectin-3 enhanced cell adhesion as in the previous reports. Likewise, when a human corneal epithelial cell line was used instead of primary corneal epithelial cells, galectin-3 enhanced cell adhesion onto ECM-coated plates after 1 hr of culture (unpublished data, Fujii et al.). However, decreased cell adhesion by galectin-3 has also been reported (Sato and Hughes, 1992; Ochieng et al., 1998). The reasons for conflicting results are not clear, but different study protocols may be responsible. Long-term culture may induce other indirect effects that increase cell attachment on ECMs. For example, an anti-apoptotic effect of galectin-3 was observed in leukemia cells and T-lymphocytes (Yang et al., 1996; Cheng et al., 2011), and may indicate increased cell attachment onto ECM-coated plates during long-term culture. ECMs produced from living cells may also increase cell attachment onto integrin-coated plates during long-term culture.
4.3 Unique distribution of galectin-3
Galectin-3 protein was highly expressed in epithelia from monkey cornea and conjunctiva (Fig. 6). Surprisingly, negligible amounts were found in tear fluid, which might be expected to provide galectin-3 to the cornea and conjunctiva. Similarly, galectin-3 was not detected in tears from healthy human volunteers (Hrdlicková-Cela et al., 2001). In contrast, galectin-3 was present in tears from the patients with ocular inflammation, which may have caused release of galectin-3 for self-healing. For example, the level of galectin-3 in tears from dry eye patients is 0.38 ng/μg total protein (Uchino et al., 2015); or 0.76–4.56 μg galectin-3/mL tear assuming 2–12 μg total protein/μL (Ng et al. 2000; Li et al, 2010). Since partially degraded galectin-3 has been observed in 50% of patients (Uchino et al., 2015), the in vivo level of intact galectin-3 might be lower than the calculated and effective levels used in our models. In our experiments, galectin-3 only facilitated 6–7% healing, because healthy epithelial cells also spontaneously covered the wounded area. However, re-epithelialization is delayed in dry eye and diabetic patients (Pfister, 1992; Schultz et al., 1981). Thus, exogenous galectin-3 may be a good candidate for topical treatment of corneal epithelial defects, ranging from mild epithelial abrasion with intact basement membrane to severe recurrent corneal erosion with basement membrane dystrophy (Chen et al., 2006).
Highlights.
Monkey corneal wound models with and without basement membrane were produced.
Exogenous galectin-3 enhanced re-epithelialization in both models.
Galectin-3 promoted cell adhesion onto major ECMs and integrins.
Galectin-3 was detected at high levels in the epithelium, but negligible in tears.
Acknowledgments
This research was supported in part by NIH grant P51 OD011092 to the Oregon National Primate Research Center.
The authors would like to thank Drs. Robert J Kayton and Lisa Dirling Vecchiarelli at the Immuno Electron Microscopy Core at Oregon Health & Science University (Portland, OR) for the electron microscopy analysis, and Dr. Rolly Perez at MedSurge Pathology (Tigard, OR) for histological preparations.
Abbreviations
- ECM
extracellular matrix
- NaOH
sodium hydroxide
- FAK
focal adhesion kinase
- HBSS
Hank’s balanced salt solution
- MEM
Minimum Essential Medium
- H & E
hematoxylin and eosin
- DMEM/F12
Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12
- DPBS
Dulbecco’s phosphate buffer saline
- BSA
bovine serum albumin
- TTBS
Tris-buffered saline with 0.05% Tween 20
- MMP
matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Shearer is a paid consultant for Senju Pharmaceutical Co., Ltd., a company that may have a commercial interest in the results of this research and technology. Dr. Azuma and Ms. Fujii are employees of Senju Pharmaceutical Co., Ltd.
This potential conflict of interest was reviewed, and a management plan approved by the OHSU Conflict of Interest in Research Committee was implemented.
References
- Ambrósio R, Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24(4):396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- Argüeso P, Panjwani N. Focus on molecules: galectin-3. Exp Eye Res. 2011;92(1):2–3. doi: 10.1016/j.exer.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213(3):565–73. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- Burling K, Seguin MA, Marsh P, Brinkman K, Madigan J, Thurmond M, Moon-Massat P, Mannis M, Murphy CJ. Effect of topical administration of epidermal growth factor on healing of corneal epithelial defects in horses. Am J Vet Res. 2000;61(9):1150–5. doi: 10.2460/ajvr.2000.61.1150. [DOI] [PubMed] [Google Scholar]
- Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277(44):42299–305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- Chahud F, Ramalho LN, Ramalho FS, Haddad A, Roque-Barreira MC. The lectin KM+ induces corneal epithelial wound healing in rabbits. Int J Exp Pathol. 2009;90(2):166–73. doi: 10.1111/j.1365-2613.2008.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Huang CW, Huang FC, Tseng SY, Tseng SH. The cleavage plane of corneal epithelial adhesion complex in traumatic recurrent corneal erosion. Mol Vis. 2006;12:196–204. [PubMed] [Google Scholar]
- Cheng YL, Huang WC, Chen CL, Tsai CC, Wang CY, Chiu WH, Chen YL, Lin YS, Chang CF, Lin CF. Increased galectin-3 facilitates leukemia cell survival from apoptotic stimuli. Biochem Biophys Res Commun. 2011;412(2):334–40. doi: 10.1016/j.bbrc.2011.07.099. [DOI] [PubMed] [Google Scholar]
- Choi JS, Joo CK. Wakayama symposium: new therapies for modulation of epithelialization in corneal wound healing. Ocul Surf. 2013;11(1):16–8. doi: 10.1016/j.jtos.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Choma DP, Pumiglia K, DiPersio CM. Integrin alpha3beta1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117(Pt 17):3947–59. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- Choma DP, Milano V, Pumiglia KM, DiPersio CM. Integrin alpha3beta1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J Invest Dermatol. 2007;127(1):31–40. doi: 10.1038/sj.jid.5700505. [DOI] [PubMed] [Google Scholar]
- Chung JH, Fagerholm P, Lindström B. The behaviour of corneal epithelium following a standardized alkali wound. Acta Ophthalmol (Copenh) 1987;65(5):529–37. doi: 10.1111/j.1755-3768.1987.tb07036.x. [DOI] [PubMed] [Google Scholar]
- Galentine PG, Cohen EJ, Laibson PR, Adams CP, Michaud R, Arentsen JJ. Corneal ulcers associated with contact lens wear. Arch Ophthalmol. 1984;102(6):891–4. doi: 10.1001/archopht.1984.01040030711025. [DOI] [PubMed] [Google Scholar]
- Hatchell DL, Magolan JJ, Jr, Besson MJ, Goldman AI, Pederson HJ, Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch Ophthalmol. 1983;101(3):469–71. doi: 10.1001/archopht.1983.01040010469029. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Osawa T, Tohyama K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J Morphol. 2002;254(3):247–58. doi: 10.1002/jmor.10030. [DOI] [PubMed] [Google Scholar]
- Hrdlicková-Cela E, Plzák J, Smetana K, Jr, Mĕlková Z, Kaltner H, Filipec M, Liu FT, Gabius HJ. Detection of galectin-3 in tear fluid at disease states and immunohistochemical and lectin histochemical analysis in human corneal and conjunctival epithelium. Br J Ophthalmol. 2001;85(11):1336–40. doi: 10.1136/bjo.85.11.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya Y, Kawamura C, Tabei T, Gu J. Bisecting GlcNAc residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J Biol Chem. 2010;285(5):3330–40. doi: 10.1074/jbc.M109.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(10):3507–12. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- Kimura K, Kawamoto K, Teranishi S, Nishida T. Role of Rac1 in fibronectin-induced adhesion and motility of human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47(10):4323–9. doi: 10.1167/iovs.05-1508. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156(10):3939–44. [PubMed] [Google Scholar]
- Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol. 2006;26(8):3181–93. doi: 10.1128/MCB.26.8.3181-3193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Chen Z, Duan F, Liang J, Wu K. Quantification of tear proteins by SDS-PAGE with an internal standard protein: a new method with special reference to small volume tears. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):853–62. doi: 10.1007/s00417-009-1275-3. [DOI] [PubMed] [Google Scholar]
- Lock JG, Wehrle-Haller B, Strömblad S. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin Cancer Biol. 2008;18(1):65–76. doi: 10.1016/j.semcancer.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mauris J, Woodward AM, Cao Z, Panjwani N, Argüeso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci. 2014;127:3141–8. doi: 10.1242/jcs.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merindano MD, Costa J, Canals M, Potau JM, Ruano D. A comparative study of Bowman’s layer in some mammals: Relationships with other constituent corneal structures. Eur J Anat. 2002;6(3):133–139. [Google Scholar]
- Ng V, Cho P, Mak S, Lee A. Variability of tear protein levels in normal young adults: between-day variation. Graefes Arch Clin Exp Ophthalmol. 2000;238(11):892–9. doi: 10.1007/s004170000165. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Leite-Browning ML, Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun. 1998;246(3):788–91. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Warfield P, Green-Jarvis B, Fentie I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem. 1999;75(3):505–14. doi: 10.1002/(sici)1097-4644(19991201)75:3<505::aid-jcb14>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Pastor JC, Calonge M. Epidermal growth factor and corneal wound healing. A multicenter study. Cornea. 1992;11(4):311–4. doi: 10.1097/00003226-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Pfister RR. Clinical measures to promote corneal epithelial healing. Acta Ophthalmol Suppl. 1992;202:73–83. doi: 10.1111/j.1755-3768.1992.tb02172.x. [DOI] [PubMed] [Google Scholar]
- Pipparelli A, Arsenijevic Y, Thuret G, Gain P, Nicolas M, Majo F. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PLoS One. 2013;8(4):e62095. doi: 10.1371/journal.pone.0062095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricci F, Leto G, Amadio L, Iacobini C, Romeo G, Cordone S, Gradini R, Barsotti P, Liu FT, Di Mario U, Pugliese G. Role of galectin-3 as a receptor for advanced glycosylation end products. Kidney Int Suppl. 2000;77:S31–9. doi: 10.1046/j.1523-1755.2000.07706.x. [DOI] [PubMed] [Google Scholar]
- Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992;267(10):6983–90. [PubMed] [Google Scholar]
- Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168(4):1813–22. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Liu FT, Gipson IK, Panjwani N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alp ha3beta1 integrin. J Cell Sci. 2009;122(Pt 20):3684–93. doi: 10.1242/jcs.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–99. [PMC free article] [PubMed] [Google Scholar]
- Su Y, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) promotes cell migration and adhesion via focal adhesion kinase. J Surg Res. 2014;189(2):222–31. doi: 10.1016/j.jss.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Saito J, Yanai R, Yamada N, Chikama T, Seki K, Nishida T. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22(2):113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013;54(9):6390–400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino Y, Mauris J, Woodward AM, Dieckow J, Amparo F, Dana R, Mantelli F, Argüeso P. Alteration of Galectin-3 in Tears of Patients With Dry Eye Disease. Am J Ophthalmol. 2015;159(6):1027–35. doi: 10.1016/j.ajo.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welf ES, Naik UP, Ogunnaike BA. A spatial model for integrin clustering as a result of feedback between integrin activation and integrin binding. Biophys J. 2012;103(6):1379–89. doi: 10.1016/j.bpj.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta C, Yano F, Fujii A, Shearer TR, Azuma M. Galectin-3 enhances epithelial cell adhesion and wound healing in rat cornea. Ophthalmic Res. 2014;51(2):96–103. doi: 10.1159/000355846. [DOI] [PubMed] [Google Scholar]
- Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and ap optosis. Proc Natl Acad Sci U S A. 1996;93(13):6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Li X, Ma J, Lv X, Zhao S, Lang W, Zhang Y. Blockade of the intermediate-conductance Ca(2+)-activated K+ channel inhibits the angiogenesis induced by epidermal growth factor in the treatment of corneal alkali burn. Exp Eye Res. 2013;110:76–87. doi: 10.1016/j.exer.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, Rhodes JM. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282(1):773–81. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Ma YQ, Liang T, Jiang T, Wang CF, Zhang YX. Animal study on expression of laminin and fibronectin in cornea during wound healing following alkali burn. Chin J Traumatol. 2003;6(1):37–40. [PubMed] [Google Scholar]


