Abstract
BACKGROUND
Among elderly patients, the management of type 2 diabetes mellitus (T2DM) is complicated by population heterogeneity and elderly-specific complexities. Few studies have been done to understand treatment intensification among elderly patients failing multiple oral antidiabetic drugs (OADs).
OBJECTIVE
To examine the association between time to treatment intensification of T2DM and elderly-specific patient complexities.
METHODS
In this observational, retrospective cohort study, elderly (aged ≥ 65 years) Medicare beneficiaries (n = 16,653) with inadequately controlled T2DM (hemoglobin A1c ≥ 8.0% despite 2 OADs) were included. Based on the consensus statement for diabetes care in elderly patients published by the American Diabetes Association and the American Geriatric Society, elderly-specific patient complexities were defined as the presence or absence of 5 geriatric syndromes: cognitive impairment; depression; falls and fall risk; polypharmacy; and urinary incontinence.
RESULTS
Overall, 48.7% of patients received intensified treatment during follow-up, with median time to intensification 18.5 months (95% CI = 17.7–19.3). Median time to treatment intensification was shorter for elderly patients with T2DM with polypharmacy (16.5 months) and falls and fall risk (12.7 months) versus those without polypharmacy (20.4 months) and no fall risk (18.6 months). Elderly patients with urinary incontinence had a longer median time to treatment intensification (18.6 months) versus those without urinary incontinence (14.6 months). The median time to treatment intensification did not significantly differ by the elderly-specific patient complexities that included cognitive impairment and depression. However, after adjusting for demographic, insurance, clinical characteristics, and health care utilization, we found that only polypharmacy was associated with time to treatment intensification (adjusted hazard ratio, 1.10; 95% CI = 1.04–1.15; P = 0.001).
CONCLUSIONS
Less than half of elderly patients with inadequately controlled T2DM received treatment intensification. Elderly-specific patient complexities were not associated with time to treatment intensification, emphasizing a positive effect of the integrated health care delivery model. Emerging health care delivery models that target integrated care may be crucial in providing appropriate treatment for elderly T2DM patients with complex conditions.
Uncontrolled diabetes, defined as high levels of hemoglobin A1c (A1c; e.g., > 7.0%), among patients with type 2 diabetes mellitus (T2DM) is often treated by intensifying current treatment, either by adding oral antidiabetes drugs (OADs) or injectable drugs (e.g., insulin), or by increasing the dose(s) of the current drug(s).1 Findings from randomized controlled trials have shown the benefits of treatment intensification among patients with T2DM with inadequately controlled diabetes with regard to reduction in A1c values.2,3 An observational study found that patients with T2DM with inadequately controlled diabetes who received treatment intensification were 67% less likely to have a 30-day hospital readmission or emergency department (ED) visit, as compared with those patients with T2DM without treatment intensification.4
However, studies in different settings, such as U.S. Veterans Affairs institutions, integrated managed care practices, and general practices, have reported that among patients with T2DM with inadequately controlled diabetes (A1c ≥ 8.0%), treatment intensification rates can be as low as 20.8%5 and as high as 64.0%.6 It must be noted that both these studies included elderly (aged ≥ 65 years) and nonelderly patients with T2DM. In addition, rates of treatment intensification have been found to be significantly lower among elderly individuals with T2DM. For example, at a U.S. Veterans Affairs institution, elderly patients with T2DM in the age groups 65–74 years and ≥ 75 years had lower treatment intensification rates compared with younger patients aged < 65 years with T2DM (18.6% and 17.3% vs. 23.3%, respectively).5
Although joint guidelines from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes suggest that treatment intensification should be “at as rapid a pace as titration of medications allows,”7 time to treatment intensification and factors associated with it among elderly patients have not been well researched. In a study, non-specific to the elderly, that used data from 2002 on all members of the Kaiser Permanente Medical Care Program of Northern California, 57% of members who had inadequately controlled T2DM (A1c ≥ 8.0%) had their pharmacological therapy modified within 3 months.8 An observational, retrospective cohort study using a General Electric Centricity electronic medical record database found that the median time to treatment intensification among individuals with T2DM who failed metformin therapy was 14 months.
Although not specifically for elderly patients, the findings from this study indicate that age may be inversely associated with time to treatment intensification (adjusted hazard ratio [aHR], 0.995; 95% confidence interval [CI] = 0.992–0.997; P <0.001).6 Other investigators studied data from a multispecialty group practice in southeast Michigan. They found that in patients with T2DM who, despite receiving monotherapy with OADs, had sustained hyperglycemia (defined as 2 A1c values of > 8.0% and no recent treatment intensification), the mean time to treatment intensification was 9.7 months.9 The mean age (± standard deviation [SD]) of the patients in this study was 58 (±12) years.9
Treatment intensification, which has a significant role in the attainment of glycemic control, can have a positive impact on A1c levels among elderly patients with T2DM; the prevalence of T2DM can be as high as 27% in the elderly population.10 There is also a need for personalized treatment intensification among elderly patients with T2DM because of the complexities associated with the aging process. Guidelines published by the American Geriatrics Society (AGS) emphasize the fact that frailties and complexities of older patients with diabetes need to be considered when managing diabetes among the elderly.11 In this context, the AGS has highlighted 6 syndromes in particular that require consideration: cognitive impairment; depression; falls and fall risk; pain; polypharmacy; and urinary incontinence. None of the studies mentioned above has studied the association between time to treatment intensification and elderly-specific patient complexities.
The primary objective of this study, therefore, was to examine the association between time to treatment intensification and elderly-specific patient complexities as novel risk factors for treatment intensification after controlling for clinical, demographic, and insurance characteristics; and health care utilization among elderly individuals who have inadequate glycemic control (A1c ≥ 8.0%) despite treatment with 2 OADs.
Methods
Study Design
An observational, retrospective cohort study design was used to estimate the median time to treatment intensification and factors associated with time to treatment intensification in a cohort of elderly patients with T2DM who, despite treatment with 2 OADs, had an A1c level ≥ 8.0%. The index date was defined as the first observed date with an A1c level ≥ 8.0%. The baseline period was defined as 6 months before the index date, and the follow-up period was defined as the period from the index date to the date of treatment intensification or the end of the study observation period (February 28, 2012), or the disenrollment date of the Medicare beneficiary.
Data Source
Data from elderly patients with T2DM who were enrolled in the Humana Medicare Advantage Prescription Drugs (MAPD) plan database between January 1, 2007, and February 28, 2012, were used. This database includes claims data from more than 12 million current and previous Humana members and contains information on nearly 1.9 million MAPD plan members.
For these members, enrollment, and medical, pharmacy, and laboratory results claims files were made available to researchers. The enrollment files contain information on year of birth, race, sex, and monthly enrollment status of Medicare beneficiaries. The medical claims files contained information on hospitalization, cost, and diagnosis (based on the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis codes, and the American Medical Association Current Procedural Terminology codes). Pharmacy claims files included information on prescription fill date, days of supply, and the national drug codes for each dispensed medication. Laboratory results were available only for a third of the total enrolled population. The key fields in the laboratory claims were laboratory test codes and laboratory test results from which A1c values could be captured.
Study Population
The study population comprised elderly patients with T2DM aged ≥ 65 years at index date. T2DM was identified according to ICD-9-CM diagnosis codes 250.x0 (Diabetes mellitus type 2) and 250.x2 (Diabetes mellitus type 2, uncontrolled). Elderly patients with at least 1 inpatient visit or ≥ 2 outpatient visits with a primary or secondary diagnosis of T2DM were considered to have diagnosed T2DM. Several other inclusion criteria were applied in order to obtain the cohort for analysis: continuous enrollment for 6 months during the baseline period; receipt of any 2 OADs (metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, alpha-glucosidase inhibitors, meglitinides) during the baseline period; and nonreceipt of pramlintide, a glucagon-like peptide-1 receptor agonist, or any insulin during the baseline period.
Dependent Variable: Time to Treatment Intensification
Among patients with T2DM in whom treatment was intensified during the observation period, time to treatment intensification was calculated as the number of months between the index date and the first observed date with intensification (defined as either initiation of insulin or addition of a third OAD). T2DM patients who did not receive treatment intensification during the observation period were considered “censored” and separated from the patient cohort with treatment intensification. Time to treatment intensification among those patients with T2DM with no treatment intensification during the follow-up period was calculated as the number of months between the index date and the study observation end date.
Independent Variables
All independent variables were measured during the baseline period.
Demographic and Insurance Characteristics
Demographic variables included age (65–74 and ≥ 75 years); sex; race (white, African American, Hispanic, and other); and U.S. region (Midwest, South, and Northeast/Northwest/other). Insurance type (health maintenance organization [HMO], preferred provider organization, fee-for-service, and other) and MAPD coverage gap (popularly known as the “donut hole”) were assessed. Patients were defined to be in the donut hole if they had the coverage gap during the baseline period, whereas those without the coverage gap were categorized as being present in the pre- or post-donut hole phase.
Health Care Utilization
Health care utilization was identified as any inpatient visits, any ED visits, or number of outpatient office visits grouped as 0, 1–2, or ≥ 3.
Clinical Characteristics
Diabetes severity was assessed as a clinical characteristic using the Diabetes Complications Severity Index (DCSI),12 which used automated diagnostic data (i.e., ICD-9-CM codes) without laboratory data, using the modified algorithm by Chang et al. (2012).13 DCSI scores were grouped into 4 categories based on quartiles. The presence of hypoglycemia was measured using the Ginde algorithm14 and baseline A1c values were categorized as follows: ≥ 8.0% to < 8.5%; ≥ 8.5% to < 9.0%; and ≥ 9.0%. Co-occurring conditions were defined using an algorithm specifically developed for “diabetes care within the context of comorbid conditions” by Piette and Kerr (2006)15 and were classified based on the following hierarchy: dominant, concordant, and discordant conditions. Because of considerable overlap between concordant conditions and DCSI and other measures of elderly-specific patient complexities, only 1 discordant condition, the presence of chronic obstructive pulmonary disease (COPD), and 1 dominant condition, the presence of cancer, were used to define co-occurring conditions.
Elderly-Specific Patient Complexities
Based on the consensus statement for diabetes care in elderly patients published by the ADA and the AGS, elderly-specific patient complexities were defined as the presence or absence of 5 geriatric syndromes: cognitive impairment; depression screening; falls and fall risk; polypharmacy; and urinary incontinence.11 These elderly-specific patient complexities were identified using the ICD-9-CM diagnosis codes presented in the Appendix. Cognitive impairment was defined as the presence of Alzheimer’s disease, dementia, Huntington’s disease, Parkinson’s disease, schizophrenia, bipolar disorder, or psychotic disorders. As depression screening was unavailable in the dataset, we used the presence of major depressive disorder as a proxy for depression screening. Falls and fall risk were captured using ICD-9-CM E-codes and V-codes.16,17 Polypharmacy was measured as the total number of drugs that were 1 SD above the mean number of drugs.18 Polypharmacy was further classified as ≤ 6 drugs and > 6 drugs for this assessment. Urinary incontinence was also captured using ICD-9-CM diagnosis codes.19
Sensitivity Analysis
We also conducted sensitivity analyses by including dose escalation as 1 of the forms of treatment intensification among elderly patients with T2DM who remained on 2 OADs. Dose was considered to have increased if the daily dose of either of the 2 active ingredients from the baseline period was increased anytime during follow-up. The active ingredient information from the baseline period was matched with that in the follow-up period, and if the 2 active ingredients remained the same, the daily doses were directly compared to assess dose escalation. However, in the case of different active ingredients but similar therapeutic classes, dose escalation was derived using the comparison with the maximum daily dose. The maximum daily doses of each medication were obtained using Lexi-Drugs files (with AHFS DI Essentials and AHFS DI for hospital and academic clients). The maximum daily dose of the active ingredient during the baseline period was compared with the other active ingredient during the follow-up period, and individuals were considered to have OAD dose escalation if they had received a maximum daily dose in the follow-up period but not during the baseline period.
Statistical Analysis
Survival analysis techniques were used to test unadjusted and adjusted associations between time to treatment intensification and independent variables. Unadjusted differences were analyzed with Kaplan-Meier product limit estimates, and log-rank tests were used to examine statistically significant differences for days to treatment intensification. In our study, time to treatment intensification is a duration variable with each individual having a start date and an end date anywhere along the time line of the study observation period. Kaplan-Meier analysis is a simpler way of examining differences in time to treatment intensification when not all the individuals received intensification at the same time or did not receive treatment intensification within the observation period (i.e., censoring).20
Multivariable Cox-proportional hazards regression was used to analyze the association between the independent variables and days to treatment intensification in the presence of censoring. The following baseline variables were included in this regression: elderly-specific patient complexities (cognitive impairment, depression, falls and fall risk, polypharmacy, and urinary incontinence); clinical characteristics (DCSI, hypoglycemia, A1c levels, discordant condition [COPD], and dominant condition [cancer]); demographic and insurance characteristics (sex, race, age, U.S. region, insurance type, and donut hole status on index date); and health care utilization (any inpatient visits, any ED visits, and the number of outpatient visits). Parameter estimates are expressed as aHRs and 95% CIs associated with the parameter estimates. Variations in treatment intensification rates were examined by varying the follow-up periods: 4, 6, 12, and 24 months.
Results
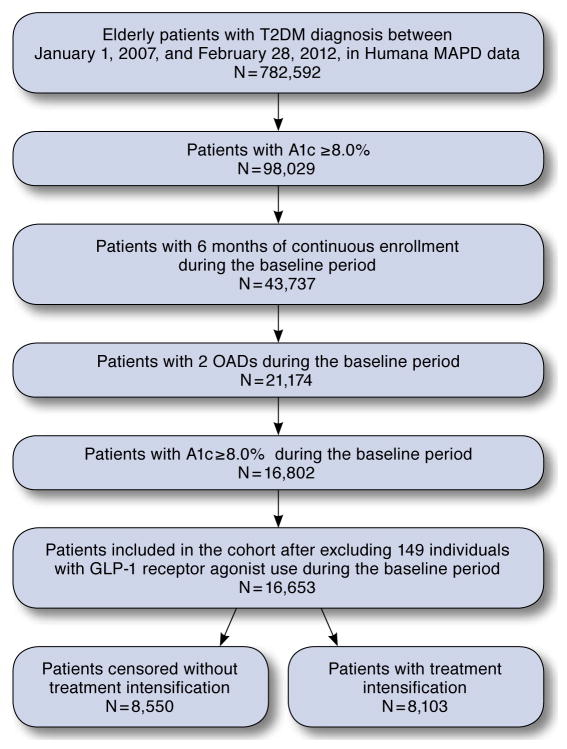
After applying all exclusion criteria, we used a study population of 16,653 elderly patients with T2DM with inadequately controlled A1c levels (≥8.0%) enrolled in the Humana MAPD plan database. Patient attrition is shown in Figure 1. A majority of the study population was male (51.3%), white (69.4%), aged 65–74 years (63.8%), living in the South of the United States (79.4%), and enrolled in HMO insurance plans (70.1%). In terms of diabetes severity, 34.2% of the study population was within the lowest category of DCSI scores and 30.2% was within the highest. A small proportion of patients had inpatient and ED visits (11.7% and 8.9%, respectively) during the baseline period. The average follow-up time in the study was around 22 months (range 0–56.9 months; median 19.6 months).
FIGURE 1.
Study Cohort Selection Chart
A1c = hemoglobin A1c; GLP-1 = glucagon-like peptide-1; MAPD = Medicare Advantage Prescription Drugs; OADs = oral antidiabetes drugs; T2DM = type 2 diabetes mellitus.
In the study population, 48.7% of elderly patients with T2DM received treatment intensification during follow-up, with a median time to treatment intensification of 18.5 months. Table 1 shows the subgroup differences in median time to treatment intensification, 95% CIs, and statistical significance based on log-rank tests. Statistically significant differences in median time to treatment intensification were found for the following variables: age, race, U.S. region, insurance type, prescription drug coverage gap on the index date (in the donut hole), inpatient visits, ED visits, number of outpatient visits, A1c, falls and fall risk, polypharmacy, and urinary incontinence.
TABLE 1.
Description of Median Time to Treatment Intensification in T2DM Patients with Uncontrolled Diabetes: Results from Kaplan-Meier Product Limit Estimates Using Humana MAPD Data 2007–2012
| N | Censored | % Censored | Median Time, Months | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| All | 16,653 | 8,550 | 51.3 | 18.5 | 17.7–19.3 | |
| Demographic and insurance characteristics | ||||||
| Age | < 0.001 | |||||
| 65–74 years | 10,620 | 5,293 | 49.8 | 17.4 | 16.5–18.3 | |
| ≥ 75 years | 6,033 | 3,257 | 54.0 | 20.9 | 19.2–22.5 | |
| Sex | 0.966 | |||||
| Women | 8,102 | 4,123 | 50.9 | 18.4 | 17.4–19.8 | |
| Men | 8,551 | 4,427 | 51.8 | 18.6 | 17.4–19.5 | |
| Race | < 0.001 | |||||
| White | 11,559 | 5,747 | 49.7 | 17.1 | 16.2–18.0 | |
| African American | 3,305 | 1,807 | 54.7 | 23.9 | 21.9–26.7 | |
| Hispanic | 664 | 334 | 50.3 | 17.3 | 14.4–23.1 | |
| Other | 1,125 | 662 | 58.8 | 20.0 | 17.1–25.2 | |
| U.S. region | < 0.001 | |||||
| Midwest | 1,861 | 932 | 50.1 | 16.8 | 14.4–18.9 | |
| South | 13,226 | 6,863 | 51.9 | 19.3 | 18.3–20.1 | |
| Northeast/Northwest/Other | 1,566 | 755 | 48.2 | 14.5 | 12.6–16.8 | |
| Insurance type | < 0.001 | |||||
| HMO | 10,986 | 5,730 | 52.2 | 20.9 | 19.8–21.9 | |
| PPO | 2,825 | 1,516 | 53.7 | 14.8 | 13.5–16.5 | |
| FFS | 2,658 | 1,201 | 45.2 | 14.8 | 13.5–16.2 | |
| Other | 184 | 103 | 56.0 | 17.2 | 12.3–23.7 | |
| Donut hole | < 0.001 | |||||
| Pre-donut hole | 15,189 | 7,938 | 52.3 | 19.6 | 18.6–20.4 | |
| In donut hole | 1,337 | 562 | 42.0 | 11.4 | 9.9–12.6 | |
| Post-donut hole | 127 | 50 | 39.4 | 11.1 | 6.0–16.8 | |
| Baseline health care utilization | ||||||
| Inpatient visits | 0.020 | |||||
| Yes | 1,947 | 984 | 50.5 | 16.6 | 15.0–18.3 | |
| No | 14,706 | 7,566 | 51.5 | 18.8 | 18.0–19.8 | |
| Emergency department visits | 0.020 | |||||
| Yes | 1,476 | 736 | 49.9 | 16.6 | 14.4–19.5 | |
| No | 15,177 | 7,814 | 51.5 | 18.6 | 18.0–19.5 | |
| Number of outpatient visits | < 0.001 | |||||
| 0 | 8,058 | 4,161 | 51.6 | 18.6 | 17.7–20.1 | |
| 1–2 | 4,209 | 2,229 | 53.0 | 20.7 | 18.9–22.5 | |
| ≥ 3 | 4,386 | 2,160 | 49.2 | 16.6 | 15.3–17.7 | |
| Clinical characteristics | ||||||
| DCSI categories | 0.308 | |||||
| 0 | 5,693 | 2,987 | 52.5 | 19.4 | 18.0–21.0 | |
| 1–2 | 2,875 | 1,425 | 49.6 | 17.5 | 15.9–19.2 | |
| 3 | 4,990 | 2,568 | 51.5 | 18.6 | 17.1–20.1 | |
| 4–12 | 3,095 | 1,570 | 50.7 | 17.7 | 16.2–19.5 | |
| Hypoglycemia | 0.952 | |||||
| Yes | 516 | 279 | 54.1 | 18.9 | 15.0–27.0 | |
| No | 16,137 | 8,271 | 51.3 | 18.5 | 17.7–19.2 | |
| A1c categories | < 0.001 | |||||
| ≥ 8.0% to < 8.5% | 8,970 | 5,000 | 55.7 | 23.2 | 22.2–24.3 | |
| ≥ 8.5% to < 9.0% | 2,575 | 1,300 | 50.5 | 16.9 | 15.3–18.9 | |
| ≥ 9.0% | 5,108 | 2,250 | 44.0 | 12.1 | 11.4–12.9 | |
| Discordant condition (COPD) | 0.352 | |||||
| Yes | 7,762 | 3,997 | 51.5 | 17.7 | 16.5–18.6 | |
| No | 8,891 | 4,553 | 51.2 | 19.2 | 18.3–20.4 | |
| Dominant condition (cancer) | 0.495 | |||||
| Yes | 1,493 | 793 | 53.1 | 18.9 | 16.5–21.9 | |
| No | 15,160 | 7,757 | 51.2 | 18.5 | 17.7–19.2 | |
| Elderly-specific patient complexities | ||||||
| Cognitive impairment | 0.449 | |||||
| Yes | 1,634 | 849 | 52.0 | 17.3 | 15.3–20.4 | |
| No | 15,019 | 7,701 | 51.3 | 18.6 | 17.7–19.5 | |
| Depression | 0.494 | |||||
| Yes | 1,072 | 546 | 50.9 | 17.4 | 15.3–20.4 | |
| No | 15,581 | 8,004 | 51.4 | 18.6 | 17.7–19.5 | |
| Falls and fall risk | 0.018 | |||||
| Yes | 217 | 97 | 44.7 | 12.7 | 10.2–16.2 | |
| No | 16,436 | 8,453 | 51.4 | 18.6 | 17.7–19.5 | |
| Polypharmacy | < 0.001 | |||||
| ≤ 6 drugs | 9,306 | 4,889 | 52.5 | 20.4 | 19.2–21.9 | |
| > 6 drugs | 7,347 | 3,661 | 49.8 | 16.5 | 15.6–17.4 | |
| Urinary incontinence | 0.028 | |||||
| Yes | 16,318 | 8,393 | 51.4 | 18.6 | 17.7–19.2 | |
| No | 335 | 157 | 46.9 | 14.6 | 10.8–18.6 | |
Note: Based on data from the Humana MAPD plan database of 16,653 elderly patients with T2DM who had treatment intensification during the assessment period (January 2007 to June 2012).
A1c = hemoglobin A1c; CI = confidence interval; COPD = chronic obstructive pulmonary disease; DCSI = Diabetes Complications Severity Index; FFS = fee-for-service; HMO = health maintenance organization; MAPD = Medicare Advantage Prescription Drugs; PPO = preferred provider organization; T2DM = type 2 diabetes mellitus.
Median time to treatment intensification was shorter for elderly patients with T2DM with polypharmacy (16.5 months) and falls and fall risk (12.7 months) versus those without polypharmacy (20.4 months) and no fall risk (18.6 months). Elderly patients with urinary incontinence had a longer median time to treatment intensification (18.6 months) versus those without urinary incontinence (14.6 months). The median time to treatment intensification did not significantly differ by the elderly-specific patient complexities of cognitive impairment and depression. Individuals with baseline A1c values ≥ 9.0% had shorter median time to treatment intensification (12.1 months) than those with A1c values of ≥ 8.0% to < 8.5% and ≥ 8.5% to < 9.0% (23.2 months and 16.9 months, respectively).
Treatment intensification rates varied by follow-up months (Table 2). For example, 23.5% of patients received treatment intensification within 4 months; 27.8% within 6 months, 36.9% within 12 months, and 44.7% within 24 months. Among those with treatment intensification, a majority received it through the addition of a third OAD (58.7%; n = 4,754) followed by insulin initiation (41.3%; n = 3,349). This finding was consistent across all lengths of follow-up. Statistically significant differences in rates of treatment intensification by age were observed for all follow-up months (P < 0.001).
TABLE 2.
Description of Rates of Treatment Intensification at Variable Lengths of Follow-up by Age Groups in T2DM Patients with Uncontrolled Diabetes: Humana MAPD Data 2007–2012a
| Follow-up Time | Treatment Intensification | Type of Treatment Intensification Among Those Intensified | ||
|---|---|---|---|---|
| N | % | Addition of Third OAD (%) | Insulin (%) | |
| All | ||||
| 4 months | 3,911 | 23.5 | 60.9 | 39.1 |
| 6 months | 4,632 | 27.8 | 60.1 | 39.9 |
| 12 months | 6,145 | 36.9 | 59.8 | 40.2 |
| 24 months | 7,445 | 44.7 | 59.3 | 40.7 |
| Overall | 8,103 | 48.7 | 58.7 | 41.3 |
| Age group: 65–74 years | ||||
| 4 months | 2,566 | 24.2 | 62.0 | 38.0 |
| 6 months | 3,044 | 28.7 | 61.6 | 38.4 |
| 12 months | 4,022 | 37.9 | 61.5 | 38.5 |
| 24 months | 4,877 | 45.9 | 60.9 | 39.1 |
| Overall | 5,327 | 50.2 | 60.3 | 39.7 |
| Age group: ≥ 75 years | ||||
| 4 months | 1,345 | 22.3 | 58.6 | 41.4 |
| 6 months | 1,588 | 26.3 | 57.2 | 42.8 |
| 12 months | 2,123 | 35.2 | 56.7 | 43.3 |
| 24 months | 2,568 | 42.6 | 56.4 | 43.6 |
| Overall | 2,776 | 46.0 | 55.5 | 44.5 |
Note: Based on data from the Humana MAPD plan database of 16,653 elderly patients with T2DM who had treatment intensification during the assessment period (January 2007 to June 2012).
Rates of treatment intensification significantly (P < 0.001) differed between age groups 65–74 years and ≥ 75 years when measured using chi-square tests.
MAPD = Medicare Advantage Prescription Drugs; OAD = oral antidiabetes drug; T2DM = type 2 diabetes mellitus.
Adjusted hazard ratios and 95% CIs from multivariable Cox-proportional hazards regression are presented in Table 3. The only elderly-specific patient complexity to significantly affect time to treatment intensification was polypharmacy. T2DM patients taking > 6 drugs had a higher aHR of treatment intensification compared with those taking ≤ 6 drugs (aHR, 1.10; 95% CI = 1.04–1.15; P < 0.001). Other elderly-specific patient complexities were not significantly associated with time to treatment intensification.
TABLE 3.
Adjusted Hazard Ratios for Multivariable Cox-Proportional Hazards Regression for Treatment Intensification in T2DM Patients with Uncontrolled Diabetes: Humana MAPD Data 2007–2012
| aHR | 95% CI | P Value | |
|---|---|---|---|
| Demographic and insurance characteristics | |||
| Age | |||
| 65–74 years | Ref. | Ref. | |
| ≥75 years | 0.90 | 0.86–0.94 | < 0.001 |
| Sex | |||
| Women | 1.04 | 0.99–1.08 | 0.345 |
| Men | Ref. | Ref. | |
| Race | |||
| White | Ref. | Ref. | |
| African American | 0.83 | 0.79–0.89 | < 0.001 |
| Hispanic | 0.97 | 0.87–1.09 | 0.870 |
| Other | 0.91 | 0.83–1.00 | 0.087 |
| U.S. region | |||
| Midwest | 0.93 | 0.85–1.03 | 0.199 |
| South | 0.88 | 0.82–0.95 | < 0.001 |
| Northeast/Northwest/other | Ref. | Ref. | |
| Insurance type | |||
| HMO | 0.85 | 0.80–0.91 | < 0.001 |
| PPO | 1.00 | 0.92–1.07 | 0.872 |
| FFS | Ref. | Ref. | |
| Other | 0.90 | 0.72–1.13 | 0.347 |
| Donut hole | |||
| Pre- and post-donut hole | 0.75 | 0.70–0.81 | < 0.001 |
| In donut hole | Ref. | Ref. | |
| Baseline health care utilization | |||
| Inpatient visits | |||
| Yes | 0.98 | 0.91–1.06 | 0.966 |
| No | Ref. | Ref. | |
| Emergency department visits | |||
| Yes | 1.02 | 0.93–1.11 | 0.479 |
| No | Ref. | Ref. | |
| Number of outpatient visits | |||
| 0 | Ref. | Ref. | |
| 1–2 | 0.96 | 0.91–1.02 | 0.150 |
| ≥ 3 | 1.09 | 1.03–1.15 | 0.021 |
| Clinical characteristics | |||
| DCSI categories | |||
| 0 | Ref. | Ref. | |
| 1–2 | 1.07 | 1.00–1.14 | 0.051 |
| 3 | 1.05 | 0.99–1.11 | 0.126 |
| 4–12 | 1.09 | 1.02–1.17 | 0.025 |
| Hypoglycemia | |||
| Yes | 0.99 | 0.87–1.13 | 0.0702 |
| No | Ref. | Ref. | |
| Baseline A1c categories | |||
| ≥ 8.0% to < 8.5% | Ref. | Ref. | |
| ≥ 8.5% to < 9.0% | 1.20 | 1.12–1.27 | < 0.001 |
| ≥ 9.0% | 1.51 | 1.44–1.59 | < 0.001 |
| Discordant condition (COPD) | |||
| Yes | 0.99 | 0.94–1.03 | 0.512 |
| No | Ref. | Ref. | |
| Dominant condition (cancer) | |||
| Yes | 1.00 | 0.92–1.08 | 0.750 |
| No | Ref. | Ref. | |
| Elderly-specific patient complexities | |||
| Cognitive impairment | |||
| Yes | 0.97 | 0.86–1.09 | 0.898 |
| No | Ref. | Ref. | |
| Depression | |||
| Yes | 1.02 | 0.88–1.18 | 0.946 |
| No | Ref. | Ref. | |
| Falls and fall risk | |||
| Yes | 1.16 | 0.96–1.39 | 0.098 |
| No | Ref. | Ref. | |
| Polypharmacy | |||
| ≤ 6 drugs | Ref. | Ref. | |
| > 6 drugs | 1.10 | 1.04–1.15 | < 0.001 |
| Urinary incontinence | |||
| Yes | 1.13 | 0.97–1.31 | 0.102 |
| No | Ref. | Ref. | |
Note: Based on data from the Humana MAPD plan database of 16,653 elderly patients with T2DM who had treatment intensification during the assessment period (January 2007 to June 2012).
A1c = hemoglobin A1c; aHR=adjusted hazard ratio; CI = confidence interval; COPD = chronic obstructive pulmonary disease; DCSI = Diabetes Complications Severity Index; FFS = fee-for-service; HMO = health maintenance organization; MAPD = Medicare Advantage Prescription Drugs; PPO = preferred provider organization; Ref. = reference; T2DM = type 2 diabetes mellitus.
Other variables associated with significantly higher aHRs of treatment intensification were ≥ 3 outpatient visits (aHR, 1.09; 95% CI = 1.03–1.15; P = 0.021), DCSI categories 1–2 (aHR, 1.07; 95% CI = 1.00–1.14; P = 0.051) and 4–12 (aHR, 1.09; 95% CI = 1.02–1.17; P = 0.025), and patients with baseline A1c values of ≥ 8.5% to < 9.0% (aHR, 1.20; 95% CI = 1.12–1.27; P < 0.001) and ≥ 9.0% (aHR, 1.51; 95% CI = 1.44–1.59; P < 0.001).
Variables associated with lower aHRs of treatment intensification were African American race (aHR, 0.83; 95% CI = 0.79–0.89; P < 0.001), aged ≥ 75 years (aHR, 0.90; 95% CI = 0.86–0.94; P < 0.001), residing in the South of the United States (aHR, 0.88; 95% CI=0.82–0.95; P < 0.001), enrollment in an HMO insurance plan (aHR, 0.85; 95% CI = 0.80–0.91; P < 0.001), and pre- or post-donut hole status (aHR, 0.75; 95% CI = 0.70–0.81; P <0.001).
Results from sensitivity analysis (including dose escalation) revealed that median time to treatment intensification decreased to 11.1 months (95% CI = 10.6–11.6). Results from multivariable Cox-proportional hazards regression remained the same as our previous analysis without dose escalation.
Discussion
This study analyzed the factors associated with time to treatment intensification among elderly Humana MAPD plan database patients with T2DM who had A1c levels ≥ 8.0% despite using 2 OADs at baseline. The study found that 51.3% of elderly patients with T2DM and A1c levels ≥ 8.0% were censored without treatment intensification, and 48.7% received treatment intensification over a period of 4.67 years. As previous studies have included both nonelderly and elderly populations,5,6 the current study findings cannot be directly compared. However, in a study that provided data on age categories and was based on author calculations, only 18.3% of elderly patients (aged ≥ 65 years) with T2DM had received treatment intensification over a period of 14 months.6 This finding suggests that the high rates of treatment intensification observed in the current study could be due to the long follow-up period. In the current study, median time to treatment intensification was 18.5 months, but because of the few studies among elderly patients with T2DM, it is not possible to compare the current study findings to existing studies on time to treatment intensification.
A noteworthy finding of the current study is the absence of an association between treatment intensification and elderly-specific patient complexities except for polypharmacy. These findings suggest that in a managed care setting, patient complexities are not associated with time to treatment intensification. It has been suggested that the quality of diabetes care among MAPD plan enrollees with T2DM and comorbid conditions is higher than in those patients without comorbid conditions.21 It is highly plausible that MAPD plan enrollees might receive appropriate treatment irrespective of complexities owing to the provision of direct or indirect integrated and coordinated care.22 Future research needs to explore whether such lack of association is due to personalized treatment goals or better management of diabetes through coordinated care among individuals with T2DM and complex chronic conditions.
In the current study, the only elderly-specific patient complexity that was significant was polypharmacy, and these patients had a shorter duration of time to treatment intensification compared with patients without polypharmacy. A previous study has reported that T2DM patients with OAD polypharmacy were more likely to have poorer glycemic control compared with those patients without polypharmacy.23 Although not specific to elderly patients with T2DM, predictive models in health care suggest that the number of medications a patient takes is predictive of medical complexity24; therefore, it is possible that health care providers may intensify current treatment(s) in order to reduce adverse outcomes in medically complex patients, for example, those with polypharmacy.
Patients aged ≥ 75 years had longer median time to intensification compared with those aged 65–74 years. We do not know of any comparable studies assessing time to intensification in elderly T2DM patients. However, existing evidence suggests that among adult patients with T2DM, younger individuals (aged < 65 years) had higher rates of treatment intensification compared with elderly individuals.5 Other studies have found that intensification of treatment decreases with increasing age.5,6,24 Because of the lack of strong evidence that tight glycemic control is beneficial to older patients with T2DM, health care providers may not intensify treatment regimens in these patients.25 Previous studies have demonstrated that older patients with T2DM had less treatment intensification compared with patients in other age groups.26,27 Future studies should examine if patient-physician factors influence treatment intensification among elderly patients with T2DM.
The current study found that the aHR for treatment intensification was lower for African American patients with T2DM compared with white patients, suggesting a significantly longer time to treatment intensification in this racial group. Again, owing to a lack of studies that focus exclusively on elderly patients with T2DM, the validity of the current study’s findings with regard to race cannot be confirmed. Specific reasons for lower treatment intensification among African Americans were not directly examined in this study and can only be speculated. It has been suggested that physician behavior may be influenced by a patient’s race/ethnicity,28 which may have resulted in the longer time to treatment intensification among African American patients as seen in the current study. Furthermore, beliefs by African American patients about differential treatment from health care providers due to their race29 and lack of trust in medical systems30 may have translated into the longer time to treatment intensification for this racial group.
Given that half of the study population in the current study was censored without treatment intensification (defined as patients without dose escalation), our findings suggest a need for programs designed to promote treatment intensification in patients with T2DM. Several initiatives have been proven to reduce clinical inertia in diabetes management, most notably educational interventions among physicians that target cognitive barriers to medication initiation and intensification; clinical decision-support interventions using electronic medical record technology; and patient activation interventions.31,32 The low rates of treatment intensification among elderly patients with T2DM in the current study warrant the use of such interventions, specifically tailored to elderly patients.
Limitations
The study findings must be interpreted in the context of some limitations. As this was an observational, retrospective cohort study, the data may be subject to confounding and selection bias. The presence of a claim for filled prescriptions does not indicate if the medication was actually consumed or if it was taken as prescribed. Our dose escalation measurement may be an overestimation because we considered dose increases of even 1 OAD or in a single prescription. The presence of an ICD-9-CM diagnosis code on a medical claim may not necessarily signify the positive presence of disease, as the diagnosis might have been incorrectly coded or included as a rule-out criterion rather than as the actual disease. Finally, the analysis was based on data from a managed care population and may not be representative of other populations or generalizable to all Medicare beneficiaries with T2DM.
However, the strengths of this study are many and include its use of a nationwide database of Humana MAPD beneficiaries, examination of factors associated with treatment intensification in elderly patients with inadequately controlled T2DM (enabled by access to laboratory data on A1c levels), adjustments to a comprehensive list of independent variables, assessment of patient complexities, and inclusion of dose escalation based on drug names and potencies.
Conclusions
The current study makes a unique contribution to the existing literature on treatment intensification for patients with inadequately controlled diabetes by exclusively focusing on elderly patients (aged ≥ 65 years) seeking care in real-world practice settings. Also, it assesses the association between treatment intensification and elderly-specific patient complexities; clinical, demographic, and insurance characteristics; and health care use. The current study found that nearly half of elderly patients with T2DM did not receive treatment intensification over a 5-year period, and that elderly-specific patient complexities did not negatively affect time to treatment intensification. Further studies are warranted to examine why some subgroups of patients with uncontrolled T2DM, such as those aged ≥ 75 years and African American patients, had lower treatment intensification rates and had to wait longer to receive treatment intensification.
What is already known about this subject
Among individuals with type 2 diabetes mellitus (T2DM), treatment intensification with insulin is needed to prevent diabetes complications among elderly patients who failed to achieve glycemic control.
Risk factors specific to elderly patients may facilitate or act as barriers to treatment intensification with insulin.
What this study adds
We show that elderly-specific patient complexities are not associated with time to treatment intensification.
These findings emphasize a positive effect on the integrated health care delivery model.
Emerging health care delivery models that target integrated care may therefore be crucial in providing appropriate treatment for elderly T2DM patients with complex conditions.
Acknowledgments
The authors received writing/editorial support in preparing this manuscript from Pim Dekker, PhD, of Excerpta Medica, funded by Sanofi U.S.
APPENDIX ICD-9-CM Diagnosis Codes
| Disease Conditions | ICD-9-CM Codes |
|---|---|
| Diabetes Complications Severity Index | |
| Retinopathy | |
| Diabetic ophthalmologic disease | 250.5x |
| Background retinopathy | 362.01 |
| Other retinopathy | 362.1 |
| Retinal edema | 362.83 |
| Cystoid macular edema/degeneration | 362.53 |
| Other retinal disorders | 362.81, 362.82 |
| Proliferative retinopathy | 362.02 |
| Retinal detachment | 361.xx |
| Blindness | 369.xx |
| Vitreous hemorrhage | 379.23 |
| Nephropathy | |
| Diabetic nephropathy | 250.4 |
| Acute glomerulonephritis | 580 |
| Nephrotic syndrome | 581 |
| Hypertension, nephrosis | 581.81 |
| Chronic glomerulonephritis | 582 |
| Nephritis/nephropathy | 583 |
| Chronic renal failure | 585 |
| Renal failure, not otherwise specificed | 586 |
| Renal insufficiency | 593.9 |
| Neuropathy | |
| Diabetic neuropathy | 356.9, 250.6 |
| Amyotrophy | 358.1 |
| Cranial nerve palsy | 951.0, 951.1, 951.3 |
| Mononeuropathy | 354.0–355.9 |
| Charcot’s arthropathy | 713.5 |
| Polyneuropathy | 357.2 |
| Neurogenic bladder | 596.54 |
| Autonomic neuropathy | 337.0, 337.1 |
| Gastroparesis/diarrhea | 564.5, 536.3 |
| Orthostatic hypotension | 458.0 |
| Cerebrovascular | |
| Transient ischemic attack | 435 |
| Stroke | 431, 433, 434, 436 |
| Cardiovascular disease | |
| Atherosclerosis | 440.xx |
| Other ischemic heart disease | 411 |
| Angina pectoris | 413 |
| Other chronic ischemic heart disease | 414 |
| Myocardial infarction | 410 |
| Ventricular fibrillation, arrest | 427.1, 427.3 |
| Atrial fibrillation, arrest | 427.4, 427.5 |
| Other atherosclerotic cardiovascular disease | 429.2 |
| Old myocardial infarction | 412 |
| Heart failure | 428 |
| Atherosclerosis, severe | 440.23, 440.24 |
| Aortic aneurysm/dissection | 441 |
| Peripheral vascular disease | |
| Diabetic peripheral vascular disease | 250.7 |
| Other aneurysm, lower extremity | 442.3 |
| Peripheral vascular disease | 443.81, 443.9 |
| Foot wound + complications | 892.1 |
| Claudication, intermittent | 443.9 |
| Peripheral vascular disease | |
| Embolism/thrombosis, lower extremity | 444.22 |
| Gangrene | 785.4 |
| Gas gangrene | 0.40 |
| Ulcer of lower limbs | 707.1 |
| Metabolic | |
| Ketoacidosis | 250.1 |
| Hyperosmolar | 250.2 |
| Other coma | 250.3 |
| Elderly-Specific Patient Complexities | |
| Cognitive impairment | |
| Delirium, dementia, and amnestic and other cognitive disorders | 290.0x, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 290.8, 290.9, 293.0, 293.1, 294.0, 294.1, 294.10, 294.11, 294.8, 294.9, 310.0, 310.2, 310.8, 310.9, 331.0, 331.1, 331.11, 331.19, 331.2, 331.82, 797.xx |
| Huntington’s disease | 333.4 |
| Parkinson’s disease | 332.xx |
| Schizophrenia and other psychotic disorders | 293.8, 293.82, 295.00, 295.01, 295.02, 295.03, 295.04, 295.05, 295.10, 295.11, 295.12, 295.13, 295.14, 295.15, 295.20, 295.21, 295.22, 295.23, 295.24, 295.25, 295.30, 295.31, 295.32, 295.33, 295.34, 295.35, 295.40, 295.41, 295.42, 295.43, 295.44, 295.45, 295.50, 295.51, 295.52, 295.53, 295.54, 295.55, 295.60, 295.61, 295.62, 295.63, 295.64, 295.65, 295.70, 29.571, 295.72, 295.73, 295.74, 295.75, 295.80, 295.81, 295.82, 295.83, 295.84, 295.85, 295.90, 295.91, 295.92, 295.93, 295.94, 295.95, 297.0, 297.1, 297.2, 297.3, 297.8, 297.9, 298.0, 298.1, 298.2, 298.3, 298.4, 298.8, 298.9 |
| Bipolar disorder | 296.00, 296.01, 296.02, 296.03, 296.04, 296.05, 296.06, 261.0, 296.11, 296.12, 296.13, 296.1, 296.15, 296.16, 296.40, 296.41, 296.42, 296.43, 296.44, 296.45, 296.46, 296.50, 296.51, 296.52, 296.53, 296.54, 296.55, 296.56, 296.60, 296.61, 296.62, 296.63, 296.64, 296.65, 296.66, 296.7, 296.80, 296.81, 296.82, 296.89, 296.90, 296.99 |
| Urinary incontinence | |
| Urinary incontinence | 788.3 |
| Urinary incontinence unspecified | 788.30 |
| Urge incontinence | 788.31 |
| Mixed incontinence, male, female | 788.33 |
| Incontinence without sensory awareness | 788.34 |
| Continuous leakage | 788.37 |
| Falls and fall risk | |
| Falls | E880 through E888 |
| Fall risk | V15.88 |
| Mental illness | |
| Depression | 293.83, 296.20, 296.21, 296.22, 296.23, 296.24, 296.25, 296.26, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36, 300.4, 311.xx |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Footnotes
DISCLOSURES
Sanofi U.S. provided research funding support, proposed and codeveloped the concept, and codeveloped the analysis plan. Sanofi was also involved in interpreting the results of the analyses, reviewing the manuscript, and providing comments on the manuscript versions. Sanofi approved the manuscript to be submitted for publication.
Ajmera, Raval, and Bhattacharya were PhD students at West Virginia University at the time this manuscript was written. Raval and Sambamoorthi have no disclosures. Zhou and Wei are employees of Sanofi U.S. Pan is an employee of Pro Unlimited, under contract with Sanofi U.S. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the organizations that supported the study.
Study design was created by Ajmera, Wei, and Sambamoorthi, assisted by Raval, Bhattacharya, and Zhou. Zhou, Pan, and Sambamoorthi took the lead in data collection, with assistance from Ajmera, Wei, and Bhattacharya. Data interpretation was performed by Ajmera, Raval, Bhattacharya, and Sambamoorthi. The manuscript was written primarily by Ajmera, along with Raval and assisted by Bhattacharya and Sambamoorthi, and revised by Ajmera, Raval, and Sambamoorthi, with assistance from Zhou, Wei, and Bhattacharya.
Contributor Information
Mayank Ajmera, Health Outcomes Scientist, RTI Health Solutions, Research Triangle Park, North Carolina.
Amit Raval, Senior Researcher, HealthCore, Wilmington, Delaware.
Steve Zhou, Senior Manager, Evidence-Based Medicine, Medical Affairs, Sanofi U.S., Bridgewater, New Jersey.
Wenhui Wei, Senior Director, Evidence-Based Medicine, Medical Affairs, Sanofi U.S., Bridgewater, New Jersey.
Rituparna Bhattacharya, Consultant, Humana-Clinical Analytics, Irving, Texas.
Chunshen Pan, Consultant, PRO Unlimited, Boca Raton, Florida.
Usha Sambamoorthi, Professor, Department of Pharmaceutical Systems and Policy, School of Pharmacy, West Virginia University, Morgantown.
References
- 1.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. [Accessed October 20, 2015];Diabetes Care. 2006 29(8):1963–72. doi: 10.2337/dc06-9912. Available at: http://care.diabetesjournals.org/content/29/8/1963.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- 2.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connor PJ, Ismail-Beigi F. Near-normalization of glucose and microvascular diabetes complications: data from ACCORD and ADVANCE. Ther Adv Endocrinol Metab. 2011;2(1):17–26. doi: 10.1177/2042018810390545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei NJ, Wexler DJ, Nathan DM, Grant RW. Intensification of diabetes medication and risk for 30-day readmission. Diabet Med. 2013;30(2):e56–62. doi: 10.1111/dme.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhry SI, Berlowitz DR, Concato J. Do age and comorbidity affect intensity of pharmacological therapy for poorly controlled diabetes mellitus? J Am Geriatr Soc. 2005;53(7):1214–16. doi: 10.1111/j.1532-5415.2005.53370.x. [DOI] [PubMed] [Google Scholar]
- 6.Fu AZ, Qiu Y, Davies MJ, Radican L, Engel SS. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13(8):765–69. doi: 10.1111/j.1463-1326.2011.01405.x. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. [Accessed October 20, 2015];Diabetes Care. 2009 32(1):193–203. doi: 10.2337/dc08-9025. Available at: http://care.diabetesjournals.org/content/32/1/193.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodondi N, Peng T, Karter AJ, et al. Therapy modifications in response to poorly controlled hypertension, dyslipidemia, and diabetes mellitus. Ann Intern Med. 2006;144(7):475–84. doi: 10.7326/0003-4819-144-7-200604040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafata JE, Dobie EA, Divine GW, Ulcickas Yood ME, McCarthy BD. Sustained hyperglycemia among patients with diabetes: what matters when action is needed? [Accessed October 20, 2015];Diabetes Care. 2009 32(8):1447–52. doi: 10.2337/dc08-2028. Available at: http://care.diabetesjournals.org/content/32/8/1447.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Statistics about Diabetes. Data from the. [Accessed October 20, 2015];National Diabetes Statistics Report. 2014 (released June 10, 2014). Available at: http://www.diabetes.org/diabetes-basics/statistics/
- 11.Kirkman SM, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. [Accessed October 20, 2015];J Am Geriatr Soc. 2012 60(12):2342–56. doi: 10.1111/jgs.12035. Available at: http://onlinelibrary.wiley.com/doi/10.1111/jgs.12035/epdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. [Accessed October 20, 2015];Am J Manag Care. 2008 14(1):15–23. Available at: http://www.ajmc.com/publications/issue/2008/2008-01-vol14-n1/Jan08-2781p15-23/ [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. [Accessed October 20, 2015];Am J Manag Care. 2012 18(11):721–26. Available at: http://www.ajmc.com/publications/issue/2012/2012-11-vol18-n11/Validating-the-Adapted-Diabetes-Complications-Severity-Index-in-Claims-Data/ [PubMed] [Google Scholar]
- 14.Ginde AA, Blanc PG, Lieberman RM, Camargo CA., Jr Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. [Accessed October 20, 2015];BMC Endocr Disord. 2008 8:4. doi: 10.1186/1472-6823-8-4. Available at: http://www.biomedcentral.com/content/pdf/1472-6823-8-4.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. [Accessed October 20, 2015];Diabetes Care. 2006 29(3):725–31. doi: 10.2337/diacare.29.03.06.dc05-2078. Available at: http://care.diabetes-journals.org/content/29/3/725.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- 16.Mehta S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using antipsychotic agents: a propensity-matched retrospective cohort study. Drugs Aging. 2010;27(10):815–29. doi: 10.2165/11537890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Tinetti ME, Gordon C, Sogolow E, Lapin P, Bradley EH. Fall-risk evaluation and management: challenges in adopting geriatric care practices. [Accessed October 20, 2015];Gerontologist. 2006 46(6):717–25. doi: 10.1093/geront/46.6.717. Available at: http://gerontologist.oxford-journals.org/content/46/6/717.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg JF, Brooks JO, 3rd, Kurita K, et al. Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: findings from the STEP-BD. J Clin Psychiatry. 2009;70(2):155–62. doi: 10.4088/jcp.08m04301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anger JT, Saigal CS, Madison R, Joyce G, Litwin MS Urologic Diseases of America Project. Increasing costs of urinary incontinence among female Medicare beneficiaries. J Urol. 2006;176(1):247–51. doi: 10.1016/S0022-5347(06)00588-X. [DOI] [PubMed] [Google Scholar]
- 20.Rich JT, Neely JG, Paniello RC, Voelker CC, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg. 2010;143(3):331–36. doi: 10.1016/j.otohns.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham JM, Marmor S, Knutson D, Zeglin J, Virnig B. Variation in diabetes care quality among Medicare advantage plans: understanding the role of case mix. Am J Med Qual. 2012;27(5):377–82. doi: 10.1177/1062860611428529. [DOI] [PubMed] [Google Scholar]
- 22.Gold M. Medicare’s private plans: a report card on Medicare Advantage. [Accessed October 20, 2015];Health Aff (Millwood) 2009 28(1):w41–54. doi: 10.1377/hlthaff.28.1.w41. Available at: http://content.healthaf-fairs.org/content/28/1/w41.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- 23.Willey CJ, Andrade SE, Cohen J, Fuller JC, Gurwitz JH. Polypharmacy with oral antidiabetic agents: an indicator of poor glycemic control. [Accessed October 20, 2015];Am J Manag Care. 2006 12(8):435–40. Available at: http://www.ajmc.com/publications/issue/2006/2006-08-vol12-n8/Aug06-2339p435-440/ [PubMed] [Google Scholar]
- 24.Higdon R, Stewart E, Roach JC, et al. Predictive analytics in healthcare: medications as a predictor of medical complexity. [Accessed October 20, 2015];Big Data. 2013 1(4):237–44. doi: 10.1089/big.2013.0024. Available at: http://online.liebertpub.com/doi/pdf/10.1089/big.2013.0024. [DOI] [PubMed] [Google Scholar]
- 25.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. [Accessed October 20, 2015];JAMA. 2009 301(15):1565–72. doi: 10.1001/jama.2009.460. Available at: http://jama.jamanetwork.com/article.aspx?articleid=183750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith NL, Savage PJ, Heckbert SR, et al. Glucose, blood pressure, and lipid control in older people with and without diabetes mellitus: the Cardiovascular Health Study. J Am Geriatr Soc. 2002;50(3):416–23. doi: 10.1046/j.1532-5415.2002.50103.x. [DOI] [PubMed] [Google Scholar]
- 27.Grover SA, Coupal L, Zowall H, Dorais M. Cost-effectiveness of treating hyperlipidemia in the presence of diabetes: who should be treated? [Accessed October 20, 2015];Circulation. 2000 102(7):722–27. doi: 10.1161/01.cir.102.7.722. Available at: http://circ.ahajournals.org/content/102/7/722.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- 28.Van Ryn M. Research on the provider contribution to race/ethnicity disparities in medical care. Med Care. 2002;40(1 Suppl):I140–51. doi: 10.1097/00005650-200201001-00015. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19(2):101–10. doi: 10.1111/j.1525-1497.2004.30262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traylor AH, Subramanian U, Uratsu CS, Mangione CM, Selby JV, Schmittdiel JA. Patient race/ethnicity and patient-physician race/ethnicity concordance in the management of cardiovascular disease risk factors for patients with diabetes. [Accessed October 20, 2015];Diabetes Care. 2010 33(3):520–25. doi: 10.2337/dc09-0760. Available at: http://care.diabe-tesjournals.org/content/33/3/520.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperl-Hillen JM, O’Connor PJ, Rush RW, et al. Simulated physician learning program improves glucose control in adults with diabetes. [Accessed October 20, 2015];Diabetes Care. 2010 33(8):1727–33. doi: 10.2337/dc10-0439. Available at: http://care.diabetesjournals.org/content/33/8/1727.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenfield S, Kaplan SH, Ware JE, Jr, Yano EM, Frank HJ. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–57. doi: 10.1007/BF02595921. [DOI] [PubMed] [Google Scholar]