Abstract
BACKGROUND
Crizotinib has antitumor activity in ALK (anaplastic lymphoma receptor tyrosine kinase)-rearranged non–small cell lung cancer (NSCLC). The current diagnostic test for ALK rearrangement is breakapart fluorescence in situ hybridization (FISH), but FISH has low throughput and is not always reflective of protein concentrations. The emergence of multiple clinically relevant biomarkers in NSCLC necessitates efficient testing of scarce tissue samples. We developed an anaplastic lymphoma kinase (ALK) protein assay that uses multiplexed selected reaction monitoring (SRM) to quantify absolute amounts of ALK in formalin-fixed paraffin-embedded (FFPE) tumor tissue.
METHODS
After validation in formalin-fixed cell lines, the SRM assay was used to quantify concentrations of ALK in 18 FFPE NSCLC samples that had been tested for ALK by FISH and immunohistochemistry. Results were correlated with patient response to crizotinib.
RESULTS
We detected ALK in 11 of 14 NSCLC samples with known ALK rearrangements by FISH. Absolute ALK concentrations correlated with clinical response in 5 of 8 patients treated with crizotinib. The SRM assay did not detect ALK in 3 FISH-positive patients who had not responded to crizotinib. In 1 of these cases, DNA sequencing revealed a point mutation that predicts a nonfunctional ALK fusion protein. The SRM assay did not detect ALK in any tumor tissue with a negative ALK status by FISH or immunohistochemistry.
CONCLUSIONS
ALK concentrations measured by SRM correlate with crizotinib response in NSCLC patients. The ALK SRM proteomic assay, which may be multiplexed with other clinically relevant proteins, allows for rapid identification of patients potentially eligible for targeted therapies.
Targeted cancer therapies designed to disrupt proteins in oncogenic signaling pathways are the current focus of cancer drug development. Several targeted therapy regimens have been approved by the US Food and Drug Administration for the treatment of advanced lung cancer. The first targeted therapy regimens are the epidermal growth factor receptor (EGFR)11 tyrosine kinase inhibitors (TKIs), such as erlotinib and afatinib, which show efficacy against tumors harboring activating mutations in EGFR (epidermal growth factor receptor)12 kinase domain. In 2013, ALK (anaplastic lymphoma receptor tyrosine kinase) 2p23 rearrangements were validated as additional molecular targets in TKI-based therapy for late-stage non–small cell lung cancer (NSCLC) with US Food and Drug Administration approval of the anaplastic lymphoma kinase (ALK) inhibitor crizotinib. This was followed by the recent approval of the second-generation ALK TKI ceritinib, which is active in the majority of patients who have acquired crizotinib resistance (1, 2). Other therapeutic agents for patients with ALK-rearranged disease are currently in clinical trials (3).
ALK rearrangements are found in 2%–5% of NSCLC patients; most patients are relatively young, with a history of never or light smoking, and have tumors with nonsquamous histology. Current evidence-based guidelines recommend testing all patients with advanced, nonsquamous NSCLC for ALK rearrangements (4). In patients with ALK-rearranged NSCLC, crizotinib yields better response rates (65% vs 20%) and higher progression-free survival (7.7 vs 3 months) than standard cytotoxic chemotherapy (5).
Under typical physiological conditions, ALK is not expressed in the adult lung, whereas it is embryonically expressed in early developmental regulation. ALK rearrangement leads to aberrant expression of the ALK fusion protein and constitutive activation of the ALK kinase domain (5, 6). Oncogenic activation of ALK occurs owing to intrachromosomal inversion in chromosome 2, leading to fusion of the tyrosine kinase domain of ALK with a 5′ end partner such as EML4 (echinoderm micro-tubule associated protein-like 4). EML4 is the most common ALK fusion partner in NSCLC; however, > 20 EML4-ALK transcript variants have been described, and ≥ 6 other partner genes have been identified (7–11). The clinical significance of different types of ALK rearrangements is under investigation (12).
Presently, fluorescence in situ hybridization (FISH) is the standard test to detect ALK rearrangements. The ALK Break-Apart FISH Probe Kit (Abbott Molecular) was used in clinical trials of crizotinib and approved by the Food and Drug Administration as a companion diagnostic in 2013. However, the presence of ALK gene rearrangement is not always reflective of ALK protein concentrations. In patients with advanced disease, a small tissue biopsy is often the only material available; hence, extracting as much phenotypic and molecular information as possible from a limited tissue sample is desirable and warranted.
Although ALK FISH detects the breakpoint of the ALK gene, ALK immunohistochemistry (IHC) detects ALK protein overexpression. Multiple studies have demonstrated ALK IHC to be tightly correlated with ALK FISH, suggesting that IHC could be used as the routine screening method to identify pathologic ALK rearrangement in NSCLC (13–16). Consequently, many groups have attempted to standardize and validate IHC methods to supplement or replace ALK FISH (17–19). Others have reported discrepancies between IHC and FISH (20 –22). A study of 3244 consecutive NSCLC cases found that FISH and IHC were discordant in 46.6% of ALK-rearranged tumors; both types of discordant cases (FISH+/IHC− and FISH−/IHC+) responded to crizotinib (22). IHC testing of a single protein requires 1–2 formalin-fixed paraffin-embedded (FFPE) sections, and testing of multiple proteins can rapidly deplete small tissue samples.
Both IHC and FISH have technical limitations. IHC is sensitive to preanalytical variables such as delayed fixation, fixation time, and the age of cut tissue sections (23). FISH is a DNA-based assay, and many preanalytical tissue handling steps may lead to DNA degradation and test failure (24). Both FISH and IHC are semiquantitative, and their interpretation can be somewhat subjective and challenging. McLeer-Florin et al. reported that, in their hands, 19 of 100 lung adenocarcinomas analyzed by FISH were uninterpretable (16).
To overcome these limitations, we have developed a proteomic assay to detect and quantify ALK expression in FFPE tumor tissue. Liquid Tissue® selected reaction monitoring (SRM) is a mass spectrometry (MS)-based multiplexed assay that allows simultaneous measurement of the absolute amount of dozens of targeted proteins in FFPE tissues, including ALK (25–27). The assay results were compared with ALK testing results by FISH and IHC and correlated with response to crizotinib therapy.
Materials and Methods
PATIENTS AND TISSUE SAMPLES
After institutional review board approval at the medical center sites, we identified FFPE tissue sections (n = 18) from 17 NSCLC patients who had been tested for ALK rearrangement in 2006–2013 (14 ALK FISH+; 4 ALK FISH−). The samples were 14 lung resection samples, 2 lymph node metastases, and 2 brain metastases. The tissues had been archived at Dartmouth-Hitchcock Medical Center, Lebanon, NH (n = 12; 11 patients); Cleveland Clinic, Cleveland, OH (n = 5); and West Virginia University (n = 1). All tissue samples and clinical annotations (extracted from medical records) were anonymized.
SAMPLE PREPARATION
Tissue sections (10 μm) were cut from FFPE blocks, placed onto the energy transfer coating of Director® microdissection slides, and deparaffinized. We used laser microdissection to isolate tumor cells from FFPE sections and prepare Liquid Tissue lysates, as previously described (25–27). Total protein concentration for each lysate was measured with a bicinchoninic acid protein assay (Micro BCA™, Thermo Fisher Scientific).
SRM ASSAY DEVELOPMENT
We used trypsin digestion mapping of recombinant ALK protein (UniProtKB acc. no. Q9UM73) to identify candidate peptides from the intracellular domain for assay development. The resulting peptides were analyzed with a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific) equipped with a nanoAcquityLC system (Waters). We used Pinpoint1.0 and Xcalibur2.1 (Thermo Scientific) software to identify the optimal tryptic peptides for SRM analysis. Peptides containing methionine or cysteine residues were excluded because of ongoing unpredictable oxidation. Candidate peptides were then screened in FFPE tumor samples and in the ALK-rearranged H3122 cell line to assess the capability of the assay for detecting ALK protein.
The selected optimal peptide, DPEGVPPLL-VSQQAK, encoded by exon 29 of the ALK gene, was C-terminal to the ALK kinase domain and found to be unique to ALK compared to the entire human proteome with the Basic Local Alignment Search Tool (BLAST), as previously described (25–27). Unlabeled (DPEGVP-PLLVSQQAK) and isotopically labeled [ ] versions of this peptide were synthesized to develop and perform the assay (Aqua Ultimate grade peptide; Thermo Scientific). SRM transitions used for quantification of the unlabeled DPEGVP-PLLVSQQAK peptide were m/z 789.43/498.22 (b5 +1), 540.82 (y10+2 ), and 1080.64 (y10+1) (Q1/Q3), and the transitions used for the isotopically labeled internal standard were m/z 793.44/498.22 (b5 +1), 544.83 (y10 +2), and 1088.65 (y10 +1) (Q1/Q3). The optimized collision energy was 24V for all 3 transitions. We used the same liquid chromatography gradient and MS parameters as those previously described (25–27), except that Pronto-SIL 200-5-C18AQ reversed-phase particle (C18, 5 μm, 200-Å pore size; Bischoff Chromatography) was used for column packing in this study.
A calibration curve was generated in a Pyrococcus furiosus complex matrix (Pfu; Agilent Technologies) to determine the assay’s limits of detection (LOD) and quantification (LOQ). Briefly, we added unlabeled peptide to 11 separate tubes containing Pfu and a constant concentration of isotopically labeled internal standard peptide (5000 amol) to provide a final concentration ranging from 75 to 25 000 amol unlabeled peptide per concentration point, including a zero sample (matrix sample processed without unlabeled peptide but with internal standard). Each concentration point was run in quintuplicate on the LC-MS system.
We identified optimal quantification peptides for 11 other proteins by trypsin digestion mapping of recombinant proteins specific for each target and similarly developed an assay for each protein.
MEASUREMENT OF ALK IN FFPE TISSUE SAMPLES AND DATA ANALYSIS
A known amount of isotopically labeled internal standard peptide was added to each lysate prepared from the microdissected tissue. We calculated the amount of ALK in each sample (attomoles per microgram of total protein) from the ratio of unlabeled to labeled peak areas multiplied by the known amount of spiked isotopically labeled internal standard, normalized to the amount of total protein injected. Unlabeled and labeled peptide peak areas were exported from Pinpoint 1.0. Lysates were analyzed in triplicate except for samples DH3 and DH5, for which measurements were from a single SRM analysis because of sample limitations.
IHC, FISH, AND DNA SEQUENCING
We selected tumor samples on which ALK IHC and/or ALK FISH had been previously performed. IHC was performed on 4- to 5-μm-thick FFPE tumor samples with the D5F3 ALK antibody (1:100, Cell Signaling Technology) at the respective institutions. Any ALK immunoreactivity within the tumor cells was interpreted as a positive result. ALK FISH results had been obtained with the ALK Break Apart FISH Probe Kit (Abbott Laboratories). A minimum of 50 cells were counted by 2 observers. The orange-green split signals and the single orange signal (with loss of the 5′ green signal) were counted as rearranged patterns; a rearrangement rate of >15% was considered positive for ALK rearrangement. DNA sequencing for the region encoding the optimal ALK peptide was performed by Macrogen (Rockville, MD). Briefly, a 210-bp DNA fragment generated by PCR amplification was analyzed by Sanger sequencing with a 3730XL DNA analyzer (Life Technologies). DNA concentrations in tumor tissue lysates were determined with a Qubit ssDNA Assay Kit (Life Technologies). Primer sequences used for PCR amplification were ALK-F (5′-ATCTCTGCAGCTGTGGGTTT-3′) and ALK-R (5′-TGTAATCAACACCGCTTTGC-3′).
Results
TISSUE AND PATIENT CHARACTERISTICS
Eighteen tissues were identified from 17 patients with adenocarcinomas (n = 15) and adenosquamous carcinomas (n = 2). All patients had been tested with both ALK FISH and ALK IHC except 1 with only FISH results. There were 14 ALK-rearranged cases (by FISH) and 12 cases with ALK protein expression (by IHC). Eight FISH-positive patients had received crizotinib treatment (Table 1).
Table 1.
Characteristics of ALK protein concentrations (by SRM), ALK sequencing results, ALK FISH, and ALK IHC in 18 non–small cell lung cancer FFPE tissues.
| Sample ID | Tumor histology | Mean ALK SRM protein concentration,amol/3g (SD) | ALK sequencing | ALK FISH | ALK IHC | Crizotinib treatment | Overall patient response |
|---|---|---|---|---|---|---|---|
| Dartmouth Medical Center | |||||||
| DH1 | ADCa | 153.9 (13.5) | WT | Positive | Positive | Yes | PFS 7 months; NED in spring 2012; lost to follow-up |
| DH2 | ADC | 261.0 (26.3) | NA | Positive | Positive | Yes | PFS 14 months; then NED |
| DH3 | ADC | 223.3b | NA | Positive | Positive | Yes | PFS 22 months; then NED; possibly independent of crizotinib because patient was on crizotinib only 5 months |
| DH4 | ADC (LN) | 453.0 (28.1) | WT | Positive | Positive | Yes | PFS 12 months; then AWD |
| DH5c | ADSCC | 216.4b | NA | Positive | Positive | No | Alive, NED |
| DH6c | ADSCC (LN) | 126.3 (12.7) | WT | Positive | Positive | No | Alive, NED |
| DH7 | ADSCC | ND | WT | Negative | Negative | No | |
| DH8 | ADC (brain) | ND | WT | Negative | Negative | No | |
| DH9 | ADC | ND | HT G->A | Positive | Negative | Yes | PD; then DOD |
| DH10 | ADC | ND | WT | Negative | Negative | No | |
| DH11 | ADC | ND | WT | Negative | Negative | No | |
| DH12 | ADC (brain) | ND | WT | Positive | Positive | Yes | PR of liver metastases for 10 months; then DOD |
| Cleveland Clinic | |||||||
| ALK002 | ADC | 336.6 (2.5) | WT | Positive | Positive | Yes | PFS 29 months; initial PR, then CR and currently NED |
| ALK081 | ADC | 138.8 (20.7) | WT | Positive | Positive | No | Alive |
| ALK083 | ADC | 153.9 (12.9) | WT | Positive | Positive | No | Alive |
| ALK516 | ADC | 106.4 (6.4) | WT | Positive | Positive | No | Alive, NED |
| ALK634 | ADC | 136.3 (24.6) | WT | Positive | Positive | No | OS 14 months; DOD |
| West Virginia University | |||||||
| CD0370 | ADC | ND | NA | Positive | NA | Yes | PFS <2 months; DOD |
ADC, adenocarcinoma; WT, wild type; PFS, progression-free survival; NED, no evidence of disease; NA, not available; LN, lymph node; AWD, alive with disease; ADSCC, adenosquamous cell carcinoma; HT G->A, heterozygous G->A point mutation on DNA region encoding for the mass-spectrometry-targeted peptide; ND, not detected; PD, progressive disease; DOD, died of disease; PR, partial response; CR, complete response; OS, overall survival.
Measurements in DH3 and DH5 were from a single SRM analysis.
Samples DH5 and DH6 are from the same patient.
SRM ASSAY DEVELOPMENT
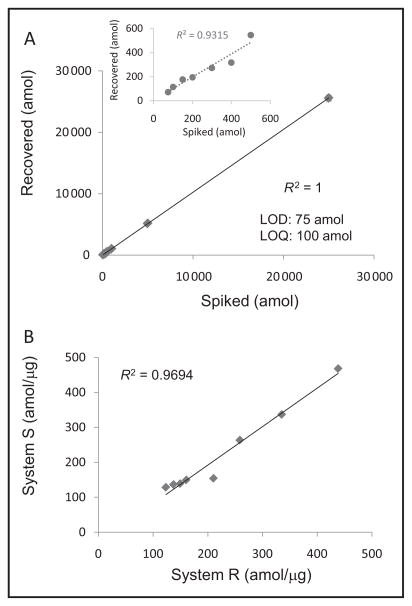
For development of the ALK SRM assay, multiple peptides obtained from a tryptic digest of recombinant ALK were measured with MS. When ALK translocation occurs, breakpoints in the N-terminal fusion partner can vary; however, the usual ALK breakpoint occurs at exon 20 of the kinase domain. Consequently, we focused on peptides within the intracellular domain including peptides in or C-terminal to the kinase domain. The resulting 2 intracellular candidate peptides, SNQEV-LEFVTSGGR and DPEGVPPLLVSQQAK, were then extensively screened in formalin-fixed cell lines and FFPE clinical samples. The H3122 cell line expressed 396 amol/μg ALK protein. Some tumors expressed amounts of ALK that were so low (<150 amol/μg) that only the DPEGVPPLLVSQQAK peptide was detected (LOD 75 amol); therefore, this peptide was selected for clinical assay development. CVs for the various concentration points in the calibration curve ranged from 1.1% to 20.1% for samples analyzed in quintuplicate. The amount of light peptide recovered (attomoles) was plotted against the amount of light peptide spiked (attomoles) to create a calibration curve (see Supplemental Table 1, which accompanies the online version of this article at http://www.clinchem.org/content/vol62/issue1). The LOD was determined by identifying the lowest concentration in the calibration curve where the CV from replicate measurements was ≤25% and accuracy from replicate measurements was ≥80%; the LOQ was determined by identifying the next-highest concentration of the calibration curve above the LOD. The LOD and LOQ were 75 and 100 amol, respectively, with a linear regression value of R2 = 1 for the calibration curve with 10 spiking concentration points (Fig. 1A), and R2 = 0.93 when excluding the 3 highest concentrations (Fig. 1A inset). The calibration curve showed linearity (R2 = 0.9) and low variations over a protein concentration range of 2 orders of magnitude.
Fig. 1. Development of ALK SRM assay and assessment of assay precision.
(A), Calibration curve generated in Pfu. Inset, Calibration curve omitting the 3 highest concentrations (25 000, 5000, and 1000 amol). (B), Assessment of assay precision and reproducibility for measuring ALK protein concentrations in 8 ALK FISH+ NSCLC FFPE tumor tissues. Each sample was analyzed on 2 different LC-MS systems.
ALK QUANTIFICATION
The SRM assay detected ALK in 11 tissue samples (from 10 patients) with concentrations of 106–453 amol/μg of tumor protein (Table 1). ALK was not detected by SRM in any of the FISH−/IHC− samples (n = 4), nor was it detected in 3 FISH+ cases. Of these 3 cases in which SRM absolute protein concentrations were discordant with FISH, 1 was IHC−, 1 was IHC+, and 1 had not been tested by IHC.
To evaluate the ALK SRM assay reproducibility and precision, ALK concentrations were measured in 8 of the 14 FISH+ tissues on 2 different LC-MS systems (systems R and S) by 2 different operators. Each sample was analyzed in triplicate on each system. The interinstrument CVs for 6 measurements from the same sample were 1.6%–17.7%. Results from systems R and S had an R2 value of 0.9694 (Fig. 1B, online Supplemental Table 2), demonstrating that the SRM assay reproducibly generated a low level of variance between the 2 systems.
RESPONSE TO CRIZOTINIB
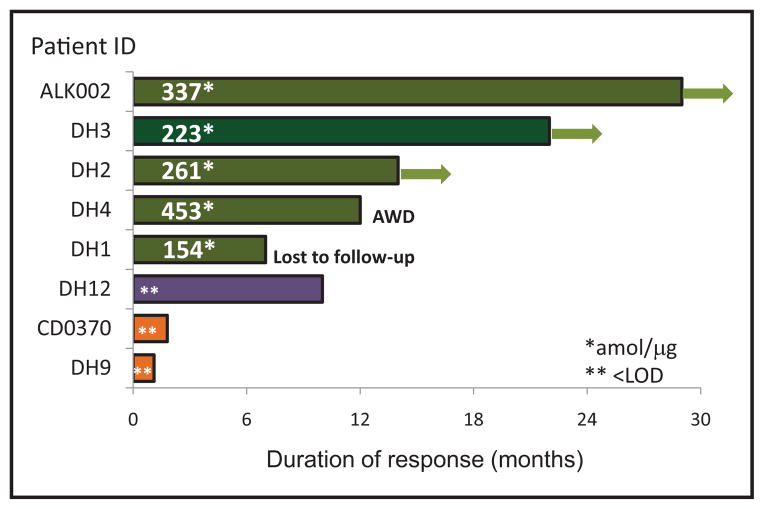
Of 8 patients treated with crizotinib, 5 had a positive tumor response (progression-free survival 7–29 months; 1 was lost to follow up); all 5 patients were ALK-positive by FISH, IHC, and SRM. The 3 remaining patients were discordant between ALK protein concentrations and ALK FISH and had nonresponse or partial response to crizotinib (Table 1; Fig. 2).
Fig. 2. Duration of response among patients treated with crizotinib.
Arrows indicate patients in complete remission (no evidence of disease). Numbers in the bar indicate ALK protein expression determined by SRM. Patients in green had positive response (progression-free survival 7–29 months), patient in purple (DH12) had partial response, and patients in orange had progressive disease while on crizotinib.
VERIFICATION OF ASSAY BY DNA SEQUENCING
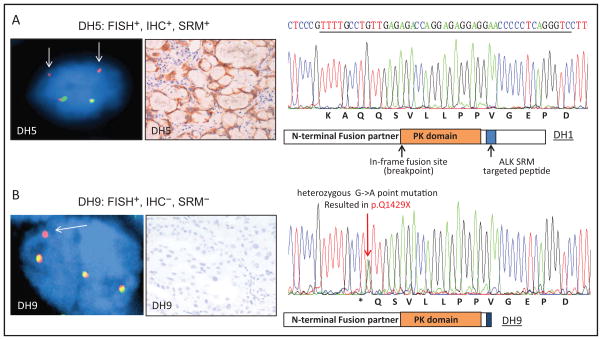
Because 3 of 14 ALK rearrangement–positive tissue samples (DH9, DH12, and CD0370) did not have detectable concentrations of ALK protein, we sought to investigate the DNA sequence of a 210-bp region on exon 29 of the ALK gene, where the ALK targeted peptide and its flanking amino acids sequences are encoded. Thirteen of the 14 samples sequenced carried no mutation within the 210-bp region tested (Fig. 3A), but sample DH9, the FISH+/IHC−/SRM− case, was found to carry a heterozygous nonsense point mutation (G->A) within ALK targeted peptide–encoding DNA sequence. The point mutation introduced a stop codon (p.Q1429X); therefore, a nonfunctional (truncated) fusion protein would likely be produced, possibly resulting in total lack of any protein product (Fig. 3B).
Fig. 3. Comparison of SRM with ALK FISH, IHC, and DNA sequencing in selective cases.
FISH and paired IHC for patient DH5 to represent FISH+/IHC+ cases; DNA sequence within the MS targeted peptide from DH1 to represent wild-type sequence. (A), ALK FISH testing shows deletion of the 5′ (green) signal with retained 3′ (orange) signal, consistent with rearrangement. Arrows indicate the rearranged red signal. (B), The FISH+, SRM−, and IHC− case (DH9) showed a nonsense point mutation that resulted in a stop codon (p.Q1429X); therefore, nonfunctional fusion protein would be produced.
Discussion
Quantitative mass spectrometry represents an emerging clinical method that is highly specific, reproducible, and quantitative and has decreased sensitivity to preanalytical variation. In addition, MS-based proteomic analysis of FFPE tissue is capable of multiplexing analysis of 100 analytes from a small amount of tissue (25–27). The cost of reagents, instrument time, and personnel are greater with the ALK-SRM assay compared with ALK IHC. However, multiplexing the ALK protein with additional predictive/prognostic proteins in a single SRM assay makes the overall cost comparable to or less than performing IHC or FISH for each of these proteins individually. Also, in contrast to IHC, this SRM method quantifies proteins on the basis of a unique sequence of amino acids, and thus does not have the same limitations as traditional antibody-based protein detection methods and is less susceptible to problems resulting from preanalytical tissue handling and time elapsed from tissue sectioning (27).
In this study, we evaluated ALK protein expression in 18 NSCLC tissues (14 FISH+ and 4 FISH−) with ALK FISH, ALK IHC, or both. Of the 14 samples with ALK rearrangement by FISH, 11 had measurable ALK protein expression by MS, whereas 3 ALK FISH+ samples had undetectable amounts of ALK protein. Among the patients who responded to crizotinib treatment, all but 1 (5 of 6) had measureable ALK protein by mass spectrometry.
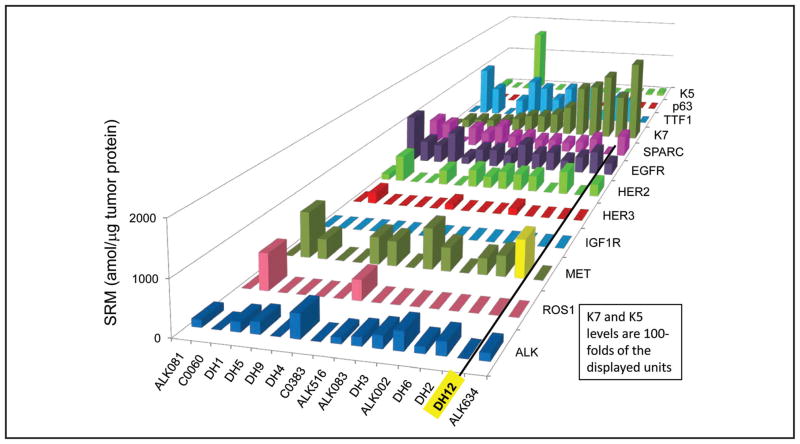
Interestingly, the 3 FISH+/SRM− instances represent 2 cases in which the ALK-targeted crizotinib therapy was ineffective and 1 in which a modest response was seen followed by prompt tumor progression. In 1 such case (DH9), the patient had immediate disease progression upon crizotinib initiation. We confirmed nonexpression of ALK protein by IHC in this patient, and with DNA sequencing identified a DNA point mutation in the ALK gene that predicts a truncated ALK protein. In this case, ALK protein quantities appeared to be more predictive of response than FISH testing. A second patient with a FISH+/SRM− ALK profile was treated with crizotinib without response for a brief period (progression free survival <2 months). Again, for this patient, the unsatisfactory crizotinib response could be predicted by the absence of ALK protein expression. The third FISH+/SRM− case was ALK IHC+; this patient with stage IV disease had a partial response of liver metastases and stabilization of lung disease on crizotinib therapy but died of progressive disease after 10 months. Of note, upon review of other proteins analyzed by multiplex SRM, this patient’s tumor expressed high concentrations of MET protein (Fig. 4), a second target of crizotinib activity, and a possible explanation for the partial response in this patient’s liver lesions; similar responses have been previously reported (28 –30).
Fig. 4. NSCLC tissue expression for each of the targets in a multiplex SRM analysis, sorted by K7 expression from low to high, left to right.
The 15 samples represent a mixture of 13 ALK rearrangement positive controls and 2 ROS1 rearrangement positive controls. Note that DH12 showed an increased expression of MET (highlighted in yellow).
Other studies have reported FISH+ cases that were negative for ALK by IHC (20 –22), supporting the conclusion that in such cases there is an ALK rearrangement that leads to a nontranslated or nonfunctional fusion protein. There are also reports of FISH− cases that showed genomically complex ALK rearrangements by next-generation sequencing. In 2 separately reported cases, ALK FISH was negative, ALK IHC was positive, and next-generation sequencing showed atypical ALK rearrangements; both patients rapidly responded to crizotinib (31, 32), again supporting the contention that ALK expression is necessary for crizotinib activity.
Of note, ALK SRM was evaluated against gold standard FISH testing for ALK rearrangement, which is inherently limited. In a large study of consecutive NSCLC cases tested with both FISH and IHC, 70 of 150 ALK-altered cases were detected by 1 method but not the other; either test alone would have missed approximately 30% of ALK-positive cases. In a small sample of these patients (n = 44), both types of discordant cases (FISH+/IHC− and FISH−/IHC+) responded to crizotinib (22). Other studies found similar discrepancies between IHC and FISH (20 –22). Although we applied the benchmark of crizotinib response/nonresponse, the results of this study should be considered in light of certain limitations. First, 1 criticism of SRM technology in FFPE tissue is its inability to identify very low expression concentrations (i.e., <LOD). Second, a potential problem with peptide-based measurements is that any deviation from the canonical sequence will prevent detection; the expansion of the assay to multiple peptides, even if the confirmatory peptides do not have the same level of diagnostic sensitivity, would help to address this problem. Finally, this study’s sample size was small; crizotinib response data from a larger group of patients will be necessary to confirm the clinical sensitivity, specificity and utility of the SRM assay.
To reduce testing costs, some have proposed hierarchical ALK screening algorithms on the basis of patient characteristics (e.g., young age, never or light smoker), histology (adenocarcinoma), and absence of other oncogenic driver mutations (33). However, ALK rearrangements have been found in older patients and smokers (34), squamous cell and adenosquamous carcinoma cases (35, 36), and rarely, patients with mutations in EGFR, Kirsten rat sarcoma viral oncogene homolog (KRAS), and B-Raf proto-oncogene, serine/threonine kinase (BRAF) (20 –22, 37, 38). Although the triage of samples based on clinicopathological features does increase the success rate of detection of ALK rearrangements (33), it is not an optimal strategy, since a portion of patients who could benefit from ALK inhibitors would unavoidably be excluded (4, 39, 40).
The ALK SRM assay was multiplexed to enable concurrent assessment of clinically actionable proteins indicative of gene rearrangement (ALK, ROS1, RET, TRK, and others), histology markers (K7, TTF1, K5, p63), receptor tyrosine kinase targets (EGFR, HER2, HER3, MET, IGF1R, FGFR2), proteins involved in immune checkpoints (PD-L1), and predictive/prognostic protein markers for chemotherapy agents. For example, as demonstrated in Fig. 4, multiplex SRM analysis of biomarkers in FFPE NSCLC tissues identified moderately high MET expression (732 amol/μg) in a brain biopsy tissue of patient who was SRM−/FISH+/IHC+ for ALK. Metastatic diseases tend to have greater tumor heterogeneity; it is possible that there was a higher expression of MET in the metastatic liver tumor than in the primary lung tumor, and this may have contributed to the partial response to crizotinib (which is active against both ALK and MET).
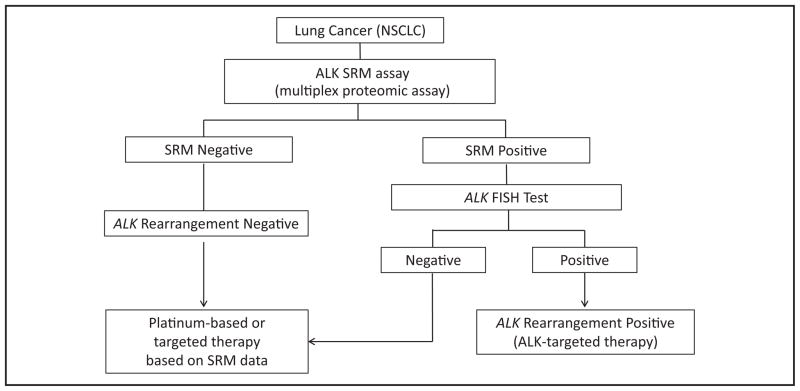
Proteomics analysis of ALK as part of a multiplexed proteomic screening of patient tissue on initial biopsy could save time, tissue, and the expense of multiple FISH or IHC testing to detect different biomarkers. In the event that ALK expression is detected by SRM, ALK-positive cases could be reflexed to FISH to confirm eligibility for treatment with ALK-targeted therapy (Fig. 5). After critical testing of the assay’s performance, the proposed testing protocol would provide clinicians with valuable diagnostic information and ensure that all patients whose lung cancers express ALK and other clinically actionable markers have the opportunity to receive treatment.
Fig. 5. Potential ALK testing algorithm in NSCLC.
The proposed strategy would allow the opportunity for patients with lung cancer to be tested for ALK expression and multiple predictive/prognostic biomarkers.
Supplementary Material
Footnotes
Nonstandard abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; NSCLC, non–small cell lung cancer; ALK, anaplastic lymphoma kinase; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; FFPE, formalin-fixed paraffin-embedded; SRM, selected reaction monitoring; MS, mass spectrometry; BLAST, Basic Local Alignment Search Tool; Pfu, Pyrococcus furiosus complex matrix; LOD, limit of detection; LOQ, limit of quantification.
Human genes: ALK, anaplastic lymphoma receptor tyrosine kinase; EML4, echinoderm microtubule associated protein-like 4; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homolog; BRAF, B-Raf proto-oncogene, serine/threonine kinase.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: T. Hembrough, NantOmics; W.-L. Liao, NantOmics; S. Thyparambil, NantOmics; E. An, NantOmics; D. Krizman, NantOmics; J. Burrows, OncoPlex Diagnostics.
Consultant or Advisory Role: None declared.
Stock Ownership: T. Hembrough, NantOmics; W.-L. Liao, Nant-Omics; S. Thyparambil, NantOmics; E. An, NantOmics; D. Krizman, NantOmics.
Honoraria: None declared.
Patents: T. Hembrough, W.-L. Liao, S. Thyparambil, and D. Krizman are coinventors of PCT/US2014/031138.
Role of Sponsor: Dartmouth and NantOmics codesigned the study. NantOmics played no role in the choice of enrolled patients. Dart-mouth and NantOmics both reviewed, interpreted data, and approved the manuscript.
Research Funding: NantOmics. Expert Testimony: None declared.
References
- 1.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:2537–9. doi: 10.1056/NEJMc1404894. [DOI] [PubMed] [Google Scholar]
- 2.Chabner BA. Approval after phase I: ceritinib runs the three-minute mile. Oncologist. 2014;19:577– 8. doi: 10.1634/theoncologist.2014-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon B, Wilner KD, Shaw AT. Current status of targeted therapy for anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Clin Pharmacol Ther. 2014;95:15–23. doi: 10.1038/clpt.2013.200. [DOI] [PubMed] [Google Scholar]
- 4.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828– 60. doi: 10.5858/arpa.2012-0720-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051– 60. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou SH, Soo RA, Kubo A, Kawaguchi T, Ahn MJ. Will the requirement by the US FDA to simultaneously co-develop companion diagnostics (CDx) delay the approval of receptor tyrosine kinase inhibitors for RTK-rearranged (ROS1-, RET-, AXL-, PDGFR-a-, NTRK1-) non-small cell lung cancer globally? Front Oncol. 2014;4:58. doi: 10.3389/fonc.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majewski IJ, Mittempergher L, Davidson NM, Bosma A, Willems SM, Horlings HM, et al. Identification of recurrent FGFR3 fusion genes in lung cancer through kinome-centred RNA sequencing. J Pathol. 2013;230:270– 6. doi: 10.1002/path.4209. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15:3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 10.Togashi Y, Soda M, Sakata S, Sugawara E, Hatano S, Asaka R, et al. KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only. PLoS One. 2012;7:e31323. doi: 10.1371/journal.pone.0031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong M, Kim RN, Song JY, Choi SJ, Oh E, Lira ME, et al. HIP1-ALK, a novel fusion protein identified in lung adenocarcinoma. J Thorac Oncol. 2014;9:419–22. doi: 10.1097/JTO.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 12.Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18:4682–90. doi: 10.1158/1078-0432.CCR-11-3260. [DOI] [PubMed] [Google Scholar]
- 13.Minca EC, Portier BP, Wang Z, Lanigan C, Farver CF, Feng Y, et al. ALK status testing in non-small cell lung carcinoma: correlation between ultrasensitive IHC and FISH. J Mol Diagn. 2013;15:341– 6. doi: 10.1016/j.jmoldx.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Hutarew G, Hauser-Kronberger C, Strasser F, Llenos IC, Dietze O. Immunohistochemistry as a screening tool for ALK rearrangement in NSCLC: evaluation of five different ALK antibody clones and ALK FISH. Histopathology. 2014;65:398– 407. doi: 10.1111/his.12399. [DOI] [PubMed] [Google Scholar]
- 15.Paik JH, Choe G, Kim H, Choe JY, Lee HJ, Lee JS, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol. 2011;6:466–72. doi: 10.1097/JTO.0b013e31820b82e8. [DOI] [PubMed] [Google Scholar]
- 16.McLeer-Florin A, Moro-Sibilot D, Melis A, Salameire D, Lefebvre C, Ceccaldi F, et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol. 2012;7:348–54. doi: 10.1097/JTO.0b013e3182381535. [DOI] [PubMed] [Google Scholar]
- 17.Cutz JC, Craddock KJ, Torlakovic E, Brandao G, Carter RF, Bigras G, et al. Canadian anaplastic lymphoma kinase study: a model for multicenter standardization and optimization of ALK testing in lung cancer. J Thorac Oncol. 2014;9:1255– 63. doi: 10.1097/JTO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 18.Conklin CM, Craddock KJ, Have C, Laskin J, Couture C, Ionescu DN. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol. 2013;8:45–51. doi: 10.1097/JTO.0b013e318274a83e. [DOI] [PubMed] [Google Scholar]
- 19.Wynes MW, Sholl LM, Dietel M, Schuuring E, Tsao MS, Yatabe Y, et al. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thoracic Oncol. 2014;9:631– 8. doi: 10.1097/JTO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallander ML, Geiersbach KB, Tripp SR, Layfield LJ. Comparison of reverse transcription-polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule-associated proteinlike 4-anaplastic lymphoma kinase fusion-positive non-small cell lung carcinoma: implications for optimal clinical testing. Arch Pathol Lab Med. 2012;136:796– 803. doi: 10.5858/arpa.2011-0321-OA. [DOI] [PubMed] [Google Scholar]
- 21.Sholl LM, Weremowicz S, Gray SW, Wong KK, Chirieac LR, Lindeman NI, Hornick JL. Combined use of ALK immunohistochemistry and FISH for optimal detection of ALK-rearranged lung adenocarcinomas. J Thorac Oncol. 2013;8:322– 8. doi: 10.1097/JTO.0b013e31827db604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabillic F, Gros A, Dugay F, Begueret H, Mesturoux L, Chiforeanu DC, et al. Parallel FISH and immunohistochemical studies of ALK status in 3244 non-small-cell lung cancers reveal major discordances. J Thorac Oncol. 2014;9:295–306. doi: 10.1097/JTO.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 23.Engel KB, Moore HM. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2011;135:537– 43. doi: 10.5858/2010-0702-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 24.Babic A, Loftin IR, Stanislaw S, Wang M, Miller R, Warren SM, et al. The impact of pre-analytical processing on staining quality for H&E, dual hapten, dual color in situ hybridization and fluorescent in situ hybridization assays. Methods. 2010;52:287–300. doi: 10.1016/j.ymeth.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Hembrough T, Thyparambil S, Liao WL, Darfler MM, Abdo J, Bengali KM, et al. Selected reaction monitoring (SRM) analysis of epidermal growth factor receptor (EGFR) in formalin fixed tumor tissue. Clin Proteomics. 2012;9:5. doi: 10.1186/1559-0275-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hembrough T, Thyparambil S, Liao WL, Darfler MM, Abdo J, Bengali KM, et al. Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J Mol Diagn. 2013;15:454– 65. doi: 10.1016/j.jmoldx.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Catenacci DV, Liao WL, Thyparambil S, Henderson L, Xu P, Zhao L, et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One. 2014;9:e100586. doi: 10.1371/journal.pone.0100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942– 6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 29.Schwab R, Petak I, Kollar M, Pinter F, Varkondi E, Kohanka A, et al. Major partial response to crizotinib, a dual MET/ALK inhibitor, in a squamous cell lung (SCC) carcinoma patient with de novo c-MET amplification in the absence of ALK rearrangement. Lung Cancer. 2014;83:109–11. doi: 10.1016/j.lungcan.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y, Minca EC, Lanigan C, Liu A, Zhang W, Yin L, et al. High MET receptor expression but not gene amplification in ALK 2p23 rearrangement positive non-small-cell lung cancer. J Thorac Oncol. 2014;9:646–53. doi: 10.1097/JTO.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 31.Peled N, Palmer G, Hirsch FR, Wynes MW, Ilouze M, Varella-Garcia M, et al. Next-generation sequencing identifies and immunohistochemistry confirms a novel crizotinib-sensitive ALK rearrangement in a patient with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7:e14– 6. doi: 10.1097/JTO.0b013e3182614ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren S, Hirsch FR, Varella-Garcia M, Aisner DL, Boyle T, Zhou C, Camidge DR. Atypical negative ALK break-apart FISH harboring a crizotinib-responsive ALK rearrangement in non-small-cell lung cancer. J Thorac Oncol. 2014;9:e21–3. doi: 10.1097/JTO.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi M, Sonobe M, Takahashi T, Yoshizawa A, Kikuchi R, Date H. Detection of ALK fusion in lung cancer using fluorescence in situ hybridization. Asian Cardiovasc Thorac Ann. 2012;20:426–31. doi: 10.1177/0218492312440700. [DOI] [PubMed] [Google Scholar]
- 34.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dragnev KH, Gehr G, Memoli VA, Tafe LJ. ALK-rearranged adenosquamous lung cancer presenting as squamous cell carcinoma: a potential challenge to histologic type triaging of NSCLC biopsies for molecular studies. Clin Lung Cancer. 2014;15:e37– 40. doi: 10.1016/j.cllc.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Chaft JE, Rekhtman N, Ladanyi M, Riely GJ. ALK-rearranged lung cancer: adenosquamous lung cancer masquerading as pure squamous carcinoma. J Thorac Oncol. 2012;7:768–9. doi: 10.1097/JTO.0b013e31824c9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou JX, Yang H, Deng Q, Gu X, He P, Lin Y, et al. Oncogenic driver mutations in patients with non-small-cell lung cancer at various clinical stages. Ann Oncol. 2013;24:1319–25. doi: 10.1093/annonc/mds626. [DOI] [PubMed] [Google Scholar]
- 38.Barlesi F, Blons H, Beau-Faller M, Rouquette I, Ouafik L, Mosser J, et al. Biomarkers (BM) France: results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts) J Clin Oncol. 2013;31:Abstract 8000. [Google Scholar]
- 39.Jokoji R, Yamasaki T, Minami S, Komuta K, Sakamaki Y, Takeuchi K, Tsujimoto M. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol. 2010;63:1066–70. doi: 10.1136/jcp.2010.081166. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida A, Tsuta K, Nakamura H, Kohno T, Takahashi F, Asamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011;35:1226–34. doi: 10.1097/PAS.0b013e3182233e06. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.