Abstract
The use of animal cell cultures as tools for studying the microsporidia of insect and mammals is briefly reviewed, along with an in depth review of the literature on using fish cell cultures to study the microsporidia of fish. Fish cell cultures have been used less often but have had some successes. Very short-term primary cultures have been used to show how microsporidia spores can modulate the activities of phagocytes. The most successful microsporidia/fish cell culture system has been relatively long-term primary cultures of salmonid leukocytes for culturing Nucleospora salmonis. Surprisingly, this system can also support the development of Enterocytozoon bienusi, which is of mammalian origin. Some modest success has been achieved in growing Pseudoloma neurophilia on several different fish cell lines. The eel cell line, EP-1, appears to be the only published example of any fish cell line being permanently infected with microsporidia, in this case Heterosporis anguillarum. These cell culture approaches promise to be valuable in understanding and treating microsporidia infections in fish, which are increasingly of economic importance.
Introduction
Historically, animal cell cultures have been essential for studying viral and microbial pathogens to humans and animals. Some pathogens require animal cells in order to complete their life, making animal cell cultures, or the in vitro approach, the most convenient avenue for producing and studying the pathogen. This is most famously true of viruses. Cell lines have been crucial for viral detection and for producing viruses, which then can be characterized for a variety of biochemical and morphological features and used in vaccines (Enders et al. 1949; Hsiung 1989). Animal cell cultures have allowed the single-cell reproductive cycle of viruses to be dissected and have contributed to studies of viral pathogenesis and development of therapeutic agents. Less well known is that some single-cell eukaryotic pathogens also require animal cells to complete their life cycle and their study is aided by the use of animal cell cultures (Wittner 1999). This is the case with microsporidia. Microsporidia survive only by living in other cells and are found outside of host cells only as spores. Here the past and future value of in vitro approaches to studies of the microsporidia infecting fish is reviewed after a brief overview of the biology of microsporidia and the in vitro success achieved with economically important microsporidia of insects and with clinically important microsporidia of humans.
Microsporidia classification
Microsporidia are currently included in the Fungi (Hibbett et al. 2007). Although organisms now known as microsporidia were originally identified as fungi, they were reclassified as protozoans by the end of the 19th century (Nageli 1857; Pasteur 1870), and this designation was accepted for over 100 years until molecular techniques to determine phylogeny returned microsporidia to the Fungi (Hirt et al. 1999; Keeling and Fast 2002; Keeling et al. 2000). The phylum Microsporidia encompass over 1200 species and almost 150 genera (Franzen and Muller 2001; Wittner 1999). They infect every major animal group, from invertebrates to all classes of vertebrates.
The type of host in which they have been found to infect has long been used as an informal categorization of microsporidia. For this classification, the principal groups have been insect and human microsporidia. These have been identified and studied because of their economic and clinical relevance. Less intensively investigated, but also of economic importance, are fish microsporidia. Amphibians, reptiles, and birds are susceptible to microsporidia, but the research is comparatively much less on these microsporidial infections (Snowden and Shadduck 1999). In the future, these informal categorizations may be difficult to maintain because growing evidence, including from in vitro approaches, demonstrates that several microsporidia have low host-specificity (Coyle et al. 2004; Lores et al. 2003; Rinder et al. 2000; Sutherland et al. 2004).
Cellular life cycle of microsporidia
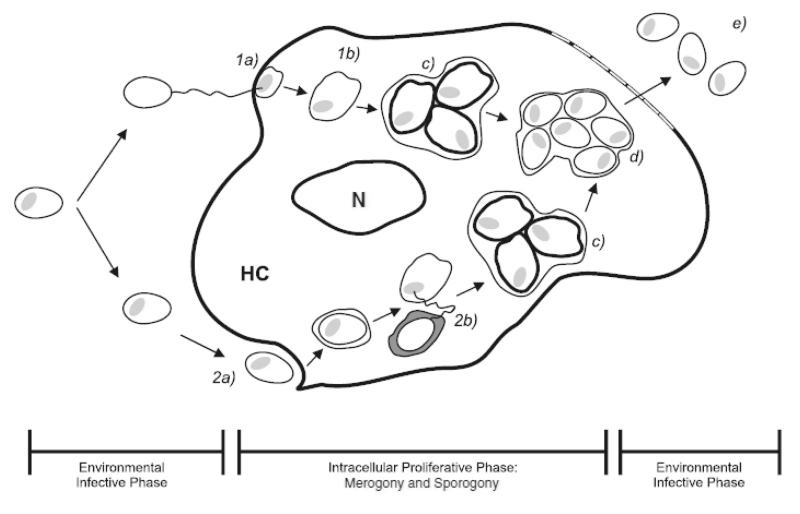
Diversity is found among the numerous microsporidian genera with respect to their life cycle in animal cells. Despite the variations, a generalized life cycle can be described (Fig 1). Microsporidia are obligate intracellular pathogens, but have an extracellular infective spore phase of development. The spore contains a specialized structure, the polar filament, which under certain conditions will eject from the spore and inject infective sporoplasm into the host cell. The injection of sporoplasm commences the proliferative, intracellular phase of the parasite’s development. The intracellular proliferative phases of the life cycle are characterized by the developmental stages of merogony and sporogony. Merogony, or the stage of meront development, originates with the infective sporoplasm of a germinated spore and typically occurs in direct contact with host cytoplasm. Meronts proliferate, often through binary fission, and differentiate into sporonts during sporogony. Sporogony is characterized by a number of morphological changes. A thickening of the electron dense plasmalemma surrounding the parasites, for example, is one indication of sporont development (Lom et al. 2000). As well, this stage can occur in direct contact with host cytoplasm but may occur within a membranous envelope that develops of host, parasite, or host-parasite origin (Cali and Takvorian 1999; Lom and Dyková 2005). The developing spore is designated a sporoblast at its last sporont division and, through metamorphosis, condenses to become a mature spore smaller than its developmental predecessors (Cali and Takvorian 1999). Liberation of mature spores into the extracellular environment occurs when the host cell dies.
Figure 1. Life cycle of generalized microsporidia in culture.
Environmental Infective Phase (left): Spores can germinate and inject infective sporoplasm (1a) or undergo phagocytosis (2a) to gain entry into the host cell. Intracellular Proliferative Phase (middle): Merogony typically occurs in direct contact with host cytoplasm (1b). With phagocytosis, sproplasm evades lysosomal destruction (2b). Sporogony may (c) or may not occur within a membrane. Spore maturation occurs through metamorphosis (d). Environmental Infective Phase (right): Liberation of infective spores (e), accompanied with host cell death. HC = host cell; N = nucleus of host cell.
Economically important microsporidia of insects
Since the mid 19th century, microsporidia have been known pathogens of economic importance. The initial impetus for scientific research of microsporidia was due to an epidemic threatening the silk industry of Europe. Characterized by black speckling of the silk worm Pasteur identified the causative agent as Nosema bombycis, and is credited with devising methods to reduce the impact of the disease (Pasteur 1870). Pasteur’s work is believed to be the first scientific investigation of the pathogenic nature of microsporidia. The apiculture industry is also negatively affected by microsporidiosis. Until recently, Nosema apis was exclusively the cause of nosemosis in the European honeybee (Apis mellifera) (Ellis and Munn 2005). Unfortunately, another microsporidian, N. ceranae, is an emerging pathogen of the European honeybee (Chen et al. 2008; Williams et al. 2008). The cost of nosemosis to the apiculture industry is unclear. However, the economics of pollination services have been estimated to provide a 600 to 700% return on investment for farmers indicating the potential decrease in profitability if pollination services are not optimal (Kevan and Phillips 2001; Olmstead and Woolen 1987).
Culturing microsporidia of insects
The breakthrough uniting cell culture techniques and microsporidia research came in 1937 and with insects (Trager 1937). A silkworm cell culture was successfully infected with N. bombycis. The infected cells were reported to have filled with spores and behaved similarly to uninfected cells. Trager (1937) speculated that the failure of previous attempts to grow microsporidia in culture were likely a result of the addition of too few spores. His speculation has been supported by subsequent research: the optimal spore to cell ratio to infect a culture is 10:1 or greater (Jaronski 1984). Despite the initial success in 1930s, the common use of insect cell cultures in microsporidia research did not begin until 1960s with the development of insect cell lines. Since then, insect cell lines have been used for two distinct purposes. One is as a basic research tool to study the life cycle of microsporidia. The other purpose is practical and is to develop large-scale cultures that produce spores that can be used as biological control agents for insect pests and for medically important insects (Jaronski, 1984). A unique feature of some combinations of insect cell line and microsporidia species is that the cultures can be subcultivated over 70 times and continue to produce spores (Iwano and Kurtti 1995; Kurtti et al. 1994). Nosema locustae, now reassigned to the genus Paranosema is a fatal parasite of over 90 species of grasshoppers and is the only commercially approved microsporidial pesticide in the United States (Canning 1953; Henry and Onsager 1982; Sokolova et al. 2003). Although propagation of P. locustae has been achieved in vitro, the spore yield is higher in vivo, which is the commercial source (Becnel 2006; Khurad et al. 1991).
Clinically important microsporidia
Human microsporidiosis was first documented in 1959 (Matsubayashi et al. 1959). Over the next 25 years incidents of microsporidia infections in humans were rare (Ashton et al. 1973; Marcus et al. 1973; Margileth et al. 1973; Sprague 1974), but in the 1980s microsporidia was found to be the causative agent of an often fatal diarrheal disorder of AIDS patients (Desportes et al. 1985; Dobbins and Weinstein 1985; Modigliani et al. 1985). Since this discovery, microsporidiosis has been found not only to affect AIDS patients, but can be an opportunistic infection of immunodepressed individuals and potentially individuals with seemingly intact immune responses (Didier and Weiss 2006). There are 14 species of microsporidia known to infect humans (Didier 2005). Enterocytozoon bieneusi and Encephalitozoon intestinalis are the most commonly identified human microsporidial infections, respectively (Didier and Weiss 2006; Kotler and Orenstein 1999). Although intestinal disease is the most prevalent consequence of human microsporidia, they can cause a broad range of conditions including keratoconjunctivitis, myositis and encephalitis (Kotler and Orenstein 1999). Once only a concern of economic importance, microsporidia suddenly had significant clinical relevance and this led to research on these microsporidia in animal cell cultures (Visvesvara 2002).
Culturing microsporidia of human diseases
Although there are some notable exceptions, species of microsporida in human diseases have been cultured effectively in vitro since 1990. The first success was with Vittaforma corneae (Shadduck et al. 1990) and made use of the approaches used years earlier to culture Encephalitozoon cuniculi, which infect rabbits and was grown in cultures of a mouse lymphosarcoma cell line (MB III) (Morris et al. 1956). Now at least a half dozen species infecting humans opportunistically have been cultured, including Encephalitozoon hellem and E. intestinalis (Didier et al. 1991; Visvesvara 2002; Visvesvara et al. 1995a). This has been done with different mammalian cell lines, and Visvesvara (2002) has compiled a list of microsporidia species, cell lines, and media that have been used together to obtain microsporidia growth in vitro. However, success is not a certainty. For the same species of microsporidia, some isolates can be maintained in culture, whereas other isolates cannot. Most significantly, at least one clinically important species, E. bieneusi, has failed to be cultured despite repeated attempts with different isolates. Only short-term cultivation with very low spore production was achieved with E. bieneusi (Visvesvara et al. 1995b). For other species, successfully infected cell cultures often can be maintained for several months to over a year. In old cultures, spores were seen by phase contrast microscopy attached to the plastic growth surface among patches of disrupted mammalian cells and in some cases spores that had extruded polar tubules were visible as spermatozoan-like structures (Visvesvara, 2002). The microsporidia/cell culture systems have been used for a variety of purposes.
The first use is to produce the pathogen in amounts to allow identification and study. Microsporidial organisms are often present in very small numbers in samples such as urine (Visvesvara, 2002). Cell cultures allow their numbers to be increased substantially, which permits their identification by a variety of techniques, such as PCR analysis of DNA extracted from the cultures. Thus cell cultures are an aid to clinical identification. Cell cultures also provide a source of pathogens for infecting animals in vivo and sufficient microsporidia for biochemical and molecular biology studies (Belkorchia et al. 2008).
A second use of microsporidia/cell culture systems is to study the early events of infection, including initial adherence, kinetics, and route of uptake. This is perhaps the most common use of microsporidia cultures, with them being examined by a variety of techniques and being the subjects for different experimental purposes. They have been used to show that the initial adherence of spores to host cells is mediated by sulftated glycosaminoglycans and is augmented by Mn++ and Mg++ but not by Ca++ (Hayman et al. 2005; Southern et al. 2007). Adherence of Encephalitozoon spp. was rapid (3-6h) (Fischer et al. 2008). Both SEM and TEM have been used to visualize the traditional route of infection, polar tube penetration and deposition of the sporoplasm into the cytoplasm (Schottelius et al. 2000; Takvorian et al. 2005). The advantages for the microscopist are the events of infection can be closely timed and the presence of infected cells usually can be assured. In vitro studies have shown that besides the traditional route of infection, two additional routes were possible (Couzinet et al. 2000; Takvorian et al. 2005). One was the phagocytosis of spores followed by germination and movement of the sporoplasm from the phagosome to the cytoplasm. The other possibility was that sporoplasms may be released extracellularly into a microenvironment close to the host cell and subsequently internalized into the host cytoplasm by phagocytosis or a type of endocytosis (Takvorian et al. 2005).
Thirdly, microsporidia/cell culture systems can be utilized to study the cell biology and kinetics of the intracellular stages of microsporidia. For this, transmission electron microscopy (TEM) is perhaps the most successful approach (Hollister et al. 1996; Lowman et al. 2000). The biochemical processes and signaling pathways of the proliferative phase that leads to meronts (merogony) and of meront conversion into sporoblasts and sporoblast differentiation into spores (sporogony) remain to be studied in detail, likely because of the complexity involved in teasing out these processes within a host cell that is also changing. An example of this would be determining the origin, fate and fusion of the parasitophorous vacuole (Fasshauer et al. 2005; Ronnebaumer et al. 2008). However, procedures have been developed for isolating sporogonial stages of E. cuniculi in cultures with Madin-Darby canine kidney (MDCK) cells (Taupin et al. 2006b). This opens up the possibility of applying many biochemical methods, including proteome studies. The in vitro systems are very amenable to cytochemical and immunocytochemical investigation. RNA in situ hybridization has been used to follow the level of mRNA for endospore-destined protein (EnP1) (Taupin et al. 2006a). Fluorescence microscopy allowed the identification and localization of a microsporidian cytoskeletal component, actin (Bigliardi et al. 1999). The in vitro approach can be used to determine the timing of intracellular events and to study the effect of variables, such as temperature, on proliferation and sporogony. The replication kinetics of E. intestinalis in a mouse intestinal cell line (CMT-93) was studied by real time PCR (Wasson and Barry 2003). Elevated temperature was shown to impeded proliferation and the onset of sporogony for B. algerae in rabbit kidney cells (Lowman et al. 2000).
A fourth use of microsporidia/cell culture systems is to characterize the responses of the host animal cells to microsporidia, which would include innate cellular mechanisms for protecting the host against infection but also modulation of the host cell to protect the pathogen and perhaps disseminate it. These responses are more conveniently studied in vitro than in vivo, but such studies are in their infancy, with only a few cellular processes examined to date. Treatment of mouse macrophages in primary culture but not of a mouse macrophage cell line with interferon gamma (IFNγ) inhibited E. cuniculi replication (Jelinek et al. 2007). On the other hand, the reorganization of microbutules and induction of multinucleation in green monkey cell line E6 by V. cornea might be away for the parasite to be protected from the host immune response (Leitch et al. 2005). To date, this has only been documented in vitro. Another focus is induction of chemokines in macrophages in vitro and the migration of naïve monocytes by Encephalitozoon spp (Fischer et al. 2007). This type of study aims to understand the dissemination of the pathogen within the host. Finally the modulation of the cell cycle and cell death has been examined (del Aguila et al. 2006). Overall these types of studies have the potential to provide unique insights into the regulation of cellular processes and to open up new avenues of treatment.
As a final application, microsporidia/cell culture systems provide convenient platforms for discovering and studying treatments that can be used to kill microsporidia. This approach was used to screen a variety of drugs for their therapeutic potential and to identify albendazole and fumagilin as effective at inhibiting the growth of E. cuniculi in vitro but at the same time causing little harm to the human cells, which in this case were MRC5 (Beauvis et al. 1994). Similar systems have been used to evaluate the antimicrosporidial activity of other classes of compounds, including fluoroquinolones (Didier et al. 2005; Didier et al. 2006). Although animals ultimately will have to be used to test the efficacy of therapeutics, the advantage of the in vitro approach is in providing preliminary screens of effectiveness rapidly and inexpensively. Furthermore, animal cell culture systems can be used to study the killing of microsporidia by chlorine and ultraviolet light (John et al. 2003; Wolk et al. 2000).
Microsporidia of fish
Microsporidiosis has been identified in fish for over a century (Moniez 1887). Since that time there are at least 156 documented microsporidia species in 14 genera recognized in fish (Lom 2002). Microsporidiosis is highly destructive to infected tissue resulting in high mortality rates in fish (Becker and Speare 2007; Shaw and Kent 1999). Some genera of microsporidia in fish are known to cause hypertrophic growth whereby a unique host-parasite complex develops called a xenoma or a xenoparasitic complex (Lom and Dyková 2005). Although microsporidia induced xenomas are found in some invertebrates and other poikilothermic vertebrates, xenomas are most commonly found in fish (Lom and Dyková 2005). The size of these hypertrophied cells are often 400 to 500 μm in diameter but have been described to be as large as 13 mm (Canning and Lom 1986; Shaw and Kent 1999). Xenoma inducing microsporidia tend to be more host-specific than non-xenoma inducing species (Lom and Dyková 2005). This is certainly true of Heterosporis sp., which is considered one of 3 pathogens on the Great Lakes Commission Priority Invasive Species List (Great Lakes Commission 2005; Sutherland et al. 2004).
Economic importance of microsporidia of fish
Microsporidiosis has substantial consequences to the profitability of aquaculture and commercial fishing. World aquaculture production has experienced an average annual growth of 8.8% since 1970, and continues to outpace all other animal food-producing sector growth (FAO, 2007) . With 75% of marine fish stocks at or above sustainable yields, aquaculture can anticipate future pressure to supply demand (UN Atlas of the Oceans 2000). Disease in wild and farmed fish increases these pressures on aquaculture production. For example, Becker and Speare (2007) suggest that mortality of farmed Chinook salmon from microsporidia has, in part, influenced the need to dramatically increase production to compensate for reduced returns. Additionally, high mortality rates from microsporidia have contributed to the weakening or collapse of several fisheries (Shaw and Kent 1999). Therefore, the economic impacts of microsporidia have a compounding effect and have a potential to cause economic hardships in these industries.
Sub-lethal infections can additionally impact commercial fishing and aquaculture. Glugea, Loma, Nucelospora and Heterosporis genera are responsible for a number of microsporidial diseases in economically important fish. Pathological expression of microsporidia varies by species of parasite and the tissue infected. Symptoms can include leukemia-like conditions, emaciation, disfigurement from xenoparasitic growths or tissue necrosis, and growth inhibition (Lom and Dyková 2005; Shaw and Kent 1999). For example, farmed salmonids, in particular, are susceptible to Loma salmonae and Nucleospora salmonis. L. salmonae causes xenoma growths on gill tissue and promotes respiratory failure, whereas N. salmonis results in leukemic symptoms. Heterosporis anguillarum causes morphological changes in the Japanese eel with necrotic depressions of trunk musculature (T’sui and Wang 1988). The resulting disfigurement is called “Beko Disease” whereby muscle tissue undergoes liquefaction and is replaced with developing spores. Glugea spp. cause disfigurement with xenoparasitic growths in other economically important fish, such as in ayu and winter flounder (Cali and Takvorian 1991; Lee et al. 2004). Reductions in the fitness of these fish correspond to a reduction in catch value. Consequently, sub-lethal microsporidial infections also impact the fishing and aquaculture industries.
As well as impacting capture fisheries and aquaculture, microsporidia can have an economic impact in the laboratory. The use of fish as models in biomedical research has dramatically increased in the last decade, largely lead by the development of the zebrafish (Danio rerio) model (Ackermann and Paw 2003). Two important microsporidian diseases afflict laboratory fishes; Pseudoloma neurophilia of zebrafish (Danio rerio) and Glugea anomala of stickleback species (Kent and Fournie 2007). Indeed, P. neurophilia is the most common pathogen in zebrafish research facilities.
Fish Cell Culture
As for cells from mammals, two general types of cultures can be used to study fish cells in vitro: primary cultures and cell lines. The two are interrelated because cell lines are developed from primary cultures. They differ in their life span. Primary cultures are initiated directly from the cells, tissues or organs of fish and typically last only a few days, but exceptions exist. The extreme is hemopoietic cultures from the rainbow trout spleen, which can be maintained for a year or more (Ganassin and Bols 1996). By convention (Schaeffer 1990), the primary culture ends and the cell line begins upon splitting or subcultivation of the primary culture into new culture vessels. In the case of mammalian cell lines, some can be propagated only a limited number of times, finite cells, whereas others can be grown indefinitely, continuous cell lines. Most fish cell lines appear to be continuous (Bols et al. 2005). Primary cultures or cell lines have been developed from most tissues and organs of fish (Bols and Lee 1991). Many mammalian cells lines express functional properties of mature cells or can be triggered to differentiate into more mature cells, whereas the differentiation status or capacity of piscine cell lines is largely unexplored (Bols et al. 2005), although some B lymphocyte and macrophage cell lines have been developed (Ganassin and Bols 1998; Miller et al. 1994). Relatively recent lists of fish cell lines have been published along with their availability in repositories (Bols et al. 2005; Fryer and Lannan 1994).
Microsporidia of fish in fish cell cultures
Very short-term primary cultures have been used to study the interactions of microsporidia spores with cells of the innate immune system, macrophages and neutrophils. The cultures usually have been used within 48 h and the focus has been on the study of phagocytic and respiratory burst capabilities of the phagocytes. With such cultures, head kidney macrophages from the ayu were shown to phagocytize Glugea plecoglossi spores and to do this by recognizing concanavalin A-reactive glycoproteins on the spore surface (Kim et al. 1999). As well, the G. plecoglossi spores were shown to inhibit the production of O2−> by ayu macrophages that had been stimulated with zymosan (Kim et al. 1998). The modulation of the macrophages behaviour might aid the establishment of G. plecoglossi infection. Another example is a comparison of short-term cultures of peritoneal-exudate adherent (PEA) cells from turbot that have been injected intraperitoneally with either sodium thioglycolate or spores of Tetramicra brevifilum (Leiro et al. 2001). For peritoneal cells from fish injected with microsporidian spores, more neutrophils were found among the PEA cells and these cells made less ROS in response to T. brevifilum spores. Thus again the microsporidian spores seemed capable of impairing the respiratory burst of phagocytes and this could aid infection. Cultures of macrophages from Chinook salmon and Atlantic salmon were compared for their ability to phagocytize Loma salmonae spores (Shaw et al. 2001). Phagocytosis was higher in Atlantic salmon macrophages. This suggests a possible cellular basis for the Atlantic salmon being resistant and Chinook salmon being susceptible to the parasite.
Perhaps the most successful microsporidia/fish cell culture system has been obtained not with cell lines but with relatively long-term primary cultures of salmonid mononuclear leukocytes and less frequently head kidney stromal epithelial cells (Table 1) (Desportes-Livage et al. 1996; Wongtavatchai et al. 1994; 1995). These cultures supported the growth of Enterocytozoon salmonis. For this, the basal medium was Iscove’s modified Dulbecco’s medium supplemented with fetal bovine serum, concanavalin A, lipopolysaccharide (LPS), and human recombinant interleukin 2. The source of infection was leukocytes from microsporidia-infected fish. Leukocytes from microsporidia-infected Chinook salmon and rainbow trout were co-cultured with leukocytes from non-infected fish to start the cultures. Cells from the initial cultures could be subcultured by adding them to new cultures of leukocytes from healthy fish. This subculturing could be done up to 17 times for almost a year. The cultures retained E. salmonis stages from early meronts to mature spores. Two mechanisms were postulated to explain the spread of the parasite among the cultured cells: microsporidia could be transferred from mother cell to two daughter cells upon division of the host cell or could directly penetrate uninfected host cells. As the cultures required the periodic addition of uninfected leukocytes, the latter mechanism appeared to be in operation.
Table 1.
Fish cell culture systems used in the cultivation of microsporidia from fish
| Characteristics of the fish cell cultures | Characteristics of the microsporidia |
Microsporidia in fish cell culture | Reference | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Type of culture | Species | Tissue origin | Species | Normal host | Initiating infection in vitro | Microsporidia growth | |
| Primary culture: leukocytes |
Chinook salmon |
Peripheral blood |
Nucleospora
salmonis |
Fish (salmonids) |
Leukocytes from infected fish (co-cultivation) |
Spore development in leukocyte cell nucleus |
Wongtavatchai et al. (1994); Wongtavatchai et al. (1995) |
| Primary culture: leukocytes |
Chinook salmon |
Infected kidney |
Nucleospora
salmonis |
Fish (salmonids) |
Leukocytes from infected fish (co-cultivation) |
Spore development in leukocyte cell nucleus |
Desportes-Livage et al. (1996) |
| Primary culture: epithelial-like |
Rainbow trout |
Kidney |
Nucleospora
salmonis |
Fish (salmonids) |
Leukocytes from infected fish (co-cultivation) |
Spore development in epithelial cell nucleus |
Desportes-Livage et al. (1996) |
| Cell line: CHSE-214, epithelial-like |
Chinook salmon |
Salmon embryo | Glugea sp. | Fish (many species) |
Spores from infected fish (inoculation ) |
Proliferation of microsporidia stopped after 48 h in culture |
Lores et al. (2003) |
| Cell lines | |||||||
| CCO: fibroblast | Channel catfish |
CCO: ovary |
Pseudoloma
neurophilia |
Fish (zebrafish) |
Spores from infected fish (inoculation) |
Develops aggregates of approximately 8 spores per cell |
Watral et al. (2006) |
| SJD.1: fibroblast | Zebrafish | SJD.1: fin | |||||
| EPC: epithelial-like | Carp | EPC: skin | |||||
| FHM: epithelial-like | Fathead minnow |
FHM: connective tissue and muscle |
|||||
| Cell line: EP-1, epithelial-like |
Japanese eel |
Infected tissues of elvers |
Heterosporis
anguillarum |
Fish (eels) | Not done; began by exposing elvers to spores |
Meront development | Kou et al. (1995) |
Inocula from these cultures were able to cause in vivo a disease that was identical to the disease observed in naturally infected Chinook salmon (Wongtavatchai et al. 1995). Although cultures stored at 4 °C in water or at −70°C in medium without a cryoprotectant lost infectivity, the cells of E. salmonis infected leukocyte cultures could be cryopreserved in liquid nitrogen with a cryoprotectant and still retain their infectivity (Wongtavatchai et al. 1994). Interestingly, cultures with many spores and cultures with few spores were both infective in fish (Wongtavatchai et al. 1995). Possibly, the prespore stages from cultures rapidly sporulated upon injection and these gave rise in vivo to infectious spores, which started the infection in fish. Alternatively, as well as spores, early proliferative forms of E. salmonis from cell cultures could be infective to Chinook salmon.
The E. salmonis/salmonid leukocyte cultures allowed the developmental stages of the microsporidia to be examined by TEM (Desportes-Livage et al. 1996; Wongtavatchai et al. 1995). The most unique feature was that all developmental stages were in direct contact with the host nucleoplasm. TEM revealed the differentiation of the polar tube precursors (PTP), the polaroplast primordium (PLP), and the granular body (GB) and their assembly into the extrusion apparatus of the spores.
Salmonid leukocyte cultures have been used to show that E. salmonis causes the leukocytes to release mitogenic factors that stimulates the proliferation of uninfected mononuclear cells (Wongtavatchai et al. 1995). The significance of this observation is that these factors could explain the principal pathological feature of the disease. Most E. salmonis infections have been observed in Chinook salmon, and a characteristic feature of the disease is the excessive proliferation of mononuclear leukocytes. The nature of the factor(s) responsible for this has yet to be determined, but the cell culture system could be a key to unraveling them.
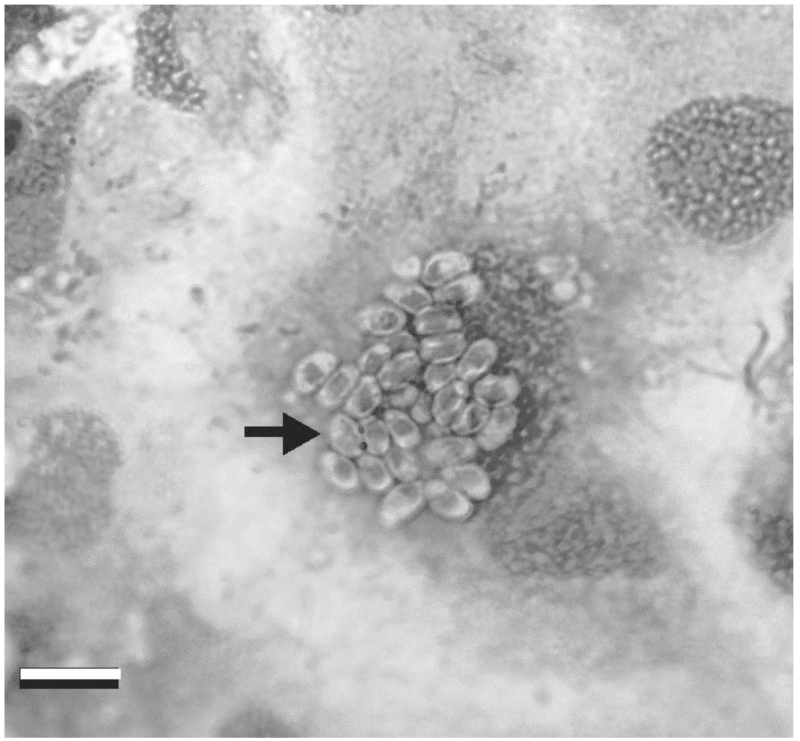
Several fish cell lines have been investigated for their capacity to support the growth of fish microsporidia. Spores of a fish microsporidia of the genus Glugea sp were internalized in 12 h by cells of the Chinook salmon embryo cell line (CHSE-214) and sporoplasms and meronts were seen in parasitophorous vacuoles (Lores et al. 2003). However, development stopped by 48 h. Another fish microsporidia that has been studied is Pseudoloma neurophilia, which in research facilities is a common pathogen of zebrafish (Danio rerio) and is found in the central nervous system (Watral et al. 2006). Spores were collected from brains and anterior spinal cords of infected zebrafish and added to cultures of Channel Catfish Ovary (CCO), Zebrafish Caudal Fin Fibroblast (SJD.1), Carp Epithelioma (EPC) and Fathead Minnow (FHM ), which were maintained at 28 °C. Aggregates of about 8 spores per cell developed. Examples are shown in Figure 2B. However, further development was limited and requires more investigation.
Figure 2. Pseudoloma neurophilia in EPC cell culture at 1 week post-exposure.
A. Low magnification through inverted microscope. B. DifQuick stain from cell culture on glass slide. Bar = 10 μm
The only other fish microsporidial pathogen that has been grown in vitro is by far the most unique of the in vitro fish microsporidia systems, and perhaps in all of in vitro microsporidia work. This is the eel (Anguilla japonica) epithelial-like cell line, EP-1, which seems to be persistently infected with Pleistophora anguillarum, now Heterosporis anguillarum (Kou et al. 1995). EP-1 was developed from infected tissues of eel elvers (young eels) that had twelve days previously been immersed in a suspension of H. anguillarium spores. The spores had been obtained from infected tissue of naturally infected eels. Several cell lines arose from the infected elver tissues but only EP-1 was characterized. EP-1 seemed immortal as the cells were passaged over 223 times and had a heteroploid karyotype. Merogonial stages of H. anguillarium were visualized in EP-1 by TEM and merozoites were detected by Feulgen staining and indirect immunofluorescent staining with antisera against H. anguillarium spores. The appearance of spores in EP-1 cultures was not specifically noted. However, after the 140th passage, EP-1 cells were injected into uninfected elvers and the young eels developed the muscle pathology of an H. anguillarium infection. Therefore, this in vitro system seemed to be producing H. anguillarium merozoites that were capable of continuing in vivo sporogonial reproduction and causing disease in the natural host, eels. This unique persistently infected cell line may prove to be an important tool to advance our knowledge of fish microsporidial pathogens and how to study microsporidia in vitro (Monaghan et al. 2008).
To date, the culturing of fish microsporidia in fish cell cultures has not achieved the success that has been accomplished with insect and mammalian microsporidia. This is not likely due to the lack of cell lines from fish because as documented in the first section below, microsporidia from one animal group can infect in vitro the cells from a very different group of animals. A possibility is that specific differentiated cell types from fish are needed to grow fish microsporidia. However, as documented in the second section below, microsporidia often appear less fastidious about the cell type in vitro.
Host animal group specificity in vitro
The precise in vivo specificity of a microsporidial species for a host animal group is difficult to state definitively. Microsporidia from diverse animal groups are being found to cause disease in animal species widely different from the hosts in which they were discovered initially. Microsporidia from mammals, insects, and even fish have been implicated in human infections. The occurrence of the four most common human microsporidial pathogens in non-human mammals raises questions regarding possible reservoirs for these parasites and their zoonotic potential (Mathis et al. 2005). A 56-year old woman died of myositis initiated apparently from a mosquito bite, and caused by the “insect” microsporidia B. algerae (Coyle et al. 2004). A fish microsporidia is thought to be the cause of myositis in an immuno-compromised man (Ledford et al. 1985). The in vitro results suggest that microsporidia can initiate at least some infection steps in cells from a wide range of vertebrates and invertebrates.
Many examples exist of microsporidia from insects being studied in mammalian cell cultures. The first successful cultivation of an insect derived microsporidian, B. algerae, in a mammalian cell culture (pig kidney) was subsequently followed by cultivation of microsporidian insect isolates in other mammalian cell cultures including rat, mouse and rabbit (Ishihara 1968; Smith and Sinden 1980; Undeen 1975). More recently, in vitro approaches using insect derived microsporidia in mammalian cell culture have furthered our understanding of parasite development and factors that influence it (Franzen et al. 2005a; Lowman et al. 2000; Takvorian et al. 2005; Trammer et al. 1999). For example, temperature imposed parameters to microsporidia growth from insect isolates have been evaluated in a number of in vitro studies using mammalian cells. Tubulinosema ratisbonensis, known only to infect fruit flies in vivo, was isolated and able to infect human lung fibroblasts (MRC-5) at 31 °C and 37 °C, yet was unsuccessful in monkey kidney cells (Vero) (Franzen et al. 2005a). T. ratisbonensis grew at both temperatures, but when compared, proliferation was reduced at 37 °C. Likewise, Lowman et al. (2000) determined that the incubation of B. algerae spores at temperatures ranging from 29 °C to 37 °C did not prevent microsporidia growth, but found that higher temperatures inhibited the rate of growth.
Another example of a species of microsporidia from invertebrates interacting in vitro with cells from mammals is Ameson michaelis, which infects blue crabs. When placed in culture media with various cell types, A. michaelis was able to inject sporoplasm into not only epithelial cells and hemocytes of the blue crab, but as well into human erythrocytes and mouse cells of various types (leukemia EL4 cells, macrophages, and neuroblastoma C1300 cells) (Weidner 1972). In these experiments proliferation of A. michaelis did not occur. Thus for A. michaelis, the first infection step showed no restriction in vitro between cells of different species and types but subsequent steps must have been limiting.
A few examples exist of microsporidia from mammals being studied in fish cell cultures (Table 2). Encephalitozoon cuniculi, of mammalian origin, was reported to develop spores in the cytoplasm of a fathead minnow cell line (Bedrnik and Vavra 1972). Also interesting was that this was accomplished at 18 °C, a temperature far cooler than any mammalian host. Enterocytozoon bieneusi, was cultivated in a primary culture from rainbow trout kidney cells (Desportes-Livage et al. 1996). This is of particular interest as E. bienusi has been very difficult to culture in mammalian cells (Visvesvara 2002). Desporte-Livage et al. (1996) observed early E. bieneusi development to be organized with cisternae of the host endoplasmic reticulum (ER). Meronts and sporonts were also found in close association with the host nucleus. In desquamating cells, mature spores were observed which indicates probable discontinuation of development (Desportes-Livage et al. 1996). The last example relates to Glugea sp. collected from the livers of Greater sand eels (Hyperoplus lanceolatus), which was unproductive in fish culture, but successful at proliferating in a mosquito larvae cell line (ECACC90100401) (Lores et al. 2003). Meront development was observed within 12 hours post-infection (pi), sporogony at 48 pi, and after 7 days a variety of developmental stages were observed demonstrating the ability of Glugea sp. to continuously proliferate in the mosquito larvae cell line (Lores et al. 2003).
Table 2.
Examples of microsporidia cultivated in animal cell culture systems different from the class or group of the apparent natural host
| Characteristics of the animal cell cultures | Characteristics of the microsporidia | Microsporidia in animal cell culture | Reference | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Type of culture | Species | Tissue origin | Species | Normal host | Initiating infection in vitro | Microsporidia growth | |
| Cell line: FHM, epithelial- like |
Fathead minnow |
Connective tissue and muscle |
Encephalitozoon
cuniculi |
Mammals; including humans |
Spores from unspecified mammalian origin (inoculation) |
Spore development | Bedrnik and Vavra (1972) |
| Primary culture: epithelial- like |
Rainbow trout |
Kidney |
Enterocytozoon
bienusi |
Mammals; including humans |
Spores from human duodenal biopsies (inoculation) |
Spore development in host cell cytoplasm |
Desportes-Livage et al. (1996) |
| Cell line: ECACC 90100401, epithelial-like |
Mosquito | Larvae | Glugea sp. | Fish; various species |
Spores from infected fish (inoculation) |
Mature spores within 72 h post-infection |
Lores et al. (2003) |
| Cell line: RK13, epithelial- like |
Rabbit | Kidney |
Brachiola
algerae |
Insect; but can infect humans |
Spores isolated from mosquito (inoculation) |
Mature spores within 72 h post-infection |
Lowman et al. (2000) |
| Cell line: MRC-5, fibroblasts | Human | Lung |
Tubulinosema
ratisbonesis |
Fruit flies | Spores isolated from fruit flies (inoculation) |
Mature spores within 72 h post-infection |
Franzen et al. (2005a) |
Currently no examples exist of microsporidia from fish being studied in mammalian cell cultures. Temperature would be anticipated to prevent microsporidia from coldwater fish being studied in mammalian cells but this might not be a barrier for microsporidia from warmwater fish. The advantages of using mammalian cells would be that more cell lines expressing differentiated functions exist, several in vitro differentiation systems are well defined, and more antibodies and molecular probes would be available to study the host cell responses.
Host tissue or cell specificity in vitro
The precise in vivo specificity of a microsporidial species for a host tissue or cell type is difficult to state definitely. While Encephalitozoon spp. are the primary cause of disseminated microsporidiosis in humans, other species of microsporidia appear to infect only specific tissue in their hosts (Mertens et al. 1997; Tosoni et al. 2002). For example, of the 14 species of microsporidia that infect humans, 3 are exclusively known to infect the eye, while 3 other species infect the eye and one other site (Didier and Weiss 2006). As well, P. ronneafiei and Brachiola vesicularum are two species that are known to infect only muscle tissue (Cali and Takvorian 2003; Didier and Weiss 2006). Interestingly, even among the same genus of microsporidial pathogens, such as Brachiola, there can be a broad range of clinical presentations. B. connori demonstrates low tissue specificity as the causative agent of systemic infections while B. vesicularum demonstrates high tissue specificity. On the other hand, E. bienusi is rarely found infecting tissue other than the epithelium of the small intestine (Didier and Weiss 2006). Nonetheless, mechanisms providing the ability for some species to invade a variety of tissues types while others are found in limited tissue types remain unclear.
Despite hints of apparent tissue tropism in vivo, the same microsporidia in vitro appear to be able to infect cells of different tissue origin and of stage of differentiation. For example, Franzen et al. (2005b) studied the internalization of 4 microsporidian species in 7 different cell lines in vitro and observed that phagocytic cells were the most proficient at internalizing spores than non-phagocytic cells. Comparing the non-phagocytic cells, the only clear result was that a pulmonary cell line, A549, was 10 times less efficient than its counterparts at internalizing the spores. Further, the intestinal cell lines were effective at internalizing spores that commonly affect the gastrointestinal tract but were also effective at internalizing spores of microsporidian species that typically do not cause gastrointestinal microsporidiosis. Because internalization patterns in the cell lines could not be correlated to clinical presentations, it was suggested that access to tissue types through various routes of infection are likely responsible for apparent tissue specificity rather than tissue tropisms (Franzen et al. 2005b).
Another example of an apparent loss of tissue tropism in vitro is with E. salmonis from rainbow trout (Desportes-Livage et al., 1996). In vivo E. salmonis was found only in leukocytes from blood and from hematopoietic tissues (kidney and spleen). By contrast, in vitro E. salmonis developed in epithelial cells of long-term head kidney cultures and the yield of spores per cell was higher for the epithelial cells than for leukocytes.
Conclusions and Future
Insect and mammalian cell culture systems pioneered the in vitro study of microsporidia leading to important observations on their infectivity and development and an evaluation of possible treatments and disinfection procedures. Fish microsporidiology in fish cell culture also could make advancements in these areas and these in turn could help in understanding how to diagnose and control microsporidia infections in aquaculture. For example, cell cultures could be a source of material for the development of vaccines. However, besides their practical value, microsporidia/fish cell culture systems might uncover some unique cell biology processes. One is the possibility that for some fish microsporidia, fluctuating temperatures rather than constant temperatures are needed for all stages to be expressed in vitro. Another is the process behind the induction of xenomas, which are exceptional animal cells because of their enormous size and are most commonly found with fish microsporidia. A final example is the possible ability of fish microsporidia to immortalize animal cells, which are hinted at in work on H. anguillarium.
Acknowledgements
The Natural Sciences and Engineering Research Council (NSERC) of Canada supported SRM through a postgraduate scholarship and LEJL and NCB through Discovery Grants. MLK, VGW and RJK would like to acknowledge support for cell culture work through a National Institutes of Health grant (NIH NCRR R24RR017386-01).
References
- Ackermann G, Paw BH. Zebrafish: a genetic model for vertebrate organogenesis and human disorders. Front Biosci. 2003;8:1227–1253. doi: 10.2741/1092. [DOI] [PubMed] [Google Scholar]
- Ashton N, Wirasinha P, Wirasinha A. Encephalitozoonosis (nosematosis) of the cornea. Br. J. Ophthalmol. 1973;60:618–631. doi: 10.1136/bjo.57.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvis B, Sarfati C, Challier S, Derouin F. In vitro model to assess the effect of antimicrobial agents on Encephalitozoon cuniculi. Antimicrob. Agents Chemother. 1994;38:2440–2448. doi: 10.1128/aac.38.10.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Speare DJ. Transmission of the microsporidian gill parasite Loma salmonae. Anim. Health Res. Rev. 2007;8(1):59–68. doi: 10.1017/S1466252307001223. [DOI] [PubMed] [Google Scholar]
- Becnel JJ. Expanding frontiers for microsporidia: A tribute to Professor Elizabeth U. Canning. J. Invert. Pathol. 2006;92:116–124. doi: 10.1016/j.jip.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Bedrnik P, Vavra J. Further observations on the maintenance of Encephalitozoon cuniculi in tissue culture. J. Protozool. 1972;19:75S. [Google Scholar]
- Belkorchia A, Biderre C, Militon C, Polonais V, Wincker P, Jubin C, Delbac F, Peyretaillade E, Peyret P. In vitro propagation of the microsporidian pathogen Brachiola algerae and studies of its chromosome and ribosomal DNA organization in the context of the complete genome sequencing project. Parasitol. Inter. 2008;57:62–71. doi: 10.1016/j.parint.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Bigliardi E, Riparbelli MG, Selmi MG, Bini L, Liberatorii S, Pallini V, Bernuzzi A, Gatti S, Scaglia M, Sacchi L. Evidence of actin in the cytoskeleton of microsporidia. J. Eukaryot. Microbiol. 1999;46:410–415. doi: 10.1111/j.1550-7408.1999.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Bols NC, Dayeh VR, Lee LEJ, Schirmer K. Use of fish cell lines in the toxicology and ecotoxicology of fish. Piscine cell lines in environmental toxicology. In: Mommsen TP, Moon TW, editors. Biochemistry and Molecular Biology of Fishes. Elsevier Science; 2005. pp. 60–84. [Google Scholar]
- Bols NC, Lee LEJ. Technology and uses of cell-cultures from the tissues and organs of bony fish. Cytotechnol. 1991;6(3):163–187. doi: 10.1007/BF00624756. [DOI] [PubMed] [Google Scholar]
- Cali A, Takvorian PM. The incidence of Glugea stephani (Protozoa: Microsporida) in winter flounder, Psudopleuronectes americanus, from the New York - New Jersy Lower Bay Complex and factors influencing it. Can. J. Zool. 1991;69:317–321. [Google Scholar]
- Cali A, Takvorian PM. Developmental morphology and life cycles of the microsporidia. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. ASM Press; Washington, D.C.: 1999. pp. 85–128. [Google Scholar]
- Cali A, Takvorian PM. Ultrastructure and development of Pleistophora ronneafiei n. sp., a microsporidium (Protista) in the skeletal muscle of an immune-compromised individual. J. Eukaryot. Microbiol. 2003;50:77–85. doi: 10.1111/j.1550-7408.2003.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Canning EU. A new microsporidian, Nosema locustae n. sp., from the fat body of the Africian migratory locust, Locusta migratoria migratorioides R. and F. Parasitol. 1953;43:287–291. doi: 10.1017/s0031182000018655. [DOI] [PubMed] [Google Scholar]
- Canning EU, Lom J. The Microsporidia of Vertebrates. Academic Press; Toronto: 1986. p. 289. [Google Scholar]
- Chen Y, Evans JD, Smith B, Pettis JS. Nosema ceranae is a long-present and wide-spred microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invert. Pathol. 2008;97:186–188. doi: 10.1016/j.jip.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S. Phagocytic uptake of Encephalitozoon cunculi by nonprofessional phagocytes. Infect. Immun. 2000;68(12):6939–6945. doi: 10.1128/iai.68.12.6939-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Weiss LM, Rhodes LV, III, Cali A, Takvorian PM, Brown DF, Visvesvara GS, Xiao L, Naktin J, Young E, Gareca M, Colasante G, Wittner M. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. N. Engl. J. Med. 2004;351(1):42–47. doi: 10.1056/NEJMoa032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Aguila C, Izquierdo F, Granja AG, Hurtado C, Fenoy S, Fresno M, Revilla Y. Encephalitozoon microsporidia modulates p53-mediated apoptosis in infected cells. Int. J. Parasitol. 2006;36:869–876. doi: 10.1016/j.ijpara.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Desportes I, Le Charpentier Y, Galian A, Bernard F, Cochand-Priolett B, Lavergne A, Ravisse P, Modigliani R. Occurrence of a new microsporidian: Enterocytozoon bieneusi n.g., n. sp., in the enterocytes of a human patient with AIDS. J. Protozool. 1985;32:250–254. doi: 10.1111/j.1550-7408.1985.tb03046.x. [DOI] [PubMed] [Google Scholar]
- Desportes-Livage I, Chilmonczyk S, Hedrick R, Ombrouck C, Monge D, Maiga I, Gentilini M. Comparative development of two microsporidian species: Enterocytozoon bieneusi and Enterocytozoon salmonis, reported in AIDS patients and salmonid fish, respectively. J. Eukaryot. Microbiol. 1996;43(1):49–60. doi: 10.1111/j.1550-7408.1996.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Didier ES. Microsporidiosis: An emerging and opportunisitc infection in humans and animals. Acta Tropica. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Didier ES, Bowers L, Stovall ME, Kuebler D, Mittleider D, Brindley PJ, Didier PJ. Antimicrosporidial activity of (fluoro)quinolones in vitro and in vivo. Folia Parasitol. 2005;52:173–181. doi: 10.14411/fp.2005.022. [DOI] [PubMed] [Google Scholar]
- Didier ES, Didier PJ, Friedberg DN, Stenson SM, Orenstein JM, Yee RW, Tio FO, Davis RM, Vossbrinck C, Millichamp N. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three patients with keratoconjunctivitis. J. Infect. Dis. 1991;163:617–621. doi: 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- Didier ES, Weiss LM. Microsporidiosis: current status. Cur. Op. Infect. Dis. 2006;19:485–492. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier PJ, Phillips JN, Kuebler D, Nasr M, Brindley PJ, Stovall ME, Bowers LC, Didier ES. Antimicrosporidial activities of fumagillin, TNP-470, ovalicin, and ovalicin derivatives in vitro and in vivo. Antimicrob. Agents Chemother. 2006;50(6):2146–2155. doi: 10.1128/AAC.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins WOI, Weinstein W. Electron microscopy of the intestine and rectum in acquired immunodeficiency syndrome. Gastroenterol. 1985;88(3):738–749. doi: 10.1016/0016-5085(85)90145-3. [DOI] [PubMed] [Google Scholar]
- Ellis JD, Munn PA. The worldwide health status of honey bees. Bee World. 2005;86:88–101. [Google Scholar]
- Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85–87. doi: 10.1126/science.109.2822.85. [DOI] [PubMed] [Google Scholar]
- Fasshauer V, Gross U, Bohne W. The parasitophorous vacuole membrane of Encephalitozoon cuniculi lacks host cell membrane proteins immediately after invasion. Eukaryot. Cell. 2005;4(1):221–224. doi: 10.1128/EC.4.1.221-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Tran D, Juneau R, Hale-Donze H. Kinetics of Encephalitozoon spp. infection of human macrophages. J. Parasitol. 2008;94(1):169–175. doi: 10.1645/GE-1303.1. [DOI] [PubMed] [Google Scholar]
- Fischer J, West J, Agochukwu N, Suire C, Hale-Donze H. Induction of host chemotactic response by Encephalitozoon spp. Infect. Immun. 2007;75(4):1619–1625. doi: 10.1128/IAI.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO: Food and Agriculture Organization of the United Nations . In: The State of World Fisheries and Aquaculture 2006. FAO Fisheries and Aquaculture Department, editor. Rome: 2007. p. 162. [Google Scholar]
- Franzen C, Fischer S, Schroeder J, Bleiss W, Schneuwly S, Scholmerich J, Salzberger B. In vitro cultivation of an insect microsporidian Tubulinosema ratisbonensis in mammalian cells. J. Eukaryot. Microbiol. 2005a;52(4):349–355. doi: 10.1111/j.1550-7408.2005.00043x. [DOI] [PubMed] [Google Scholar]
- Franzen C, Hosl M, Salzberger B, Hartmann P. Uptake of Encephalitozoon spp. and Vittaforma corneae (Microsporidia) by different cells. J. Parasitol. 2005b;91(4):745–749. doi: 10.1645/GE-468R.1. [DOI] [PubMed] [Google Scholar]
- Franzen C, Muller A. Microsporidiosis: human diseases and diagnosis. Microb. Infect. 2001;3(5):389–400. doi: 10.1016/s1286-4579(01)01395-8. [DOI] [PubMed] [Google Scholar]
- Fryer JL, Lannan CN. Three decades of fish cell culture: A current listing of cell lines derived from fishes. J. Tiss. Cult. Meth. 1994;16:87–94. [Google Scholar]
- Ganassin RC, Bols NC. Development of long-term rainbow trout spleen cultures that are haemopoietic and produce dendritic cells. Fish Shellfish Immunol. 1996;6:17–34. [Google Scholar]
- Ganassin RC, Bols NC. Development of a monocyte/macrophage-like cell line, RTS11, from rainbow trout spleen. Fish Shellfish Immunol. 1998;8:457–476. [Google Scholar]
- Great Lakes Commission Great Lakes Panel Priority Invasive Species List, 2005. 2005 http://www.glc.org/ans/pdf/priority-species.pdf Cited 26 Aug 2008.
- Hayman JR, Southern TR, Nash TE. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect. Immun. 2005;73:841–848. doi: 10.1128/IAI.73.2.841-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JE, Onsager JA. Large-scale test of control grasshoppers on rangland with Nosema locustae. J. Econ. Entomol. 1982;75:31–35. [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Lumbsch HT, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai Y-C, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Koljalg U, Maidlikowska J, Miller A, Moncalvo J-M, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schubler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao Y-J, Zhang N. A higher-level phylogenetic classification of the Fungi. Mycolog. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Hirt RP, Logsdon JM, Healy B, Dorey MW, Doolittle WF, Embley TM. Microsporidia are related to Fungi: Evidence from the largest subunit of RNA polymerase II and other proteins. Proc. Natl. Acad. Sci. USA. 1999;96(580-585) doi: 10.1073/pnas.96.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister WS, Canning EU, Anderson CL. Identification of microsporidia causing human disease. J. Eukaryot. Microbiol. 1996;43(5):S104–S105. doi: 10.1111/j.1550-7408.1996.tb05026.x. [DOI] [PubMed] [Google Scholar]
- Hsiung GD. The impact of cell culture sensitivity on rapid viral diagnosis: a historical perspective. Yale J. Biol. Med. 1989;62:79–88. [PMC free article] [PubMed] [Google Scholar]
- Ishihara R. Growth of Nosema bombycis in primary cell cultures of mammalian and chick embryos. J. Invert. Pathol. 1968;11(2):328. [Google Scholar]
- Iwano H, Kurtti TJ. Identification and isolation of dimorphis spores from Nosema furnacalis (Microspora: Nosematidae) J. Invert. Pathol. 1995;65:230–236. [Google Scholar]
- Jaronski ST. Microsporidia in Cell Culture. Adv. Cell Cult. 1984;18:183–229. [Google Scholar]
- Jelinek J, Salat J, Sak B, Kopecky J. Effects of interferon gamma and specific polyclonal antibody on the infection of murine peritoneal macrophages and murine macrophage cell line PMJ2-R with Encephalitozoon cuniculi. Folia Parasitol. 2007;54(3):172–176. [PubMed] [Google Scholar]
- John DE, Nwachuku N, Pepper IL, Gerba CP. Development and optimization of a quantitative cell culture infectivity assay for the microsporidium Encephalitozoon intestinalis and application to ultraviolet light inactivation. J. Microbiol. Meth. 2003;52:183–196. doi: 10.1016/s0167-7012(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Keeling PJ, Fast NM. Microsporidia: Biology and evolution of highly reduced intracellular parasites. Annu. Rev. Microbiol. 2002;56:93–116. doi: 10.1146/annurev.micro.56.012302.160854. [DOI] [PubMed] [Google Scholar]
- Keeling PJ, Luker MA, Palmer JD. Evidence from Beta-tubulin phylogeny that microsporidia evolved from within the Fungi. Mol. Biol. Evol. 2000;17(1):23–31. doi: 10.1093/oxfordjournals.molbev.a026235. [DOI] [PubMed] [Google Scholar]
- Kent ML, Fournie JW. Parasites of Fishes. In: Baker DG, editor. Flynn’s Parasites of Laboratory Animals. Blackwell Publ.; 2007. pp. 69–117. [Google Scholar]
- Kevan PG, Phillips TP. The economic impacts of pollinator declines: an approach to assessing consequences. Conservation Ecology. 2001;5(1):8. [online] http://wwwconsecol.org/vol5/iss1/art8/ [Google Scholar]
- Khurad AM, Raina SK, Pandharipande TN. In vitro propagation of Nosema locustae using fat body cell line derived from Mythimna convecta (Lepidoptera: Noctuidae) J. Protozool. 1991;38:91S–93S. [PubMed] [Google Scholar]
- Kim JH, Ogawa K, Wakabayashi H. Respiratory burst assay of head kidney macrophages of ayu, Plecoglossus altivelis, stimulated with Glugea plecoglossi (Protozoa: Microspora) spores. J. Parasitol. 1998;84(3):552–556. [PubMed] [Google Scholar]
- Kim JH, Ogawa K, Wakabayashi H. Lectin-reactive components of the microsporidian Glugea plecoglossi and their relation to spore phagocytosis by head kidney macrophages of ayu Plecoglossus altivelis. Dis. Aquat. Org. 1999;39(1):59–63. doi: 10.3354/dao039059. [DOI] [PubMed] [Google Scholar]
- Kotler DP, Orenstein JM. The Microsporidia and Microsporidiosis. ASM Press; Washington, D.C.: 1999. Clinical syndromes associated with microsporidiosis. [DOI] [PubMed] [Google Scholar]
- Kou G-H, Wang C-H, Hung H-W, Jang Y-S, Chou C-M, Lo C-F. A cell line (EP-1 cell line) derived from “Beko disease” affected Japanese eel elver (Anguilla japonica) persistently infected with Pleistophora anguillarum. Aquaculture. 1995;132:161–173. [Google Scholar]
- Kurtti TJ, ross SE, Liu Y, Munderloh UG. In vitro developmental biology and spore production in Nosema furnacalis (Microspora: Nosematidae) J. Invert. Path. 1994;63:188–196. [Google Scholar]
- Ledford DK, Overman MD, Gonzalvo A, Cali A, Mester SW, Lockey RF. Microsporidiosis myositis in a patient with the acquired immunodeficiency syndrome. Ann. Intern. Med. 1985;102(5):628–629. doi: 10.7326/0003-4819-102-5-628. [DOI] [PubMed] [Google Scholar]
- Lee S-J, Yokoyama H, Ogawa K. Modes of transmission of Glugea plecoglossi (Microspora) via the skin and digestive tract in an experimental infection model using rainbow trout, Oncorhynchus mykiss (Walbum) J. Fish Dis. 2004;27:435–444. doi: 10.1111/j.1365-2761.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Leiro J, Iglesias R, Parama A, Sanmartin ML, Ubeira FM. Effect of Tetramicra brevifilum (Microspora) infection on respiratory-burst responses of turbot (Scophthalmus maximus L.) phagocytes. Fish Shellfish Immunol. 2001;11:639–652. doi: 10.1006/fsim.2001.0340. [DOI] [PubMed] [Google Scholar]
- Leitch GJ, Shaw AP, Colden-Stanfield M, Scanlon M, Visvesvara GS. Multinucleate host cells induced by Vittaforma corneae (Microsporidia) Folia Parasitol. 2005;52:103–110. doi: 10.14411/fp.2005.013. [DOI] [PubMed] [Google Scholar]
- Lom J. A catalogue of described genera and species of microsporidians parasitic in fish. Syst. Parasitol. 2002;53:81–99. doi: 10.1023/a:1020422209539. [DOI] [PubMed] [Google Scholar]
- Lom J, Dyková I. Microsporidian xenomas in fish seen in wider perspective. Folia Parasitol. 2005;52:69–81. [PubMed] [Google Scholar]
- Lom J, Dyková I, Wang C-H, Lo C-F, Kou G-H. Ultrastructural justification for the transfer of Pleistophora anguillarum Hoshina, 1959 to the genus Heterosporis Schubert, 1969. Dis. Aquat. Org. 2000;43:225–231. doi: 10.3354/dao043225. [DOI] [PubMed] [Google Scholar]
- Lores B, Rosales MJ, Mascaro C, Osuna A. In vitro culture of Glugea sp. Vet. Parasitol. 2003;112:185–196. doi: 10.1016/s0304-4017(02)00411-9. [DOI] [PubMed] [Google Scholar]
- Lowman PM, Takvorian PM, Cali A. The effects of elevated temperatures and various time-temperature combinations on the development of Brachiola (Nosema) algerae N. Comb. in mammalian cell culture. J. Eukaryot. Microbiol. 2000;47(3):221–234. doi: 10.1111/j.1550-7408.2000.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Marcus PB, van der Watt JJ, Burger PJ. Human tumor microsporidiosis. Arch. Pathol. 1973;95:341–343. [PubMed] [Google Scholar]
- Margileth AM, Strano AJ, Chandra R, Neafie R, Blum M, McCully RM. Disseminated nosematosis in an immunogically compromised infant. Arch. Pathol. 1973;95:145–150. [PubMed] [Google Scholar]
- Mathis A, Weber R, Deplazes P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005;18:423–445. doi: 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H, Koike T, Mikata I, Takei H, Hagiwara S. A case of Encephalitozoon-like body in man. Arch. Pathol. 1959;67:181–187. [PubMed] [Google Scholar]
- Mertens RB, Didier ES, Fishbein MC, Bertucci DC, Rogers LB, Orenstein JM. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands and urinary bladder in a patient with AIDS. Mod. Pathol. 1997;10:68–77. [PubMed] [Google Scholar]
- Miller NW, Rycyzyn MA, Wilson MR, Warr GW, Naftel JP, Clem LW. Development and characterization of channel catfish long term B cell lines. J. Immunol. 1994;152:2180–2189. [PubMed] [Google Scholar]
- Modigliani R, Bories C, Le Charpentier Y, Salmeron M, Messing b, Galian A, Rambaud JC, Lavergne A, Cochand-Priolett B, Desportes I. Diarrhoea and malabsorption in acquired immunodeficiency syndrome: a study of four cases with special emphasis on opportunistic protozoan infestation. Gut. 1985;26(2):179–187. doi: 10.1136/gut.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan SR, Lo C-F, Bols NC, Lee LEJ. Evaluation of EP-1, a cell line from Anguilla japonica, to study the life cycle of the microsporidian Heterosporis anguillarum; World Congress on In Vitro Biology; Tucson, AZ: Vitro Cell. Dev. Biol. - Animal. 2008.p. 297. [Google Scholar]
- Moniez R. Observations pour la revision des microsporidies. C.R. Acad. Sci. Paris. 1887;104:1312–1314. [Google Scholar]
- Morris JA, McCown JM, Blount RE. Acites and hepatosplenomegaly in mice associated with protozoan-like cytoplasmic structures. J. Infect. Dis. 1956:306–311. doi: 10.1093/infdis/98.3.306. [DOI] [PubMed] [Google Scholar]
- Nageli K. Ueber die neue Krankheit die Seidenraupe und vervandte Organismen. Bot. Zietung. 1857;15:760–761. [Google Scholar]
- Olmstead A, Woolen DB. Bee pollination and productivity growth: the case of alfalfa. Amer. J. Agr. Econ. 1987;69:56–63. [Google Scholar]
- Pasteur L. Etude sur la maladie des vers a soie. Paris, France: 1870. [Google Scholar]
- Rinder H, Thomschke A, Dengjel B, Gothe R, Loscher T, Zahler M. Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J. Parasitol. 2000;86:185–188. doi: 10.1645/0022-3395(2000)086[0185:CGRBEB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ronnebaumer K, Gross U, Bohne W. The nascent parasitophorous vacuole membrane of Encephalitozoon cuniculi is formed by host cell lipids and contains pores which allow nutrient uptake. Eukaryot. Cell. 2008;7(6):1001–1008. doi: 10.1128/EC.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer WI. Terminology associated with cell, tissue and organ culture, molecular biology and molecular genetics. In Vitro Cell. Dev. Biol. - Animal. 1990;26(1):97–101. doi: 10.1007/BF02624162. [DOI] [PubMed] [Google Scholar]
- Schottelius J, Schmetz C, Kock NP, Schuler T, Sobottka I, Fleischer B. Presentation by scanning electron microscopy of the life cycle of microsporidia of the genus Encephalitozoon. Microb. Infect. 2000;2:1401–1406. doi: 10.1016/s1286-4579(00)01293-4. [DOI] [PubMed] [Google Scholar]
- Shadduck JA, Meccoli RA, Davis R, Font RL. Isolation of a microsporidian from a human patient. J. Infect. Dis. 1990;162:773–776. doi: 10.1093/infdis/162.3.773. [DOI] [PubMed] [Google Scholar]
- Shaw RW, Kent ML. Fish Microsporidia. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. ASM Press; Washington, D.C.: 1999. [Google Scholar]
- Shaw RW, Kent ML, Adamson ML. Phagocytosis of Loma salmonae (Microsporidia) spores in Atlantic salmon (Salmo salar), a resistant host, and chinook salmon (Oncorhynchus tshawytscha), a susceptible host. Fish Shellfish Immunol. 2001;11:91–100. doi: 10.1006/fsim.2000.0298. [DOI] [PubMed] [Google Scholar]
- Smith JE, Sinden RE. A technique for the culture of Nosema algerae in primary cultures of rat brain. J. Protozool. 1980;27:A59–A59. [Google Scholar]
- Snowden KF, Shadduck JA. Microsporidia in higher vertebrates. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. ASM Press; Washington, D.C.: 1999. pp. 393–417. [Google Scholar]
- Sokolova YY, Dolgikh VV, Morzhina EV, Nassonova ES, Issi IV, Terry RS, Ironside JE, Smith JE, Vossbrinck CR. Establishment of the new genus Paranosema based on the ultrastructure and molecular phylogeny of the type species Paranosema grylli Gen. Nov. comb. Nov. (Sokolova, Selezniov, Dolgikh, Issi 1994), from the cricket Gryllus bimaculatus Deg. J. Invert. Pathol. 2003;84:159–172. doi: 10.1016/j.jip.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Southern TR, Jolly CE, Lester ME, Hayman JR. EnP1, a microsporidian spore wall protein that enables spores to adhere to and infect host cells in vitro. Eukaryot. Cell. 2007;6:1354–1362. doi: 10.1128/EC.00113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague V. Nosema connori n. sp.: a microsporidian parasite of man. Trans. Am. Microsc. Soc. 1974;93(3):400–403. [PubMed] [Google Scholar]
- Sutherland DR, Cooper S, Stelzig P, Marcquenski S, Marcino J, Lom J, Dyková I, Nilsen F, Hsu H-M, Jahns W, Hoyle J, Penney R. Heterosporis sp. (Microspora): A new parasite from yellow perch (Perca flavescens) and walleye (Stizostedion vitreum) in Minnesota, Wisconsin and Lake Ontario, North America; In 13th International Conference on Aquatic Invasive Species; Ennis, County Clare, Ireland. 2004. [Google Scholar]
- T’sui W-H, Wang C-H. On the Plistophora infection in eel II. The development of Plistophora anguillarum in experimentally infected elvers, Anguilla japonica. Bull. Instit. Zool. 1988;27(4):249–258. [Google Scholar]
- Takvorian PM, Weiss LM, Cali A. The early events of Brachiola algerae (Microsporidia) infection: spore germination, sporoplasm structure, and development within host cells. Folia Parasitol. 2005;52:118–129. doi: 10.14411/fp.2005.015. [DOI] [PubMed] [Google Scholar]
- Taupin V, Metenier G, Delbac F, Vivares CP, Prensier G. Expression of two cell wall proteins during the intracellular development of Encephalitozoon cuniculi: an immunocytochemical and in situ hybridization study with ultrathin frozen sections. Parasitol. 2006a;132:815–825. doi: 10.1017/S0031182005009777. [DOI] [PubMed] [Google Scholar]
- Taupin V, Metenier G, Vivares CP, Prensier G. An improved procedure for Percoll gradient separation of sporogonial stages in Encephalitozoon cuniculi (Microsporidia) Parasitol. Res. 2006b;99(6):708–714. doi: 10.1007/s00436-006-0231-y. [DOI] [PubMed] [Google Scholar]
- Tosoni A, Nebuloni M, Ferri A, Bonetto S, Antinori S, Scaglia M, Xiao L, Moura H, Visvesvara GS, Vago L, Costanzi G. Disseminated microsporidiosis caused by Encephalitozoon cuniculi III (Dog Type) in an Italian AIDS patient: a retrospective study. Mod. Pathol. 2002;15(5):577–583. doi: 10.1038/modpathol.3880566. [DOI] [PubMed] [Google Scholar]
- Trager W. The hatching of spores of Nosema bombycis Nageli and the partial development of the organism in tissue culture. J. Parasitol. 1937;23:226–227. [Google Scholar]
- Trammer T, Chioralia G, Maier WA, Seitz HM. In vitro replication of Nosema algerae (Microsporidia), a parasite of anopheline mosquitoes, in human cells above 36 °C. J. Eukaryot. Microbiol. 1999;46:464–468. doi: 10.1111/j.1550-7408.1999.tb06062.x. [DOI] [PubMed] [Google Scholar]
- UN Atlas of the Oceans Fisheries and Aquaculture. 2000 http://www.oceansatlas.org. Cited 26 Aug 2008.
- Undeen AH. Growth of Nosema algerae in pig kidney cell cultures. J. Protozool. 1975;22(1):107–110. doi: 10.1111/j.1550-7408.1975.tb00951.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS. In vitro cultivation of microsporidia of clinical importance. Clin. Microbiol. Rev. 2002;15(3):401–413. doi: 10.1128/CMR.15.3.401-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara GS, Da Silva AJ, Croppo GP, Pieniazek NJ, Leitch GJ, Ferguson D, De Moura H, Wallace S, Slemenda SB, Tyrrel I, Moore DF, Meador J. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J. Clin. Microbiol. 1995a;33:930–936. doi: 10.1128/jcm.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara GS, Leitch GJ, Pieniazek NJ, Da Silva AJ, Wallace S, Slemenda SB, Weber R, Schwartz DA, Gorelkin L, Wilcox CM, Bryan RT. Short-term in vitro culture and molecular analysis of the microsporidian, Enterocytozoon bieneusi. J. Eukaryot. Microbiol. 1995b;42(5):506–510. doi: 10.1111/j.1550-7408.1995.tb05896.x. [DOI] [PubMed] [Google Scholar]
- Wasson K, Barry P. Molecular characterization of Encephalitozoon intestinalis (Microspora) replication kinetics in a murine intestinal cell line. J. Eukaryot. Microbiol. 2003;50(3):169–174. doi: 10.1111/j.1550-7408.2003.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Watral VG, Kauffman RB, Kent ML. In vitro culture of Pseudoloma neurophilia, a common microsporidian of zebrafish (Danio rerio); In IX International Workshops on Opportunistic Protists and the International Society of Protistologist 57th Annual Meeting; Lisbon, Portugal. June 20-24 2006.2006. p. 86. [Google Scholar]
- Weidner E. Ultrastructural study of microsporidian invasion into cells. Z. Parasitenkd. 1972;40:227–242. doi: 10.1007/BF00329623. [DOI] [PubMed] [Google Scholar]
- Williams GR, Shafer ABA, Rogers REL, Shutler D, Stewart DT. First detection of Nosema ceranae, a microsporidian parasite of European honey bees (Apis mellifera), in canada and central USA. J. Invert. Pathol. 2008;97:189–192. doi: 10.1016/j.jip.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Wittner M. In: Historic Perspective on the Microsporidia: Expanding Horizons. The Microsporidia and Microsporidiosis. Wittner M, Weiss LM, editors. ASM Press; Washington, D.C.: 1999. pp. 1–6. [Google Scholar]
- Wolk DM, Johnson CH, Rice EW, Marshall MM, Grahn KF, Plummer CB, Sterling CR. A spore counting method and cell culture model for chlorine disinfection studies of Encephalitozoon syn. Septata intestinalis. Appl. Environ. Microbiol. 2000;66(4):1266–1273. doi: 10.1128/aem.66.4.1266-1273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongtavatchai J, Conrad PA, Hedrick RP. In vitro cultivation of the microsporidian: Enterocytozoon salmonis using a newly developed medium for salmonid lymphocytes. J. Tiss. Cult. Meth. 1994;16:125–131. [Google Scholar]
- Wongtavatchai J, Conrad PA, Hedrick RP. In vitro characteristics of the microsporidian: Enterocytozoon salmonis. J. Eukaryot. Microbiol. 1995;42(4):401–405. doi: 10.1111/j.1550-7408.1995.tb01602.x. [DOI] [PubMed] [Google Scholar]