Abstract
Background
miR-126 has been reported to be differentially expressed in various malignancies, whereas its role in the pathogenesis of tongue squamous cell carcinoma (TSCC) remains largely unknown.
Material/Methods
In this study, we collected 21 pairs of TSCC cancerous and adjacent non-cancerous tissue samples, with which we performed real-time PCR to determine and compare the expression of 6 candidate miRNAs that are reportedly associated with tumorigenesis of TSCC, including miR-100, miR-451, miR-221, let-7a, miR-21, and miR-126. We further performed luciferase assay to validate KRAS as a target of miR-126, and conducted transfection to study the effect of miR-126 on proliferation and apoptosis of the cells.
Results
We identified that miR-126 was significantly downregulated in the cancerous tissue samples compared with the non-cancerous control tissue samples. By using computational analysis, we identified that KRAS is a virtual target of miR-126, and such association was verified by using luciferase assay. In addition, we found that mRNA and protein expression level of KRAS was significantly higher in the tumor tissue than the control tissue samples.
Conclusions
The following in vitro experiment showed that both mRNA and protein KRAS expression were significantly decreased in SCC-15 cells in which miR-126 was overexpressed, in comparison with similar cells transfected with a negative control, while downregulation of miR-126 by transfecting the cells with miR-126 inhibitors significantly upregulated the mRNA and protein expression of KRAS. Conclusions: miR-126 might be a promising diagnostic and therapeutic target in the prevention and management of TSCC patients.
MeSH Keywords: Apoptosis; Carcinoma, Adenosquamous; MicroRNAs
Background
Neck and head squamous cell carcinoma, including tumors of oropharynx, oral cavity, hypopharynx and larynx, is the sixth leading solid cancer worldwide [1]. The most frequent type of oral cancer is tongue squamous cell carcinoma (TSCC), which is well-recognized for its high rate of proliferation and metastasis of lymph nodes [2]. Previous studies showed that approximately 50% of patients were already at stage III and IV on onset, although TSCC obviously presents in the oral cavity [3,4]. A better understanding of the molecular pathogenesis of the disease may help with development of new diagnostic methods, and discovery of new therapeutic targets.
MicroRNAs (miRNAs), a class of small noncoding RNAs that are expressed endogenously, suppress gene expression via binding the “seed sequence” within the 3′-untranslated regions (3′-UTRs) of their target messenger RNAs (mRNAs) [5]. miRNAs are believed to play important roles in the control of biological processes such as cell growth, metastasis, apoptosis, and transformation due to their extensive regulation of gene expression [6–8]. In TSCC, miR-184 functions as an oncogene as evidenced by the overexpression of miR-184 in tumor tissue [9]. miR-138 has been reported to be crucial in regulating migration and invasion of cells [10], and miR-21 signals activation was thought to be associated with poor prognosis in patients with TSCC [11].
Recent studies have shown that the expression of miR-126 was significantly reduced in a variety of malignancies, ranged from adult T cell leukemia, breast cancer, colorectal cancer (CRC) and malignant mesothelioma [12–14] compared with nonmalignant tissue. However, miR-126 was significantly upregulated in some other tumor types such as adrenocortical adenomas [15] and breast cancer [16]. Nevertheless, the clinical importance and prognostic potential of miR-126 in TSCC remained to be further studied.
Li et al. performed miRNA microarray analysis to compare the expression profiles between the TSCC tumor tissue and adjacent non-cancerous control, and reported a list of differentially expressed miRNAs [17]. In this study, we collected 21 pairs of TSCC cancerous and adjacent non-cancerous tissue samples, with which we performed real-time PCR to determine and compare the expression of 6 candidate miRNAs that have been reportedly associated with tumorigenesis of TSCC including miR-100, miR-451, miR-221, let-7a, miR-21 and miR-126, and identified that miR-126 was significantly downregulated in the cancerous tissue samples compared with the non-cancerous control tissue samples. In addition, we validated KRAS as a target of miR-126, and verified the regulatory association between miR-126 and KRAS and their roles in the development of TSCC.
Material and Methods
Tissue specimens
Paired primary TSCC samples from anterior regions of the tongue and nearby tissues that were histological normal were collected from 21 patients (age: 58.24±8.35, male vs. female: 16 vs. 5), who received surgical treatment in Oral Cavity College of Shandong University. None of patients had been treated prior to operation. Tumor tissues as well as nearby normal tissues at minimum 1.5 cm distant to the tumor edges were collected, followed by being frozen in liquid nitrogen and placed at −80°C for use. The study protocol was approved by the Institutional Ethics Committee of Shandong University, and all patients who were collected for samples signed informed consent for approval of the application of their tissues for the study after operation.
RNA isolation and quantitative reverse-transcription PCR (qRT-PCR)
Total RNA was isolated from tissues or cells using TRIzol reagent (Invitrogen, Carlsbad, CA) as per the instructions of the manufacturer. Primer sets for amplification of KRAS, miRNAs and U6 were designed and supplied by Sangon Biotech (Shanghai, China). Quantitative PCR was performed in an ABI 7500 real-time PCR system, at 95°C for 10 min, and then 95°C for 15 sec at a total of 40 cycles, followed by 60°C for 60 sec. U6 was used an internal control, and the expression of miRNA and mRNA were normalized to the expression of U6. Gene expression changes were quantified using the delta-delta CT method.
Cell cultures and transfection
SCC-15 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), and the cells were incubated at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) containing 100 U/ml penicillin, 100 mg/ml streptomycin and 10% fetal calf serum (Invitrogen, Carlsbad, CA). Before transfection, SCC-15 cells were incubated in 6-well plates to make sure the cells grown to 80% confluence. miR-126 mimics/inhibitors, and the small interfering RNA (siRNA) that acted on siRNA control and human KRAS transcripts were obtained from Integrated Biotech Solutions Company (Ibsbio, Shanghai, China). Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used to transfect cells as per the instruction of the manufacturer.
Cell proliferation assays
The Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) was used to determine the impacts of upregulation or downregulation of miR-126 and downregulation of KRAS on proliferation of SCC-15 cells. In brief, the cells were plated into 96-well plates (103 cells/well). miRNA analogues or suppressor were used to transfect cells. After transfection, CCK-8 was pipetted to all wells and cultured at 37°C for 3 h. The microplate spectrophotometer (Bio-Tek Instruments Inc, Winooski, VT) was used to determine the absorbance at 450 nm.
Apoptosis analysis
The apoptosis status was evaluated using flow cytometry. The SCC-15 cells were transfected as described previously in this methods section. 48 h after transfection, the cells were harvested and re-suspended in phosphate-buffered saline (PBS) and then blocked in ethanol under room temperature overnight. After wash with PBS, the cells were re-suspended in staining solution containing 1 mg/ml RNase A, 50 mg/ml propidium iodide, and annexin-V-FLUOS Staining kit (Roche, Mannheim, Germany). The apoptosis of stained cells were then analyzed using The FACSCanto II (BD Biosciences, San Jose, CA), and CellQuest software (BD Biosciences, San Jose, CA).
Luciferase reporter assay
PCR was used to amplify the human KRAS 3′-UTR containing estimated targeting sites of miR-126. After amplification, they were cloned into a pcDNA3.1(+) with modification containing a firefly luciferase reporter gene where at downstream of the luciferase reporter. SCC-15 cells were incubated in a 48-well plate. 24 h after co-transfection with 40 ng of the firefly luciferase reporter plasmid that contained the 3′-UTR of the target gene, 400 ng of pcDNA3.0 or miR-126, and 4 ng of pRL-TK which was a plasmid that expressed Renilla luciferase (Promega, Madison, WI).
Western blot analysis
Cells were lysed with RIPA lysis buffer (250 mM NaCl, 1% NP40, 50 mM Tris/HCl, pH 8.0, 0.1% sodium dodecylsulfate 0.5%, (w/v) sodium deoxycholate). 12% SDS polyacrylamide gel electrophoresis was performed, and the separated protein extracts were transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membranes were incubated with primary antibodies (anti-KRAS antibody 1: 2000, and anti-β-actin antibody 1:10000, purchased from Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for one night. After washing thoroughly, the membranes were incubated with horseradish peroxidase-conjugated second antibodies at 1:10,000 dilution (Zhongshan Goldenbridge, Beijing, China) for 1 h at room temperature and determined using ECL kit (Applygen, Beijing, China).
Statistical analysis
The comparison between the groups was performed using Student’s t test and one-way ANOVA. SPSS 16.0 (SPSS, Chicago, IL) was used for all statistical analyses. Experiments were performed at least three times. Data were indicated as mean ± standard deviation (SD). The statistical significance was set at a two-tailed value of P<0.05.
Results
miR-126 Expression was downregulated in TSCC
In this study, we collected 21 pairs of TSCC cancerous and adjacent non-cancerous tissue samples, with which we performed real-time PCR to determine and compare the expression of 6 candidate miRNAs that have been reportedly associated with tumorigenesis of TSCC including miR-100, miR-451, miR-221, let-7a, miR-21 and miR-126, and identified that miR-126 was significantly downregulated in the cancerous tissue samples compared with the non-cancerous control tissue samples, as shown in Figure 1.
Figure 1.
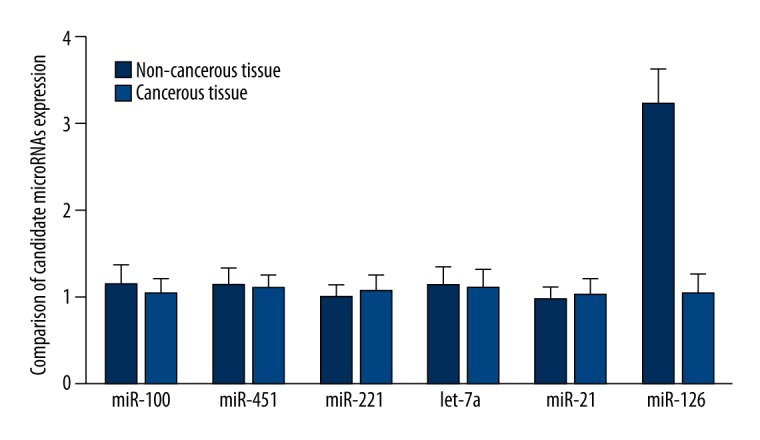
Real-time PCR was performed to screen for the differentially expressed miRNAs, and miR-126 was found to be significantly downregulated in cancerous tissue of TSCC compared with non-cancerous controls.
miR-126 targets KRAS in STCC cells
By using computational analysis, we identified that KRAS is a virtual target of miR-126 with multiple hits in the 3′UTR of the gene, as shown in Figure 2. To further confirm the interactive between KRAS 3′TUR and miR-126, we subcloned the full length of KRAS 3′UTR into a vector and cotransfected it with miR-126 mimics or inhibitors, and we found that the luciferase activity of the cells cotransfected with KRAS 3′UTR and miR-126 mimics were significantly lower than that of the cells transfected with KRAS 3′UTR and scramble controls, while the luciferase activity of the cells transfected with KRAS 3′UTR and miR-126 inhibitors were substantially higher than that of the cells transfected with scramble controls and KRAS 3′UTR (Figure 3).
Figure 2.

Computational analysis showed that there were there virtual seed sequences of miR-126 in the 3′UTR of KRAS.
Figure 3.
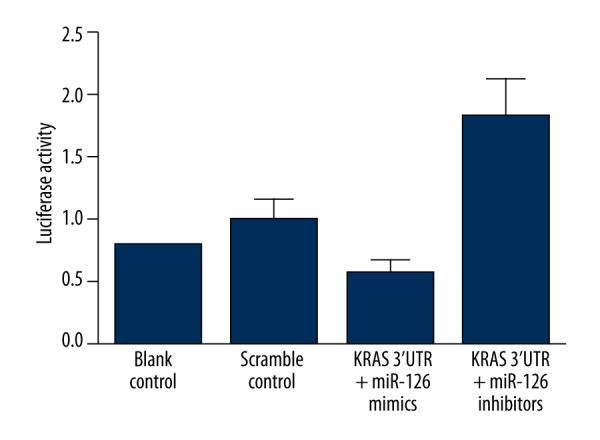
The results of luciferase reporter assay showed that the luciferase activity of the cells cotransfected with KRAS 3′UTR and miR-126 mimics were significantly lower than that of the cells transfected with KRAS 3′UTR and scramble controls, while the luciferase activity of the cells transfected with KRAS 3′UTR and miR-126 inhibitors were substantially higher than that of the cells transfected with scramble controls and KRAS 3′UTR.
Determination and comparison of mRNA and protein expression of KRAS in the cancerous and non-cancerous tissue samples
To further confirm the relationship between miR-126 and KRAS, we evaluated the mRNA and protein expression of KRAS in the cancerous and non-cancerous tissue samples by using real-time PCR and western blot. As shown in Figure 4, we found that mRNA expression level of KRAS was significantly higher in the tumor tissue than the control tissue samples. Consistently, the protein expression level of KRAS was also significantly higher in the cancerous groups than the non-cancerous group. The results further confirmed the regulatory relationship between the miR-126 and KRAS in the STCC cells.
Figure 4.
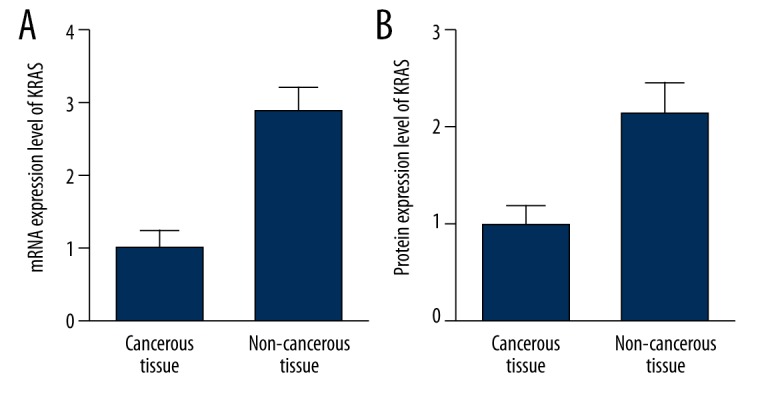
mRNA (A) and protein (B) expression levels of KRAS were significantly higher in the tumor tissue than the control tissue samples.
Overexpression of miR-126 decreased expression of KRAS in STCC cells
To further establish the regulatory relationship between the miR-126 and KRAS, we examined the effects of upregulation and downregulation of miR-126 on the endogenous expression of KRAS mRNA and proteins in a STCC cell line. Both mRNA and protein KRAS expression were significantly decreased in SCC-15 cells in which miR-126 was overexpressed, in comparison with similar cells transfected with a negative control (Figure 5A). On the other hand, downregulation of miR-126 by transfecting the cells with miR-126 inhibitors significantly upregulated the mRNA and protein expression of KRAS (Figure 5B). These findings further demonstrated that KRAS is a direct target of miR-126 in TSCC cell lines.
Figure 5.
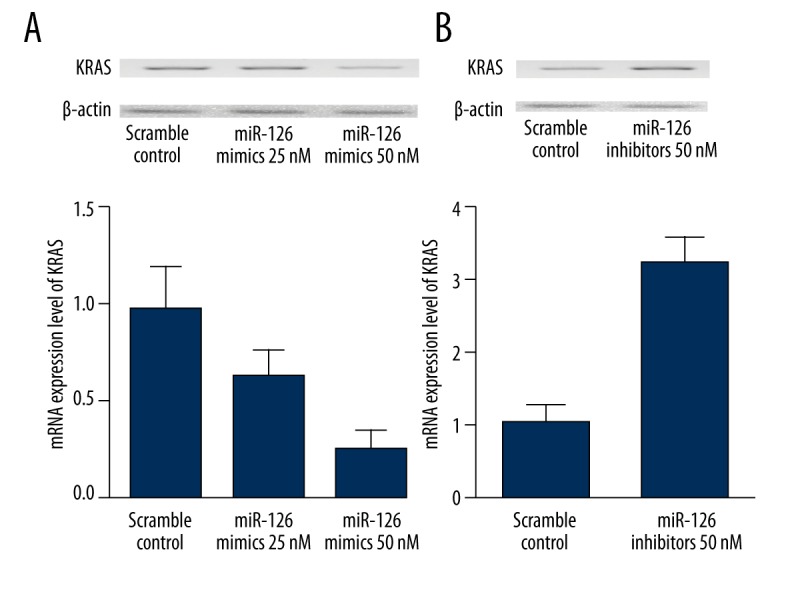
Both mRNA and protein KRAS expression were significantly decreased in SCC-15 cells in which miR-126 was overexpressed, in comparison with similar cells transfected with a negative control (A). On the other hand, downregulation of miR-126 by transfecting the cells with miR-126 inhibitors significantly upregulated the mRNA and protein expression of KRAS (B).
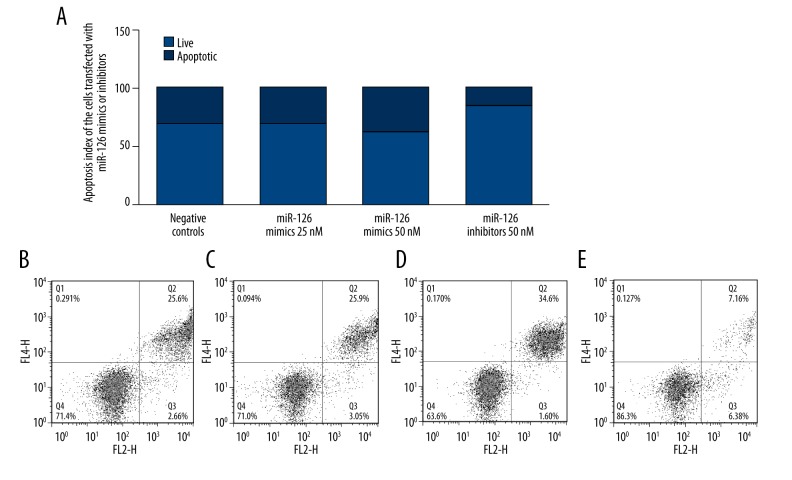
Overexpression of miR-126 suppressed proliferation and induced apoptosis, and downregulation of mIR-126 promoted proliferation and inhibited apoptosis in TSCC cell lines
To understand the biological function of miR-126 in TSCC, miR-126 was upregulated and downregulate by transfecting miR-126 mimics or inhibitors in TSCC cell lines. In the first of these experiments, we transfected TSCC cells with miR-126 mimics or inhibitors and performed proliferation assays to evaluate the effects of miR-126 expression on cell proliferation and viability. Overexpression of miR-126 inhibited the viability of SCC-15, while downregulation of miR-126 significantly promoted the proliferation of SCC-15 (Figure 6). Meanwhile, to explore the molecular mechanism underlying, we evaluated the apoptosis status of the differently treated SCC-15 cells, and found that overexpression of miR-126 induced the apoptosis of SCC-15, while downregulation of miR-126 significantly promoted the apoptosis of SCC-15 (Figure 7).
Figure 6.
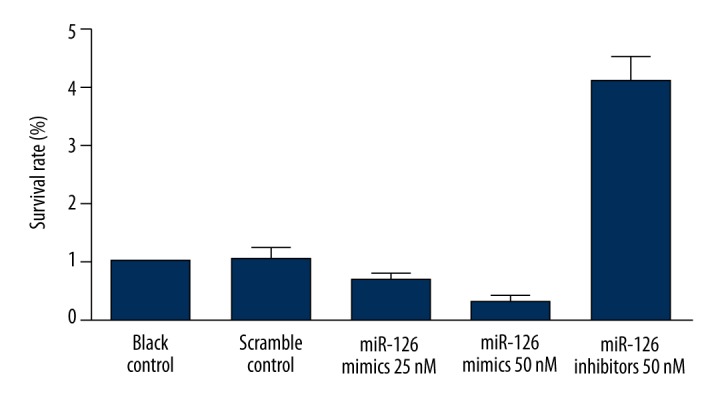
Overexpression of miR-126 inhibited the viability of SCC-15, while downregulation of miR-126 significantly promoted the proliferation of SCC-15.
Figure 7.
Summary of the effects of miR-126 mimics and inhibitors on the apoptosis status in SCC-15 cells: (A) Schematic summary of the effects of miR-126 mimics and inhibitors on the apoptosis status; (B) Scramble controls; (C) transfection of 25 nM miR-126 mimics had minimal effect on apoptosis of the cells; (D) transfection of 50 nM miR-126 mimics substantially promoted apoptosis of the cells; (E) transfection of 50 nM miR-126 inhibitors substantially suppressed apoptosis of the cells.
Discussion
Although miRNAs are not directly associated with coding of the protein, it is believed that they regulate the expression of over one-third of the genes that codes the protein in the human genome [18–20]. By binding to the certain sequences in the target mRNA molecules on the basis of ‘imperfect complementarity’, each miRNA has the potential to modulate numerous genes at post-transcriptional levels. But the accurate relationships between miRNA and its target genes remain to be further determined from the perspective of the micro-environment and cell types. In this study, we collected 21 pairs of TSCC cancerous and adjacent non-cancerous tissue samples, with which we performed real-time PCR to determine and compare the expression of 6 candidate miRNAs that have been reportedly associated with tumorigenesis of TSCC including miR-100, miR-451, miR-221, let-7a, miR-21 and miR-126, and identified that miR-126 was significantly downregulated in the cancerous tissue samples compared with the non-cancerous control tissue samples.
MicroRNA-126 (miR-126) was derived from a typical precursor structure that was present within intron 7 of epidermal growth factor-like domain 7 (EGFL7) [21]. The expression of miR-126 is high in vascular endothelial cells and acts as a crucial regulator in the control of angiogenesis, which responds to angiogenic growth factors by inhibiting negative modulators of signal transduction pathways [22]. Recently, in vivo and in vitro experiments have verified the influence of miR-126 on the tumorigenesis and progression of human malignant cancers. For instance, non-small cell lung tumor tissues showed a significant downregulation of miR-126, which was possibly associated with increased microvessel density and clinical outcomes [23]; In breast cancer, the reduced expression of miR-126 may be associated with unfavorable metastasis-free survival [24]; Insufficiency of miR-126 is more likely common in metastatic colorectal cancers [25]. On the contrary, miR-126 has been shown to be a crucial negative indicator for prognosis of squamous cell carcinomas, so it may become an oncogene [26]. These debatable results indicated that there may be discrepancy in the effects of miR-126 in different cancer types, which could be attributed to its high tissue-specificity. In this study, we identified that KRAS is a virtual target of miR-126 with multiple hits in the 3′UTR of the gene (790–796 bp, 1393–1399 bp, and 4877–4883 bp) by searching the online miRNA database (www.mirdb.org) and using computational analysis, which was further confirmed by luciferase reporter system, showing that the luciferase activity of the cells cotransfected with KRAS 3′UTR and miR-126 mimics were significantly lower than that of the cells transfected with KRAS 3′UTR and scramble controls, while the luciferase activity of the cells transfected with KRAS 3′UTR and miR-126 inhibitors were substantially higher than that of the cells transfected with scramble controls and KRAS 3′UTR. In addition, we found that mRNA expression level of KRAS was significantly higher in the tumor tissue than the control tissue samples. Consistently, the protein expression level of KRAS was also significantly higher in the cancerous groups than the non-cancerous group. All these findings supported our hypothesis that KRAS was a target of miR-126 and confirmed such regulatory relationship between miR-126 and KRAS.
As one of the three members of the Ras oncogene family (consisted of H-ras, K-ras and N-ras), V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (K-ras) encodes small GTPases that play an important role in the control of cellular activities [27]. Ras signal activation induces complicated downstream signaling cascade, including the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and protein kinase (MAPK) pathway activated by the mitogen, thereby modulating a variety of cellular processes, ranged from cell growth to differentiation, survival, migration, and apoptosis [27]. K-ras is a member of the Ras family and its mutation and excess expression are believed to lead to malignant cell transformation. The stimulation of K-ras in combination with Akt in neural progenitors could trigger generation of the glioma in mice [28]. Almost all high-grade malignancies showed activation of the MAPK pathway and the PI3K/Akt pathway, and maintenance of malignancies requires MAPK signaling [29]. Several miRNAs including miR-18a*, miR-143, miR-96, miR-217,and miR-181a have been shown to be involved in the control of oncogenesis via targeting the expression of K-ras, suggesting that miRNAs that target K-ras are very crucial in carcinogenesis and act as possible therapeutic options for human cancer [30–34]. In this study, we examined the effects of upregulation and downregulation of miR-126 on the endogenous expression of KRAS mRNA and proteins in a STCC cell line. Both mRNA and protein KRAS expression were significantly decreased in SCC-15 cells in which miR-126 was overexpressed, in comparison with similar cells transfected with a negative control (Figure 5A). On the other hand, downregulation of miR-126 by transfecting the cells with miR-126 inhibitors significantly upregulated the mRNA and protein expression of KRAS (Figure 5B). In addition, we transfected TSCC cells with miR-126 mimics or inhibitors and performed proliferation assays to evaluate the effects of miR-126 expression on cell proliferation and viability. Overexpression of miR-126 inhibited the viability of SCC-15, while downregulation of miR-126 significantly promoted the proliferation of SCC-15 (Figure 6). Meanwhile, to explore the molecular mechanism underlying, we evaluated the apoptosis status of the differently treated SCC-15 cells, and found that overexpression of miR-126 inhibited the apoptosis of SCC-15, while downregulation of miR-126 significantly promoted the apoptosis of SCC-15 (Figure 7).
Conclusions
In conclusion, our findings suggest that miR-126 modulates KRAS signaling at multiple levels. The results shown in the present paper, in combination with previous studies on miR-126 and other proto-oncogenes, indicate that miR-126 is very important in tumorigenesis. The administration of miR-126 as a therapeutic agent may be a potent approach for the treatment of this disease, contributing to the central role of the KRAS signaling pathway in TSCC.
Footnotes
Source of support: Departmental sources
Conflicts of interest
Authors claim no conflict of interest.
References
- 1.Pancari P, Mehra R. Systemic therapy for squamous cell carcinoma of the head and neck. Surg Oncol Clin N Am. 2015;24:437–54. doi: 10.1016/j.soc.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Yuen PW, Lam KY, Chan AC, et al. Clinicopathological analysis of local spread of carcinoma of the tongue. Am J Surg. 1998;175:242–44. doi: 10.1016/s0002-9610(97)00282-1. [DOI] [PubMed] [Google Scholar]
- 4.Po Wing Yuen A, Lam KY, Lam LK, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002;24:513–20. doi: 10.1002/hed.10094. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Thai TH, Calado DP, Casola S, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–8. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 7.Brennecke J, Hipfner DR, Stark A, et al. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 8.Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–30. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 9.Wong TS, Liu XB, Wong BY, et al. Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2008;14:2588–92. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Liu X, Kolokythas A, et al. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int J Cancer. 2010;127:505–12. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Huang H, Sun L, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Zhou Y, Feng X, et al. Low expression of microRNA-126 is associated with poor prognosis in colorectal cancer. Genes Chromosomes Cancer. 2014;53:358–65. doi: 10.1002/gcc.22146. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara K, Sasaki D, Tsuruda K, et al. Impact of miR-155 and miR-126 as novel biomarkers on the assessment of disease progression and prognosis in adult T-cell leukemia. Cancer Epidemiol. 2012;36:560–65. doi: 10.1016/j.canep.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Tomasetti M, Staffolani S, Nocchi L, et al. Clinical significance of circulating miR-126 quantification in malignant mesothelioma patients. Clin Biochem. 2012;45:575–81. doi: 10.1016/j.clinbiochem.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Soon PS, Tacon LJ, Gill AJ, et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res. 2009;15:7684–92. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 16.Heneghan HM, Miller N, Lowery AJ, et al. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251:499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 17.Jiang LH, Ge MH, Hou XX, et al. miR-21 regulates tumor progression through the miR-21-PDCD4-Stat3 pathway in human salivary adenoid cystic carcinoma. Lab Invest. 2015 doi: 10.1038/labinvest.2015.105. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Lewis BP, Shih IH, Jones-Rhoades MW, et al. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 19.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 20.Xie X, Lu J, Kulbokas EJ, et al. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–45. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Giorgio A, Castellano L, Krell J, Stebbing J. Crosstalk-induced loss of miR-126 promotes angiogenesis. Oncogene. 2014;33:3634–35. doi: 10.1038/onc.2013.317. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang X, Xu B, et al. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol Rep. 2013;30:1976–84. doi: 10.3892/or.2013.2633. [DOI] [PubMed] [Google Scholar]
- 23.Markou A, Sourvinou I, Vorkas PA, et al. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer. 2013;81:388–96. doi: 10.1016/j.lungcan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Wang CZ, Yuan P, Li Y. miR-126 regulated breast cancer cell invasion by targeting ADAM9. Int J Clin Exp Pathol. 2015;8(6):6547–53. [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee N, Kim H, Talcott S, Mertens-Talcott S. Pomegranate polyphenolics suppressed azoxymethane-induced colorectal aberrant crypt foci and inflammation: possible role of miR-126/VCAM-1 and miR-126/PI3K/AKT/mTOR. Carcinogenesis. 2013;34:2814–22. doi: 10.1093/carcin/bgt295. [DOI] [PubMed] [Google Scholar]
- 26.Sasahira T, Kurihara M, Bhawal UK, et al. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br J Cancer. 2012;107:700–6. doi: 10.1038/bjc.2012.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall A. The cellular functions of small GTP-binding proteins. Science. 1990;249:635–40. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- 28.Abel TW, Clark C, Bierie B, et al. GFAP-Cre-mediated activation of oncogenic K-ras results in expansion of the subventricular zone and infiltrating glioma. Mol Cancer Res. 2009;7:645–53. doi: 10.1158/1541-7786.MCR-08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson JP, VanBrocklin MW, Guilbeault AR, et al. Activated BRAF induces gliomas in mice when combined with Ink4a/Arf loss or Akt activation. Oncogene. 2010;29:335–44. doi: 10.1038/onc.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Guo X, Zhang H, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–92. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 31.Shin KH, Bae SD, Hong HS, et al. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochem Biophys Res Commun. 2011;404:896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 32.Tsang WP, Kwok TT. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30:953–59. doi: 10.1093/carcin/bgp094. [DOI] [PubMed] [Google Scholar]
- 33.Yu S, Lu Z, Liu C, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–25. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 34.Zhao WG, Yu SN, Lu ZH, et al. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31:1726–33. doi: 10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]