Abstract
NGcGM3 ganglioside is a tumor-specific antigen expressed in human breast tumors. The NGcGM3/VSSP vaccine, consisting in very small-sized proteoliposomes (VSSP) obtained by the incorporation of NGcGM3 into the outer membrane protein complex of Neisseria meningitidis, has been previously tested in a Phase II trial in patients with metastatic breast cancer (MBC) but emulsified with Montanide ISA 51. An Expanded Access study was carried out in MBC patients aiming to find if a nonemulsive formulation of NGcGM3/VSSP, without Montanide ISA 51, could be more safe and effective. A total of 104 patients were vaccinated with the nonemulsive formulation (900 μg), subcutaneously (SC), or with the emulsive formulation (200 μg), intramuscularly (IM). An intent-to-treat analysis of efficacy was performed with all patients, and 93 patients were split off according to the site of metastases (visceral/nonvisceral). Of note, SC-treated patients exhibited a superior median overall survival (OS) than IM-treated patients (23.6 vs. 8.2 months; log rank P = 0.001). Even though in the subset of patients with nonvisceral metastases SC vaccination duplicated the median OS compared to the alternative option (31.6 vs. 16.5 months), this difference did not reach statistical significance (log rank P = 0.118). Curiously, in patients with visceral metastases, the advantage of the nonemulsive formulation was more apparent (median OS 21.0 vs. 6.2 months; log rank P = 0.005). The vaccine was safe for both formulations.
Keywords: breast cancer, NGcGM3/VSSP vaccine, survival
Introduction
Breast cancer is the most frequent cause of death in women in less-developed regions, which is different from more developed countries where it is the second cause of death (cancer related), after lung tumors.1 Median survival in patients with metastatic breast cancer (MBC) varies from 18 to 24 months,2–6 even with the availability of several options of chemotherapy and biological therapy.7 At present, immunotherapy is probably the most promising option for expanding life expectations with the highest quality in these patients.
Gangliosides containing glycolylated sialic acid are not expressed in normal cells, but the presence of NGcGM3 has been reported in different human tumors, particularly in human breast tumors and metastasis,8,9 privileging this target for active-specific immunotherapy approaches due to its tumor-specific character.8,10
Currently, an NGcGM3-based cancer vaccine, developed by the Center of Molecular Immunology by solubilization of the hydrophobic outer membrane proteins of Neisseria meningitidis with the ganglioside to form nanoparticles (very small-sized proteoliposomes (VSSP)),11 has been studied in two large Phase III trials with Stage III or MBC patients. For these clinical trials, an emulsive formulation of the NGcGM3/VSSP vaccine (containing Montanide ISA 51) is used, based on the previous evidences of safety and efficacy.12,13 In the meantime, an alternative nonemulsive formulation of the vaccine with less toxicity and probably increased efficacy was obtained.14 Then, we needed to decide quickly which formulation will continue the development process, and for this purpose, we conducted an Expanded Access (EA) program study, assuming all the limitations of this type of trial. The objective of the study was to reasonably find if the nonemulsive (SC) NGcGM3/VSSP vaccine could be significantly superior in safety and impact the overall survival (OS) of MBC patients, in comparison with the alternative emulsive (IM) formulation.
Material and Methods
NGcGM3/VSSP vaccine
NGcGM3/VSSP vaccine consists in VSSP obtained by the incorporation of NGcGM3 into the outer membrane protein complex of N. meningitidis. NGcGM3/VSSP has been studied in patients with advanced breast cancer as an emulsive formulation when used with Montanide ISA 51, at a dose of 200 μg IM.12,13 Recently, this vaccine has also been tested as a nonemulsive formulation (without Montanide ISA 51) at a dose of 900 μg SC in a study that included MBC patients.14 This optimal biological dose for the nonemulsive formulation was selected according to the results of efficacy, safety, and immunogenicity obtained in the study.14
Study design
An EA program study was designed to compare the results of OS and safety in patients treated with two different formulations of the vaccine. A total of 104 patients with progressive disease after the oncospecific first-line treatment for MBC were recruited at six clinical sites of investigation; 51 patients were treated with the nonemulsive formulation and 53 patients received the emulsive variant.
Randomization list was generated with the system Random Assignment ASAL (ASignación ALeatoria in Spanish) for Windows, version 1.2, and patients were randomly assigned to treatment groups according to the inclusion and exclusion criteria in the protocol. The randomization was centralized and not stratified by control variables.
Treatment consisted in the administration of NGcGM3/VSSP (nonemulsive formulation) or NGcGM3/VSSP/Montanide ISA 51 (emulsive formulation), by subcutaneous (900 μg/dose) or intramuscular (200 μg/dose) route, respectively. For both formulations, patients received the first five doses every two weeks and subsequent doses every four weeks.
Inclusion criteria
Patients with cytohistological confirmation of breast cancer
Patients in progression after the oncospecific first-line treatment
Patients older than 18 years
Patients who consent to participate in the study by signing the informed consent model
Patients with ECOG Scale of Performance Status of ≤3.
Exclusion criteria
Patients receiving other investigational product
Patients who have criteria to be included in any ongoing clinical trial for this indication
Patients who are pregnant or breastfeeding.
Ethical considerations
The study was approved by the Ministry of Public Health (Cuba) and complied with the principles of the Declaration of Helsinki and Good Clinical Practice. A written consent for participating was required from each patient before his/her inclusion.
Evaluation during the study
Fisher’s exact test for 2 × 2 tables and the chi-square test for 3 × 2 tables were used to assess the baseline patients’ characteristics.
OS was estimated for all patients and was defined as the time from randomization until death from any cause; the results were analyzed by the Kaplan–Meier method. Common Toxicity Criteria to Evaluate Adverse Events, version 4.0, was used to classify according to the intensity of adverse events (AEs). AEs were also classified according to the System Organ Class (SOC) affected.
Results
Patient population
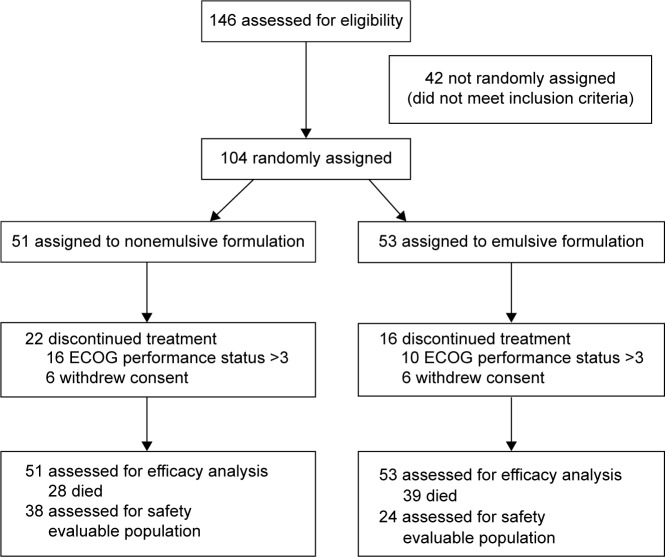
A total of 104 patients were recruited in the study (Table 1; Fig. 1). There was no statistical difference between both groups at the baseline, with the exception of the amount of patients who received chemotherapy during the primary tumor treatment (P = 0.0202) and the expression of estrogen (P = 0.0491) and progesterone (P = 0.0302) receptors. We consider that the statistical difference evidenced in the hormonal receptors’ status was due to the lack of information about this expression, with a greater percentage of patients in the nonemulsive formulation group in which the data were not available.
Table 1.
Patients’ characteristics.
| PATIENTS CHARACTERISTICS | FORMULATION | FISHER OR CHI-SQUARE TEST (P-VALUE) | |
|---|---|---|---|
| NON EMULSIVE-SC (n = 51) | EMULSIVE-IM (n = 53) | P-VALUE | |
| Age (mean/range) | 60.9 (41–81) | 56.3 (36–84) | 0.065 |
| Stage | |||
| Stage I | 8 (15.6%) | 7 (13.2%) | 0.1403 |
| Stage II | 22 (43.1%) | 13 (24.5%) | |
| Stage III | 13 (25.4%) | 16 (30.1%) | |
| Stage IV | 3 (5.8%) | 3 (5.6%) | |
| Not available | 5 (9.8%) | 14 (26.4%) | |
|
| |||
| Histopathological type | |||
| Ductal | 39 (76.4%) | 35 (66.03%) | 0.2822 |
| Lobular | 0 (0%) | 2 (3.7%) | |
| Medullary | 0 (0%) | 1 (1.8%) | |
| Other | 6 (11.7%) | 4 (7.5%) | |
| Not available | 6 (11.7%) | 11 (20.7%) | |
|
| |||
| Treatment of primary tumor | |||
| Surgery | 44 (86.2%) | 39 (73.5%) | 0.0545 |
| Chemotherapy | 42 (82.3%) | 34 (64.1%) | 0.0202 |
| Radiotherapy | 35 (68.6%) | 31 (58.4%) | 0.0917 |
|
| |||
| Treatment of metastatic disease | |||
| Surgery | 3 (5.8%) | 7 (13.2%) | 0.1230 |
| Chemotherapy | 39 (76.4%) | 36 (67.9%) | 0.1092 |
| Radiotherapy | 9 (17.6%) | 12 (22.6%) | 0.1589 |
|
| |||
| Expression of estrogen receptor | |||
| Positive | 3 (5.8%) | 9 (16.9%) | 0.0491 |
| Negative | 8 (15.6%) | 14 (26.4%) | |
| Not available | 40 (78.4%) | 30 (56.6%) | |
|
| |||
| Expression of progesterone receptor | |||
| Positive | 3 (5.8%) | 9 (16.9%) | 0.0302 |
| Negative | 7 (13.7%) | 14 (26.4%) | |
| Not available | 41 (80.3%) | 30 (56.6%) | |
|
| |||
| HER 2 expression | |||
| 1+ | 1 (1.9%) | 4 (7.5%) | 0.4047 |
| 2+ | 1 (1.9%) | 0 (0%) | |
| 3+ | 3 (5.8%) | 4 (7.5%) | |
| Not available | 46 (90.1%) | 45 (84.9%) | |
Figure 1.
CONSORT diagram.
The mean age of patients was 60.9 and 56.3 years for the nonemulsive and emulsive formulations, respectively. In both arms of treatment, more than half of the patients were classified as nonstage IV disease (84.3%—nonemulsive formulation—and 67.9%—emulsive formulation). The most frequent histopathological type was ductal carcinoma, with 76.4% and 66.03% of cases for the nonemulsive and emulsive variants, respectively.
Most patients had received treatment when the primary tumor had been diagnosed and consisted of surgical resection, chemotherapy, and radiotherapy. For the metastatic disease, the most frequent treatment was chemotherapy. In both arms of the study, chemotherapy included the following:
For the treatment of primary tumor: cyclophosphamide/methotrexate/5-fluorouracil (CMF), AC (doxorubicin/cyclophosphamide), docetaxel/carboplatin, and others.
For the treatment of metastatic disease: CMF, docetaxel or paclitaxel/carboplatin, and others.
The expression of HER 2 and hormonal receptors was unavailable for most of the cases.
Efficacy results
In the intent-to-treat (ITT) analysis with the 104 patients, median OS was higher for patients treated with the nonemulsive formulation of the vaccine than for those treated IM, with 23.6 and 8.2 months, respectively (log rank P = 0.001; HR 95% CI: 0.450; Table 2; Fig. 2).
Table 2.
Median survival for vaccinated patients.
| FORMULATION | MEDIAN (MONTHS) |
|---|---|
| Non emulsive-SC (900 μg) n = 51 | 23,633 |
| Emulsive-IM (200 μg) n = 53 | 8,233 |
| Global n = 104 | 15,833 |
| Log rank P = 0.001/HR (95% CI): 0.450 (0.273–0.740) | |
Figure 2.
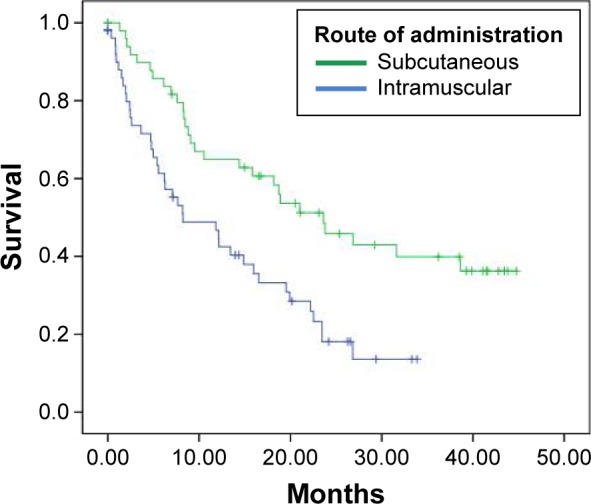
Kaplan–Meier curves for vaccinated patients.
An efficacy analysis was performed with the data from 93 patients according to the site of metastases, whether they were visceral or nonvisceral. In this analysis, median OS was superior for patients treated SC, in both the groups of patients with nonvisceral (log rank P = 0.118; HR 95% CI: 0.535) and visceral (log rank P = 0.005; HR 95% CI: 0.375) metastases (Table 3; Figs. 3 and 4). In the subset of patients with nonvisceral metastases, median OS was 31.633 and 16.533 months for subcutaneous and intramuscular routes, respectively. Patients with visceral metastases had median OS of 21.033 and 6.267 months when treated by subcutaneous and intramuscular routes, respectively.
Table 3.
Median survival (months) for patients vaccinated with either the nonemulsive or the emulsive formulation according to the site of metastases.
| SITE OF METASTASES | FORMULATION | GLOBAL | |
|---|---|---|---|
| NON EMULSIVE-SC | EMULSIVE-IM | ||
| Non visceral n = 45 | 31,633 (n = 24) | 16,533 (n = 21) | 22,533 |
| Visceral n = 48 | 21,033 (n = 24) | 6,267 (n = 24) | 8,800 |
| Non visceral (log rank P = 0.118)/HR (95% CI): 0.535 (0.241–1.186) Visceral (log rank P = 0.005)/HR (95% CI): 0.375 (0.184–0.766) | |||
Figure 3.
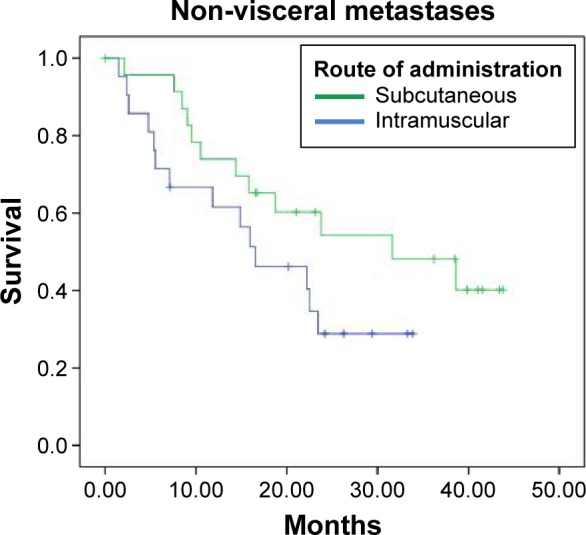
Kaplan–Meier curves for vaccinated patients with nonvisceral metastases.
Figure 4.
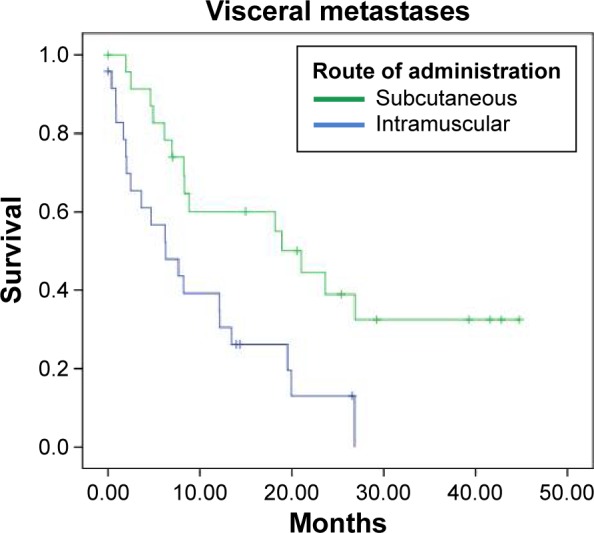
Kaplan–Meier curves for vaccinated patients with visceral metastases.
Another efficacy analysis was done with 85 patients by grouping them in patients diagnosed with Stage I or II, and patients with Stage III or IV (Table 4; Figs. 5 and 6). Median OS was superior for patients treated with the nonemulsive formulation in both groups of patients. Interestingly, there was a significant statistical difference (log rank P = 0.002; HR 95% CI: 0.311) in the subset of patients with Stage I or II.
Table 4.
Median survival (months) for patients vaccinated with either the nonemulsive or the emulsive formulation according to the disease stage.
| STAGE | FORMULATION | GLOBAL | |
|---|---|---|---|
| NON EMULSIVE-SC | EMULSIVE-IM | ||
| Stage I or II n = 50 | 26.667 (n = 30) | 11.833 (n = 20) | 19.3 |
| Stage III or IV n = 35 | 9.533 (n = 16) | 6,267 (n = 19) | 8,8 |
| Stage I or II (log rank P = 0.002)/HR (95% CI): 0.311 (0.140–0.688) Stage III or IV (log rank P = 0.263)/HR (95% CI): 0.618 (0.264–1.447) | |||
Figure 5.
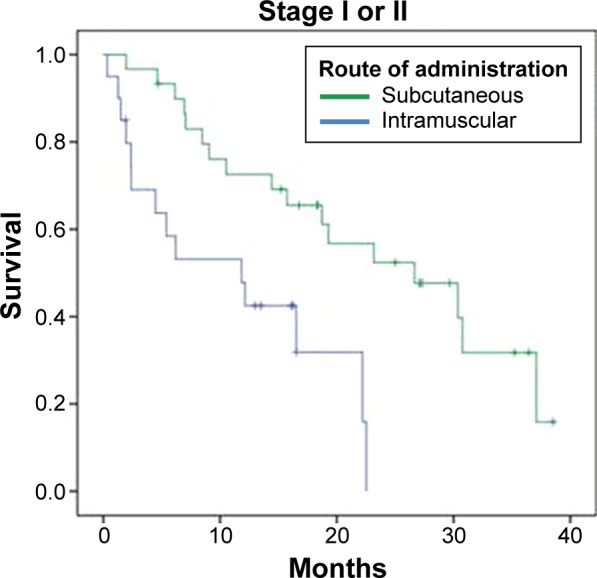
Kaplan–Meier curves for vaccinated patients with Stage I or II.
Figure 6.
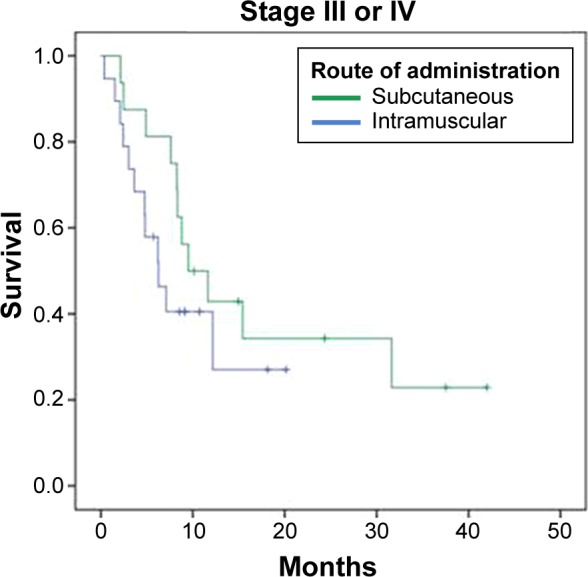
Kaplan–Meier curves for vaccinated patients with Stage III or IV.
Safety results
A total of 524 AEs were reported: 416 for the nonemulsive formulation and 108 for the emulsive variant. The majority of AEs were classified into the General Disorders and Administration Site Conditions according to the SOC. Most frequent AEs (Table 5) for both formulations were site reaction, asthenia, fever, headache, and chills. Other AEs included tachycardia (cardiac disorders), cough (reproductive system and breast disorders), vomiting (gastrointestinal disorders), and insomnia (psychiatric disorders). Most AEs were classified as grade I (Table 6) and nonserious.
Table 5.
Most frequent AEs according to the formulation.
| SYSTEM ORGAN CLASS | ADVERSE EVENT | FORMULATION | |||||
|---|---|---|---|---|---|---|---|
| NON EMULSIVE-SC | EMULSIVE-IM | TOTAL | |||||
| # | % | # | % | # | % | ||
| General disorders and administration site conditions | Site reaction | 176 | 42.3 | 27 | 25 | 203 | 38.7 |
| Asthenia | 27 | 6.5 | 5 | 4.6 | 32 | 6.1 | |
| Fever | 26 | 6.2 | 1 | 0.9 | 27 | 5.2 | |
| Chills | 15 | 3.6 | 5 | 4.6 | 20 | 3.8 | |
|
| |||||||
| Nervous system disorders | Headache | 25 | 6 | 4 | 3.7 | 29 | 5.5 |
| Metabolism and nutrition disorders | Anorexia | 4 | 0.9 | 3 | 2.7 | 7 | 1.3 |
| Gastrointestinal disorders | Diarrhea | 2 | 0.4 | 1 | 0.9 | 3 | 0.5 |
| Musculoskeletal and connective tissue disorders | Muscle pain | 3 | 0.7 | 8 | 7.4 | 11 | 2.1 |
| Arthralgia | 5 | 1.2 | 1 | 0.9 | 6 | 1.1 | |
| Other | Other | 133 | 31.9 | 53 | 49.1 | 186 | 35.4 |
Table 6.
Intensity of AEs.
| GRADE | FORMULATION | |||||
|---|---|---|---|---|---|---|
| NON EMULSIVE-SC | EMULSIVE-IM | TOTAL | ||||
| # | % | # | % | # | % | |
| 1 | 381 | 91.6 | 84 | 77.8 | 465 | 88.7 |
| 2 | 24 | 5.8 | 7 | 6.5 | 31 | 5.9 |
| 3 | 6 | 1.4 | 9 | 8.3 | 15 | 2.9 |
| 4 | 0 | 0 | 1 | 0.9 | 1 | 0.2 |
| 5 | 5 | 1.2 | 7 | 6.5 | 12 | 2.3 |
Discussion
Since the announcement of the failure of Theratope in June 2003, introducing a cancer vaccine for MBC patients, treatment remains as a dream for immunoncologists. Although this STn-KLH-conjugated vaccine was well tolerated and able to induce a high rate of IgM and IgG seroconversion, the time to progression was 3.4 months in the treatment group and 3.0 months in the control group, and the median survival was 23.1 and 22.3 months, respectively.15
To our knowledge at present, the most advanced active immunotherapy project in MBC is NGcGM3/VSSP. A first version of this vaccine comprised an additional emulsification with Montanide ISA 51, and its safety and immunogenicity was demonstrated in advanced breast cancer patients.12 The efficacy of this formulation was further assessed in a Phase II clinical trial in 80 MBC patients, achieving at least stable disease to the first-line therapy. While in the ITT analysis a modest trend toward a survival benefit for the vaccine group was observed, the most suggestive result came when patients were analyzed by the primary site of metastasis (visceral vs. nonvisceral). For patients with nonvisceral metastases, the median OS was 26.2 months for the vaccinated group and 12.2 months for the control group, but for patients with visceral metastases, no advantage in survival was associated with vaccination with the emulsive variant of NGcGM3/VSSP.13
These results fueled the initiation of an ongoing controlled, double blinded, Phase III clinical study with this vaccine in the MBC patients. In the meantime, encouraging preclinical16 and preliminary clinical data14 were obtained with a nonemulsive formulation of the product, SC administered, introducing the need to decide which formulation should be chosen for continuing the product development.
For this purpose, a new study was designed under EA to Investigational Drugs’ Principles, according to the Cuban regulatory agency (CECMED) regulation number 62 of 2012. A total of 104 MBC patients, who were nonresponders to the first-line oncospecific treatment, were divided to receive either the emulsive or the nonemulsive formulation of the NGcGM3/VSSP vaccine. Apart from the logical limitations imposed by the lack of information about hormonal receptors and HER 2 expression in the vast majority of the sample (due to the characteristics of EA), the striking difference in OS observed in these patients is more probably associated with the treatment. Median OS in the group of patients vaccinated with the nonemulsive variant of NGcGM3/VSSP almost triplicated the value in the alternative group of patients treated with the emulsive formulation. Moreover, the same notable advantage in survival in favor of patients SC vaccinated with the nonemulsive formulation was reproduced, irrespective if they have nonvisceral or visceral metastasis. Curiously, the insignificant impact on the survival of patients with visceral metastasis vaccinated with the emulsive variant of NGcGM3/VSSP, reported here, exactly reproduced the previous result obtained in the Phase II clinical trial.13 It is also to be noted that median OS was superior (log rank P = 0.002; HR 95% CI: 0.311) in patients treated with the nonemulsive formulation in the subset of patients with Stage I or II at diagnosis, irrespective of the site of metastasis after recurrence. It could be of interest to verify this finding in the future clinical trials.
In terms of safety, apart from the obvious more friendly condition of the nonemulsive formulation of the vaccine (with the removal of Montanide), 416 AEs were reported in patients injected with this variant, while 108 AEs were associated with the administration by the intramuscular route. A possible explanation of this difference could be that patients treated with the nonemulsive formulation notably survived longer and, for this reason, they received more number of injections. Besides, most AEs were classified as grade I and nonserious events. Most frequent AEs reported in patients treated with the two variants of the vaccine were reaction at the injection site, headache, asthenia, fever, and chills. This results reproduced previous ones,12–14 confirming that the NGcGM3/VSSP vaccine is safe.
The results of this EA study sustained the decision to continue the NGcGM3/VSSP vaccine development with the nonemulsive formulation.
Acknowledgments
This work was supported by the Center of Molecular Immunology, Celestino Hernández Robau Hospital, José Ramón López Tabranes Hospital, Ramón González Coro Hospital, Vladimir Ilich Lenin Hospital, María Curie Hospital, and Conrado Benítez Hospital. We thank Dr. Yamilka Alberteri for her contribution to the study by including and treating patients. She is not listed as an author because she could not be contacted to give final approval for publication.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1187 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: The authors were invited to submit this paper.
Author Contributions
Conceived and designed the trial: AT, KP, and CEV. Included and treated patients: AT, ES, RR, LH, and DD. Delivered the product of investigation to clinical sites: YD and YGM. Monitored the trial: MA, MC, MD, LC, JLS, JJH, and ARV. Analyzed the data: CEV, LS, AMV, and MÁ. Wrote the manuscript: AMV, AT, and LEF. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.GLOBOCAN . Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Breast Cancer [database in internet]; 2012. [Accessed April 2014]. Available at: http://globocan.iarcfr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- 2.Khadakban D, Gorasia-Khadakban T, Vijaykumar DK, et al. Factors associated with better survival after surgery in metastatic breast cancer patients. Indian J Surg Oncol. 2013;4(1):52–58. doi: 10.1007/s13193-012-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Medical Records and Health Statistics. Ministry of Public Health . Leading Causes of Death in People Aged 80 and Older. Cuba: Medical Sciences; 2012. Health Statistics Yearbook 2012. [Google Scholar]
- 4.Fumoleau P, Delgado FM, Delozier T, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 1993;11(7):1245–1252. doi: 10.1200/JCO.1993.11.7.1245. [DOI] [PubMed] [Google Scholar]
- 5.Rugo HS, Chien AJ, Franco SX, et al. A phase II study of lapatinib and bevacizumab as treatment for HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2012;134(1):13–20. doi: 10.1007/s10549-011-1918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuentes H, Calderillo G, Alexander F, et al. Phase II study of gemcitabine plus cisplatin in metastatic breast cancer. Anticancer Drugs. 2006;17(5):565–570. doi: 10.1097/00001813-200606000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Parnes HL, Cirrincione C, Aisner J, et al. Phase III study of cyclophosphamide, doxorubicin, and fluorouracil (CAF) plus leucovorin versus CAF for metastatic breast cancer: Cancer and Leukemia Group B 9140. J Clin Oncol. 2003;21(9):1819–1824. doi: 10.1200/JCO.2003.05.119. [DOI] [PubMed] [Google Scholar]
- 8.Marquina G, Waki H, Fernández LE, et al. Gangliosides expressed in human breast cancer. Cancer Res. 1996;56:5165–5171. [PubMed] [Google Scholar]
- 9.Carr A, Mullet A, Mazorra Z, et al. A mouse IgG1 monoclonal antibody specific for N-glycolyl GM3 ganglioside recognized breast and melanoma tumors. Hybridoma. 2000;19(3):241–247. doi: 10.1089/02724570050109639. [DOI] [PubMed] [Google Scholar]
- 10.Fernández LE, Alonso DF, Gomez DE, et al. Ganglioside-based vaccines and anti-idiotype antibodies for active immunotherapy against cancer. Expert Rev Vaccines. 2003;2(6):817–823. doi: 10.1586/14760584.2.6.817. [DOI] [PubMed] [Google Scholar]
- 11.Estevez F, Carr A, Solorzano L, et al. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP) Vaccine. 1999;18(1–2):190–197. doi: 10.1016/s0264-410x(99)00219-4. [DOI] [PubMed] [Google Scholar]
- 12.Carr A, Rodríguez E, Arango MC, et al. Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine. J Clin Oncol. 2003;21(6):1015–1021. doi: 10.1200/JCO.2003.02.124. [DOI] [PubMed] [Google Scholar]
- 13.Mulens V, de la Torre A, Marinello P, et al. Immunogenicity and safety of a NeuGcGM3 based cancer vaccine. Results from a controlled study in metastatic breast cancer patients. Human Vaccin. 2010;6(9):736–744. doi: 10.4161/hv.6.9.12571. [DOI] [PubMed] [Google Scholar]
- 14.De la Torre A, Hernández J, Ortiz R, et al. NGlycolylGM3/VSSP vaccine in metastatic breast cancer patients: results of phase I/IIa clinical trial. Breast Cancer (Auckl) 2012;6:151–157. doi: 10.4137/BCBCR.S8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles D, Roché H, Martin M, et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-Keyhole Limpet Hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–1100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labrada M, Clavell M, Bebelagua Y, et al. Direct validation of NGcGM3 ganglioside as a new target for cancer immunotherapy. Expert Opin Biol Ther. 2010;10:153–162. doi: 10.1517/14712590903443084. [DOI] [PubMed] [Google Scholar]