Abstract
We evaluated the safety and effectiveness of adjunctive tacrolimus therapy with conventional immunosuppression in patients with severe connective tissue disease-related interstitial lung disease (CTD-ILD).
We included patients from our interstitial lung disease (ILD) registry with CTD-ILD, in whom tacrolimus was added to corticosteroids and an additional immunosuppressive agent. Demographic data, clinical features, lung function, radiographic images, and pathologic findings were reviewed. Effectiveness was assessed by comparing pulmonary function tests (PFTs) closest to tacrolimus initiation to PFTs approximately 6–12 months later. Corticosteroid dose at these time points was also evaluated. We report adverse events attributed to tacrolimus.
Seventeen patients with CTD-ILD were included in adverse event analysis; twelve were included in efficacy analysis. Length of tacrolimus therapy ranged from 6 to 110 months (mean 38.8 months ± 31.4). The mean improvement in percent predicted total lung capacity was 7.5% ± 11.7 (p=0.02). Forced vital capacity mean improvement was 7.4% ± 12.5 (p=0.06). The average decrease in corticosteroid dose at follow-up was 20.3mg ± 25.2 (p=0.02) with complete discontinuation in six patients. No patients experienced a life-threatening adverse event attributed to tacrolimus.
Tacrolimus can be effective and is well tolerated as an adjunctive therapy and allows tapering of corticosteroids.
1. INTRODUCTION
Interstitial lung disease (ILD) related to connective tissue disease (CTD) causes significant morbidity and mortality (1–3). Dermatomyositis (DM) and polymyositis (PM) are idiopathic inflammatory myopathies associated with ILD that may be rapidly progressive, difficult to treat, and require multiple therapeutic agents (1, 4, 5). Anti-synthetase syndrome (ASA) is a form of the inflammatory myopathies characterized by an anti-aminoacycl transfer RNA synthetase antibody along with myositis, arthritis, Raynaud’s phenomenon or mechanic’s hands, with associated ILD that is often severe (3, 6). Some patients with ILD have autoimmune features suggested by clinical findings, serological studies or morphology, but do not fulfill criteria for a specific CTD (1, 2, 7), defined here as undifferentiated connective tissue disease (UCTD).
Corticosteroids have been the mainstay of treatment in CTD-ILD, with the addition of an immunosuppressive agent when prolonged corticosteroid administration is necessary or fails to control disease (1, 8, 9). Treatment is challenging when patients with CTD-ILD progress rapidly, have evidence of fibrosis, are refractory to “conventional therapy”, or are unable to tolerate corticosteroid tapering. ILD can be the primary manifestation of ASA, and lung disease in these patients is often more aggressive, requiring multiple immunosuppressive agents (10–14).
Because T cells may play a role in ILD pathogenesis in many CTDs (15), tacrolimus has been utilized as a therapy (16). Tacrolimus is a calcineurin inhibitor with multiple immunomodulating mechanisms, including the inhibition of T-lymphocyte proliferation and IL-2 transcription (17). Several studies conducted in transplant patients have demonstrated that tacrolimus is more potent that cyclosporine with a more favorable side effect profile, and may allow discontinuation of corticosteroids (18–20). Potentially serious side effects of tacrolimus include hypertension, kidney injury and renal failure, diabetes, cytopenias, and neurotoxicity (21). Toxicity may be limited by avoiding high serum levels (22). Previous studies have demonstrated benefit from tacrolimus in addition to corticosteroids for treatment of both refractory myositis and ILD in patients with PM, DM, or ASA (15, 16, 23–27).
This case series reports seventeen patients with CTD-ILD in whom adjunctive tacrolimus was used in addition to conventional immunosuppressive therapy because of progressive or severe ILD. We hypothesize that using two immunosuppressive agents with different mechanisms of action allows for multi-mechanistic immunosuppression, permits administration at doses that limit toxicity, avoids or limits exposure to cyclophosphamide (28), and diminishes the length and dosage of treatment with corticosteroids. We show benefit in ILD treatment not only in patients with DM, PM, and ASA, but also with UCTD as well. Some of the results of these studies have been previously reported in abstract form. (29, 30)
2. METHODS
2.1 Study design
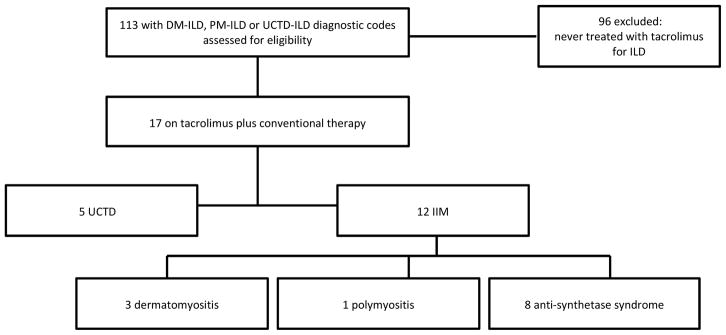
Our ILD registry (IRB 14163A) was queried for ICD-9 diagnostic codes of dermatomyositis (710.3), polymyositis (710.4), and UCTD (710.9) over the period from 2005 to May 2014 (figure 1). We then searched each patient’s electronic medical record using the terms “tacrolimus” and “Prograf” to find all patients treated with tacrolimus and conventional therapy, defined here as corticosteroids plus an additional immunosuppressive agent. Ninety-six patients were excluded, as they had not received treatment with tacrolimus for ILD, leaving seventeen patients for further analysis. All seventeen patients were assessed for adverse events during the time they were treated with tacrolimus. Five of these patients were excluded from efficacy analysis due to significant missing data, such as unknown length of tacrolimus treatment and lack of followup pulmonary function test (PFT) data. Two of these patients were excluded for insufficient length of treatment with tacrolimus; patient 13 terminating the drug due to nausea and vomiting and patient 14 terminating tacrolimus due to rapid clinical progression of ILD despite tacrolimus therapy. This left twelve patients for efficacy analysis. We performed a retrospective analysis of demographic data, medical history, clinical features, lung function, six-minute walk distance (6MWD), radiographic images, pathologic findings, laboratory data, adverse events, and immunosuppressive therapy including prednisone dosing. This study’s rheumatologist (JC) confirmed the CTD diagnoses based on clinical presentation and laboratory data. Primary ILD type was determined by review of lung biopsy findings when available and high-resolution CT scan (HRCT) by our institution’s multidisciplinary ILD team, which includes a dedicated pulmonary pathologist and chest radiologists. Evidence for fibrosis was assessed on pathology by identifying usual interstitial pneumonia (UIP) or fibrotic non-specific interstitial pneumonia (fNSIP) and on HRCT by finding traction bronchiectasis or a UIP-like pattern including honeycombing.
Figure 1.
Enrollment
DM: dermatomyositis; PM: polymyositis; IIM: idiopathic inflammatory myopathy; UCTD: undifferentiated connective tissue disease; ILD: interstitial lung disease
Five of seventeen patients (one with dermatomyositis, one with anti-synthetase syndrome, three with UCTD) were excluded from efficacy analysis due to incomplete data. These patients were included in adverse event analysis.
2.2 Tacrolimus prescribing practice and safety monitoring
Tacrolimus was added to each patient’s regimen of corticosteroids and an additional immunosuppressive agent to control severe and/or progressive ILD, avoid or limit use of cyclophosphamide, and/or diminish the length and dosage of treatment with corticosteroids. In the majority of patients (n=15), tacrolimus was started in the outpatient setting. Tacrolimus was started initially at a dose of 1 mg twice daily titrated by 1–2 mg daily with the goal of at least seven days between dose adjustment until target trough levels were reached, with final doses ranging from 1 mg twice daily to 6 mg twice daily. Laboratory monitoring included a complete metabolic panel, complete blood count and tacrolimus trough level drawn after initiation and 10–14 days after each dose adjustment and blood pressure was monitored. To limit toxicity, target 12-hour tacrolimus trough levels were 5–8 ng/mL, which is lower than that used in most post-transplant patients (31). Prophylaxis for pneumocystis jiroveci pneumonia was prescribed, usually with sulfamethoxazole/trimethoprim, which has been demonstrated to be safe and effective against this pathogen (32).
2.3 Statistical analysis
Continuous variables are presented as mean with standard deviation using Wilcoxon signed rank test to determine significance. Categorical variables are reported as counts. The pulmonary function tests (PFTs) chosen for evaluation were those performed closest to the initiation of tacrolimus as compared to those 6–12 months following tacrolimus initiation. If more than one full set of PFTs was available during this time frame, the PFTs closest to 6 months were selected. Ten of twelve patients had baseline PFT data available before tacrolimus was started (ranging from two months to the day of initiation). Patient 7’s baseline PFTs were collected 8 days after initiation and patient 10’s baseline PFTs were two months after initiation. Likewise, prednisone dosages were selected nearest to the baseline and follow-up PFTs. If the patient was on an alternate corticosteroid (e.g. methylprednisolone or cortisone acetate) these dosages were converted to equivalent prednisone dosages. All statistical analyses were performed using STATA with the assistance of a University of Chicago biostatistician. P values ≤ 0.05 were considered statistically significant.
3. RESULTS
3.1 Clinical features
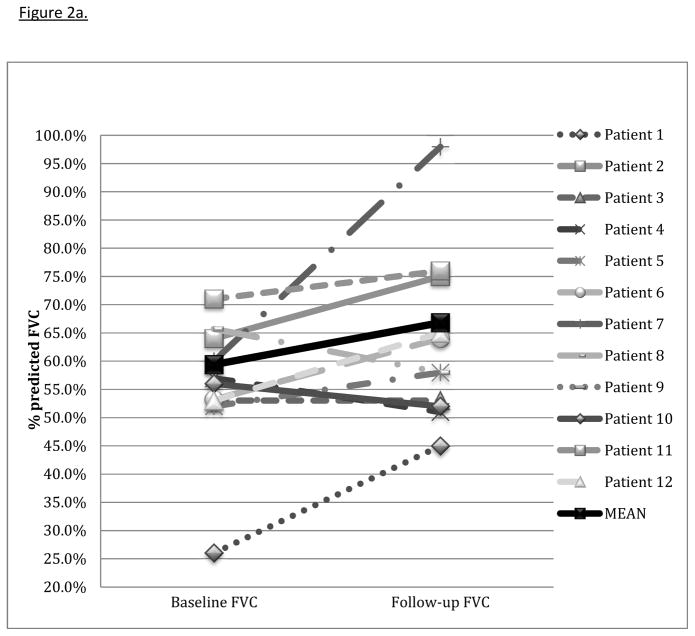
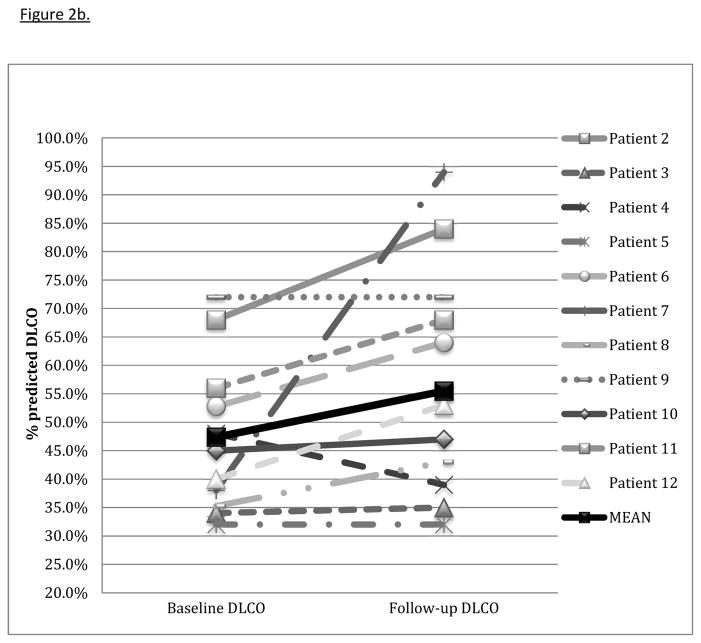
Seventeen patients were evaluated, twelve with an idiopathic inflammatory myopathy and five with UCTD (figure 1). Twelve patients received tacrolimus for greater than or equal to six months and had follow-up data sufficient for inclusion in the efficacy analysis. Demographic information, CTD features, ILD morphology, and concurrent immunosuppressive therapy are presented in table 1. All but one of twelve patients had evidence for fibrosis either by HRCT or pathology. Length of tacrolimus treatment ranged from 6 to 110 months (mean 38.8 months ± 31.4). The average time from ILD diagnosis to the initiation of tacrolimus was 18.8 months (data unavailable for one patient). Most patients (7 of 12) were treated with azathioprine concurrently with tacrolimus and corticosteroids. Dose of conventional non-steroidal immunosuppressive therapy prescribed with tacrolimus is reported in table 1. Baseline mean percent predicted FVC was 59.4% ± 17.4 (figure 2a). Baseline mean percent predicted DLCO was 47.4% ± 13.6 (data unavailable for one patient) (figure 2b).
Table 1.
Baseline characteristics of patients treated with tacrolimus
| Patient | Age/Sex | Race | CTD | Serum auto-antibody | HRCT pattern | Pathologic diagnosis | Length of tacrolimus therapy (months) | Concurrent therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | 47/F | AA | UCTD | ANA, dsDNA, anti-RNP | fNSIP/OP | OP | 27 | azathioprine 150mg daily |
| 2 | 47/M | C | UCTD | ANA, anti-Ro | fNSIP | None | 25 | azathioprine 150mg daily |
| 3 | 66/M | C | DM | none | OP/fNSIP | OP/fNSIP | 64 | MMF 1500mg BID |
| 4 | 63/M | C | DM | ANA, anti-MI-2 | UIP | fNSIP/UIP | 6 | MMF 500mg BID |
| 5 | 49/F | AA | PM | ANA | OP/fNSIP | OP/NSIP | 53 | azathioprine 50mg |
| 6 | 60/F | C | ASA | Anti-Jo-1 | fNSIP | None | 12 | MMF 500mg BID |
| 7 | 50/F | C | ASA | ANA, anti-Ro, Jo-1 | DAD | None | 26 | IV cyclophosphamide 500mg weekly |
| 8 | 71/M | C | ASA | ANA, dsDNA, anti-Ro, RF, anti-Jo-1 | fNSIP/DAD | OP/DAD | 17 | Azathioprine 150mg |
| 9 | 50/M | C | ASA | ANA, dsDNA, anti-Ro, RF, anti-Jo-1 | NSIP | cNSIP with interstitial fibrosis | 34 | PO cyclophosphamide 150mg daily |
| 10 | 41/F | AA | ASA | dsDNA, anti-Ro, anti-Jo-1 | fNSIP | None | 110 | azathioprine 150mg daily |
| 11 | 57/F | AA | ASA | ANA, anti-Ro, anti-Jo-1 | fNSIP | UIP | 78 | azathioprine 50mg daily |
| 12 | 73/F | C | ASA | Anti-Ro, anti-Jo-1 | fNSIP | None | 14 | azathioprine 150mg daily |
| 13* | 66/F | C | UCTD | ANA | fNSIP | UIP | 1 | azathioprine 150mg daily |
| 14* | 78/F | C | UCTD | ANA, anti-Ro | fNSIP | UIP | 1 | MMF 500mg BID |
| 15* | 53/M | AA | UCTD | ANA, anti-RNP, RF, anti-CCP | UIP | none | unknown | azathioprine 50mg daily |
| 16* | 63/F | AA | ASA | Anti-Jo-1 | fNSIP/cNSIP | UIP/OP | unknown | azathioprine 50mg daily |
| 17* | 63/M | C | DM | SSA | NSIP | OP | 10 | azathioprine 100mg daily |
AA: African American; ANA: anti-nuclear antibody; ASA: anti-synthetase syndrome; BID: twice a day; C: Caucasian; cNSIP: cellular non-specific interstitial pneumonia; CTD: connective tissue disease; DAD: diffuse alveolar damage; DM: dermatomyositis; F: female; fNSIP: fibrosing non-specific interstitial pneumonia; HRCT: high-resolution computed tomography; IV: intravenous; M: male; MMF: mycophenolate mofetil; OP: organizing pneumonia; PM: polymyositis; PO: by mouth; RF: rheumatoid factor; RNP: ribonuclear protein; UCTD: undifferentiated connective tissue disease; UIP: usual interstitial pneumonia.
excluded from efficacy analysis due to significant missing data
Figure 2.
Figure 2a. Change in FVC with tacrolimus
Change in forced vital capacity (FVC) from baseline PFTs to those at follow-up 4–13 months after tacrolimus start. Mean baseline FVC was 59.4% ± 17.4 with a mean improvement of 7.4% ± 12.5 (p = 0.06).
Figure 2b. Change in DLCO with tacrolimus
Change in diffusion capacity of the lung for carbon monoxide (DLCO) percent predicted from baseline PFTs to those at follow-up 4–13 months after tacrolimus start. Mean baseline DLCO was 47.4% ± 13.6 with a mean improvement of 10.0% ± 17.0 (p = 0.02).
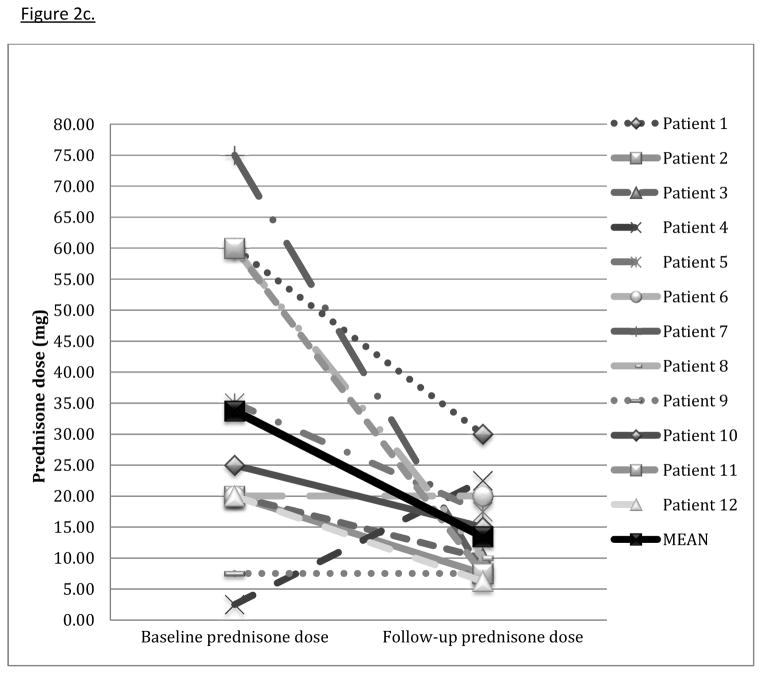
Figure 2c. Change in prednisone dose with tacrolimus
Change in prednisone dose (mg) from the clinic visit nearest the baseline PFTs to the visit nearest follow-up PFTs 4–13 months after tacrolimus start. Mean average decrease in prednisone dose at follow-up was 20.3 ± 25.2 (p=0.02).
Of note, patient 4’s increase in prednisone dose is a result of comparing dosages from the clinic visit nearest baseline and follow-up PFTs. His baseline PFT was performed two months before tacrolimus initiation, and his clinical course necessitated an increase in prednisone to 40 mg by the time of tacrolimus initiation.
3.2 Effect of treatment
All but one of twelve patients experienced stabilization or improvement in TLC (table 2). The mean change in percent predicted TLC was 7.5% ± 11.7; p = 0.02. Three patients experienced a mild decline in FVC; the rest had stabilization or improvement. The average improvement in percent predicted FVC was 7.4% ± 12.5 (p=0.06) (figure 2a). Ten of eleven patients had stabilization or improvement in percent predicted DLCO (one patient was unable to perform the DLCO maneuver), with a mean improvement of 10.0% ± 17.0 (p=0.02) (figure 2b). In all but one patient, corticosteroid dose was decreased at the time of follow-up PFTs (table 2). The average decrease in prednisone dose at follow-up was 20.3 mg ± 25.2 (p=0.02) (figure 2c). Six patients discontinued prednisone altogether. One patient eventually required lung transplantation. One patient died from progressive ILD, likely related to refractory gastroesophageal reflux with aspiration, four years after treatment with tacrolimus was started.
Table 2.
Change in PFTs and corticosteroid doses in patients treated with tacrolimus
| Patient | PFT follow-up from tacrolimus start (months) | Change in TLC (%) | Change in FVC (%) | Change in DLCO (%) | Change in prednisone dose (mg) | Prednisone discontinued? |
|---|---|---|---|---|---|---|
| 1 | 7 | +16 | +19 | n/a | −30 | Y |
| 2 | 8 | +9 | +11 | +16 | −12.5 | Y |
| 3 | 8 | +8 | 0 | +1 | −10 | Y |
| 4 | 4 | +1 | −6 | −9 | +20 | N* |
| 5 | 7 | +4 | +6 | 0 | −17.5 | N |
| 6 | 6 | +1.8 | +10.8 | +11.2 | 0 | N |
| 7 | 10 | +38 | +38 | +56 | −67.5 | Y |
| 8 | 8 | −9.3 | −7.7 | +7.7 | −50 | N |
| 9 | 4 | 0 | +5 | 0 | 0 | Y |
| 10 | 13 | +6 | −4 | +2 | −10 | N |
| 11 | 7 | +2 | +5 | +12 | −52.5 | Y |
| 12 | 7 | +14 | +12 | +13 | −13.75 | N |
| p | p=0.02 | p=0.06 | p=0.02 | p=0.02 |
TLC: total lung capacity; FVC: forced vital capacity; DLCO: diffusing capacity for carbon monoxide
prednisone for immunosuppression after lung transplantation
3.3 Adverse events
None of the seventeen patients had a life-threatening adverse event attributed to tacrolimus (table 3). The most common adverse event was pneumonia. Two patients were hospitalized with adverse events that might have been related to tacrolimus. One patient was hospitalized for pneumonia six days after starting tacrolimus. Three patients had transient acute kidney injury that resolved, one attributed to hypovolemia caused by illness (peak creatinine 2.2, resolved after two days with fluids, tacrolimus level was in target range) and two possibly attributable to tacrolimus, both with peak Cr of 1.6 but without supratherapeutic tacrolimus levels. Subsequent creatinine values were normal without dose adjustment. Other side effects were mild and self-limited. Only one patient discontinued due to intolerance (nausea and vomiting; patient 13, table 1).
Table 3.
Adverse events possibly related to tacrolimus
| Event | Number of Patients |
|---|---|
| Pneumonia | 4 |
| Acute kidney injury | 3 |
| Hospitalization | 2 |
| Herpes zoster | 2 |
| Nausea/vomiting | 2 |
| Tremor | 3 |
| Leukopenia | 1 |
| Abdominal pain | 1 |
| Diarrhea | 1 |
| Hypertension (new-onset) | 1 |
| Sinusitis | 1 |
| Death | 0 |
4. SELECTED PATIENT VIGNETTES
4.1 Patient 1
47-year-old woman with UCTD presented with progressive ILD despite increasing doses of prednisone and azathioprine 150 mg daily (table 3). HRCT showed patchy ground glass opacities bilaterally with areas of traction bronchiectasis consistent with fNSIP and organizing pneumonia (OP). Surgical lung biopsy revealed OP. (figure 3a). The addition of tacrolimus resulted in improvement in respiratory symptoms, oxygenation, serologies, lung function and HRCT (table 4, figure 3b). While on tacrolimus, she experienced one episode of leukopenia, which resolved with transient cessation of tacrolimus, herpes zoster, and one episode of diarrhea and abdominal pain. Hyperglycemia resolved with corticosteroid discontinuation. Tacrolimus was discontinued 27 months after initiation. She remains in remission on azathioprine 125 mg daily with significant improvement in HRCT and PFTs.
Figure 3.
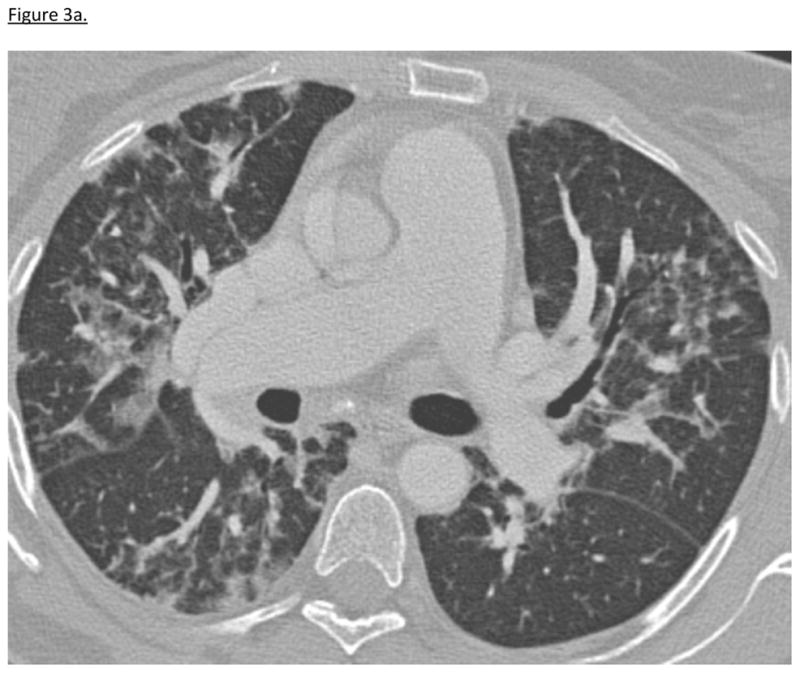
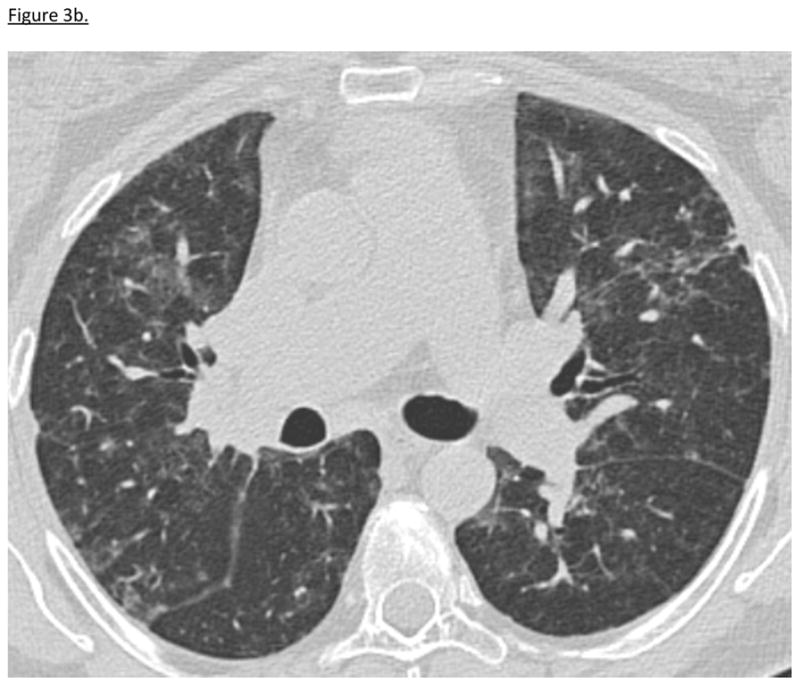
Figure 3a. Patient 1 HRCT Before Tacrolimus Treatment
12/2008: HRCT during patient 1’s initial hospitalization demonstrates reticulonodular interstitial abnormalities and patchy ground-glass opacities bilaterally.
Figure 3b. Patient 1 HRCT After Tacrolimus Treatment
10/2009: HRCT 6 months post-tacrolimus initiation reveals that the reticular interstitial opacities are less coarse and ground-glass opacities are decreased.
Table 4.
Effect of addition of tacrolimus on course of Patient 1
| 2008 | 2009 | 2011 | 2012 | |
|---|---|---|---|---|
| December | March | March | March | |
| Months of tacrolimus therapy | Pre-treatment | 0 months (date of initiation) | 24 months | 8 months after discontinuation |
| Medications | Prednisone 30 mg daily | Prednisone 60 mg daily Azathioprine 150 mg daily | Prednisone 5 mg daily Tacrolimus 2 mg BID Azathioprine 125 mg daily | Azathioprine 125 mg daily |
| TLC (% predicted) | 52% | 40% | 62% | 70% |
| FVC (% predicted) | 43% | 26% | 58% | 66% |
| DLCO (% predicted) | 37% | Unable to perform | 58% | 68% |
| O2 saturation | 100 on room air | On 2L/min oxygen Rest: 98% Post-walk: 84% | On room air Rest: 97% Post-walk: 100% | On room air Rest: 99% Post-walk: 100% |
| 6 minute walk distance | Not performed | 164.6 meters | 457.2 meters | 508.7 meters |
| CRP (mg/L) Normal: <5 mg/L | 21 → 161 | 4 | ||
| ANA (titer) Normal: 0–80 | 1280 | 160 | ||
| Aldolase (U/L) Normal: 2–8 U/L | 11.3 | 5.0 |
4.2 Patient 2
47 year-old man with UCTD-ILD initially improved on azathioprine and corticosteroids. HRCT revealed fNSIP (table 1). Approximately one year after diagnosis with ILD, his exercise tolerance and 6MWD began to decline as prednisone was tapered despite azathioprine 150 mg daily, so tacrolimus was added. Eight months later, his lung function markedly improved as well as his exercise tolerance allowing significant reduction in prednisone dose followed by discontinuation (table 2). Tacrolimus was discontinued 25 months after initiation. Complications possibly related to tacrolimus were recurrent sinusitis and new hypertension, both of which have been successfully treated. He remains in remission on azathioprine 150 mg daily.
4.3 Patient 3
66 year-old man with DM and OP/fNSIP on HRCT and lung pathology (table 1) experienced worsening pulmonary symptoms coincident with prednisone tapering from 30 mg daily despite mycophenolate mofetil 1500 mg twice daily. He had previously received a six-month course of intravenous cyclophosphamide and intravenous immunoglobulin G (IVIG). Azathioprine could not be used due to an acute hypersensitivity reaction. Eight months after initiation of tacrolimus, pulmonary symptoms and lung function improved allowing prednisone tapering. Herpes zoster complicated tacrolimus therapy. He remains in remission off prednisone for 3 years. He has been treated with tacrolimus for 64 months, as attempts at tapering either tacrolimus or mycophenolate mofetil result in ILD flares.
4.4 Patient 7
50 year-old woman transferred to our intensive care unit with acute hypoxemic respiratory failure requiring mechanical ventilation. Prior to this hospitalization, she had arthralgias, progressive dry cough, and shortness of breath. HRCT showed diffuse ground glass opacities suggestive of diffuse alveolar damage (DAD) and Jo-1 antibody was positive (table 1), leading to the diagnosis of ASA. Despite antibiotics and methylprednisolone 125 mg every 8 hours, her respiratory failure progressed, so IV cyclophosphamide and tacrolimus were initiated four days after transfer. Shortly thereafter, the patient was extubated to BiPAP and later discharged on home oxygen. Following a final dose of IV cyclophosphamide, azathioprine was added to tacrolimus and prednisone. Ten months later, her lung function improved to normal with excellent exercise ability (table 2). She has been treated with tacrolimus for 26 months, along with azathioprine tapered from 150 mg to 50 mg daily. Prednisone was tapered off. She remains in remission with normal lung function and no longer requires supplemental oxygen.
4.5 Patient 8
71 year-old man with ASA, fNSIP/DAD on HRCT and OP/DAD on lung pathology was transferred to our institution with acute hypoxemic respiratory failure requiring high flow supplemental oxygen despite azathioprine 150 mg daily and prednisone 40 mg daily (table 1). Prednisone was increased to 60 mg daily and tacrolimus was added which resulted in improvement in hypoxemia such that he could be discharged from the hospital. At 8-month follow-up, pulmonary function remained severely reduced but stable with a decreased dose of prednisone. He has been on tacrolimus for 17 months, azathioprine has been decreased to 125 mg daily, and prednisone has been tapered to 10 mg daily. He has experienced three isolated and transient elevations in creatinine; peak of 1.6 possibly related to tacrolimus, though without associated supratherapeutic tacrolimus levels.
5. DISCUSSION
In this investigation, we report the chronic use of tacrolimus in addition to conventional therapy with prednisone and another immunosuppressive agent, in a cohort of patients with severe and/or progressive CTD-ILD. We demonstrate stabilization or improvement at initial follow-up, as well as a significantly decreased corticosteroid dose, despite severe ILD including fibrosis and markedly reduced lung function at baseline. In a subset of patients, tacrolimus allowed for complete cessation of corticosteroids, likely avoiding significant side effects from prolonged corticosteroid exposure. Tacrolimus was well tolerated; only one of seventeen patients terminated therapy due to drug intolerance.
It is our hypothesis that tacrolimus co-administration with conventional therapy (corticosteroids and another immunosuppressive agent) augments clinical response by suppressing multiple immunologic pathways responsible for ILD in these patients. Among posttransplant patients, this “triple immunosuppressive therapy” is standard of care, and patients are routinely treated with corticosteroids, an anti-metabolite (azathioprine) or inhibitor of nucleotide synthesis (mycophenolate mofetil) and calcineurin inhibitor (tacrolimus or cyclosporine) immediately post-transplant (21). As in the post-transplant population, we demonstrated that multi-modality immunosuppression could be both safe and effective as rescue therapy in patients with severe ILD related to CTD. Data is mixed on the interaction between tacrolimus and mycophenolate mofetil in particular, but it is known in the transplant population that the addition of mycophenolate mofetil to tacrolimus causes a lower incidence of acute rejection (33).
Current therapy for CTD-ILD is based on expert opinion and practitioner comfort with existing agents. There are few prospective randomized trials to guide treatment in patients with severe or rapidly progressive ILD, especially those with an idiopathic inflammatory myopathy where mortality has historically been high (13, 34). Since the initial report by Oddis of response to tacrolimus in patients with refractory ILD related to the idiopathic inflammatory myopathies (16), several case reports and case studies have proposed the use of tacrolimus in addition to corticosteroids in this population. In 2012, Matsubara and colleagues summarized the published data on treatment of DM/PM patients with tacrolimus and at that time, only twentyseven such patients had been reported (35).
More recently, Kurita et al demonstrated improved event and disease-free survival with the addition of tacrolimus to corticosteroids and occasionally cyclophosphamide in twenty-five patients with DM/PM-ILD as compared to matched controls (27). Compared to this study, our patients had significantly worse baseline lung function (FVC mean 59.4% vs. 77.9% and DLCO mean 47.4% vs. 53.2%). As such, our study adds credence to the mounting evidence for the effectiveness of tacrolimus in patients with severe ILD.
Rituximab has been proposed as rescue therapy for severe CTD-ILD. Keir et al demonstrated, in a case series of eight patients, that most patients experienced improved lung function at follow-up 9–12 months after treatment with rituximab (36). As in our study, some patients were initiated on concurrent immunosuppressive therapies (corticosteroids and/or cyclophosphamide in addition to the study agent) due to disease severity. Given factors limiting access to rituximab including high cost and requirements for insurance prior authorization, we propose that tacrolimus may be a more readily available rescue therapy for some patients.
This study is limited by its retrospective design, which does not permit controlling for multiple variables in the clinical course of each patient (e.g. lack of standardization of concurrent treatment with other immunosuppressive agents). The study is also limited by its lack of a control arm to demonstrate the natural history of severe CTD-ILD without an additional immunosuppressive agent. As is the case in a retrospective study, baseline and follow-up PFT data could not be strictly standardized. Additionally, it was not possible to evaluate decline in lung function in the months prior to tacrolimus initiation, as many patients were referred from other institutions and this data was not uniformly available for analysis.
Randomized, prospective and controlled studies are difficult to perform in this patient population, therefore data is limited in the treatment of CTD-ILD. Of note, most patients in this series had evidence of fibrosis on pathology or HRCT, yet experienced improvement with immunosuppression. We note that despite the presence of UIP in some of our patients, immunosuppression was able to be used both safely and effectively in contrast to patients with idiopathic pulmonary fibrosis (37). This argues for careful phenotyping of fibrotic CTD-ILD before initiating immunosuppression. Only individuals familiar with the pharmacology and potential toxicities of these immunosuppressive agents should administer them, with careful monitoring.
In conclusion, this case series demonstrates that tacrolimus can be safe and effective for the treatment of severe, progressive CTD-ILD, even in the setting of acute hypoxemic respiratory failure or fibrosis. Careful monitoring of tacrolimus trough levels, blood pressure, electrolytes, and renal function permitted the chronic, safe co-administration of tacrolimus. Tacrolimus should be considered in patients who have failed conventional therapy or with severe and/or rapidly progressive ILD related to an idiopathic inflammatory myopathy or UCTD with careful clinical monitoring as outlined in the ACCP guidelines (31).
Acknowledgments
We gratefully acknowledge the University of Chicago Interstitial Lung Disease Research Group and Biostatistics Clinic, and Nancy Trojan for assistance with figures and tables. Funding support is provided by the NIH funded Research Training in Respiratory Biology grant at the University of Chicago (T32 HL007605).
Footnotes
Previous publication: Patient 1 has been previously presented in abstract form at ATS in May 2013. The preliminary data from this case series was presented in abstract form at CHEST in October 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. CHEST Journal. 2013;143:814–824. doi: 10.1378/chest.12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 3.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest. 2010;138:1464–1474. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 4.Marie I, Hachulla E, Cherin P, Dominique S, Hatron PY, Hellot MF, Devulder B, Herson S, Levesque H, Courtois H. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Care & Research. 2002;47:614–622. doi: 10.1002/art.10794. [DOI] [PubMed] [Google Scholar]
- 5.Cottin V, Thivolet-Bejui F, Reynaud-Gaubert M, Cadranel J, Delaval P, Ternamian PJ, Cordier JF Groupe d’Etudes et de Recherche sur les Maladies “Orphelines P. Interstitial lung disease in amyopathic dermatomyositis, dermatomyositis and polymyositis. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2003;22:245–250. doi: 10.1183/09031936.03.00026703. [DOI] [PubMed] [Google Scholar]
- 6.Marguerie C, Bunn CC, Beynon HLC, Bernstein RM, Hughes JMB, So AK, Walport MJ. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-trna synthetase enzymes. QJM. 1990;77:1019–1038. doi: 10.1093/qjmed/77.1.1019. [DOI] [PubMed] [Google Scholar]
- 7.Kinder BW, Collard HR, Koth L, Daikh DI, Wolters PJ, Elicker B, Jones KD, King TE., Jr Idiopathic nonspecific interstitial pneumonia: Lung manifestation of undifferentiated connective tissue disease? American journal of respiratory and critical care medicine. 2007;176:691–697. doi: 10.1164/rccm.200702-220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oddis CV. Idiopathic inflammatory myopathies: A treatment update. Current rheumatology reports. 2003;5:431–436. doi: 10.1007/s11926-003-0053-1. [DOI] [PubMed] [Google Scholar]
- 9.Mira-Avendano IC, Parambil JG, Yadav R, Arrossi V, Xu M, Chapman JT, Culver DA. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respiratory medicine. 2013;107:890–896. doi: 10.1016/j.rmed.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Koreeda Y, Higashimoto I, Yamamoto M, Takahashi M, Kaji K, Fujimoto M, Kuwana M, Fukuda Y. Clinical and pathological findings of interstitial lung disease patients with antiaminoacyl-trna synthetase autoantibodies. Internal medicine. 2010;49:361–369. doi: 10.2169/internalmedicine.49.2889. [DOI] [PubMed] [Google Scholar]
- 11.Marie I, Josse S, Hatron P, Dominique S, Hachulla E, Janvresse A, Cherin P, Mouthon L, Vittecoq O, Menard JF. Interstitial lung disease in anti–jo-1 patients with antisynthetase syndrome. Arthritis care & research. 2013;65:800–808. doi: 10.1002/acr.21895. [DOI] [PubMed] [Google Scholar]
- 12.Spath M, Schroder M, Schlotter-Weigel B, Walter MC, Hautmann H, Leinsinger G, Pongratz D, Muller-Felber W. The long-term outcome of anti-jo-1-positive inflammatory myopathies. Journal of neurology. 2004;251:859–864. doi: 10.1007/s00415-004-0449-5. [DOI] [PubMed] [Google Scholar]
- 13.Marie I, Hachulla E, Cherin P, Dominique S, Hatron PY, Hellot MF, Devulder B, Herson S, Levesque H, Courtois H. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis and rheumatism. 2002;47:614–622. doi: 10.1002/art.10794. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita T, Hasegawa M, Fujimoto M, Hamaguchi Y, Komura K, Hirano T, Horikawa M, Kondo M, Orito H, Kaji K. Clinical evaluation of anti-aminoacyl trna synthetase antibodies in japanese patients with dermatomyositis. The Journal of rheumatology. 2007;34:1012–1018. [PubMed] [Google Scholar]
- 15.Takada K, Nagasaka K, Miyasaka N. Polymyositis/dermatomyositis and interstitial lung disease: A new therapeutic approach with t-cell-specific immunosuppressants. Autoimmunity. 2005;38:383–392. doi: 10.1080/08916930500124023. [DOI] [PubMed] [Google Scholar]
- 16.Oddis CV, Sciurba FC, Elmagd KA, Starzl TE. Tacrolimus in refractory poly myositis with inter stitial lung disease. The Lancet. 1999;353:1762–1763. doi: 10.1016/S0140-6736(99)01927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson A, Bonham C, Zeevi A. Mode of action of tacrolimus (fk506): Molecular and cellular mechanisms. Therapeutic drug monitoring. 1995;17:584–591. doi: 10.1097/00007691-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Randomised trial comparing tacrolimus (fk506) and cyclosporin in prevention of liver allograft rejection. The Lancet. 1994;344:423–428. [PubMed] [Google Scholar]
- 19.Margreiter R. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: A randomised multicentre study. The Lancet. 2002;359:741–746. doi: 10.1016/S0140-6736(02)07875-3. [DOI] [PubMed] [Google Scholar]
- 20.Kramer BK, Montagnino G, del Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, Kruger B, Ortuno J, Kohler H, Kunzendorf U, Stummvoll H-K, Tabernero JM, Muhlbacher F, Rivero M, Arias M Group ftETvCMRTS. Efficacy and safety of tacrolimus compared with cyclosporin a microemulsion in renal transplantation: 2 year follow-up results. Nephrology Dialysis Transplantation. 2005;20:968–973. doi: 10.1093/ndt/gfh739. [DOI] [PubMed] [Google Scholar]
- 21.Halloran PF. Immunosuppressive drugs for kidney transplantation. New England Journal of Medicine. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 22.Bottiger Y, Brattstrom C, Tyden G, Sawe J, Groth C. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. British journal of clinical pharmacology. 1999;48:445. doi: 10.1046/j.1365-2125.1999.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochi S, Nanki T, Takada K, Suzuki F, Komano Y, Kubota T, Miyasaka N. Favorable outcomes with tacrolimus in two patients with refractory interstitial lung disease associated with polymyositis/dermatomyositis. Clinical and experimental rheumatology. 2005;23:707. [PubMed] [Google Scholar]
- 24.Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetaseassociated interstitial lung disease with tacrolimus. Arthritis and rheumatism. 2005;52:2439–2446. doi: 10.1002/art.21240. [DOI] [PubMed] [Google Scholar]
- 25.Ando M, Miyazaki E, Yamasue M, Sadamura Y, Ishii T, Takenaka R, Ito T, Nureki S, Kumamoto T. Successful treatment with tacrolimus of progressive interstitial pneumonia associated with amyopathic dermatomyositis refractory to cyclosporine. Clinical rheumatology. 2010;29:443. doi: 10.1007/s10067-009-1358-x. [DOI] [PubMed] [Google Scholar]
- 26.Guglielmi S, Merz TM, Gugger M, Suter C, Nicod LP. Acute respiratory distress syndrome secondary to antisynthetase syndrome is reversible with tacrolimus. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2008;31:213–217. doi: 10.1183/09031936.00014707. [DOI] [PubMed] [Google Scholar]
- 27.Kurita T, Yasuda S, Oba K, Odani T, Kono M, Otomo K, Fujieda Y, Oku K, Bohgaki T, Amengual O, Horita T, Atsumi T. The efficacy of tacrolimus in patients with interstitial lung diseases complicated with polymyositis or dermatomyositis. Rheumatology. 2014 doi: 10.1093/rheumatology/keu166. [DOI] [PubMed] [Google Scholar]
- 28.Meyer KC, Bierach J. Immunosuppressive therapy for autoimmune lung diseases. Immunology and allergy clinics of North America. 2012;32:633–669. doi: 10.1016/j.iac.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Witt L, Demchuk C, Curran J, Strek M. Safety and efficacy of tacrolimus in connective tissue disease-related interstitial lung disease. CHEST Journal. 2013;144:470A–470A. [Google Scholar]
- 30.Curran J, Strek M, Witt L. Successful treatment of dermatomyositis-related interstitial lung disease with tacrolimus plus azathioprine. American journal of respiratory and critical care medicine. 2013;187:A5776. [Google Scholar]
- 31.Baughman RP, Meyer KC, Nathanson I, Angel L, Bhorade SM, Chan KM, Culver D, Harrod CG, Hayney MS, Highland KB, Limper AH, Patrick H, Strange C, Whelan T. Monitoring of nonsteroidal immunosuppressive drugs in patients with lung disease and lung transplant recipients: American college of chest physicians evidence-based clinical practice guidelines. CHEST Journal. 2012;142:e1S–e111S. doi: 10.1378/chest.12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Seminars in arthritis and rheumatism. Elsevier; 2011. Primary prophylaxis for pneumocystis jirovecii pneumonia in patients with connective tissue diseases; pp. 497–502. [DOI] [PubMed] [Google Scholar]
- 33.Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clinical pharmacokinetics. 2002;41:813–851. doi: 10.2165/00003088-200241110-00003. [DOI] [PubMed] [Google Scholar]
- 34.Douglas WW, Tazelaar HD, Hartman TE, Hartman RP, Decker PA, Schroeder DR, Ryu JH. Polymyositis-dermatomyositis-associated interstitial lung disease. American journal of respiratory and critical care medicine. 2001;164:1182–1185. doi: 10.1164/ajrccm.164.7.2103110. [DOI] [PubMed] [Google Scholar]
- 35.Matsubara S, Kondo K, Sugaya K, Miyamoto K. Effects of tacrolimus on dermatomyositis and polymyositis: A prospective, open, non-randomized study of nine patients and a review of the literature. Clinical rheumatology. 2012 doi: 10.1007/s10067-012-2044-y. [DOI] [PubMed] [Google Scholar]
- 36.Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Renzoni EA. Severe interstitial lung disease in connective tissue disease: Rituximab as rescue therapy. European Respiratory Journal. 2012;40:641–648. doi: 10.1183/09031936.00163911. [DOI] [PubMed] [Google Scholar]
- 37.Behr J. Prednisone, azathioprine, and n-acetylcysteine for pulmonary fibrosis. The New England journal of medicine. 2012;367:870–871. doi: 10.1056/NEJMc1207471. [DOI] [PubMed] [Google Scholar]