Abstract
Standard replacement therapy for Addison's disease (AD) does not restore a normal circadian rhythm. In fact, hydrocortisone replacement in AD patients likely induces disrupted sleep. Given that healthy sleep plays an important role in improving quality of life, optimizing cognition, and ensuring affect regulation, the aim of this study was to investigate whether poor quality of life, mood alterations, and memory complaints reported by AD patients are associated with their disrupted sleep patterns. Sixty patients with AD and 60 matched healthy controls completed a battery of self-report questionnaires assessing perceived physical and mental health (Short-Form 36), mood (Beck Depression Inventory—II), sleep quality (Pittsburgh Sleep Quality Index), and cognition (Cognitive Failures Questionnaire). A latent variable model revealed that although AD had a significant direct effect on quality of life, the indirect effect of sleep was significantly greater. Furthermore, although AD had no direct effect on cognitive functioning, the indirect effect of sleep was significant. The overall model showed a good fit (comparative fit index = 0.91, root mean square of approximation = 0.09, and standardized root mean square residual = 0.05). Our findings suggest that disrupted sleep, and not the disease per se, may induce poor quality of life, memory impairment, and affect dysregulation in patients with AD. We think that improving sleep architecture may improve cognitive, affective, and physical functioning.
Keywords: Addison's disease, Cognition, Hydrocortisone, Quality of life, Sleep
1. Introduction
Addison's disease (AD) results from destruction of the adrenal cortex, with subsequent decreased production of glucocorticoids and mineralocorticoids. If left untreated, the disease can be life-threatening. The typical medication regime consists of oral hydrocortisone or alternative preparations to replace cortisol, and mineralocorticoid (fludrocortisone) to control sodium and potassium balance [1,2].
Despite current replacement therapy, patients with AD report relatively poor quality of life, with reduced general health perception (both emotional and physical), decreased vitality, memory impairment, increased prevalence of affective disorders, sleep disturbances, and fatigue [3–7]. Despite known and independent relationships between sleep and (a) general health, (b) memory, and (c) mood, no studies in AD patients have explored contemporaneous associations between these four variables. We postulate that poor sleep in AD patients is a biological mechanism underlying self-reported disturbances in quality of life, mood, and cognition.
Lovas, Loge, and Husebye [3] suggest that fatigue is a feature of adrenal failure, that it persists despite replacement therapy, and that it is a major contributor to self-reported impaired health in AD. Patients with AD also demonstrate increased daytime fatigue, which may be a consequence of poor quality of sleep [8]. Although few studies report on sleep impairments in AD, patients appear to experience disrupted sleep that is of poor quality [5,8,9]. Lovas, Husebye, Holsten, and Bjorvatn [5] found that 34% of their sample of patients with AD reported frequent sleep disturbances, including difficulty falling asleep and repeated and early morning awakenings.
Sleep plays important roles in memory consolidation and affect regulation [10]. Rapid eye movement (REM) sleep and non-REM sleep provide optimal conditions for consolidation of different forms of memory [11]. Furthermore, REM sleep has a mood regulatory function [12], with research demonstrating that patients with mood disorders have, relative to healthy individuals, altered REM intensity and integrity [13]. Because cortisol plays a key role in initiating and maintaining these different sleep stages, it has an important influence on the memory consolidation and affect regulation that take place during normal, healthy sleep [14].
Regarding cognitive functioning in AD, a small number of studies report that, even when on replacement therapy, declarative memory is worse in patients than in healthy controls [15]. Increased levels of anxiety and depression have also been reported in AD populations [6]. None of these studies, however, explored disrupted sleep as a possible mechanism underlying the observed deficits.
No studies in AD patients have explored the relationship between sleep and general health, mood, and memory. Our objectives were therefore to characterize self-reported quality of life in a sample of South African AD patients, and to investigate whether sleep disturbances correlate with poor quality of life, cognitive impairment, and affective dysregulation. We hypothesize that AD patients will report poor quality of life (marked by impaired cognition and mood alterations), and that this may be explained by sleep disturbances. Our hypothesis is based on literature showing that (a) cortisol plays a key role in sleep maintenance and integrity, (b) sleep plays an important role in cognitive functioning and affect regulation, and (c) hydrocortisone replacement medication used by AD patients does not restore the natural circadian rhythm and has direct effects on sleep architecture.
2. Patients and methods
2.1. Research and ethics
The research ethics committees from the Department of Psychology and Faculty of Health Sciences at University of Cape Town, both of which adhere to the Declaration of Helsinki, approved the study procedures. All participants gave informed consent.
2.2. Patients and healthy controls
Sixty adult patients with a diagnosis of AD (recruited from the South African Addison's disease (SAAD) database [16]) completed the self-administered survey described below. The diagnosis of AD was made on the basis of the suggestive clinical presentation, low basal cortisol level and simultaneously elevated ACTH concentration, or, where indicated, a peak cortisol level following 250 μg ACTH stimulation, of less than 550 nmol/L associated with a basal raised plasma ACTH level, exceeding 10.1 pmol/L. There was confirmed etiology for 49 of the 60 AD patients: autoimmune (82%; n = 40), idiopathic (12%; n = 6), tuberculosis (4%; n = 2), and X-linked adrenal hypoplasia (2%; n = 1). For all AD patients, clinical and demographic data were extracted by interview and from patient folders.
Sixty healthy controls, recruited using advertisements placed at the University of Cape Town and in large corporations in the Cape Town metropolitan area, also completed the survey.
We used a case–control design, matching groups by age, sex, ethnicity, and household income. We restricted enrolment to individuals between the ages of 18 and 75 years.
2.3. Procedure
A member of the research team systematically inspected the SAAD database and then contacted AD patients telephonically to invite them to participate. Healthy control participants were enrolled in the study after they responded to advertisements by contacting the research team.
All potential participants received a consent form and the study questionnaire in the post, and were asked to return the completed consent form and questionnaires by way of return post. Fig. 1 presents the flow of participants through the recruitment and study processes.
Fig. 1.
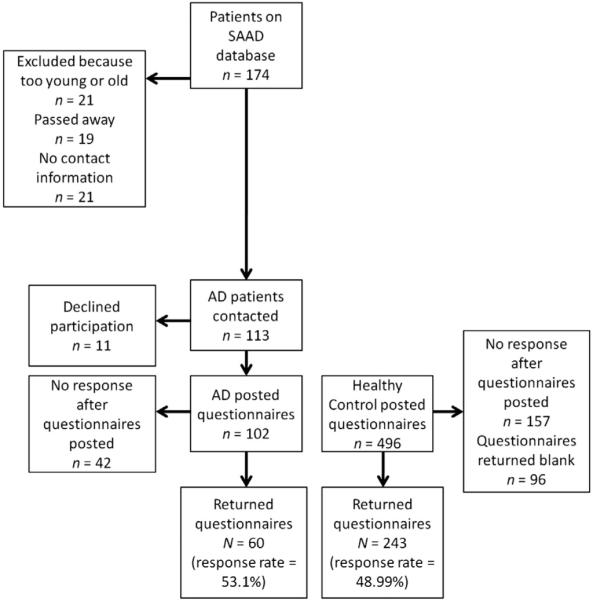
Flow of participants through the recruitment and study processes. To facilitate a case–control design, we selected, based on age, sex, race, and household income, 60 participants from the pool of 243 possible healthy controls. SAAD—South African Addison's disease database and AD—Addison's disease.
2.4. Measures
A sociodemographic and medical questionnaire elicited data about (a) demographic variables (e.g., age, race, and household income), (b) medical history, and (c) type and dosage of current medication, and length of time since diagnosis (AD patients only).
The Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) [17] assesses eight health-related concepts: a) physical functioning; b) role limitations due to physical health; c) pain; d) general health; e) role limitations due to emotional problems; f) energy/fatigue; g) emotional well-being; and h) social functioning. On each of these concepts, the range of scores is 0–100, with higher scores representing better QoL. Four of these concepts (a–d, listed above) are averaged to produce a global Physical QoL score, and the other four (e–h, listed above) are averaged to produce a global Mental QoL score.
The Beck Depression Inventory—Second Edition (BDI-II) [18] is a 21-item instrument that measures intensity, severity, and depth of depression in respondents. Possible scores range from 0 to 63, with higher scores representing more depressive symptomatology.
The Pittsburgh Sleep Quality Index (PSQI) [19] assesses sleep quality and disturbances over the previous 1 month. It comprises 19 items that relate to seven components: sleep quality, sleep latency, sleep duration, sleep disturbances, habitual sleep efficiency, the use of sleeping medication, and daytime dysfunction due to disrupted sleep. The score on each component ranges from 0 to 3; hence, the total PSQI score ranges from 0 to 21, with higher scores representing more disrupted sleep.
The Cognitive Failures Questionnaire (CFQ) [20] is a frequently used 12-item measure of cognitive lapses and slips, over the previous 6 months. Each of the 12 items loads onto one of three factors: Clumsiness (i.e., failures related to motor awkwardness, such as bumping into objects inadvertently), Retrieval (i.e., failures of both retrospective memory and prospective memory, such as forgetting to transfer a message to somebody when requested to do so), and Intention forgotten (i.e., failures related to maintaining intentions, such as forgetting what you came to the shops to buy). The CFQ total score ranges from 0 to 48, with higher scores indicating more impaired cognitive functioning.
2.5. Data management and statistical analysis
We scored each of the measures using standard procedures, detailed in the relevant scoring manuals. After comparing sample characteristics and between-group comparisons, we examined zero-order correlations using Pearson's r correlation coefficients, and created latent variable models to test predictions about the direction and strength of relationships between AD, quality of life, sleep, and cognition. Specifically, we hypothesized that these four latent variables would relate to one another in the way depicted in Fig. 2. Regarding the manifest variables in that figure: We included in our modeling (a) in the case of the SF-36, two composite variables (Physical and Mental), each of which summarized four of the instrument's eight subscales; and (b) in the case of the PSQI, three variables (subjective sleep quality, sleep efficiency, and sleep disturbance) that, both empirically and theoretically, best described sleep disruptions in this sample.
Fig. 2.
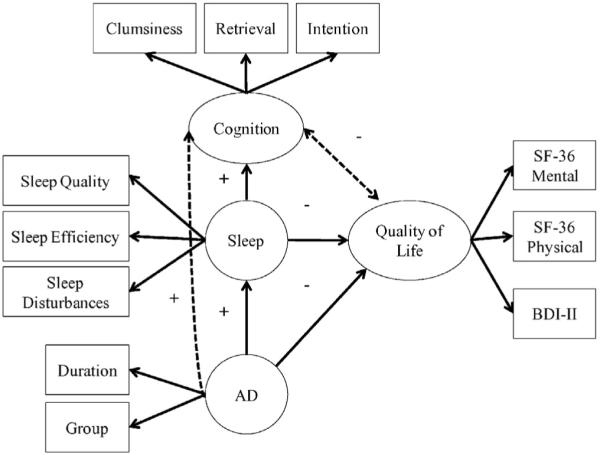
Initial latent variable model showing hypotheses we wished to examine (represented by solid lines) and plausible hypotheses (dashed lines). Plus signs (+) indicate a positive relationship, whereas minus signs (−) indicate a negative relationship. We predicted that having Addison's disease would result in poorer sleep and impaired cognition (represented by higher scores on the Pittsburgh Sleep Quality Inventory and Cognitive Failures Questionnaire), and poorer quality of life (represented by lower scores on the SF-36 Mental and Physical scales). We also predicted that having poorer sleep (i.e., higher PSQI scores) would result in worse cognition (i.e., higher CFQ scores) and poorer quality of life (i.e., lower SF-36 scores). Finally, we predicted that having poorer cognition (i.e., higher CFQ scores) may result in poorer quality of life (i.e., lower SF-36 scores), or vice-versa.
Fig. 2, then, shows the set of hypotheses that we wished to examine (solid lines), as well as plausible alternative hypotheses (dashed lines). We created a latent variable model to describe the complete set of relationships (both dashed and solid lines) depicted in Fig. 2; then, we created a second model to describe the set of relationships depicted by the solid lines only in Fig. 2. In other words, this second model removed parameters that, initially, represented plausible alternative hypotheses. We compared the two models to see which provided the best fit for the data.
Regarding sample size, conventional guidelines suggest that a minimum of 5 participants is required for every free parameter included in a model [21]. It is possible to run latent variable models with 100 participants [22]. Obtaining larger samples can be challenging when studying rare diseases such as AD (a global population prevalence of 39–117 per million [23], and a prevalence in South Africa of 3.1 per million [24]). We believe that, given our study population, there were a sufficient number of participants in our final sample (N = 120).
We completed all analyses using R (the sem package), and SPSS (version 22). Unless noted otherwise, we set the threshold for statistical significance (α) at 0.05.
3. Results
3.1. Sample characteristics
Table 1 presents the clinical characteristics of AD patients. Of note is that six participants (one man aged 37 years, and five women aged 21, 38, 47, 50, and 64 years, respectively) were prescribed prednisone; the remainder were prescribed hydrocortisone. The average daily prednisone dose was 9.06 mg (SD = 4.01), and the average daily prednisone/kg dose was 0.12 mg/kg (SD = 0.08).
Table 1.
Sample characteristics (N=120).
| Variable | Addison's disease |
Healthy controls |
t / χ2 | p | ESE |
|---|---|---|---|---|---|
| (n=60) | (n=60) | ||||
| Age at diagnosis (years)a | 31.03 (14.41) 3–67 |
||||
| Duration of AD (years)a | 19.33 (13.34) 1–54 |
||||
| Total hydrocortisone doseb | 22.02 (7.23) 5–35 |
||||
| Hydrocortisone/kgc | 0.31 (0.14) 0.10–0.70 |
||||
| Number of doses per dayd | 1.95 (0.70) 1–3 |
||||
| Concurrent co-morbid diseases |
1.63 (1.71) 0–7 |
||||
| Age | 50.57 (14.37) 20–74 |
49.90 (14.70) 19–77 |
−0.26 | .792 | 0.05 |
| Body mass index | 26.80 (6.02)e | 24.82 (4.02)f | −1.99 | .049* | 0.39 |
| 18.37–42.19 | 18.20–37.81 | ||||
| Income (in ZAR)g | 4.92 | .426 | 0.21 | ||
| 1000–2499 | 4 | 1 | |||
| 2500–5499 | 8 | 6 | |||
| 5500–9999 | 11 | 7 | |||
| 10,000–19,999 | 10 | 10 | |||
| 20,000–30,000 | 6 | 11 | |||
| >30,000 | 15 | 19 |
Note. Body mass index was calculated by dividing the participant's weight by height2 (information obtained from the sociodemographic questionnaire). For all of the variables except Income, means (with standard deviations in parentheses) are presented on the top line, and range is presented below that. Regarding the variable Income, ZAR=South African rands; when the study was conducted, the ZAR:US$ exchange rate was 8.65:1. For that variable, actual numbers of participants in each cell are presented. ESE=effect size estimate; in this case, Cohen's d (for the t-tests), or Cramer's V (for the χ2 test).
Data based on 58 participants.
Data based on 52 participants; 2 patients did not provide the relevant answers on the sociodemographic questionnaire, and the remaining 6 were prescribed prednisone.
Data based on 51 participants; 3 patients did not provide the relevant answers on the sociodemographic questionnaire, and the remaining 6 were prescribed prednisone.
Data based on 59 participants.
Data based on 48 participants.
Data based on 55 participants.
Data based on 54 participants in each group.
p < .05.
Overall, the patients and controls were matched for age (AD: M = 50.57 ± 14.37 years, range = 20–74 years; controls: M = 49.90 ± 14.70 years, range = 19–77 years; p = .792), sex distribution (14 males and 46 females in each group), ethnicity distribution (53 white, 2 Asian, and 5 colored [mixed black–white ancestry] individuals in each group), and monthly household income (p = .426). Table 1 also shows that, on average, patients' BMI was significantly greater than that of controls. The most prevalent co-morbid illnesses were primary hypothyroidism (n = 18 patients) and diabetes mellitus (n = 12 patients).
3.2. Between-group comparisons: patients versus controls
Table 2 presents the results of independent-samples t-tests comparing the scores of AD patients and controls on all measures of quality of life, depression, sleep disruption, and cognitive functioning. Even after the Bonferroni correction to control for inflated familywise error associated with multiple comparisons (in this case, α = .05 / 21 = .002), most between-group differences remained statistically significant. AD patients reported poorer quality of life, more depressive symptomatology, more disrupted sleep, and more cognitive impairment than controls.
Table 2.
Self-reported quality of life, depression, sleep quality, and cognitive functioning (N=120).
| Variable | Addison's disease |
Healthy controls |
t | p | ESE |
|---|---|---|---|---|---|
| (n=60) | (n=60) | ||||
| SF-36 | |||||
| Physical | 67.58 (28.58) | 89.33 (18.97) | 4.91 | <.001** | 0.90 |
| Role limitations – physical |
62.5 (41.69)a | 92.50 (22.22) | 4.90 | <.001** | 0.90 |
| Role limitations – emotional |
62.22 (43.17) | 93.89 (20.81) | 5.12 | <.001** | 0.93 |
| Energy | 48.53 (22.18)a | 67.45 (14.36)a | 5.45 | <.001** | 1.01 |
| Emotional | 68.48 (19.50) | 81.83 (9.89) | 4.73 | <.001** | 0.86 |
| Social | 72.73 (27.48) | 92.92 (12.47) | 5.18 | <.001** | 0.95 |
| Pain | 65.63 (32.36) | 88.13 (15.51) | 4.86 | <.001** | 0.89 |
| General health | 50.20 (24.01) | 78.05 (14.17)b | 7.72 | <.001** | 1.41 |
| Overall physical | 61.08 (26.83)a | 87.04 (12.48)b | 6.69 | <.001** | 1.24 |
| Overall mental | 62.49 (22.60)a | 83.98 (10.84)a | 6.53 | <.001** | 1.21 |
| BDI-II | 12.36 (10.27)a | 3.37 (3.76) | −6.28 | <.001** | 1.17 |
| PSQI | |||||
| Sleep quality | 1.18 (0.93) | 0.72 (0.61) | −3.25 | .002* | 0.58 |
| Sleep latency | 1.28 (1.13) | 0.71 (0.87)b | −3.08 | .003* | 0.56 |
| Sleep duration | 1.02 (0.87) | 0.72 (0.56) | −2.25 | .027* | 0.41 |
| Sleep efficiency | 0.93 (1.06) | 0.42 (0.67) | −3.20 | .002* | 0.58 |
| Sleep disturbances | 1.42 (0.70) | 0.97 (0.52) | −4.01 | <.001** | 0.73 |
| Use of sleep medication | 1.02 (1.40) | 0.20 (0.55) | −4.22 | <.001** | 0.77 |
| Daytime dysfunction | 1.02 (0.85) | 0.50 (0.50) | −4.04 | <.001** | 0.75 |
| CFQ | |||||
| Clumsiness | 4.12 (3.68) | 2.75 (2.17) | −2.48 | .015* | 0.45 |
| Intention forgotten | 3.17 (2.66) | 2.08 (1.73) | −2.65 | .009* | 0.49 |
| Retrieval | 8.46 (4.30)b | 5.15 (3.40) | −4.66 | <.001** | 0.85 |
Note. SF-36=Medical Outcomes Study 36-Item Short-Form Health Survey; BDI-II=Beck Depression Inventory–Second Edition; PSQI=Pittsburgh Sleep Quality Index; CFQ=Cognitive Failures Questionnaire; and ESE=effect size estimate; in this case, Cohen's d.
Data based on 58 participants.
Data based on 59 participants.
p < .05.
p < .002 (i.e., statistically significant after the Bonferroni correction).
3.3. Latent variable models
We utilized statistical models that sought to determine the set of relationships between our latent variables: AD (estimated by its presence or absence, and its duration), sleep disruption (estimated by three components on the PSQI), quality of life (estimated by two components on the SF-36 plus the BDI-II), and cognition (estimated by three components on the CFQ). Table 3 presents the correlations among manifest variables.
Table 3.
Correlation matrix for all manifest variables included in the latent variable models (N=120).
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. SF-36: Physical | 1.00 | ||||||||
| 2. SF-36: Mental | 0.778** | 1.00 | |||||||
| 3. BDI-II | −0.733** | −0.797** | 1.00 | ||||||
| 4. PSQI: Sleep quality | −0.459** | −0.458** | 0.458** | 1.00 | |||||
| 5. PSQI: Sleep efficiency | −0.365** | −0.320** | 0.438** | 0.459** | 1.00 | ||||
| 6. PSQI: Sleep disturbances | −0.597** | −0.527** | 0.589** | 0.506** | 0.414** | 1.00 | |||
| 7. CFQ: Clumsiness | −0.426** | −0.465** | 0.436** | 0.331** | 0.290** | 0.368** | 1.00 | ||
| 8. CFQ: Retrieval | −0.460 | −0.555** | 0.511** | 0.296** | 0.323** | 0.489** | 0.675** | 1.00 | |
| 9. CFQ: Intention forgotten | −0.391** | −0.459** | 0.356** | 0.276** | 0.348** | 0.452** | 0.668** | 0.732** | 1.00 |
Note. Values presented are Pearson's r correlation coefficients. SF-36=Medical Outcomes Study 36-Item Short-Form Health Survey; BDI-II=Beck Depression Inventory–Second Edition; PSQI=Pittsburgh Sleep Quality Index; and CFQ=Cognitive Failures Questionnaire.
p < .01.
We utilized a two-step procedure. First, we tested a measurement model, using confirmatory factor analysis (CFA), and then we tested two structural causal models designed to test theory. In all three models, most of the fit indices reflected adequate fit.
3.3.1. Measurement model
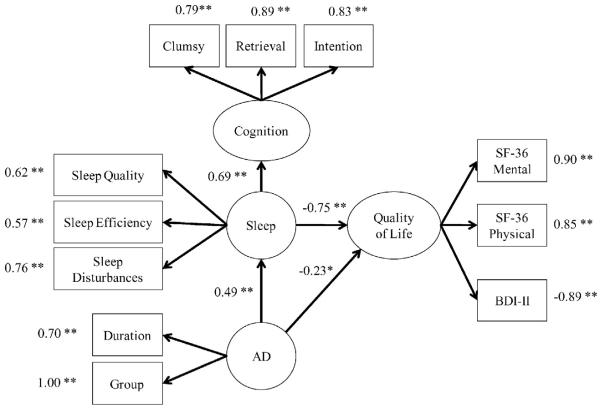
Our model estimated the relationships between four hypothesized latent variables (AD, sleep disruption, quality of life, and cognition). Fig. 3 summarizes the results of the measurement model. The CFA fit well according to all but one of the tested fit indices; only the χ2 statistic was statistically significant. This statistic is used to test the null hypothesis that the model fits the data, and hence its statistical significance might be a cause for concern. However, this statistic is quite sensitive to sample size and to departures from multivariate normality, and often rejects otherwise well-fitting models [25].
Fig. 3.
Measurement model. Model chi-square = 74.97406, df = 38, p = .0003, goodness-of-fit index = 0.91, root-means-square error of approximation (RMSEA) = 0.09, Bentler–Bonett Normed Fit Index (NFI) = 0.91, Bentler Comparative Fit Index (CFI) = 0.95, Bentler Relative Noncentrality Index (RNI) = 0.95, Bollen Incremental Fit Index (IFI) = 0.95, and standardized root mean square residual (SRMR) = 0.05. *p < .05. **p < .01.
3.3.2. Structural model
The two theoretical models that we tested (see Fig. 2) were identical except that one (indicated using dashed lines) included two plausible alternative causal hypotheses. These two hypotheses were not predicted by the theory being tested, but served as alternatives that could have either disconfirmed the theory or provided evidence that it was not complete. The more inclusive model had adequate fit, but the two alternative causal paths were not statistically significant, with a standardized path coefficients of −0.147, p = .16 (the causal pathway connecting cognition to quality of life) and 0.05, p = .64 (the causal pathway connecting AD to cognition). Hence, the final model omitted these non-significant causal pathways and fit just as well (see Fig. 3).
3.4. Additional analyses
We sought to determine whether certain demographic and disease characteristics played a role in determining outcome variable scores. Such information may be useful in establishing (a) across the sample, whether individual differences affected quality of life, and (b) within the sample of AD patients, which disease characteristics affected quality of life.
First, a series of independent-samples t-tests sought to determine whether, within each group separately, sex had a significant influence on the outcome variable scores. The analyses detected no significant sex differences within either the AD or control groups, with this exception: On average, male controls reported, on the PSQI, a greater number of sleep disturbances than their female counterparts (men: M = 1.21 ± 0.43, women: M = 0.89 ± 0.53; t(58) = 2.09, p = .041, d = 0.63).
Second, correlational analyses sought to determine whether, within each group separately, age had a significant influence on the outcome variable scores. Among controls, increasing age was associated with worse scores on the SF-36 Physical (r = − 0.27, p = .036), CFQ Retrieval (r = 0.29, p = .023), and CFQ Intention Forgotten (r = 0.35, p = .005) scales. In the AD group, increasing age was associated with worse scores on the SF-36 Physical (r = −0.26, p = .050) and PSQI Sleep disturbances (r = 0.30, p = .022) scales.
Third, correlational analyses sought to determine whether, in the AD group, there were significant associations between disease characteristics and outcome variable scores. These analyses suggested that none of the disease characteristics (viz., duration of illness, total hydrocortisone dose, dosage/kg, and number of doses per day) correlated significantly with any of the outcome scores.
Fourth, a series of Fisher's Exact Tests sought to determine whether, in the AD group, there was an association between timing of the last dose (before noon, n = 18; between noon and 18 h00, n = 16; after 18 h00, n = 20; 6 AD participants did not report on the timing of their last dose of the day) and outcome variable scores. The analyses suggested that timing of the last dose was associated with scores on (a) the SF-36 Physical scale, p < .001 (b) the BDI-II, p = .037, and (c) the PSQI, p = .026. Overall, these analyses suggested that (a) patients who took their last dose between noon and 18 h00 and after 18 h00 were more likely to report shorter sleep duration, (b) patients who took their last dose before noon and after 18 h00 were more likely to report severe depression, and (c) patients who took their last dose after 18 h00 were more likely to report poor QoL.
Finally, we wished to determine whether, within the AD group, the characteristic of certain co-morbid illnesses influenced outcome scores. Because the most common co-morbid diseases were hypothyroidism (present in 18 patients) and diabetes (present in 12 patients), separate independent samples t-tests compared scores on the outcome variables in patients who had hypothyroidism versus those who did not, and in patients who had diabetes versus those who did not. Regarding the hypothyroidism comparison, there were no significant between-group differences (all p's > .105). However, the presence of diabetes was associated with better self-reported mental health (SF-36 Mental: t(56) = 2.06, p = .044, d = 0.69), lower levels of depression (BDI-II: t(56) = −2.41, p = .019, d = 0.78), and better sleep quality (PSQI Sleep disturbances: t (58) = −3.50, p = .002, d = 0.95).
4. Discussion
A cohort of South African patients with Addison's disease reported poorer quality of life, increased mood disturbance, more disrupted sleep, and a greater degree of memory impairment than matched healthy controls. Our data are consistent with previous European studies suggesting that, despite being on replacement therapy, AD patients report experiencing poor quality of life [3,6]. The novel contribution of this study, however, is that it links disrupted sleep and poor quality of life in AD patients to both cognitive dysregulation and affective dysregulation. Using a series of latent variable models, we describe specific associations between sleep, affect, memory, and quality of life in AD. These models suggested that (a) the indirect effect of sleep disturbances on quality of life was greater than that of the disease status, and (b) the presence of AD did not directly influence cognition; however, (c) the relationship between AD and cognition was significant when mediated by sleep. Hence, we suggest that sleep disturbances are a plausible, but not yet confirmed, biological mechanism underlying poor quality of life, mood disturbances, and impaired cognitive functioning in AD patients.
Sleep has a restorative function on the body (both physically and neurologically), and sleep and circadian rhythms play an important role in daily physiological functions [26]. Disrupted sleep and circadian rhythms attenuate quality of life and well-being [27], and lead to energy imbalances, metabolic dysregulation, and fatigue [26]. Hence, it is not surprising that AD patients who report frequent sleep disturbances and reduced sleep quality also experience poorer subjective health, fatigue, and reduced vitality.
A plethora of research has shown that both slow wave sleep (SWS) and rapid eye movement (REM) sleep, and the successful transition between these sleep stages, are vital for healthy memory consolidation across the night [11]. Cortisol plays a vital role in ensuring the smooth transition from one sleep stage to the next [28]. AD patients on replacement therapy show patterns of cortisol secretion that are non-physiological: They experience lower than normal cortisol concentrations in the early hours of the morning and before taking their medication, and experience a peak in cortisol levels soon after medication ingestion. They may also have higher-than-normal cortisol levels prior to sleep onset and in the early hours of the sleep cycle [29].
Previous studies suggest that replacement regimens may influence Addison's disease patients' sleep architecture directly. For instance, García-Borreguero et al. [8] reported that AD patients who took hydrocortisone before bedtime had significantly decreased REM latency and increased REM sleep time; in contrast, and in confirmation of previous results [9], those who were deprived of glucocorticoid medication 1.5 h prior to bedtime showed increased REM latency and decreased REM sleep time.
The extant literature has not, however, explored the implications for cognition, and for overall quality of life, of these altered circadian cortisol patterns, and of consequent disrupted sleep architecture, in AD patients. Although previous studies have shown that AD patients self-report sleep disruptions and experience memory impairment [5,15], we show a definite association between sleep disruption and memory impairment.
Similarly, previous studies have found, as we did, that AD patients experience more symptoms of affective disorder than do healthy controls [6,7], but no published study has explored the relationship between affect dysregulation and sleep disturbances in AD. It is our contention that disrupted sleep is a key biological mechanism underlying depressed mood in AD patients. In general, depressed patients self-report sleep disturbances (e.g., difficulty falling and staying asleep, and early morning awakenings) [30], and on polysomnographic measure appear to experience both SWS and REM disruptions [13]. Following treatment for depression, mood improves after REM sleep is selectively inhibited [31]. Of interest here is that individuals who take hydrocortisone before bedtime have significantly decreased REM latency and increased REM sleep time, a pattern similar to that found in depressed patients. Hence, altered circadian rhythms in AD patients may explain the high presence of affective disorders in this population.
Consistent with previous studies, in the current patient cohort the clinical characteristics of Addison's disease did not impact on measures of quality of life, sleep disruption, mood, and cognition [32]. Counterintuitively, however, AD patients with diabetes reported significantly fewer sleep disturbances, better mental health, and lower levels of depression than those without. This unexpected finding cannot be explained by between-group differences with regard to clinical or sociodemographic characteristics: The groups of AD patients with and without diabetes were not significantly different in terms of, for instance, age, age at diagnosis, duration of illness, BMI, and the number of doses per day. Hence, there remain at least two possible explanations for the observed pattern of data. The first is that the statistical analyses have produced a Type I error. The likelihood of such an error arising is particularly strong given the unequal sample sizes, and the small size of the diabetes group (n = 12). A second possible explanation for our findings, however, is that AD patients with diabetes report better mental health and lower levels of depression because they experience less disturbed sleep. This explanation would be consistent with our findings that sleep plays an important role in quality of life. This finding requires replication; certainly, the current data do not permit speculation as to causal direction, and, as noted above, we cannot rule out the possibility of a Type I error.
Regarding the effects of demographic variables on the measured outcomes, our findings are consistent with known associations between advancing age and poorer physical well-being, increased cognitive impairment (in healthy controls), and increased sleep disturbances. In contrast to some previous studies reporting that female AD patients have more impaired quality of life than their male counterparts [33], we detected no sex differences in our patient cohort. This lack of effect might be attributed to our relatively small sample size.
A major limitation of our study is the lack of objective measurements. Although our results suggest that disrupted sleep is a mechanism underlying poor quality of life, impaired memory, and affective dysregulation in AD patients, all of the information is self-reported; to corroborate our findings, studies using objective measures should be conducted. For instance, the specific memory deficits experienced by AD patients must be assessed using standardized neuropsychological instruments. Similarly, polysomnographic studies are needed to confirm the presence, and nature, of disrupted sleep architecture in AD patients.
Another limitation of our study is its small sample size, particularly in relation to the statistical analyses that we used. However, sample size in structural equation modeling is a complex topic on which there is no general consensus. Estimates for minimum sample size range from 50 to 500 participants [34]. The larger estimates ensure sufficient statistical power, generalizability, and accuracy of parameter estimates across a range of conditions that impact statistical power. To get the most out of our rare participants we used reliable measures, tested a relatively simple model, and shied away from empirically modifying the models a posteriori. These tactics maximized statistical power in our relatively small sample. In such cases, it has been argued that 100 participants is sufficient to obtain a convergent solution.
Moreover, our small patient population was extremely heterogeneous. They were sampled across a large age range; some women had experienced menopause, and others were using oral contraceptives, and they were not screened for neuropsychiatric illness. All of these variables might affect sleep quality and architecture [35].
Finally, we note that our results might have been influenced by the presence of survivor and volunteer biases in our sample. Regarding the former, 19 of the 174 patients registered in the SAAD database had died before the onset of our study. We have no information about these individuals' cause of death, or about their quality of life, mood, sleep, and cognition in the years preceding their death. Regarding possible volunteer biases, 11 potential AD participants declined involvement in the study, 42 did not respond after a questionnaire had been posted to them, and 21 were not contactable. Again, we have no information about why these individuals either were not willing to participate, or why the database contained no accurate contact information for them. There did not appear to be any systematic reason (e.g., age, race/ethnicity, language, and time since diagnosis) for these individuals' non-participation, however.
5. Conclusion
In summary, we found that, consistent with previously published studies, South African AD patients report poorer quality of life, sleep quality, mood, and cognition than matched healthy controls. The novel contribution of our study, however, is that it reports data suggesting that disrupted sleep may be a biological mechanism underlying the abovementioned deficits. Intervention studies and clinical trials might seek to confirm this suggestion. Such confirmation has important implications for the treatment of AD, in that it will encourage health professionals to identify whether disrupted sleep is present, and to prioritize its treatment.
HIGHLIGHTS.
In latent variable models, Addison's disease directly affected quality of life.
The indirect effect of sleep on quality of life was significantly greater, however.
AD had no direct effect on memory, but the indirect effect of sleep did.
Disrupted sleep may underlie behavioral, cognitive, and affective complaints in AD.
Acknowledgments
This study was funded by the University of Cape Town's Doctoral Scholarship, the National Research Foundation of South Africa, the South African Medical Research Council, and the Ernst and Ethyl Eriksen Trust. The project described was also partially supported by Award Numbers T32 DA017629 and P50 DA10075 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Conflicts of interest and financial disclosure
We declare no financial or other conflicts of interest.
References
- [1].Tytherleigh MY, Vedhara K, Lightman SL. Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison's disease. Psychoneuroendocrinology. 2004;29:712–723. doi: 10.1016/S0306-4530(03)00103-3. http://dx.doi.org/10.1016/S0306-4530(03)00103-3. [DOI] [PubMed] [Google Scholar]
- [2].Ten S, New M, Maclaren N. Addison's disease 2001. J. Clin. Endocrinol. Metab. 2001;86:2909–2922. doi: 10.1210/jcem.86.7.7636. http://dx.doi.org/10.1210/jcem.86.7.7636. [DOI] [PubMed] [Google Scholar]
- [3].Lovas K, Loge JH, Husebye ES. Subjective health status in Norwegian patients with Addison's disease. Clin. Endocrinol. 2002;56:581–588. doi: 10.1046/j.1365-2265.2002.01466.x. http://dx.doi.org/10.1046/j.1365-2265.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- [4].Lovas K, Curran S, Oksnes M, Husebye ES, Huppert FA, Chatterjee VK. Development of a disease-specific quality of life questionnaire in Addison's disease. J. Clin. Endocrinol. Metab. 2010;95:545–551. doi: 10.1210/jc.2009-1711. http://dx.doi.org/10.1210/jc.2009-1711. [DOI] [PubMed] [Google Scholar]
- [5].Lovas K, Husebye ES, Holsten F, Bjorvatn B. Sleep disturbances in patients with Addison's disease. Eur. J. Endocrinol. 2003;148:449–456. doi: 10.1530/eje.0.1480449. http://dx.doi.org/10.1530/eje.0.1480449. [DOI] [PubMed] [Google Scholar]
- [6].Hahner S, Loeffler M, Fassnacht M, Weismann D, Koschker AC, Quinkler M, Decker O, Arlt W, Allolio B. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J. Clin. Endocrinol. Metab. 2007;92:3912–3922. doi: 10.1210/jc.2007-0685. http://dx.doi.org/10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]
- [7].Thomsen AF, Kvist TK, Andersen PK, Kessing LV. The risk of affective disorders in patients with adrenocortical insufficiency. Psychoneuroendocrinology. 2006;31:614–622. doi: 10.1016/j.psyneuen.2006.01.003. http://dx.doi.org/10.1016/j.psyneuen.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [8].García-Borreguero D, Wehr TA, Larrosa O, Granizo JJ, Hardwick D, Chrousos GP, Friedman TC. Glucocorticoid replacement is permissive for rapid eye movement sleep and sleep consolidation in patients with adrenal insufficiency 1. J. Clin. Endocrinol. Metab. 2000;85:4201–4206. doi: 10.1210/jcem.85.11.6965. http://dx.doi.org/10.1210/jcem.85.11.6965. [DOI] [PubMed] [Google Scholar]
- [9].Gillin J, Jacobs L, Snyder F, Henkin R. Effects of ACTH on the sleep of normal subjects and patients with Addison's disease. Neuroendocrinology. 1974;15:21–31. doi: 10.1159/000122289. http://dx.doi.org/10.1159/000122289. [DOI] [PubMed] [Google Scholar]
- [10].Diekelmann S, Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. http://dx.doi.org/10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- [11].Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol. Psychiatry. 2006;60:1324–1330. doi: 10.1016/j.biopsych.2006.03.051. http://dx.doi.org/10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- [12].Walker MP. The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. http://dx.doi.org/10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- [13].Cartwright R, Baehr E, Kirkby J, Pandi-Perumal S, Kabat J. REM sleep reduction, mood regulation and remission in untreated depression. Psychiatry Res. 2003;121:159–167. doi: 10.1016/s0165-1781(03)00236-1. http://dx.doi.org/10.1016/S0165-1781(03)00236-1. [DOI] [PubMed] [Google Scholar]
- [14].Payne JD, Nadel L. Sleep, dreams, and memory consolidation: the role of the stress hormone cortisol. Learn. Mem. 2004;11:671–678. doi: 10.1101/lm.77104. http://dx.doi.org/10.1101/lm.77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Henry M, Thomas KG. Episodic memory impairment in Addison's disease: results from a telephonic cognitive assessment. Metab. Brain Dis. 2014;1-10 doi: 10.1007/s11011-014-9511-x. http://dx.doi.org/10.1007/s11011-014-9511-x. [DOI] [PubMed] [Google Scholar]
- [16].Ross I, Boulle A, Soule S, Levitt N, Pirie F, Karlsson A, Mienie J, Yang P, Wang H, She JX. Autoimmunity predominates in a large South African cohort with Addison's disease of mainly European descent despite long-standing disease and is associated with HLA DQB* 0201. Clin. Endocrinol. 2010;73:291–298. doi: 10.1111/j.1365-2265.2010.03807.x. http://dx.doi.org/10. 1111/j.1365-2265.2010.03807.x. [DOI] [PubMed] [Google Scholar]
- [17].Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30:473–483. [PubMed] [Google Scholar]
- [18].Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- [19].Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. http://dx.doi.org/10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [20].Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Brit. J. Clin. Psychol. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. Pt 1. http://dx.doi.org/10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- [21].Bentler PM, Chou C-P. Practical issues in structural modeling. Sociol. Methods Res. 1987;16:78–117. http://dx.doi.org/10.1177/0049124187016001004. [Google Scholar]
- [22].Gorsuch RL. Factor Analysis. Lawrence Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- [23].Lovas K, Husebye ES. High prevalence and increasing incidence of Addison's disease in western Norway. Clin. Endocrinol. 2002;56:787–791. doi: 10.1046/j.1365-2265.2002.t01-1-01552.x. http://dx.doi.org/10.1046/j.1365-2265.2002.t01-1-01552.x. [DOI] [PubMed] [Google Scholar]
- [24].Ross IL, Levitt NS. Addison's disease symptoms—a cross sectional study in urban South Africa. PLoS One. 2013;8:e53526. doi: 10.1371/journal.pone.0053526. http://dx.doi.org/10.1371/web.pone.0053526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hatcher L. A Step-by-step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. SAS Institute; 1994. [Google Scholar]
- [26].Depner CM, Stothard ER, Wright KP., Jr. Metabolic consequences of sleep and circadian disorders. Curr. Diab. Rep. 2014;14:1–9. doi: 10.1007/s11892-014-0507-z. http://dx.doi.org/10.1007/s11892014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Winzeler K, Voellmin A, Schäfer V, Meyer AH, Cajochen C, Wilhelm FH, Bader K. Daily stress, presleep arousal, and sleep in healthy young women: a daily life computerized sleep diary and actigraphy study. Sleep Med. 2014;15:359–366. doi: 10.1016/j.sleep.2013.09.027. http://dx.doi.org/10.1016/j.sleep.2013.09.027. [DOI] [PubMed] [Google Scholar]
- [28].Stickgold R. Sleep, Memory and Learning, An Issue of Sleep Medicine Clinics. Elsevier Health Sciences; 2011. [Google Scholar]
- [29].Harbeck B, Kropp P, Mönig H. Effects of short-term nocturnal cortisol replacement on cognitive function and quality of life in patients with primary or secondary adrenal insufficiency: a pilot study. Appl. Psychophy. Biof. 2009;34:113–119. doi: 10.1007/s10484-009-9082-5. http://dx.doi.org/10.1007/s10484-009-9082-5. [DOI] [PubMed] [Google Scholar]
- [30].Almeida OP, Pfaff JJ. Sleep complaints among older general practice patients: association with depression. Brit. J. Gen. Pract. 2005;55:864–866. [PMC free article] [PubMed] [Google Scholar]
- [31].Cartwright R, Luten A, Young M, Mercer P, Bears M. Role of REM sleep and dream affect in overnight mood regulation: a study of normal volunteers. Psychiatry Res. 1998;81:1–8. doi: 10.1016/s0165-1781(98)00089-4. http://dx.doi.org/10.1016/S0165-1781(98)00089-4. [DOI] [PubMed] [Google Scholar]
- [32].Bleicken B, Hahner S, Loeffler M, Ventz M, Decker O, Allolio B, Quinkler M. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin. Endocrinol. 2010;72:297–304. doi: 10.1111/j.1365-2265.2009.03596.x. http://dx.doi.org/10.1111/j.1365-2265.2009.03596.x. [DOI] [PubMed] [Google Scholar]
- [33].Kluger N, Matikainen N, Sintonen H, Ranki A, Roine RP, Schalin-Jäntti C. Impaired health-related quality of life in Addison's disease—impact of replacement therapy, co-morbidities and socioeconomic factors. Clin. Endocrinol. 2014;81:511–518. doi: 10.1111/cen.12484. http://dx.doi.org/10.1111/cen.12484. [DOI] [PubMed] [Google Scholar]
- [34].Iacobucci D. Structural equations modeling: fit indices, sample size, and advanced topics. J. Consum. Psychol. 2010;20:90–98. http://dx.doi.org/10.1016/j.jcps.2009.09.003. [Google Scholar]
- [35].Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]