Abstract
Men and women may differ in their sensitivity to the mood-modulating properties of nicotine. Male and female adult smokers were exposed to four sessions crossing two nicotine deprivation conditions (12-hr deprived vs. nondeprived) with two drug conditions (nicotine vs. placebo nasal spray). Acoustic probes elicited startle eyeblink responses while viewing affective and cigarette-related slides. In-session mood ratings were collected to gauge self-reported negative affect, positive affect, and craving. Nicotine nasal spray reduced startle amplitude in both men and women following 12-hr deprivation compared with smoking nondeprivation. During nondeprivation, nicotine nasal spray increased startle amplitude in women compared with placebo spray, whereas no difference was found for men. The startle results suggest that both men and women are responsive to the hedonic properties of nicotine.
Keywords: nicotine, gender differences, startle, affect, smoking deprivation
1. Introduction
Women may be less likely than men to abstain from smoking after trying to quit on their own or after participating in smoking cessation therapy (e.g., Fiore et al., 2000; Perkins, 1996; Wetter et al., 1999), though this gender difference in abstinence rates has not been universally found (e.g., Gourlay, Forbes, Marriner, Pethica, & McNeil, 1994; Stapleton et al., 1995). Other evidence suggests that this gender difference in abstinence rates following nicotine replacement therapy does not emerge until long-term follow-up (Bjornson et al., 1995; Cepeda-Benito, Reynoso, & Erath, 2004). Several hypotheses have been forwarded to explain this gender discrepancy, including greater perceived benefits of weight control due to smoking (Gritz, 1986; Waldron, 1991), lower socioeconomic status (Bjornson et al., 1995), stronger withdrawal symptoms (Shiffman, 1979), differential sensitivity to or metabolism of nicotine (Grunberg, Winders, & Wewers, 1991), and increased impact of negative affect on smoking outcomes (Borelli, Bock, King, Pinto, & Marcus, 1996) in women than men.
Negative affect has been linked to nicotine use and relapse (e.g., Baker, Piper, McCarthy, Majeskie, & Fiore, 2004), and explanations involving negative affect may be particularly important as women are at greater risk to experience negative affect than men (Angst, 1992). The presence of negative affect may accompany over 50% of all smoking lapses, and high levels of negative mood are predictive of failure to quit and relapse after short-term abstinence (e.g., Baer & Lichtenstein, 1988; Brandon, Tiffany, Obremski, & Baker, 1990; Piasecki, Kenford, Smith, Fiore, & Baker, 1997; Shiffman, 1986). Additionally, the use of nicotine to control negative affect and depression is often postulated as the reason that many smokers find it difficult to remain abstinent (see Brandon, 1994; Hall, Reus, Munoz, & Sees, 1993, for review), and expectancies for negative affect reduction has been found to predict relapse (Wetter et al., 1994). Therefore, it is important to investigate the role affect regulation in gender patterns of nicotine use and relapse.
Results from studies examining various nicotine reinforcement explanations for the gender discrepancy in quitting and relapsing have largely been equivocal. Most (e.g., Breslau, Kilbey, & Andreski, 1992; Pomerleau & Pomerleau, 1994) but not all (Shiffman, 1979) studies have found no gender differences in nicotine withdrawal symptoms. Nicotine administration has been found to be less effective in ameliorating withdrawal symptoms in women than in men (Hatsukami, Skoog, Allen, & Bliss, 1995; Killen, Fortmann, Newman, & Varady, 1990), despite findings that women tend to consume less nicotine (e.g., Goldberg et al., 1993; Killen et al., 1990) and report less nicotine dependence than men (Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994; Perkins, 1996). Additionally, compared with males, female smokers have shown decreased nicotine metabolism (Beckett, Gorrod, & Jenner, 1971; Benowitz & Jacob, 1984), decreased accuracy in discriminating among nicotine dosages (Perkins, 1999), and decreased ability to regulate nicotine intake in the absence of exteroceptive nicotine cues (Perkins, 1996) than men.
These seemingly contradictory findings suggest that differences in nicotine reinforcement alone cannot account for gender differences in nicotine response. Perkins (1996) proposed that men and women might differ in the extent to which they smoke for non-nicotine reinforcement. Women may be more responsive than men to stimuli associated with the act of smoking or to secondary social reinforcement rather than for nicotine reinforcement (e.g., socializing, weight loss). Thus, women’s responses to nicotine may be mediated by the cues surrounding drug delivery to a greater extent than men.
The ability to identify gender differences in the effects of nicotine on affect may be limited by how these differences are measured. Most studies have largely relied on self-report measures following short-term deprivation (e.g., Hall, Muñoz, & Reus, 1994; Hutchison, Niaura, & Swift, 1999) or retrospective self-report following relapse (e.g., Marlatt & Gordon, 1980; Shiffman, 1982). These studies have yielded important findings regarding the relationship between smoking behavior, relapse, and negative mood. However, self-report measures require extensive cognitive processing and may not be sensitive to the subtle yet important changes in affect that are brought about by nicotine. Additionally, self-report may be subject to gender differences in terms of awareness of bodily responses (Roberts & Pennebaker, 1995) or in terms of biases in the approach to self-report (Waldron, 1983).
A more informative means of determining gender differences in the effects of nicotine on affect may be measuring emotion-relevant modalities in addition to self-report, such as physiological reactions to emotional stimuli. One such measure is the acoustic startle eyeblink response, which is sensitive to ambient emotional cues (see Bradley, Cuthbert, & Lang, 1999, for review). Startle responses to emotional stimuli are typically assessed by measuring the strength of the orbicularis oculi electromyogram (EMG) following an unexpected acoustic probe (80–115 dB). The strength of the eyeblink response to the acoustic probe varies in accordance with the emotional content (valence) of the slide. For example, blinks are larger when subjects view unpleasant rather than pleasant pictures (e.g., Cook, Davis, Hawk, Spence, & Gautier, 1992; Vrana, Spence, & Lang, 1988) and are smaller when participants view pictures of positive as opposed to negative or neutral valence (Cuthbert, Bradley, & Lang, 1996). The acoustic startle response has been used to study the affective properties of smoking cues compared with positive and negative cues, though gender analyses have not typically been reported (e.g., Elash, Tiffany, & Vrana, 1995; Geier, Mucha, & Pauli, 2000).
This paper is based on secondary analyses of data from a study in which startle probe methodology was used to examine the effects of nicotine administration and deprivation on emotional processes associated with motivation (Cinciripini et al., in press). In that study, smokers completed four laboratory sessions that counterbalanced deprivation (12-hour deprived vs. nondeprived) with nicotine spray (active vs. placebo). During these sessions smokers viewed affective pictures (positive, negative, neutral) and pictures involving cigarette cues while intermittent startle probes were presented. The results showed that smoking deprivation decreased startle response to cigarette cues, that nicotine nasal spray administration suppressed overall startle response during smoking deprivation, and that during deprivation, decreased adaptation of the startle response occurred during negative pictures. These results suggested that withdrawal may both increase the reinforcement salience of smoking stimuli and sensitize smokers to negative emotional cues.
In this paper, we describe the differential effects of nicotine by gender by assessing the affective impact of acute nicotine administration and deprivation on both the startle eyeblink response and self-report measures of affect in adult male and female smokers. First, we examined the effects of gender, acute nicotine administration using nasal spray, and 12-hr nicotine deprivation on startle eyeblink response to affective slides in cigarette smokers. Second, we examined the effects of gender, nicotine nasal spray, and smoking deprivation on self-reported mood and craving during affective slide viewing. Third, we evaluated the effects of gender, acute nicotine administration, and 12-hr nicotine deprivation on saliva nicotine. Finally, we examined whether gender interacted with nicotine deprivation to predict self-reported withdrawal symptoms.
2. METHOD
2.1. Design
This study used a within-subjects design with manipulations of pre-session nicotine deprivation and within-session nicotine administration to evaluate affective response using startle eyeblink amplitude and self-report. Smokers attended an orientation session and four 90-min laboratory assessment sessions scheduled approximately three days apart. The four sessions provided a complete crossing of two pre-laboratory deprivation conditions (12-hour deprived vs. nondeprived) with two drug conditions (nicotine vs. placebo nasal spray). Self-reports of negative affect and nicotine craving were obtained before each laboratory session. Two blocks of trials were presented in each session. The first was a habituation block, which was used to acclimate the participant to the novel effects of the startle procedure, nasal spray administration, and general laboratory surroundings. All participants received placebo spray before this block. The second (test) block was similar to the habituation block in terms of slide content. Smokers received either nicotine or placebo nasal spray before the test block of trials. During each session, smokers viewed two blocks of affective slides and were administered acoustic probes to elicit startle eyeblink responses during viewing of positive, negative, neutral, and cigarette slides. In-session mood and craving ratings were measured after every third slide during picture viewing.
2.2. Participants
One hundred fifteen cigarette smokers (63 men, 52 women) were recruited using newspaper advertisements from the Houston metropolitan area and included in these analyses. They were paid $125 for attending one orientation and four laboratory sessions. Smokers who were between the ages of 18 and 59, smoked 10 or more cigarettes per day, produced an expired carbon monoxide (CO) level greater than 8 ppm, were fluent in English, and had no uncontrolled medical illness were included in the study. Individuals were excluded if they were taking psychotropic or narcotic medication, met criteria for a current psychiatric disorder, reported hearing loss, or were involved in current smoking cessation activity. Twenty-four additional participants met the inclusionary criteria but were excluded from the analyses due to experimental noncompliance, including use of exclusionary substances, failure to abstain from smoking on their deprivation sessions, completion of only a single lab session, or because of a startle nonresponse/missing rate greater than 30%.
2.3. Procedure
2.3.1. Screening and Orientation
All smokers were initially screened in a 40-min telephone interview to establish their eligibility for the study. Potential participants were administered a telephone version of the PRIME-MD semi-structured interview (Spitzer et al., 1994), which screened for major mental disorders and substance use. Participants who were deemed eligible after the telephone screening attended an orientation session and completed questionnaires concerning their demographic, health, mood, and smoking history. These questionnaires included the Center for Epidemiologic Studies’ Depression Scale (CES-D; Radloff, 1977) and the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Out of 263 eligible callers, 193 were screened and 115 completed more than one laboratory session (109 completed all 4 sessions).
2.3.2. Laboratory Sessions
Figure 1 depicts the timeline of events for a typical laboratory session.
Figure 1.

Diagram of a typical laboratory session.
2.3.2.1. Smoking deprivation and assessment
Before the smoking deprivation sessions, the participants were instructed to refrain from smoking for 12 hr before their laboratory visit, and compliance with these nicotine deprivation instructions was assessed using expired CO to confirm abstinence. Smokers were required to produce a CO level below 10 ppm or 50% of their baseline level, which was assessed during the orientation session. Participants nonabstinent during deprived sessions were rescheduled. Smoking was unrestricted before the nondeprived sessions, with subjects being told to smoke their typical amount. To ensure similar conditions of nondeprivation before the startle probe trials, participants in the nondeprived session smoked one cigarette of their own brand. Participants next completed the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) during all laboratory sessions. Participants were asked to limit their intake of caffeinated beverages to no more than two cups taken before 8:00 AM on the day of the laboratory session1. All sessions were run at either 9 AM or 11 AM.
2.3.2.2. Physiological measurement
After the participants completed the WSWS, electrodes (Ag-AgCl) filled with saline gel were attached in each participant’s right orbicularis oculi region (Fridlund & Cacioppo, 1986) to record startle eyeblink EMG. This signal was acquired and amplified with BIOPAC Systems’ (Goleta, CA) EMG100A module connected to BIOPAC Systems’ MP100WSW bioamplifier with a time constant of 1000-ms. Data were recorded and displayed using BIOPAC Systems’ AcqKnowledge III data acquisition software. After the sensors were attached, participants rested quietly for 5 minutes to become habituated to the environment. Startle response was manually scored offline in peak microvolts (µV) using the data acquisition software. Trials with clear movement artifact or excessive baseline activity were marked as missing as were trials with no blink (zero startle trials). Overall, 6.8 % of the participants’ startle responses (range, 0% - 27%) were excluded from analysis because they were scored as either a nonresponse or missing because of an unstable baseline.
2.3.2.3. Affective slide viewing
Forty-eight color slides were presented, twelve each from the categories of positive, neutral, negative, and cigarette. The positive, neutral, and negative slides were selected from the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention, 1999). The negative slides were selected from the negatively valent, high arousal (interest) category, the neutral slides were selected from the mildly positively valent, low arousal category, and the positive slides were selected from the positively valent, high arousal category. High arousal positive and negative slides were chosen because differences between the two valences appear strongest for highly arousing stimuli (Cuthbert et al., 1996; Witvliet & Vrana, 1995). The cigarette slides, consisting of smoking cues such as images of burning cigarettes and people smoking in a social context, were created for this experiment and are validated elsewhere (Carter et al., in press). A PC using Psychology Tools’ Eprime software (Pittsburgh, PA) digitally projected a 91.5 cm x 122 cm image of the slides, on a screen approximately 1.5 m from the participant.
Two separate blocks of trials (habituation and test), each consisting of 24 slides, were presented using procedures similar to those used in previous studies (Cuthbert et al., 1996; Smith, Bradley, & Lang, 2005). Each block of 24 slides included 6 slides of each valence. Each slide was presented for 6 s and was then followed by a randomly determined inter-slide interval that varied from 10 to 20 s. Four sessions, each comprised of a distinct sequence of slides, were counterbalanced between subjects. The order of slide presentation in each slide sequence was counterbalanced so that participants saw each picture equally often in the first through sixth positions within each block and so that each block occurred in the first or second position with equal frequency. Slide viewing with accompanying startle probes took approximately 45 minutes. At the fourth session, after both blocks of slides were completed, the slides were repeated in a free-viewing procedure and rated2.
2.3.2.4. Startle probe presentation
The acoustic startle stimulus consisted of a 50-ms 100-dB(A) presentation of white noise with instantaneous rise time. The startle stimuli were generated and amplified by Psychology Tools’ Eprime software. The noise burst was presented binaurally with Sennheiser (Wedemark, Germany) HD500 headphones. A startle probe was presented during 16 of the 24 slides in each block, at a random time of 2.5 to 5 s after slide onset, such that a probe was presented during 4 of the 6 slides within each valence condition. Counterbalancing for slide sequence was arranged so that, across subjects, each slide would be associated with a startle probe equally often. Each session began with four test startle probe trials executed before the first slide of each session. These trials were not included in the data analysis. In addition, to enhance the unpredictability of the startle presentation, eight startle probes were presented during inter-slide intervals in each block.
2.3.2.5. In-session mood and craving ratings
After every third slide, the participants were asked to use a five-point scale to rate their agreement with three statements that were presented in a random order on the same screen as the affective slides. The first statement asked about positive mood state; the second about negative mood state; and the third about craving to smoke3. Within each block, two ratings were collected for each slide valence to provide self-report measure of the impact of the slide stimuli. The participants were instructed to base their ratings on how they felt “at that moment.” The three ratings took less than 15 s to complete followed by the immediate resumption of the slide viewing task.
2.3.2.6. Nasal spray administration
The participants were administered nasal spray twice during each session, once before each of the two slide blocks. After the initial 5-min rest period before block 1, the habituation block, the participants always received placebo nasal spray. The habituation block allowed the participants to adapt to the aversiveness of the nasal spray and startle probes. This was followed by a 5-min absorption period. After this absorption period, the first block of slides was presented and physiological recording was initiated. The smokers received either nicotine nasal spray (1 mg) or placebo spray (0 mg), depending on the condition, 5 min before block 2 (the test block). The participants self-administered one spray per nostril (either 0 or 0.5 mg nicotine per spray). The study used a single-blind design; participants were blind to the content of the spray. The nicotine spray is commercially available from Pharmacia & Upjohn, Inc. (Peapack, NJ) and marketed as a nicotine replacement product (Nicotrol NS). Peak arterial levels of nicotine of about 10 ng/ml occur within 5 min of a single application (0.5 mg/nostril) of the nasal spray (Gourlay & Benowitz, 1997). The placebo spray, also provided by Pharmacia & Upjohn, contained piperine.
2.3.2.7. Saliva Nicotine Levels
Salivary nicotine samples were obtained on each of the four sessions 5 min after spray administration, before the habituation block (block 1, placebo only) and to the test block (block 2, placebo or nicotine). We used these samples to evaluate whether there were any systematic gender differences in nicotine saliva levels following overnight deprivation and nicotine nasal spray administration and whether the nicotine spray increased nicotine saliva levels compared to both placebo spray and smoking ad lib. The samples were analyzed for nicotine using the High Performance Liquid Chromatography method of Hariharan & VanNoord (1991). The assay provides sensitivity to <1 ng/ml of nicotine.
2.4. Data Reduction and Analysis
Four types of data were collected during the laboratory session: startle response, in-session mood ratings, saliva nicotine values, and WSWS scores. With the startle response, each cell of the 2 (Gender) x 2 (Nasal Spray) x 2 (Deprivation Status) x 4 (Slide Valence) design was composed of four observations. Each set of in-session mood ratings occurred eight times per block, with the slide shown immediately before the ratings counterbalanced across all four picture types. Analyses for the in-session mood ratings were generated using the model described above for the startle analyses. Saliva nicotine was collected once before the experimental block (block 2) during each of the four sessions of the 2 (Nasal Spray) x 2 (Deprivation Status) design. The WSWS questionnaire scales administered before each laboratory session were subject to separate 2 (Gender) x 2 (Deprivation Status) analyses.
Additional analyses were performed on the above models that included the habituation block (block 1) as a covariate. The habituation block was included in the design to allow the participant to become accustomed to the laboratory environment, the potential novelty effects of the startle procedure, and the aversiveness of the nasal spray. These covariate analyses were used to examine adaptation in the startle response across the two blocks, which in this case could be influenced by either nicotine spray or placebo (given in block 2) or deprivation. This approach has several methodological advantages over computing a difference score between the two blocks (Mulligan & Wiesen, 2003).
Repeated measures data analyses on startle amplitude, in-session mood and craving ratings, and WSWS scores were conducted using the SAS procedure Proc Mixed (version 8.2, SAS Institute Inc, Cary, NC) with subjects as a random effect. The mixed model approach is a form of the generalized linear model that allows for more specific estimation of the correlation structure of the residuals. Unlike traditional ANOVA, mixed models uses a maximum likelihood approach and does not exclude cases with missing observations, thereby allowing the use of all available data (Bageilla, Sloan, & Heitjan, 2000). All descriptions of differences between means following a significant mixed model multilevel effect were the result of comparisons of least-square means of fixed effects using SAS contrast statements.
3. RESULTS
The main outcome and deprivation verification results are presented in Cinciripini et al. (in press). The following analyses only include secondary analyses of gender interactions and main effects.
3.1. Participant Characteristics
The sample was compared by gender on baseline measures of age, number of daily cigarettes smoked, expired carbon monoxide (CO), body mass index (BMI), body mass, CES-D and the FTND using univariate ANOVA. Table 1 shows that men and women differed only on baseline CO and BMI, with men producing larger CO readings and having a smaller BMI than women.
Table 1.
Participant Demographic and Baseline Smoking Data.
| Measure | Male | Female | Total |
|---|---|---|---|
| Mean Age (SD) | 38.76 (10.79) | 35.29 (10.89) | 37.18 (10.88) |
| Ethnicity/Race | |||
| Euro-American | 25 (39.7%) | 23 (44.2%) | 48 (41.7%) |
| African-American | 34 (54.0%) | 22 (42.3%) | 56 (48.7%) |
| Other | 4 (6.3%) | 7 (13.5%) | 11 (9.6%) |
| Occupation | |||
| Employed | 40 (63.5%) | 32 (61.5%) | 72 (62.6%) |
| Unemployed | 23 (36.5%) | 20 (38.5%) | 37 (37.4%) |
| Education | |||
| High School or less | 26 (41.3%) | 17 (32.7%) | 43 (37.4%) |
| Some College | 30 (47.6%) | 31 (59.6%) | 61 (53.0%) |
| College Degree | 7 (11.1%) | 4 (7.7%) | 11 (9.6%) |
| Mean Cigs (SD) | 23.34 (11.21) | 20.98 (9.81) | 22.26 (10.26) |
| Mean CO (SD) | 22.29 (9.53) | 18.42 (9.66) | 20.56 (9.70)* |
| Mean FTND (SD) | 4.82 (2.12) | 5.22 (2.27) | 5.00 (2.18) |
| Mean CES-D (SD) | 10.98 (7.95) | 11.32 (8.70) | 11.07 (8.69) |
| Mean BMI (SD) | 25.86 (4.23) | 28.41 (7.61) | 27.03 (6.11)* |
| Mean Mass, kg (SD) | 82.48 (14.56) | 78.84 (22.93) | 80.81 (18.79) |
| Total Subjects | 63 (54.8%) | 52 (45.2%) | 115 |
Note.: p<05
As can be seen in Table 1, 58.3% of the participants were members of a minority group, with 48.7% being African-American. Education and occupation data suggested that a significant portion of the participants came from lower socioeconomic backgrounds, with 37.4 % of the participants currently unemployed. On average, the participants in this study smoked about a pack a day.
3.2. Saliva Nicotine Levels
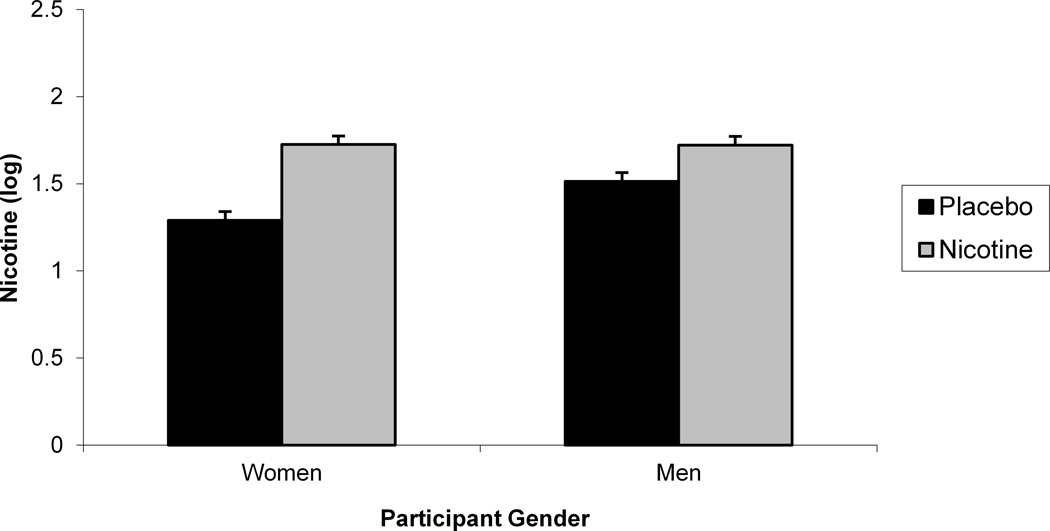
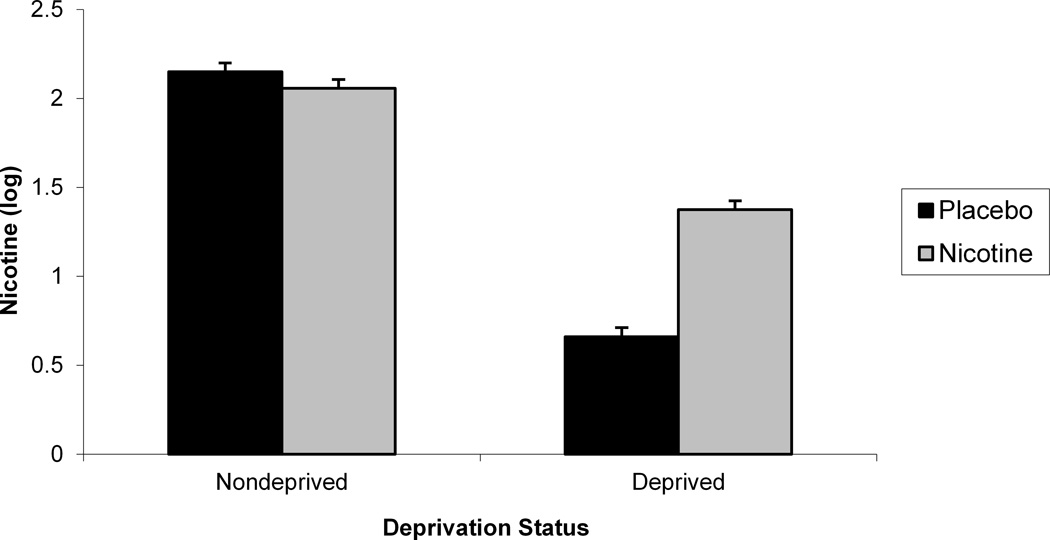
We evaluated whether there were any systematic gender differences in nicotine saliva levels following overnight deprivation and nicotine nasal spray administration and whether the nicotine spray increased nicotine saliva levels compared to both placebo spray and smoking ad lib by evaluating the interaction of gender, deprivation status, and nasal spray on block 2 nicotine (see Table 2). Upon examination of the distribution, nicotine values were found to be positively skewed and kurtotic (M = 221.71 ng/ml, SD = 376.64, range = 0–3610). To reduce the impact of outliers, we log transformed the nicotine values for this analysis. An interaction between gender and nasal spray was found, F (1, 108) = 6.02, p < .02. (see Figure 2). Post hocs indicated that nicotine nasal spray increased nicotine level for both men, F (1, 108) = 11.29, p < .002, and women, F (1, 108) = 39.80, p < .0001, but that women had lower nicotine values following placebo than men, F (1, 108) = 6.88, p = .01. We also found a significant Nasal Spray x Deprivation Status interaction on block 2 nicotine, F (1, 94) = 98.84, p < .0001 (see Figure 2). Post hocs indicated that during deprivation, nicotine nasal spray produced larger nicotine values compared with placebo spray, F (1, 94) = 153.95, p < .0001. However, during nondeprivation, nicotine values did not vary by nasal spray, likely due to the variability of nicotine values following ad libitum smoking (Cinciripini et al., in press). The significant deprivation status and nasal spray main effects are not discussed in light of their interaction. The results were not altered by inclusion of block 1 nicotine as a covariate.
Table 2.
Gender results of the saliva nicotine levels and startle eyeblink response analyses.
| Analysis | Df | F | p |
|---|---|---|---|
| Nicotine Results | |||
| Gender x Deprivation Status x Nasal Spray | 1,93 | 2.37 | <.13 |
| Gender x Deprivation Status | 1,108 | 0.09 | <.76 |
| Gender x Nasal Spray | 1,108 | 6.02 | <.02 |
| Deprivation Status x Nasal Spray | 1,94 | 98.84 | <.0001 |
| Gender | 1,112 | 2.45 | <.13 |
| Deprivation Status | 1,109 | 542.55 | <.0001 |
| Nasal Spray | 1,109 | 44.36 | <.0001 |
| Startle Results | |||
| Gender x Deprivation Status x Nasal Spray x Slide Valence | 3,318 | 0.67 | <.58 |
| Gender x Deprivation Status x Nasal Spray | 1,106 | 13.25 | <.0005 |
| Gender x Deprivation Status x Slide Valence | 3,336 | 0.34 | <.80 |
| Gender x Nasal Spray x Slide Valence | 3,339 | 0.97 | <.41 |
| Gender x Deprivation Status | 1,112 | 4.34 | <.04 |
| Gender x Nasal Spray | 1,113 | 9.78 | <.003 |
| Gender x Slide Valence | |||
| Gender | 1,113 | 4.96 | <.03 |
Figure 2.
A significant Gender x Nasal Spray interaction for block 2 saliva nicotine (a). A significant 2-way Deprivation Status x Nasal Spray interaction for block 2 saliva nicotine (b).
3.3. Startle Eyeblink Response
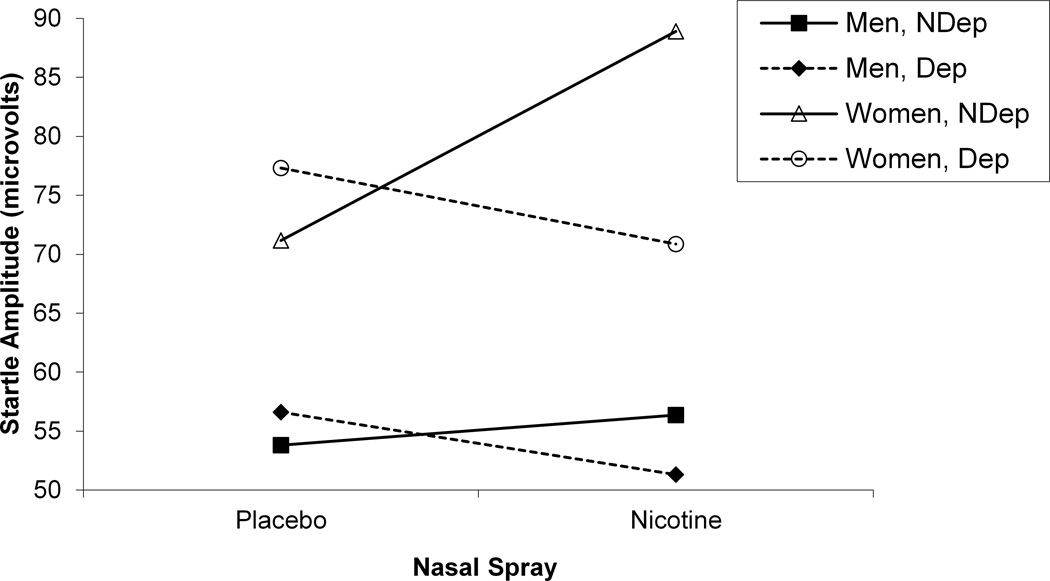
We examined the effects of gender, acute nicotine administration using nasal spray, and 12-hr nicotine deprivation on startle eyeblink response to affective slides in cigarette smokers using a 2 (Gender) x 2 (Nasal Spray) x 2 (Deprivation Status) x 4 (Slide Valence) mixed models analysis on block 2 startle amplitude (see Table 2). A significant 3-way Gender x Deprivation Status x Nasal Spray interaction was found, F (1, 106) = 13.25, p < .0005 (see Figure 3). During smoking deprivation, both men, F (1, 106) = 6.18, p < .02, and women, F (1, 106) = 7.69, p < .007, produced smaller startle amplitude following nicotine nasal spray compared with placebo nasal spray. However, during nondeprivation, nicotine nasal spray produced elevated startle amplitude compared with placebo in women, F (1, 106) = 56.62, p < .0001, while no difference was found for spray in nondeprived men. Significant Gender x Nasal Spray and Gender x Deprivation Status interactions were found, as was a gender main effect, but were not interpreted in light of the 3-way interaction.
Figure 3.
A significant 3-way Gender x Deprivation Status x Nasal Spray interactions for startle amplitude.
A second model, identical to the 4-way model described above, with the addition of block 1 startle response as a covariate, was also examined for the gender analyses reported above. As a habituation block, block 1 provided a within-session control for the nonspecific effects of the recording environment, novelty of the startle procedure, and the potential aversiveness of the nasal spray itself (everyone received placebo spray before this block). Covarying block 1 startle amplitude did not change the startle gender results observed for the test block alone.
To determine whether baseline differences in age, number of daily cigarettes smoked, level of expired CO, FTND score, CES-D score, BMI, and body mass could account for the gender differences in the startle response reported above, each of these seven variables, individually and as a group, was introduced into the mixed model analysis of the startle response. None of these baseline variables altered the significant 3-way interaction on startle response depicted in Figure 3.
3.4. In-Session Mood Ratings
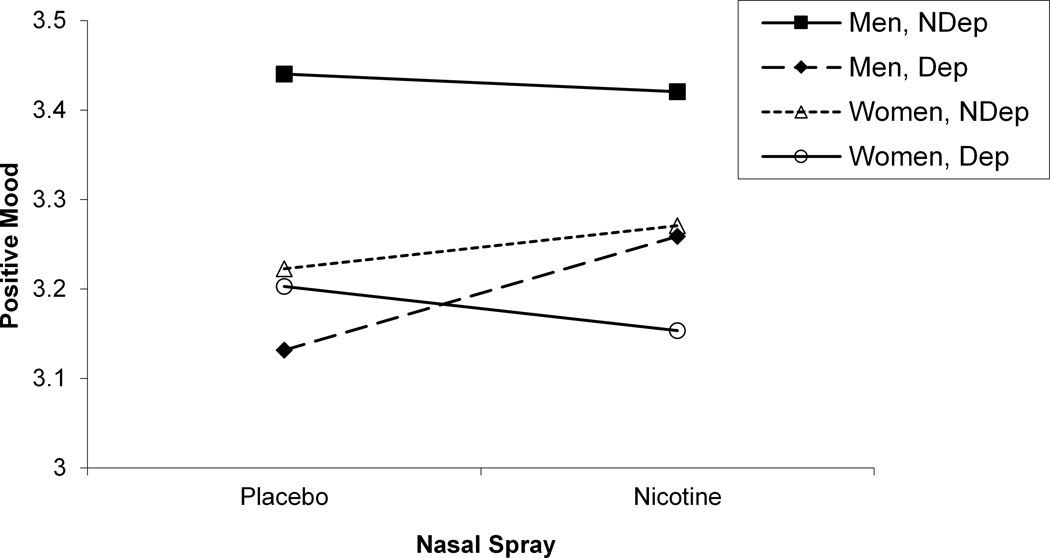
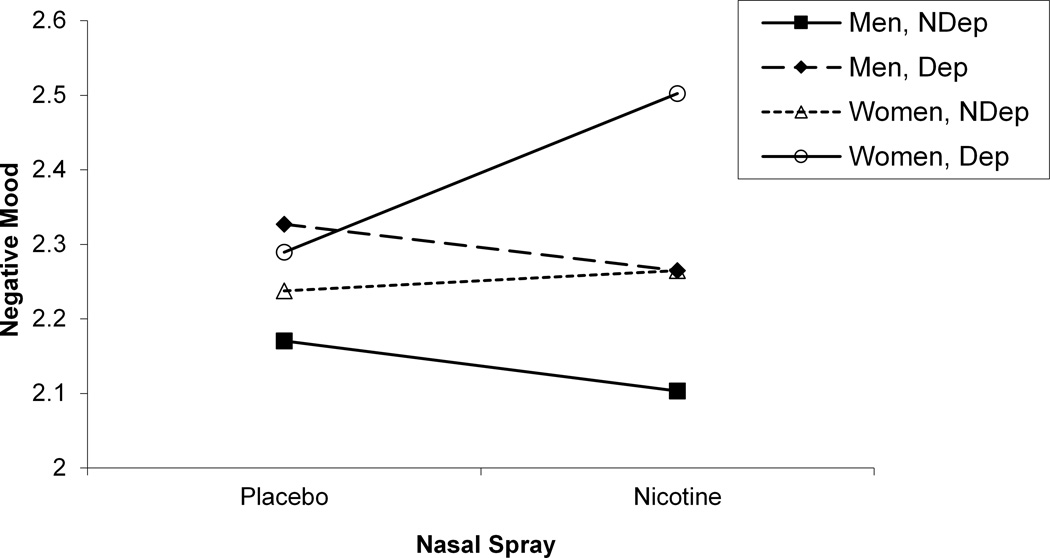
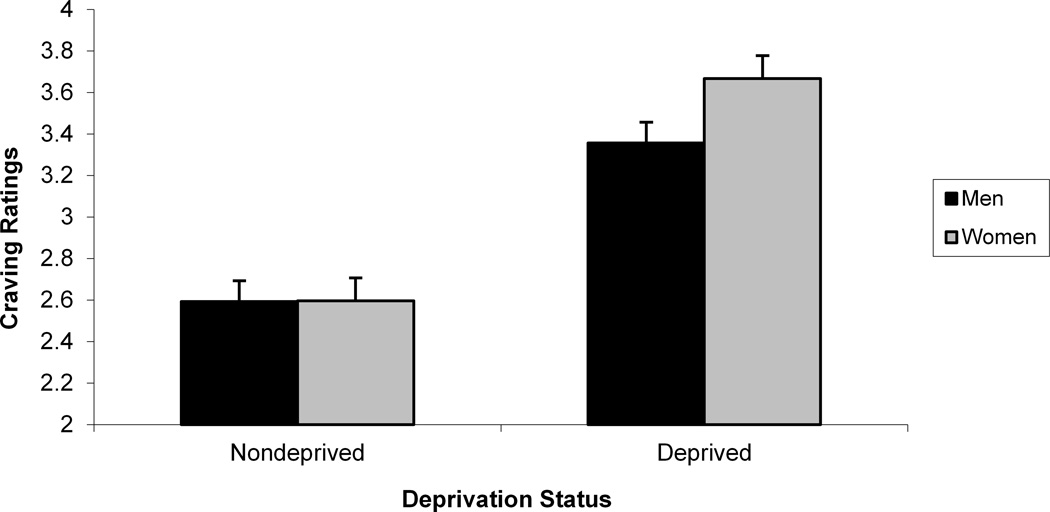
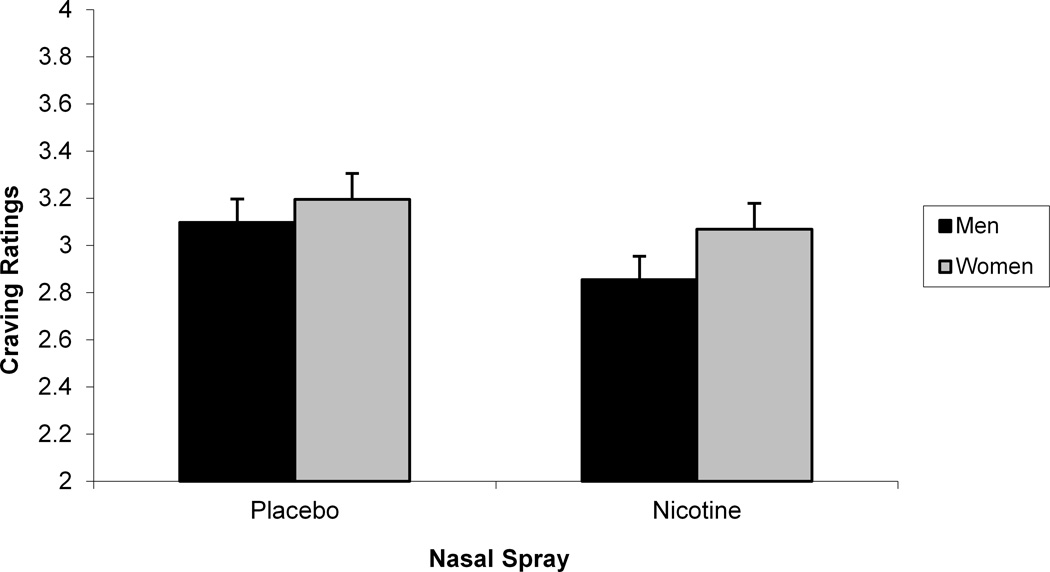
We next sought to determine whether self-reported mood and craving were sensitive to gender differences. As we did in our startle analyses, we evaluated the interaction between gender, deprivation status, nasal spray, and slide type separately for block 2 negative mood, positive mood, and craving ratings (see Table 3). For positive mood ratings, a significant Gender x Deprivation Status x Nasal Spray interaction was found, F (1, 105) = 9.04, p < .004 (see Figure 4). Post hoc tests of simple main effects indicated that for deprived men, nicotine nasal spray resulted in an increase in positive mood compared with placebo spray, F (1, 105) = 10.98, p < .002. No significant contrasts were found for women. For negative mood ratings, a significant Gender x Deprivation Status x Nasal Spray interaction was also found, F (1, 105) = 4.57, p < .04 (see Figure 4). Post hoc tests of simple main effects indicated that for deprived women, nicotine nasal spray resulted in an increase in negative mood compared with placebo spray, F (1, 105) = 22.38, p < .0001. No significant contrasts were found for men for the negative mood ratings. For craving ratings, Gender interacted with Deprivation Status, F (1, 112) = 36.15, p < .0001, with post hocs indicating that deprivation increased craving for both men, F (1, 112) = 501.55, p < .0001, and women, F (1, 112) = 800.55, p < .0001, and that women reported greater craving during deprivation than men, F (1, 112) = 4.36, p < .04 (see Figure 5). There was also a significant Gender x Nasal Spray interaction for craving ratings, F (1, 1113) = 5.09, p < .03, with post hoc tests of simple main effects indicating that nicotine spray decreased craving for both men, F (1, 113) = 50.30, p < .0001, and women, F (1, 105) = 11.14, p < .002 (see Figure 5). Additional analyses were run using block 1 in-session ratings as a covariate, but none of the above results was altered.
Table 3.
Gender results of the in-session mood and craving ratings analyses.
| Analysis | Df | F | p |
|---|---|---|---|
| In-Session Negative Mood Ratings Results | |||
| Gender x Deprivation Status x Nasal Spray x Slide Valence | 3,315 | 0.81 | <.50 |
| Gender x Deprivation Status x Nasal Spray | 1,105 | 4.57 | <.04 |
| Gender x Deprivation Status x Slide Valence | 3,336 | 0.24 | <.88 |
| Gender x Nasal Spray x Slide Valence | 3,339 | 0.62 | <.61 |
| Gender x Deprivation Status | 1,112 | 0.12 | <.73 |
| Gender x Nasal Spray | 1,113 | 17.78 | <.0001 |
| Gender x Valence | 3,339 | 0.08 | <.99 |
| Gender | 1,113 | 4.96 | <.03 |
| In-Session Positive Mood Ratings Results | |||
| Gender x Deprivation Status x Nasal Spray x Slide Valence | 3,315 | 0.86 | <.47 |
| Gender x Deprivation Status x Nasal Spray | 1,105 | 9.04 | <.004 |
| Gender x Deprivation Status x Slide Valence | 3,336 | 0.44 | 0.73 |
| Gender x Nasal Spray x Slide Valence | 3,339 | 0.22 | 0.86 |
| Gender x Deprivation Status | 1,112 | 16.92 | <.0001 |
| Gender x Nasal Spray | 1,113 | 1.77 | <.19 |
| Gender x Valence | 3,339 | 0.24 | <.88 |
| Gender | 1,113 | 0.84 | <.37 |
| In-Session Craving Ratings Results | |||
| Gender x Deprivation Status x Nasal Spray x Slide Valence | 3,315 | 0.33 | <.81 |
| Gender x Deprivation Status x Nasal Spray | 1,105 | 0.01 | <.91 |
| Gender x Deprivation Status x Slide Valence | 3,336 | 0.66 | <.58 |
| Gender x Nasal Spray x Slide Valence | 3,339 | 0.13 | <.95 |
| In-Session Craving Ratings Results | |||
| Gender x Deprivation Status | 1,112 | 36.15 | <.0001 |
| Gender x Nasal Spray | 1,113 | 5.09 | <.03 |
| Gender x Valence | 3,339 | 0.26 | <.86 |
| Gender | 1,113 | 1.11 | <.35 |
Figure 4.
Significant 3-way Gender x Deprivation Status x Nasal Spray interactions for in-session positive mood (a) and in-session negative mood (b).
Figure 5.
Significant 2-way Gender x Deprivation Status (a) and Gender x Nasal Spray (b) interactions for in-session craving ratings.
3.5. Session WSWS
We examined whether gender interacted with nicotine deprivation to predict self-reported withdrawal symptoms using a Gender x Deprivation Status model to separately analyze each scale of the WSWS (see Table 4). There were neither significant Gender x Deprivation Status interactions nor gender main effects for any of the seven WSWS scales. Additional analyses were carried out using WSWS ratings from the screening visit as covariates, but none of the results were altered.
Table 4.
Gender results of the session WSWS scales analyses.
| Analysis | Df | F | p |
|---|---|---|---|
| WSWS Anger Scale Results | |||
| Gender x Deprivation Status | 1,113 | 0.18 | <.68 |
| Gender | 1,113 | 0.41 | <.53 |
| WSWS Anxiety Scale Results | |||
| Gender x Deprivation Status | 1,113 | 1.92 | <.17 |
| Gender | 1,113 | 1.30 | <.26 |
| WSWS Concentration Scale Results | |||
| Gender x Deprivation Status | 1,113 | 1.54 | <.22 |
| Gender | 1,113 | 0.37 | <.55 |
| WSWS Craving Scale Results | |||
| Gender x Deprivation Status | 1,113 | 0.76 | <.11 |
| Gender | 1,113 | 0.66 | <.42 |
| WSWS Hunger Scale Results | |||
| Gender x Deprivation Status | 1,113 | 0.13 | <.72 |
| Gender | 1,113 | 0.10 | <.76 |
| WSWS Sadness Scale Results | |||
| Gender x Deprivation Status | 1,113 | 1.91 | <.18 |
| Gender | 1,113 | 0.11 | <.75 |
| WSWS Sleep Scale Results | |||
| Gender x Deprivation Status | 1,113 | 0.07 | <.79 |
| Gender | 1,113 | 1.66 | <.21 |
4. DISCUSSION
The results suggest that the effects of nicotine nasal spray during 12-hr deprivation did not affect men and women differently as both men and women displayed suppressed startle responding after receiving nicotine nasal spray compared with placebo spray. However, nondeprived women receiving an acute dose of nicotine spray experience an increased startle response, suggesting enhanced activation of defensive pathways in connection with the aversive stimulation of the startle probe. Responses of nondeprived men were unaltered by the administration of nasal spray. In contrast to our startle results, our in-session mood ratings indicate that the effects of nasal spray and deprivation status on self-reported affect varied by gender. A 3-way interaction involving gender, deprivation status, and nasal spray was found for positive and negative in-session mood ratings. The positive mood ratings were concordant with the startle results for men, showing that nicotine nasal spray was associated with increased positive affect ratings compared with placebo during smoking deprivation. However, the negative mood ratings and startle results were discordant for women, in that the reduction in startle amplitude found for deprived women given nicotine compared with placebo spray was not matched by a decrease in in-session negative mood ratings. Instead, nicotine nasal spray was associated with increased in-session ratings of negative mood compared with placebo spray for deprived women. Additionally, while smoking-nondeprived women given nicotine spray produced larger startle responses compared with placebo spray, we found no differences for either positive or negative in-session ratings. In terms of craving, we found that nicotine nasal spray reduced in-session craving ratings for women and men compared to placebo spray.
Our startle findings support negative reinforcement conceptualizations of smoking dependence which postulate that many smokers use nicotine to reduce increases in negative affect due to smoking deprivation (e.g., Baker et al., 2004; Parrott, 1999; Perkins, Grobe, Caggiula, Wilson, & Stiller, 1997). These results showed that nicotine nasal spray given to smoking-deprived men and women caused a decrease in startle response, suggesting activation of approach/reward pathways, which are possibly associated with alleviation of the negative-affect state caused by withdrawal. In other words, this suggests that nicotine may attenuate defensive activation to an aversive stimulus (the startle probe) during smoking deprivation. The fact that this attenuation occurred regardless of the valence of foreground cues (i.e., the slides) indicates that this motivational shift in defensive activation is global in nature and not specific to particular affective states. Given that the saliva nicotine results during deprivation corresponded with the startle results, and that the nasal spray was blindly administered, this decrease in startle response appears to be attributable to the hedonic properties of the drug, rather than to expectancy effects or secondary reinforcement associated with nicotine use.
Our startle results during overnight deprivation also suggest that female smokers are as responsive to the hedonic properties of nicotine as are male smokers. This is interesting because previous research suggests that women are less responsive to the reinforcing properties of nicotine administered without exteroceptive smoking cues than men (Perkins, 1996). However, our in-session mood self-report results are consistent with the nicotine self-administration studies that found women were less responsive than men to the hedonic effects of nicotine administered through nasal spray, in the absence of exteroceptive smoking cues (Perkins et al., 1994; Perkins et al., 1996; Perkins, 1996; Perkins, Donny, & Caggiula, 1999). The increase in deprived women’s self-report of in-session negative mood found here may reflect increased reactance to the aversive sensory properties of the nicotine nasal spray compared to men. In the absence of exteroceptive cues, deprived women may have been responding to the salient aversive sensory qualities of the nicotine nasal spray rather than to its underlying activation of appetitive pathways reflected in the central nervous systems structures mediating the startle response (e.g., the amygdala, reticular nuclei, and the anterior cingulate cortex; Lang, 1985). This could account for the apparently opposite results found for women during smoking deprivation, in which startle indicated activation of appetitive pathways whereas self-report indicated increased negative mood (and decreased positive mood, albeit nonsignificantly) during nicotine compared with placebo spray. However, this explanation should be taken with caution, given the lack of a similar pattern of in-session results for women during nondeprivation.
Making comparisons between the in-session mood ratings and the startle response should be done with reservation. Our in-session self-report measures of mood and craving were given after every third slide, while startle was collected for 4 of 6 of each type of slide. Also, the participants were instructed to make in-session ratings in response to how they felt “at that moment” rather than in response to the previously viewed slide. Additionally, multimodal measure of emotion is often marked by response desynchrony and discordance (e.g., Hodgson & Rachman, 1974). It is also possible that differences in response between startle and self-reported measures of affect emerge over differing time courses (e.g., desynchrony) that may vary by gender and level of nicotine dependence.
The increase in startle response to nicotine compared with placebo nasal spray in nondeprived women suggests that the additional dose of nicotine provided by the spray may have been aversive to nondeprived women, who may have already been sated from ad lib smoking. That is, women typically report smoking less than men (Goldberg et al., 1993), and providing nondeprived women who smoked before and just prior to the lab session with more nicotine in the form of nicotine nasal spray may have exceeded some internal upper threshold. However, this explanation is questionable given the fact that nicotine nasal spray produced no discernable increase in saliva nicotine levels or self-report of negative mood compared with placebo spray during nondeprivation for either men or women. This suggests that an absolute increase in nicotine levels was not the cause of the increase in startle response to nicotine spray in nondeprived women. However, we cannot entirely rule out that increased levels of nicotine in the CNS were in fact responsible for the increased startle response of nondeprived women administered nicotine nasal spray. Although arterial nicotine from nasal spray has been found to peak within 5 min (Gourlay & Benowitz, 1997), it is unknown whether saliva nicotine follows a similar concentration-time curve. Studies have found that female smokers have decreased nicotine metabolism (Beckett et al., 1971; Benowitz & Jacob, 1984), and saliva nicotine measured at 5 min following nasal spray administration may not reflect this metabolic difference. However, both men and women produced smaller startle responses with nicotine nasal spray than with placebo spray when they were smoking deprived, which suggests that the nicotine nasal spray dose was sufficient to increase appetitive motivation in the absence of previous exposure.
Although this study showed that there are gender differences in startle reactivity to nicotine deprivation, the period of nicotine deprivation was relatively brief (12 hr). Studies finding differential rates of success with smoking cessation treatment between the genders suggest that gender-specific affective responses do not quickly dissipate (Hatsukami et al., 1995), even for successfully abstinent women (e.g., Brandon & Baker, 1991). Other work has suggested that smokers may experience variable withdrawal symptoms over time (Piasecki, Fiore, & Baker, 1998). The effects of nicotine and gender on affective processing following longer nicotine deprivation need to be addressed.
The findings and limitations of this study suggest several avenues for further investigation. First, examinations of the physiological differences in response to nicotine would benefit by varying the nicotine dose. Second, the incorporation of measures of smoking topography would allow for precise assessment of tobacco-use behavior and would allow for replication of these results using cigarette-administered nicotine. Third, the use of placebo cigarettes would allow for further evaluation of postulated gender differences to non-nicotine reinforcement of smoking. Finally, the generalizability of these findings to smokers attempting to quit may be limited by our specifically selecting smokers who were not attempting to quit.
Acknowledgments
Portions of this paper were completed by the first author as partial fulfillment of the requirements to obtain a doctorate degree from Purdue University, West Lafayette, IN. Portions of this paper were presented at the annual meeting of the Society for Research on Nicotine and Tobacco, New Orleans, LA, February 2003.
Supported by grants from the Texas Tobacco Settlement, the National Cancer Institute (1R21CA81649) and the National Institute on Drug Abuse (R01DA1182-01) to Dr. Cinciripini, and a M. D. Anderson Education Program in Cancer Prevention Postdoctoral Fellowship Grant (R25CA57730) to Dr. Robinson. Pharmacia & Upjohn, Inc. provided the nicotine and placebo nasal spray. We thank Terry Blumenthal and Margaret Bradley for their technical assistance and Tracy Long, Cathy Sanders, Renata Benjamin, and Deena Martinez for their assistance in data collection.
Footnotes
Three smokers reported smoking within 9 to 11 hr of a deprivation session. Excluding these sessions did not affect any of the analyses involving the deprivation status variable. Additionally, we conducted analyses where we excluded sessions where smokers produced CO above 10 ppm on deprived days. This more stringent definition of deprivation also did not alter the results. Thus, the decision was made by the authors to not exclude any of these sessions in the analyses.
The following IAPS slides were used: Positive, 4220, 4652, 4658, 4659, 4660, 4670, 5621, 5629, 8030, 8370, 8490, and 8500; Neutral, 7000, 7010, 7020, 7030, 7040, 7050, 7060, 7080, 7090, 7100, 7150, and 7170; Negative, 3010, 3060, 3100, 3120, 3130, 3150, 3170, 3500, 6230, 6350, 6560, and 9410. The subject were asked to view each slide and then rate the slide on affective valence (positive, negative), arousal (low, high), and craving (low, high) by selecting a box on a Likert scale ranging from 1 to 9. Results are presented in Cinciripini et al. (2005).
The statement about positive mood state was “I am feeling happy, joyful, or pleased,” the statement about negative mood state “I am depressed, angry, worried or frustrated”, and the statement about craving “I am craving a cigarette right now”. The participants were asked to indicate to what extent they agreed or disagreed with these statements: Strongly agree, agree, neutral, disagree, and strongly disagree.
References
- Angst J. Epidemiology of depression. Psychopharmacology. 1992;106:S71–S74. doi: 10.1007/BF02246240. [DOI] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: An exploration of individual differences. Journal of Consulting and Clinical Psychology. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Bageilla E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37:13–20. [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beckett AH, Gorrod JW, Jenner P. The effect of smoking on nicotine metabolism in vivo in man. Journal of Pharmacy and Pharmacology. 1971;23:55S–61S. doi: 10.1111/j.2042-7158.1971.tb08769.x. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Jacob P. Daily intake of nicotine during cigarette smoking. Clinical Pharmacology and Therapeutics. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, Lindgren P, Wides M, Pope F, Buist SA, Hoppe-Ryan C, O'Hara P. Gender differences in smoking cessation after three years in the Lung Health Study. American Journal of Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. American Journal of Preventive Medicine. 1996;12:378–387. [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. New York: Cambridge University Press; 1999. pp. 157–183. [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Current Directions in Psychological Science. 1994;3:33–37. [Google Scholar]
- Brandon TH, Baker TB. The smoking consequences questionnaire: The subjected expected utility of smoking in college students. Psychological Assessment. 1991;3:484–491. [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemiologic study of young adults. American Journal of Psychiatry. 1992;149:464–469. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, Cinciripini PM. Toward a standardized set of cigarette stimuli for use in cue reactivity research. Nicotine & Tobacco Research. doi: 10.1080/14622200600670215. in press. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: NIMH-Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: Differences between men and women. Journal of Consulting and Clinical Psychology. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, De Moor CA, Baile WS, Wetter DW. The Effects of Smoking Deprivation and Nicotine Administration on Emotional Reactivity. Nicotine & Tobacco Research. doi: 10.1080/14622200600670272. in press. [DOI] [PubMed] [Google Scholar]
- Cook EW, Davis TL, Hawk LW, Spence EW, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33:103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure: Self-report, psychophysiological, and startle probe responses. Experimental and Clinical Psychopharmacology. 1995;3:156–162. [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Holbrook J, Jaen CR, Kottke TE, Lando HA, Mecklenbur R, Mullen PD, Nett LM, Robinson L, Stitzer M, Tommasello AC, Villejo L, Wewers ME. Treating tobacco use and dependence. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000. [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha R, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Ockene JK, Kristeller J, Kalan K, Landon J, Hosmer DW. Factors associated with heavy smoking among men and women: The physician-delivered smoking intervention project. American Heart Journal. 1993;125:818–823. doi: 10.1016/0002-8703(93)90176-a. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Benowitz NL. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clinical Pharmacology and Therapeutics. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. British Medical Journal. 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER. Gender and the teenage smoker. NIDA Research Monographs. 1986;65:70–79. [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Wewers ME. Gender differences in tobacco use. Health Psychology. 1991;10:143–153. [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive history smokers. Journal of Consulting and Clinical Psychology. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL. Nicotine, negative affect, and depression. Journal of Consulting and Clinical Psychology. 1993;61(5):761–767. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- Hariharan M, VanNoord T. Liquid-chromatographic determination of nicotine and cotinine in urine from passive smokers: Comparison with gas chromatography with a nitrogen-specific detector. Clinical Chemistry. 1991;37:1989–1993. [PubMed] [Google Scholar]
- Hatsukami D, Skoog K, Allen S, Bliss R. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Experimental and Clinical Psychopharmacology. 1995;3:163–173. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hodgson R, Rachman S. Desynchrony in measures of fear. Behavior Research and Therapy. 1974;12:319–326. doi: 10.1016/0005-7967(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Experimental and Clinical Psychopharmacology. 1999;3:250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. Journal of Consulting and Clinical Psychology. 1990;58:85–92. doi: 10.1037//0022-006x.58.1.85. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The cognitive psychophysiology of emotion: Fear and anxiety. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Hillsdale, NJ: Erlbaum; 1985. pp. 131–170. [Google Scholar]
- Marlatt G, Gordon B. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavior medicine: Changing health lifestyles. New York: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- Mulligan NW, Wiesen C. Using the analysis of covariance to increase the power of priming experiments. Canadian Journal of Experimental Psychology. 2003;57:152–166. doi: 10.1037/h0087422. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Cigarette smoking does cause distress. American Psychologist. 1999;55:1159–1160. doi: 10.1037//0003-066x.55.10.1159. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Experimental and Clinical Psychopharmacology. 1996;4:166–177. [Google Scholar]
- Perkins KA. Nicotine discrimination in men and women. Pharmacology, Biochemistry and Behavior. 1999;64:295–299. doi: 10.1016/s0091-3057(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine & Tobacco Research. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Caggiula AR, Wilson AS, Stiller RL. Acute reinforcing effects of low dose nicotine nasal spray in humans. Pharmacology, Biochemistry and Behavior. 1997;56:235–241. doi: 10.1016/s0091-3057(96)00216-x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, D'Amico D, Fonte C, Wilson AS, Stiller RL. Low-dose nicotine nasal spray use and effects during initial smoking cessation. Experimental and Clinical Pharmacology. 1996;4:157–165. [Google Scholar]
- Perkins KA, Sexton JE, Reynolds WA, Grobe JE, Fonte C, Stiller RL. Comparison of acute subjective and heart rate effects of nicotine intake via tobacco smoking versus nasal spray. Pharmacology, Biochemistry and Behavior. 1994;47:295–299. doi: 10.1016/0091-3057(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: Two studies of variability in the time course of smoking withdrawal symptoms. Journal of Abnormal Psychology. 1998;107:238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Kenford SL, Smith SS, Fiore MC, Baker TB. Listening to nicotine: Negative affect and the smoking withdrawal conundrum. Psychological Science. 1997;8:184–189. [Google Scholar]
- Pomerleau CS, Pomerleau OF. Gender differences in frequency of smoking withdrawal symptoms. Annals of Behavioral Medicine. 1994;16(Suppl.):S118. [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roberts TA, Pennebaker JW. Gender differences in perceiving internal state: Toward a his-and-hers model of perceptual cue use. Advances in Experimental Social Psychology. 1995;27:143–175. [Google Scholar]
- Shiffman S. The tobacco withdrawal syndrome. In: Krasnegor NM, editor. Cigarette Smoking as a Dependence Process. Monograph 23. Rockville, Maryland: National Institute on Drug Abuse, United States Department of Health, Education, and Welfare; 1979. pp. 158–184. [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S. A cluster-analytic typology of smoking relapse episodes. Addictive Behaviors. 1986;11:295–307. doi: 10.1016/0306-4603(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bradley MM, Lang PJ. State anxiety and affective physiology: Effects of sustained exposure to affective pictures. Biological Psychology. 2005;69:247–260. doi: 10.1016/j.biopsycho.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Kroenke K, Linzer M, deGruy FV, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: The PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- Stapleton JA, Russell MA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, Wiseman D. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction. 1995;90:31–42. doi: 10.1046/j.1360-0443.1995.901316.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Waldron I. Sex differences in illness incidence, prognosis and mortality. Social Science and Medicine. 1983;17:1107–1123. doi: 10.1016/0277-9536(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Waldron I. Patterns and causes of gender differences in smoking. Social Science and Medicine. 1991;32:989–1005. doi: 10.1016/0277-9536(91)90157-8. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–61. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Smith SS, Kenford SL, Jorenby DE, Fiore MC, Hurt RD, Offord KP, Baker TB. Smoking outcome expectancies: Factor structure, predictive validity, and discriminant validity. Journal of Abnormal Psychology. 1994;103:801–811. doi: 10.1037//0021-843x.103.4.801. [DOI] [PubMed] [Google Scholar]
- Witvliet CVO, Vrana SR. Psychophysiological responses as indices of affective dimensions. Psychophysiology. 1995;32:436–443. doi: 10.1111/j.1469-8986.1995.tb02094.x. [DOI] [PubMed] [Google Scholar]