Abstract
Background
Antacid treatments decrease the serum concentrations of first-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs), although it is unknown whether antacids affect clinical outcomes. As cerebrospinal fluid concentrations of TKIs are much lower than serum concentrations, we hypothesized that this drug-drug interaction might affect the prognosis of patients with de novo brain metastases.
Materials and Methods
This retrospective study evaluated 269 patients with EGFR-mutant non-small cell lung cancer (NSCLC) who had been diagnosed between December 2010 and December 2013, and had been treated using first-line first-generation EGFR-TKIs. Among these patients, we identified patients who concurrently used H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) as antacids. Patients who exhibited >30% overlap between the use of TKIs and antacids were considered antacid users.
Results
Fifty-seven patients (57/269, 21.2%) were antacid users, and antacid use did not significantly affect progression-free survival (PFS; no antacids: 11.2 months, H2RAs: 9.4 months, PPIs: 6.7 months; p = 0.234). However, antacid use significantly reduced overall survival (OS; no antacids: 25.0 months, H2RAs: 15.5 months, PPIs: 11.3 months; p = 0.002). Antacid use did not affect PFS for various metastasis sites, although antacid users with de novo brain metastases exhibited significantly shorter OS, compared to non-users (11.8 vs. 16.3 months, respectively; p = 0.041). Antacid use did not significantly affect OS in patients with bone, liver, or pleural metastases.
Conclusion
Antacid use reduced OS among patients with EGFR-mutant NSCLC who were treated using first-line first-generation EGFR-TKIs, and especially among patients with de novo brain metastases.
Introduction
The incidence of lung cancer is increasing in Taiwan, and lung cancer is the leading cause of cancer-related deaths worldwide.[1–4] Relatively high incidences of epidermal growth factor receptor (EGFR) mutations have been reported among patients with an Asian lineage, never smokers, and cases of adenocarcinoma.[5–7] Nevertheless, EGFR-tyrosine kinase inhibitors (TKIs) improve progression-free survival (PFS), overall survival (OS), and quality of life outcomes among non-small cell lung cancer (NSCLC) patients harboring EGFR mutation. Furthermore, EGFR-TKIs are less toxic than platinum-based doublet chemotherapy.[8–10]
Antacids decrease the area under the plasma drug concentration-time curve and peak plasma concentration of first-generation EGFR-TKIs by 33–70% among healthy volunteers,[11] although the effects of this drug-drug interaction on the survival outcomes of previous studies remains debatable.[12, 13] Nevertheless, the concentrations of TKIs in the cerebrospinal fluid (CSF) are less than the serum concentrations,[14–17] and EGFR-TKIs are only effective for a portion of patients with brain metastases from NSCLC.[18] Therefore, we hypothesized that patients with de novo brain metastases from NSCLC would be more likely to be affected (i.e., experience less tumor control and/or new metastases) by the interaction between antacids and EGFR-TKIs.
Material and Methods
Patient and Clinical Characteristics
This retrospective study evaluated patients with NSCLC who were diagnosed between December 2010 and December 2013 at Kaohsiung Chang Gung Memorial Hospital in Taiwan. All patients were subsequently followed-up until June 2015. The inclusion criteria were age of >18 years, histologically or cytologically confirmed advanced-stage NSCLC with EGFR mutations, and first-line treatment with first-generation EGFR-TKIs. Patients were excluded if they had previously received any targeted therapy, chemotherapy, or immunotherapy. This study’s design was approved by the institutional review board of Kaohsiung Chang Gung Memorial Hospital, and the requirement for informed consent was waived, due to the retrospective design.
Baseline assessments were performed within 4 weeks of treatment initiation, including clinical characteristics and findings from chest radiography, chest computed tomography, bone scan, and brain magnetic resonance imaging. The clinical characteristics included age, sex, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status (PS), diabetes mellitus, EGFR mutations, and sites and symptoms of distant metastases. We also recorded whether the patient was concomitantly using antacids (proton pump inhibitors [PPIs] or H2 receptor antagonists [H2RAs]) while also receiving TKI treatment, and the duration of concomitant use as a proportion of the TKI-treatment period. Patients who exhibited an overlap of >30% between antacids and TKIs usage days were defined as antacid users. Among patients who used more than one antacid, we only considered the antacid with the greatest overlap.
EGFR Mutation Testing
Tumor specimens were obtained from biopsy samples that were obtained via bronchoscopy, computed tomography-guided biopsy, or surgical procedures. Tumor specimens from pleural effusion cytology were also considered acceptable. The genetic analyses were performed using Scorpion primers and genomic DNA that was extracted from the paraffin-embedded tissues (QIAGEN EGFR RGQ PCR Kit), which was subjected to amplification refractory mutation system-polymerase chain reaction.[19] Deletions in exon 19 and the L858R mutations were defined as “common” mutations, and all other mutations (rare and/or compound) were defined as “uncommon” mutations.[20]
Evaluating Response to EGFR-TKI Treatment
To evaluate the tumor response, patients underwent chest radiography every 2–4 weeks and chest computed tomography every 2–3 months. Disease status was determined by the attending clinician according to Response Evaluation Criteria in Solid Tumors guidelines (version 1.1).[21] PFS was defined as the period from the first day of EGFR-TKI treatment until disease progression, death before documented progression, or the last visit during the follow-up period. OS was defined as the period from the first day of EGFR-TKI treatment until death, loss to follow-up, or the last follow-up. Patients with various sites of metastases (e.g., brain, bone, liver, and pleura) were further subdivided into antacids users or non-users, and their PFS and OS were compared.
Statistical Analyses
Statistical analyses were performed using MedCalc software (version 14.10.2) and receiver operating characteristic curves. The PFS and OS analyses were performed using the Kaplan-Meier method and the log-rank test. A Cox proportional hazards regression model was used to evaluate independent factors that affected the survival outcomes. A p-value of <0.05 was considered statistically significant.
Results
Patient Characteristics
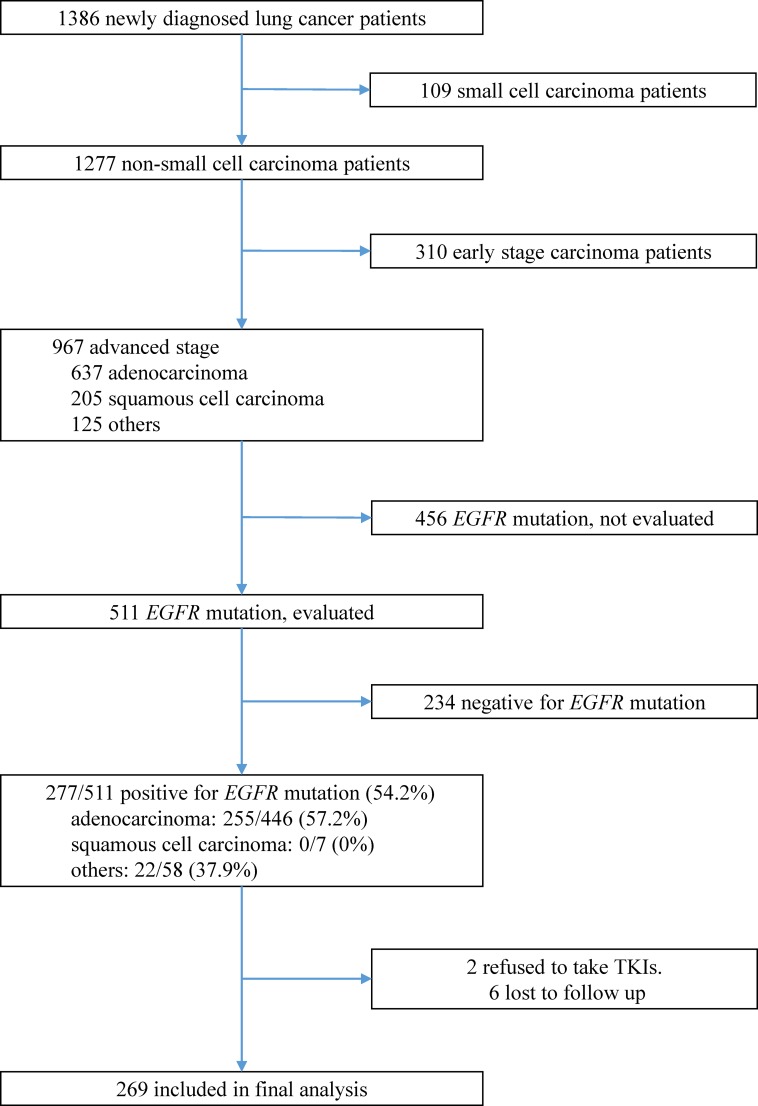
Among 1,386 patients who were diagnosed with lung cancer between December 2010 and December 2013, we identified 511 patients with advanced NSCLC who were screened for EGFR mutations (Fig 1). Among these patients, 277 (57.2%) patients had EGFR-mutant NSCLC. However, 2 patients refused to receive TKI treatment and 6 patients were lost to follow-up; therefore, 269 patients were included in the final analysis. The mean patient age was 65.1 ± 12.3 years, 42.0% (113/269) of the patients were male, 23.8% (64/269) of the patients had de novo brain metastases, 44.2% (119/269) of the patients had de novo bone metastases, 13.0% (35/269) of the patients had de novo liver metastases, and 48.0% (129/269) of the patients had de novo pleural metastases (Table 1).
Fig 1. The inclusion, screening, and group assignments for this study.
Among 1,386 patients who were diagnosed with non-small-cell lung cancer between January 2011 and January 2014, 269 patients were included in the final analysis.
Table 1. The clinical characteristics of patients who did and did not use antacids.
| All patients (n = 269) | Used antacids (N = 57, 21.2%) | No antacids (N = 212, 78.8%) | P-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Clinical characteristics | ||||
| Age, years | 65.1 ± 12.3 | 66.6 ± 14.2 | 64.6 ± 11.7 | 0.286 |
| Sex | 0.133 | |||
| Male | 113 (42.0) | 29 (50.9) | 84 (39.6) | |
| Female | 156 (58.0) | 28 (49.1) | 128 (60.4) | |
| ECOG performance status | 0.052 | |||
| 0 | 50 (18.6) | 3 (5.3) | 47 (22.2) | |
| 1 | 167 (62.1) | 41 (71.9) | 126 (59.4) | |
| 2 | 28 (10.4) | 6 (10.5) | 22 (10.4) | |
| 3 | 19 (7.1) | 5 (8.8) | 14 (6.6) | |
| 4 | 5 (1.9) | 2 (3.5) | 3 (1.4) | |
| EGFR mutation | 0.890 | |||
| Common | 242 (90.0) | 51 (89.5) | 191 (90.1) | |
| Uncommon | 27 (10.0) | 6 (10.5) | 21 (9.9) | |
| Tumor type | 0.466 | |||
| Adenocarcinoma | 247 (91.8) | 51 (89.5) | 196 (92.5) | |
| Non-adenocarcinoma | 22 (8.2) | 6 (10.5) | 16 (7.5) | |
| No. of distant metastasis | 0.122 | |||
| 0 | 31 (11.5) | 4 (7.0) | 27 (12.7) | |
| 1 | 133 (49.4) | 23 (40.4) | 110 (51.9) | |
| 2 | 62 (23.0) | 18 (31.6) | 44 (20.8) | |
| 3 | 32 (11.9) | 7 (12.3) | 25 (11.8) | |
| ≥4 | 11 (4.0) | 5 (8.8) | 6 (2.9) | |
| Brain metastasis | 64 (23.8) | 18 (31.6) | 46 (21.7) | 0.120 |
| Bone metastasis | 119 (44.2) | 25 (43.9) | 94 (44.3) | 0.948 |
| Liver metastasis | 35 (13.0) | 12 (21.1) | 23 (10.8) | 0.074 |
| Pleura metastasis | 129 (48.0) | 33 (57.9) | 96 (45.3) | 0.091 |
| Adverse events | ||||
| Skin rash | 116 (43.1) | 19 (33.3) | 97 (45.8) | 0.102 |
| Diarrhea | 33 (12.3) | 4 (7.0) | 29 (13.7) | 0.253 |
| Interstitial lung disease | 2 (0.1) | 0 (0.0) | 2 (0.9) | 1.000 |
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
At the last follow-up, disease progression was noted in 84.8% (228/269) of the patients, and 48.3% (130/269) of the patients were alive. The median PFS was 10.3 ± 0.7 months, the median OS was 22.0 ± 1.6 months, the partial tumor response rate was 79.2%, and the disease control rate was 88.5% (stable disease was detected in 25 of the 269 patients). The median follow-up was 24.5 months, and the longest follow-up was 47.2 months.
Antacid Use
Among the 269 patients, 57 (21.2%) patients were considered antacid users (>30% overlap between antacid and TKI treatment). Thirty-nine (14.5%) patients had received H2RAs, and 18 (6.7%) patients had received PPIs. Among the 57 antacid users, 32 (56.1%) patients exhibited an overlap of >80%, 9 (15.8%) patients exhibited an overlap of 51–80%, and 16 (28.1%) patients exhibited an overlap of 31–50%.
Survival Analysis
In the univariable analysis, prolonged PFS was significantly associated with an ECOG PS of ≤2 (p < 0.001), common EGFR mutations (p < 0.001), no brain metastasis (p = 0.001), no bone metastasis (p < 0.001), no liver metastasis (p < 0.001), and no pleural metastasis (p = 0.007) (Table 2). Age, sex, diabetes mellitus, smoking, tumor histology, and antacid use (Fig 2) were not significantly associated with PFS. In the multivariable analysis, prolonged PFS was independently associated with an ECOG PS of ≤2 (p = 0.006), common EGFR mutations (p < 0.001), no bone metastasis (p < 0.001), no liver metastasis (p = 0.005), and no pleural metastasis (p = 0.004) (Table 2).
Table 2. Univariable and multivariable analysis of progression-free survival.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| n | PFS (months) | P-value | Hazard ratio | P-value | 95% CI | |
| Age, years | 0.204 | |||||
| >65 | 134 | 11.3 | ||||
| ≤65 | 135 | 10.0 | ||||
| Sex | 0.702 | |||||
| Male | 113 | 10.3 | ||||
| Female | 156 | 10.5 | ||||
| Diabetes mellitus | 0.602 | |||||
| Yes | 51 | 10.3 | ||||
| No | 218 | 10.3 | ||||
| Smoking history | 0.683 | |||||
| Never | 182 | 10.6 | ||||
| Former / current | 87 | 9.8 | ||||
| Performance status | <0.001 | 0.006 | ||||
| ECOG 0–2 | 242 | 11.3 | 1 | |||
| ECOG 3–4 | 27 | 2.9 | 2.16 | 1.20–2.98 | ||
| EGFR mutation | <0.001 | <0.001 | ||||
| Common | 242 | 11.3 | 1 | |||
| Uncommon | 27 | 5.1 | 2.76 | 1.77–4.32 | ||
| Tumor type | 0.104 | |||||
| Adenocarcinoma | 247 | 10.6 | ||||
| Non-adenocarcinoma | 22 | 9.0 | ||||
| Brain metastasis | 0.001 | 0.180 | ||||
| Yes | 64 | 7.4 | 1.25 | 0.90–1.73 | ||
| No | 205 | 12.0 | 1 | |||
| Bone metastasis | <0.001 | <0.001 | ||||
| Yes | 119 | 7.8 | 1.78 | 1.32–2.40 | ||
| No | 150 | 13.5 | 1 | |||
| Liver metastasis | <0.001 | 0.005 | ||||
| Yes | 35 | 6.7 | 1.80 | 1.20–2.71 | ||
| No | 234 | 11.3 | 1 | |||
| Pleura metastasis | 0.007 | 0.004 | ||||
| Yes | 129 | 9.0 | 1.49 | 1.14–1.94 | ||
| No | 140 | 11.8 | 1 | |||
| Antacid | 0.234 | |||||
| Proton pump inhibitor | 18 | 6.7 | ||||
| H2 receptor antagonists | 39 | 9.4 | ||||
| None | 212 | 11.2 | ||||
PFS, progression-free survival; CI, confidential interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
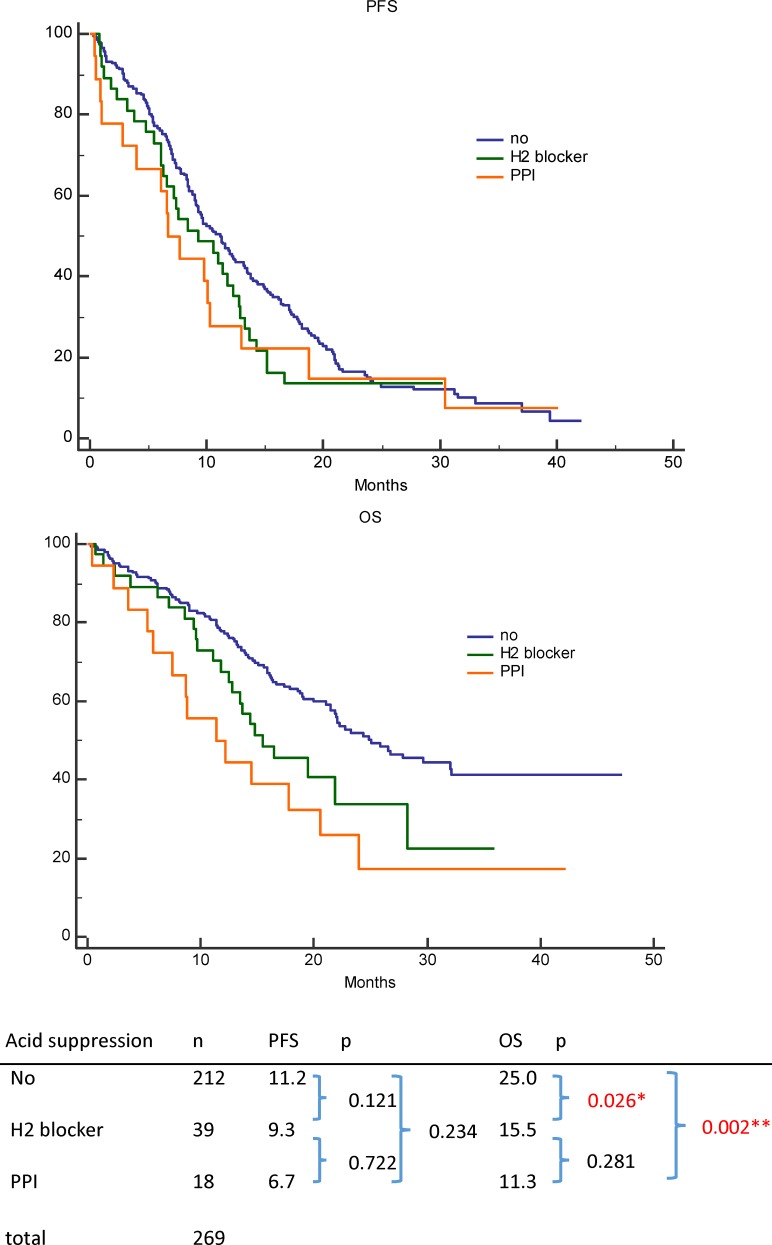
Fig 2. Progression-free survival (PFS) and overall survival (OS) according to antacid use among patients with epidermal growth factor receptor-mutant non-small-cell lung cancer who were treated using first-line tyrosine kinase inhibitors.
(top) PFS among patients who were receiving proton pump inhibitors, histamine H2-receptor antagonists, or no antacid. (bottom) OS among patients who were receiving proton pump inhibitors, histamine H2-receptor antagonists, or no antacid.
In the univariable analysis, prolonged OS was significantly associated with an ECOG PS of ≤2 (p < 0.001), adenocarcinoma histology (p = 0.007), no brain metastasis (p < 0.001), no bone metastasis (p < 0.001), no liver metastasis (p < 0.001), and concomitant use of antacids (p = 0.002) (Fig 2) (Table 3). Age, sex, diabetes mellitus, smoking, EGFR mutation type, and pleural metastasis were not significantly associated with OS. In the multivariable analysis, prolonged OS was independently associated with an ECOG PS of ≤2 (p = 0.006), no brain metastasis (p = 0.037), no bone metastasis (p = 0.002), no liver metastasis (p = 0.013), and concomitant use of antacids (p = 0.014) (Table 3).
Table 3. Univariable and multivariable analysis of overall survival.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| n | OS (months) | P-value | Hazard ratio | P-value | 95% CI | |
| Age, years | 0.311 | |||||
| >65 | 134 | 21.8 | ||||
| ≤65 | 135 | 22.8 | ||||
| Sex | 0.389 | |||||
| Male | 113 | 21.9 | ||||
| Female | 156 | 24.4 | ||||
| Diabetes mellitus | 0.917 | |||||
| Yes | 51 | 24.0 | ||||
| No | 218 | 22.0 | ||||
| Smoking history | 0.567 | |||||
| Never | 182 | 22.0 | ||||
| Former / current | 87 | 22.8 | ||||
| Performance status | <0.001 | 0.008 | ||||
| ECOG 0–2 | 242 | 23.3 | 1 | |||
| ECOG 3–4 | 27 | 7.3 | 2.08 | 1.22–3.56 | ||
| EGFR mutation | 0.334 | |||||
| Common | 242 | 22.2 | ||||
| Uncommon | 27 | 14.0 | ||||
| Tumor type | 0.007 | 0.087 | ||||
| Adenocarcinoma | 247 | 25.0 | 1 | |||
| Non-adenocarcinoma | 22 | 15.9 | 1.54 | 0.94–2.52 | ||
| Brain metastasis | <0.001 | 0.037 | ||||
| Yes | 64 | 14.3 | 1.51 | 1.02–2.23 | ||
| No | 205 | 25.9 | 1 | |||
| Bone metastasis | <0.001 | 0.002 | ||||
| Yes | 119 | 15.8 | 1.77 | 1.23–2.54 | ||
| No | 150 | 32.1 | 1 | |||
| Liver metastasis | <0.001 | 0.013 | ||||
| Yes | 35 | 12.2 | 1.82 | 1.14–2.92 | ||
| No | 234 | 24.4 | 1 | |||
| Pleura metastasis | 0.115 | |||||
| Yes | 129 | 21.4 | ||||
| No | 140 | 24.4 | ||||
| Antacid | 0.002 | 0.014 | ||||
| Proton pump inhibitor | 18 | 11.3 | 2.27 | 1.26–4.11 | ||
| H2 receptor antagonists | 39 | 15.5 | 1.46 | 0.92–2.33 | ||
| None | 212 | 25.0 | 1 | |||
OS: overall survival; CI, confidential interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
The Effect of Antacids among Patients with Different Metastasis Sites
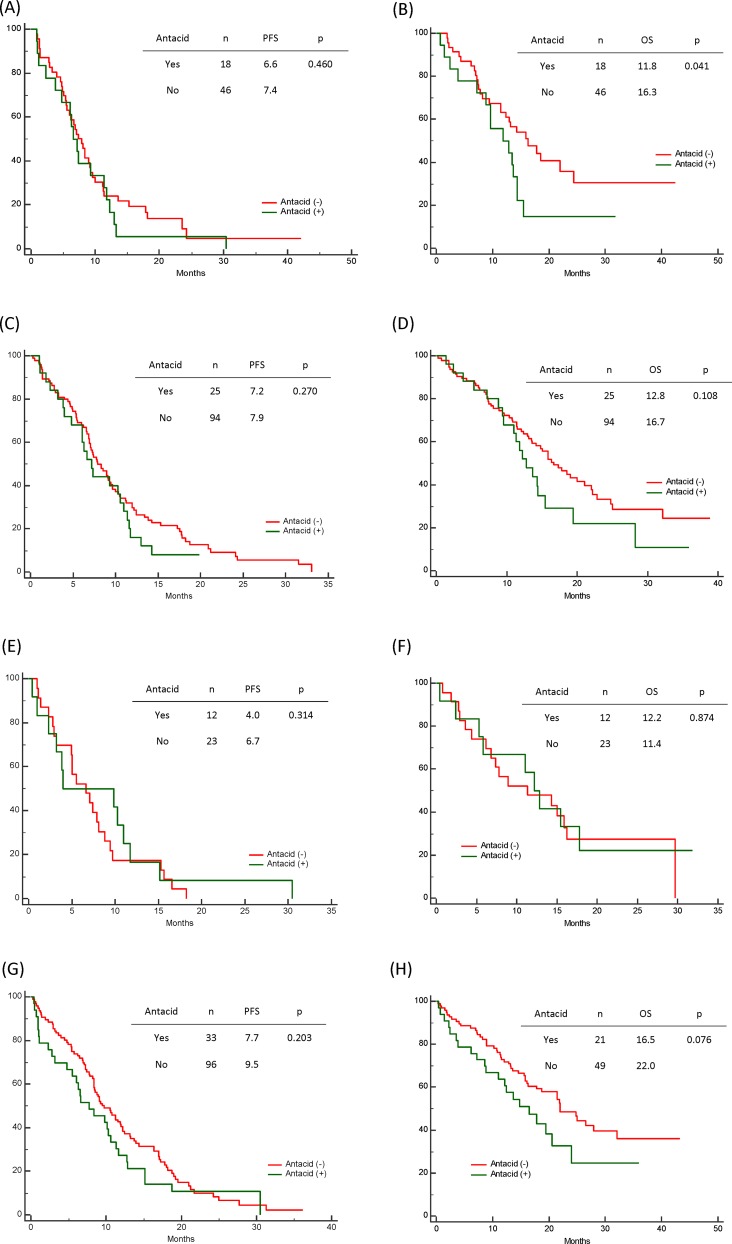
We also evaluated the effect of antacids among patients with different distant organ metastases (i.e., brain, bone, liver, or pleural metastases). The use of antacids did not significantly affect PFS in this analysis (Fig 3), although antacid users with de novo brain metastases exhibited significantly shorter OS (antacid users: 11.8 months, non-users: 16.3 months; p = 0.041). No significant differences in OS were observed when we compared antacid users and non-users among patients with bone, liver, or pleural metastases.
Fig 3. The effect of antacids use and metastasis sites on progression-free survival (PFS) and overall survival (OS) among patients with epidermal growth factor receptor-mutant non-small-cell lung cancer who were treated using first-line tyrosine kinase inhibitors.
(A) PFS and (B) OS among patients with brain metastases according to antacid use. (C) PFS and (D) OS among patients with bone metastases according to antacid use. (E) PFS and (F) OS among patients with liver metastases according to antacid use. (G) PFS and (H) OS among patients with pleural metastases according to antacid use.
Discussion
In the present study, we found that 21.0% of our patients with NSCLC exhibited a >30% overlap between their prescriptions for antacids and EGFR-TKIs.
There is controversy regarding the effect of antacids on clinical outcomes among patients with EGFR-mutant NSCLC who are receiving first-generation TKIs. For example, Hilton et al. reported that antacids did not adversely affect PFS and OS among patients who were receiving TKIs in the second line or later line.[13] In contrast, another study reported that the concomitant use of antacids and EGFR-TKIs shortened the PFS and OS among patients with NSCLC (only 4% of patients were receiving first-line TKIs).[12] However, both studies did not examine the patients’ EGFR mutation status, and most of the patients were receiving TKIs after receiving a different first-line treatment. Therefore, the present study only included patients who were receiving first-line TKIs, in order to eliminate any confounding that was related to the line of treatment.
In the present study, we found that antacids significantly shortened OS among patients who were treated using first-line EGFR-TKIs, although there was no significant effect on PFS. However, the mechanism for this phenomenon is not clear, and may be multifactorial. One relevant hypothesis is the second hit theory, whereby the drug-drug interaction’s effect becomes enhanced during re-challenge with the TKI. For example, we found that 25% (67/269) of the EGFR-mutant patients were re-challenged with TKIs after second line of chemotherapy. During both the initial and re-challenge treatments, 11 of 67 patients using antacids, and 49 of 67 patients not using antacids; the concordance rate for antacid status at the initial and re-challenge treatments was 89.6% (60/67). Moreover, antacid use during the TKI re-challenge exhibited a trend towards shortening the PFS, although this difference was not statistically significant (antacid users: 1.4 months, non-users: 2.0 months; p = 0.52). Therefore, studies are needed to evaluate whether antacid-related reductions in PFS during each TKI treatment period might significantly affect OS. Another hypothesis is related to a legacy effect for the drug-drug interaction between antacids and TKIs. In this context, we hypothesize that the reduced serum levels of the TKI might not affect control of the main tumor, although it might affect the control of microscopic or gross metastases. The subclinical effect on the distance metastases would not likely affect PFS during the first line of therapy, although it might affect OS beyond the first line. Therefore, we evaluated PFS among the patients who were eligible to receive second-line chemotherapy (n = 123), and found that the PFSs were 2.9 months (n = 24) for antacid users and 4.2 months (n = 99) for non-users (p = 0.159).
Previous studies have reported that the CSF penetration rate of first-generation TKIs ranges from 1% to 3%.[14–17] Therefore, we examined patients with de novo brain metastases, and found that the concomitant use of antacids and TKIs significantly shortened their OS. Based on this finding, we hypothesized that the interaction between antacids and TKIs might further reduce the already insufficient levels of TKIs in the CSF of patients with brain metastases. Another interesting issue is whether antacid use might increase the incidence of new brain metastases during TKI treatment in patients without de novo brain metastases. Among patients with NSCLC who were receiving platinum-based chemotherapies, CNS metastasis was observed in 7% of patients at the 1-year follow-up,[22] and we also observed that 6.3% (17/269) of our patients exhibited new brain metastases. However, there was no significant difference in the incidences of brain metastases among patients who were and were not using antacids (antacid users: 1/57 [1.8%], non-users: 16/212 [7.5%]; p = 0.111). Therefore, further studies are needed to address this issue.
The present study had several limitations. First, we did not have access to serial data regarding the patients’ serum TKI levels, and cannot confirm that antacid use affected prognosis by reducing TKI levels. Second, our study used a retrospective design with a small patient population, and prospective studies are needed to validate our findings. Nevertheless, to the best of our knowledge, this is the first study to demonstrate that antacid use adversely affected OS among patients with advanced-stage EGFR-mutant NSCLC who were treated using first-line EGFR-TKIs.
Conclusion
Antacid use reduced OS among patients with EGFR-mutant NSCLC who were treated using first-line first-generation EGFR-TKIs, and this result was especially pronounced among patients with de novo brain metastases.
Acknowledgments
We thank Tsui-Ping Tang and I-Chun Lin for data collection.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Henley SJ, Richards TB, Underwood JM, Eheman CR, Plescia M, McAfee TA, et al. Lung cancer incidence trends among men and women—United States, 2005–2009. MMWR Morbidity and mortality weekly report. 2014;63(1):1–5. . [PMC free article] [PubMed] [Google Scholar]
- 2.Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in taiwan: a population-based cancer registry. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8(9):1128–35. 10.1097/JTO.0b013e31829ceba4 . [DOI] [PubMed] [Google Scholar]
- 3.Lie CH, Chang HC, Chao TY, Chung YH, Wang JL, Wang CC, et al. First- or second-line gefitinib therapy in unknown epidermal growth factor receptor mutants of non-small-cell lung cancer patients treated in Taiwan. Clinical lung cancer. 2011;12(2):116–24. 10.1016/j.cllc.2011.03.006 . [DOI] [PubMed] [Google Scholar]
- 4.Kogure Y, Saka H, Oki M, Saito TI, Ahmed SN, Kitagawa C, et al. Post-Progression Survival after EGFR-TKI for Advanced Non-Small Cell Lung Cancer Harboring EGFR Mutations. PloS one. 2015;10(8):e0135393 10.1371/journal.pone.0135393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13306–11. 10.1073/pnas.0405220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokumo M, Toyooka S, Kiura K, Shigematsu H, Tomii K, Aoe M, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11(3):1167–73. . [PubMed] [Google Scholar]
- 7.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer research. 2004;64(24):8919–23. 10.1158/0008-5472.CAN-04-2818 . [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Feng J, Zhou C, Wu YL, Liu XQ, Wang C, et al. Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomised, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC). Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24(6):1615–22. 10.1093/annonc/mdt012 . [DOI] [PubMed] [Google Scholar]
- 9.Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6(11):1872–80. 10.1097/JTO.0b013e31822adaf7 . [DOI] [PubMed] [Google Scholar]
- 10.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. The Lancet Oncology. 2015;16(2):141–51. 10.1016/S1470-2045(14)71173-8 . [DOI] [PubMed] [Google Scholar]
- 11.Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clinical pharmacology and therapeutics. 2012;92(2):203–13. 10.1038/clpt.2012.73 . [DOI] [PubMed] [Google Scholar]
- 12.Chu MP, Ghosh S, Chambers CR, Basappa N, Butts CA, Chu Q, et al. Gastric Acid suppression is associated with decreased erlotinib efficacy in non-small-cell lung cancer. Clinical lung cancer. 2015;16(1):33–9. 10.1016/j.cllc.2014.07.005 . [DOI] [PubMed] [Google Scholar]
- 13.Hilton JF, Tu D, Seymour L, Shepherd FA, Bradbury PA. An evaluation of the possible interaction of gastric acid suppressing medication and the EGFR tyrosine kinase inhibitor erlotinib. Lung cancer. 2013;82(1):136–42. 10.1016/j.lungcan.2013.06.008 . [DOI] [PubMed] [Google Scholar]
- 14.Deng Y, Feng W, Wu J, Chen Z, Tang Y, Zhang H, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Molecular and clinical oncology. 2014;2(1):116–20. 10.3892/mco.2013.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer chemotherapy and pharmacology. 2012;70(3):399–405. 10.1007/s00280-012-1929-4 . [DOI] [PubMed] [Google Scholar]
- 16.Fukudo M, Ikemi Y, Togashi Y, Masago K, Kim YH, Mio T, et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clinical pharmacokinetics. 2013;52(7):593–609. 10.1007/s40262-013-0058-5 . [DOI] [PubMed] [Google Scholar]
- 17.Togashi Y, Masago K, Fukudo M, Terada T, Fujita S, Irisa K, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5(7):950–5. 10.1097/JTO.0b013e3181e2138b . [DOI] [PubMed] [Google Scholar]
- 18.Gurpide A, Perez-Gracia JL, Lopez-Picazo JM, Moreno M, Zubieta JL, Martin-Algarra S, et al. Activity of gefitinib in central nervous system metastases in patients with non-small-cell lung cancer: two case reports and a review of the literature. Clinical lung cancer. 2005;7(2):138–40. . [DOI] [PubMed] [Google Scholar]
- 19.Horiike A, Kimura H, Nishio K, Ohyanagi F, Satoh Y, Okumura S, et al. Detection of epidermal growth factor receptor mutation in transbronchial needle aspirates of non-small cell lung cancer. Chest. 2007;131(6):1628–34. 10.1378/chest.06-1673 . [DOI] [PubMed] [Google Scholar]
- 20.Chen YM, Lai CH, Chang HC, Chao TY, Tseng CC, Fang WF, et al. Baseline and Trend of Lymphocyte-to-Monocyte Ratio as Prognostic Factors in Epidermal Growth Factor Receptor Mutant Non-Small Cell Lung Cancer Patients Treated with First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. PloS one. 2015;10(8):e0136252 10.1371/journal.pone.0136252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer. 2009;45(2):228–47. 10.1016/j.ejca.2008.10.026 . [DOI] [PubMed] [Google Scholar]
- 22.Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(23):5873–82. 10.1158/1078-0432.CCR-10-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.