Abstract
Background and Objective
Sentinel lymph node (SLN) metastasis size is an important predictor of non-SLN involvement. The goal of this study was to construct a nomogram incorporating SLN metastasis size to accurately predict non-SLN involvement in patients with SLN-positive disease.
Methods
We identified 509 patients with invasive breast cancer with a positive SLN who underwent completion axillary lymph node dissection (ALND). Clinicopathologic data including age, tumor size, histology, grade, presence of multifocal disease, estrogen and progesterone receptor status, HER2/neu status, presence of lymphovascular invasion (LVI), number of SLN(s) identified, number of positive SLN(s), maximum SLN metastasis size and the presence of extranodal extension were recorded. Univariate and multivariate logistic regression analyses identified factors predictive of positive non-SLNs. Using these variables, a nomogram was constructed and subsequently validated using an external cohort of 464 patients.
Results
On univariate analysis, the following factors were predictive of positive non-SLNs: number of SLN identified (p<.001), number of positive SLN (p<.001), SLN metastasis size (p<.001), extranodal extension (p<.001), tumor size (p=.001), LVI (p=.019) and histology (p=.034). On multivariate analysis, all factors remained significant except LVI. A nomogram was created using these variables (AUC=0.80; 95% CI:0.75–0.84). When applied to an external cohort, the nomogram was accurate and discriminating with an AUC=0.74 (95% CI:0.68 – 0.77).
Conclusion
SLN metastasis size is an important predictor for identifying non-SLN disease. In this study, we incorporated SLN metastasis size into a nomogram that accurately predicts the likelihood of having additional axillary metastasis and can assist in personalizing surgical management of breast cancer.
INTRODUCTION
Determination of the axillary lymph node status remains important for breast cancer patients because it is an essential prognostic factor and guides adjuvant therapy decisions. For patients who present with clinically node negative disease, sentinel lymph node (SLN) biopsy is widely used for nodal staging. Multiple studies have confirmed the accuracy of SLN biopsy for axillary staging and the reduced morbidity when compared with a complete axillary lymph node dissection (ALND). 1–3 In approximately 60–80% of patients, the SLN will be negative and no further axillary surgery will be performed. 4–12 In patients found to have a positive SLN, the reported incidence of non-SLN involvement varies greatly with individual centers reporting additional disease in between 40% and 60% of patients (reviewed by Grube and Giuliano). 13 In highly select patient populations, such as the patients reported in the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, this percentage is lower (27%).14
Differences in the rates of finding additional axillary disease may be in part due to the methods by which patients are evaluated preoperatively to include whether axillary ultrasound and fine needle aspiration biopsy is used. These procedures are being increasingly used in breast centers to clinically stage the axilla in breast cancer patients.15 Finding metastatic disease preoperatively on ultrasound would stage the patient as having clinically node-positive disease and would preclude the use of SLN biopsy. The routine use of axillary ultrasound has been reported to decrease SLN biopsy procedures by between 8% and 26%. 16–18 Because preoperative axillary ultrasound selects out patients with greater nodal burden, it is also likely that its routine use would reduce the overall axillary disease burden in those undergoing SLN biopsy.
Given the variability in the rates of additional axillary disease, there remains debate as to which patients with a positive SLN should undergo completion ALND. Several groups have addressed this issue by developing mathematical models to predict non-SLN status in patients with a positive SLN. 6, 19–24 Proponents of these models suggest that completion ALND would only be valuable in those patients likely to have additional nodal disease. The most widely used model is the nomogram developed by Van Zee and colleagues at Memorial Sloan-Kettering Cancer Center (MSKCC). 24 This nomogram has been validated using several external cohorts and is based on 8 clinicopathological variables including pathologic tumor size, histology, nuclear grade, lymphovascular invasion (LVI), multifocality, estrogen receptor (ER) status, method of SLN metastasis detection, number of positive SLNs and number of negative SLNs. The MSKCC nomogram does not incorporate the size of the SLN metastasis; however, it has been suggested that the method of detection serves as a surrogate for this variable. Our group has also published a predictive model for additional non-SLN disease based on a multivariate analysis that identified four factors: primary tumor size > 2cm, SLN metastasis > 2 mm, and the presence of LVI were predictors of non-SLN involvement while the number of SLNs harvested was a significant negative predictor. 6 In this model, the size of the SLN metastasis was the single most important factor in predicting the presence of additional nodal involvement. Multiple other groups have also identified the size of the SLN metastasis as a powerful independent predictor of non-SLN involvement. 4, 8–11, 19, 20, 25 These previous studies have all evaluated size as a dichotomized variable.
The 6th edition of the American Joint Committee on Cancer staging manual emphasized the size of lymph node metastases in breast cancer staging. 26 In this iteration of the staging system, as well as the subsequent 7th edition, the categorization of lymph nodes was based on the maximum size of the metastasis. Foci of disease ≤ 0.2 mm are referred to as isolated tumor cells (pN0(i+)), > 0.2 – 2.0 mm are micrometastases (pN1mi) and > 2.0 mm are macrometastases. This system implies that larger foci of metastatic disease portend a worse prognosis. The College of American Pathologists has recommended reporting the size of the largest metastatic deposit in their protocol for examination of specimens from patients with invasive breast carcinoma. 27 Given the importance of the SLN metastasis size and the availability of these data in routine pathology reports, we sought to determine whether including SLN metastasis size as a continuous variable could be added to standard clinicopathologic factors to construct a predictive nomogram that predicts non-SLN involvement. Importantly, this nomogram was constructed using data from a contemporary cohort of patients, the majority of whom had been evaluated preoperatively with axillary ultrasound and fine needle aspiration biopsy.
METHODS
Patients
A prospectively maintained database of breast cancer patients undergoing surgery at The University of Texas MD Anderson Cancer Center was used to identify patients with clinically node negative breast cancer who had a positive SLN and underwent completion ALND from December 1996 through August 2007. Patients receiving neoadjuvant chemotherapy were excluded. Demographic data including patient age and gender were noted. The following clinicopathologic data were recorded: primary tumor size, presence of multifocal or multicentric disease, histology (invasive ductal, invasive lobular, mixed invasive, or other), tumor grade (modified Black’s nuclear grade), estrogen and progesterone receptor (ER and PR) and HER2/neu (HER2) status, presence of lymphovascular invasion (LVI), number of SLNs identified, number of positive SLNs, maximum SLN metastasis size (mm) and presence of extranodal extension. Histology was recorded as “other” for tubular, mucinous, papillary and medullary tumors. For hormone receptor status, we considered >10% staining of the cells by immunohistochemistry (IHC) to be positive. Tumors overexpressed HER2 if they were 3+ by IHC or positive by fluorescence in-situ hybridization. Complete data were available for 509 patients who comprised the study population used to construct the nomogram. To externally validate the nomogram, a second cohort of 464 patients with clinically node-negative breast cancer who had a positive SLN and underwent completion ALND at the Mayo Clinic between November 1997 and June 2004 was also identified. The institutional review boards at the MD Anderson Cancer Center and the Mayo Clinic approved this study.
Sentinel Lymph Node Biopsy
SLN biopsy was performed as previously described. 28 Briefly, intraoperative lymphatic mapping was performed using isosulfan blue dye (Lymphazurin, US Surgical Corporation, Norwalk, CT), 99mTc-labeled sulfur colloid, or a combination of the two agents. Mapping agents were injected in the subdermal, subareolar, or peritumoral location at the discretion of the operating surgeon. 99mTc-labeled sulfur colloid was injected on the day prior to surgery (2.5 mCi) or on the day of surgery (0.5 mCi). Blue dye was injected at the time of surgery at a volume of 3 to 5 cc. SLNs were detected by the uptake of radiolabeled colloid detected with a handheld gamma detection probe (Neoprobe, US Surgical Corporation), the visualization of blue dye, or both.
Sentinel Lymph Node Pathologic Assessment
Prior to April 2000, SLNs were serially sectioned along the short axis at 2- to 3-mm intervals; sections were embedded in paraffin blocks, and one level from each block was stained with hematoxylin and eosin (H&E). Beginning in April 2000, SLNs were grossly processed in the same manner, and each paraffin block was then serially sectioned at 5-μm intervals with two levels evaluated by routine H&E staining and one level analyzed for cytokeratin by IHC. 29 SLNs were considered positive if any focus of metastatic disease was identified using either H&E or IHC. For positive SLNs, the size of the largest metastasis was recorded. For SLNs described only as containing isolated tumor cells (ITCs), size was recorded as 0.1 mm. For a prior study, we had identified all patients who had undergone SLND prior to April 2000 and had a histologically negative SLN. SLNs from these patients were reanalyzed by a senior breast pathologist who resectioned the paraffin blocks at 5-μm intervals. In addition to H&E staining, IHC was used to analyze one level for cytokeratin. 30 The results of the repeat pathologic evaluation for these patients were included for the current study.
Data Analysis
Clinicopathologic data were tabulated for both the MD Anderson and Mayo Clinic cohorts. Differences in clinicopathologic factors between the groups were assessed using t-tests for continuous variables and the chi-squared test for categorical variables. Univariate logistic regression analyses were performed on the data from the MD Anderson cohort to determine which factors were predictors of positive non-SLNs. Factors that were statistically significant at p<0.05 were fit into a multivariate logistic regression model. All of these factors except LVI were jointly statistically significant in multivariate analysis, and no model selection was performed.
A nomogram to predict the likelihood of non-SLN metastasis was developed on the basis of the results of this model. The discrimination of the model was measured using the area under the receiver operating characteristic (ROC) curve (AUC). The nomogram was validated internally with 10,000 bootstrap samples, and the bootstrap-adjusted AUC was estimated along with a 95% confidence interval (CI). Calibration (the association between the observed outcome frequencies and the predicted probabilities) was performed by categorizing patients on the basis of their predictive probability from the model of having a non-SLN metastasis. The observed outcome frequency was determined for each category, and the relationship between the actual and predicted probability is shown graphically. A logistic regression model that considered a non-SLN metastasis as the event was fit with a single covariate, the linear predictor from the multivariate regression model. A standard measurement of calibration, the unreliability [U] statistic, which is a likelihood ratio statistic testing the joint hypothesis that the intercept from this logistic regression model equals zero and the slope equals one, was calculated and tested using a chi-square test. The average and maximal errors between predictions and observed frequencies were also obtained from this model.
The nomogram was subsequently validated using the external cohort from the Mayo Clinic. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC) and R 2.10.1 (http://www.r-project.org) software.
RESULTS
Clinicopathologic characteristics of the MD Anderson and Mayo Clinic cohorts are listed in Table 1. Table 2 shows the results of the univariate analysis performed on data from the MD Anderson cohort to determine which factors were predictive of positive non-SLNs. Factors found to be significant in this analysis were primary tumor size, histology, the presence of LVI, number of SLNs found, number of positive SLNs, the maximum SLN metastasis size measured as a continuous variable and the presence of extranodal extension (p<.05 for each). These significant variables were then incorporated into a multivariate logistic regression model (Table 3).
Table 1.
Clinicopathologic Characteristics for MD Anderson and Mayo Clinic Patient Cohorts
| Characteristic | MD Anderson (n=509) | Mayo Clinic (n=464) | P-value |
|---|---|---|---|
|
| |||
| Age | .02 | ||
| median | 54 | 56 | |
| range | 22–84 | 26–88 | |
|
| |||
| Pathologic tumor size (cm) | .04 | ||
| mean | 2.3 | 2.6 | |
| range | 0.1–12.0 | 0.1–15.0 | |
|
| |||
| Histology | <.001 | ||
| IDC | 388 (76%) | 357 (77%) | |
| ILC | 42 (8%) | 95 (20%) | |
| mixed IDC/ILC | 66 (13%) | 12 (3%) | |
| other | 13 (3%) | 0 | |
|
| |||
| Grade | <.001 | ||
| I | 51 (10%) | 107 (23%) | |
| II | 274 (54%) | 261 (56%) | |
| III | 184 (36%) | 96 (21%) | |
|
| |||
| Multifocal disease | 1.0 | ||
| yes | 85 (17%) | 78 (17%) | |
| no | 424 (83%) | 386 (83%) | |
|
| |||
| ER | .46 | ||
| positive | 435 (85%) | 405 (87%) | |
| negative | 74 (15%) | 59 (13%) | |
|
| |||
| PR | <.001 | ||
| positive | 348 (68%) | 380 (82%) | |
| negative | 161 (32%) | 84 (18%) | |
|
| |||
| HER2 | |||
| positive | 96 (19%) | Data not | |
| negative | 413 (81%) | available | |
|
| |||
| LVI | .02 | ||
| present | 189 (37%) | 206 (44%) | |
| absent | 320 (63%) | 258 (56%) | |
|
| |||
| Number of SLN identified | <.001 | ||
| median | 3 | 2 | |
| range | 1–13 | 1–11 | |
|
| |||
| Number of positive SLN | <.001 | ||
| median | 1 | 1 | |
| range | 1–6 | 1–8 | |
|
| |||
| SLN maximum metastasis size (mm) | .36 | ||
| median | 4 | 4.2 | |
| range | .1–25 | .1–26 | |
|
| |||
| Extranodal extension | <.001 | ||
| present | 115 (23%) | 211 (45%) | |
| absent | 394 (77%) | 253 (55%) | |
|
| |||
| Status of non-SLN | <.001 | ||
| positive | 149 (29%) | 202 (44%) | |
| negative | 360 (71%) | 262 (56%) | |
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor ; PR, progesterone receptor, HER2, HER2/neu ; LVI, lymphovascular invasion ; SLN, sentinel lymph node
Table 2.
Univariate Analysis of Clinicopathologic Factors Predictive of Non-SLN Metastases
| Characteristic | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Age (per year) | 1.002 | 0.985–1.019 | .83 |
|
| |||
| Pathologic tumor size (cm) (per unit) | 1.199 | 1.074–1.337 | .001 |
|
| |||
| Histology | .03 | ||
| IDC | Reference | - | |
| ILC | 1.731 | 0.905–3.310 | |
| mixed IDC/ILC | 0.513 | 0.265–0.994 | |
| other | 0.420 | 0.092–1.923 | |
|
| |||
| Grade | .53 | ||
| I | Reference | - | |
| II | 1.317 | 0.655–2.645 | |
| III | 1.496 | 0.730–3.067 | |
|
| |||
| Multifocal disease | .28 | ||
| yes | 1.312 | 0.799–2.154 | |
| no | Reference | - | |
|
| |||
| ER | .65 | ||
| positive | 1.138 | 0.655–1.978 | |
| negative | Reference | - | |
|
| |||
| PR | .15 | ||
| positive | 0.744 | 0.497–1.114 | |
| negative | Reference | - | |
|
| |||
| HER2 | .64 | ||
| positive | 1.123 | 0.694–1.817 | |
| negative | Reference | - | |
|
| |||
| LVI | .02 | ||
| present | 1.594 | 1.080–2.353 | |
| absent | Reference | ||
|
| |||
| Number of SLN identified | 0.765 | 0.666–0.879 | <.001 |
|
| |||
| Number of positive SLN | 1.706 | 1.323–2.200 | <.001 |
|
| |||
| SLN maximum metastasis size (mm) | 1.186 | 1.129–1.246 | <.001 |
|
| |||
| Extranodal extension | <.001 | ||
| present | 3.388 | 2.195–5.230 | |
| absent | Reference | - | |
Abbreviations: CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor ; PR, progesterone receptor, HER2, HER2/neu ; LVI, lymphovascular invasion ; SLN, sentinel lymph node
Table 3.
Multivariate Analysis of Clinicopathologic Factors with Non-SLN Metastases
| Characteristic | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Pathologic tumor size (cm) (per unit) | 1.227 | 1.070–1.408 | .004 |
|
| |||
| Histology | .03 | ||
| IDC | Reference | - | |
| ILC | 1.331 | 0.598–2.962 | |
| mixed IDC/ILC | 0.336 | 0.149–0.756 | |
| other | 0.463 | 0.090–2.379 | |
|
| |||
| LVI | .14 | ||
| present | 1.594 | 0.8292–2.279 | |
| absent | Reference | ||
|
| |||
| Number of SLN identified | 0.604 | 0.502–0.727 | <.001 |
|
| |||
| Number of positive SLN | 2.120 | 1.486–3.026 | <.001 |
|
| |||
| SLN maximum metastasis size (mm) | 1.136 | 1.076–1.199 | <.001 |
|
| |||
| Extranodal extension | .01 | ||
| present | 1.917 | 1.163–3.160 | |
| absent | Reference | ||
Abbreviations: CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LVI, lymphovascular invasion ; SLN, sentinel lymph node
The significant variables were also used to develop a nomogram to predict positive non-SLNs (Figure 1). The overall predictive accuracy of the nomogram, as measured by the AUC was 0.80 (95% CI: 0.75–0.84) (Figure 2A). The bootstrap-adjusted AUC was 0.78. To further investigate the utility of including SLN metastasis size as a continuous variable, we also constructed a nomogram using SLN metastasis size as a categorical variable, i.e., ITC, micrometastasis or macrometastasis (not shown). The overall predictive accuracy of this second nomogram was 0.71, suggesting that using size as a continuous variable allowed for improved prediction of non-SLN disease.
Figure 1. Nomogram to predict likelihood of additional, non-sentinel lymph node metastases in a patient with a positive SLN.
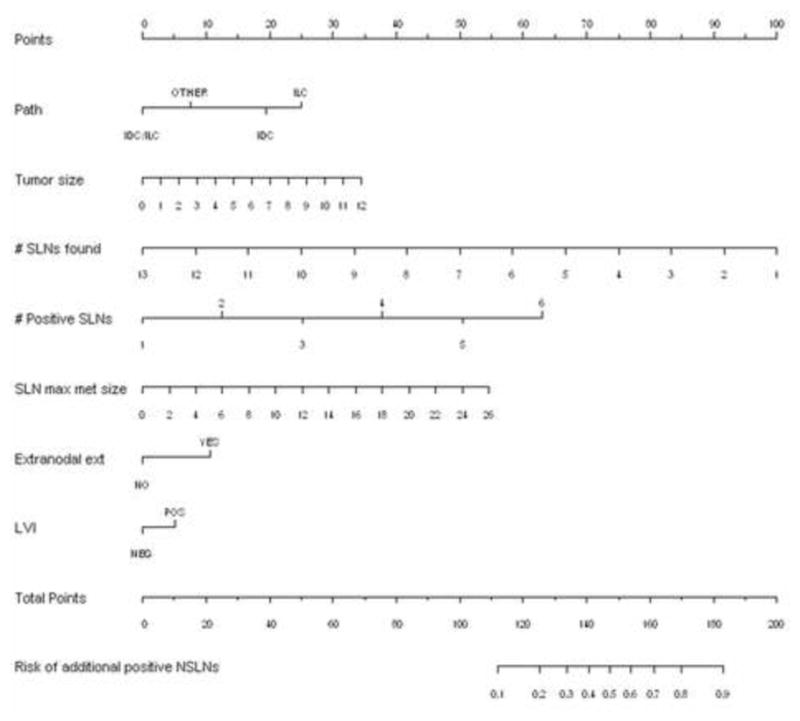
The first row (Points) is the point assignment for each variable. Rows 2–8 contain the variables included in the model. For an individual patient, each variable is assigned a point value based on the characteristic. A vertical line is made between the appropriate variable value and the Points line. The assigned points for the 7 variables are summed and the total is found in row 9 (Total Points). Once the total points value is determined, a vertical line is made between row 9 and row 10 to determine the risk of additional positive non-SLNs. SLN maximum metastasis (max met) size is measured in millimeters.
Figure 2. Validation of nomogram performance.
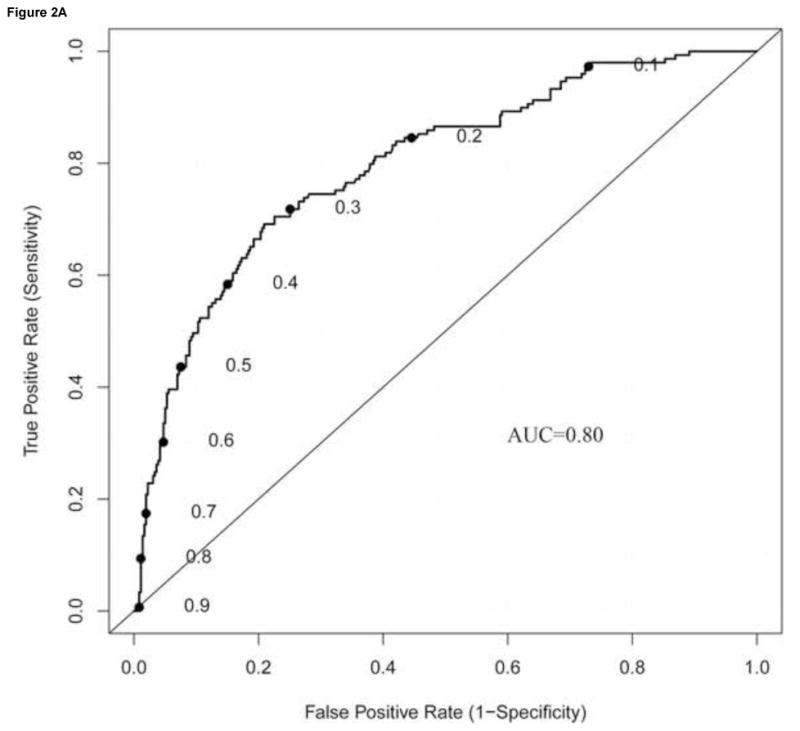
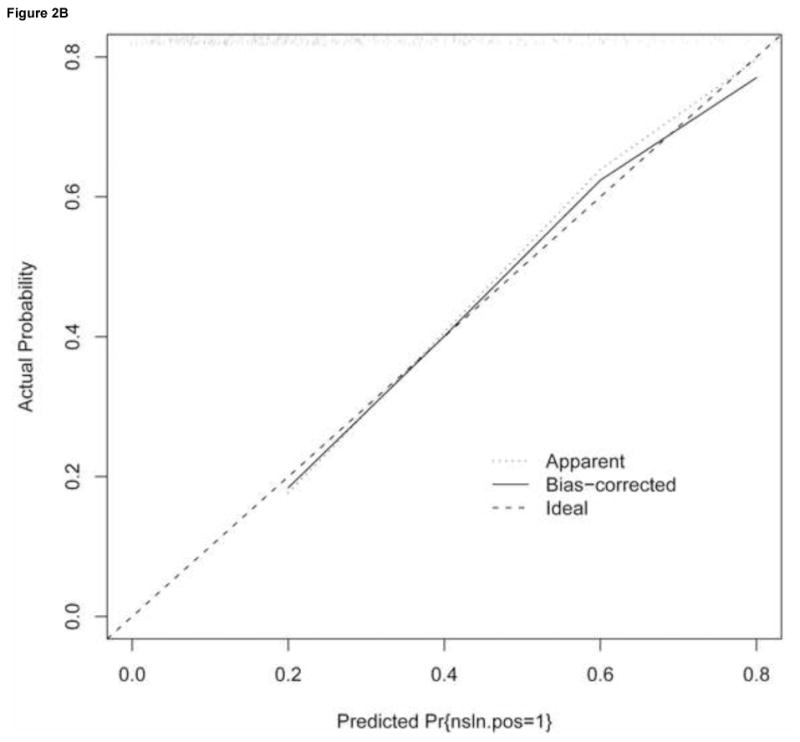
The performance of the nomogram was quantified with respect to (A) discrimination and (B) calibration. Discrimination was quantified with the area under the receiver operating characteristic (ROC) curve (AUC). To construct the calibration curve, a histogram of the probabilities calculated using the nomogram was plotted along the horizontal axis. The vertical axis represents the actual incidence on non-SLN positivity.
A calibration curve for the nomogram using SLN metastasis size as a continuous variable was generated to evaluate the agreement between observed outcome frequencies and predicted probabilities. As shown in Figure 2B, the nomogram was well calibrated with an average absolute difference in predicted and calibrated probabilities of 1.4% and a maximum error of 3.9%. The unreliability statistic was 0.4%, with no evidence of a difference between the predicted and the observed probabilities (p=0.99).
To further investigate the clinical utility of the nomogram, we determined the false-negative rate. A cutoff value of 10% or less was considered to define a subgroup of patients with a low predicted probability of metastatic disease in non-SLNs. This value was chosen because it was previously published by Coutant and colleagues in a prospective multicenter study conducted to compare models for predicting non-SLN involvement in patients with positive SLNs. 31 Among the 101 patients in our study population predicted to have a probability of non-SLN metastasis of ≤ 10%, 4 patients had a positive non-SLN, for a 4% false negative rate.
To determine if the nomogram had utility outside of our institution, we performed additional analyses using data from a cohort of 464 patients from the Mayo Clinic (Table 1). When compared with the MD Anderson cohort, this external cohort had a greater percentage of positive non-SLNs (44% versus 29%, p<.001). This was consistent with the Mayo Clinic cohort having larger primary tumors, a greater percentage of tumors with LVI, and a greater percentage of SLNs with extranodal involvement (p<.05 for each factor, Table 1). When the nomogram was applied to this external cohort, it was accurate and discriminating, with an AUC of 0.74 (95% CI: 0.70–0.79) (Figure 3). During the time period during which the Mayo Clinic cohort was evaluated (November 1997 to June 2004), HER2/neu status was not routinely assessed and thus, this data was not available.
Figure 3. External validation.
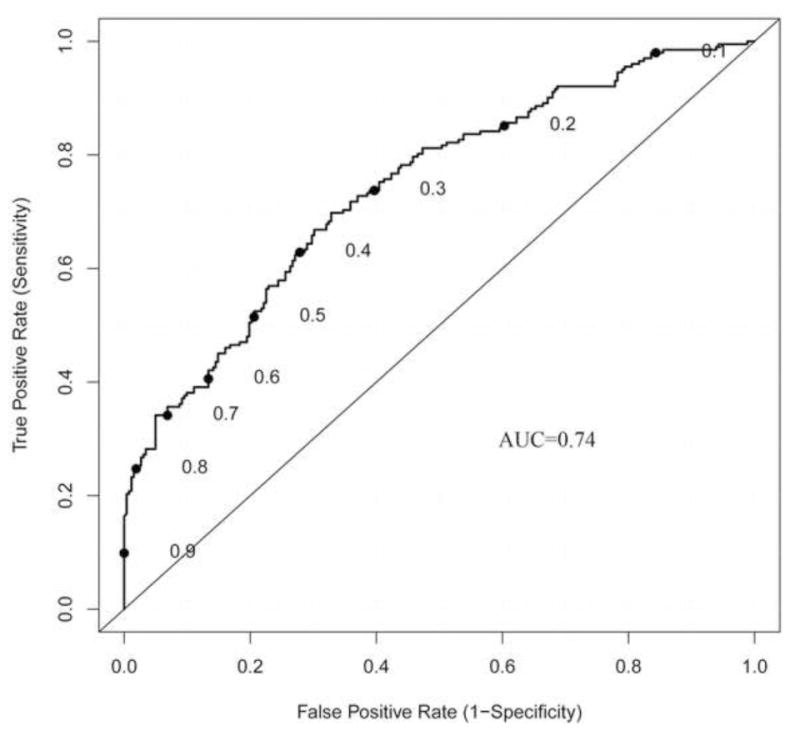
To determine if the nomogram is generalizable to external patient populations we used a cohort of patients from the Mayo Clinic. The AUC of 0.74 confirmed that the nomogram has broad applicability.
DISCUSSION
We describe here the development and validation of a predictive nomogram that incorporates the size of the SLN metastatasis as a continuous variable. This nomogram was developed using a contemporary cohort of patients, the majority of whom were evaluated by axillary ultrasound preoperatively. The nomogram is also reliable for use at other institutions and among patients with varying clinicopathologic factors, as evidenced by the analysis of the nomogram’s performance using patient data from the Mayo Clinic. Importantly, during the time the patients evaluated in this study were treated, preoperative ultrasound was not routinely used at the Mayo Clinic which further strengthens the validation.
The nomogram utilizes clinicopathologic data that are readily available, including the size of the SLN metastasis, which in accordance with recommendations from the College of American Pathologists, is now routinely recorded in pathology reports at many institutions. Importantly, to confirm the value of incorporating SLN metastasis size as a continuous variable, we developed a second nomogram using metastasis size as a categorical variable and found that it was not as discriminating. Data obtained by applying this nomogram to a patient with a positive SLN can facilitate further conversation with that patient regarding the potential for additional axillary disease and the utility of performing a completion ALND.
Predictive models such as nomograms have become increasingly popular in the field of oncology. 32 The appeal of a nomogram is that it provides reliable prognostic information that is individualized to a given patient. This information is in the form of a numerical probability of a clinical event, such as the identification of additional metastases in non-SLNs. In order for a nomogram to have clinical utility, it must predict this probability as accurately as possible. The ability of a nomogram to separate patients with different outcomes is referred to as discrimination, an evaluation criterion for predictive models that is most often quantified using the AUC. 32, 33 The AUC can range from 0 to 1, with 1 being perfect concordance and 0 indicating perfect discordance. An AUC of 0.5 would indicate that the nomogram is no better than chance at predicting the likelihood of an outcome. In reality, discrimination as measured by the AUC, has limited clinical significance as it provides a measure of whether the relative ranking of individual predictions is in the correct order. 33 In contrast, calibration, which determines how far the predictions are from actual outcomes, has high clinical significance. In our nomogram, both discrimination and calibration are excellent. In a prospective validation of models designed to predict non-SLN status in patients with a SLN metastasis, Coutant and colleagues used a cohort of 561 SLN-positive patients who underwent ALND to evaluate the AUC and calibration of 9 previously published predictive models, including 4 nomograms (Table 4). 31 In that study, they calculated an AUC for the nomograms ranging from 0.72 to 0.78. The AUC of our nomogram applied to both an internal cohort (AUC=0.80) and to the external validation cohort (AUC=0.74) compares favorably. With respect to calibration, Coutant et al. found that two of the four nomograms were well calibrated, whereas two others showed differences between the predicted and the observed probability. They also determined that the average differences between the predicted and calibrated probabilities for these nomograms ranged from 3% to 25%. For our nomogram, the average difference between predicted and calibrated probabilities was 1.4%, with a maximum difference of 3.9%. This information has clinical utility because it affords the clinician the opportunity to provide the patient with information regarding both the predicted probability of additional non-SLN metastases and the range of probability. If a patient had a predicted probability of 12% of additional non-SLN metastases, we could counsel them that this risk may vary between 8% and 16%.
Table 4.
Validation of Nomograms for Predicting Positive Non-Sentinel Lymph Nodes in Patients with a Positive Sentinel Lymph Node*
| Nomogram | AUC | 95% CI | Calibration Plot P value |
|---|---|---|---|
| Memorial Sloan Kettering24 | 0.78 | 0.76 to 0.81 | < 0.001 |
| Mayo Clinic20 | 0.74 | 0.71 to 0.76 | 0.08 |
| Cambridge22 | 0.73 | 0.70 to 0.75 | 0.1 |
| Stanford21 | 0.72 | 0.70 to 0.74 | <0.001 |
Table modified from Coutant et al. 31 Data reflects evaluation of four nomograms using a prospective cohort of 561 patients.
To further investigate the clinical utility of our nomogram, we looked at the false-negative rate. The previously published study by Coutant et al used a 10% or less cutoff value to identify a subgroup of patients with a low predicted probability of metastatic non-SLNs. 31 Use of this cutoff value was also supported by a previous study from our institution in which we validated the MSKCC nomogram. In that study, we used a cohort of 141 patients and found that overall the nomogram was valid with an AUC of 0.69. 34 There was a good correlation between the observed percentage of non-SLN metastases and the average predicted probability of non-SLN metastases. However, we demonstrated that the nomogram was least robust for patients who had a predicted probability of ≤ 10%. For the 28 patients in that risk group, the actual percentage of patients with positive non-SLN metastases was > 20%. Another group evaluating the accuracy of the MSKCC nomogram using a population of 588 patients, including 213 with micrometastases in their SLN, found that it was reliable for patients with macrometastases in the SLN (AUC=.72) but not for those with micrometastases (AUC=.54). 35 In the current study, we found that only 4 (4%) out of 101 patients with a predicted probability of ≤ 10% had a positive non-SLN. These data suggest that our nomogram is reliable for patients with small-volume SLN metastases.
Recent studies reporting data from large databases have demonstrated a trend towards omitting completion ALND in patients with a positive SLN. Using the National Cancer Data Base (NCDB) data from 1998–2005, Bilimoria et al found that 21% of patients with a positive SLN did not undergo completion ALND. 36 Similarly, our group has reviewed data from the Surveillance, Epidemiology, and End Results (SEER) database (1998–2004) and reported that 16% of SLN-positive patients did not undergo completion ALND. 37 The trend has been seen most often in older patients with low-grade, ER-positive tumors. In that report, we suggested that one reason for the decrease in performing completion ALND was the increased use of predictive nomograms. We believe that nomograms can play an important role in identifying patients in whom a completion ALND may safely be omitted. The most recent report of the Early Breast Cancer Trialists’ Collaborative group demonstrated that for every four local recurrences avoided by 5 years, one breast cancer related death was avoided at 15 years. 38 Morrow suggested that, to reach this level of difference in recurrence by omitting axillary dissection, the risk of additional nodal involvement would need to be 30% or greater. 39 Of the 509 patients in the cohort used to establish this nomogram, 197 had a predicted probability of non-SLN metastases of ≥ 30% suggesting that approximately 40% of our patients would have hit that threshold.
Data published by the American College of Surgeons Oncology Group from the ACOSOG Z0011 trial have questioned the need to perform a completion ALND regardless of the predicted probability of finding additional nodal disease. 40 In that study, patients with clinical T1/T2N0 disease who were undergoing breast conserving surgery and whole breast radiation, and had a positive SLN, were randomized to either completion ALND or no further surgery. At a median follow-up of 6.3 years, the rate of axillary recurrence was less then 1% in both arms. 40 There are important aspects of this trial that limit its general applicability to all breast cancer patients. All patients in Z0011 underwent breast conserving therapy and whole breast irradiation and it is known that the tangential fields for whole breast irradiation cover a significant portion of the axilla. 41, 42 Therefore, the data are not applicable to patients undergoing mastectomy. In addition, the Z0011 trial had a low proportion of patients with invasive lobular cancer (7%), over 60% of patients were 50 years of age or older, approximately 70% had T1 tumors, 83% were ER positive and 35% had micrometastases in the SLN. Therefore, the trial enrolled a highly select population of patients. We have implemented the Z0011 data into practice at our institution, however there are patients who meet Z0011 inclusion criteria that were not well represented in the trial population and in whom we use predicted probabilities of additional disease as facilitated by this nomogram to counsel and assist in making the decision as to whether to recommend completion ALND.
In conclusion, there continues to be discussion and debate among breast cancer surgeons regarding the need to perform completion ALND in patients with a positive SLN. This nomogram incorporating SLN metastasis size is another tool that can be utilized by surgeons to more effectively counsel individual patients, thereby helping to personalize the surgical treatment of their breast cancer. The nomogram is available at http://www3.mdanderson.org/app/medcalc/bc_nomogram2.
References
- 1.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222(3):394–9. doi: 10.1097/00000658-199509000-00016. discussion 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 3.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 4.Bolster MJ, Peer PG, Bult P, et al. Risk factors for non-sentinel lymph node metastases in patients with breast cancer. The outcome of a multi-institutional study. Ann Surg Oncol. 2007;14(1):181–9. doi: 10.1245/s10434-006-9065-1. [DOI] [PubMed] [Google Scholar]
- 5.Chu KU, Turner RR, Hansen NM, et al. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229(4):536–41. doi: 10.1097/00000658-199904000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang RF, Krishnamurthy S, Hunt KK, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10(3):248–54. doi: 10.1245/aso.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Joseph KA, El-Tamer M, Komenaka I, et al. Predictors of nonsentinel node metastasis in patients with breast cancer after sentinel node metastasis. Arch Surg. 2004;139(6):648–51. doi: 10.1001/archsurg.139.6.648. [DOI] [PubMed] [Google Scholar]
- 8.Ozmen V, Karanlik H, Cabioglu N, et al. Factors predicting the sentinel and non-sentinel lymph node metastases in breast cancer. Breast Cancer Res Treat. 2006;95(1):1–6. doi: 10.1007/s10549-005-9007-9. [DOI] [PubMed] [Google Scholar]
- 9.Schrenk P, Konstantiniuk P, Wolfl S, et al. Prediction of non-sentinel lymph node status in breast cancer with a micrometastatic sentinel node. Br J Surg. 2005;92(6):707–13. doi: 10.1002/bjs.4937. [DOI] [PubMed] [Google Scholar]
- 10.Wada N, Imoto S, Yamauchi C, et al. Predictors of tumour involvement in remaining axillary lymph nodes of breast cancer patients with positive sentinel lymph node. Eur J Surg Oncol. 2006;32(1):29–33. doi: 10.1016/j.ejso.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Weiser MR, Montgomery LL, Tan LK, et al. Lymphovascular invasion enhances the prediction of non-sentinel node metastases in breast cancer patients with positive sentinel nodes. Ann Surg Oncol. 2001;8(2):145–9. doi: 10.1007/s10434-001-0145-y. [DOI] [PubMed] [Google Scholar]
- 12.Wong SL, Edwards MJ, Chao C, et al. Predicting the status of the nonsentinel axillary nodes: a multicenter study. Arch Surg. 2001;136(5):563–8. doi: 10.1001/archsurg.136.5.563. [DOI] [PubMed] [Google Scholar]
- 13.Grube BJ, Giuliano AE. Observation of the breast cancer patient with a tumor-positive sentinel node: implications of the ACOSOG Z0011 trial. Semin Surg Oncol. 2001;20(3):230–7. doi: 10.1002/ssu.1038. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 252(3):426–32. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boughey JC, Moriarty JP, Degnim AC, et al. Cost modeling of preoperative axillary ultrasound and fine-needle aspiration to guide surgery for invasive breast cancer. Ann Surg Oncol. 17(4):953–8. doi: 10.1245/s10434-010-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deurloo EE, Tanis PJ, Gilhuijs KG, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer. 2003;39(8):1068–73. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 17.Mathijssen IM, Strijdhorst H, Kiestra SK, Wereldsma JC. Added value of ultrasound in screening the clinically negative axilla in breast cancer. J Surg Oncol. 2006;94(5):364–7. doi: 10.1002/jso.20590. [DOI] [PubMed] [Google Scholar]
- 18.van Rijk MC, Deurloo EE, Nieweg OE, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol. 2006;13(1):31–5. doi: 10.1245/ASO.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Barranger E, Coutant C, Flahault A, et al. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91(2):113–9. doi: 10.1007/s10549-004-5781-z. [DOI] [PubMed] [Google Scholar]
- 20.Degnim AC, Reynolds C, Pantvaidya G, et al. Nonsentinel node metastasis in breast cancer patients: assessment of an existing and a new predictive nomogram. Am J Surg. 2005;190(4):543–50. doi: 10.1016/j.amjsurg.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Kohrt HE, Olshen RA, Bermas HR, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. doi: 10.1186/1471-2407-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal A, Provenzano E, Duffy SW, et al. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95(3):302–9. doi: 10.1002/bjs.5943. [DOI] [PubMed] [Google Scholar]
- 23.Saidi RF, Dudrick PS, Remine SG, Mittal VK. Nonsentinel lymph node status after positive sentinel lymph node biopsy in early breast cancer. Am Surg. 2004;70(2):101–5. discussion 105. [PubMed] [Google Scholar]
- 24.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Viale G, Maiorano E, Pruneri G, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241(2):319–25. doi: 10.1097/01.sla.0000150255.30665.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual. 6. New York (NY): Springer-Verlag; 2002. [Google Scholar]
- 27. [accessed Sep 16]; www.cap.org.
- 28.Hunt KK, Yi M, Mittendorf EA, et al. Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy is Accurate and Reduces the Need for Axillary Dissection in Breast Cancer Patients. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 29.Yared MA, Middleton LP, Smith TL, et al. Recommendations for sentinel lymph node processing in breast cancer. Am J Surg Pathol. 2002;26(3):377–82. doi: 10.1097/00000478-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Mittendorf EA, Sahin AA, Tucker SL, et al. Lymphovascular invasion and lobular histology are associated with increased incidence of isolated tumor cells in sentinel lymph nodes from early-stage breast cancer patients. Ann Surg Oncol. 2008;15(12):3369–77. doi: 10.1245/s10434-008-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutant C, Olivier C, Lambaudie E, et al. Comparison of models to predict nonsentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: a prospective multicenter study. J Clin Oncol. 2009;27(17):2800–8. doi: 10.1200/JCO.2008.19.7418. [DOI] [PubMed] [Google Scholar]
- 32.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 33.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 34.Lambert LA, Ayers GD, Meric-Bernstam F. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann Surg Oncol. 2007;14(8):2422–3. doi: 10.1245/s10434-007-9419-3. [DOI] [PubMed] [Google Scholar]
- 35.Alran S, De Rycke Y, Fourchotte V, et al. Validation and limitations of use of a breast cancer nomogram predicting the likelihood of non-sentinel node involvement after positive sentinel node biopsy. Ann Surg Oncol. 2007;14(8):2195–201. doi: 10.1245/s10434-006-9331-2. [DOI] [PubMed] [Google Scholar]
- 36.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–53. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 37.Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17(Suppl 3):343–51. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 39.Morrow M. Patterns of care with a positive sentinel node: echoes of an opportunity missed. Ann Surg Oncol. 2009;16(9):2429–30. doi: 10.1245/s10434-009-0552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–32. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reznik J, Cicchetti MG, Degaspe B, Fitzgerald TJ. Analysis of axillary coverage during tangential radiation therapy to the breast. Int J Radiat Oncol Biol Phys. 2005;61(1):163–8. doi: 10.1016/j.ijrobp.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 42.Schlembach PJ, Buchholz TA, Ross MI, et al. Relationship of sentinel and axillary level I–II lymph nodes to tangential fields used in breast irradiation. Int J Radiat Oncol Biol Phys. 2001;51(3):671–8. doi: 10.1016/s0360-3016(01)01684-4. [DOI] [PubMed] [Google Scholar]


