Significance
Bile acids bind to the nuclear hormone receptor, farnesoid X receptor (FXR). This bile acid–FXR interaction regulates bile acid synthesis, transport, and cholesterol metabolism. Recently, drugs targeting FXR activation have been reported to treat both liver and intestinal inflammatory diseases in both animal models and human clinical trials. Experimental autoimmune encephalomyelitis (EAE) is an inflammatory demyelinating disease of the central nervous system and serves as an animal model for multiple sclerosis (MS). In this study, we found that FXR knockout mice had more severe EAE, and treatment of mice with established EAE with a synthetic FXR agonist, obeticholic acid (6α-ethyl-chenodeoxycholic acid, 6-ECDCA), reduced EAE severity. Thus, we provide an FXR target for development of a previously unidentified disease-modifying therapy for MS.
Keywords: experimental autoimmune encephalomyelitis, farnesoid X receptor, bile acid agonist, obeticholic acid, multiple sclerosis
Abstract
Bile acids are ligands for the nuclear hormone receptor, farnesoid X receptor (FXR). The bile acid–FXR interaction regulates bile acid synthesis, transport, and cholesterol metabolism. Recently, bile acid–FXR regulation has been reported to play an integral role in both hepatic and intestinal inflammation, and in atherosclerosis. In this study, we found that FXR knockout mice had more disease severity in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). Obeticholic acid (6α-ethyl-chenodeoxycholic acid, 6-ECDCA), a synthetic FXR agonist, is an orally available drug that is currently in clinical trials for the treatment of inflammatory diseases such as alcoholic hepatitis, nonalcoholic steatohepatitis, and primary biliary cirrhosis. When we treated mice exhibiting established EAE with 6-ECDCA, or the natural FXR ligand chenodeoxycholic acid (CDCA), clinical disease was ameliorated by (i) suppressing lymphocyte activation and proinflammatory cytokine production; (ii) reducing CD4+ T cells and CD19+ B cell populations and their expression of negative checkpoint regulators programmed cell death protein 1 (PD1), programmed death-ligand 1 (PD-L1), and B and T lymphocyte attenuator (BTLA); (iii) increasing CD8+ T cells and PD1, PDl-1, and BTLA expression; and (iv) reducing VLA-4 expression in both the T- and B-cell populations. Moreover, adoptive transfer of 6-ECDCA– or CDCA-treated donor cells failed to transfer disease in naive recipients. Thus, we show that FXR functions as a negative regulator in neuroinflammation and we highlight that FXR agonists represent a potential previously unidentified therapy for MS.
Bile acid synthesis plays a major role in cholesterol homeostasis in the liver and small intestine (1, 2). Within the liver, bile acids are synthesized from cholesterol and serve as the final end products of cholesterol catabolism. Bile acids are also essential for the adsorption and solubilization of dietary cholesterol and fat-soluble vitamins. When high levels of bile acids accumulate in the body, the nuclear bile acid receptor, farnesoid X receptor (FXR), is activated by its natural bile acid ligands that include chenodeoxycholic acid (CDCA), deoxycholic (DCA), and lithocholic (LCA) (3, 4). This negatively regulates the transcription of the rate-limiting enzyme, cholesterol 7-alpha-hydroxylase (CYP7A1) to inhibit further bile acid synthesis and uptake and to increase the export of bile acids out of the cells (1). CDCA is the highest affinity natural ligand for FXR (4).
FXR is mainly expressed in liver, intestine, kidneys, and adrenal gland, with less expression in adipose tissue and heart (5). FXR was originally identified as a receptor for the cholesterol precursor farnesol, but is now recognized as the primary receptor for bile acid (3, 6). The bile acid–FXR interaction plays an integral role in regulating hepatic inflammation and regeneration, and FXR activation has been shown to attenuate liver injury in a rodent model of autoimmune hepatitis (7). In the intestinal tract, bile acids and FXR prevent bacterial overgrowth and regulate the extent of inflammatory responses and pathology from a compromised barrier (8, 9). Furthermore, FXR activation results in preservation of the intestinal barrier in a rodent model of inflammatory bowel disease (10). Activation of FXR has been reported to inhibit vascular smooth muscle cell (VSMC) inflammation by down-regulating the proinflammatory enzymes inducible nitric oxide synthase and cyclooxygenase-2 expression as well as cell migration into VSMCs (11). This finding suggests that FXR may be a potential target for the progressive inflammatory disease, atherosclerosis (11, 12). Recently, 12 nuclear receptors, including FXR, were shown to be expressed on highly purified CD4+, CD8+, CD19+, and CD14+ cells by quantitative real-time PCR, suggesting that FXR may be coregulated in human immune cells (13).
Obeticholic acid (6α-ethyl-chenodeoxycholic acid, 6-ECDCA), an orally active synthetic FXR agonist, has ∼100-fold greater FXR agonistic activity than CDCA (14) and does not activate other nuclear receptors (15). In animal models, 6-EDCA has been shown to treat cholestatic liver disease and decrease insulin resistance and hepatic steatosis (16–18). Obeticholic acid is currently in phase I clinical trials for the treatment of alcoholic hepatitis (19), phase II clinical trials for nonalcoholic steatohepatitis (NASH) (20) and type 2 diabetes mellitus (18), and phase III clinical trials of primary biliary cirrhosis (PBC) (19, 21).
In this study, we explored the role of FXR in an animal model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE). EAE is an inducible inflammatory disease of the central nervous system (CNS) mediated by myelin-specific CD4+ T cells that is augmented by circulating myelin autoantibodies (22–24). Here we show that FXR knockout (FXR-KO) mice exhibit enhanced EAE disease severity. Moreover, we found that the synthetic FXR agonist, 6-ECDCA, inhibits both active and passive EAE more effectively than the natural FXR ligand, CDCA, in part by reducing IFN-gamma production and modulating both T and B trafficking and checkpoint inhibitors. Taken together, these findings suggest that FXR may serve as a negative regulator of CNS autoimmune inflammation.
Results
FXR Knockout Mice Have More Severe EAE.
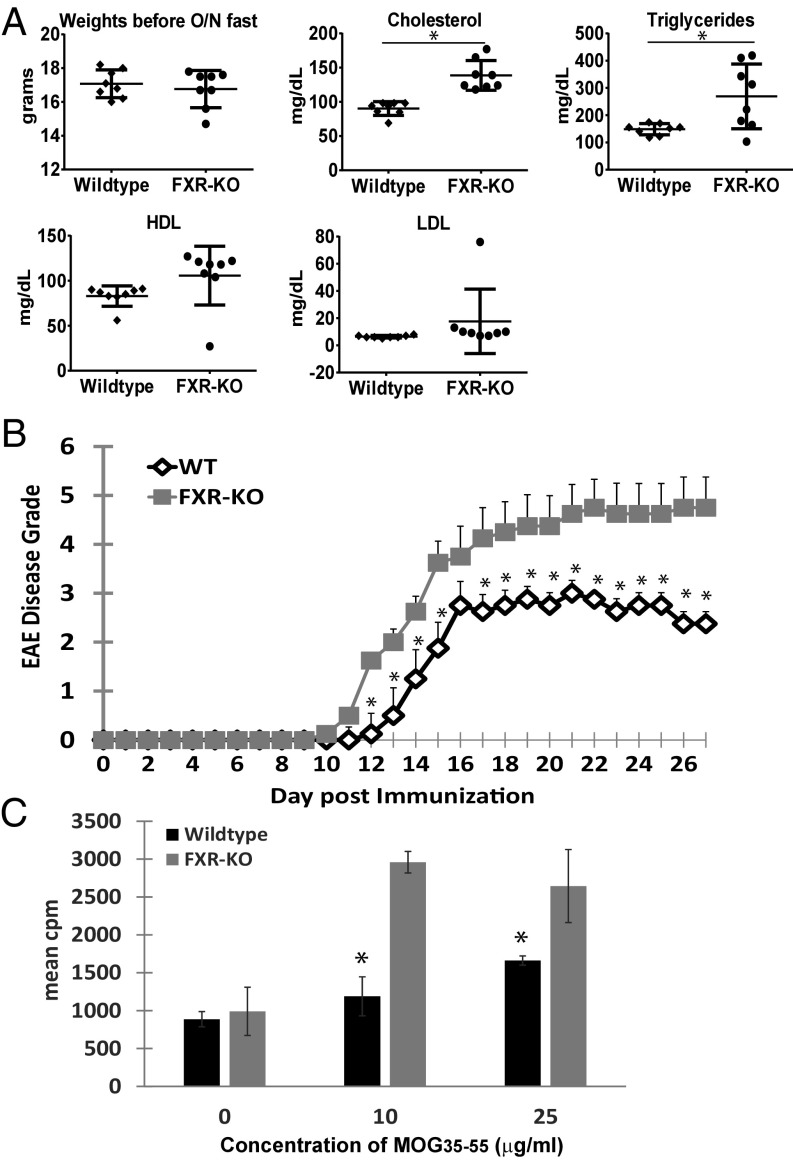
Mice that are homozygous for the targeted Nr1h4 allele (FXR-KO) have been reported to display a proartherogenic serum lipoprotein profile that is characterized by elevated levels of serum cholesterol, triglycerides, and bile acids (4). Before induction of EAE, we performed a lipid panel analysis of both wild-type and FXR-KO naive mice that had been fasted overnight. As shown in Fig. 1A, we confirmed that the FXR-KO mice had statistically elevated levels of cholesterol and triglycerides and a trend toward increased high-density lipoprotein (HDL) levels compared with the wild-type mice. When both groups were immunized with myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55), the FXR-KO mice had significantly more EAE disease severity than the wild-type mice (Fig. 1B). Proliferation assay of splenocytes indicated that splenocyte T cells from FXR-KO mice responded more robustly to MOG35–55 restimulation than from wild-type mice (Fig. 1D).
Fig. 1.
FXR knockout mice have more severe EAE. (A) Serum lipid panel analysis confirms that FXR-KO mice have elevated serum cholesterol and triglyceride levels after fasting overnight. *P < 0.05 by Student’s t test. (B) Clinical EAE scores of wild-type (WT, n = 8) versus FXR-knockout (FXR-KO, n = 8) mice immunized with MOG35–55 in CFA with pertussis toxin. Each point represents mean ± SEM, *P < 0.05 by Mann–Whitney u test. (C) Seventy-two-hour proliferation assay of harvested splenocytes with increasing concentrations of MOG35–55. 3H-thymidine incorporation is measured in triplicate wells. Values are the mean + SEM of triplicates, *P < 0.05 by Student’s t test. These experiments were repeated once with similar results.
Oral Administration of Obeticholic Acid Is Effective in Treating EAE.
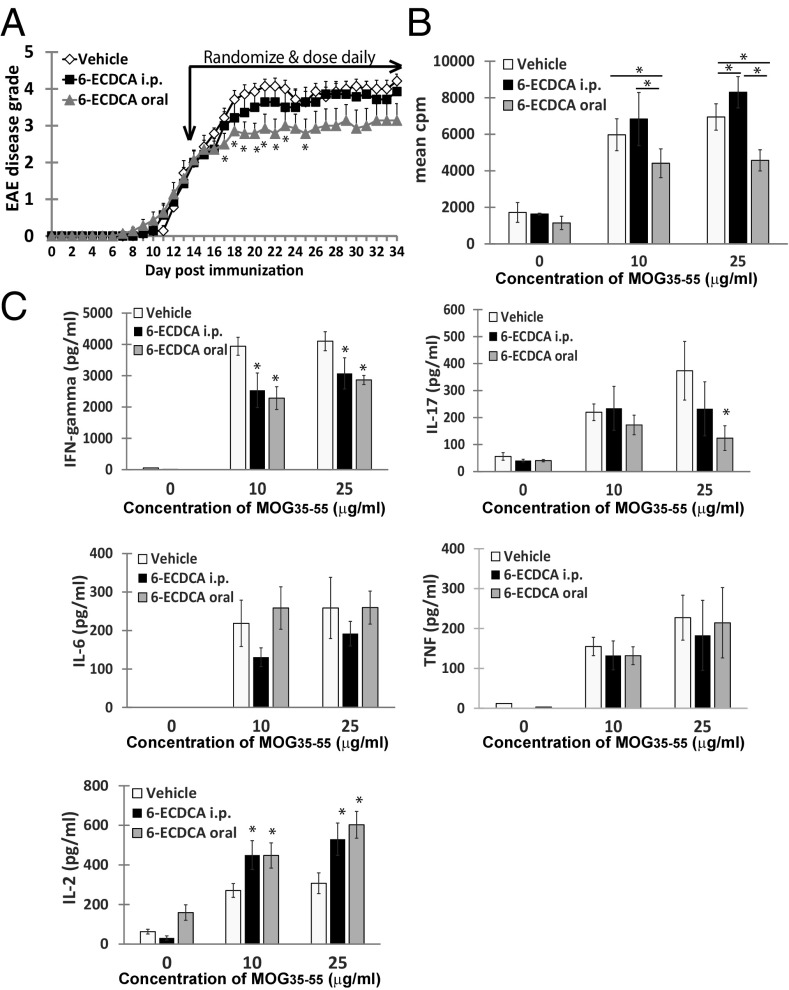
Given that the lack of FXR expression worsened disease course of EAE, we wanted to determine if treatment of EAE with an FXR agonist would in turn be beneficial. Because obeticholic acid (6-ECDCA) was recently reported as a promising oral therapeutic for the treatment of NASH and PBC, both recognized as inflammatory diseases, we selected this synthetic FXR agonist as our treatment drug. We initially compared the route of administration of 6-ECDCA intraperitoneally versus orally to determine if one or both routes would be effective in treating established EAE. Additionally, to keep the study consistent, all treatment groups were dosed both orally and intraperitoneally with the vehicle alone or a combination of vehicle and 6-ECDCA. We found that following randomization of mice at the peak of disease, daily oral dosing of 6-ECDCA was most effective in attenuating established EAE (Fig. 2A).
Fig. 2.
Oral administration of obeticholic acid is more effective in treating EAE than intraperitoneally (i.p.) injections. (A) Clinical EAE scores of mice administered 6-ECDCA or vehicle. Mice with established EAE were randomized into three groups (n = 9) and given daily doses of vehicle or 5 mg/kg of 6-ECDCA either i.p. or orally (oral). Each point represents mean ± SEM, *P < 0.05 by Mann–Whitney u test. (B) Seventy-two-hour proliferation assay of harvested splenocytes with increasing concentrations of MOG35–55. 3H-thymidine incorporation is measured in triplicate wells. Values are the mean + SEM of triplicates, *P < 0.05 by Student’s t test. (C) Cytokine production by harvested splenocytes with increasing concentrations of MOG35–55. Values are the mean + SEM of triplicates, *P < 0.05 by Student’s t test.
T-cell proliferation to MOG35–55 was significantly reduced in mice treated orally with 6-ECDCA compared with mice treated with the vehicle control or treated intraperitoneally with 6-ECDCA (Fig. 2B). Splenocytes harvested from both orally and intraperitoneally 6-ECDCA-treated mice had reduced IFN-gamma production in response to MOG35–55 compared with the vehicle control group, whereas oral treatment with 6-ECDCA reduced IL-17 production (Fig. 2C). IL-6 and TNF production were not significantly altered by 6-ECDCA, although production of IL-2 was enhanced.
The Synthetic FXR Agonist, Obeticholic Acid, Is More Effective Than the Natural FXR Ligand, Chenodeoxycholic Acid, in Treating EAE.
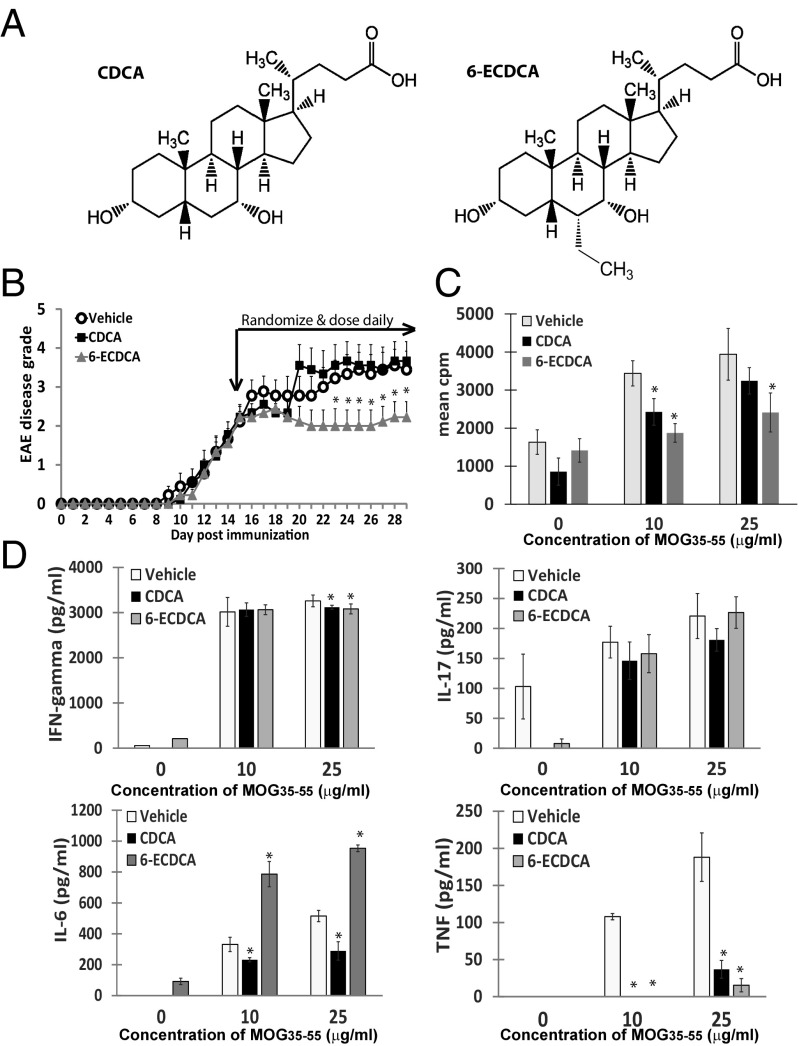
We next compared the efficacy of treating EAE with the natural bile acid FXR ligand, CDCA, versus 6-ECDCA, a CDCA derivative that contains an additional ethyl group (Fig. 3A). The synthetic FXR ligand, 6-ECDCA, has ∼100-fold greater FXR agonistic activity than CDCA (17). Mice with established EAE were randomized and treated orally with daily doses of either vehicle, CDCA, or 6-ECDCA. As shown in Fig. 3B, daily oral treatment with 6-ECDCA was more effective than CDCA in ameliorating the average EAE disease grade in mice.
Fig. 3.
Obeticholic acid is more effective than chenodeoxycholic acid in treating EAE. (A) Chemical structures of CDCA and 6-ECDCA. (B) Clinical EAE scores of mice administered 6-ECDCA, CDCA, or vehicle. Mice with established EAE were randomized into three groups (n = 9) and given daily oral doses of vehicle or 5 mg/kg of 6-ECDCA or CDCA. Each point represents mean ± SEM, *P < 0.05 by Mann–Whitney u test. (C) Seventy-two-hour proliferation assay of harvested splenocytes with increasing concentrations of MOG35–55. 3H-thymidine incorporation is measured in triplicate wells. Values are the mean + SEM of triplicates, *P < 0.05 by Student’s t test. (D) Cytokine production by harvested splenocytes with increasing concentrations of MOG35–55. Values are the mean + SEM of triplicates, *P < 0.05 by Student’s t test. These experiments were repeated twice with similar results.
T-cell recall responses to MOG35–55 were significantly suppressed in both 6-ECDCA– and CDCA-treated mice (Fig. 3C). Both FXR agonists decreased IFN-gamma and TNF production (Fig. 3D). Interestingly, IL-6 production was decreased from CDCA treatment but increased with 6-ECDCA treatment, and IL-17 production was not significantly altered in this experiment.
Obeticholic Acid and Chenodeoxycholic Acid Alter the Lymphocyte Activation Profile in EAE and Prevent Disease Transfer.
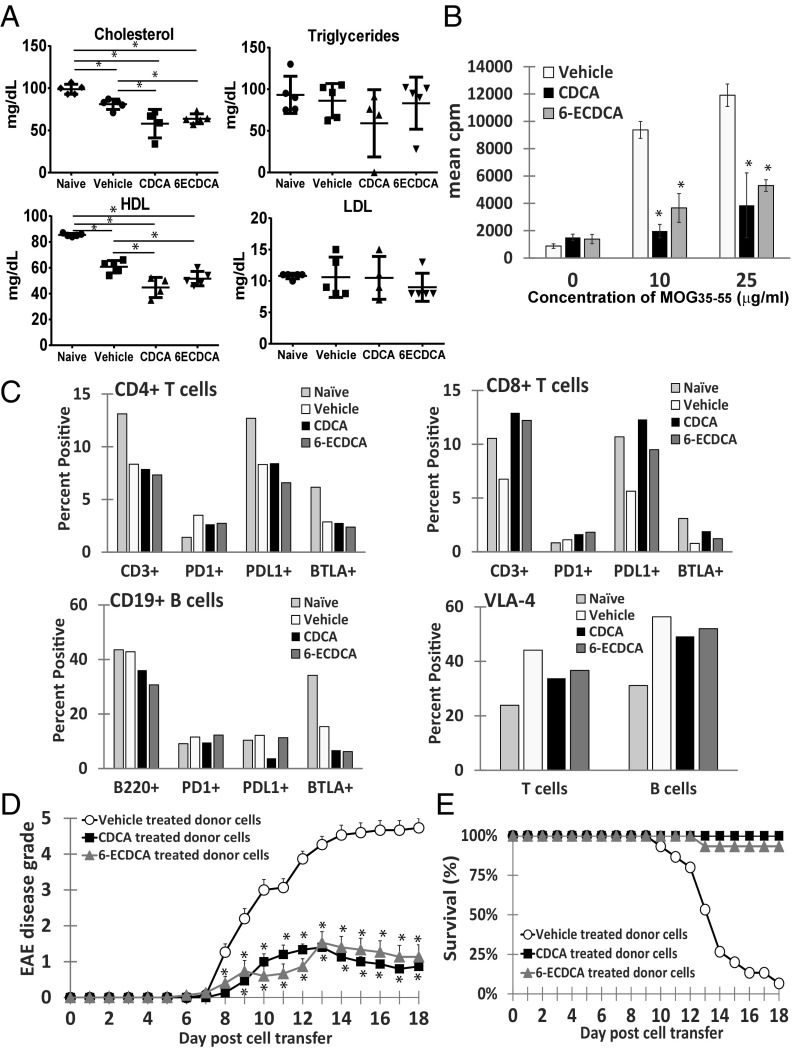
To investigate the underlying mechanism by which 6-ECDCA alters the immune cells in vivo during EAE, we examined several aspects following EAE immunization and 11 d of oral dosing with 6-ECDCA or CDCA compared with vehicle and naive control mice. First, we assessed the serum lipid panel following an overnight fast. Both cholesterol levels and HDL levels were decreased in mice treated with 6-ECDCA and CDCA (Fig. 4A). The triglycerides and low-density lipoprotein (LDL) levels were not significantly altered. We next performed a proliferation assay with the combined splenocytes and lymphocytes harvested from each group. Both 6-ECDCA and CDCA treatment greatly reduced the recall responses of the MOG35–55-specific lymphocytes compared with the vehicle treatment (Fig. 4B).
Fig. 4.
FXR agonists alter the lymphocyte activation profile in EAE and prevent EAE disease transfer. (A) Serum lipid panel analysis comparing naive (n = 5), vehicle (n = 5), CDCA- (n = 4), and 6-ECDCA– (n = 5) treated MOG35–55-immunized mice given 11 doses daily and fasted overnight. *P < 0.05 by Student’s t test. (B) Seventy-two-hour proliferation assay of harvested splenocytes with increasing concentrations of MOG35–55. 3H-thymidine incorporation is measured in triplicate wells. Values are the mean + SEM of triplicates, *P < 0.05 by Student’s t test. (C) Graphs represent summarized FACS analysis of harvested splenocytes profiling CD4+ T cells, CD8+ T cells, CD19+ B cells, and their expression of PD1, PD-L1, BTLA, and VLA-4 (CD29+CD49d+) expression on T and B cells. (D) Clinical EAE scores of naive recipient mice (each group, n = 15) administered 3 × 107 donor spleen and lymph node cells from mice treated with either 6-ECDCA, CDCA, or vehicle for 11 days before in vitro restimulation with 20 μg/mL MOG35–55 and 10 ng/mL rIL-12 for 72 h. Each point represents mean ± SEM, *P < 0.05 by Mann–Whitney u test. (E) Survival curve of EAE mice shown in D.
FACScan analysis of the same splenocytes revealed a reduction in both CD3+CD4+ T cells (8.34% WT, 7.89% CDCA, 7.33% 6-ECDCA) and CD19+B220+ B cells (42.82% WT, 36.07% CDCA, 30.69% 6-ECDCA), whereas CD3+CD8+ T cells increased with treatment of 6-ECDCA and CDCA (6.75% WT, 12.92% CDCA, 12.22% 6-ECDCA) (Fig. 4C). Because the MOG35–55 proliferative responses in 6-ECDCA– and CDCA-treated cells were reduced, we hypothesized that the lymphocytes were potentially more prone to apoptosis. To address this possibility, we also stained for cell surface expression of three molecules involved in down-regulating the immune system by preventing the activation of lymphocytes and promoting apoptosis: programmed cell death protein 1 (PD1), programmed death-ligand 1 (PD-L1), and B and T lymphocyte attenuator (BTLA).
CD4+ T cells from spleens of 6-ECDCA–treated mice had relatively reduced expression of PD1 (2.74%), PD-L1 (6.59%), and BTLA (2.38%) compared with CD4+ T cells from vehicle-treated mice (3.5%, 8.32%, and 2.87%, respectively). CD4+ T cells from spleens of CDCA-treated mice had similarly reduced PD1 expression (2.64%) as 6-ECDCA treatment, but PD-L1 (8.43%) and BTLA (2.77%) expression were relatively unchanged. In contrast, with the increase in the CD8+ T-cell population following treatment with 6-ECDCA and CDCA, the expression of PD1 (1.12% WT, 1.63% CDCA, 1.82% 6-ECDCA), PD-L1 (5.63% WT, 12.3% CDCA, 9.5% 6-ECDCA), and BTLA (0.78% WT, 1.92% CDCA, 1.21% 6-ECDCA) were all relatively increased compared with the CD8+ T cells from the vehicle control mice. As for the CD19+ B cells, CDCA treatment was more effective in reducing expression of PD1 (11.58% WT, 9.58% CDCA, 12.3% 6-ECDCA) and PD-L1 (12.21% WT, 3.79% CDCA, 11.34% 6-ECDCA). Both CDCA and 6-ECDCA appear to similarly decrease BTLA expression on CD19+ B cells (6.76% CDCA, 6.23% 6-ECDCA) compared with vehicle treatment (12.21%).
We also stained for VLA-4 (CD29+CD49d+) expression on both T and B cells. The expression of VLA-4 (α4β1 integrin) on lymphocytes is necessary for the effective extravasation across the blood vessel endothelium into target organs such as the CNS. Indeed, Tysabri, a monoclonal antibody directed against the α4-integrin subunit of VLA-4 is currently one of the most effective immunosuppressive drugs currently available for the treatment of MS (25). Here, we observed that VLA-4 expression was reduced in both T cells (44.12% WT, 33.78% CDCA, 36.67% 6-ECDCA) and B cells (56.35% WT, 49.16% CDCA, 51.98% 6-ECDCA) following treatment with 6-ECDCA and CDCA (Fig. 4C).
Together these results show that treatment with 6-ECDCA and CDCA can alter the activation and migration of both T and B cells. Finally, to determine if these treated cells can effectively transfer EAE disease, naive recipients were injected with cells from donor mice that had been treated with either vehicle, CDCA, or 6-ECDCA for 11 d followed by an additional 3 d of MOG35–55 restimulation in the presence of recombinant IL-12 (rIL-12). Fig. 4D summarizes the EAE clinical scores, whereby mice receiving activated lymphocytes from 6-ECDCA– or CDCA-treated donor mice had significantly less severe disease than mice receiving activated lymphocytes from vehicle-treated donor mice.
Discussion
The objective of this study was to determine if the expression of the bile acid receptor, FXR, plays a critical role in the autoimmune demyelinating disease EAE. Here we found that loss of functional FXR exacerbated EAE disease in FXR-KO mice, whereas activation of FXR via a synthetic FXR-specific agonist, 6-ECDCA, effectively ameliorated both active and passive EAE. Administration of 6-ECDCA rendered MOG35–55-specific lymphocytes to be less reactive to MOG35–55 restimulation and suppressed several proinflammatory cytokines including IFN-gamma, IL-17, and TNF. Physiologically, 6-ECDCA and CDCA reduced serum cholesterol and HDL levels in vivo. Immunologically, FXR agonists appear to decrease both activated CD4+ T-cell and CD19+ B-cell percentages but increase CD8+ T cells. The expression of PD1 is decreased in these CD4+ T cells, with 6-ECDCA having a more pronounced effect in decreasing PD-L1 expression on CD4+ T cells, whereas CDCA was more effective in reducing PD-L1 expression on CD19+ B cells. BTLA expression was also decreased on both CD4+ T cells and CD19+ T cells in mice treated with the FXR agonists. In contrast, FXR agonists appear to increase the percentages of MOG35–55-activated CD8+ T-cells. Moreover, PD1, PD-L1, and BTLA expression were all up-regulated in these activated CD8+ T cells. VLA-4 expression, the cell surface molecule critical for lymphocyte extravasation from blood vessels into target organs such as the CNS, was decreased in both T and B cells following treatment with the FXR agonists. Finally, adoptive transfer of MOG35–55-specific lymphocytes treated in vivo with the FXR agonists failed to induce EAE in naive recipients.
The costimulatory pathway of PD1 and PD-L1/PD-L2 delivers inhibitory signals that regulate the balance among T-cell activation, tolerance, and immune-mediated tissue damage during interactions with self-antigens, chronic viral infections, and tumors (26–28). PD1 expression is normally up-regulated on T cells upon activation, whereas the effectors of PD1 ligation on T cells is evident as early as 2 h after activation (29, 30). Binding of PD1 with either of its ligands during TCR signaling can block T-cell proliferation, cytokine production, and cytolytic function, and impair T-cell survival (28, 31). BTLA is another negative checkpoint regulator with expression limited to B cells, T cells, mature dendritic cells, and macrophages (32). BTLA negatively regulates T-cell activation, and BTLA engagement leads to peripheral tolerance and prosurvival function (33). Our observation that both CD4+ T cells and CD19+ B cells had decreased expression of PD1, PD-L1, and BTLA following treatment with 6-ECDCA and CDCA indicates that modulation of these two MOG35–55-activated cell populations leads to decreased T-cell proliferation and that these populations may be more prone to apoptosis. Further studies are currently underway to confirm this.
MS is characterized by an inflammatory infiltrate that includes CD4+ and CD8+ T cells (34). Clonal expansion of CD8+ T cells appears to persist within active lesions and in the cerebrospinal fluid and blood in MS (35–37). Myelin-specific CD8+ T cells have been shown to play a pathogenic role in EAE (38, 39). However, more recently, myelin-specific autoregulatory CD8+ T cells have been shown to inhibit EAE, through the suppression of CD4+ T-cell and/or antigen-presenting cell function by direct killing in an antigen-specific manner (40, 41). The fact that both FXR agonists increased the CD8+ T-cell population in vivo concurrently with increased PD1, PD-L1, and BTLA expression, suggests that a subset of autoregulatory CD8+ T cells may be enhanced.
Arresting lymphocyte trafficking has been the approach for two currently approved MS disease-modifying therapies. Tysabri (natalizumab), a monoclonal antibody directed against α4 integrin, prevents the extravastion of circulating activated lymphocytes across the blood–brain barrier into the CNS (25). In contrast, the first orally available MS therapeutic Gilenya (fingolimod), a sphingosine 1-phosphate receptor agonist, sequesters activated lymphocytes within the secondary lymphoid organs, resulting in severe lymphopenia (42). Here we show that treating MOG35–55-immunized mice with an FXR agonist also reduces the expression of VLA-4 on both T and B cells, implying a role in modulating cell trafficking during lymphocyte activation.
Regarding orally available treatments of MS, the finding here that intraperitioneal injection was less effective than oral administration was unexpected. The oral activity of obeticholic acid suggests further modulation of the drug by gut flora or gut enzymes or the importance of hepatic uptake.
Inflammation impairs reverse cholesterol transport by cholesterol efflux from peripheral cells, such as lipid-laden foam cells, onto circulating HDL for transport to the liver for secretion into bile and feces (43). Impairment of reverse cholesterol transport may contribute to atherosclerosis in chronic inflammatory diseases, including obesity, metabolic syndrome, and type 2 diabetes. The bile acid biosynthesis pathway plays an important role in maintaining cholesterol homeostasis in mammals (1). The conversion of cholesterol to bile acids accounts for the catabolism of about 50% of cholesterol in the body, and bile acids are also required for the disposal of 40% of cholesterol in feces (1). Bile acids are reabsorbed in the ileum by active transport systems and are transported back to the liver through the portal venous circulation (44). The enterohepatic circulation of bile acids is extremely efficient, with only 5% bile acids lost in feces, which is compensated for by biosynthesis from cholesterol. This feedback mechanism not only regulates bile acid synthesis and bile flow, but is also important in regulating cholesterol synthesis by inhibiting the rate-limiting enzyme, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and hepatic uptake of LDL cholesterol by LDL receptor (1, 12).
We have previously reported on the benefits of using cholesterol-lowering HMG-CoA reductase inhibitors, statins, for the treatment of EAE (45–48). Blockade of HMG-CoA reductase inhibits proinflammatory T helper cell response during EAE by regulating isoprenoid availability through the mevalonate pathway (45). Targeting the bile acid synthesis pathway may be another approach in blocking HMG-CoA reductase to further inhibit the proinflammatory T-cell response in EAE and MS.
FXR is not the first nuclear hormone receptor involved in regulating cholesterol turnover and targeted for controlling proinflammatory responses in EAE. Peroxisome proliferator-activated receptors (PPARs), another member of the nuclear hormone receptor subfamily, also regulates whole body lipid and glucose homeostasis and controls inflammatory responses (49, 50). We have reported that PPAR alpha and PPAR delta serve as important molecular brakes on T-cell immunity for the control of CNS inflammation (51, 52). Furthermore, several PPAR agonists directed toward PPAR alpha (53), PPAR beta/delta (51, 54), and PPAR gamma (55–57) have all shown efficacy in treating EAE.
Finally, 6-ECDCA was recently reported to be remarkably successful in treating noncirrhotic, nonalcoholic steatohepatitis (NASH) in its phase II (FLINT) clinical trial (18). However, concerns for its long-term benefits and safety were brought to light when 23% of patients in the 6-ECDCA–treated group developed pruritus. Further, there were unexpected increases in total cholesterol and LDL cholesterol, as well as a slight decrease in HDL cholesterol within 12 wk of beginning treatment. These changes diminished in magnitude while on treatment, and were not sustained when treatment was discontinued after 72 wk (20). Nevertheless, changes in serum cholesterol and insulin resistance while on treatment with 6-ECDCA could lead to the increased risk of developing atherosclerosis. Because disease management for MS also requires long-term comprehensive treatment, further studies of utilization of FXR agonists as a potential MS therapy are necessary to address these safety concerns. One possibility may be to combine a statin with 6-ECDCA. Nevertheless, our data provide a previously unidentified approach in treating MS through the bile acid–FXR interaction.
Materials and Methods
Mice and EAE Induction.
C57BL/6J and FXRKO (B6.129 × 1(FVB)-Nr1h4tm1Gonz/J, no. 0007214) female mice were purchased from The Jackson Laboratory. Animal experiments were approved by, and performed in compliance with, the National Institutes of Health guidelines of the Institutional Animal Care and Use Committee at Stanford University. For induction of active and adoptive transfer of EAE, 8- to 12-wk-old female mice were immunized s.c. with 100 µg of MOG35–55 emulsified in complete Freund’s adjuvant (CFA), consisting of incomplete Freund’s adjuvant and 0.4 mg of heat-inactivated Mycobacterium tuberculosis, strain H37 RA (Difco Laboratories). On days 0 and 2, mice were also injected i.v. or intraperitoneally with 400 ng pertussis toxin in 0.2 mL 1× PBS. Adoptive transfer requires harvesting lymph nodes and spleens 10 d after immunization. Combined lymphocytes and splenocytes are cultured in bulk, at 5 × 107 cells per milliliter for 72 h in the presence of MOG35–55 (20 μg/mL) and rIL-12 (10 ng/mL, R&D Systems). Cells were harvested and washed twice before transfer of 3 × 107 cells in 0.2 mL 1× PBS into naive 6- to 7-wk-old female mice, intraperitoneally. Clinical disease was monitored daily using the following scoring system: 0, no disease; 1, limp tail; 2, hind limb weakness; 3, hind limb paralysis; 4, hind limb and forelimb paralysis; and 5, death.
Reagents.
MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) was obtained from Genemed Synthesis. CDCA (Sigma) and 6-ECDCA (Caymen Chemicals or AdipoGen International) were solubilized in 100% dimethyl sulfoxide (DMSO) at a stock concentration of 25 mg/mL and further diluted with PBS (1×). Mice were given 200 μL of 6-ECDCA (5 mg/kg) or CDCA (5 mg/kg) daily, either intraperitoneally or orally where indicated. Vehicle control contained equivalent amounts of DMSO and 1× PBS.
Proliferation and Cytokine Assays.
Splenocytes and lymph nodes were harvested and stimulated with increasing concentrations of MOG35–55 for a total of 72 h. For assessment of proliferation, 1 μCi of 3H-thymidine was added to each well for the final 18–24 h of culture, and incorporation of radioactivity was measured by using a Betaplate scintillation counter. Cytokine assays were performed on culture supernatants after 72 h of culture by using the IL-2, IL-6, IFN-gamma, and TNF BD OptEIA Mouse ELISA kits (BD Biosciences) or Mouse IL-17 DuoSet ELISA Development kit (R&D Systems).
Flow Cytometry.
Cells were stained according to standard protocols, run on a FACScan flow cytometer (BD Biosciences), and analyzed with CellQuest software (BD Immunocytometry Systems). The antibody conjugates used were PerCP-Cy5.5 anti-CD3, FITC anti-CD4, PE anti-CD8, PE anti-B220, PerCp-Cy5.5 anti-B220, FITC anti-CD19, PE anti-PD1, PE anti-PD-L1, PE anti-BTLA, PE anti-CD29, and FITC anti-CD49d (BD Biosciences and eBioscience).
Serum Lipid Panel Analysis.
Serum from mice fasted overnight was submitted to the Diagnostic Laboratory at Stanford University, Department of Comparative Medicine for a full lipid panel analysis.
Acknowledgments
This work was funded in part by the National Multiple Sclerosis Society (L.S.).
Footnotes
Conflict of interest statement: P.P.H. and L.S. have filed an invention disclosure with the Stanford Office of Licensing and Technology.
References
- 1.Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–d193. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 2.Lambert G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278(4):2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 3.Makishima M, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 5.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA. 2004;101(10):3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman BM, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 7.Mencarelli A, et al. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J Immunol. 2009;183(10):6657–6666. doi: 10.4049/jimmunol.0901347. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki T, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103(10):3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183(10):6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 10.Gadaleta RM, et al. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801(7):683–692. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27(12):2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 12.Hageman J, Herrema H, Groen AK, Kuipers F. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(8):1519–1528. doi: 10.1161/ATVBAHA.109.197897. [DOI] [PubMed] [Google Scholar]
- 13.Schote AB, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: Expression and correlations. Mol Immunol. 2007;44(6):1436–1445. doi: 10.1016/j.molimm.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Pellicciari R, et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47(18):4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo G, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78(4):617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103(4):1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellicciari R, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45(17):3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 18.Mudaliar S, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3(1):5. doi: 10.3978/j.issn.2305-5839.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuschwander-Tetri BA, et al. NASH Clinical Research Network Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trivedi PJ, Hirschfield GM, Gershwin ME. Obeticholic acid for the treatment of primary biliary cirrhosis. Expert Rev Clin Pharmacol. 2016;9(1):13–26. doi: 10.1586/17512433.2015.1092381. [DOI] [PubMed] [Google Scholar]
- 22.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 23.Lassmann H, Brunner C, Bradl M, Linington C. Experimental allergic encephalomyelitis: The balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol. 1988;75(6):566–576. doi: 10.1007/BF00686201. [DOI] [PubMed] [Google Scholar]
- 24.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- 25.McCormack PL. Natalizumab: A review of its use in the management of relapsing-remitting multiple sclerosis. Drugs. 2013;73(13):1463–1481. doi: 10.1007/s40265-013-0102-7. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 27.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: Crucial controls of immune tolerance and function. Immunol Rev. 2009;229(1):88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 30.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 33.Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol. 2015;6:418. doi: 10.3389/fimmu.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 35.Babbe H, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192(3):393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skulina C, et al. Multiple sclerosis: Brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA. 2004;101(8):2428–2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauser SL, et al. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986;19(6):578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- 38.Huseby ES, et al. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194(5):669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun D, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166(12):7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 40.York NR, et al. Immune regulatory CNS-reactive CD8+T cells in experimental autoimmune encephalomyelitis. J Autoimmun. 2010;35(1):33–44. doi: 10.1016/j.jaut.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortega SB, Kashi VP, Cunnusamy K, Franco J, Karandikar NJ. Autoregulatory CD8 T cells depend on cognate antigen recognition and CD4/CD8 myelin determinants. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e170. doi: 10.1212/NXI.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 43.McGillicuddy FC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119(8):1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myant NB, Mitropoulos KA. Cholesterol 7 alpha-hydroxylase. J Lipid Res. 1977;18(2):135–153. [PubMed] [Google Scholar]
- 45.Dunn SE, et al. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med. 2006;203(2):401–412. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youssef S, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 47.Stüve O, et al. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J Clin Invest. 2006;116(4):1037–1044. doi: 10.1172/JCI25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanislaus R, Pahan K, Singh AK, Singh I. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci Lett. 1999;269(2):71–74. doi: 10.1016/s0304-3940(99)00414-0. [DOI] [PubMed] [Google Scholar]
- 49.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 50.Barbier O, et al. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22(5):717–726. doi: 10.1161/01.atv.0000015598.86369.04. [DOI] [PubMed] [Google Scholar]
- 51.Dunn SE, et al. Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity. J Exp Med. 2010;207(8):1599–1608. doi: 10.1084/jem.20091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunn SE, et al. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204(2):321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lovett-Racke AE, et al. Peroxisome proliferator-activated receptor alpha agonists as therapy for autoimmune disease. J Immunol. 2004;172(9):5790–5798. doi: 10.4049/jimmunol.172.9.5790. [DOI] [PubMed] [Google Scholar]
- 54.Polak PE, et al. Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;168(1-2):65–75. doi: 10.1016/j.jneuroim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Klotz L, et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206(10):2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diab A, et al. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168(5):2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- 57.Feinstein DL, et al. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol. 2002;51(6):694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]