Significance
Hodgkin’s lymphoma (HL) manifests activation of Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) signaling pathway and CD30 expression on Reed–Sternberg cells. Additivity/synergy inhibiting effects were noted with combination treatments in vitro with HL cell line, HDLM-2, when treated with JAK 1/2 inhibitor; ruxolitinib with B-cell lymphoma 2 (Bcl-2)/B-cell lymphoma-extra large) (Bcl-xL) inhibitor, Navitoclax; or with anti-CD30 toxin conjugate, brentuximab vedotin (BV). Either ruxolitinib combined with Navitoclax or BV alone prolonged survival period but did not cure HDLM-2 tumor-bearing mice whereas BV combined with ruxolitinib and/or with Navitoclax resulted in sustained complete remissions in this model of HL. These studies provide scientific support for clinical trials to evaluate BV combined with ruxolitinib in select patients with HL.
Keywords: Hodgkin's lymphoma, ruxolitinib, Navitoclax, brentuximab vedotin
Abstract
Despite relative success of therapy for Hodgkin’s lymphoma (HL), novel therapeutic agents are needed for patients with refractory or relapsed disease. Recently, anti-PD1 immunotherapy or treatment with the anti-CD30 toxin conjugate brentuximab vedotin (BV) have been associated with remissions; however, the median responses of complete responses (CRs) with the latter were only 6.7 mo. To obtain curative therapy, other effective agents, based on HL biology, would have to be given in combination with BV. Hodgkin’s Reed–Sternberg (HRS) cells secrete cytokines including IL-6 and -13, leading to constitutive activation of JAK/STAT signaling. In the present study the JAK1/2 inhibitor ruxolitinib reduced phosphorylation of STAT3 and STAT6 and expression of c-Myc in the HL cell line HDLM-2. These changes were enhanced when, on the basis of a matrix screen of drug combinations, ruxolitinib was combined with the Bcl-2/Bcl-xL inhibitor Navitoclax. The combination augmented expression of Bik, Puma, and Bax, and attenuated Bcl-xL expression and the phosphorylation of Bad. The use of the two-agent combination of either ruxolitinib or Navitoclax with BV or the three-agent combination strongly activated Bax and increased activities of cytochrome c and caspase-9 and -3 that, in turn, led to cleavage of poly(ADP ribose) polymerase and Mcl-1. Either ruxolitinib combined with Navitoclax or BV alone prolonged survival but did not cure HDLM-2 tumor-bearing mice, whereas BV combined with ruxolitinib and/or with Navitoclax resulted in a sustained, complete elimination of the HDLM-2 HL. These studies provide scientific support for a clinical trial to evaluate BV combined with ruxolitinib in select patients with HL.
Hodgkin’s lymphoma (HL) is a relatively common lymphoid neoplasm with a bimodal incidence curve involving ∼9,000 cases diagnosed annually in the United States (1). The neoplastic cells in classical HL, termed Hodgkin’s Reed–Sternberg (HRS) cells (2), comprise only a minority of cells in the tumor mass. Such HRS express the CD30 surface antigen. Although HL has remained a largely curable disease, ∼20% of patients with relapsed and refractory disease will not be cured with currently available therapy and will require subsequent treatments (3). HL patients whose disease relapses after autologous stem-cell transplantation are rarely cured with current treatment modalities. Treatment with anti-PD1 immunotherapy or the anti-CD30 toxin conjugate brentuximab vedotin (BV) have resulted in remissions in refractory and relapsed HL. However, the median complete response (CR) after BV therapy was only 6.7 mo. It is likely that combination therapies involving BV will be required to obtain a curative strategy. Thus, new drugs and novel treatment strategies are required, based on our understanding of HL biology and signaling pathways (4). Novel combination therapies are possible that take advantage of insights concerning the disorders of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway in patients with HL. Malignant HRS cells secrete various cytokines, leading to activation of signaling pathways such as the NF-κB pathway and the JAK/STAT pathway (5, 6). Constitutive phosphorylation of STAT1, STAT3, STAT5, and STAT6 have been found in several HL cell lines, as well as primary HRS cells (7–10). Cytokines secreted by HL cell lines and primary tumors include well-known activators of the JAK/STAT pathway IL-13 and -6 (11). JAK2 showed chromosomal gains in ∼20% of HL and in rare cases was translocated (12, 13). JAK2 functions in HRS cells as an activator of STAT signaling and is also involved in epigenetic regulation, because it can phosphorylate histone H3 (14). Novel JAK2 inhibitors such as AZD1480 and SB1518 have been investigated in both the preclinical and clinical settings involving HL (15, 16). Our group has initiated a clinical trial using the FDA-approved JAK1/2 inhibitor ruxolitinib in the treatment of adult T-cell leukemia (ATL). To use such an agent for HL, we investigated ruxolitinib with other agents in both in vitro studies with HL cells and in a murine model with the HL cell line HDLM-2. Because it was clear that multielement combinations would be optimal, we performed a matrix screen of ruxolitinib drug combinations with HL cell lines and identified Navitoclax as the most effective partner.
The targets of Navitoclax, BcL-2 and BcL-xL, are the main effector molecules in the survival pathways downstream of activation of JAK/STAT signaling (17). Inhibiting the oncogenic JAK2 signaling network by targeting JAK2 and BcL-2/BcL-xL provided an augmented therapeutic benefit in mutant JAK2-driven malignancies (17–19). In our studies with the HDLM-2 murine model, the combination of ruxolitinib and Navitoclax attenuated progression of HL, but did not cure the disease. Therefore, we explored a third agent, BV, for further therapeutic trials.
CD30 is a transmembrane member of the TNF cell receptor superfamily that is highly expressed in HRS cells, but is highly restricted in normal cells (20). Therefore, CD30 is considered an ideal target for monoclonal antibody therapy of HL (21). BV is an antibody-drug conjugate (ADC) comprising an anti-CD30 antibody coupled to the antitubulin monomethyl auristatin E (MMAE). Binding of MMAE to tubulin disrupts the microtubule network, induces cell-cycle arrest, and results in apoptotic death of the CD30-expressing tumor cells (22). Phase I and II trials demonstrated its impressive efficacy in relapsed HL and anaplastic large cell lymphoma (23–25). In the present study, an additive/synergistic effect yielding complete depletions of HL cells was observed when BV was combined with either ruxolitinib or Navitoclax. Moreover, combination therapy of BV and ruxolitinib or Navitoclax led to complete remissions of established HDLM-2 lymphoma-bearing mice.
Results
Matrix Screening Highlights Ruxolitinib Drug Combinations that Block Proliferation and Induce Apoptosis in Human HL Cells.
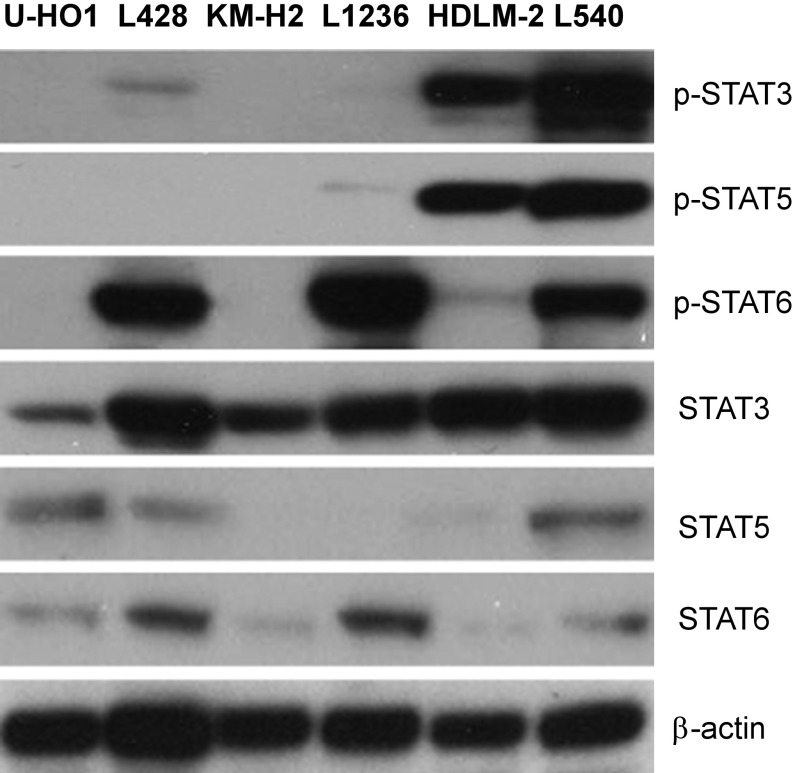
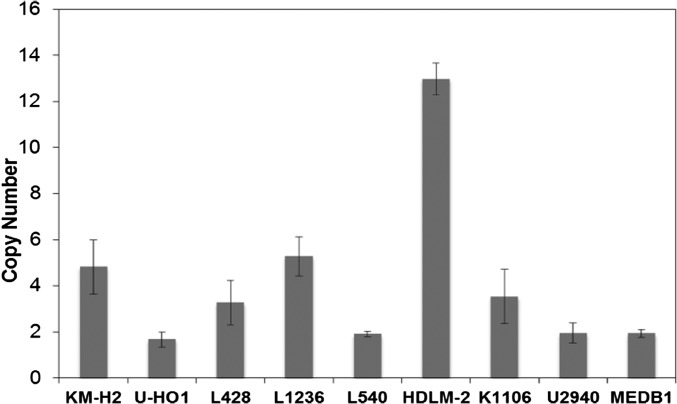
Aberrant activities of JAK/STAT signaling pathways have been observed in several hematologic malignancies, including HL (26). Western blot analysis showed that four of the six HL cell lines expressed phosphorylated STAT3, STAT5, or STAT6 (Fig. S1). Furthermore, there was selective 9p24.1 amplification with increased JAK2 induction in three of the six HL lines analyzed (KM-H2, L1236, and HDLM-2; Fig. S2). We examined the response of the six HL cell lines KM-H2, L428, U-HO1, L1236, L540, and HDLM-2 to a large collection of 1,912 approved and investigational drugs using a common cell viability assay that incorporated numerous JAK/STAT inhibitors, including ruxolitinib. The results of this single-agent viability screen are freely available at https://tripod.nih.gov/matrix-client/. Although most or all of the cell lines were inhibited by relatively nonspecific JAK inhibitors, such as Degrasyn and Merck-5, only two of the cell lines, HDLM-2 and L1236, responded to the more specific U.S. Food and Drug Administration (FDA)-approved JAK inhibitors, ruxolitinib (JAK1, 2) and tofacitinib (pan-JAK).
Fig. S1.
Western blot analysis of expressions of phosphorylated STATs in HL cell lines. Data represent one of three independent experiments.
Fig. S2.
The DNA copy number of JAK2 in HL cell lines. Genomic DNAs from six HL lines and three Primary Mediastinal B-Cell Lymphoma (PMBL) Lines were extracted by using the AllPrep DNA/RNA Mini Kit (Qiagen). A total of 20 ng of gDNA were added to the MicroAmp Reaction Plate (Thermo Fisher) tubes containing 15 μL of reaction mix of the following: human JAK2 Copy Number assay (Hs01533212_cn; Thermo Fisher), human RNase P, TaqMan Copy Number Reference Assay (catalog no. 4403326, Thermo Fisher), and 2× Taqman Genotyping Master Mix (catalog no. 4371353, Thermo Fisher). JAK2 amplifications were performed by using an ABI7500 Real Time Sequence Detection System (Life Technologies) at the following cycling conditions: 1 cycle at 95 °C followed by 40 cycles of denaturation at 95 °C (15 s) and an annealing/extension step at 60 °C for 1 min. Reactions were kept on hold at 4 °C. All samples and standards were run in triplicate. DNA Copy Number variation in HL and PMBL cell lines were analyzed by using the CopyCaller Software (Version 2.1; Life Technologies). Data are representative of two independent experiments.
To improve on the antitumor activity of ruxolitinib we next sought out appropriate combinations for HL therapy. Therefore, we evaluated ruxolitinib combined with the same 1,912 therapeutic agents with the L1236 cell line using a previously described 6 × 6 dose–response matrix screening platform. Synergistic drug combinations from the 6 × 6 screen where then repeated in the L1236, L428, U-HO1, and HDLM-2 cell lines. The results of these experiments are freely available at https://tripod.nih.gov/matrix-client/. Additive/synergistic effects were demonstrated using the HDLM-2 cell line with ruxolitinib combined with inhibitors of Bcl-2/Bcl-xL, HDACs, Heat shock protein 90, PI3K, and mTOR. Previously, we have defined a strong synergistic effect for JAK inhibitors when combined with Bcl-2/Bcl-xL in ATL. Based upon these studies we chose to more deeply evaluate the combination effects of ruxolitinib and Navitoclax (ABT-263) in HL HDLM-2 cell line.
Blockade of JAK2/STATs Signal with Ruxolitinib and Inhibition of BcL-xL with Navitoclax Yielded Antiproliferative Actions in Human HL Cell Lines.
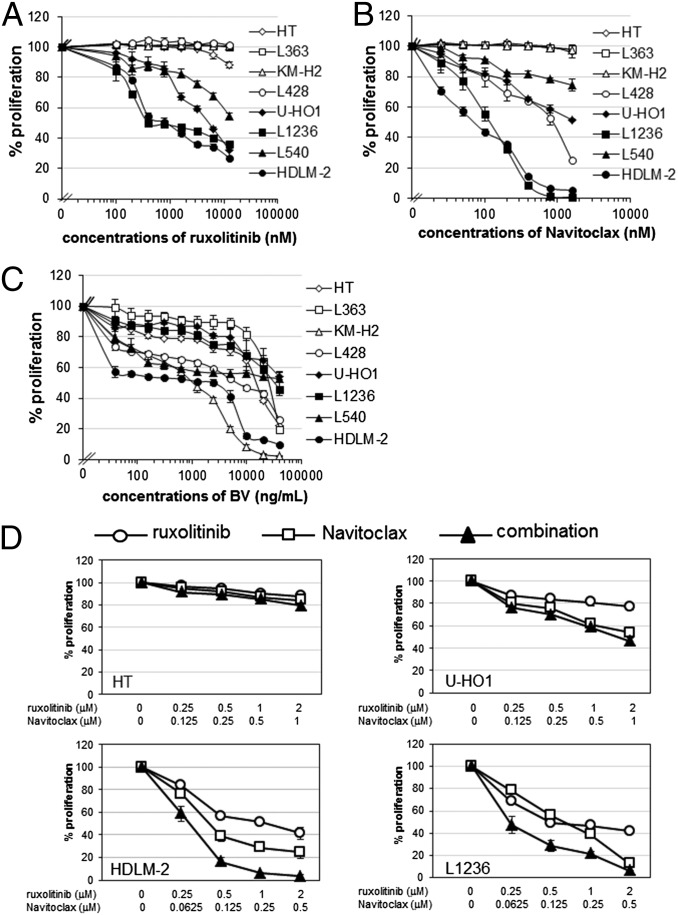
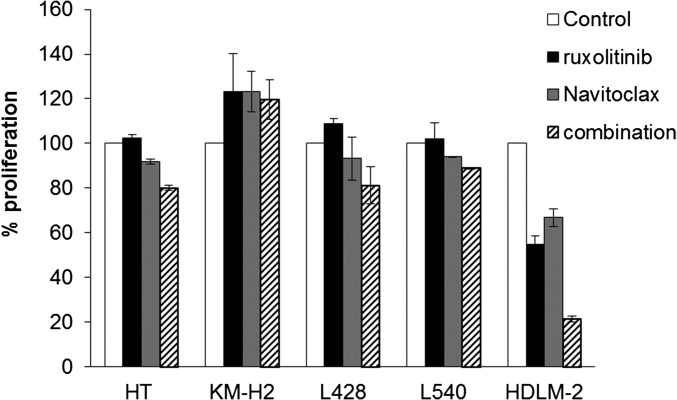
To determine whether ruxolitinib and Navitoclax are effective at reducing the viability of HL cells, we evaluated them on six human HL cell lines, KM-H2, L428, U-HO1, L1236, L540, and HDLM-2; one human B-cell lymphoma cell line, HT; and one human multiple myeloma cell line, L363. Cells were cultured with or without increasing concentrations of ruxolitinib or Navitoclax for 48 h and analyzed for 3H-thymidine incorporation. Ruxolitinib inhibited proliferation of four HL cell lines—U-HO1, L1236, L540, and HDLM-2—in a concentration-dependent manner, but did not affect KM-H2, L428, and L363 lines up to the highest dose of 10 μM (Fig. 1A). Navitoclax also inhibited the proliferation of four HL cell lines—L428, U-HO1, L1236, and HDLM-2—but did not affect the KM-H2, HT, or L363 lines up to the highest dose of 1.6 μM. Navitoclax had a modest effect on the proliferation of L540 cells (Fig. 1B). Based upon these data, we evaluated the combination regimen of ruxolitinib with Navitoclax for its capacity to inhibit cell proliferation of U-HO1, L1236, HDLM-2, and the control HT line. Compared with a single reagent alone, HDLM-2 and L1236 lines treated with the combination regimens responded in an additive/synergistic fashion (Fig. 1D), with a meaningful induction of apoptosis as judged by a companion CaspaseGlo analysis (Fig. S3). In contrast, the combination regimens did not possess significant inhibiting activity with HT and U-HO1 cell lines (Fig. 1D) or with the KM-H2 and L540 cell lines (Fig. S4).
Fig. 1.
The antiproliferative activity of ruxolitinib, Navitoclax, and BV was demonstrated in human HL cell lines. (A–C) Proliferation assay of HL cell lines treated with increasing doses of ruxolitinib (A), Navitoclax (B), or BV (C) for 48 h. HT and L363 cell lines were used as a control. Data are presented as mean ± SD. (D) Proliferation assay of the HT cell line and HL cell lines U-HO1, HDLM-2, and L1236 treated with ruxolitinib, Navitoclax, or in combinations in a selective dose–response plot for 48 h. Data represent one of three independent experiments.
Fig. S3.
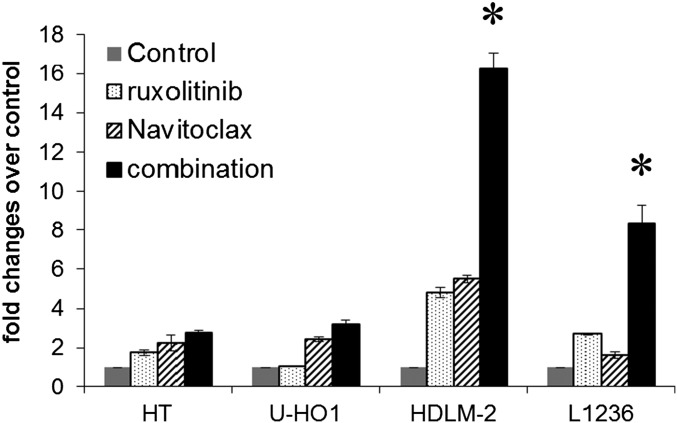
The combination of ruxolitinib and Navitoclax results in enhanced activation of caspase-3/7. Caspase-3/7 activities were measured by using the Caspase-Glo assay kit (Promega) at 48 h after different treatments (ruxolitinib 400 nM and Navitoclax 200 nM) with multiple cell lines as indicated. The experiments were performed in triplicate and repeated on two separately initiated cultures. Data are presented as mean ± SD. *P < 0.01 compared with single reagent treatment.
Fig. S4.
The combination of ruxolitinib and Navitoclax did not result in significant inhibiting activity in all HL cell lines. HL cell lines were treated with or without ruxolitinib (400 nM), Navitoclax (200 nM), or their combinations for 48 h. The cells were pulsed after 42 h of culture for 6 h with 3H-thymidine. These experiments were performed in duplicate or triplicate in three independent experiments. Data are presented as mean ± SD. Data represent one of the three independent experiments.
Ruxolitinib Combined with Navitoclax and/or BV Enhanced the Antiproliferative Action and Induction of Apoptosis with HDLM-2 Cells.
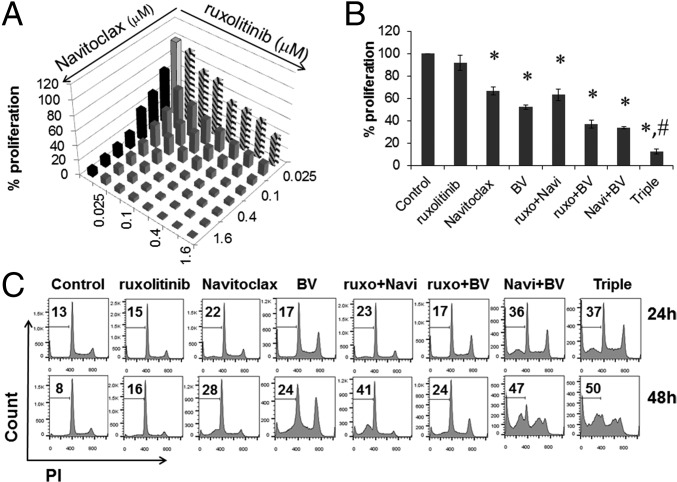
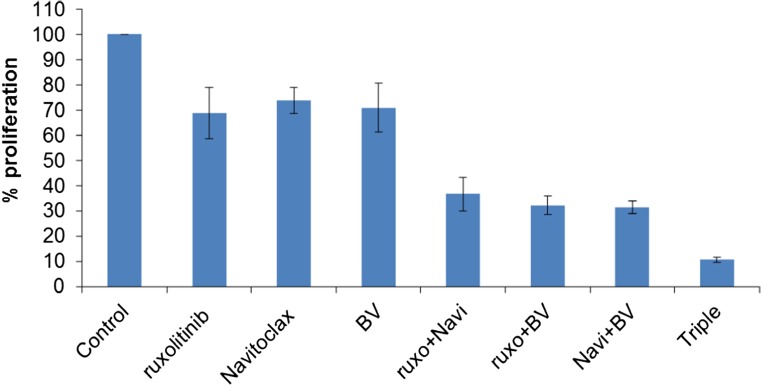
We further investigated the combination of ruxolitinib and Navitoclax at multiple doses and their combination with the JAK inhibition-sensitive HDLM-2 cell line (Fig. 2A). The additive/synergistic efficacy for the inhibition of proliferation was demonstrated again with this cell line. Unfortunately, although ruxolitinib or Navitoclax possessed antiproliferative action in vitro, these reagents alone or their combination did not prevent tumor progression in a xenograft mouse model with HDLM-2 HL (Fig. S5). To identify a more effective therapeutic strategy, we applied a third reagent—BV. BV had antiproliferative action with all HL cell lines (Fig. 1C). Furthermore, the combination of BV with either ruxolitinib or Navitoclax resulted in additive/synergistic cell killing, and the simultaneous application of all three drugs achieved the greatest additivity/synergy (Fig. 2B). This additivity/synergy was reproducible with the L1236 cell line (Fig. S6). To better understand this antiproliferative action, we evaluated cell-cycle changes in HDLM-2 cells with different treatments as indicated by analyzing their DNA content. Previous studies indicated that BV induced G2/M-phase growth arrest and cell death through the induction of apoptosis (27). Compared with untreated cells, cells treated with ruxolitinib, Navitoclax, or BV alone showed an increased percentage of the sub-G1 population; BV alone demonstrated an arrested G2/M population, whereas combinations with BV enhanced the arrest of G2/M population. The two combinations and the triple combination showed additive/synergistic efficacy with an enhanced induction of apoptosis (Fig. 2C).
Fig. 2.
The proapoptotic activity of ruxolitinib, Navitoclax, and BV in combination was additive/synergistic in HDLM-2 cells. (A) Proliferation assay of HDLM-2 cells treated with multiple doses of ruxolitinib, Navitoclax, or combinations for 48 h. (B) Proliferation assay of HDLM-2 cells treated with ruxolitinib (250 nM), Navitoclax (50 nM), BV (100 ng/mL), or their two- or three-agent combinations for 48 h. Data are presented as mean ± SD. *P < 0.001 compared with untreated controls; #P < 0.001 compared with double reagent treatment. (C) DNA content of HDLM-2 cells treated as indicated (ruxolitinib, 250 nM; Navitoclax, 50 nM; and BV, 100 ng/mL) for 24 or 48 h. Data represent one of three independent experiments.
Fig. S5.
In vivo therapeutic study involving the combination of ruxolitinib and Navitoclax did not prevent tumor progression in a murine model of HDLM-2 HL. There were four groups of mice, with three mice per group. Mice of the control group received vehicle alone. Mice of the ruxolitinib group received ruxolitinib at a dose of 50 mg/kg per day by s.c. inserted osmotic pumps for 2 wk. Mice of the Navitoclax group received Navitoclax at a dose of 30 mg/kg per day orally every day for 14 d. Mice of the combination group received a combination of ruxolitinb with Navitoclax at the same doses and dosing schedules as those in the ruxolitinib and Navitoclax groups. Data are presented as mean ± SD. *P < 0.01 compared with control group; #P < 0.05 compared with single reagent treatment groups.
Fig. S6.
Proliferation assay of L1236 cells treated with ruxolitinib (200 nM), Navitoclax (200 nM), BV (2.5 μg/mL), or their two- or three-agent combinations for 48 h. These experiments were performed in triplicate in three independent experiments. Data are presented as mean ± SD.
Combination of Ruxolitinib and Navitoclax and BV Enhanced Apoptosis in HDLM-2 Cells.
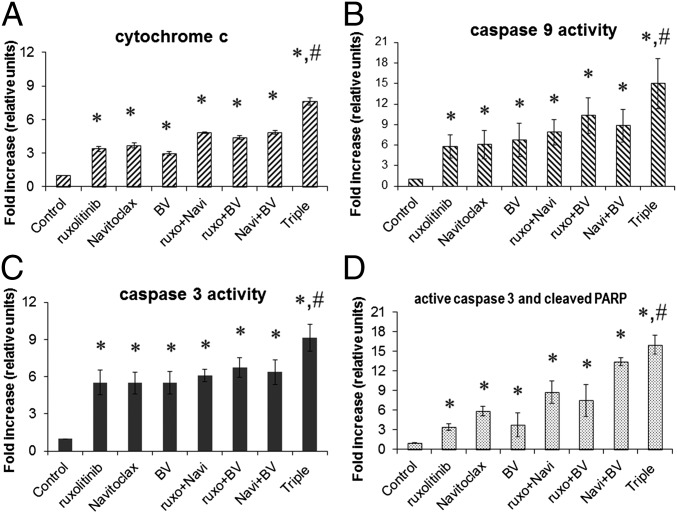
To further understand the additivity/synergy mediated by the combinations in inducing apoptosis of HDLM-2 cells, we investigated the apoptotic related molecules cytochrome c and caspase-9 and -3. In response to apoptotic signals, cytochrome c can be released from mitochondria into the cytosol, which in turn activates an apoptotic program via caspase-driven cascades (28). More specifically, the complex of Apaf 3/caspase-9 is activated with the release of cytochrome c, which leads to the downstream activation of caspase-3, -7, and -9. This process is followed by the activation of additional caspases, including caspase-1, -2, -4, -8, -10, and -13, that ultimately lead to cellular apoptosis (29, 30). After single agent treatment, there is a modest 3- to 3.7-fold increase in cytochrome c, a fivefold to sevenfold increase with caspase-9, and a fivefold increase with caspase-3. Higher increases in these apoptotic elements were seen with two- and three-drug combinations (Fig. 3 A–C). The double-positive population of active caspase-3/cleaved poly(ADP ribose) polymerase (PARP) cells was observed to be modestly increased (threefold to sixfold) in single agent treatment, significantly increased (7- to 13-fold) in two reagent combinations, whereas it was dramatically increased (16-fold) in the triple combination (Fig. 3D). These results indicated that there was enhanced apoptosis mediated by the combinations of the two or three agents with HDLM-2 cells.
Fig. 3.
The combination of ruxolitinib, Navitoclax, and BV resulted in enhanced activation of caspase-3 and -9 and apoptosis in HDLM-2 cells. The concentrations used in the experiments were ruxolitinib, 250 nM; Navitoclax, 50 nM; and BV, 100 ng/mL. (A) Cytochrome c activities were measured after 8 h of treatment as indicated. *P < 0.01 compared with untreated control; #P < 0.01 compared with double reagents treatment. (B) Caspase-9 activities were measured after 48 h of treatment as indicated. *P < 0.01 compared with controls; #P < 0.05 compared with double reagent treatment. (C) Caspase-3 activities were measured after 48 h of treatment as indicated. *P < 0.001 compared with controls; #P < 0.01 compared with double reagent treatment). (D) Cleaved PARP and active caspase-3 were measured by FACS after 48 h of treatment as indicated and analyzed as fold changes. *P < 0.001 compared with controls; #P < 0.05 compared with double reagent treatment. The data are representative of three independent experiments.
Blockade of JAK/STAT Signaling Pathway and Regulation of Bcl-2 Family Members Participated with Antiapoptotic and Proapoptotic Actions and Contributed to Apoptosis of HDLM-2 Cells.
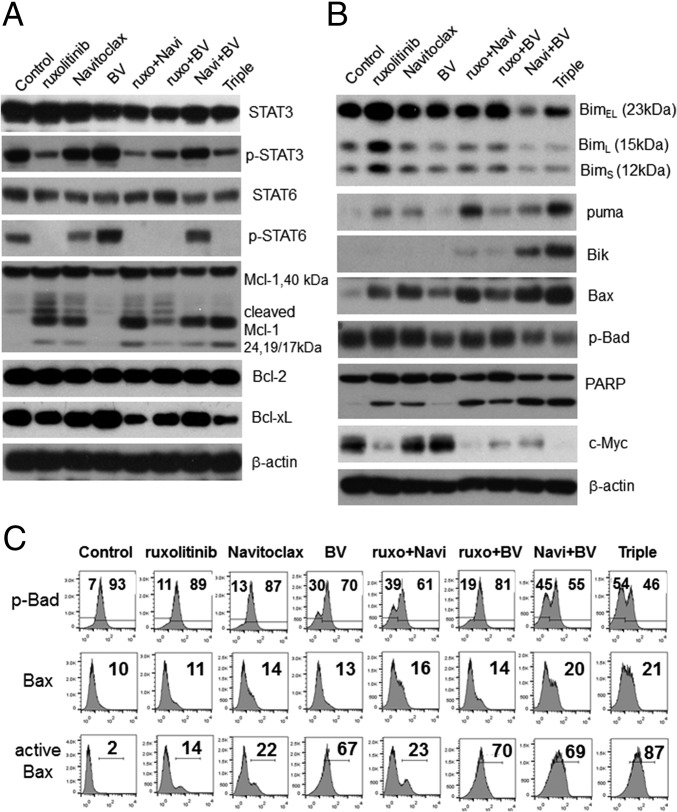
Because the JAK/STAT pathway is constitutively activated in HDLM-2 cells (31), we examined how blockade of this signaling axis by ruxolitinib modified key apoptosis-related Bcl-2 family proteins. As expected, the phosphorylation status of both STAT3 and STAT6 were dramatically reduced with ruxolitinib treatment (Fig. 4A). By directly targeting antiapoptotic Bcl-2 family members Bcl-2 and Bcl-xL, Navitoclax effectively amplifies the apoptotic response in HDLM-2 cells. To further understand the effector mechanism of the combination, Bcl-2 family members that are key elements of the apoptosis machinery were examined. Ruxolitinib, Navitoclax, and the combination of these agents triggered the cleavage of antiapoptotic protein Mcl-1, resulting in 24- and 17/19-kDa products, whereas BV had no effect on the Mcl-1 cleavage, but slightly suppressed its expression. The application of all three drugs alone or in combination did not affect Bcl-2 levels after 48-h treatment; however, ruxolitinib alone or in combination did modestly suppress Bcl-xL levels (Fig. 4A).
Fig. 4.
The combination of ruxolitinib, Navitoclax, and BV triggered the apoptosis pathway and blocked the JAK/STAT pathway in HDLM-2 cells. (A) Western blot analysis of expressions of STAT3/p-STAT3 and STAT6/p-STAT6 as well as antiapoptotic members (Bcl-2, Bcl-xL, and Mcl-1) after 48 h of treatments. (B) Western blot analysis of expressions of proapoptotic proteins (Bim, Puma, Bik, Bax, and p-Bad) as well as caspase cascade molecules (PARP and c-Myc) after 48 h of treatments. β-Actin was used as an input control. (C) Flow-cytometric analysis of intracellular p-Bad, Bax, and active Bax at 48 h after different treatments as indicated: ruxolitinib (250 nM), Navitoclax (50 nM), and BV (100 ng/mL). Numbers in top and bottom panels represent percentage and numbers in middle panel represent median fluorescence intensity (MFI). Data represent one of three independent experiments.
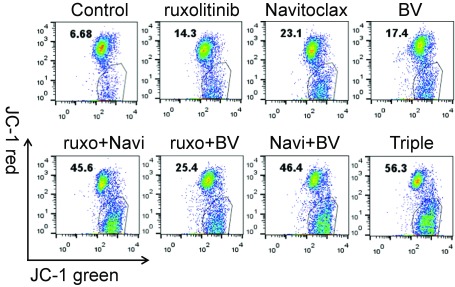
BH3-only members of the Bcl-2 family—which include Bim, Bmf, Bik, Bad, Bid, Puma, Noxa, and Hrk—mediate many developmentally programmed and induced cytotoxic signals (32). The levels of proapoptotic Bim complex of Bim extralong (BimEL), Bim long (BimL), and Bim short (BimS) were increased with ruxolitinib treatment, but decreased after the combination of Navitoclax and BV (Fig. 4B). Puma (33) levels increased after ruxolitinib or Navitoclax single treatment and significantly increased with their combination. Bik was expressed with the combination regimen with the greatest expression detected with the triple combination. Modest down-regulation of phospho-Bad was noted after BV treatment, whereas profound suppression was observed with the triple-combination treatment (Fig. 4 B and C). In response to apoptotic signals, Bax-mediated destabilization may trigger mitochondrial swelling and the release of cytochrome c into the cytosol, which could result in the acceleration of cell death. Some BH3-only proteins (specifically Bim) can directly activate Bax and/or Bad to translocate to the outer mitochondrial membrane and thereby lead to caspase activation. To evaluate the effect of the three reagents and their combinations on this process, we investigated Bax, active Bax (Fig. 4 B and C), and the mitochondrial membrane potential (Fig. S7). Increased Bax and active Bax levels were noted after treatment with each drug individual with profound elevations for all BV-related combination regimens (Fig. 4 B and C). Mitochondrial depolarization was also noted after administration of each drug, with more profound effects noted for the drug combinations (Fig. S7).
Fig. S7.
The combination of ruxolitinib, Navitoclax, and BV triggers a mitochondrial apoptosis pathway in HDLM-2 cells. The mitochondrial membrane potential was measured by the membrane-permeant JC-1 dye staining. After treatment (ruxolitinib, 250 nM; Navitoclax, 50 nM; and BV, 100 ng/mL) for 48 h, the HDLM-2 cells were incubated with JC-1 at a concentration of 10 µg/mL in medium at 37 °C, 5% CO2 for 10 min and then washed with PBS. The samples were collected on a FACScan flow cytometer. Data are representative of three independent experiments.
Cytochrome c release from mitochondria initiates a caspase cascade, which, in turn, cleaves a series of substrates including PARP. PARP is a universal marker of the apoptotic process and one of the main cleavage targets of caspase-3. The cleaved PARP was seen with both ruxolitinib and Navitoclax alone treatments and was further enhanced in the double or triple combinations (Fig. 4B). Furthermore, previous studies demonstrated that Myc, as a major target of JAK2-mediated histone phosphorylation, was silenced after JAK2 inhibition in primary mediastinal B-cell lymphoma and HL (14). We examined the expression of c-Myc, a main member of the Myc family in HDLM-2 cells, and noted a dramatic suppression after ruxolitinib treatment and, moreover, complete elimination with the three-reagent combinations (Fig. 4B). These results confirmed that the inhibition of JAK/STAT signaling pathway with ruxolitinib affected the relative abundance and functional activity of Bcl-2 family members in response to Navitoclax-induced apoptosis and BV-induced cell toxicity.
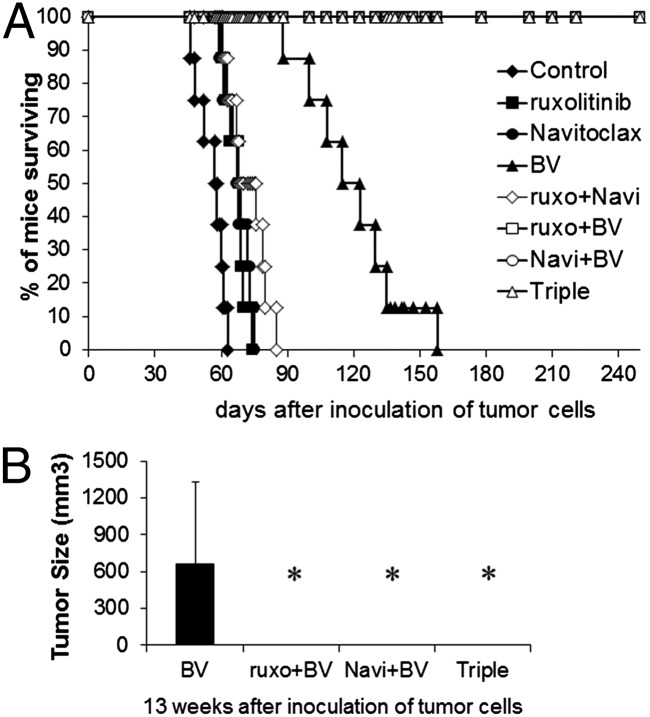
BV Combined with Ruxolitinib, Navitoclax, or Both Led to an Apparent Cure of the HDLM-2 HL.
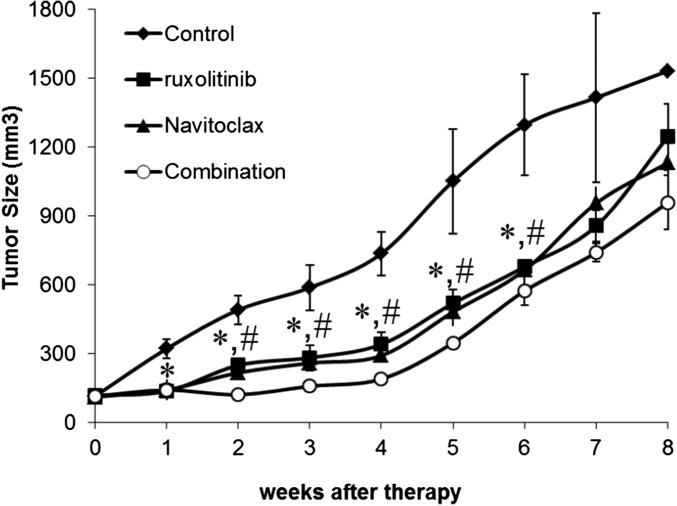
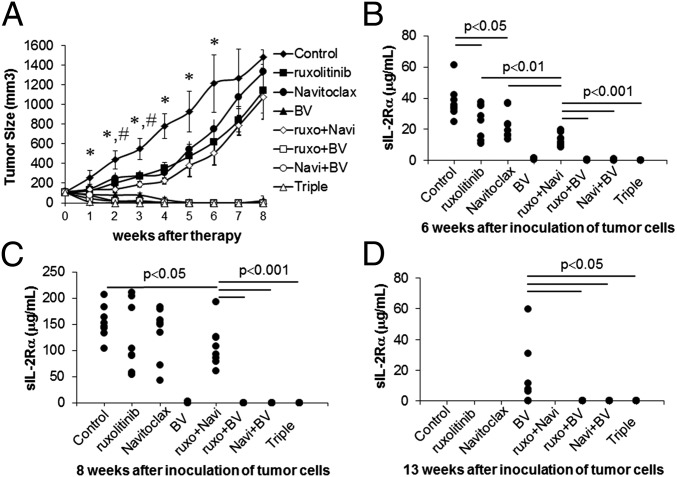
Constitutive activation of JAK/STAT was manifested in HDLM-2 cells that express CD30 on their cell surfaces. We investigated the therapeutic efficacy of all possible combination regimens of ruxolitinib, Navitoclax, and BV in the HDLM-2 xenograft mouse model of human HL. Therapy was initiated when the average volume of s.c. tumors reached 100 mm3. HDLM-2 tumor-bearing mice were randomly divided into eight groups with comparable mean tumor volumes, and the experiment was terminated on day 250 after tumor cell inoculation. Treatment with either ruxolitinib or Navitoclax significantly inhibited the tumor growth as demonstrated by a reduction in tumor volume compared with controls (Fig. 5A; P < 0.01). Although the treatment involving ruxolitinib combined with Navitoclax yielded a greater therapeutic efficacy (P < 0.01) compared with either single agent, HDLM-2 tumors manifested accelerate growth when the treatments were terminated (Fig. 5A). Treatment with BV dramatically inhibited tumor growth compared with the other two single-agent groups (P < 0.01). The BV group appeared tumor-free 5 wk after starting therapy (Fig. 5A). However, tumor recurred in the mice of the BV-treated group 1 mo after tumor initially became undetectable. Treatments with BV combined the other two individual agents or all three together yielded a greater reduction in tumor volume than observed with BV alone. After 4 wk of therapy, the double-combination groups yielded a complete response that was maintained for the 250 d of observation (Fig. 5A). After 10 d of therapy, the triple group yielded a complete response, and the mice remained tumor-free over the same time period.
Fig. 5.
The combination therapy of ruxolitinib, Navitoclax, and BV significantly limited HDLM-2 tumor growth in vivo. (A) Average tumor volumes following the therapeutic course for each group. Data are presented as mean ± SD. *P < 0.001 compared with control; #P < 0.01 compared with single agent treatment. (B) Serum levels of human sIL-2Rα 6 wk after inoculation of HDLM-2 tumor cells. (C) Serum levels of human sIL-2Rα 8 wk after inoculation of tumor cells. (D) Serum levels of human sIL-2Rα 13 wk after inoculation of tumor cells.
Meanwhile, the surrogate tumor marker serum levels of soluble IL-2R alpha (sIL-2Rα) paralleled the clinical observations and aided in prediction of tumor recurrence. Compared with the control group, there were reductions of sIL-2Rα in both ruxolitinib and Navitoclax individual groups (P < 0.05 compared with control group) at 6 wk, but the reductions were no longer evident at 8 or 13 wk after tumor inoculation. There were greater reductions in sIL-2Rα with the combination group (P < 0.01) compared with single-agent treatment groups. Furthermore, very low but detectable levels in the BV group of sIL-2Rα (range 23–2,121 pg/mL) were present at 6 and 8 wk (P < 0.001), which rose by the 13-wk time point. In contrast to other groups, the sIL-2Rα levels in all combination groups involving BV with ruxolitinib and/or Navitoclax became and remained undetectable (compared with BV, P < 0.05; Fig. 5 B–D).
BV in Combination with Ruxolitinib and/or Navitoclax Significantly Inhibited Tumor Growth and Resulted in Complete Tumor Elimination from the HDLM-2 Tumor-Bearing Mice.
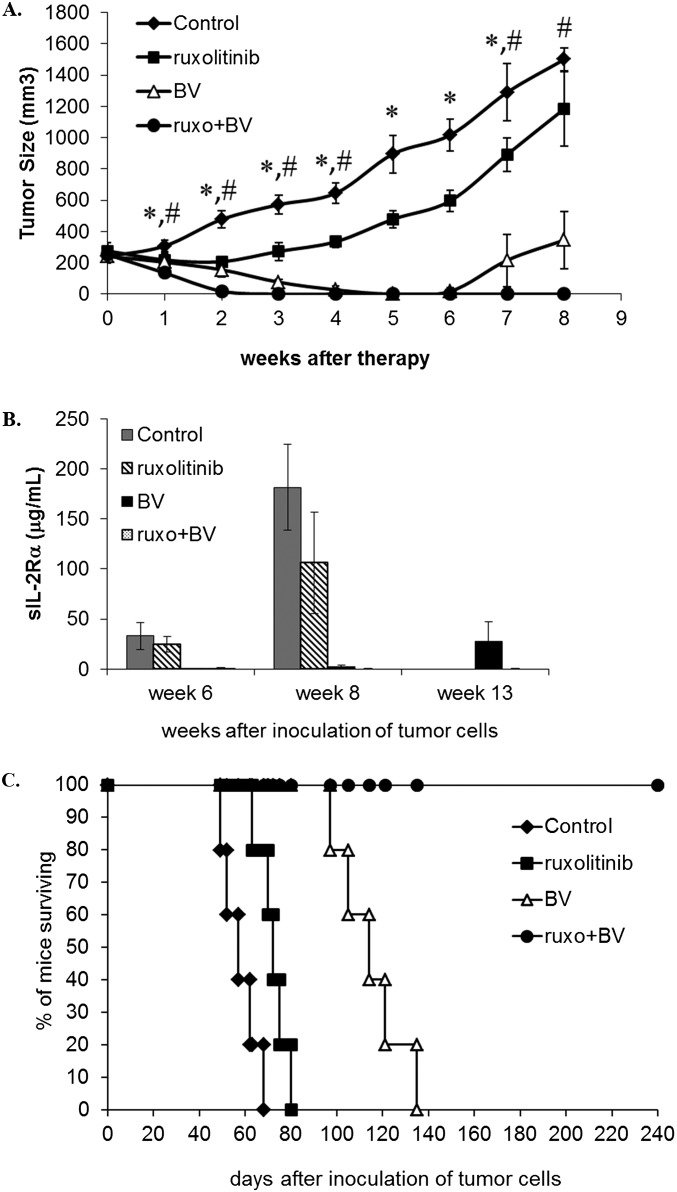
HDLM-2 tumor-bearing mice that received no treatment died between days 46 and 63, with a median survival of 57.5 d. A modest increase in survival was noted after treatments with either ruxolitinib (mice died between days 60 and 74, median survival: 68.5 d, P < 0.001 compared with control), Navitoclax (mice died between days 59 and 75, median survival: 67.5 d, P < 0.001), or the combination (mice died between days 61 and 85, median survival: 72 d, P < 0.001). Treatment with BV yield a greater prolongation of survival (mice died between days 88 and 158, median survival: 119 d, P < 0.0001) (Fig. 6A). At 13 wk after inoculation of tumor cells, the mice treated with BV alone had a tumor recurrence with a measurable tumor mass compared with combination treatment groups with BV (Fig. 6B). All combination-treatment groups with BV manifested a sustained complete response in the tumor-bearing mice that was confirmed by survival of all mice for the total 250-d period of observation (combination vs. BV, P < 0.0001; Fig. 6A). The therapeutic study was repeated with four groups of HDLM-2 tumor-bearing mice that included a control group, ruxonitinib-treated group, BV-treated group, and ruxonitinib plus BV-treated group, respectively. The results of the second study were comparable to those observed in the initial study (Fig. S8).
Fig. 6.
The combination therapy of ruxolitinib, Navitoclax, and BV resulted in a sustained complete response in HDLM-2 tumor-bearing mice. (A) Kaplan–Meier analysis of the survival of the tumor-bearing mice in the therapeutic studies. (B) Average tumor volumes for each group of mice 13 wk after inoculation of tumor cells. Data are presented as mean ± SD. *P < 0.01 compared with BV alone treatment.
Fig. S8.
The repeat therapeutic study demonstrated that the combination of BV and ruxolitinib prevented tumor progression in HDLM-2-bearing mice. In this study, there were five male, 2-mo-old, NOD/SCID mice in each group. Mice of the control group received vehicle alone. Mice of the ruxolitinib group received ruxolitinib at a dose of 50 mg/kg per day by s.c. inserted osmotic minipumps for 2 wk. Mice of the BV group received BV at 4 mg/kg by i.v. injection every 4 d for three injections. Mice of the combination group received a combination of ruxolitinb with BV at the same doses and dosing schedules as those in the ruxolitinib and BV groups. (A) Data are presented as mean ± SD. *P < 0.01 compared with control group; #P < 0.05 compared with single reagent treatment groups. (B) Serum levels of human sIL-2Rα at different time points after inoculation of HDLM-2 tumor cells. Data are presented as mean ± SD. (C) Kaplan–Meier analysis of the survival of the tumor-bearing mice in the therapeutic studies.
Discussion
Recently, there have been major advances in the treatment of HL relapsing after autologous bone marrow transplantation. Amplifications of the 9p24 location in HL caused high amounts of JAK2 and PD-L1 to be made in HL cells (34). Taking advantage of this observation, anti-PD1 immunotherapy has been associated with remissions in refractory and relapsed HL (35). In particular the anti-PD1 monoclonal antibody blocked the immune privilege that PD-L1 confers on HL cells, allowing T cells to kill malignant RS cells (35). Furthermore, the FDA has approved the anti-CD30 toxin conjugate BV for the treatment of relapsed HL after failure of autologous stem cell transplantation. There were 32% CRs, with a median response of 6.7 mo. Nevertheless, because such single-agent therapy is not curative, there is a need for combination therapy involving BV.
In parallel with these clinical observations using a murine xenograft model with the HDLM-2 HL cell line, in the present study we examined the action of BV alone and in selected drug combinations. Single-agent therapy with BV led to an initial marked tumor response, but did not cure the disease, with recurrences developing in each of the 13 mice studied. Therefore, we evaluated the administration of the FDA-approved JAK1/2 inhibitor ruxolitinib, with BV in this model. The scientific basis for the addition of ruxolitinib was that in HL, there is a frequent selective 9p24.1 amplification inducing augmented expression of JAK2―a target of ruxolitinib. Furthermore, the expression of IL-6 and -13 leading to constitutive activation of JAK/STAT signaling has been observed in HRS cell lines. In our two preclinical trials, in the HDLM-2 model, the addition of ruxolitinib to BV led to the long-term persistent elimination of the established HL xenografts. It should be noted that single agent ruxolitinib profoundly inhibited the proliferation of two (L1236 and HDLM-2) of the six HL lines studied. This finding suggests that the development of biomarkers would be of value to differentiate between HL tumors responsive to ruxolitinib from tumors that do not respond. In terms of such biomarkers L1236 and HDLM-2 produced IL-6, -6R, and -13; manifested phosphorylation of STAT3, STAT5, and STAT6; and, as analyzed by Taqman PCR, had evidence for increased JAK2 via chromosome 9p24.1 amplification (Fig. S2). Although the combination of BV and ruxolitinib was sufficient in the HDLM-2 murine model, in refractory and relapsed HL, additional agents would probably be required. To further develop a combination therapy, we evaluated the three-drug combination of BV, ruxolitinib, and the Bcl-2/Bcl-xL inhibitor Navitoclax. Previously we demonstrated that the combination of ruxolitinib and Navitoclax showed additivity/synergy when evaluated in a murine model of ATL. Furthermore, this combination showed additivity with the HL cell line HDLM-2. A number of observations support this combination. When the selective JAK1/2 inhibitor ruxolitinib was examined in a high-throughput matrix screen combined with 1,912 potential therapeutic agents with the cell line HDLM-2, and the Bcl-2/Bcl-xL inhibitor, Navitoclax, was identified as a strong candidate for multicomponent therapy because the drug combination blocked the proliferation and induced cell death of this HL cell line. The Bcl-2 specific inhibitor ABT-199 showed markedly less activity than Navitoclax in the inhibition of the proliferation of both ATL and HL cell lines. In prior studies, the combination of ruxolitinib and Navitoclax induced enhanced permeabilization of the mitochondrial membrane in IL-2–dependent ATL cell lines. Furthermore, in both ATL and HL cell lines, the combination of ruxolitinib and Navitoclax induced caspases-3/-7 activation, which resulted in cleavage of PARP and Mcl-1from its 40-kDa antiapoptotic to a 24-kDa proapoptotic form. Critically, the combination of ruxolitinib and Navitoclax inhibited tumor growth and resulted in prolonged survival of HL tumor-bearing mice. Furthermore, when used in combination with BV, it led to the persistent elimination of tumors.
In summary, the present study demonstrated that the combination of BV with ruxolitinib and Navitoclax or with ruxolitinib alone provided additive/synergistic activity in a mouse-xenograft model of human HL. These findings provide support for a therapeutic trial for select patients with refractory or with relapsed HL that would follow autologous bone marrow transplantation and/or anti-PD1 therapy with a combination regimen that involves BV with ruxolitinib and Navitoclax or ruxolitinib alone.
Materials and Methods
Reagents and Cells.
Ruxolitinib and Navitoclax were purchased from Selleckchem. BV was purchased from Seattle Genetics. Further details are included in SI Materials and Methods.
Cell Proliferation Assay.
Aliquots of 1 × 104 cells were seeded in 96-well culture plates and incubated with medium alone or with serial dilutions of ruxolitinib or Navitoclax or BV or their combinations. The cells were pulsed after 42 h of culture for 6 h with 1 μCi (0.037 MBq) 3H-thymidine. Then, the cells were harvested with a cell harvester (Tomtec) and counted in a MicroBeta2 microplate counter (PerkEmer). These assays were performed in duplicate or triplicate in three independent experiments.
Immunoblotting.
Whole HDLM-2 cell lysates were prepared after 48 h of treatment. Equal protein amounts from each sample were electrophoresed on SDS/PAGE gels and transferred to PVDF membranes (Invitrogen). Proteins were detected by immunoblotting. Further details are included in SI Materials and Methods.
Flow Cytometric Analysis.
After 48 h of treatment, the HDLM-2 cells were washed with cold PBS and fixed with 2% (vol/vol) paraformaldehyde for 10 min. The cells were intracellularly stained, collected on a FACScan flow cytometer (BD Biosciences), and analyzed by using FlowJo cytometry analysis software (Tree Star). Further details are included in SI Materials and Methods.
Measurement of Activities of Caspase-3 or -9 or Cytochrome c.
The caspase-3 and -9 and cytochrome c activities were measured by using caspase-3, -9, and cytochrome c assays from R&D Systems. The experiments were performed in triplicate and repeated on two separately initiated cultures. Further details are included in SI Materials and Methods.
Therapeutic Study in a Mouse Model with HDLM-2 HL.
The xenograft tumor model of human HL HDLM-2 was established by s.c. injection of 2 × 107 HDLM-2 cells into the right flank of female, 8-wk-old, NOD/SCID mice (Jackson Labs). Ten days after injection of the tumor cells, the average tumor volume reached >100 mm3, and the therapy was started. All animal experiments were approved by the National Cancer Institute Animal Care and Use Committee (NCI ACUC) and were performed in accordance with NCI ACUC guidelines. Further details are included in SI Materials and Methods.
SI Materials and Methods
Reagents and Cells.
For in vitro studies, stock solutions of ruxolitinib and Navitoclax were made in DMSO and subsequently diluted in RPMI medium 1640 for use. For in vivo studies, ruxolitinib was dissolved in polyethylene glycol (PEG) 300 (VWR) and continuously administrated via a s.c. miniosmotic pump (Alzet). Navitoclax was formulated in 10% (vol/vol) ethanol, 30% (vol/vol) PEG 400, and 60% (vol/vol) Phosal 50 PG. BV was dissolved in distilled water. Human HL cell lines KM-H2, L428, U-HO1, L1236, L540, and HDLM-2 were from the DSMZ collection and were maintained in RPMI medium 1640 containing 20% (vol/vol) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin in an atmosphere containing 5% CO2. The human B-cell lymphoma cell line HT and the human multiple myeloma cell line L363 were obtained from ATCC and maintained in complete RPMI medium 1640 containing 10% (vol/vol) FBS. All cell lines were authenticated by genomic approaches, including gene-expression profiling and array CGH analysis, as described (14).
Cell-Cycle and Apoptosis Analysis.
Cells were collected and washed with PBS containing 0.5% BSA (PBS/BSA) and 0.02% sodium azide after treatment for 48 h. The cells were fixed with 70% (vol/vol) ethanol on ice for 20 min. Then the cells were incubated with 100 µL (50 µg/mL) of DNase-free RNase (Roche Applied Science) at 37 °C for 30 min and stained with 300 µL (50 µg/mL) of propidium iodide (Roche Applied Science). The DNA content was measured by a FACScan flow cytometer (Becton Dickinson).
Immunoblotting.
Antibodies to phospho-STAT3, total STAT3, phospho-STAT6, total STAT6, Bim, Puma, phospho-Bad (pS112), Bik, Bax, Bcl-xL, Mcl-1, PARP, and β-actin were purchased from Cell Signaling Technology. Antibody to c-Myc was purchased from Abcam. Antibody to Bcl-2 was purchased from Santa Cruz.
Flow-Cytometric Analysis.
Then cells were intracellularly stained with the primary anti-Bax antibody, 6A7 (Santa Cruz), phospho-Bad or Bax (Cell Signaling Technology), or isotype control (2 µg per 100 mL) at room temperature for 45 min. The cells were washed and then stained with a FITC-labeled goat anti-mouse IgG antibody (SouthernBiotech). Active caspase-3 and cleaved PARP were also evaluated by intracellular staining with a phycoerythrin (PE)-conjugated anti-human active caspase-3 and an allophycocyanin (APC)-conjugated anti-human PARP (BD Biosciences). The samples were collected on a FACScan flow cytometer and analyzed. The active caspase-3 and cleaved PARP double-positive population was expressed as fold increase in the treated cells over that of nontreated cells.
Measurement of Activities of Caspase-3 or -9 or Cytochrome c.
After treatments, cell numbers were counted, and the cells were collected by centrifugation. The cell pellet was lysed by lysis buffer at 25 μL of 1 × 106 cells. The cell lysates were incubated on ice for 10 min and then centrifuged. The supernatants were collected and kept on ice. A total of 50 μL of cell lysate was moved into a 96-well flat bottom plate, and then 50 μL of 2× Reaction Buffer 3 or 9, and 5 μL of caspase-3 or -9 colorimetric substrates were added. The plate was incubated at 37 °C for 2 h and then read with a microplate reader. For measurement of cytochrome c activity, the cell pellet was lysed by Cell Lysis Buffer at 1 mL of 1.5 × 106 cells. The cell lysates were incubated at room temperature for 1 h and then centrifuged. The activity was measured using an ELISA kit (R&D Systems) according to manufacturer’s instructions. The activity was expressed as fold increase with the ruxolitinib alone, Navitoclax alone, BV alone, or the combination of two or three of the reagents over that of nontreated cells. The background values were subtracted from the experimental results before calculating the fold induction.
Therapeutic Study in a Mouse Model with HDLM-2 HL.
The HDLM-2 tumor-bearing mice were divided into eight groups, with eight mice per group and with comparable average tumor volumes among groups. Group 1 of mice that received vehicle alone served as a control. Group 2 received ruxolitinib at a dose of 50 mg/kg per day by s.c. inserted osmotic pumps for 2 wk. Group 3 received Navitoclax at a dose of 30 mg/kg per day orally every day for 14 d. Group 4 received BV at 4 mg/kg by i.v. injection every 4 d for three injections. Group 5 received a combination of ruxolitinb with Navitoclax at the same doses and dosing schedules as those in the ruxolitinib and Navitoclax groups. Group 6 received a combination of ruxolitinb with BV at the same dose and dosing schedules as those in the ruxolitinib and BV groups. Group 7 received a combination of Navitoclax with BV. Group 8 received a combination of ruxolitinb with Navitoclax and BV. Throughout the experiments, survival of the mice was recorded and the tumor growth was monitored by measuring tumor size in two orthogonal dimensions. The tumor volume was calculated by using the formula 1/2(long dimension)(short dimension)2. Measurements of the serum concentrations of the surrogate tumor marker human sIL-2Rα were performed by using ELISAs as indicated by the manufacturer (R&D Systems). The therapeutic study was repeated (Fig. S4).
Statistical Analysis.
Serum levels of human sIL-2Rα were analyzed at different time points among different treatment groups by using the Student’s t test for unpaired data. Comparison of cell proliferation, caspase-3 or -9 activity, cytochrome c activity, and tumor size among different treatment groups was analyzed by using Student’s t test. Statistical significance of differences in survival of mice in different groups was determined by the log-rank test using GraphPad Prism program (GraphPad Software).
SI Results
Matrix screening highlights ruxolitinib drug combinations that block proliferation and induce apoptosis in human HL cells. Of particular interest were actions of known JAK inhibitors, including approved drugs ruxolitinib and tofacitinib and investigational drugs AZD-1480, TG101209, Degrasyn, NVP-BSK805, TG-46, SB1518, CEP-33779, AZ 960, TG-101348, LY2784544, and Merck-5. Because no phosphorylation of STATs was detected in U-HO1 cells, it was anticipated that KM-H2, L428, L1236, L540, and HDLM-2 cell lines, but not the U-HO1 cell line, would be sensitive to JAK inhibition. However, the results of these agents showed that all six cell lines responded to Merck 5 [KM-H2: AC50 = 5.9 μM; L428: AC50 = 6.62 μM; U-HO1: AC50 = 1.05 μM; L1236: AC50 = 1.32 μM; L540: AC50 = 2.09 μM; and HDLM-2: AC50 = 1.05 μM], whereas they had different response to the other JAK inhibitors. The KM-H2 cell line responded to TG101209, Degrasyn, and TG-46; L428 cell line responded to TG-101348 and LY2784544; the U-HO1 cell line responded to SB1518, TG101209, Degrasyn, and NVP-BSK805; the L1236 cell line responded to Degrasyn, NVP-BSK805, TG-46, CEP-33779, AZ 960, tofacitinib, and ruxolitinib; the L540 cell line responded to SB1518, Degrasyn, TG101209, NVP-BSK805, and AZ 960; and the HDLM-2 cell line responded to the TG101209, NVP-BSK805, TG-46, CEP-33779, AZD-1480, tofacitinib, and ruxolitinib.
Additive/synergistic effects were demonstrated by using the L1236 cell line with ruxolitinib combined with inhibitors of Bcl-2/Bcl-xL (TW-37, Obatoclax, ABT-737, Navitoclax, and ABT-199), HDACs (Vorinostat, Panobinostat, Pracinostat, and Romidepsin), PI3K (GDC-0941 and GSK-2126458), mTORC1/2 (Torin-1, AZD-8055, and torin-2), Heat Shock Protein 90 (Alvespimycin hydrochloride, CNF-2024, and Geldanamycin), Proteasome (MLN-2238, Carfilzomib, and Bortezomib), and EGFR (HER1; erbB1) (Neratiniband Afatinib). We further evaluated these combination regimens for their capacity to inhibit cell proliferation with the other three HL cell lines: U-HO1, L428, and HDLM-2. The combination regimens showed additive/synergistic effects with the HDLM-2 cell line compared with single drugs alone. Significant additivity/synergy was noted with the cell lines with the combinations of ruxolitinib with Navitoclax, Rapamycin (mTOR inhibitor), Givinostat (HDAC inhibitor), or NVP-BGT226 (PI3K inhibitor).
The cell lines also responded to Bcl-2/Bcl-xL inhibitors, in particular the Bcl-2/Bcl-xL inhibitors including approved drug Obatoclax and investigational drugs Chelerythrine chloride, TW-37, Navitoclax, ABT-737, and ABT-199. The sensitivities of the six HL cell lines varied: KM-H2 cell line responded to Chelerythrine chloride, Obatoclax, and TW-37, with individual AC50 of 2.35, 6.62, and 6.62 μM, respectively. L428 cell line responded to Navitoclax, ABT-737, and Obatoclax, with individual AC50 of 0.47, 4.69, and 5.9 μM, respectively. U-HO1 cell line responded to Chelerythrine chloride and Navitoclax, with AC50 of 0.74 and 0.33 μM, respectively. L1236 cell line responded to Navitoclax, ABT-737, Obatoclax, and TW-37, with individual AC50 of 0.1, 0.93, 5.26, and 3.32 μM, respectively. L540 cell line responded to Chelerythrine chloride, Navitoclax, Obatoclax, and TW-37, with individual AC50 of 4.18, 2.35, 0.83, and 1.32 μM, respectively. HDLM-2 cell line responded to Navitoclax, ABT-737, ABT-199, and Obatoclax, with individual AC50 of 0.08, 0.59, 0.59, and 5.9 μM, respectively.
Acknowledgments
This work was supported by the Division of Preclinical Innovation, National Center for Advancing Translational Sciences; the Intramural Research Program of the National Human Genome Research Institute; and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524668113/-/DCSupplemental.
References
- 1.Chen X, Soma LA, Fromm JR. Targeted therapy for Hodgkin lymphoma and systemic anaplastic large cell lymphoma: focus on brentuximab vedotin. Onco Targets Ther. 2013;7:45–56. doi: 10.2147/OTT.S39107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanzler H, Küppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184(4):1495–1505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batlevi CL, Younes A. Novel therapy for Hodgkin lymphoma. Hematol Am Soc Hematol Educ Program. 2013;2013(1):394–399. doi: 10.1182/asheducation-2013.1.394. [DOI] [PubMed] [Google Scholar]
- 4.Diaz T, et al. Lestaurtinib inhibition of the Jak/STAT signaling pathway in Hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One. 2011;6(4):e18856. doi: 10.1371/journal.pone.0018856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baus D, Pfitzner E. Specific function of STAT3, SOCS1, and SOCS3 in the regulation of proliferation and survival of classical Hodgkin lymphoma cells. Int J Cancer. 2006;118(6):1404–1413. doi: 10.1002/ijc.21539. [DOI] [PubMed] [Google Scholar]
- 6.Staudt LM. The molecular and cellular origins of Hodgkin’s disease. J Exp Med. 2000;191(2):207–212. doi: 10.1084/jem.191.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García JF, et al. Spanish Hodgkin Lymphoma Study Group Hodgkin and Reed-Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood. 2003;101(2):681–689. doi: 10.1182/blood-2002-04-1128. [DOI] [PubMed] [Google Scholar]
- 8.Skinnider BF, et al. Interleukin 13 and interleukin 13 receptor are frequently expressed by Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2001;97(1):250–255. doi: 10.1182/blood.v97.1.250. [DOI] [PubMed] [Google Scholar]
- 9.Kube D, et al. STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001;98(3):762–770. doi: 10.1182/blood.v98.3.762. [DOI] [PubMed] [Google Scholar]
- 10.Hinz M, et al. Nuclear factor kappaB-dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med. 2002;196(5):605–617. doi: 10.1084/jem.20020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapp U, et al. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed-Sternberg cells. J Exp Med. 1999;189(12):1939–1946. doi: 10.1084/jem.189.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joos S, et al. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res. 2000;60(3):549–552. [PubMed] [Google Scholar]
- 13.Van Roosbroeck K, et al. JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood. 2011;117(15):4056–4064. doi: 10.1182/blood-2010-06-291310. [DOI] [PubMed] [Google Scholar]
- 14.Rui L, et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. 2010;18(6):590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derenzini E, et al. The JAK inhibitor AZD1480 regulates proliferation and immunity in Hodgkin lymphoma. Blood Cancer J. 2011;1(12):e46. doi: 10.1038/bcj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes A, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol. 2012;30(33):4161–4167. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waibel M, et al. Combined targeting of JAK2 and Bcl-2/Bcl-xL to cure mutant JAK2-driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell Reports. 2013;5(4):1047–1059. doi: 10.1016/j.celrep.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, et al. JAK2V617F drives Mcl-1 expression and sensitizes hematologic cell lines to dual inhibition of JAK2 and Bcl-xL. PLoS One. 2015;10(3):e0114363. doi: 10.1371/journal.pone.0114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc Natl Acad Sci USA. 2015;112(40):12480–12485. doi: 10.1073/pnas.1516208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dürkop H, et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell. 1992;68(3):421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 21.Younes A. CD30-targeted antibody therapy. Curr Opin Oncol. 2011;23(6):587–593. doi: 10.1097/CCO.0b013e32834bb8a7. [DOI] [PubMed] [Google Scholar]
- 22.Bradley AM, Devine M, DeRemer D. Brentuximab vedotin: an anti-CD30 antibody-drug conjugate. Am J Health Syst Pharm. 2013;70(7):589–597. doi: 10.2146/ajhp110608. [DOI] [PubMed] [Google Scholar]
- 23.Younes A, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 24.Pro B, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 25.Younes A, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mottok A, Renné C, Willenbrock K, Hansmann ML, Bräuninger A. Somatic hypermutation of SOCS1 in lymphocyte-predominant Hodgkin lymphoma is accompanied by high JAK2 expression and activation of STAT6. Blood. 2007;110(9):3387–3390. doi: 10.1182/blood-2007-03-082511. [DOI] [PubMed] [Google Scholar]
- 27.Francisco JA, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 28.Tschopp J, Thome M, Hofmann K, Meinl E. The fight of viruses against apoptosis. Curr Opin Genet Dev. 1998;8(1):82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 29.Pan G, Humke EW, Dixit VM. Activation of caspases triggered by cytochrome c in vitro. FEBS Lett. 1998;426(1):151–154. doi: 10.1016/s0014-5793(98)00330-5. [DOI] [PubMed] [Google Scholar]
- 30.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94(3):325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 31.Cochet O, Frelin C, Peyron J-F, Imbert V. Constitutive activation of STAT proteins in the HDLM-2 and L540 Hodgkin lymphoma-derived cell lines supports cell survival. Cell Signal. 2006;18(4):449–455. doi: 10.1016/j.cellsig.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17(6):617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 34.Green MR, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]